Abstract
Fibrin gels are a promising material for use in promoting bone repair and regeneration due to their ease of implant formation, tailorability, biocompatibility, and degradation by natural processes. However, these materials lack necessary osteoconductivity to nucleate calcium, integrate with surrounding bone, and promote bone formation. Polymeric substrata formed from poly(lactide-co-glycolide) (PLG) are widely used in bone tissue engineering. A carbonated apatite layer of bone-like mineral can be successfully grown on the surface of PLG microspheres after a multiday incubation process in modified simulated body fluid. Such coatings improve the osteoconductivity of the polymer, provide nucleation sites for cell-secreted calcium, and enhance the potential osseointegration with host tissue. We examined the capacity of mineralized polymeric microspheres suspended within fibrin hydrogels to enhance the osteoconductivity of fibrin gels and increase the osteogenic potential of these materials. The inclusion of microparticles, both nonmineralized and mineralized, reduced the capacity of mesenchymal stem cells (MSCs) to contract the gel. When cultured in osteogenic media, we detected a near linear increase in both calcium and phosphate incorporation in gels containing mineralized microspheres and entrapped MSCs. The osteoconductivity of acellular fibrin gels with mineralized and nonmineralized microspheres was assessed in a rodent calvarial bone defect over 12 weeks. Compared to untreated rodent calvarial bone defects, we detected significant increases in early vascularization when treated with fibrin gels, with greater vascularization, on average, occurring with gels containing microspheres. We detected a trend for increased bone mineral density in gels containing mineralized microspheres after 12 weeks. These findings demonstrate that the osteoconductivity of fibrin gels can be increased by inclusion of mineralized microspheres, but additional signals may be required to rapidly accelerate bone repair.
Introduction
Fibrin is a promising material for bridging bone defects, as it facilitates cell attachment and proliferation, utilizes natural wound-healing mechanisms for remodeling and repair, and may be harvested in an autologous manner.1–4 Commercially available fibrin sealants have been examined for orthopedic repair; yet these constructs lack appropriate osteoconductivity and exhibit rapid gelation times that compromise the viability of encapsulated progenitor cells.5–7 Methods to tailor gel architecture frequently utilize high concentrations of fibrinogen or thrombin,8–10 yet large quantities of these clotting proteins are often not available from hemostatically compromised patients. As an alternative, we recently demonstrated that fibrin gels with increased mechanical properties, stable morphology, and the capacity to promote osteogenic differentiation of entrapped mesenchymal stem cells (MSCs) can be produced by supplementing the fibrinogen solution with NaCl before gelation.11 Despite producing hydrogels with increased stiffness that enhance osteogenesis, these materials require greater osteoconductivity to nucleate calcium, integrate with surrounding bone, and promote bone formation.
Polymeric substrata formed from poly(lactide-co-glycolide) (PLG) are widely used in bone tissue engineering and repair and have functioned as cell microcarriers, delivery devices for growth factors and drugs, and building blocks for three-dimensional scaffolds.12–14 A carbonated apatite layer of bone-like mineral can be successfully grown on the surface of PLG microspheres after a multiday incubation process in modified simulated body fluid (mSBF).15 Such coatings improve the osteoconductivity of the polymer, provide nucleation sites for cell-secreted calcium, and enhance the potential osseointegration with host tissue. Furthermore, they can bind osteoinductive molecules and modulate their controlled release.16–18 However, microsphere-based formulations tend to lose efficacy as they spatially disperse when delivered within a defect.
The formation of composite implants derived from fibrin and PLG microspheres may further enhance the advantages offered by each material for bone repair. The inclusion of a mineralized phase within fibrin constructs has exhibited a synergistic response with fibrin's hemostatic, mitogenic, and angiogenic attributes.18–21 However, the variations in density between the fibrin solution and bioceramics create challenges in maintaining the homogeneous distribution of the ceramic component. Polymeric microspheres are less dense than bioceramics such as β-tricalcium phosphate or hydroxyapatite, thus allowing for improved distribution throughout the gel and improved spatial interaction with osteogenic cells.
We hypothesized that the inclusion of mineralized polymeric microspheres within fibrin hydrogels would enhance the osteoconductivity of fibrin gels and increase the osteogenic potential of these materials. We supplemented fibrin hydrogels, formed from a formulation of salt and fibrinogen determined previously to possess the greatest osteogenic potential for human osteoprogenitor cells,11 with apatite-coated polymeric microspheres. We characterized the physical properties of these composite gels, as well as the osteogenic response of human MSCs in vitro. Finally, we examined the ability of composite fibrin hydrogels to promote bone repair using a rodent calvarial bone defect model.
Materials and Methods
Materials
Human fibrinogen and thrombin were purchased from Calbiochem (Gibbstown, NJ). Dulbecco's phosphate-buffered saline (PBS) (without calcium and magnesium) was obtained from Cellgro (Mediatech, Inc., Manassas, VA) and had the following formulation (g/L): KCl: 0.20; KH2PO4: 0.20; NaCl: 8.00; Na2HPO4 (anhydrous): 1.15. Aprotinin was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). PLG pellets (lactide:glycolide=85:15 DL High IV) were purchased from Lakeshore Biomaterials (Birmingham, AL). mSBF was prepared as previously described17,22,23 and consisted of the following reagents dissolved in distilled H2O: 141 mM NaCl, 5.0 mM CaCl2, 4.2 mM NaHCO3, 4.0 mM KCl, 2.0 mM KH2PO4, 1.0 mM MgCl2, and 0.5 mM MgSO4. The solution was held at pH=6.8 to avoid homogeneous precipitation of CaP phases. Human MSCs (hMSCs) were purchased from Lonza (Walkersville, MD) at passage 2 and expanded in the αMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (JR Scientific, Woodland, CA) and 1% penicillin/streptomycin (Mediatech, Inc.) until use at passage 4–6. All chemicals were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise stated.
Preparation of apatite-coated microspheres
PLG microspheres were formed using a standard double emulsion technique.24 To prepare mineralized polymeric substrates, microspheres were hydrolyzed for 10 min in 0.5 M NaOH to functionalize the polymer surface and rinsed in distilled H2O. Microspheres were immediately placed in mSBF and incubated at 37°C for 7 days, making sure to exchange the solution daily to maintain appropriate ion concentrations, frozen overnight at −80°C, and lyophilized for 3 days. Both nonmineralized and mineralized microspheres were strained through a testing sieve to collect particles with diameters less than 250 μm to avoid microsphere clumping. Microspheres were sterilized under ultraviolet light for 15 min before use.
