Abstract
It has been previously suggested that overexpression of mitochondrial superoxide dismutase (SOD) attenuates cancer development; however, the exact mechanism remains unclear. In this work, we have studied the direct effect of the mitochondria-targeted superoxide scavenger, (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mitoTEMPO), on B16-F0 mouse melanoma cells and tumor growth in a nude mouse model of human melanoma. We show that scavenging of mitochondrial superoxide inhibited cell growth, reduced viability, and induced apoptosis in melanoma cells, but did not affect nonmalignant skin fibroblasts. Diminished mitochondrial superoxide inhibited redox-dependent Akt, restored activity of mitochondrial pyruvate dehydrogenase, and reduced HIF1-α and lactate dehydrogenase expression in cancer cells. Suppression of glycolysis in mitoTEMPO-treated melanoma cells resulted in a significant drop of cellular adenosine-5′-triphosphate and induced cell death. In vivo mitoTEMPO treatment effectively suppressed growth of established tumor in the mouse model of human melanoma. Therefore, our data lead to the hypothesis that scavenging of mitochondrial superoxide selectively inhibits redox-sensitive survival and metabolic pathways, resulting in cancer cell death. In contrast to existing anticancer therapies, inhibition of mitochondrial superoxide may represent a novel specific anticancer treatment with reduced cytotoxic side effects. Antioxid. Redox Signal. 19, 344–349.
Introduction
Many types of cancer are known to have reduced expression of mitochondrial superoxide dismutase (SOD2) and altered mitochondrial functions (6). Reduced SOD2 rises mitochondrial superoxide (O2•−) and increases reactive oxygen species (ROS) both in the mitochondria and cytoplasm (4). These changes in cancer cells do not cause cell death, but lead to a series of redox-sensitive adaptive responses, including switch from mitochondrial metabolism to glycolysis for accelerated adenosine-5′-triphosphate (ATP) synthesis that facilitates cell survival and cell growth. Cytoplasmic glycolysis generates ATP much faster compared to mitochondrial respiration-driven oxidative phosphorylation. This phenomenon known as the Warburg effect leads to high glucose consumption by cancer cells (9). Recent studies confirmed that Warburg effect is not associated with mitochondrial dysfunctions and showed that malignant cells depend on a number of mitochondrial metabolic functions (9). Since mitochondrial ROS are essential both for cancer proliferation (9) and ROS-induced apoptosis (5), we need more detailed understanding of ROS-mediated cell signaling to develop new antioxidant interventions.
It has been previously reported that overexpression of SOD2, but not SOD1, attenuates malignant transformation and inhibits tumor development (8). Alteration of SOD2 in the Val16Ala polymorphism has been associated with reduced cancer patient survival. These data suggest that diminished SOD2 activity can promote and sustain cancer development, while scavenging of mitochondrial O2•− may suppress cancer. The exact molecular mechanism of SOD2 anticancer activity is not clear, but it may be associated with redox-dependent cell signaling mediated by mitochondrial ROS.
Innovation.
Numerous currently used cancer treatments are aimed to induce cancer cell apoptosis by increased reactive oxygen species (ROS) production. Cancer cells are ROS resistant and use ROS to maintain a malignant proliferative phenotype; thus, increasing ROS may have a limited efficacy and cause off-target cytotoxicity. In our study, we show that scavenging mitochondrial O2•− in melanoma, known to be resistant to many pharmacological treatments, efficiently prevents cell growth and tumor development by inhibition of ROS-dependent survival and metabolic signaling pathways.
