Abstract
An area near the city of Chihuahua has been traditionally irrigated with wastewater to grow forage crops. It has been hypothesized that metal levels could be found in these soils high enough to cause potential health problems to the population. The objective of this study was to determine heavy metal concentrations in different soils due to irrigation practices. Four soil types were evaluated; a soil with a past and present history of wastewater irrigation (S1), a soil with a history of wastewater irrigation until 2003 (S2), a soil with no irrigation history (S3), and a soil similar to S1 and adjacent to the river where the wastewater is transported (S11). Three soil depths were evaluated; 0–15, 15–30 and 30–50 cm. Consequently, a total of 150 soil samples were analyzed evaluating pH, EC, OM and the following elements; Na, K, Cd, Pb, Ni, Cr, Cu and Fe. The pH (P=0.000) and EC (P=0.000) were different for each soil type but no differences were noted for soil depth and the interaction. Maximum pH levels were noted in S3 with a value of 8.74 while maximum EC was observed in S1 with a value of 0.850 dSm−1. The OM level was different for soil type (P=0.000), soil depth (P=0.005) and the interaction (P=0.014). S1 and S11 obtained maximum levels of OM while minimum levels were noted in S3. Maximum OM levels were observed at the 0–15 cm depth followed by the 15–30 cm depth and finally at the 30–50 cm depth. The highest concentration of metals was as follows: K in S1 (359.3 mg kg−1); Cd in S1 (4.48 mg kg−1); Pb in S11 (155.83 mg kg−1); Ni in S1 (10.74 mg kg−1); Cu in S1 (51.36 mg kg−1); B in S3 (41.5 mg kg−1); Fe in S3 (20,313.0 mg kg−1), Cr in S3 (44.26 mg kg−1) and Na in S3 (203.0 mg kg−1). The conclusion is that some metals are present in the soils due to anthropogenic activities but others are present in natural forms.
Keywords: Contamination, metals, environment, soil, wastewater, irrigation
Introduction
Soil is an essential natural resource for support of human life; but with time, its degradation has been constantly increasing due to the deposition of pollutants. The background concentration of metals in virgin soil depends primarily on the bedrock type from which the soil parent material was derived [1]. In addition, anthropogenic inputs may increase metal concentrations, especially in highly industrialized parts of the world producing rare and heavy metals [2]. Until recently, most studies concerning soils related to plant nutrition, with most studies published overseas.
In the particular case of Mexico, one of the environmental problems that require immediate attention is that of metal contaminated soils [3]. Due mainly to industrial activity in recent years, the deposition of a variety of elements in the natural environment has increased. As a result, the concern over water contaminated from human activities has also increased [4]. This concern takes into account a variety of elements referred to in the scientific literature as toxic metals. These metals might be found in waste materials, which in the future will likely be incorporated to the soil resource. The final outcome is an accumulation in the biota, followed by a transfer to humans through the food chain, and causing a potential risk to human health [5, 6]. According to the USDA [7], the accumulation of heavy metals in soil is toxic for humans as well as animals in general.
In agricultural areas, the addition of metals into the system is primarily due to commercial fertilizer applications [8], organic manures [9–11], sediments produced by wastewater [12], irrigation water from industrial and urban areas [13] and atmospheric deposition [14,15]. Still, these results are controversial after researchers like Takeda et al. [4] who did not find an increase of elements in soils with the application of soil amendments and Adeli et al. [16] who concluded that broiler litter application did not threaten the ecosystem. Regardless, once in the soil, contaminants may be further transported throughout the soil profiles to groundwater.
Holmgren et al. [17] created a database after analyzing 3,045 soil samples collected from 307 soil series in the United Sates and found differences between land regions. Dudal et al. [18] calculated the influence of some environmental parameters such as residue incorporation, soil temperature and soil events over the quantity of metals that the organic matter could hold. They found that high precipitation events might be responsible for transferring metals held in the soluble organic matter. In other study, Cuevas and Walter [19] concluded that the mobility and bioavailability of heavy metals were factors that depended largely on physical and chemical soil characteristics. Also, the mobility of metals in soil depended not only on its concentration, but it was affected by soil properties, metal properties and environmental factors [20]. The latter factors are related to processes such as adsorption-desorption, occlusion, and precipitation reactions [21]. Therefore, the metals in soil may accumulate in different forms [20] and are the foremost factors impeding the soil microbial process [22].
Ejido Tabalaopa, an area near the city of Chihuahua, has been producing crops and irrigating the land with water from the Chuviscar River. This river, a tributary of the Río Conchos, contains the wastewater of the city of Chihuahua, a city which has grown in terms of industry and includes a very dynamic human population. Several studies like those of Gutierrez et al. [23], Rubio et al. [24], Holguin et al. [25] and Gutierrez and Borrego [26] reported heavy metal concentrations in the water of the Rio Conchos; thus, we expected contamination of the soil resource being irrigated with this water. Yet, in the Tabalaopa area, there is no information concerning the level of soil contamination as an outcome of wastewater irrigation. The objective of this study was to determine the level of contamination from heavy metals, in four types of soil and at three soil depths. The results offer an overall assessment of the contamination degree of the soil due to irrigation with wastewater. It is also an attempt to understand the accumulation of some elements in soil that are not biodegradable and immobile [27] and its possible transfer to the food chain and persistence in the environment as a whole.
Materials and Methods
The study was conducted during 2006, within Ejido Tabalaopa, an area of 1,094 ha located near the southeastern part of the city of Chihuahua, Mexico. The soil was an Orthid Aridisol with well developed pedogenic horizons, low organic matter in the virgin soil, and dry more than six months a year. An ejido is a community where Mexican law has established that the land must be used collectively or shared. The Ejido Tabalaopa was traditionally used to grow various crops, among them vegetables, until 1996, and using wastewater from the city of Chihuahua to irrigate the land. In 1996, the production of vegetables or any edible crop using wastewater irrigation throughout Mexico was prohibited. At present, this agricultural area is producing different sorts of forage for cattle production and some irrigated land has been abandoned.
