Abstract
Impaired healing of cortical bone grafts represents a significant clinical problem. Cadaveric bone grafts undergo extensive chemical processing to decrease the risk of disease transmission; however, these processing techniques alter the bone surface and decrease the osteogenic potential of cells at the healing site. Extensive work has been done to optimize the surface of bone grafts, and hydroxyapatite (HAP) and nanotopography both increase osteoblastic differentiation. HAP is the main mineral component of bone and can enhance osteoblastic differentiation and bone implant healing in vivo, while nanotopography can enhance osteoblastic differentiation, adhesion, and proliferation. This is the first study to test the combined effects of HAP and nanotopographies on bone graft healing. With the goal of identifying the optimized surface features to improve bone graft healing, we tested the hypothesis that HAP-based nanotopographic resurfacing of bone grafts improves integration of cortical bone grafts by enhancing osteoblastic differentiation. Here we show that osteoblastic cells cultured on processed bones coated with specific-scale (50–60 nm) HAP nanotopographies display increased osteoblastic differentiation compared to cells on uncoated bone, bones coated with poly-l-lactic acid nanotopographies, or other HAP nanotopographies. Further, bone grafts coated with 50–60-nm HAP exhibited increased formation of new bone and improved healing, with mechanical properties equivalent to live autografts. These data indicate the potential for specific HAP nanotopographies to not only increase osteoblastic differentiation but also improve bone graft incorporation, which could significantly increase patient quality of life after traumatic bone injuries or resection of an osteosarcoma.
Introduction
Cadaveric bone grafts undergo chemical processing and irradiation to reduce the risk of disease transmission. These processing procedures not only render the graft avascular and acellular, but also alter surface characteristics of bone,1,2 and decrease the ability of osteoblasts to adhere and differentiate on the surface.1 Because the surface of cadaveric bone is altered during chemical processing1,2 and surfaces of many synthetic implants are not osteoconductive,3,4 a good deal of research has been focused on surface modification of bone grafts and implants.5–10 Biomaterial surface characteristics that can affect cell behavior include surface topography, chemistry, and energy. Surface topography, that is, the relative roughness and pattern of peaks and valleys at the micro- and nanoscale, can regulate many aspects of cell behavior, including osteoblastic adhesion,11 proliferation,12 and differentiation.13–16 In vitro studies have chemically/mechanically altered surface roughness, which results in enhanced osteoblastic differentiation.6–8 Other studies have focused on the role of hydroxyapatite (HAP), which is one of the most common coatings for bone implants17–19 and results in increased bone formation and healing.9,10 The in vitro and in vivo osteoconductivity of HAP17–21 is likely due in part to the bone biomimetic properties of HAP, since it accounts for 70% of the dry weight of bone.22
To determine the effects of both surface chemistry and specific nanoscale topographies, we examined osteoblastic differentiation on chemically processed bone grafts coated with nanotopographic films composed of either (1) poly-l-lactic acid (PLLA) using a polymer demixing process or (2) HAP using a physical vapor deposition process, an approach that has not previously been taken to coat nonplanar surfaces such as bone. We chose to examine nanotopographies in the range of 10–120 nm, because this range is similar to that of bone surfaces in vivo,12 and has been shown to enhance bone cell, including stem cell, osteoblastic differentiation in vitro.12,15,23,24 We tested the hypothesis that preosteoblastic cells cultured on processed bone surfaces coated with unique nanoscale topographies composed of either PLLA or HAP display increased osteoblastic differentiation, compared to cells cultured on uncoated processed bone. We also tested whether HAP, which has the same surface chemistry as bone, would enhance osteogenesis to a greater degree than polymers that have a different surface chemistry than bone. In vivo healing of bone grafts was also assessed using polymer and HAP nanotopographies that were found to be more osteogenic in vitro, to test the hypothesis that enhanced osteoblastic differentiation in vitro would correlate with enhanced integration of coated graft bone in vivo.
Our results show that 50–60-nm HAP nanotopographies enhance osteoblastic differentiation and bone graft healing with mechanical properties comparable to live autografts. This is the first study to test the effectiveness of HAP coating on bones, with a specific focus on the effect of the nanotopography scale on osteoblastic differentiation and bone graft healing.
Materials and Methods
Bone processing
A 4-mm segment of the mid-shaft was removed from female C57Bl6/J mice femurs, and soft tissue and bone marrow were removed. Bones were processed in 95% ethanol for 3 h, and 100% ethanol for 2 h, as previously described,1 and frozen at −80°C for one week.
Cell culture
Preosteoblastic MC3T3-E1, subclone 4 cells (ATCC No. CRL-2593), were cultured in an osteoblastic differentiation medium α-minimum essential medium, 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 50 μg/mL ascorbic acid, 10 nM dexamethasone, and 10 mM β-glycerol phosphate]. Cells were cultured in 0.05% FBS growth medium for 16 h before culturing on bones. Processed bones were incubated in a growth medium containing 0.05% FBS for 3 h before cell cultures. One million cells were then seeded on to each bone in growth medium with 0.05% FBS for 3 h. After incubation, cells/bones were cultured in a complete osteogenic medium for 14 days.