It is important that the malignant phenotype is associated with stimulation of prosurvival signaling pathways, including enhanced Akt and Erk activity, hallmarked by their phosphorylation. These pathways are stimulated by production of cellular ROS. One of the main sources of cellular O2•− and H2O2 is NADPH oxidase (4). It stimulates cell proliferation facilitated by the Akt and Erk ROS-sensitive cell signaling pathways. Melanoma cell proliferation is reduced by inhibition of NADPH oxidase (2). The other major source of cellular ROS is the mitochondria. It has been shown that mitochondrial O2•− activates Akt. We have recently shown that overexpression of SOD2 or treatment with mitochondria-targeted SOD mimetic, (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mitoTEMPO), significantly reduces cellular ROS and inhibit NADPH oxidase activity (4). In this work, we have investigated if scavenging of mitochondrial ROS by mitoTEMPO blocks ROS-sensitive cell signaling in cancer cells.
Moderate increase in ROS production in cancer cells does not cause oxidative stress and cell death. Instead, cells adapt to these conditions and employ increased ROS production for maintenance of the malignant phenotype and cell survival. In fact, malignant cells are highly resistant to ROS, particularly drug-resistant cancer (5). Meanwhile, many modern anticancer therapies rely on ROS-induced apoptosis (5), but this approach results in significant off-target cell apoptosis leading to pathological conditions, including cardiotoxicity, neurotoxicity, nephrotoxicity, and hepatotoxicity (5). To reduce cytotoxic side effects of anticancer therapy, we have tested an alternative antioxidant strategy to inhibit mitochondrial ROS to attenuate redox-sensitive signaling, thereby inhibiting cancer metabolism and inducing cancer cell death.
In this work, we have determined if scavenging of mitochondrial ROS by mitoTEMPO selectively inhibits redox-sensitive regulation of metabolic and cell survival pathways in melanoma.
mitoTEMPO Inhibits Melanoma Cell Growth Leading to Senescence and Apoptosis
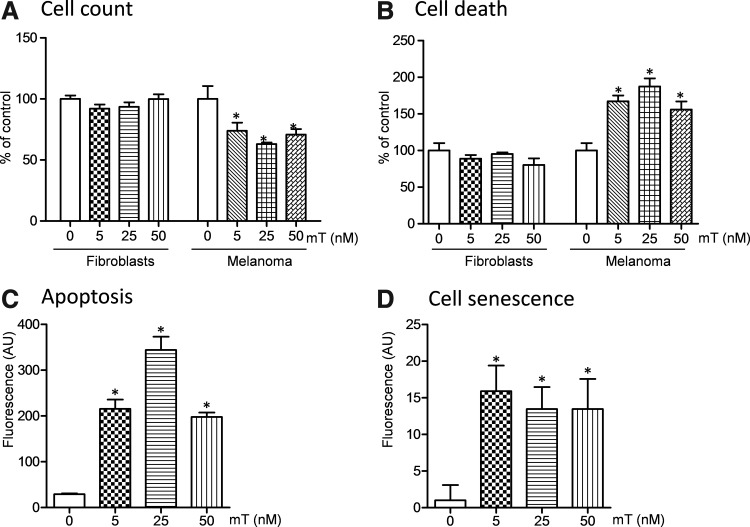
The effect of the mitochondria-targeted superoxide scavenger on melanoma cells was tested by 24-h incubation with saline as a vehicle (control), 5 nM, 25 nM, or 50 nM mitoTEMPO (Fig. 1A). We found that mitoTEMPO at 5 nM concentration significantly decreased number of melanoma cell, while mitoTEMPO did not affect mouse skin fibroblasts. Increasing dose of mitoTEMPO to 50 nM reduced the cell count by 40%, indicating on melanoma cell death. To confirm these results, we have investigated cell death, apoptosis, and cell senescence. Figure 1B shows that mitoTEMPO increased melanoma cell death in a dose-dependent fashion with a maximum effect at 25 nM, but did not increase cell death in nonmalignant fibroblasts. Further, treatment of melanoma cells with mitoTEMPO significantly increased cellular apoptosis measured by the early apoptosis marker, Annexin V, and induced cell senescence as indicated by Beta-gal staining (Fig. 1C, D). These data clearly demonstrate the specific anticancer effect of the mitochondria-targeted superoxide scavenger mitoTEMPO.