Our study area was divided into four zones; a zone having soils with a past and present history of wastewater irrigation (S1), a zone with a history of wastewater irrigation until 2003 (S2), a soil with no irrigation history (S3), and a soil similar to S1 and adjacent to the river where the wastewater is transported (S11). This division was performed using the geographic package Idrisi Kilimanjaro. Using the map included in the package, a total of fifty points were randomly selected resulting in 18 sampling points for S1, 12 sampling points for S11, 10 sampling points for S2 and 10 sampling points for S3. At the Tabalaopa stakeholder′s office, using Autocad software, each site was properly located. At each point, three composite soil samples were taken at 0–15, 15–30, and 30–50 cm depths. Hence, fifty-four soil samples were taken in S1, 36 soil samples in S11, 30 soil samples in S2 and 30 soil samples in S3, totaling 150 soil samples.
The soil was collected in plastic bags and transported to the lab. The samples were dried, ground and passed through a 0.355 mm sieve to remove rocks, roots and any larger particles. The digestion of the soil samples was accomplished with aqua regia following the Analysis protocol of Canada (MAF). The metal concentrations were determined using an Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) Perkin Elmer 2100. The pH and EC values were determined by a saturated paste using a standard glass electrode for pH (Hanna) and a conductivity meter for EC (Hanna). The OM was calculated following the standard methodology. Soil texture was calculated using the Boyoucous method. The statistical model included the zone as factor A with four levels (S1, S2, S3 and S11) and the soil profile as factor B with three levels (0–15, 15–30 and 30–50 cm).
Results and Discussion
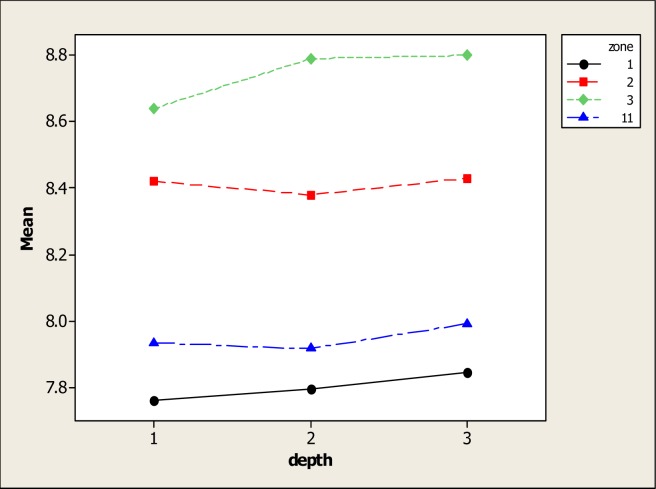
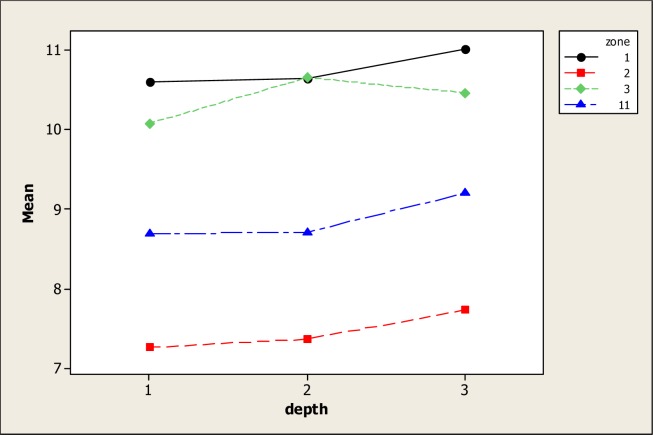
Concerning parameter pH, ANOVA showed statistical differences for soil type (P=0.000) but no differences were found for soil depth and the interaction. It is well known that soil pH plays an important role in the mobility of metals as in their bioavailability for plants [28, 29]. Figure 1 shows the pH levels in the four types of soil and its noted that high pH values with an average of 8.74 were in S3. Minimum pH values were noted in S11 with 7.93 as well as in S1 with 7.80. In S2 soil, the pH was 8.41. It is also apparent that in the four soil types the pH was moderately alkaline. Additionally, a tendency of the pH to increase with depth was observed even though no statistical differences were found. This tendency of the pH to increase below soil profile disagrees with the findings of Ilg et al. [30] which noted that pH values diminished with increasing depth after phosphorous fertilizations.
Figure 1:
Soil pH in four different types of soil and three depths
The average pH value in the above soil profile (0–15 cm) was 8.11 followed by the 15–30 depth with 8.14 and profile 30–50 with a pH average of 8.18. These results concur with those presented by other researchers [31]. It is generally accepted and well documented that organic matter applications reduce pH levels [32–34] although Percival [9] did not find this effect in a soil amended with sewage sludge as did Lee et al. [35] who found an increase in the surface soil with manure compost applications. Moreover, He and Singh [36] discovered that organic matter applications reduced the pH levels while McLaren et al. [37] found a decrease in the pH levels of four soil types after applications of sewage sludge.
In this study, the minimum pH levels found in S1 and S11 may be explained by the fact that organic matter, in its initial phase forms organic acids; but with time, these acids are consumed or transformed and so the pH tends to increase [38]. Even so, other authors like Lee et al. [35] noted that pig litter applications to a soil in Taiwan tended to increase the pH in the upper soil horizons. These tests confirmed the results of Shen and Shen [39] who also noted pH increases in soil with pig compost applications. The results obtained by Adeli et al. [16] found that broiler litter applications increased the pH values. Interestingly, these researchers also determined that the pH values decreased in soil receiving only commercial fertilizing applications.