Fabrication of fibrin gels
A solution of 40 mg/mL fibrinogen and 4.40% (w/v) NaCl (solution F, 1 mL) was mixed in equal volume with a human thrombin solution (solution T, 1 mL) at a concentration of 5 U/mL in 40 mM CaCl2, all in PBS, and allowed to gel for 1 h at 37°C in cylindrical nylon washers (0.953-cm inner diameter).11 This fabrication process resulted in gels with a fibrinogen concentration of 20 mg/mL, 2.60% (w/v) supplemental NaCl concentration, and CaCl2 and thrombin concentrations of 20 mM and 2.5 U/mL, respectively. Composite gels were fabricated in the same manner with the addition of 10 mg/mL microspheres to solution F, resulting in gels with a total concentration of 5 mg/mL of either nonmineralized (NON) or mineralized (MIN) microspheres.
Characterization of gel morphology and physical properties
The morphological characteristics of composite fibrin gels were observed by scanning electron microscopy. Briefly, fibrin gels were produced in nylon washers as described above and allowed to gel for 1 h at 37°C. The gels were carefully removed from their molds using a spatula and placed in PBS for 1 h to swell. Gels were then bisected and placed in 1 mL of 3% glutaraldehyde overnight at 4°C. Gels were dehydrated in ethanol in 20-min intervals using the following ethanol dilutions in distilled H2O: 25%, 35%, 50%, 60%, 70%, 80%, 90%, 95%, and 100%. After critical point drying, substrates were gold coated using a sputter coater (Pelco Auto Sputter Coater SC-7, Redding, CA) and imaged with a scanning electron microscope (Philips XL30 TMP).
The ability of hMSCs to contract composite fibrin gels was analyzed with gels containing 250 KIU/mL aprotinin to prevent fibrinolysis using a previously described technique.25,26 Briefly, 250 μL of a gel solution was added to each well of a 24-well dish to create a thin film and allowed to incubate at 37°C for 1 h. To examine the cellular contribution toward gel contraction, 5×104 hMSCs were uniformly seeded on the films' surfaces and allowed to adhere for 1 h, and the seeding distribution was visually confirmed before initiating the assay. Afterward, a 25G needle was inserted between the wall and gel and encircled several times to release the gel from the wall and allow for uniform contraction. Media were added and changed after 3 days of culture. At 7 days, gels were washed in PBS and fixed with 10% buffered paraformaldehyde for 30 min. About 500 μL of the Ponceaue S solution was added to each well to enhance the contrast for imaging and measurement, and gels were imaged using a Brother DCP-8080DN Scanner. The average diameter of the contracted area was derived from one width and length measurement of the image using ImageJ (http://rsb.info.nih.gov/nih-image) and compared to the average diameter of acellular gels processed identically to calculate percent contraction.
Changes in turbidity resulting from the combination of microspheres with solutions F and T (100 μL of each) were quantified spectrophotometrically with a microplate reader (BIO-TEK Synergy HTTR, Wisnooski, VT) every 12 s at 550 nm for 1 h.5,11 Gelation time was determined by differentiating each kinetic curve to determine the maximum values.
The compressive moduli of acellular fibrin gels were measured using an Instron 3345 compressive testing system (Norwood, MA) as described.11 Briefly, gels were incubated for 1 h in PBS, blotted, and then loaded between two flat platens and compressed at 1 mm/min. Compressive moduli were calculated from the linear portions of the force–displacement graph for a strain ranging from 0% to 10% (n=5 for each group).
Measurement of cell migration and apoptosis within gels
The ability of hMSCs to migrate through composite fibrin gels was evaluated using a modified Boyden chamber assay. Cell culture inserts (BD Falcon FluoroBlok inserts, 6.5-mm diameter, 8-μm pore size) were dipped into a thin layer of a gelatin solution (0.1% bovine skin gelatin, Sigma) and used to form dual compartments in a 24-well tissue culture plate. About 100 μL of the fibrin gel solution was placed into the transwell insert and allowed to incubate for 1 h at 37°C, resulting in 1-mm-thick gels. For comparison of cellular invasion without impedence, empty transwells were treated in the same manner as the fibrin construct wells. Inserts were then placed into their companion plates, cells were seeded on top of the hydrogel layer (or empty transwell) at 50,000 cells/insert, and cells were allowed to adhere for 1 h. A complete medium (αMEM with 10% FBS/1% penicillin/streptomycin) was added to the insert, and the well and the plates were incubated at 37°C with 5% CO2 for 24 h. After culture, cells from below the insert membrane were lysed in a passive lysis buffer (n=4 per group), and the DNA quantity was determined using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen).
To determine apoptosis of hMSCs within fibrin gels, gels were finely minced after 7 days in culture and lysed in the protein lysis buffer containing 0.1% Triton X-100 (Sigma), 1% Tris-EDTA (Sigma), and 1% Protease Inhibitor Cocktail Set I (EMD Chemicals, Darmstadt, Germany). Apoptosis was quantitatively assessed by analyzing 100 μL of lysate per sample using a Caspase-Glo 3/7 assay (Promega, Madison, WI). Luminescence was detected on a microplate reader and normalized to the DNA content.
In vitro osteogenic response
To determine the osteogenic potential of composite fibrin gels, hMSCs were seeded in fibrin constructs at 1.5×106 cells/mL of the total gel solution. Cells and aprotinin were added to the solution F phase before mixing with solution T and immediately mixed, with all remaining steps as described above. The resulting gels were cultured in 12-well culture dishes with osteogenic media containing standard supplements (10 mM β-glycerophosphate, 50 μg/mL ascorbate-2-phosphate, and 10 nM dexamethasone) in a standard CO2 incubator on an XYZ shaker at 25 rpm. At 1, 7, and 21 days, gels were collected and minced before the addition of 500 μL of the passive lysis buffer (Promega). The mixture was incubated for 10 min on ice before brief sonication to break up the content, and then centrifuged at 10,000 rpm for 5 min at 4°C. The supernatant was assayed for the DNA content and the intracellular alkaline phosphatase (ALP) activity using p-nitrophenyl phosphate (PNPP) as described.17
Total RNA from gels was collected after 7, 14, or 21 days in culture. Gels were rinsed 1×in sterile PBS, minced with a sterile scalpel, and placed in 350 μL of the RLT buffer (Qiagen, Valencia, CA) supplemented with 10 μL/mL β-mercaptoethanol. Total RNA was purified using the RNeasy Mini kit (Qiagen) and 400 ng of total RNA reverse-transcribed with the QuantiTect Reverse Transcription Kit (Qiagen). qPCR was performed using TaqMan1 Universal PCR Master Mix (Applied Biosystems, Foster City, CA) on a Mastercycler1 realplex2 (Eppendorf, Westbury, NY); primers and probes for MRPL13 (Hs00204173_m1), RUNX2 (Hs00231692_m1), SP7 (Hs01866874_s1), and VEGFA (Hs00900055_m1) were purchased from Applied Biosystems. Amplification conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Quantitative PCR results were normalized to the RPL13 transcript level to yield ΔCt values.