Polymer demixing and two-stage replication
To make 45-nm topographies, PLLA (Polysciences, Warrington, PA) and polystyrene (Sigma Aldrich, St. Louis, MO) were mixed (70/30 w/w) in chloroform for a total polymer concentration of 2%. One percent PLLA was used as a flat control. Twenty-five-mm-diameter glass coverslips were spin-cast at 4000 rpm for 30 s as previously described.23 After spin casting, the coverslip was coated with polydimethyl siloxane (PDMS) to create a negative, and the PDMS mold was carefully peeled off coverslips.25 Processed bones were briefly dipped in 1% PLLA in chloroform, and the PDMS mold was wrapped around the bone to create a replicon of the original flat or 45-nm nanotopography. The PDMS negative was removed, and the bones were sterilized by ultraviolet light for one hour before use.
Physical vapor deposition of HAP
Calcium phosphate tribasic or HAP powder (Alfa Aesar, Ward Hill, MA) was pressure-compacted into a disk in a circular copper mold and was mounted as a sputtering target in the low-pressure chamber of a pilot plant for direct current/radio frequency (DC/RF)-sputtering plant custom-designed and manufactured by K. J. Lesker (Clairton, PA). Before RF sputtering,26 a physical vapor deposition technique was started, and the base pressure was set at 2×10−6 Torr by a helium-compressor cryopump. During RF sputtering, the sputtering target was cooled by chilled water, and 99.995%-pure argon and 99.994%-pure oxygen were pumped into the chamber. Oxygen was used for a better stoichiometry of the HAP film deposited on the murine femurs.27 Deposition parameters during sputtering were as follows: argon flow rate 140 sccm, oxygen flow rate 10 sccm, chamber pressure 6.4×10−3 Torr, and input power level 140 W. HAP was deposited at a rate of 25–30 nm/h. Murine femurs were mounted at their ends by a vacuum tape to a planar steel platform 150 mm from the sputtering target; after deposition to the desired thickness, each femur was rotated about its longitudinal axis by 180°, and the entire deposition process was repeated. We found that denaturation of murine femurs during long-time sputtering can be avoided by interspersing 90-min sputtering periods and 30-min fallow periods.
Surface characterization
A detailed geometrical surface morphology of the sample was characterized with an atomic-force microscope (Dimension 3100/VEECO). Three-dimensional atomic-force micrographs with topographic information were obtained using a silicon cantilever (PPP-NCH/Nanosensors) under a tapping mode at scan frequencies of 1–2 Hz with a typical resonance frequency around 250 kHz. The sharp tip on the cantilever was scanned laterally across the sample surface, and vertical movement of the tip was continuously recorded by a computer, which used the data to construct a quantitative three-dimensional topographic map.
X-ray photoelectron spectroscopy and Fourier-transform infrared analysis
HAP was sputtered on a polished silicon substrate for characterization, because it is a better choice than glass slides for infrared (IR) waves due to silicon's near transparency in the IR regime.
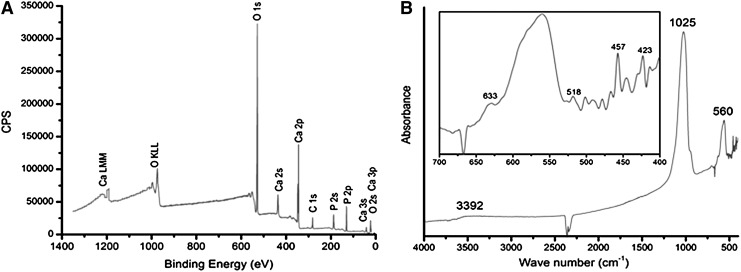
X-ray photoelectron spectroscopy (XPS) was performed using Kratos Ultra XPS with a monochromatic Al Kα source, and the plot of number of electrons detected per second versus the binding energy of electrons is shown in Figure 2A. Fourier-transform infrared (FTIR) spectroscopy was performed using a Bruker IFS 66/S FT-IR Spectrometer to determine the nature of bonds present in the film.
FIG. 2.
(A) Counts per second (CPS) versus the binding energy of electrons collected by the detector in X-ray photoelectron spectroscopy. The peaks have been labeled by the most-likely candidate transition by comparing the peaks with the standard peaks of elements. (B) Absorbance versus the wave number of incident infrared light on the sample. Inset shows a part of the plot in fine detail.
Alkaline phosphatase activity assay
Alkaline Phosphatase (AP) activity was quantified by the conversion of p-nitrophenyl phosphate to p-nitrophenol. Cells were removed from bones using 0.025% trypsin–ethylenediaminetetraacetic acid, and lysed with Triton X. AP buffer [1:1 0.75/M 2-amino-2-methyl-1-propanol (Sigma Aldrich) and 2/mg/mL p-nitrophenol phosphate (Sigma Aldrich)] and 0.1 N sodium hydroxide (NaOH) were added to the cell lysates and incubated at 37°C for 15 min. Absorption was measured at 410 nm, and the enzyme activity was estimated from a p-nitrophenol standard curve. AP activity was normalized to total protein concentration using a detergent-compatible protein assay (Bio-Rad, Hercules, CA).