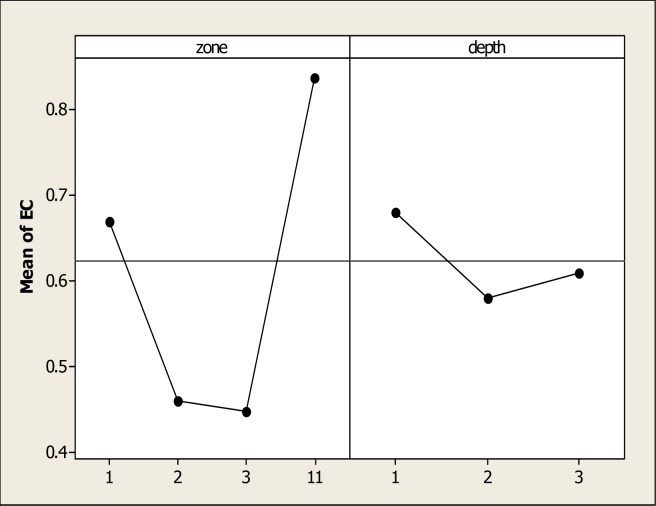
Similar to the results for soil pH, ANOVA detected statistical differences for parameter EC for soil type (P=0.000) but no differences were found for soil depth or the interaction. In Figure 2, the main effects of the EC values are observed, and it is evident that S11 obtained maximum levels while the minimum values were noted in S3 with a mean of 0.44 dSm−1. It is well documented that OM may increase the levels of EC in soils and the results of this study showed the maximum amount of the OM in S1. Abbaspour et al. [40] reported an increase in levels of EC from 2.3 to 2.7 dSm−1 when 50 mmol NaCl−1 was applied to the soil. It is important to acknowledge that the four zones showed acceptable levels of salinity. The literature has reported that high salinity levels may have increased the metal mobility in soils. This process is complete when some cations like Na and K, substitute the heavy metal cations in the absorption places.
Figure 2:
Levels of electrical conductivity (dSm−1) in four different types of soil and three depths
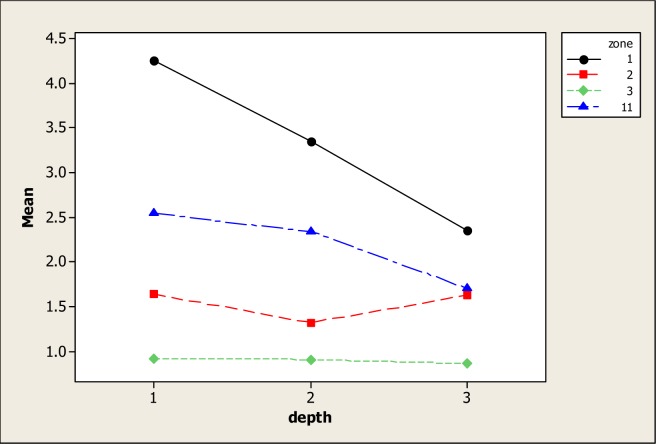
Soil OM is a complex and heterogeneous medium that consists of both fractions; the humus fraction (humic acid, fulvic acid, humin) and the particulate fraction, known as particulate organic matter [41]. In this study, there were statistical differences in OM for soil type (P=0.000), soil depth (P=0.005) and for the interaction (P=0.014). OM is critical because of its tendency to form metal composite OM-metals [42, 43]. Previous studies have demonstrated that OM contains functional groups like –COOH and –OH that serve as a union of OM with metals [44, 45]. Other studies have exemplified the increment of OM levels in soils with the application of organic matter [46–49], resulting in the improvement of the soil structure [50]. Figure 3 identifies the different levels of OM, according to soil type. As expected, S1 and S11 obtained maximum levels of OM with means of 3.31 and 2.19 mg kg−1 in comparison with S2 and S3, which presented means of 1.52 and 0.89 mg kg−1, respectively. For S1 and S11, a negative linear tendency of OM content as affected by soil profile was clear. The maximum amount of OM was found in the 0–15 cm soil profile with a mean of 2.65 mg kg−1, followed by the soil profile 15–30 cm with an OM mean of 2.21 mg kg−1, and finally, the soil profile 30–50 cm with a mean of 1.75 mg kg−1. It is essential to point out that OM has proven the most important soil component controlling the sorption and desorption of metals, being most clear this effect in the metal cadmium [51].
Figure 3:
Soil OM in four different types of soil and three depths
The K concentration was different for soil type (P=0.000) and no differences were found for soil depth or the interaction. Maximum K levels were noted in S1 with 359.3 mg kg−1 and S11 with 349.2 mg kg−1 when compared to levels of 321.8 mg kg−1 for S2 and 331.0 mg kg−1 for S3. Even though there were no statistical differences in soil depth, a decrease in K was observed in the way the soil horizon was lower. Lee et al. [35] in a study with pink compost applications, found the maximum K concentration in the higher soil horizons. The results of these authors agree with those of Bulluck et al. [52] who recorded K increments in the above soil profile after bovine cattle manure applications. In this research, soils S1 and S11 are obviously receiving this element in a dynamic form, hence their presence in the above strata.
The amount of Na was different for soil type (P=0.000) but no differences were noted for soil depth or the interaction. The highest level of this element was found in S3 with 203.0 mg kg−1 while the lowest amount was noted in S1 with 197.6 mg kg−1 and S11 with 197.8 mg kg−1. The Na levels in soil depth were similar and no tendency was noted, obtaining 199.7 mg kg−1 in the 0–15 cm depth, 199.6 mg kg−1 in the 15–30 cm depth and 198.9 mg kg−1 in 30–50 cm soil profile.