To quantify cell-secreted mineral by entrapped hMSCs, fibrin gels fabricated as described above were washed twice with PBS, flash frozen in liquid nitrogen, and subsequently lyophilized for 3 days. The constructs were then exposed to 0.9 N H2SO4 for 24 h and the calcium content was analyzed using cresolphthalein complexone as described after subtracting the calcium contribution of acellular gels of each composition.11 Cell-secreted phosphate was measured using the QuantiChrom™ Phosphate Assay Kit (BioAssay Systems, Hayward, CA) as instructed, with the phosphate contribution of acellular gels subtracted from each experimental group.
In vivo osteogenic response
Treatment of experimental animals was in accordance with the UC Davis animal care guidelines and all National Institutes of Health animal-handling procedures. Bilateral calvarial defects were created in skeletally mature 10-week-old male nude rats.27 Animals were anesthetized and maintained using an isoflurane/O2 mixture delivered through a mask. A midlongitudinal incision was made on the dorsal surface of the cranium, and care was taken to ensure that the periosteum was completely cleared from the surface of the cranium by scraping. A trephine burr (Ace Dental Implant System, Brockton, MA) was used to create two circular 3.5-mm-diameter defects in the rat cranium, one on each side of the sagittal suture. The full thickness of the cranial bone was removed. Fibrin gels were prepared and allowed to sit for 4 min to achieve an adequate viscosity for injection. About 15 μL of the control fibrin solution, NON or MIN acellular solution was then pipetted into the defect, while other animals remained untreated to examine the natural healing response (n=6 per group per time point). To ensure sufficient gelation in the defect, the incision was closed after 5 min with a continuous suture (5–0 nylon; Ethicon, Somerville, NJ), and animals were allowed access to food and water ad libitum.
Three weeks postimplantation, animals were euthanized and calvaria were removed, fixed in 10% formalin for 24 h, and transferred to 70% ethanol. Bone healing at the defect sites was assessed radiographically. Briefly, the interior of the calvaria were placed in the supine position to ensure the defects remained flat against the film, and images were collected using a cabinet x-ray (model 43805N Faxitron X-ray Corp., Lincolnshire, IL) at 22kVp, 1.5 min, 3 mA with Kodak “Portal Pack for Localization” film. Each radiograph was digitized by a scanner, areas were calculated with ImageJ, and the radiological healing (%) was calculated as 100×(1-(area of the radiological defect/initial defect area). After imaging, calvaria were decalcified in EDTA, rinsed in PBS, and processed for histology using standard techniques. Paraffin-embedded tissue sections were cut at 5-μm thickness for subsequent analysis.
Vessel density was quantified as previously described.28,29 Briefly, H&E-stained histological sections were imaged at 100×magnification, and blood vessels within the margins of randomly selected, representative areas of each section were counted manually and normalized to the tissue area with the use of Adobe Photoshop software (Adobes Systems Incorporated, San Jose, CA). Vessels were defined as tubular structures with well-defined lumens containing at least one erythrocyte.
Cranial defects were also monitored noninvasively to follow bone formation longitudinally at 3, 7, and 12 weeks postimplantation using a commercially available computed tomography (CT) scanner, microCAT II (Siemens, Knoxville, TN).17,30 Animals were anesthetized and maintained with isoflurane (3.5% induction, 2.5% maintenance at 2.0 L/min). Three hundred sixty projections were acquired during a full rotation around the animal with the following scan parameters: 70 KvP, 500 mA, 200 ms per frame, and 30 calibration images (bright and dark fields). The image was reconstructed using the Feldkamp reconstruction algorithm as a 512×512×768 array with a corresponding voxel size of 0.097×0.097×0.097 mm. Image resolution was 68 μm.
After 12 weeks, all remaining animals were euthanized and ex vivo microCT was performed on formalin- and ethanol-fixed samples to corroborate in vivo microCT results. Setup and analyses were performed as described with a cylindrical region of interest with a 3.5-mm diameter and a 1.5-mm length centered in the defect (diameter fit to be core diameter plus kurf loss, the length fit to include all visible material on either side of the cortex).29
Statistical analysis
Data are presented as mean±standard deviation for at least three replicates. The statistical significance was assessed by one-way analysis of variance (ANOVA) followed by a Newman–Keuls post hoc test, and a p-value of less than 0.05 was considered statistically significant. Data in Figures 3, 4, and 5 were assessed by two-way ANOVA with Bonferroni post testing. Statistical analysis was performed using GraphPad Prism® 4 analysis software (GraphPad Software, San Diego, CA).
FIG. 3.
(A) Migration of human mesenchymal stem cells (hMSCs) across fibrin gels within transwells. (B) Quantification of the caspase 3/7 activity and normalized to the DNA content within each fibrin gel formulation. Chart values represent mean±SD; n=4; *p<0.001 versus empty. **p<0.01 versus control.
FIG. 4.
The alkaline phosphatase activity of hMSCs entrapped in fibrin gels. Chart values represent mean±SD; n=4; *p<0.05 versus control, #p<0.05 versus NON.
FIG. 5.