Bone isograft surgery
All animal experiments were conducted in accordance with Penn State Hershey Institutional Animal Care and Use Committee (IACUC). Ten-week-old female C57Bl6/J mice underwent femoral isograft surgery as described.28 After preparation of the surgical site and incision in skin along the femur, the mid-shaft of the femur was exposed by blunt dissection of muscles. A 4-mm mid-diaphyseal segment of femur was removed as previously described.28 Nanotopography-coated and uncoated grafts were transplanted into recipient mice using a 22-gauge pin in the intramedullary cavity to secure the graft. Autograft recipients underwent the same procedure; however, the 4-mm femoral segment was removed, briefly bathed in warm saline, and reinserted into the same mouse, using a 22-gauge metal pin to secure the graft.
Microcomputed tomography
Grafts within the host bone were scanned at an isometric resolution of 10.5 μm using a vivaCT 40 microCT (Scanco Medical AG, Brüttisellen, Switzerland). Reconstructed images were segmented and analyzed using the Image Processing Library supplied by the manufacturer. Changes in the total callus volume (TCV) and mineralized callus volume (MCV) were quantified for autografts, isografts, and nanotopography-coated grafts. All slices containing graft bone were included in the analysis. Slices were contoured to define the outer border of callus, and to exclude the cortical bone and medullary cavity. All voxels within callus were used to calculate the TCV. Images were thresholded to include soft tissue and cartilage, and the voxels considered mineralized were used to calculate MCV.
Biomechanical torsion testing
After harvest and removal of the intramedullary pin, femurs were potted in square aluminum pots using an automotive body filler (Dynalite; 3M, St. Paul, MN), and rehydrated in phosphate-buffered saline for at least 3 h before testing. Femurs were tested in torsion at a rate of 1°/s until failure using a 177 N-mm load cell. Torsional rigidity was calculated as the slope of the linear region of torque (N*mm) versus rotational deformation (radians), with the latter normalized to the measured gage length (mm).
Statistical analysis
Data are presented as mean±standard error of the mean. Significant differences between groups were determined by nonparametric analysis of variance and Bonferroni post hoc test. Differences were considered significant at p (probability value) <0.05.
Results
Altering the bone surface at the nanoscale
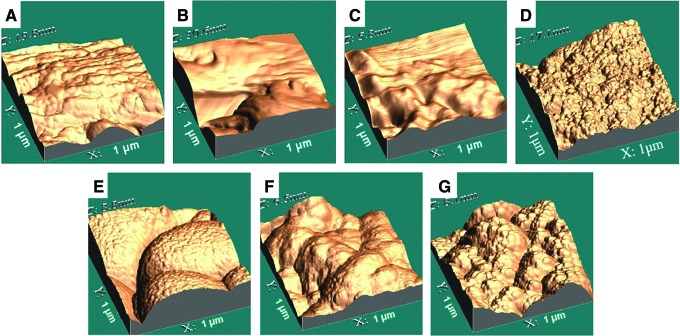
After chemical processing of murine bones with ethanol as previously described,1 physical vapor deposition of HAP and two-stage replication were used to create unique nanoscale topographies on the bone surface. Atomic-force microscopy (AFM) of native bone revealed a very complex surface (Fig. 1A). Coating bone with a flat polymer film resulted in a relatively flat surface at the nanoscale, with native nanotopography of the bone slightly masked (Fig. 1B). Coating bone with a polymer biofilm with 45-nm islands resulted in a surface with easily identifiable nanoislands (Fig. 1C) compared to flat coating.
FIG. 1.
Three-dimensional atomic-force micrographs of (A) processed, uncoated bones, and processed bones coated with (B) flat (0 nm) poly-l-lactic acid (PLLA) nanotopography, (C) 45-nm PLLA nanotopography, (D) 10–15-nm hydroxyapatite (HAP) nanotopography, (E) 25–30-nm HAP nanotopography, (F) 50–60-nm HAP nanotopography, and (G) 100–120-nm HAP nanotopography. Color images available online at www.liebertpub.com/tea
The thickness of the HAP coating was directly proportional to deposition time, and increasing time of deposition also changed specific characteristics of the surface topography (summarized in Table 1). AFM images of HAP nanotopographies in Figure 1D–G showed that the maximum trough-to-crest height was 17.1 nm for 10–15-nm-thick HAP deposit (Fig. 1D), 8.8 nm for 25–30-nm-thick HAP deposit (Fig. 1E), 6.5 nm for 50–60-nm HAP deposit (Fig. 1F), and 8.1 nm for 100–120-nm HAP deposit (Fig. 1G). The anomalous height in Figure 1D can be explained by the fact that very thin HAP deposition was unable to completely hide the rugosity of bone itself. Once the deposit was sufficiently thick, the maximum trough-to-crest heights in Figure 1E–G were not significantly different from one another. However, the top-surface geographies in Figure 1E–G appear very different from each other due to the fractal nature of the coatings formed by physical vapor deposition of HAP. The 25–30-nm-thick HAP deposit in Figure 1E was made of islands of 900 nm in diameter. Primary islands in the 50–60-nm-thick HAP deposit in Figure 1F were decorated with secondary islands of about 200-nm diameter. Tertiary islands, even smaller at about 40 nm in diameter, appeared in the 100–120-nm-thick HAP deposit in Figure 1G. Thus, the doubling thickness of HAP deposited reduced small-scale geography by a factor of about 4.5, indicating that the multiscale surface geography of sufficiently thick HAP deposits had a fractal nature26,29 with a similarity dimension of about (log 4.5)/(log 2)=2.17.
Table 1.