The levels of Cd were different for soil type (P=0.000) but not differences were noted for soil depth and the interaction. The lowest levels of Cd were in S2 with 1.62 mg kg−1 while maximum levels were obtained in S1 (4.88 mg kg−1) and S3 (4.41 mg kg−1). The Cd level found in S3 which identified a soil under natural condition is higher than the results presented by Rubio et al. [53] in a study of natural soil also in the state of Chihuahua. As well, the results obtained in the present study were higher than those presented in soils of India with levels of 0.37 mg kg−1[54], England with 0.8 mg kg−1[55], China with 0.07 mg kg−1[56], Spain with amounts in the range of 0.4–0.8 mg kg−1[57], Germany with levels in a range of 0.6–1–4 mg kg−1[58] and Japan with 0.41 mg kg−1[59]. Although no statistical differences were noted for soil depth, a slight decrement of Cd amounts in lower soil profiles was observed. These results may be explained by the fact that Cd is more soluble and mobile in soil when compared with other elements [60, 61]. Plus, OM levels in soil are the most important component that controls the absorption and desorption processes [51]. Yet, researchers like McLaren et al. [62] reported that Cd was the element less mobile when compared to other elements like Cr, Cu and Pb. These researchers explained their results by which OM influences the soil environment. Our original hypothesis was that differences will be evident in S1 and S3; one with wastewater irrigation and the other natural soil without any irrigation background. It was surprising that both soils presented similar levels of Cd. So, it is assumed that the Cd in the natural soil is coming from natural sources or due to anthropogenic pollution from sources other than irrigation. Near the study area, one of the larger smelter/refinery complexes in the world, named Avalos, operated for approximately 90 until the past several years when it ceased to operate, and we assumed that the amount of Pb in S3 might be explained by that fact. To date, there are no geological studies to acknowledge the Cd levels in the zone; however, the results of this study are higher than other results overseas (<1.0 mg kg−1) for a mineral soil [63].
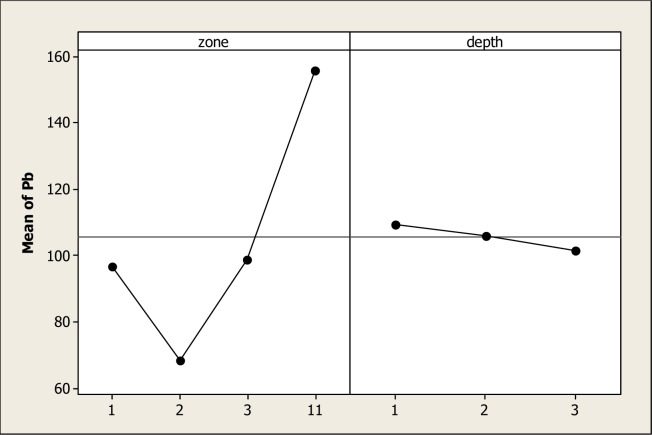
Figure 4 shows the main effects of Pb in the four soil types and in the three depths. ANOVA detected statistical differences for soil type but not differences for soil depth and the interaction. In Figure 4, it is evident that the greatest amount of Pb was in S11 with a mean of 155.83 mg kg−1. Contrary to our original hypothesis, S2 obtained the lowest Pb concentration with a mean of 68.47 mg kg−1, followed by S3 with 98.75 mg kg−1. The results in S2 might be explained by the lixiviation of the element in soil due to precipitation events after wastewater irrigation ceased [64, 65]. The presence of Pb in S3 may be explained by the background of the smelter/refinery complex (Avalos); in fact, Ornelas et al. [66] reported values of 400 ppm of this element in the vicinity of the complex. We must make clear that in 1994, Mexico produced 163,700 tons of lead [67] and was considered at the top of the world′s countries in lead production and the State of Chihuahua was considered the major producer in Mexico [68]. Studies of Pb pollution have reported accumulation of this metal in soils even far from the source [69]. For instance, Billett et al. [70] found significant increments in forestry soils where the contamination source was about 120 km away. Ultimately, the levels of Pb reported in this study must be regarded as high, when considering that a normal soil contains approximately 10 mg kg−1, but some contaminated soils may contain 1,000 mg kg−1[71]. A tendency of the Pb levels to decrease with depth was observed. These results support the hypothesis proposed by Miller and Friedland [72] which established that colloidal particles are of great importance in the Pb movement from the upper to lower soil horizons. This study has noted that all levels of OM were higher in the upper soil horizon and possibly, the levels in the lower soil horizons are a result of this movement [73]. The concentrations obtained in this study are higher than those reported by Rubio et al. [53] in Chihuahua, Mexico; likewise, in soils of India with 10.4 mg kg−1[54] and Japan with 21 mg kg−1[59]. It must be known that high levels of Pb in soils may produce a significant decrement on the enzymatic activity, meaning that this metal has a toxic effect in the different biochemical reaction occurring in the soil environment [22, 74–76]. Accordingly, plant productivity and microbiological communities in the soil environment decrease [77].
Figure 4:
Soil Pb levels in four different types of soil and three depths
In the particular case of Ni, ANOVA detected statistical differences by soil type (P=0.000) but no differences were found for soil depth or the interaction (Figure 5). Again, the highest level of Ni was observed in the 30–50 cm depth (9.81 mg kg−1) with the exception of soil S3. These results are consistent with those of McLaren et al. [62] who found that about 57% of Ni applications were lixiviated from upper soil horizons to lower soil horizons. The lowest Ni concentrations were detected in S2 with a mean of 7.45 mg kg−1 and S11 with a mean of 8.86 mg kg−1.
Figure 5:
Soil Ni levels in four different types of soil and three depths
The Cr level was superior in S3 with a mean of 44.27 mg kg−1 while the lowest values were noted in S2 with a mean of 37.10 mg kg−1. Intermediate values were observed in S1 with 40.06 mg kg−1 and S11 with 38.74 mg kg−1. It was clear that Cr has a tendency to be increased with depth.
