Abstract
Significant evidence has indicated that poly(L-lactide)-co-(ɛ-caprolactone) [(poly(LLA-co-CL)] scaffolds could be one of the suitable candidates for bone tissue engineering. Oxygen-terminated nanodiamond particles (n-DP) were combined with poly(LLA-co-CL) and revealed to be positive for cell growth. In this study, we evaluated the influence of poly(LLA-co-CL) scaffolds modified by n-DP on attachment, proliferation, differentiation of bone marrow stromal cells (BMSCs) in vitro, and on bone formation using a sheep calvarial defect model. BMSCs were seeded on either poly(LLA-co-CL)- or n-DP-coated scaffolds and incubated for 1 h. Scanning electron microscopy (SEM) and fluorescence microscopy were used in addition to protein and DNA measurements to evaluate cellular attachment on the scaffolds. To determine the effect of n-DP on proliferation of BMSCs, cell/scaffold constructs were harvested after 3 days and evaluated by Bicinchoninic Acid (BCA) protein assay and SEM. In addition, the osteogenic differentiation of cells grown for 2 weeks on the various scaffolds and in a dynamic culture condition was evaluated by real-time RT-PCR. Unmodified and modified scaffolds were implanted into the calvaria of six-year-old sheep. The expression of collagen type I (COL I) and bone morphogenetic protein-2 (BMP-2) after 4 weeks as well as the formation of new bone after 12 and 24 weeks were analyzed by immunohistochemistry and histology. Scaffolds modified with n-DP supported increased cell attachment and the mRNA expression of osteopontin (OPN), bone sialoprotein (BSP), and BMP-2 were significantly increased after 2 weeks of culture. The BMSCs had spread well on the various scaffolds investigated after 3 days in the study with no significant difference in cell proliferation. Furthermore, the in vivo data revealed more positive staining of COL I and BMP-2 in relation to the n-DP-coated scaffolds after 4 weeks and presented more bone formation after 12 and 24 weeks. n-DP modification significantly increased cell attachment and differentiation of BMSCs on poly(LLA-co-CL) scaffolds in vitro and enhanced bone formation in vivo.
Introduction
Selection of the appropriate biomaterial as a scaffold for application to bone tissue engineering is an important step in determining the essential properties of the construct. Ceramic-based scaffolds such as tricalcium phosphates, hydroxyapatites (HA)1 resemble the inorganic bone matrix and are currently widely used bone substitute materials. Degradable polymers have had widespread applications in life science,2 and synthetic polymers like poly-L-lactide have been applied clinically for over 45 years. So far, low mechanical stability compared to ceramic-based scaffold materials is limiting the clinical use of such degradable polymers in the field of bone regeneration. The ability to support cellular function as well as the chemical versatility of degradable polymers are extraordinary properties of these materials. Copolymers with improved mechanical properties and increased hydrophilicity promoting cell attachment and differentiation have been suggested as appropriate candidates for bone tissue-engineering applications.3–5
The ability of a scaffold material to serve as a substrate for living cells is to a large extent determined by the properties of the surface. Surfaces coated with diamond films at the nanolevel are used widely in a range of technological fields, tribology, and tool industry,6,7 and the unique chemical stability, mechanical properties, and biocompatibility of a diamond have led to its introduction into medical science.8–10 Promising results have been presented for improving the integration and durability of biomedical implants through coating with diamond films,11,12 and due to newly developed production methods and functionalization techniques such modified surfaces have a variety of potential biological applications.8 Through modification of medical biomaterials with nanodiamond particles (n-DP), surfaces are equipped with diamonds and extraordinary properties can be created without compromising the overall structure of the materials. These surfaces could immobilize growth factors,7 and this advantage might be potential for enhanced regeneration.
Bone cells derive from distinct progenitor cells during prenatal organogenesis, and a preferred differentiation of bone marrow stromal cells (BMSCs) toward the osteogenic lineage has been suggested.13 The osteogenic potential of BMSCs was proven many years ago from classical experiments with bone marrow transplantation and subsequent ectopic bone formation.14,15 The multipotency of BMSCs is also well recognized in the field of regenerative medicine,16 and BMSCs are considered as appropriate cellular sources for tissue regeneration, including bone. The ability of cells to attach, proliferate, and differentiate on a material depends on both the chemical properties and the surface roughness as well on the micro- as on the nanolevel.17 Investigations on the biological response of osteoblasts on surfaces equipped with nanoscale diamond coatings have shown improved adhesion and proliferation,12 suggesting the surface topography to influence the cellular response directly even without modification with osteoinductive growth factors.