Surface Characterization of Hydroxyapatite Nanotopographies
| Time of deposition | Thickness of hydroxyapatite (nm) | Max. peak-to-trough height (nm) | Diameter of islands (nm) |
|---|---|---|---|
| 30 min | 10–15 | 17.1 | – |
| 1 h | 25–30 | 8.8 | 900 |
| 2 h | 50–60 | 6.1 | 200, 900 |
| 4 h | 100–120 | 8.1 | 40, 200, 900 |
Physical vapor deposition does not alter the composition of HAP
To further characterize surface composition of HAP nanotopographies, the chemical composition of HAP deposited onto murine bones to pure HAP was compared by elemental analysis using XPS.30 Elemental analysis of the top 10 nm of the surface was possible with XPS30; however, XPS cannot directly determine the presence of hydrogen atoms in the film.31 HAP topographies were composed of an equal proportion of calcium and phosphate (15% each), while oxygen made up 60% of the coating. Minimal contamination (10%) with carbon occurred using physical vapor deposition (Fig. 2A).
FTIR was used to determine the nature of chemical bonds in HAP nanotopographic films. Correlation of vibrational modes represented by peaks in absorbance curves obtained with FTIR with those for hydroxyl ions and phosphate ions in pure HAP32 showed the presence of both hydroxyl and phosphate ions in the film (Fig. 2B). However, quantification of elements detected using XPS revealed that the ratio of oxygen to phosphorous is four to one, suggesting that x is slightly <4 in POx ions, because some of the oxygen atoms would be bonded to hydrogen atoms in hydroxyl ions. Additionally, XPS showed that the ratio of calcium and phosphorous atoms was one to one, whereas the same ratio was 10 to 6 in the target. Further, FTIR showed the absence of carbonate ions in the film; therefore, detection of carbon in the film surface by XPS is due to bonding of atmospheric carbon with the surface.
Nanoscale deposition of HAP increases osteogenic differentiation in vitro
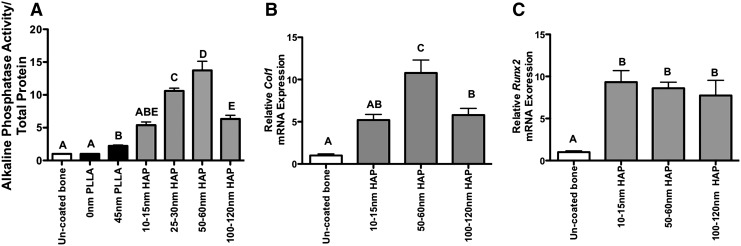
To determine the ability of PLLA or HAP nanotopographies to enhance osteoblastic differentiation of cells on chemically processed bones, bones were coated with either PLLA or HAP nanotopographies as described above and AP activity and expression of markers of osteoblastic differentiation compared to cells grown on uncoated processed bones. Cells cultured on bones with 45-nm PLLA islands had a significant 2.2-fold increase in AP activity compared to cells cultured on bones with a flat polymer nanotopography (p=0.0007) and uncoated bones (p=0.0007). Cells cultured on bones with 50–60-nm islands of HAP had significantly higher levels of AP activity compared to all other groups, with a 13-fold increase over cells on uncoated bones (p=0.0004). Interestingly, cells cultured on 10–15-nm (p=0.004), 25–30-nm (p=0.019), and 100–120-nm (p<0.0001) HAP nanotopographies had a significantly decreased AP activity compared to 50–60-nm nanotopographies, while AP activity on 45-nm PLLA topographies was significantly decreased compared to 25–30-nm HAP (p<0.0001) and 50–60-nm HAP (p=0.0008) (Fig. 3A).
FIG. 3.
(A) Alkaline phosphatase activity of MC3T3-E1 cells cultured on uncoated bones, bones coated with flat (0 nm) or 45-nM biofilms composed of PLLA by two-stage replication, or bones coated with HAP by physical vapor deposition. HAP deposition resulted in nanoisland heights of 10–15, 25–30, 50–60, and 100–120 nm. Alkaline phosphatase activity, a marker of osteoblast differentiation, was quantified and normalized to total protein and alkaline phosphatase activity of cells cultured on uncoated bones. (B) Col1a1 and (C) Runx2 mRNA expression of cells cultured on uncoated and HAP nanotopography-coated bones. Cells were cultured in an osteogenic medium for 14 days. N=4–5/group; different letters indicate p<0.05, while groups with the same letters are not statistically different from each other. Data are presented±standard error of the mean.
Collagen I, a marker of immature osteoblasts, was significantly increased in cells cultured on 50–60-nm HAP topographies compared to all other groups. Col1a1 expression in cells on 100–120-nm HAP was also significantly increased (p=0.004) relative to uncoated bones, but was not different than cells on 10–15-nm HAP (p=0.6, Fig. 3B). Expression of the transcription factor Runx2, which is required for osteoblastic differentiation, was significantly decreased in cells cultured on uncoated bones relative to cells on all HAP nanotopography-coated bones. No differences in Runx2 expression were observed between HAP nanotopography-coated groups (Fig. 3C).