Contrary to the findings of Ni and Cr, the lowest concentrations of Cu were detected in S3 and S2 with means of 7.17 and 10.6 mg kg−1, respectively, while maximum values were noted in S1 with 51.35 mg kg−1 and S11 with 38.61 mg kg−1. Li et al. [78] reported that levels of Cu in soil above 10–25 mg kg−1 might reduce plant productivity. Thus, S1 and S11 might prove toxic for plant production. Besides, the Cu concentration has a tendency to increase with depth. This tendency agrees with a report by Hu et al. [79] that found Cu concentrations in a range of 0.01 to 0.26 mg L−1 in the 20 cm depth and of 0.01 to 0.33 mg L−1 in the 50 cm depth. In other study, McLaren et al. [62] mentioned that Cu soil applications have certain mobility from upper horizons to lower horizons. These authors specified that Cu, Cr and Pb had a mobility of 35% from the above soil profile to deeper soil profiles. These results also agree with Dowdy et al. [80] which speculated that metals could be transported from upper zones to lower zones through macropores present in soils. For this reason, most of the metals evaluated in this research have a tendency to increase in lower horizons and coincide with other studies previously reported [81, 82].
Fe level was different due to soil type (P=0.000) but no differences were found for soil depth or the interaction. The highest levels of Fe were noted in S3 with 20,313.0 mg kg−1 while the lowest amount was observed in S2 with a mean of 17,865.33 mg kg−1. As noted in the other evaluated metals, the levels of Fe also increased with depth. The B concentration was different due to soil type (P=0.000) but no differences were found for soil depth and the interaction. The S3 showed the highest amount of this element with a mean of 41.47 mg kg−1, while the lower amount was detected in S2 with a mean of 14.70 mg kg−1. In contract with other elements, B levels were superior in the upper soil horizon.
Conclusions and Recommendations
The analysis demonstrated that among the variables, soil type was the only showing a statistical difference, which indicates that the resulting concentrations can be largely explained by the type of irrigation the soil had at the time. It was noted that concentrations of nickel, chromium, copper, iron and boron concentrated in deeper soil layers while potassium, sodium, cadmium, and lead showed the opposite effect.
In areas where elements were expected to be present in lesser concentrations as in the case of S3, the opposite effect was observed with respect to S1, S11 and S2. Instead, sodium, cadmium, chrome, iron and boron, showed higher concentrations in S3, which is contradictory to the established hypothesis for being an area lacking in irrigation. This may be explained by natural concentrations of said elements or, in the case of cadmium, by airborne contamination from the Avalos smelter. It was also noted that organic material is an important variable and that it can influence the mobility of metals in those areas where high concentrations such as S1 and S11, which coincide with constant irrigation. Clearly, the area has been constantly exposed to certain health hazardous metals. More attention is recommended, even though at this time a wastewater treatment plant has been built and part treated water is used to irrigate the crops. Still, residual water is used for forage irrigation, and this practice could generate illnesses in the population near the study area.
Acknowledgments
We are deeply grateful with the National Committee of Science and Technology of Mexico (CONACYT) which provided a grant for carrying out the research reported here.
References
- 1.Donahue RL, Miller RW, Shickluna JC. Soils, and introduction to soils and plant growth. Fifth edition. Prentice, Hall, Inc.; Englewood Cliffs, New Jersey: 1983. pp. 1–45. [Google Scholar]
- 2.USGS, United States Geological Survey Statistics and information. 2008. Available in: http://mineral.usgs.gov/minerals/pubs/commodity. Accessed July 2008.
- 3.INE, Instituto Nacional de Estadística. Dirección de investigación en residuos y suelos contaminados. 2004. Available in: www.ine.gob.mx.
- 4.Takeda A, Tsukada H, Nanzyo M, Takaku Y, Uemura T, Hisamatsu S, Inaba J. Effect of long-term fertilizer application on the concentration and solubility of major and trace elements in a cultivated andisol. J. Soil Science. Plant Nutr. 2005;51(2):251–260. [Google Scholar]
- 5.Arnt J, Rudnitski K, Schmidt B, Speelman L, Nobouphasavanh S. Environmental risk assessment of spraying landfill leachate on the Guelph Turfgrass Institute (GTI) site: Focus on Pb and As. Earth and Atmosphere Field Camp 87-411. University of Guelph; Guelph, ON: 1997. [Google Scholar]
- 6.Franklin RE, Duis L, Smith BR, Brown R, Toler JE. Elemental concentration in soils of south Carolina. J. Soil Science. 2003;168:280–291. [Google Scholar]
- 7.USDA, United States Department of Agriculture Heavy metals soil contamination. 2000. Available in; www.soil.usda..gov/sqi/files/u03d.pdf. Acceded March 8, 2006.