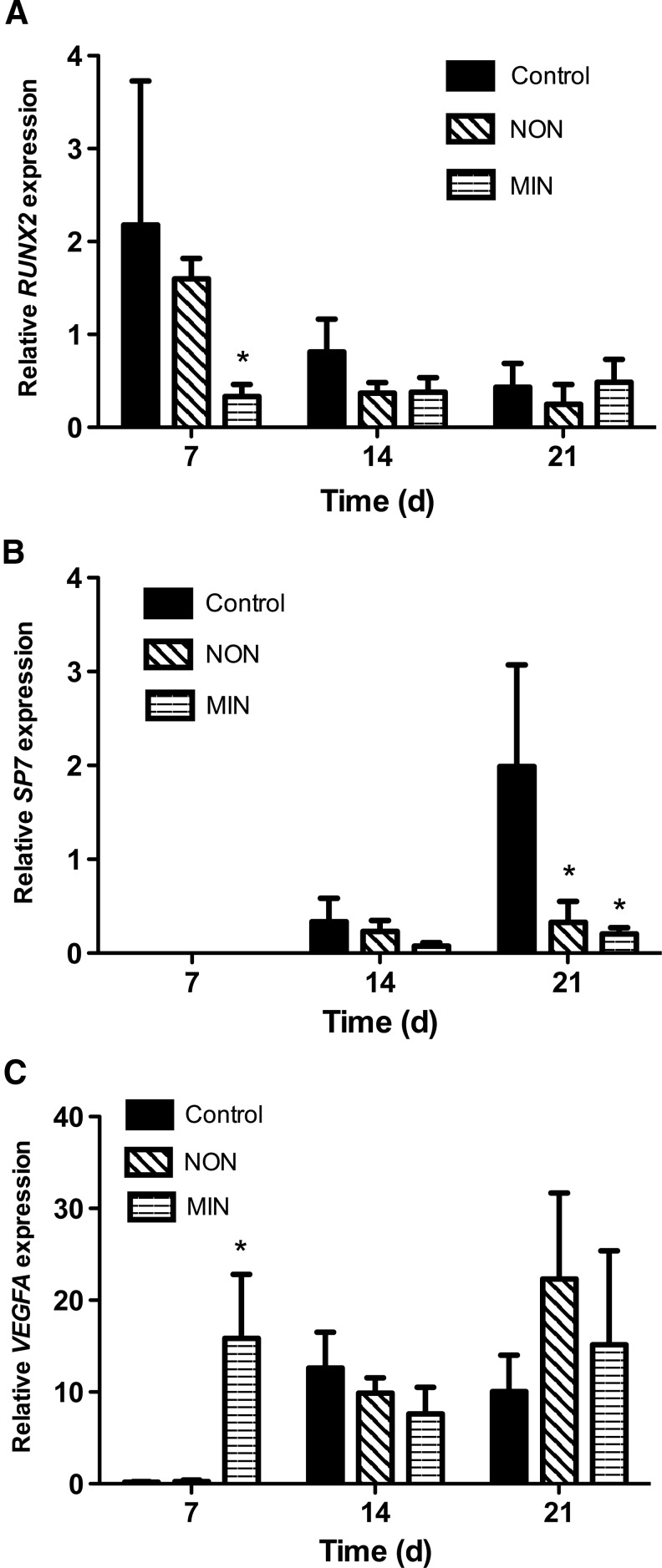
Gene expression for hMSCs entrapped in fibrin gels after 7-, 14- and 21-day culture. (A) RUNX2; (B) SP7; and (C) VEGFA. Chart values represent mean±SD; n=4; *p<0.05 versus control.
Results
Characterization of gel morphology and cellular response
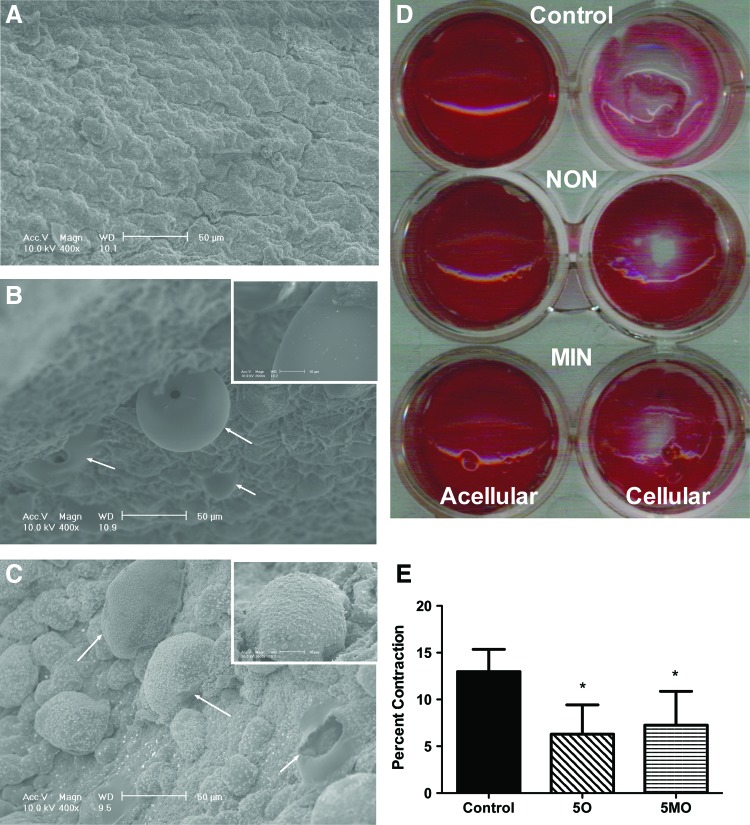
SEM imaging confirmed the inclusion of either nonmineralized or mineralized microspheres in the NON and MIN fibrin gel formulations, respectively (Fig. 1A–C). The density of microspheres per view appeared comparable for both gel composites. The presence of pores in microsphere walls was observed for both nonmineralized and mineralized groups. At high magnification, the appearance of apatite was visible on mineralized microspheres (Fig. 1C, insert).
FIG. 1.
SEM micrographs of fibrin gel cross sections of (A) control gels, (B) nonmineralized (NON), and (C) mineralized (MIN). Substrates imaged at 400×magnification; scale bar represents 50 μm. Arrows indicate the presence of microspheres. Insets imaged at 2000×; scale bar represents 10 μm. (D) Representative films after culture, fixation, and Ponceau staining to determine gel film contraction. (E) Quantification of the cellular gel diameter normalized to acellular gels. Chart values represent mean±SD; n=4; *p<0.05 versus control gels. Color images available online at www.liebertpub.com/tea
To assess the inherent ability of hMSCs to contract each gel, a modified two-dimensional contraction assay was used (Fig. 1D, E). After initial seeding, there were no apparent differences between acellular or cellular films, with NON and MIN films appearing slightly more opaque due to microsphere entrapment (data not shown). After 7 days, acellular gels for all groups remained unchanged from their initial morphology and filled the entire well area. However, cellular control gels contracted during the incubation period and were more transparent. Control gels contracted significantly more (13.0%±2.4% contraction) than the NON and MIN groups. Cells on NON and MIN films resulted in limited and comparable contraction (6.3%±3.1% and 7.3%±3.6%, respectively).