Specific-scale HAP nanotopographies enhance mineralized callus formation during bone graft healing
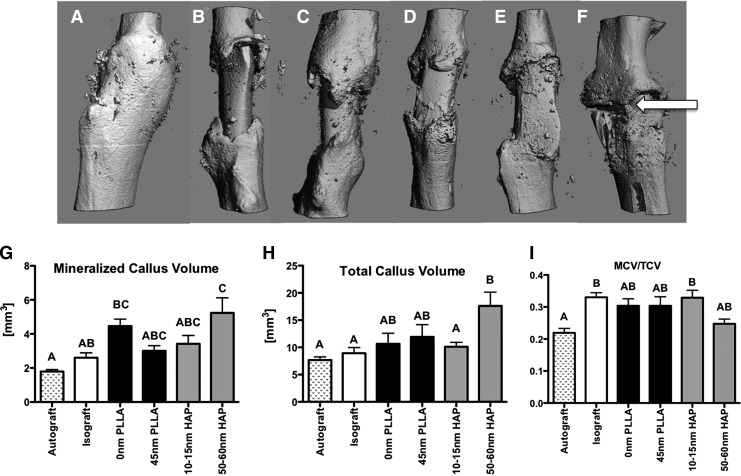
After transplantation, healing was assessed at 42 days postsurgery by microcomputed tomography (μCT). Three-dimensional μCT reconstructions (Fig. 4A–F) show that autografts heal with complete bridging of the callus (Fig. 4A), while uncoated isografts remain poorly healed with limited callus formation (Fig. 4B). Grafts coated with PLLA (0 nm, 45 nm; Fig. 4C, D) displayed formation of a creeping callus that did not bridge the two sides of the host bone, and grafts coated with 10–15-nm HAP also had callus formation that was confined to both graft–host junctions (Fig. 4E). In contrast, grafts coated with 50–60-nm HAP displayed robust callus formation with bridging occurring at sites around graft (white arrow, Fig. 4F).
FIG. 4.
(A–F) Representative three-dimensional microcomputed tomography (μCT) reconstructions of (A) autografts, (B) isografts, or bone grafts coated with (C) 0-nm PLLA, (D) 45-nm PLLA, (E) 10–15-nm HAP, or (F) 50–60-nm HAP nanotopographies at 42 days post-surgery. White arrow indicates bridging of the callus. (G–I) μCT quantification of healing bone grafts. (G) Mineralized callus volume (MCV), (H) Total callus volume (TCV), (I) MCV/TCV. N=6–9/group; different letters indicate p<0.05, while groups with the same letters are not statistically different from each other. Data are presented±standard error of the mean.
To quantify changes in new bone formation, μCT images were semimanually segmented to exclude graft and host bone, while including only mineralized tissue in the healing callus. Autograft MCV was not significantly different than isografts, bones coated with 45-nm PLLA, or bones coated with 10–15-nm HAP. MCV was significantly decreased in autografts compared to bones coated with 0-nm PLLA (p<0.0001) and 50–60-nm HAP (p=0.001). Bones coated with 50–60-nm HAP also had a significant increase in MCV compared to isografts (p=0.003, Fig. 4G).
TCV, a measure of both mineralized and nonmineralized tissue in the callus, was also significantly increased in bones coated with 50–60-nm HAP compared to autografts (p=0.0023), isografts (p=0.027), and bones coated with 10–15-nm HAP (p=0.01). No changes in TCV were observed in bones coated with a flat PLLA nanotopography, or 45-nm PLLA, compared to any other group (Fig. 4H). The MCV/TCV ratio represents the proportion of total callus that is composed of mineralized tissue. MCV/TCV was significantly decreased in autografts compared to isografts (p<0.0001), and bones coated with 10–15-nm HAP (p=0.0026). Bones coated with 50–60-nm HAP were not significantly different than any other group, indicating that 50–60-nm HAP results in a larger callus with more mineralized tissue; however, the proportion of mineralized tissue to total tissue area is not significantly different than any other group (Fig. 4I).
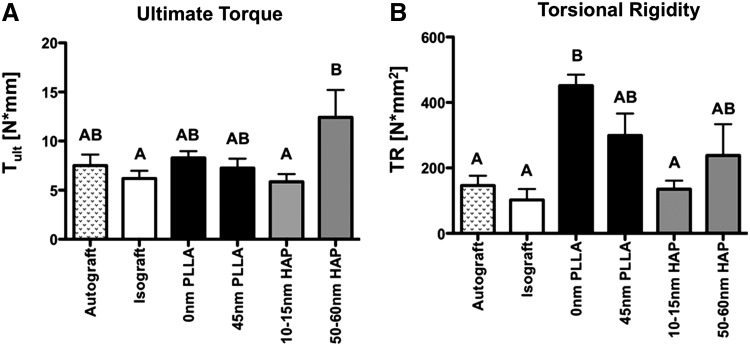
Osteointegration of bone grafts is improved by 50–60-nm HAP nanotopographic coating
The degree of osteointegration of graft bone was determined by torsional biomechanical testing. Ultimate torque was not significantly different between autografts, isografts, both PLLA-coated groups, and bones coated with 10–15-nm HAP. Grafts coated with 50–60-nm HAP had significantly increased ultimate torque compared to isografts (p=0.024) and 10–15-nm HAP (p=0.025); however, 50–60 nm was not significantly different than autografts (p=0.13) (Fig. 5A). Torsional rigidity, or graft stiffness, was significantly increased in bones coated with 0-nm PLLA and 45-nm PLLA, compared to isografts, autografts, and bones coated with 10–15-nm HAP. Torsional rigidity of bones coated with 50–60-nm HAP was not significantly different than any other group (Fig. 5B).