- 8.Gray CW, McLaren RG, Roberts AHC, Condron LM. Sorption and desorption of cadmium from New Zealand soil: effect of pH and contact time. Australian Journal of Soil Research. 1998;36:199–216. [Google Scholar]
- 9.Percival HJ. Soil and soil solution chemistry of a New Zealand pasture soil amended with heavy metal-containing sewage sludge. Australian Journal of Soil Research. 2003;41:1–17. [Google Scholar]
- 10.Payne GG, Martens DC, Winarko C, Perera NF. Availability and form of copper in three soils following eight annual applications of copper. enriched swine manure. J. Environ. Quality. 1988;17:740–746. [Google Scholar]
- 11.Han FX, Kingery WL, Selim HM, Derard PD. Accumulation of heavy metals in a long-term poultry waste-amended soil. Soil Science. 2000;165:260–268. [Google Scholar]
- 12.Assadian NW, Vogel Ch, Sheng Z, Figueroa UV, Palomo M. Heavy metal distribution in open canals and drains in the upper Rio Grande Basin. Soil and Sediment Contamination. 2003;12(3):305–323. [Google Scholar]
- 13.Adriano DC. Trace elements on terrestrial environments. 2nd Ed. Springer-Verlag; New York, Berlin, and Heidelberg: 2001. [Google Scholar]
- 14.Blake L, Goulding KWT. Effects of atmospheric deposition, soil pH and acidification on heavy metals contents in soils and vegetation of semi-natural ecosystems at Rothamsted Experimental Station, UK. Plant Soil. 2002;240:235–251. [Google Scholar]
- 15.Senesi GS, Baldassarre G, Senesi N, Radina R. Trace elements inputs into soils by anthropogenic activities and implications for human health. Chemosphere. 1999;39:343–377. doi: 10.1016/s0045-6535(99)00115-0. [DOI] [PubMed] [Google Scholar]
- 16.Adeli A, Sistani KR, Tewolde H, Rowe DE. Broiler litter application effects on selected trace elements under conventional and no-till system. Soil Science. 2007;172(5):349–365. [Google Scholar]
- 17.Holmgren G, Meyer MW, Chaney RL, Daniels RB. Cadmium, lead, zinc, copper and nickel in agricultural soils of the United States of America. J. of Environ. Quality. 1993;22:335–348. [Google Scholar]
- 18.Dudal Y, Sévenier G, Dupont L, Guillon E. Fate of the metal-binding soluble organic matter throughout a soil profile. J. Soil Science. 2005;170:707–715. [Google Scholar]
- 19.Cuevas G, Walter I. Metales pesados en maíz (Zea maiz L) cultivado en un suelo enmendado con diferentes dosis de composta de lodo residual. Rev. Internacional de contaminación ambiental. 2004;5:19–21. [Google Scholar]
- 20.He ZL, Zhang MK, Calvert DV, Stofella PJ, Yang XE, Yu S. Transporte de metales pesados en el escurrimiento superficial de campos de vegetales y cítricos. J. Soil Science. Soil & Water Management & Conservation. 2004;68:1662–1669. [Google Scholar]
- 21.Li J, Rate AW, Gilkes RJ. Fractionation of trace elements in some non-agricultural Australian soils. Australian Journal of Soil Research. 2003:1389–1402. [Google Scholar]
- 22.Stuczynskiel TI, McCarty GW, Siebielec G. Response of soil microbiological activities to cadmium, lead, and zinc salt amendments. J. of Environ. Quality. 2003;32:1346–1355. doi: 10.2134/jeq2003.1346. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez LR, Rubio-Arias H, Quintana R, Ortega JA, Gutierrez M. Heavy metals in water of the San Pedro River in Chihuahua, Mexico and its potential health risk. International Journal of Environmental Research and Public Health. 2008;5(2):91–98. doi: 10.3390/ijerph5020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio AH, Wood K, Alanis HE. Water pollution in the Rio Conchos of Northern Mexico. Develpment and application of computer techniques to Environmental Studies. Editores; G. Latini, G. Passerini y C.A. Brebbia. Witpress. 2004:167–176. [Google Scholar]
- 25.Holguín C, Rubio AH, Olave MA, Saucedo TR, Gutiérrez M, Bautista R. Calidad del agua del Río Conchos en la región de Ojinaga, Chihuahua: Parámetros fisicoquímicos, metales y metaloides. Universidad y Ciencia. 2006;22(1):51–63. [Google Scholar]
- 26.Gutierrez M, Borrego P. Water quality assessment of the Rio Conchos, Chihuahua, Mexico. Environmental International. 1999;25(5):573–583. [Google Scholar]
- 27.Mermut AR, Jain JC, Song L, Kerrich R, Kozak L, Jana S. Trace element concentration of selected soils and fertilizers in Saskatchewan. Canadian Journal of Environ. Quality. 1996;25:845–853. [Google Scholar]
- 28.Gambrell RP. Trace and toxic metals in wetlands: A review. J. Environ Quality. 1994;23:883–891. doi: 10.2134/jeq1994.00472425002300050005x. [DOI] [PubMed] [Google Scholar]
- 29.Sparling DW, Lowe TP. Metal concentrations in aquatic macrophytes as influenced by soil and acidification. Water Air Soil Pollut. 1998;108:203–221. [Google Scholar]
- 30.Ilg K, Siemens J, Kaupenjohann M. Colloidal and dissolved phosphorous in sandy soils as affected by phosphorous saturation. J. of Environ. Quality. 2005;34:926–935. doi: 10.2134/jeq2004.0101. [DOI] [PubMed] [Google Scholar]
- 31.Smith LM, Hall KJ, Lavkulich LM, Schreier H. Trace metals concentrations in an intensive agricultural watershed in British Columbia, Canada. Journal of the American Water Resources Association. 2007;2007;43(6):1455–1467. [Google Scholar]
- 32.Chang C, Sommerfeldt TG, Entz T. Rates of Soil Chemical Changes with 11 Annual Applications of Cattle Feedlot Manure. Canadian Journal of Soil Science. 1990;70:673–681. [Google Scholar]