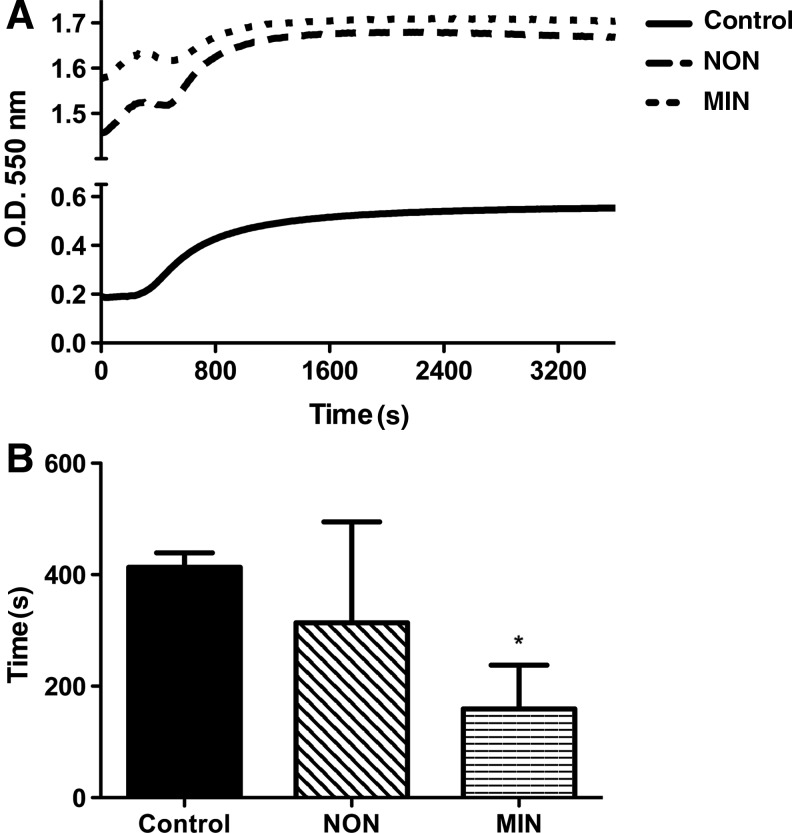
The addition of microspheres to fibrin gels increased the optical density compared to pregel solutions; yet all groups exhibited a similar increase in turbidity over the course of gelation (Fig. 2). The kinetic curves for the NON and MIN groups were not as smooth as the control group, as both experienced a lag in turbidity gain before reaching their final optical density. Gels formed with mineralized microspheres possessed significantly shorter gelation time than the other groups. We did not detect differences in compressive moduli between gel groups (approximately 13±5 kPa for all groups; data not shown).
FIG. 2.
(A) Optical density of fibrin gels as a function of time. (B) Gelation time of fibrin gels. Chart values represent mean±SD; n=4; *p<0.05 versus control.
The inclusion of microspheres in fibrin gels did not impair migration of hMSCs. As expected, hMSC migration was faster through empty wells, yet no differences in the cell number on the underside of the transwell were observed among any of the gel formulations after 24 h (Fig. 3A). When examining apoptosis of cells within gels, we detected a significant reduction in the caspase activity for hMSCs suspended in NON and MIN gels compared to fibrin gels without microspheres, but caspase 3/7 levels were similar between microsphere-containing gels (Fig. 3B). The DNA content was nearly identical within each gel formulation (data not shown).
The ALP activity, an early indicator of osteogenic differentiation, was significantly higher in hMSCs entrapped in fibrin gels without microspheres compared to composite gels after 7 days (Fig. 4). Additionally, cells entrapped in NON gels had a significantly higher ALP activity compared to cells in the MIN formulation. The ALP activity was not significantly different between constructs at 1 or 21 days. We did not detect differences in the DNA content within MSC-loaded gels at any time point, and absolute values of DNA did not change significantly over time (data not shown).
The expression of mRNA encoding osteogenic and angiogenic molecules was quantified with qPCR for hMSCs seeded in control, NON, and MIN fibrin gels (Fig. 5). RUNX2, a transcription factor necessary for osteogenesis and bone development, followed a similar trend as the ALP activity on day 7, with significantly lower expression in cells seeded in MIN gels than control gels. At day 21, RUNX2 expression was similar among all fibrin constructs. Osterix (SP7), another early-stage osteoblastic marker, was undetectable at day 7 for all fibrin constructs. By day 21, cells within NON and MIN formulations had significantly lower osterix expression than cells in control gels. VEGFA, a proangiogenic molecule responsible for proliferation and chemotaxis of endothelial cells, was significantly increased in hMSCs for the MIN gels at day 7, yet no differences were apparent at later time points.
We quantified the deposition of cell-secreted calcium and phosphate by entrapped hMSCs within each formulation over 21 days (Fig. 6). We measured both ions due to our addition of calcium to solution T and the presence of phosphate in the culture medium. Both curves were in good stoichiometric accordance for carbonated-substituted apatite. The mineral content in MIN formulations was significantly higher at day 21 than both the control and NON groups, and mineral contained within NON gels was greater than control gels. Interestingly, mineral accumulation (both calcium and phosphate) within the MIN composite gel occurred in a nearly linear fashion over time (R2=0.998 and 0.999, respectively).
FIG. 6.
Calcium (A) and phosphate (B) within hMSC-seeded fibrin gels. Chart values represent mean±SD; n=4; *p<0.05 versus control.
In vivo osteogenic response
Vascularization of regenerating bone is a critical factor in enhancing the production and maturity of new bone in the defect. Calvarial defects treated with microsphere-loaded gels exhibited similar and significantly increased vascularization compared to empty defects, with a trend for more vessels compared to defects treated with control gels (Fig. 7). We did not appreciate any statistical differences in vessel density for defects treated with MIN or NON gels. No differences in the vessel diameter between treatment groups were detectable (data not shown).
FIG. 7.