FIG. 5.
Biomechanical properties of bone grafts at 42 days post-surgery. (A) Ultimate torque at failure, and (B) torsional rigidity of autografts, isografts, and bone grafts coated with 0- or 45-nm PLLA topography, 10–15-nm HAP, or 50–60-nm HAP. N=8–9; different letters indicate p<0.05, while groups with the same letters are not statistically different from each other. Data are presented±standard error of the mean.
Discussion
Critical-size defects treated with allografts are prone to complications and are plagued by poor outcomes, most notably lack of integration with host bone. Here we show that physical vapor deposition of HAP on bone results in enhanced osteoblastic differentiation and incorporation of bone grafts. Our results demonstrate that coating bone with PLLA polymer nanotopographies, which have a different surface chemistry than bone, resulted in a subtle, yet statistically significant increase in AP activity in vitro, but no change in bone formation or ultimate torque in vivo. However, coating bone with a specific nanotopography (50–60 nm) composed of HAP, which has a surface chemistry similar to bone, resulted in increased AP activity and Col1a1 expression in vitro and callus formation and bone strength in vivo. Interestingly, Runx2 was uniformly elevated in cells on HAP-coated bones relative to uncoated, suggesting that the surface chemistry of the coating, rather than topography, may be a potent stimulus for Runx2 expression. The decreased osteoblast differentiation of cells on other HAP nanotopographies compared to 50–60 nm emphasizes that specific-scale nanotopographies are more osteogenic than others. Thus, both surface chemistry and topography contribute to graft healing and osteogenesis.
Healthy bone has a complex surface topography that facilitates adhesion and maturation of osteoblasts and osteoclasts to maintain bone homeostasis. This delicate balance is altered when cadaveric bone is processed in preparation for transplantation. Chemical processing and irradiation decreases risk of disease transmission; however, the osteogenic potential of the graft is diminished in part by altering the surface features. Standard chemical processing protocols (NaOH and hydrogen peroxide) eradicate osteoid staining in human trabecular bone samples and alter the structure and organization of collagen fibrils.1 These surface changes led to decreased adhesion of osteoblasts and decreased AP activity relative to normal bone.1 In addition, gamma-irradiation, which is often the terminal event in graft sterilization, can produce free radicals that target collagen and alter structural properties of bone.33 These studies indicate the importance of a resurfacing approach to improve allograft healing, and both HAP9,10 and nanotopography6 have been identified as potential approaches.
The ability of nanotopography to regulate osteoblast behavior, including proliferation,12 adhesion,11 and maturation,13–15 has been well studied. Using polymer topographies, we have previously demonstrated that 11-nm topographies increase osteoblast differentiation to a greater degree than larger nanoislands and flat topographies.14,15 While others have shown that 35- and 95-nm topographies increase cell adhesion to a greater degree than 13-nm or flat topographies.11 The specific topographies that we found to be most osteogenic in this study are slightly different than in other studies; however, intrinsic differences in the cell lines may account for these differences. Interestingly, the root mean square roughness of bone is ∼32 nm, and the cross-striation pattern of collagen fibers is 67 nm,12 which is within the scale of the HAP topographies that we found to be most osteogenic. We have chosen to focus on 45-nm PLLA topographies in this study based on the data from preliminary in vitro studies that suggested that 45-nm PLLA was more osteogenic than other PLLA topographies (data not shown). It is possible that other PLLA topographies may enhance osteogenesis in vivo to a greater degree than 45 nm; however, based on the dramatic increases in osteoblastic differentiation and graft healing that we observe with HAP nanotopographies, we do not believe that other polymer topographies would result in this dramatic improvement in healing due to the non-bone-biomimetic surface chemistry of the PLLA topographies. We have used 0-nm PLLA topographies as a polymer control group. However, AFM micrographs indicate that this coating does not result in a completely flat surface, but rather masks some of the surface features of bone. Additionally, we have not compared the ability of HAP nanotopographies to enhance bone healing relative to a flat HAP coating, which is an inherent limitation of our physical vapor deposition technique. However, several studies suggest that resurfacing grafts with a flat or smooth topography would not enhance healing, because these surfaces are not conducive to osteoblastic adhesion, differentiation, or graft integration.3,4,34 Our future studies will determine short- and long-term effects of optimized nanotopographies on graft healing and remodeling.
Synthetic implants, including titanium, are routinely roughened to improve implant integration,3,34–36 and roughening enhances cell adhesion, proliferation, and osteoblastic differentiation.4 Chemical roughening by acid etching can improve integration. However, greater increases in push-out force of trabecular bone implants and new bone formation on the implant surface occur with roughening by HAP coating compared to implants roughened by high-temperature acid etching.35 This study is consistent with our data, suggesting that HAP coating increases biomechanical properties of bone grafts by increasing new bone formation. It also supports the concept that bone biomimetic surface chemistries such as HAP play an important role in enhancing osteoblastic differentiation. This explains why HAP is commonly used to improve the osteoconductive potential of bone implants.37
While this study provides the first evidence of the exciting potential for HAP nanotopographies on bone graft healing, there are several limitations. These results are based on isograft rather than the more clinically applicable allograft model. However, Tiyapatanaputi et al. have shown no significant differences in healing between isograft- and allograft-treated bone defects, including new bone formation on graft and host.28 Additionally, the potential for delamination is an important consideration for resurfacing bone grafts. Although we have not used the AFM to analyze the surface of bone grafts after healing, we have analyzed HAP-coated bones after 14 days in culture and do not see a significant alteration in surface topography relative to grafts that have not been used in vitro.