Recently, the developed poly(L-lactide-co-ɛ-caprolactone) [(poly(LLA-co-CL)] has been shown to promote attachment, proliferation, and differentiation of bone-forming cells.4,18–20 The present study was aimed to further improve the cellular responses by modifying the surface of poly(LLA-co-CL) scaffolds with n-DP. Attachment, proliferation, and differentiation of BMSCs on the modified scaffolds were investigated in vitro. Furthermore, the effect of this surface modification on the formation of new bone was evaluated using a sheep calvarial defect model.
Materials and Methods
Production of scaffolds
The copolymer poly(LLA-co-CL) was polymerized from ɛ-Caprolactone (CL; Sigma-Aldrich) and L-LA (LLA; Boehringer Ingelheim) by ring-open polymerization as described before.18 The number average molecular weight (Mn) of the purified copolymer was approximately 100,000 and the polydispersity index was around 1.3, determined by Size Exclusion Chromatography (SEC, Polymer Laboratories) using chloroform and polystyrene standards. The copolymer was composed of 75 mol% LLA and 25 mol% CL, confirmed by 1H-NMR (Bruker Avance 400).
Porous scaffolds were prepared by a previously described solvent casting particulate leaching method.18 Two geometric types of samples were made: disc-shaped scaffolds (diameter≈12 mm, thickness≈1.3 mm) for the in vitro analysis and cylinders (diameter≈10 mm, thickness≈10 mm) for the animal experiment. Hence, three-dimensional porous scaffolds were produced in both geometric types. Porosities of above 83% were obtained for all polymer scaffolds investigated in the study as calculated by the method described earlier18 and by a Micro-CT (SkyScan 1172 scanner) using 40 kV and 2.4 micron voxel.
Scaffold modification
Colloidal n-DPs were prepared as described in.21 Briefly, a milling technique was used to achieve narrow size distribution and low agglomeration of the diamond particles, followed by ultrasonic redispersion to further reduce agglomeration of the colloidal particle solution.
The hydrophilic surface was achieved by increasing the OH and COOH groups through acid etching with sulfuric acid/nitric acid/perchloric acid (1:1:1) at 80°C and subsequent washing with water for neutralization prior to milling.
Fourier-transformed infrared spectrometry showed the presence of several carbon-oxygen groups (C-O, C=O, O-C-O), and a very low concentration of carbon-hydrogen (CHx) groups. C=O vibrations confirmed the presence of significant amounts of carboxyl groups (COOH), allowing attachment of organic molecules with amine groups (NH2) on the surface.22 A dip coating of the n-DP solution was prepared on Teflon and Silicone, and the hydrophilicity of the n-DP was determined through contact angle measurements and the wettability of the dip coating. Contact angles for pure Teflon and Silicone compared to n-DP dip coating were 119°–6,8° and 26,5°–8,4°, respectively. Modification of the poly(LLA-co-CL) scaffolds was performed with a combination of a manual perfusion technique under pressure with a defined flow. A vacuum pump for drying was applied subsequently to ensure uniform distribution of n-DP throughout the material. The concentration in the n-DP solution was 1017 n-DP/mL.
Cell culture
Human bone marrow-derived mesenchymal stem cells (BMSCs) were purchased from StemCell Technologies, Inc. Cells were routinely cultured in a 37°C humidified atmosphere containing 5% CO2, and only cells below passage 6 were used according to the manufacturer's suggestion.23 The culture medium consisted of the Mesencult® MSC Basal medium with added Stimulatory Supplements and Penicillin–Streptomycin, all purchased from StemCell Technologies™ Inc.
Cell attachment
A 500 μL cell suspension containing 2×105 cells/scaffold was added to the top of either poly(LLA-co-CL) scaffolds (CL group) or n-DP-coated poly(LLA-co-CL) scaffolds (CL+DP group) (n=5 in each group). Cell/scaffold constructs were harvested 1 h after seeding, rinsed in phosphate-buffered saline (PBS), and the Bicinchoninic Acid (BCA) Protein Assay (The Thermo Scientific Pierce) was used to determine the total protein level. Briefly, a 50 μL lysis buffer from each sample was processed for testing. Absorbance at 562 nm was measured on a microplate reader (BMG LABTECH). To confirm the results, protein and DNA contents were measured directly using a Nanodrop Spectrophotometer (Thermo Fisher Scientific, Inc.).