FIG. 1.
Effect of the mitochondria-targeted superoxide dismutase mimetic (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mitoTEMPO) on cell growth (A), cell viability (B), apoptosis (C), and cell senescence (D). Skin fibroblasts and melanoma B16 cells were incubated for 24 h with a vehicle or various concentrations of mitoTEMPO. *p<0.05 versus vehicle.
mitoTEMPO Reduces Cellular Superoxide and Inhibits the Activity of Redox-Sensitive Akt and Erk
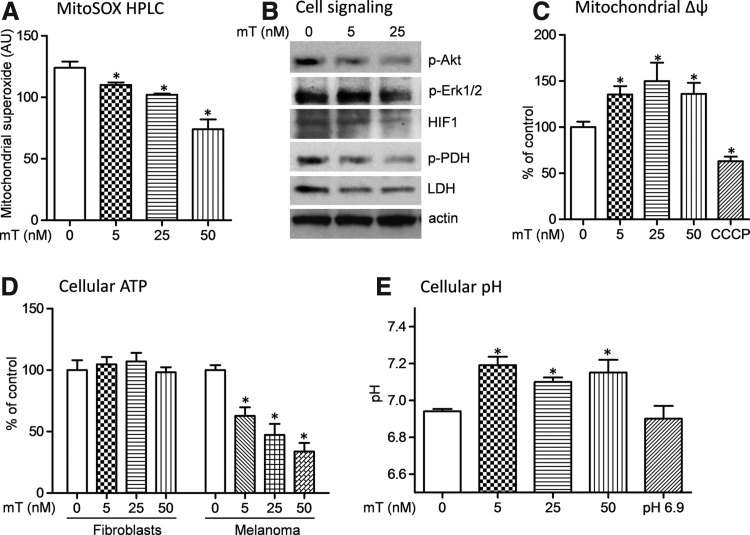
To further investigate the molecular mechanisms of anticancer activity of mitoTEMPO, we measured cellular ROS and ROS-sensitive prosurvival cell signaling mediated by Akt and Erk phosphorylation. We found that 25 nM mitoTEMPO decreased mitochondrial O2•− both acutely and after 24 h of mitoTEMPO treatment (Fig. 2A). Measurements of cytoplasmic O2•− by high-performance liquid chromatography (HPLC) (4) and detection cellular H2O2 by the Amplex Red assay showed reduced ROS production in mitoTEMPO-treated melanoma cells (data not shown). These data confirmed inhibition of ROS production in mitoTEMPO-treated melanoma cells.
FIG. 2.
Role of mitochondrial reactive oxygen species (ROS) in redox-sensitive cell signaling and metabolic function. (A) Detection of mitochondrial O2•− using high-performance liquid chromatography (HPLC) and mitoSOX (4). (B) Western blot analysis of phosphorylation of Akt, Erk1/2, and pyruvate dehydrogenase (PDH) and expression of HIF1-α and lactate dehydrogenase compared to the actin loading control in B16 melanoma cells incubated for 24 h with a vehicle or mitoTEMPO. (C) Measurements of the mitochondrial membrane potential (Δψ) in B16 melanoma cells incubated for 24 h with a vehicle or mitoTEMPO. Cells treated with the mitochondrial uncoupling agent carbonyl cyanide m-chlorophenyl hydrazone (1 μM) were used as a negative control. (D) Analysis of cellular adenosine-5′-triphosphate level in skin fibroblasts and B16 melanoma cells incubated for 24 h with a vehicle or mitoTEMPO. (E) Analysis of cellular pH using SNARF-l-AM. *p<0.05 versus vehicle.