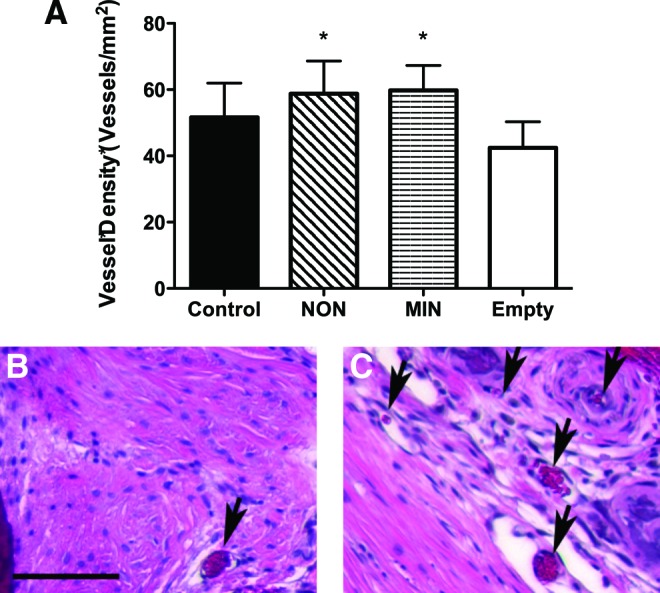
Vessel quantification in implanted fibrin gels. (A) Quantification of vascular density (vessels/mm2) from random histological sections 3 weeks after implantation. *p<0.05 versus empty defect. Representative H&E staining of (B) empty and (C) MIN fibrin gel-treated defect. Magnification is 100×; scale bar represents 100 μm. Arrows indicate blood vessels. Color images available online at www.liebertpub.com/tea
Bone healing was assessed radiographically after 3 weeks as an early indicator of the osteoconductive contribution to bone repair for composite fibrin gels (Fig. 8A, B). Defects treated with any of the gel formulations exhibited healing greater than untreated (empty) defects, with significant increases in healing observed for control and NON gels.
FIG. 8.
Analysis of osteogenic potential in vivo. (A) X-ray examination of the acellular empty, control, NON, and MIN-treated defects 3 weeks after implantation. (B) Percent area radiological defect healing at 3 weeks. (C) Bone mineral density within regenerating defects at 3, 7, and 12 weeks. Chart values represent mean±SD; n=6 per time point; *p<0.05 versus empty defect.
In vivo microCT was used to screen for bone formation over time in a constant cohort of animals at 3, 7, and 12 weeks (Fig. 8C). We observed a similar trend as data from the radiological assessment at 3 weeks, yet significant differences between groups were not apparent. CT imaging revealed increases in bone density within the defect over the 12-week duration for all groups, with defects treated with MIN gels exhibiting greater mineralized bone densities at 12 weeks. Reconstructed microCT images were obtained from the scanning of explanted calvarial tissue at 12 weeks to corroborate in vivo microCT results. We did not detect significant differences between groups in either the bone volume fraction or bone mineral density, although the MIN group had increased average values for both measures (data not shown).
Discussion
In this study, we describe the development and characterization of composite fibrin gels containing apatite-coated polymeric microspheres and their suitability as biomaterials for bone tissue engineering. In light of their weak physical properties, fibrin gels and other hydrogel systems have great promise for use in nonweight bearing bone healing such as the head and neck region. The gel may serve as an effective carrier vehicle for transplanting cells and bridging tissue defects. Although promising results have been reported previously using a fibrin-PLG microsphere composite for cartilage engineering,31 the system remained relatively uncharacterized regarding applications for bone repair. We hypothesized that the addition of mineral in substrates for bone repair would enhance the osteoconductivity of fibrin gels and potentially improve osseointegration with surrounding native tissue, leading to more seamless transitions between stable and remodeling or new bone. Our findings demonstrated that the inclusion of mineralized microspheres provided nucleation sites for calcium and phosphate secreted by entrapped osteogenic cells. However, the implantation of acellular materials did not result in significant increases in bone formation. Collectively, these data demonstrate the promise of incorporating mineralized substrata in fibrin gels for enhancing osteoconductivity, while revealing the need for additional contributions to drive bone formation in vivo.
Scanning electron microscopy confirmed the successful incorporation of both nonmineralized and mineralized microspheres and provided evidence that microspheres did not simply diffuse out of the construct during hydrogel swelling. Surprisingly, the incorporation of both types of microspheres appeared similar in distribution within the gels and displacement above the surface, even though nonmineralized microspheres presented a hydrophobic surface and fibrin is a hydrophilic natural polymer.
During bone growth, osteogenic cells remodel matrices to deposit new material in an organized manner conducive to nucleating mineral. If a material does not provide enough resistance against cell contractile forces, the material will degrade too rapidly before a proper wound-healing response can be initiated, or cells might differentiate down an undesirable lineage.32,33 In a prior study, three-dimensional fibrin constructs were used to analyze cell-mediated contraction, and no significant contraction in both acellular and cell encapsulated gels was observed after 7 days in culture.11 In this study, we used a well-described two-dimensional setup to analyze cell-mediated forces against our constructs to eliminate the contribution of destructive interference of forces along the z-axis. Similar to the prior study, none of the acellular gels contracted during incubation. For cell-laden gels, control gels exhibited significant contractions during the culture duration compared to microsphere-containing gels. Resistance to cellular contraction was dependent on microsphere inclusion and not mineralization, as both NON and MIN groups had comparative, limited contraction. The inclusion of biodegradable microspheres thus offers an approach for maintaining the volume of hydrogels.
The inclusion of polymer microspheres, regardless of mineral, resulted in higher optical densities than the control group at time zero due to the dispersion of microspheres within the otherwise transparent gel. Additionally, a lag was noted for both groups during the steep rise in optical density that was not present in the control group's kinetic curve. Zhao et al. attributed this pause to settling of microspheres in gel.31 However, the kinetic curve generated with the least thrombin and fibrinogen, which was the most comparable to our formulations, resulted in large dips in optical density compared to the lag we observed. This is suggestive that microsphere settling in the NON and MIN systems contributed to a limited extent compared to Zhao's study, perhaps due to the use of PBS instead of distilled water to form gels. The inclusion of mineralized microspheres decreased the gelation time compared to the other two groups. Calcium shortens the time necessary to convert monomeric fibrinogen to fibrin in the presence of thrombin, thereby reducing the gelation time.34–36 The calcium present in the apatite coatings of the mineralized microspheres in the MIN group may act to stabilize the fibrinogen in a configuration more accessible to thrombin.