In addition, four samples from each group were rinsed in PBS, fixed with 4% paraformaldehyde (PFA) for 1 h, and processed with 4′,6-diamidino-2-phenylindole (DAPI) staining. The samples were rinsed and observed by a fluorescence microscope (Nikon 80i). The area fraction of the DAPI staining was analyzed by the NIS-Elements 3.07® software (Nikon).
Cell proliferation
About 1×105 cells in 500 μL of the culture medium were added to the top of each scaffold (n=5 in each group). The cell/scaffold constructs were incubated overnight, and then transferred to spinner flasks, a procedure described in.24 The scaffolds loaded with cells were put on the pins of the metal holder in the spinner flask. A magnetic stirrer was used with a speed of 50 rpm during culture. The constructs were cultured for 3 days, and then harvested for BCA assay. The protein and DNA level were also measured directly on a Nanodrop® Spectrophotometer as described above.
Scanning electron microscopy
To investigate cellular morphology on different materials, the samples loaded with BMSCs for 1 h and 3 days were prepared for SEM analyses as described earlier.4 The samples were fixed in glutaraldehyde, dehydrated, critical point dried, and coated with a gold-platinum layer before imaging. The samples were examined by a SEM (JSM 7400F Jeol) using secondary electrons and a voltage applied of 10 kV.
Quantitative real-time RT-PCR analysis
To investigate the influence of n-DP on differentiation of BMSCs, constructs were cultured in spinner flasks for 2 weeks before harvesting. An osteogenic stimulatory medium, containing dexamethasone, ascorbic acid, and β-glycerophosphate was added to the cells after 3 days of culture in the spinner flasks. Total RNA was isolated and real-time RT-PCR was performed as described earlier.24 TaqMan® gene expression assays (Applied Biosystems™); osteopontin (OPN), bone sialoprotein (BSP), bone morphogenetic protein 2 (BMP-2), alkaline phosphatase (ALP), integrin, alpha 2 (ITGA2), osteocalcin (OC), and GAPDH. The data were analyzed using a comparative Ct method.
Surgical procedure
Three healthy 6-year-old female sheep weighing 75±5 kg were fasted overnight, while having free access to water. About 0.5 mg atropine was administered and anesthesia was induced with ketamin (Ketavet® 7–8 mg/kg).7 The sheep were ventilated with a ventilator (Draeger EV-A) with 35% O2/air at 14–18 breaths/min and a tidal volume of 800 mL after fiberoptic intubation during spontaneous respiration. Anesthesia was maintained with Isoflurane (Forane®; Abbot) and the Ringer's solution (6 mL/kg/h). A sagittal incision was made on the forehead to access the frontal bone. In each animal, critical-size defects with 1 cm in diameter were created using a trephine burr. During trepanation, special care was taken to avoid damage to the dura.7 Defects were filled either with poly(LLA-co-CL) scaffolds (CL group), n-DP-coated poly(LLA-co-CL) scaffolds (CL+DP group), or left empty (control group), nine defects were created in one sheep and three defects were randomly distributed for each group. Before suturing, the periosteum was removed above the defects. The sheep were sacrificed after 4, 12, and 24 weeks.
Histological preparation, immunohistochemistry, and histomorphometry
All samples were first fixed in 4% paraformaldhyde (Merck) overnight, and then dehydrated using a series of ethanol with a gradient of 70, 96, and 100% (1 day per concentration). Host bone together with inserted scaffolds was embedded in Technovit® 9100 New as described previously.25 Evaluation of bone formation was performed on sawing sections with 25 μm thickness with Toludine Blue O staining.
Immunohistochemistry staining was performed according to the protocols reported previously with some modifications.26,27 Briefly, sections with 5 μm thickness were obtained with a rotary microtome (Leica RM2255). The samples were deplasticized in 2-methoxyethylacetate (Meck) overnight at RT, followed by immersing in Proteinase K (Dako), EDTA, 10% H2O2, Protein Block (Dako) for 15 (RT), 60 (37°C), 30 (RT), and 20 (RT) min, respectively. Then, samples were incubated with the following primary antibodies: BMP-2 (Santa Cruz Biotechnology, Inc.; 1:50 dilution) and Collagen I (Sigma; 1:1000 dilution) for 60 min at RT and overnight at 4°C, respectively. After washing with Tri-buffer saline, peroxidase-labeled secondary antibodies (Einvision+ Dual Link System-HRP; Dako) were applied for 30 min at RT. The 3-amino-9-ethylcarbazole (AEC) substrate chromogen system (Dako) was used to visualize peroxidase activities The samples were mounted with the aqueous mounting medium (Aquatex) for observations.