High levels of mitochondrial ROS those are observed in cancer cells may contribute to signaling events that facilitates their highly proliferative phenotype. Decrease in cellular ROS may attenuate the activity of redox-sensitive Akt and Erk. Therefore, we tested if scavenging mitochondrial O2•− will attenuate proliferative and antiapoptotic signaling hallmarked by Akt and Erk phosphorylation. Indeed, we found that mitoTEMPO significantly decreased activation of Akt and Erk 1/2 as measured by their phosphorylation (Fig. 2B).
mitoTEMPO Inhibits Glycolysis and Improves Mitochondrial Metabolism
One of the most important features of melanoma cell is utilization of glycolysis as a main ATP source matching the high-energy demand that is required for aggressive proliferation and cell growth. In many cancer cells, phosphorylation of mitochondrial pyruvate dehydrogenase (PDH) inhibits the conversion of pyruvate to acetyl-CoA, thereby limits mitochondrial metabolism. These metabolic processes are regulated by ROS-sensitive HIF1-α. In this work, we tested if scavenging of mitochondrial superoxide affects HIF1-α expression, phosphorylation of mitochondrial PDH, and cellular ATP level. We found that mitoTEMPO decreased HIF1-α expression and inhibited PDH phosphorylation (Fig. 2B). These data suggest that mitoTEMPO improved mitochondrial function by reduction of mitochondrial O2•− and increased activity of PDH. Analysis of mitoTEMPO-treated melanoma cells showed a dose-dependent increase in the mitochondrial membrane potential (Fig. 2C). Further, mitoTEMPO decreased expression of cytoplasmic lactate dehydrogenase (LDH), which is a hallmark of glycolytic cancer metabolism. Diminished contribution of glycolysis to overall metabolism should result in decreased ATP levels in these cells. Indeed, our data show that mitoTEMPO significantly decreased the total ATP content in melanoma, but did not affect nonmalignant skin fibroblasts (Fig. 2D). Glycolytic metabolism increases outflow of protons due to intense generation of lactate; therefore, melanoma cells may have significantly lower pH. We tested if mitoTEMPO would affect pH in melanoma cells. Intracellular pH in melanoma cells was significantly lower compared with skin fibroblasts (pH 6.9 vs. pH 7.2), and mitoTEMPO treatment significantly increased pH in melanoma, but not in nonmalignant cells (Fig. 2E). These results further support metabolic changes in mitoTEMPO-treated cancer cells.
mitoTEMPO Inhibits Growth of Established Tumor in the Mouse Model of Human Melanoma
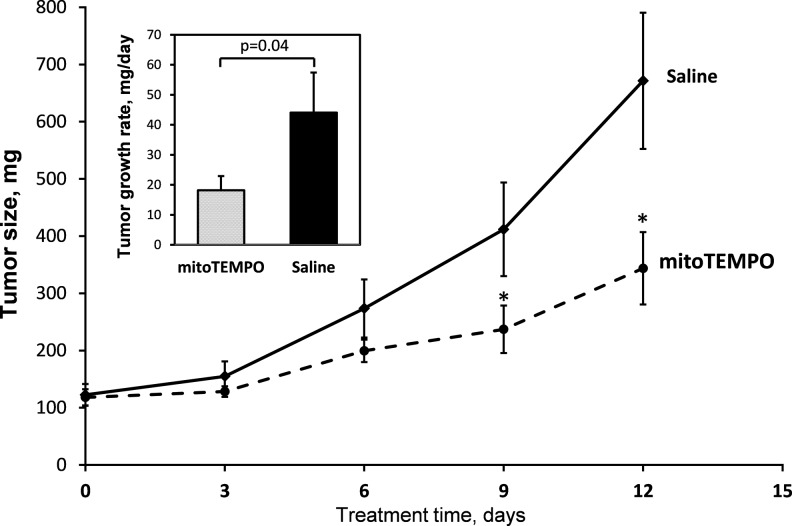
We performed in vitro and in vivo studies on the human melanoma cell line A375 to evaluate if described above effects are not limited to mouse species and confirmed the in vivo mitoTEMPO anticancer effect. Our preliminary experiments showed that similarly to B16 melanoma cells, A375 human melanoma cell growth, ATP production, and phosphorylation of key regulatory proteins, including Akt and PDH, were also inhibited by mitoTEMPO (not shown). Therefore, in the next step, we tested the effects of mitoTEMPO on A375 human cell tumor growth in a mouse model. First, we injected subcutaneously A375 human melanoma cells into nude mice to induce tumor. When tumor was well established and growing we implanted the pumps with saline (vehicle) or with mitoTEMPO. It was found that administration of mitoTEMPO for 12 days led to significant attenuation of tumor growth (Fig. 3, insert). After 9 days of mitoTEMPO treatment, the difference in the tumor size reached statistical significance (Fig. 3). These data show that scavenging of mitochondrial O2•− has impaired not only the growth of cultured melanoma cells but also human tumor progression in vivo.