In agreement with similar studies, the ALP activity of hMSCs encapsulated within the fibrin constructs was not increased in the presence of apatite-coated substrates.17,37 However, the addition of nonmineralized microspheres also significantly decreased ALP levels compared to the control group. We and others have reported that the ALP activity correlates with compressive moduli of fibrin constructs,8,11 yet we did not observe differences in Young's moduli between these groups. RUNX2 and osterix (SP7) expression followed similar trends as the ALP activity, with cells entrapped in microsphere-containing gels exhibiting lower levels of gene expression compared to control gels. Others have reported that matrix mineralization does not consistently follow the same trend as RUNX2, as there are other pathways that act alongside, or independent of, RUNX2 during osteogenesis.38,39 However, cell-secreted calcium and phosphate were significantly greater for MIN gels at day 21. Thus, the presentation of mineralized microspheres within the fibrin gel provides nucleation sites for mineral accumulation and demonstrates that more osteoconductive implants may be formed by simple inclusion of mineralized substrates. We also detected reduced apoptosis for hMSCs in gels containing either microsphere formulation compared to control gels. Previously, the inclusion of gelatin microspheres within agarose gels enhanced cell viability and reduced apoptosis for entrapped hMSCs by presenting additional binding sites to facilitate cell adhesion and enabling cells to spread out to their natural morphology.40 Although anchorage-dependent cells adhere to fibrin gels better than many other hydrogels, the addition of microspheres in these formulations may act similarly to enhance adhesion and spreading.
VEGFA expression was significantly increased for the MIN group at day 7 and all groups expressed similar amounts by day 14 and 21. In other studies, VEGF secretion by hMSCs was increased by the presence of mineral.29 hMSCs produce trophic factors to recruit other cell populations to drive tissue repair and enhance angiogenesis and neovascularization, critical processes for nutrient transport in the early stages of regeneration.41,42 Thus, the MIN group may demonstrate increased utility at the early stages of bone formation by increasing blood vessel counts, particularly when used as a delivery vehicle for hMSCs or other progenitor cell populations.
The in vivo studies suggest the possibility of the constructs supporting early bone formation, as radiological healing was increased over the empty defect after 3 weeks. The addition of fibrin gels, regardless of composition, enhanced healing compared to empty defects, and gels loaded with nonmineralized microspheres yielded the greatest defect closure, on average. Defects treated with fibrin gels exhibited increased neovascularization compared to untreated defects, and gels loaded with microspheres further increased vessel density compared to control gels. Despite a trend in bone repair for fibrin gel-treated defects at 3 weeks, we failed to detect significant differences between the experimental groups and the empty defect after 12 weeks. Using fibrin gel containing particulates of PLG-hydroxyapatite substrates coated with bone-like mineral to treat 8-mm calvarial defects, Kim et al. reported significant increases in bone formation after 8 weeks compared to fibrin gel alone.18 Although no details are presented about the fibrin gel, the presence of additional hydroxyapatite in the system may provide sufficiently greater osteoconductivity to nucleate the calcium secreted by invading host cells. In light of the protein concentration used to fabricate gels in these studies, fibrin implants likely degraded in situ within the first weeks after implantation. Therefore, these constructs may elicit a more robust osteogenic response by implanting cell-seeded constructs or entrapping a potent osteoinductive or chemoattractant molecule for local, sustained release. Importantly, the defects used in this study were not critical sized, and thus, an improvement in early bone repair might translate to a larger enhancement of regeneration in an irradiated or critical-sized defect model.
With regard to bone formation, the entrapment of apatite-coated microspheres within fibrin gels offers several unique advantages. Given the potential to adsorb proteins to the mineral coat or entrap them within polymer microspheres, the inclusion of microspheres may enable a multisignal, temporally regulated drug delivery scheme that can be further modulated by the properties of the fibrin gel. Recent studies demonstrate the utility of mineral coatings to provide a platform for temporally controlled protein release, and protein release can be predictably controlled by regulating mineral morphology and dissolution rate.43 By controlling the physical properties of fibrin gels (i.e., protein concentration and crosslinking density), protein release may be further tailored for the desired presentation at the defect site. Thus, composite hydrogels formed from the inclusion of apatite-coated microspheres entrapped in fibrin may hold great utility to the field of bone repair.
Conclusions
Fibrin–apatite-PLG-microsphere composites were fabricated and evaluated for use in bone repair. The presence of microspheres alone decreased cell-mediated contraction and modulated other osteogenic signals. The inclusion of apatite-coated microspheres significantly increased mineralization of fibrin gels in vitro. All gel groups demonstrated the potential for improving the early bone formation response in vivo. The lack of a generally robust bone-healing response suggests these composite gel substrates may have greater utility when used in combination with other osteogenic stimuli.
Acknowledgments
This project was supported by NIH Grant R03-DE021704, USAMRAA Grant W81XWH-10-1-0984, and the CMF Clinical Priority Program of the AO Foundation (Project no. C10-39L) to JKL. HED was partially supported by a NASA Harriett G. Jenkins Predoctoral Fellowship (NNX08AY31G) and an Achievement Rewards for College Scientists (ARCS) scholarship.
Disclosure Statement
No competing financial interests exist.