Analyses of histological sections were performed with Image-Pro Plus® software. Images were taken with the Nikon Eclipse 80i microscope coupled with a CCD camera with a fixed exposure and light intensity. The region of interest was defined as the area of the defect containing tissues/scaffolds, but reaching from the periphery of the host bone on both sides. The new bone formation was quantified using a positively selected pixel area divided by the total area of defect, which was then presented as the percentage of new bone formation (area).
Statistics
The in vitro experiments were repeated using BMSCs derived from two different donors. Statistical analysis was performed using SigmaStat 3.1 package software and a Student's t-test. The in vivo experiment's data were processed with the Kruskal–Wallis test. All values in bar diagrams were presented as mean±standard deviation.
Results
Cell attachment
After 1 h of incubation, the unattached BMSCs were gently washed away from the scaffold surface. The analysis performed by the BCA Protein Assay demonstrated higher values on the surfaces functionalized by n-DP (CL+DP group). Nanodrop measurements also showed the same tendency with a higher protein content and DNA level on the CL+DP group (Fig. 1). This indicated that more cells were attached on the n-DP functionalized scaffolds.
FIG. 1.

Evaluation of cell/scaffold constructs after 1 h. Bicinchoninic Acid (BCA) protein assay showed higher values from the CL+DP group (A); direct measurement of protein using the Nanodrop spectrophotometers (B); the DNA content measured with Nanodrop spectrophotometers (C). CL, ɛ-caprolactone; DP, diamond particles. *p<0.05.
Nuclei stained with DAPI on both types of scaffolds could be observed under the fluorescence microscope. The image analysis revealed a higher nuclear fraction in the CL+DP group, which clearly indicated that more BMSCs were attached to these surfaces (Fig. 2).
FIG. 2.
Fluorescence images showed DAPI stainings from the CL group (A) and the CL+DP group (B). Image analysis showed a higher nucleus area fraction from the CL+DP group (C) (Scale bar=200 μm). *p<0.05. Color images available online at www.liebertpub.com/tea
The SEM results showed the same tendency, which was obtained by the fluorescence microscopy, that BMSCs were attached and spread well on the surface of n-DP scaffolds. (Fig. 3A, B).
FIG. 3.
Scanning electron microscopy images from both groups after 1 h (A, B) and 3 days (C, D) (Scale bar=10 μm). Good cell spreading could be observed from the CL+DP group after 1 h (B). Both groups have good cell growth on scaffolds after 3 days (C, D).
Cell proliferation
After culturing cells for 3 days, the results did not show significantly higher values on the CL+DP than on the CL groups. Measurements from the Nanodrop Spectrophotometer also showed that the protein content and DNA levels on the two groups were similar (Fig. 4).
FIG. 4.
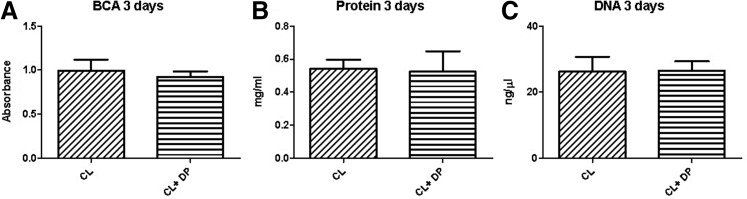
Evaluation of proliferation after 3 days. No significant difference between groups from BCA protein assay (A). Direct measurement of proteins using the Nanodrop spectrophotometers (B); the DNA content measured with Nanodrop spectrophotometers (C).
The SEM images showed that BMSCs could spread well on both types of scaffolds after 3 days. No obvious difference between the groups could be observed (Fig. 3C, D).
Cell differentiation
To investigate the influence on cell differentiation, long-term dynamic culture was performed in spinner flask bioreactors. The data clearly demonstrated that the n-DP-coated scaffolds promoted the expression of genes involved in the process of bone formation. In human BMSCs grown on surfaces of poly(LLA-co-CL) scaffolds modified with n-DP, there were significant increases in OPN, BSP, and BMP-2 mRNA expression compared to those with unmodified surfaces (p<0.05) (Fig. 5). There was a higher tendency of mRNA expression levels of ITGA2 or OC by the cells grown on the n-DP-modified scaffolds compared to those with unmodified surfaces, although the differences were not statistically significant.
FIG. 5.
Real-time RT-PCR results from two groups after 2 weeks. Significant higher expression of osteopontin, (OPN) bone sialoprotein (BSP), and bone morphogenetic protein-2 (BMP-2) could be detected from the CL+DP group. *p<0.05. ALP, alkaline phosphatase; OC, osteocalcin.