FIG. 3.
Effect of mitoTEMPO on human melanoma growth in nude mice. Nude mice were injected with A375 human melanoma cells, and after tumor was well established and growing, we have implanted osmotic pumps with saline (vehicle) or with mitoTEMPO (1.5 mg/kg/day). MitoTEMPO significantly attenuated the tumor size at days 9 and 12 compared with the saline group (*p<0.05 vs. saline, n=7). Insert demonstrates significant inhibition of the tumor growth rate by 12-day mitoTEMPO infusion.
Conclusions and Future Directions
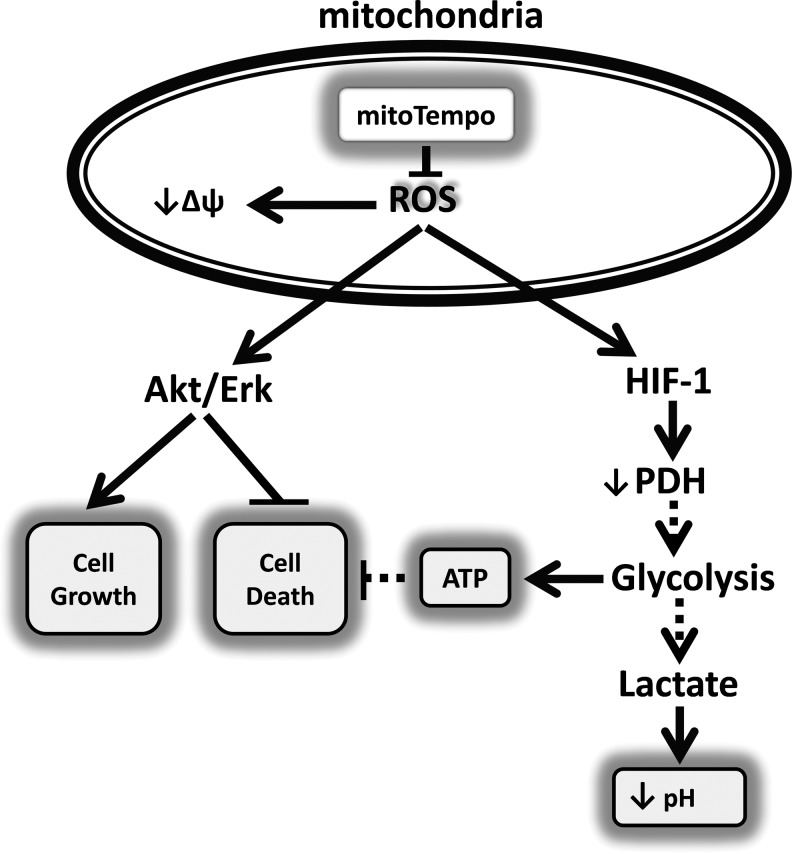
In this work, we have investigated the potential role of ROS-sensitive survival and metabolic pathways in anticancer activity of mitochondria-targeted antioxidants. For the first time, we show that the mitochondria-targeted SOD mimetic, mitoTEMPO, inhibits melanoma cell growth, reduces melanoma cell viability, and induces apoptosis, but does not affect nonmalignant skin fibroblasts. Our data show that diminished ROS production in mitoTEMPO-treated melanoma cells alters cell signaling mediated by ROS-sensitive Akt, Erk1/2, and HIF1-α and induces cell death (Fig. 4). We show that attenuation of these signaling pathways leads to dramatic changes in melanoma cell metabolism due to inhibition of glycolysis and decreased ATP. Our data therefore support the hypothesis that the anticancer activity of mitochondria-targeted antioxidants is likely mediated by inhibition of the ROS-sensitive Akt/Erk survival signaling pathways and glycolysis.