In summary, this work uses the novel approach of coating processed bone with HAP using physical vapor deposition to enhance osteoblastic differentiation and bone formation. This technique results in a bone that is coated with an osteogenic, biomimetic, resorbable material, in a predictable and reproducible manner that improves osteointegration of bone grafts. HAP nanotopography can be scaled-up for large-bone grafts, has a long shelf-life, and would be able to be used at the point of care, unlike stem cell38 or gene therapy39,40 approaches. Additionally, HAP nanotopographies could potentially be used in conjunction with recent cell-based methods to enhance bone formation.41 This therapeutic approach has the potential to improve patient function and quality of life after bone transplantation and enhance osteointegration of a wide variety of artificial musculoskeletal implants.
Acknowledgments
This work was supported by a Grace Woodward Grant from the Pennsylvania State University, the Musculoskeletal Transplant Foundation, and a grant R01AR54937 from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases. A.L. thanks the Charles Godfrey Binder Endowment at Penn State for ongoing support. We acknowledge Vincent J. Bojan, Joshua J. Stapleton, and Greg D. Barner at Pennsylvania State University for assistance with nanotopography surface characterization experiments.
Disclosure Statement
No competing financial interests exist.
References
- 1.Dumas A. Gaudin-Audrain C. Mabilleau G. Massin P. Hubert L. Basle M.F., et al. The influence of processes for the purification of human bone allografts on the matrix surface and cytocompatibility. Biomaterials. 2006;27:4204. doi: 10.1016/j.biomaterials.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen H. Morgan D.A. Forwood M.R. Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2007;8:93. doi: 10.1007/s10561-006-9020-1. [DOI] [PubMed] [Google Scholar]
- 3.Buser D. Schenk R.K. Steinemann S. Fiorellini J.P. Fox C.H. Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 4.Deligianni D.D. Katsala N. Ladas S. Sotiropoulou D. Amedee J. Missirlis Y.F. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22:1241. doi: 10.1016/s0142-9612(00)00274-x. [DOI] [PubMed] [Google Scholar]
- 5.Bell B.F. Schuler M. Tosatti S. Textor M. Schwartz Z. Boyan B.D. Osteoblast response to titanium surfaces functionalized with extracellular matrix peptide biomimetics. Clin Oral Implants Res. 2011;22:865. doi: 10.1111/j.1600-0501.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gittens R.A. McLachlan T. Olivares-Navarrete R. Cai Y. Berner S. Tannenbaum R., et al. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32:3395. doi: 10.1016/j.biomaterials.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivares-Navarrete R. Gittens R.A. Schneider J.M. Hyzy S.L. Haithcock D.A. Ullrich P.F., et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12:265. doi: 10.1016/j.spinee.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivares-Navarrete R. Hyzy S.L. Hutton D.L. Erdman C.P. Wieland M. Boyan B.D., et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31:2728. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Ghannam A. Amin H. Nasr T. Shama A. Enhancement of bone regeneration and graft material resorption using surface-modified bioactive glass in cortical and human maxillary cystic bone defects. Int J Oral Maxillofac Implants. 2004;19:184. [PubMed] [Google Scholar]
- 10.Soballe K. Mouzin O.R. Kidder L.A. Overgaard S. Bechtold J.E. The effects of hydroxyapatite coating and bone allograft on fixation of loaded experimental primary and revision implants. Acta Orthop Scand. 2003;74:239. doi: 10.1080/00016470310014139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riehle M.O. Dalby M.J. Johnstone H. MacIntosh A. Affrossman S. Cell behaviour of rat calvaria bone cells on surfaces with random nanometric features. Mater Sci Eng C. 2003;23:337. [Google Scholar]
- 12.Palin E. Liu H.N. Webster T.J. Mimicking the nanofeatures of bone increases bone-forming cell adhesion and proliferation. Nanotechnology. 2005;16:1828. [Google Scholar]
- 13.Dalby M.J. McCloy D. Robertson M., et al. Osteoprogenitor response to semi-ordered and random nanotopographies. Biomaterials. 2006;24:2980. doi: 10.1016/j.biomaterials.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Lim J.Y. Hansen J.C. Siedlecki C.A. Hengstebeck R.W. Cheng J. Winograd N., et al. Osteoblast adhesion on poly(L-lactic acid)/polystyrene demixed thin film blends: effect of nanotopography, surface chemistry, and wettability. Biomacromolecules. 2005;6:3319. doi: 10.1021/bm0503423. [DOI] [PubMed] [Google Scholar]
- 15.Lim J.Y. Hansen J.C. Siedlecki C.A. Runt J. Donahue H.J. Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. J R Soc Interface. 2005;2:97. doi: 10.1098/rsif.2004.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J.Y. Shaughnessy M.C. Zhou Z. Noh H. Vogler E.A. Donahue H.J. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials. 2008;29:1776. doi: 10.1016/j.biomaterials.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Venugopal J. Low S. Choon A.T. Sampath Kumar T.S. Ramakrishna S. Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers. J Mater Sci Mater Med. 2008;19:2039. doi: 10.1007/s10856-007-3289-x. [DOI] [PubMed] [Google Scholar]