Immunohistochemistry
After 4 weeks of implantation, defects were harvested for immunohistochemical evaluation of osteogenic progression in the center of the scaffolds. As shown in Figure 6, the expression of collagen type I (COL 1) and BMP-2 was more prevalent in the CL+DP scaffolds compared to the empty defects and the unmodified poly(LLA-co-CL) scaffolds.
FIG. 6.
CL+DP scaffolds enhanced COL I and BMP-2 production within the defect site. COL I and BMP-2 staining are more prevalent in the CL+DP scaffolds compared with the empty defect and the CL scaffolds. Images were taken with 20×objective showing the center of the scaffolds. Arrows indicated the positive staining of COL I and BMP-2. COL I, collagen type I. Color images available online at www.liebertpub.com/tea
New bone formation
Undecalcified methyl methacrylate-based resin sections were stained with Toluidine Blue O to evaluate new bone formation within the defect site after 12 and 24 weeks of implantation. As shown in Figure 7, at 12 weeks, the new bone formation within the empty defects commenced from the periphery of the host bone and grew toward the center; however, the new bone did not bridge those defects and there was still a gap in the middle of the defect filled with dense fibrous tissue. In the CL group, the new bone formation was minimal with a similar pattern of ingrowth as in the control group. Only the edges of the poly(LLA-co-CL) scaffold were in contact with the new bone. By contrast, markedly more new bone ingrowth could be observed in the CL+DP group. The newly formed bone was interconnected across the defect area with close contact to the surfaces of the scaffolds. At 24 weeks, a dramatical increase of new bone formation was observed for all the tested materials. For the CL+DP group, closely packed mineralized bone was massively presented within the defect and the entire defect was filled by newly formed bone. It was noteworthy that the CL+DP scaffold was almost completely degraded by 24 weeks. By converse, only a few bony islands, which presented at the edges of the defect were observed for the CL scaffold. The majority of scaffold fragments could still be seen. For the empty defect, it still showed nonunion from the host bone after 24 weeks. Histomorphometry results indicated higher new bone formation areas from the CL+DP group (Fig. 8).
FIG. 7.
Histology of the CL+DP group indicates enhanced bone healing after 12 and 24 weeks of implantation. Dotted lines represent edges of the original defect. Longitudinal sections stained with Toluidine Blue O are presented (NB=new bone, HB=host bone, and FT=fibrous tissue). Color images available online at www.liebertpub.com/tea
FIG. 8.
New bone formation areas in sheep cavarial defect after 12 weeks and 24 weeks. *p<0.05.
Discussion
In this study, the cellular response of BMSCs to copolymer poly(LLA-co-CL) scaffolds, where the surface had been modified with n-DP, was evaluated in a dynamic culture system in vitro, and the effects on bone healing were evaluated in a sheep calvarial defect model.
Surface characteristics of biomaterials greatly influence the behavior of cells upon contact, and cell adhesion is a particularly important factor concerning biocompatibility of materials.28 The first attachment phase in the process of cell adhesion is dominated by relatively unspecific cell-surface physicochemical interactions, whereas the following phase includes a more complex interplay between cellular receptors and extracellular ligands that lead to altered downstream signaling influencing gene expression by the adhering cells.28,29
After 1 h, more cells were attached to the n-DP-coated scaffold, which might be explained by altered hydrophilicity of the surface. It is known that both a positively or negatively charged copolymer surface will increase the number of attached cells.17,30,31 Cell attachment on biomaterials is a process where adhesion proteins fibronectin and vitronectin are involved, both favoring hydrophilic surfaces.32 This can be generated by functionalization with n-DP providing multiple binding sites for proteins such as the aforementioned adhesion molecules.9 Cell attachment is also influenced by the production of integrins, and surface wettability has been shown not only to influence the physicochemical attachment, but also to promote integrin-associated adhesion in human osteoblasts.10 Although the difference was not statistically significant, upregulated expression of ITGA2 was observed for cells grown on the surface modified with n-DP, which might also have contributed to the enhanced cell attachment.
Biomaterials coated with nanocrystalline diamond films (NCD) have previously been shown to increase cell adhesion and proliferation in vitro, as well as improving bone healing in sheep calvarial defects.7 In the present work, the diamond coating was achieved through particles rather than films, but it would be reasonable to conclude that through surface modifications with diamonds on the nanoscale, positive cellular responses can be achieved using both methods. A potential difference between NCD and n-DP is the microroughness. Zhao et al.33 found that the combination of a certain microroughness and a surface with increased wettability had a synergistic effect and concluded that biomaterials with higher surface energy would improve the biological response by the host tissue.33 However, the data presented in this study are not sufficient to determine the relative contribution of the wettability and the microroughness to the improved cell attachment.