- 33.Couillard D, Li JF. Assessment of Manure Application Effects Upon the Runoff Water Quality by Algal Assays and Chemical Analyses. Environmental Pollution. 1993;80(3):273–279. doi: 10.1016/0269-7491(93)90048-s. [DOI] [PubMed] [Google Scholar]
- 34.Karthikeyan KG, Kalbasi M, Miller PS. Nitrogen and Solution Dynamics in Soils Receiving Chemically Treated Dairy Manure. Transactions of the ASAE. 2005;48:601–610. [Google Scholar]
- 35.Lee ChH, Wu MY, Asio VB, Chen ZS. Using a soil quality index to assess the effects of applying swine manure compost on soil quality under a crop rotation system in Taiwan. Soil Science. 2006;171(3):210–222. [Google Scholar]
- 36.He QB, Singh BR. Effect of organic matter on the distribution, extractability and uptake of Cd in soils. J. Soil Science. 1993;44:641–650. [Google Scholar]
- 37.McLaren RG, Clucas LM, Taylor MD, Hendry T. Leaching of macronutrients and metals form undisturbed soils treated with metal-spiked sewage sludge. 2. Leaching of metals. Australian Journal of Soil Research. 2004;42:459–471. [Google Scholar]
- 38.Simandi P, Takayanagi M, Inubushi K. Changes in the pH of two different composts are dependent on the production of organic acids. Soil Science and Plant Nutrition. 2005;51(5):771–774. [Google Scholar]
- 39.Shen QR, Shen ZG. Effects of pigs manure and wheat straw on growth of mungbean seedlings grown in aluminum toxic soil. Bioresour. Technol. 2001;76:235–240. doi: 10.1016/s0960-8524(00)00109-7. [DOI] [PubMed] [Google Scholar]
- 40.Abbaspour A, Kalbasi M, Hajrasuliha S, Golchin A. Effects of plant residue and salinity on factions of Cadmium and Lead in three soils. Soil and Sediment Contamination. 2007;16(6):539–556. [Google Scholar]
- 41.Elliott ET. Rationale for developing bioindicators of soil health. In: Pankhurst CE, Doube BM, Gupta VVSR, editors. Biological Indicators of Soil Health. Commonwealth Agricultural Bureau, International. Wallingford; Oxon, UK: 1997. pp. 49–78. [Google Scholar]
- 42.Halim M, Conte P, Piccolo A. Potential availability of heavy metals to phytoextraction from contaminated soils induced exogenous humic substances. Chemosphere. 2003;52:265–275. doi: 10.1016/S0045-6535(03)00185-1. [DOI] [PubMed] [Google Scholar]
- 43.Almas AR, Mc Bride MB, Singh BR. Solubility and lability of cadmium and zinc in two soils treated with organic matter. Soil Science. 2000;165:250–259. [Google Scholar]
- 44.Heredia W, Peirano P, Borie G, Aguilera M. Soil organic matter-metal interactions in Chilean volcanic soils under different agronomic management. Commun Soil Sd Plant Anal. 2002;33:2083–2099. [Google Scholar]
- 45.Weng LP, Temminghoff EJM, Lofts S, Tipping E, Van Riemsdijk WH. Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ Sd Technol. 2002;36:4804–4810. doi: 10.1021/es0200084. [DOI] [PubMed] [Google Scholar]
- 46.Warman PR. Soil fertility, yield and nutrient contents of vegetable crops after 12 years of compost amendments. Biological Agriculture and Horticulture. 2005;2005;23:85–96. [Google Scholar]
- 47.Crecchio C, Curci M, Mininni R, Ricciuti P, Ruggiero P. Short-term effects of municipal soil waste compost ammendments on soil carbon and nitrogen content, some enzyme activities and genetic diversity. Biology and Fertility of Soils. 2001;34:311–318. [Google Scholar]
- 48.Garcia-Gill JC, Plaza C, Soler-Rovira P, Polo A. Long-term effect of municipal waste compost application on soil enzyme activities and microbial biomass. Soil Biology and Biochemistry. 2000;32:1907–1913. [Google Scholar]
- 49.Gagnon B, Lalande R, Fahmy S. Organic matter and aggregation in a degraded potato soil as affected by raw and composted pulp residue. Biology and Fertility of Soil. 2001;34:441–447. [Google Scholar]
- 50.Shepherd MA, Harrison R, Webb J. Managing soil organic matter - implications for soil structure on organic farms. Soil Use and Management. 2002;18:284–292. [Google Scholar]
- 51.Gray CW, McLaren RG, Roberts AHC, Condron LM. The effect of long-term phosphatic fertilizer applications on the amounts and forms of cadmium in soils under pasture in New Zealand. Nutrient Cycling in Agroecosystems. 1999;54:267–277. [Google Scholar]
- 52.Bulluck LR, III, Brosius M, Evanylo GK, Ristaino JB. Organic and synthetic fertility amendments influence soil microbial physical and chemical properties on organic and conventional farms. Appl. Soil Ecol. 2002;19:147–160. [Google Scholar]
- 53.Rubio AH, Saucedo TR, Bautista R, Wood K, Holguin C, Jimenez J. In: Are crop and range land being contaminated with cadmium and lead in sediments transported by wind from an adjacent contaminated shallow lake? Geo-environment and Landscape Evolution II. Martin.Duque JF, Brebbia CA, Emmanouloudis DE, Mander U, editors. Witpress; 2006. pp. 135–141. [Google Scholar]
- 54.Roychowdhury T, Uchino T, Tokunaka H, Abd Ando M. Arsenic and other heavy metals in soils from an arsenic-affected area of West Bengal, India. Chemosphere. 2002;49(6):605–618. doi: 10.1016/s0045-6535(02)00309-0. [DOI] [PubMed] [Google Scholar]
- 55.McGrath SP, Loveland PJ. Heavy metals in soils. In: Alloway BJ, editor. In the soil geochemical atlas of England. Blackie Academic and Professional; Glasgow, Scotland: 1992. [Google Scholar]
- 56.Wang Y, Wei FS, editors. Soil environmental element chemistry. Chinese Environmental Science Press; Peking, People′s Republic of China: 1995. [Google Scholar]