FIG. 4.
Proposed scheme of attenuation of cancer prosurvival signaling pathways by mitochondria-targeted antioxidants. Mitochondrial ROS decrease the Δψ, activates cytoplasmic redox-dependent Akt/Erk1/2 signaling promoting cell growth and survival, and increases expression of HIF1-α leading to PDH inactivation and activation of the glycolytic pathway. Blocking mitochondrial ROS by mitochondria-targeted antioxidants prevents Akt/Erk activation and decreases HIF1-α expression, leading to inhibition of glycolysis and reduced cancer cell growth and survival.
Previous studies suggested that the combinations of mitochondria targeted antioxidants with an antiglycolytic agent 2-deoxy-d-glucose or other anticancer drugs (3). In contrast to our work, these studies have not showed cancer cell death induced by antioxidants alone. Our work shows anticancer activity of mitoTEMPO at low nanomolar concentrations, but descending potential to suppress the melanoma cell proliferation and to induce apoptosis at high levels (Fig. 1). We think that high concentrations of mitochondria-targeted antioxidants may fail to inhibit redox signaling due to potential oxidation of the mitochondrial matrix and off-target effects of excessive mitochondria-targeted nitroxides or quinones.
It is conceivable that mitochondria-targeted antioxidants act on cancer cells not only by scavenging of O2•− but also through regulation of the redox status of the mitochondria. Indeed, MitoQ attenuated ROS production and destabilized HIF1-α in a human hepatoblastoma cell line (7). In experiments with p53 KO mice, Skulachev's group showed that SkQ1 attenuated development of cancer (1). Our work further supports an idea that mitochondria-targeted antioxidants, such as mitoTEMPO, inhibit redox-dependent HIF1-α-mediated glycolysis and cancer prosurvival signaling pathways. Further, mitoTEMPO without combination with other drugs caused cancer cell death and inhibited tumor growth in vivo.
It is important that melanoma cell death was not caused by mitoTEMPO-induced mitochondrial dysfunction. Indeed, our work shows that mitoTEMPO reduced mitochondrial ROS, increased mitochondrial membrane potential, and restored PDH activity, which is likely to improve mitochondrial metabolism. Attenuation of cytoplasmic glycolysis, ATP deprivation, and inhibition of Akt and Erk1/2 are likely the causes of cancer cell death. Upregulation of the prosurvival Akt and Erk1/2 signaling axis is commonly observed in various cancer cells, and targeting these signaling pathways by highly specific inhibitors arrests growth and triggers apoptosis (5). We suggest that mitochondria-targeted antioxidants may have therapeutic potential for cancer treatment because of their specific effect on an ROS-sensitive mechanisms regulating prosurvival signaling pathways (Fig. 4).