BMSCs spread well on the coated scaffolds, suggesting that the surface modification generated was captivating for cells. It has been reported that changes in surface energy not only influence the adsorption of proteins and subsequently cell attachment, but as a consequence of this, cellular spreading and proliferation is altered as well. Studies on hydrophilic biomedical implant surfaces have also demonstrated increased maturation and differentiation of osteogenic cells.34,35 In the present study, the proliferation of cells was not different between the groups after 3 days. This result could be explained by the functional reciprocal relationship between cell growth and differentiation-related gene expression, where in order for the extracellular matrix to develop, cells have to stop proliferating allowing differentiation into more mature phenotypes.36,37 Indeed, the relative gene expression of osteogenic biomarkers was influenced by the surface modification with n-DP.
OPN and BSP are both phosphoproteins that bind to HA in the bone matrix and are therefore increasingly expressed in the phase of matrix mineralization.37 After 2 weeks of dynamic culture, an increased expression on the n-DP surface was observed, suggesting a higher level of extracellular matrix maturation. These findings are in accordance with Yang et al.,38 who could find that osteoblasts cultured on NCD-surfaces presented increased deposition of extracellular calcium when compared to silicon and borosilicate glass surfaces.
Kalbacova et al.39 cultured osteoblasts on NCD surfaces with different roughness and found that the ALP activity and calcium accumulation were increased on diamond surfaces after 11 days. However, the ALP activity seemed to depend on the surface roughness, where increased roughness led to decreased activity.39 In the present study, we could observe a tendency toward ALP downregulation and OC upregulation after 2 weeks of dynamic culture. As ALP is considered an early marker for osteoblast differentiation, a lower expression is to be expected in later stages of differentiation. This is in accordance with the upregulated expression of both OPN and BSP, supporting the hypothesis that extracellular matrix maturation had reached a late stage for the cells grown on the modified surface.
To verify the in vitro findings, an in vivo investigation for bone regeneration was performed using a sheep model. Such a model has the advantage of being of similar weight to humans and having a similar pattern of bone remodeling.40 BMP-2 expression was higher in the CL+DP group in vivo, a result in accordance with the significant higher fold change demonstrated by the RT-PCR in vitro. Stable functionalization of BMP-2 on endosseous implants with NCD has been shown in previous work,7 where immobilization of BMP-2 molecules was achieved through strong physisorption.41 The ability of surfaces to bind BMP-2, after being modified in a manner comparable to the one used in the present work, should therefore be considered as a possible contributing factor to the higher levels found in the n-DP scaffolds.
COL I is widely distributed in most connective tissues, and is the principal organic component of mineralized bone where it comprises around 80% of the total protein content.42 We found that COL I was highly produced in the n-DP scaffolds compared to the nonmodified scaffolds, thus showing accumulation of more bone matrix in the CL+DP group. This conclusion was further supported by the histological evaluation, where more bone formation was found in the n-DP group. In combination, these observations suggest that n-DP-modified scaffolds exhibit considerable osteoconductive properties and bioactivity leading to enhanced bone healing in sheep calvarial defects.
Conclusions
Modification of poly(L-lactide)-co-(ɛ-caprolactone) scaffolds by n-DP promoted cell attachment and osteogenic differentiation of BMSCs in vitro and enhanced bone formation in vivo.
Acknowledgments
The authors would like to acknowledge Prof. Bruce Stuart for English revision of the manuscript. This work was supported by the VascuBone project, European Union FP7; Grant Number: 242175 and The Research Council of Norway; Stem Cell; Grant Number: 180383/V40.
Disclosure Statement
No competing financial interests exist.