- 18.Ito Y. Hasuda H. Kamitakahara M. Ohtsuki C. Tanihara M. Kang I.K., et al. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J Biosci Bioeng. 2005;100:43. doi: 10.1263/jbb.100.43. [DOI] [PubMed] [Google Scholar]
- 19.Venugopal J.R. Low S. Choon A.T. Kumar A.B. Ramakrishna S. Nanobioengineered electrospun composite nanofibers and osteoblasts for bone regeneration. Artif Organs. 2008;32:388. doi: 10.1111/j.1525-1594.2008.00557.x. [DOI] [PubMed] [Google Scholar]
- 20.Shu R. McMullen R. Baumann M.J. McCabe L.R. Hydroxyapatite accelerates differentiation and suppresses growth of MC3T3-E1 osteoblasts. J Biomed Mater Res A. 2003;67:1196. doi: 10.1002/jbm.a.20021. [DOI] [PubMed] [Google Scholar]
- 21.Liao S.S. Cui F.Z. Zhang W. Feng Q.L. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater. 2004;69:158. doi: 10.1002/jbm.b.20035. [DOI] [PubMed] [Google Scholar]
- 22.Palmer L.C. Newcomb C.J. Kaltz S.R. Spoerke E.D. Stupp S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 2008;108:4754. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim J.Y. Dreiss A.D. Zhou Z. Hansen J.C. Siedlecki C.A. Hengstebeck R.W., et al. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials. 2007;28:1787. doi: 10.1016/j.biomaterials.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Lim J.Y. Loiselle A.E. Lee J.S. Zhang Y. Salvi J.D. Donahue H.J. Optimizing the osteogenic potential of adult stem cells for skeletal regeneration. J Orthop Res. 2011;11:1627. doi: 10.1002/jor.21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milner K.R. Siedlecki C.A. Fibroblast response is enhanced by poly(L-lactic acid) nanotopography edge density and proximity. Int J Nanomedicine. 2007;2:201. [PMC free article] [PubMed] [Google Scholar]
- 26.Lakhtakia A. Messier R. Bellingham, WA: SPIE Press; 2005. Sculptured Thin Films: Nanoengineered Morphology and Optics. [Google Scholar]
- 27.van dijk K.J. Verhoeven C.H.M. Maree F.H. Habraken P.M. Jansen J.A. Study of the influence of oxygen on the composition of thin films obtained by r.f. sputtering from a Ca5(PO4)3OH target. Thin Solid Films. 1997;304:191. [Google Scholar]
- 28.Tiyapatanaputi P. Rubery P.T. Carmouche J. Schwarz E.M. O'Keefe R.J. Zhang X. A novel murine segmental femoral graft model. J Orthop Res. 2004;22:1254. doi: 10.1016/j.orthres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Messier R. Yehoda J.E. Geometry of thin-film morphology. J Appl Physiol. 1985;58:3739. [Google Scholar]
- 30.Moulder J.F. Stickle W.F. Sobol P.E. Bomben K.D. Minnesota: Perkin-Elmer Corp.; 1979. Handbook of x-ray photoelectron spectroscopy: a reference book of standard data for use in x-ray photoelectron spectroscopy. [Google Scholar]
- 31.Kerber S.J. Brucker J.J. Wozniak K. Seal S. Hardcastle S. Barr T.L. The nature of hydrogen in x-ray photoelectron spectroscopy: general patterns from hydroxides to hydrogen bonding. J Vac Sci Technol A. 1996;14:1314. [Google Scholar]
- 32.Rehman I. Bonfield W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J Mater Sci Mater Med. 1997;8:1. doi: 10.1023/a:1018570213546. [DOI] [PubMed] [Google Scholar]
- 33.Akkus O. Belaney R.M. Das P. Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res. 2005;23:838. doi: 10.1016/j.orthres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K. Aoki K. Ohya K. Effects of surface roughness of titanium implants on bone remodeling activity of femur in rabbits. Bone. 1997;21:507. doi: 10.1016/s8756-3282(97)00204-4. [DOI] [PubMed] [Google Scholar]
- 35.Wong M. Eulenberger J. Schenk R. Hunziker E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J Biomed Mater Res. 1995;29:1567. doi: 10.1002/jbm.820291213. [DOI] [PubMed] [Google Scholar]
- 36.Cooper L.F. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000;84:522. doi: 10.1067/mpr.2000.111966. [DOI] [PubMed] [Google Scholar]
- 37.Laurencin C.T. Kumbar S.G. Nukavarapu S.P. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:6. doi: 10.1002/wnan.25. [DOI] [PubMed] [Google Scholar]
- 38.Dragoo J.L. Choi J.Y. Lieberman J.R. Huang J. Zuk P.A. Zhang J., et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res: official publication of the Orthopaedic Research Society. 2003;21:622. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 39.Koefoed M. Ito H. Gromov K. Reynolds D.G. Awad H.A. Rubery P.T., et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Ito H. Koefoed M. Tiyapatanaputi P. Gromov K. Goater J.J. Carmouche J., et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yazici C. Takahata M. Reynolds D.G. Xie C. Samulski R.J. Samulski J., et al. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther: the journal of the American Society of Gene Therapy. 2011;19:1416. doi: 10.1038/mt.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]