Previous reports have shown that mitochondrial O2•− may play a critical role in tumor development (6). These articles have reported that overexpression of SOD2 attenuates malignant transformation and inhibits tumor development, suggesting that targeting mitochondrial O2•− may be used for cancer treatment. Meanwhile, most of the currently used cancer treatments increase cellular ROS to induce cell apoptosis. Cancer cells however are well adapted to oxidative stress, and this approach may trigger cell proliferation through the ROS-sensitive pathways. This would explain drug resistance of many types of cancer. We propose a novel approach to treatment cancer, such as melanoma, by inhibition of mitochondrial O2•− that attenuates tumor as presented in our in vitro experiments and in vivo mouse model of human melanoma. Since mitoTEMPO treatment selectively affected cancer cell survival and metabolic pathways, it is conceivable that mitochondria-targeted antioxidants will not have deleterious side effects commonly observed as a consequence of anticancer therapy.
Notes
Materials and Methods
Reagents
MitoSOX was supplied by Roche Molecular Biochemicals. Actin and LDH rabbit polyclonal antibodies were obtained from Santa Cruz Biotechnology. Phospho-PDH polyclonal antibodies, phospho-Akt (Ser-473, Thr-308), and phospho-Erk1/2 (Thr-202/Tyr-204) antibodies were from Cell Signaling Technology, Inc. mitoTEMPO was purchased from Enzo Life Sciences. All other reagents were from Sigma.
Cell culture and cell count
Both mouse skin fibroblasts and B16 mouse melanoma cells (ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. The medium was supplemented with a vehicle or mitoTEMPO. The concentration of mitoTEMPO has been selected based on its effects. We choose 25 nM mitoTEMPO instead of standard 30 nM due to the maximal in vitro effects at 25 nM mitoTEMPO. Application of mitoTEMPO above 50 nM or below 5 nM did not improve the outcomes of experiments. For cell count, cells grown on 100-mm dishes were tripsinized, washed with phosphate-buffered saline (PBS), and counted using a Millipore Scepter automated cell counter.
Cell viability assay
Adherent cells were stained according to the Molecular Probes protocol (Viability test kit L-3224). Fluorescence was measured by a fluorescence microplate reader BioTek Synergy H1.
Cell apoptosis assay
Early apoptosis was measured in adherent cells by detection of externalized Annexin V using a commercial kit from BD Pharmingen. In early apoptosis, phosphatidylserine is translocated to outer site of the plasma membrane. In this assay, apoptotic cells are detected by Annexin V labeled with a fluorophore binding to phosphatidylserine translocated to the outer layer of the plasma membrane. Fluorescence was recorded with the fluorescence plate reader BioTek Synergy H1.
Cell senescence
Cells plated at a low density in 12-well plates were grown with a medium supplemented with a vehicle or various concentrations of mitoTEMPO. Cells were then washed with PBS, and β-galactosidase assay reagent was added to each well according to the manufacturer's instructions (Thermo Scientific). Absorbance at 405 nm was measured using the plate reader BioTek Synergy H1.
HPLC superoxide detection
Mitochondrial O2•− production was measured using a mitochondrion-targeted fluorescent probe MitoSOX (excitation/emission: 510/580 nm) as described (4). Cells were incubated with 2 μM MitoSOX in a Krebs–Hepes buffer for 20 min at 37°C in a CO2 incubator. Next, the cells were washed and collected for analysis. Cytoplasmic O2•− was measured by dihydroethydium (4). Fluorescent products were detected by HPLC with a fluorescence detector.
Western blots
Cells were lysed in a lysis buffer (50 mM Hepes, pH 7.4, 50 mM NaCl, 1% Triton X-100, 5 mM EDTA, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, and 1 mM sodium orthovanadate) plus anti-protease cocktail (Sigma), and total homogenates separated on 4%–20% PAGE-SDS Criterion precast gels (Bio-Rad). Protein expression was determined by enhanced chemiluminescence using specific antibodies.
Membrane potential
Adherent cells were stained for 20 min at 37°C with 40 nM tetramethylrhodamine methyl ester fluorescence dye. Then, the cells were washed with the Krebs–Hepes buffer, and the fluorescence was measured with the microplate fluorescence reader BioTek Synergy H1.