References
- 1.Salgado A.J. Coutinho O.P. Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 2.Freed L.E. Vunjak-Novakovic G. Biron R.J. Eagles D.B. Lesnoy D.C. Barlow S.K., et al. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y) 1994;12:689. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 3.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 4.Idris S.B. Arvidson K. Plikk P. Ibrahim S. Finne-Wistrand A. Albertsson A.C., et al. Polyester copolymer scaffolds enhance expression of bone markers in osteoblast-like cells. J Biomed Mater Res A. 2010;94:631. doi: 10.1002/jbm.a.32726. [DOI] [PubMed] [Google Scholar]
- 5.Odelius K. Plikk P. Albertsson A.C. Elastomeric hydrolyzable porous scaffolds: copolymers of aliphatic polyesters and a polyether-ester. Biomacromolecules. 2005;6:2718. doi: 10.1021/bm050190b. [DOI] [PubMed] [Google Scholar]
- 6.Schneider A. Steinmueller-Nethl D. Roy M. Franek F. Enhanced tribological performances of nanocrystalline diamond film. Int J Refract Met H. 2010;28:40. [Google Scholar]
- 7.Kloss F.R. Gassner R. Preiner J. Ebner A. Larsson K. Hachl O., et al. The role of oxygen termination of nanocrystalline diamond on immobilisation of BMP-2 and subsequent bone formation. Biomaterials. 2008;29:2433. doi: 10.1016/j.biomaterials.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Kloss F.R. Najam-Ul-Haq M. Rainer M. Gassner R. Lepperdinger G. Huck C.W., et al. Nanocrystalline diamond—an excellent platform for life science applications. J Nanosci Nanotechnol. 2007;7:4581. [PubMed] [Google Scholar]
- 9.Lechleitner T. Klauser F. Seppi T. Lechner J. Jennings P. Perco P., et al. The surface properties of nanocrystalline diamond and nanoparticulate diamond powder and their suitability as cell growth support surfaces. Biomaterials. 2008;29:4275. doi: 10.1016/j.biomaterials.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Lim J.Y. Taylor A.F. Li Z. Vogler E.A. Donahue H.J. Integrin expression and osteopontin regulation in human fetal osteoblastic cells mediated by substratum surface characteristics. Tissue Eng. 2005;11:19. doi: 10.1089/ten.2005.11.19. [DOI] [PubMed] [Google Scholar]
- 11.Fries M.D. Vohra Y.K. Properties of nanocrystalline diamond thin films grown by MPCVD for biomedical implant purposes. Diam Relat Mater. 2004;13:1740. [Google Scholar]
- 12.Yang L. Sheldon B.W. Webster T.J. Orthopedic nano diamond coatings: control of surface properties and their impact on osteoblast adhesion and proliferation. J Biomed Mater Res A. 2009;91:548. doi: 10.1002/jbm.a.32227. [DOI] [PubMed] [Google Scholar]
- 13.Bianco P. Robey P.G. Simmons P.J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedenstein A.J. Piatetzky S., II Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381. [PubMed] [Google Scholar]
- 15.Tavassoli M. Crosby W.H. Transplantation of marrow to extramedullary sites. Science. 1968;161:54. doi: 10.1126/science.161.3836.54. [DOI] [PubMed] [Google Scholar]
- 16.Tuan R.S. Boland G. Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Wachem P.B. Schakenraad J.M. Feijen J. Beugeling T. van Aken W.G. Blaauw E.H., et al. Adhesion and spreading of cultured endothelial cells on modified and unmodified poly (ethylene terephthalate): a morphological study. Biomaterials. 1989;10:532. doi: 10.1016/0142-9612(89)90058-6. [DOI] [PubMed] [Google Scholar]
- 18.Danmark S. Finne-Wistrand A. Wendel M. Arvidson K. Albertsson A.C. Mustafa K. Osteogenic differentiation by rat bone marrow stromal cells on customized biodegradable polymer scaffolds. J Bioact Compat Pol. 2010;25:207. doi: 10.1002/jbm.a.32945. [DOI] [PubMed] [Google Scholar]
- 19.Schander K. Arvidson K. Mustafa K. Hellem E. Bolstad A.I. Finne-Wistrand A., et al. Response of bone and periodontal ligament cells to biodegradable polymer scaffolds in vitro. J Bioact Compat Pol. 2010;25:584. [Google Scholar]
- 20.Xue Y. Danmark S. Xing Z. Arvidson K. Albertsson A.C. Hellem S., et al. Growth and differentiation of bone marrow stromal cells on biodegradable polymer scaffolds: an in vitro study. J Biomed Mater Res Part A. 2010;95A:1244. doi: 10.1002/jbm.a.32945. [DOI] [PubMed] [Google Scholar]
- 21.Kruger A. Kataoka F. Ozawa M. Fujino T. Suzuki Y. Aleksenskii A.E., et al. Unusually tight aggregation in detonation nanodiamond: identification and disintegration. Carbon. 2005;43:1722. [Google Scholar]