- 57.Gil C, Boluda R, Ramos J. Determination and evaluation of cadmium, lead and nickel in greenhouse soils of Almeria (Spain) Chemosphere. 2004;55(7):1027–1034. doi: 10.1016/j.chemosphere.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Munch D. Soil contamination beneath asphalt roads by polynuclear aromatic hydrocarbons zinc, lead and cadmium. Science of the Total Environments. 1992;126(1–2):49–60. [Google Scholar]
- 59.Akira T, Hirofumi T, Masami N, Yuichi T, Toyokazu U, Shunñichi H, Jiro I. Effect of long-term fertilizer application on the concentration and solubility of major and trace elements in a cultivated andisol. Soil Sci. Plan. Nutr. 2005;51(2):251–260. [Google Scholar]
- 60.Chen ZS, Lee GJ, Liu JC. The effects of chemical remediation treatments on the extractability and speciation of cadmium and lead in contaminated soils. Chemosphere. 2000;2000;41:235–242. doi: 10.1016/s0045-6535(99)00416-6. [DOI] [PubMed] [Google Scholar]
- 61.Massadeh AM, Tahat M, Jaradat QM, Al-Momani IF. Lead and cadmium contamination in roadside soils in Irbid city, Jordan. A case study. Soil Sediment Contamination. 2004;13:347–359. [Google Scholar]
- 62.McLaren RG, Clucas LM, Taylor MD. Leaching of macronutrients and metals from undisturbed soils treated with metal-spiked sewage sludge. 3. Distribution of residual metals. Australian Journal of Soil Research. 2005;43:159–170. [Google Scholar]
- 63.Traina SJ. The environmental chemistry of cadmium. In: McLaughlin MJ, Singh BR, editors. Cadmium in soil and plants. Lower, Academic Publ; Dordrecht, The Netherlands: 1999. pp. 11–37. [Google Scholar]
- 64.Stevens DP, McLaughlin MJ, Heinrich T. 2003: Determining toxicity of lead and zinc runoff in soils: Salinity effects on metal partitioning and on phytotoxicity. Environ Toxicol Chem. 2003;22:2017–3024. doi: 10.1897/02-290. [DOI] [PubMed] [Google Scholar]
- 65.Bongers M, Rusch B, Van Gestel CAM. The effect of counterion and percolation on the toxicity of lead for the springtail Folsomia candida in soil. Environ Toxicol Chem. 2004;23:195–199. doi: 10.1897/02-508. [DOI] [PubMed] [Google Scholar]
- 66.Ornelas HM, Sanin ALH, Diaz-Barriga F, Reza LSA, Romieu I. 2007: Evaluacion de riesgo de intoxicación por plomo en la zona urbana aledaña a una fundidora en Chihuahua, Mexico. Tecnociencia, Chihuahua. 2007;1(1):26–35. [Google Scholar]
- 67.FRD, Federal Research Division, Library of the Congress Mexico - A country study. 1996. Energy and Mining. Edited by Tim L. Merrill and Ramon Miró. http://www.country-data.com/cgi-bin/query/r-8677.html.
- 68.Gobierno del Estado de Chihuahua. Informe de Gobierno. Chihuahua, Chihuahua, Mexico. 2004 [Google Scholar]
- 69.Tyler G. Heavy metals pollute nature-may reduce productivity. Ambio. 1972;1:52–59. [Google Scholar]
- 70.Billett MF, Fitzpatrick EA, Cresser MS. Long-term changes in the Cu, Pb, and Zn content of forest soils organic horizons form North-East Scotland. Water Air Soil Pollut. 1991;59:179–191. [Google Scholar]
- 71.Pichtel J, Kuroiwa K, Sawyerr H. Distribution of Pb, Cd, and Ba in soils and plants of two contaminated sites. Environ. Pollut. 2000;110:171–178. doi: 10.1016/s0269-7491(99)00272-9. [DOI] [PubMed] [Google Scholar]
- 72.Miller EK, Friedland AJ. Lead migration in forest soils: Response to changing atmospheric inputs. Environ. Sci. Technol. 1994;28:662–669. doi: 10.1021/es00053a020. [DOI] [PubMed] [Google Scholar]
- 73.McBride MB, Richards B, Steenhuis T, Russo JJ, Sauve S. Biobility and solubility of toxic metals and nutrients in soil fifteen years after sludge application. Soil Science. 1997;162:487–500. [Google Scholar]
- 74.Tejada M, Hernandez MT, Garcia C. Application of two organic wastes in a soil polluted by lead; Effects on the soil enzymatic activities. J. of Environ. Quality. 2007;36:216–225. doi: 10.2134/jeq2006.0252RA. [DOI] [PubMed] [Google Scholar]
- 75.Chlopecka A, Bacon JR, Wilson MJ, Kay J. Forms of Cadmium, lead, and zinc in contaminated soils from southwest Poland. J. of Environ. Quality. 1996;25:69–79. [Google Scholar]
- 76.Marzadori C, Ciaviatta C, Montecchio D, Gressa C. Effects of lead pollution on different soil enzyme activities. Biol. Fertil. Soils. 1996;22:53–58. [Google Scholar]
- 77.Guiller KE, Witter E, McGrath SP. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils. A review. Soil Biol. Biochem. 1998;30:1389–1414. [Google Scholar]
- 78.Li GC, Wang YP, Chang JM. Heavy metals concentrations in soils of Taiwan. Publication by the Environmental Protection Administration (EPA) of Taiwan; Taipei, Taiwan: 1987. [Google Scholar]
- 79.Hu N, Luo Y, Longhua W, Song J. A field lysimeter study of heavy metals movement down to the profile soils with a multiple metal pollution during chelate-enhanced phytoremediation. International Journal of Phytoremediation. 2007;9(4–6):257–269. doi: 10.1080/15226510701473476. [DOI] [PubMed] [Google Scholar]
- 80.Dowdy RH, Latterell JJ, Hinesly TD, Grossman RB, Sullivan DL. Trace elements movement in an Aeric Ochraquaf following 14 years of annual sludge applications. J. of Environ. Quality. 1991;20:119–123. [Google Scholar]
- 81.Camobreco VJ, Richards BK, Steenhuis TS, Peverly JH, McBride MB. Movement of heavy metals through undisturbed and homogenized soil columns. Soil Science. 1996;161:740–750. [Google Scholar]
- 82.Sidle RC, Kardos L. Transport of heavy metals in a sludge-treated forested area. J. of Environ. Quality. 1977;6:431–437. [Google Scholar]