Cellular ATP
The levels of ATP were measured in lysed cells using a commercial kit from Abcam. This assay is used to determine the total levels of cellular ATP in cells. The ATP assay is based on the production of light released in the reaction of intracellular ATP with added luciferase and d-luciferin. The emitted light is proportional to the ATP concentration. Luminescence was detected by the microplate reader BioTek Synergy H1.
Cellular pH
Cells were loaded with a pH-sensitive 5 μM fluorescent probe, SNARF-l-AM, for 20 min at 37°C before fluorescence reading. Intracellular pH was calibrated according to the Molecular Probes protocol.
Mouse model of human melanoma
Human melanoma cells A375 were subcutaneously injected into 10 weeks old nude mice. We monitored progression of tumor growth, and once tumor was well established, mice were randomly assigned into the control or mitoTEMPO-treated group. Osmotic pumps with saline (vehicle) or 1.5 mg/kg were implanted into the mice, and the tumor volume was recorded every third day.
Statistics
All data are depicted as an average of at least three independent experiments±standard deviation of the mean. Statistical significance was determined and accepted at p<0.05 (*).
Abbreviations Used
- ATP
adenosine-5′-triphosphate
- CCCP
carbonyl cyanide m-chlorophenyl hydrazone
- DHE
dihydroethydium
- DMEM
Dulbecco's modified Eagle's medium
- LDH
lactate dehydrogenase
- mitoTEMPO
(2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride
- O2•−
superoxide
- PDH
pyruvate dehydrogenase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TMRM
tetramethylrhodamine methyl ester
Acknowledgment
This work was supported by funding from National Institutes of Health grant RO1 HL094469.
Author Disclosure Statement
Authors have no conflicts of interest to disclose.
References
- 1.Agapova LS. Chernyak BV. Domnina LV. Dugina VB. Efimenko AY. Fetisova EK. Ivanova OY. Kalinina NI. Khromova NV. Kopnin BP. Kopnin PB. Korotetskaya MV. Lichinitser MR. Lukashev AL. Pletjushkina OY. Popova EN. Skulachev MV. Shagieva GS. Stepanova EV. Titova EV. Tkachuk VA. Vasiliev JM. Skulachev VP. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 3. Inhibitory effect of SkQ1 on tumor development from p53-deficient cells. Biochemistry (Mosc) 2008;73:1300–1316. doi: 10.1134/s0006297908120031. [DOI] [PubMed] [Google Scholar]
- 2.Brar SS. Kennedy TP. Sturrock AB. Huecksteadt TP. Quinn MT. Whorton AR. Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:C1212–C1224. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 3.Cheng G. Zielonka J. Dranka BP. McAllister D. Mackinnon AC., Jr. Joseph J. Kalyanaraman B. Mitochondria targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012;72:2634–2644. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dikalova AE. Bikineyeva AT. Budzyn K. Nazarewicz RR. McCann L. Lewis W. Harrison DG. Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou GY. Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberley LW. Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 7.Sanjuan-Pla A. Cervera AM. Apostolova N. Garcia-Bou R. Victor VM. Murphy MP. McCreath KJ. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. FEBS Lett. 2005;579:2669–2674. doi: 10.1016/j.febslet.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 8.Trimmer C. Sotgia F. Whitaker-Menezes D. Balliet RM. Eaton G. Martinez-Outschoorn UE. Pavlides S. Howell A. Iozzo RV. Pestell RG. Scherer PE. Capozza F. Lisanti MP. Caveolin-1 and mitochondrial SOD2 (MnSOD) function as tumor suppressors in the stromal microenvironment: a new genetically tractable model for human cancer associated fibroblasts. Cancer Biol Ther. 2011;11:383–394. doi: 10.4161/cbt.11.4.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg F. Hamanaka R. Wheaton WW. Weinberg S. Joseph J. Lopez M. Kalyanaraman B. Mutlu GM. Budinger GR. Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]