- 22.Ghodbane S. Haensel T. Coffinier Y. Szunerits S. Steinmuller-Nethl D. Boukherroub R., et al. HREELS investigation of the surfaces of nanocrystalline diamond films oxidized by different processes. Langmuir. 2010;26:18798. doi: 10.1021/la1032652. [DOI] [PubMed] [Google Scholar]
- 23.Xue Y. Xing Z. Hellem S. Arvidson K. Mustafa K. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed Eng Online. 2009;8:34. doi: 10.1186/1475-925X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing Z. Xue Y. Danmark S. Schander K. Ostvold S. Arvidson K., et al. Effect of endothelial cells on bone regeneration using poly(L-lactide-co-1,5-dioxepan-2-one) scaffolds. J Biomed Mater Res A. 2010;96:349. doi: 10.1002/jbm.a.32989. [DOI] [PubMed] [Google Scholar]
- 25.Yang R. Davies C.M. Archer C.W. Richards R.G. Immunohistochemistry of matrix markers in Technovit 9100 New-embedded undecalcified bone sections. Eur Cell Mater. 2003;6:57–71. doi: 10.22203/ecm.v006a06. discussion. [DOI] [PubMed] [Google Scholar]
- 26.Rippstein P. Black M.K. Boivin M. Veinot J.P. Ma X. Chen Y.X., et al. Comparison of processing and sectioning methodologies for arteries containing metallic stents. J Histochem Cytochem. 2006;54:673. doi: 10.1369/jhc.5A6824.2006. [DOI] [PubMed] [Google Scholar]
- 27.Willbold E. Witte F. Histology and research at the hard tissue-implant interface using Technovit 9100 New embedding technique. Acta Biomater. 2010;6:4447. doi: 10.1016/j.actbio.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 29.Klein M.O. Bijelic A. Toyoshima T. Gotz H. von Koppenfels R.L. Al-Nawas B., et al. Long-term response of osteogenic cells on micron and submicron-scale-structured hydrophilic titanium surfaces: sequence of cell proliferation and cell differentiation. Clin Oral Implants Res. 2010;21:642. doi: 10.1111/j.1600-0501.2009.01883.x. [DOI] [PubMed] [Google Scholar]
- 30.van Wachem P.B. Hogt A.H. Beugeling T. Feijen J. Bantjes A. Detmers J.P., et al. Adhesion of cultured human endothelial cells onto methacrylate polymers with varying surface wettability and charge. Biomaterials. 1987;8:323. doi: 10.1016/0142-9612(87)90001-9. [DOI] [PubMed] [Google Scholar]
- 31.van Kooten T.G. Schakenraad J.M. van der Mei H.C. Busscher H.J. Influence of substratum wettability on the strength of adhesion of human fibroblasts. Biomaterials. 1992;13:897. doi: 10.1016/0142-9612(92)90112-2. [DOI] [PubMed] [Google Scholar]
- 32.Wilson C.J. Clegg R.E. Leavesley D.I. Pearcy M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 33.Zhao G. Raines A.L. Wieland M. Schwartz Z. Boyan B.D. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28:2821. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy S.B. Washburn N.R. Simon C.G., Jr. Amis E.J. Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials. 2006;27:3817. doi: 10.1016/j.biomaterials.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson C. Nygren H. Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials. 2004;25:4759. doi: 10.1016/j.biomaterials.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Lian J.B. Stein G.S. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995;15:118. [PMC free article] [PubMed] [Google Scholar]
- 37.Lian J.B. Stein G.S. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med. 1992;3:269. doi: 10.1177/10454411920030030501. [DOI] [PubMed] [Google Scholar]
- 38.Yang L. Sheldon B.W. Webster T.J. The impact of diamond nanocrystallinity on osteoblast functions. Biomaterials. 2009;30:3458. doi: 10.1016/j.biomaterials.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Kalbacova M. Rezek B. Baresova V. Wolf-Brandstetter C. Kromka A. Nanoscale topography of nanocrystalline diamonds promotes differentiation of osteoblasts. Acta Biomater. 2009;5:3076. doi: 10.1016/j.actbio.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Pearce A.I. Richards R.G. Milz S. Schneider E. Pearce S.G. Animal models for implant biomaterial research in bone: a review. Eur Cells Mater. 2007;13:1. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 41.Steinmuller-Nethl D. Kloss F.R. Najam-U-Haq M. Rainer M. Larsson K. Linsmeier C., et al. Strong binding of bioactive BMP-2 to nanocrystalline diamond by physisorption. Biomaterials. 2006;27:4547. doi: 10.1016/j.biomaterials.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 42.Viguet-Carrin S. Garnero P. Delmas P.D. The role of collagen in bone strength. Osteoporos Int. 2006;17:319. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]