References
- 1.Victor W.M.H. Annemie C. Pieter K. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 2.Janmey P.A. Winer J.P. Weisel J.W. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6:1. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breen A. O'Brien T. Pandit A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng Part B-Rev. 2009;15:201. doi: 10.1089/ten.TEB.2008.0527. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed T.A.E. Dare E.V. Hincke M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng Part B-Rev. 2008;14:199. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H.G. Ma L. Zhou J. Mao Z.W. Gao C.Y. Shen J.C. Fabrication and physical and biological properties of fibrin gel derived from human plasma. Biomed Mater. 2008;3:9. doi: 10.1088/1748-6041/3/1/015001. [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y. Seong Boo J. Ozawa R. Nagasaka T. Okazaki Y. Hata K.-I., et al. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Craniomaxillofac Surg. 2003;31:27. doi: 10.1016/s1010-5182(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 7.Dickneite G. Metzner H. Pfeifer T. Kroez M. Witzke G. A comparison of fibrin sealants in relation to their in vitro and in vivo properties. Thromb Res. 2003;112:73. doi: 10.1016/j.thromres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Ho W. Tawil B. Dunn J.C.Y. Wu B.M. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 9.Duong H. Wu B. Tawil B. Modulation of 3D fibrin matrix stiffness by intrinsic fibrinogen and thrombin compositions and by extrinsic cellular activity. Tissue Eng Part A. 2009;15:1865. doi: 10.1089/ten.tea.2008.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catelas I. Sese N. Wu B.M. Dunn J.C.Y. Helgerson S. Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 11.Davis H.E. Miller S.L. Case E.M. Leach J.K. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011;7:691. doi: 10.1016/j.actbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Davis H. Leach J. Designing bioactive delivery systems for tissue regeneration. Ann Biomed Eng. 2011;39:1. doi: 10.1007/s10439-010-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K. Silva E.A. Mooney D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malda J. Frondoza C.G. Microcarriers in the engineering of cartilage and bone. Trends Biotechnol. 2006;24:299. doi: 10.1016/j.tibtech.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Jongpaiboonkit L. Franklin-Ford T. Murphy W.L. Growth of hydroxyapatite coatings on biodegradable polymer microspheres. ACS Appl Mater Interfaces. 2009;1:1504. doi: 10.1021/am9001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jongpaiboonkit L. Franklin-Ford T. Murphy W.L. Mineral-coated polymer microspheres for controlled protein binding and release. Adv Mater. 2009;21:1960. [Google Scholar]
- 17.Davis H.E. Rao R.R. He J. Leach J.K. Biomimetic scaffolds fabricated from apatite-coated polymer microspheres. J Biomed Mater Res A. 2009;90:1021. doi: 10.1002/jbm.a.32169. [DOI] [PubMed] [Google Scholar]
- 18.Kim S.S. Gwak S.J. Kim B.S. Orthotopic bone formation by implantation of apatite-coated poly(lactide-co-glycolide)/hydroxyapatite composite particulates and bone morphogenetic protein-2. J Biomed Mater Res A. 2008;87:245. doi: 10.1002/jbm.a.31782. [DOI] [PubMed] [Google Scholar]
- 19.Haiguang Z. Lie M. Changyou G. Jinfu W. Jiacong S. Fabrication and properties of injectable beta -tricalcium phosphate particles/fibrin gel composite scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2009;29:836. [Google Scholar]
- 20.Le Nihouannen D. Le Guehennec L. Rouillon T. Pilet P. Bilban M. Layrolle P., et al. Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering. Biomaterials. 2006;27:2716. doi: 10.1016/j.biomaterials.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 21.Osathanon T. Linnes M.L. Rajachar R.M. Ratner B.D. Somerman M.J. Giachelli C.M. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials. 2008;29:4091. doi: 10.1016/j.biomaterials.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy W.L. Kohn D.H. Mooney D.J. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J Biomed Mater Res. 2000;50:50. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Davis H.E. Case E.M. Miller S.L. Genetos D.C. Leach J.K. Osteogenic response to BMP-2 of hMSCs grown on apatite-coated scaffolds. Biotechnol Bioeng. 2011;108:2727. doi: 10.1002/bit.23227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen S. Yoshioka T. Lucarelli M. Hwang L.H. Langer R. Controlled delivery systems for proteins based on poly(lactic glycolic acid) microspheres. Pharm Res. 1991;8:713. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 25.Even-Ram S. Fibrin gel model for assessment of cellular contractility. In: Even-Ram S., editor; Artym V., editor. Extracellular Matrix Protocols. New York, NY: Humana Press; 2009. pp. 1–9. [DOI] [PubMed] [Google Scholar]
- 26.Ghajar C.M. Kachgal S. Kniazeva E. Mori H. Costes S.V. George S.C., et al. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res. 2010;316:813. doi: 10.1016/j.yexcr.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leu A. Stieger S.M. Dayton P. Ferrara K.W. Leach J.K. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng Part A. 2009;15:877. doi: 10.1089/ten.tea.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J. Decaris M.L. Leach J.K. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng Part A. 2012;18:1520. doi: 10.1089/ten.tea.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J. Genetos D.C. Leach J.K. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng Part A. 2010;16:127. doi: 10.1089/ten.tea.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung W.K. Working D.M. Galuppo L.D. Leach J.K. Osteogenic comparison of expanded and uncultured adipose stromal cells. Cytotherapy. 2010;12:554. doi: 10.3109/14653241003709694. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H. Ma L. Gao C. Shen J. A composite scaffold of PLGA microspheres/fibrin gel for cartilage tissue engineering: Fabrication, physical properties, and cell responsiveness. J Biomed Mater Res B Appl Biomater. 2009;88B:240. doi: 10.1002/jbm.b.31174. [DOI] [PubMed] [Google Scholar]
- 32.Engler A.J. Sweeney H.L. Discher D.E. Schwarzbauer J.E. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact. 2007;7:335. [PubMed] [Google Scholar]
- 33.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Ratnoff O.D. Potts A.M. The accelerating effect of calcium and other cations on the conversion of fibrinogen to fibrin. J Clin Invest. 1954;33:206. doi: 10.1172/JCI102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada M. Blombäck B. Factors Influencing fibrin gel structure studied by flow measurement. Ann N Y Acad Sci. 1983;408:233. doi: 10.1111/j.1749-6632.1983.tb23248.x. [DOI] [PubMed] [Google Scholar]
- 36.Okada M. Blomback B. Calcium and fibrin gel structure. Thromb Res. 1983;29:269. doi: 10.1016/0049-3848(83)90039-7. [DOI] [PubMed] [Google Scholar]
- 37.Murphy W.L. Hsiong S. Richardson T.P. Simmons C.A. Mooney D.J. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials. 2005;26:303. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 38.Celil A.B. Hollinger J.O. Campbell P.G. Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J Cell Biochem. 2005;95:518. doi: 10.1002/jcb.20429. [DOI] [PubMed] [Google Scholar]
- 39.Ling L. Nurcombe V. Cool S.M. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433:1. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Wang C. Gong Y. Zhong Y. Yao Y. Su K. Wang D.A. The control of anchorage-dependent cell behavior within a hydrogel/microcarrier system in an osteogenic model. Biomaterials. 2009;30:2259. doi: 10.1016/j.biomaterials.2008.12.072. [DOI] [PubMed] [Google Scholar]
- 41.Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 42.Hoch A.I. Binder B.Y. Genetos D.C. Leach J.K. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J.S. Suarez-Gonzalez D. Murphy W.L. Mineral coatings for temporally controlled delivery of multiple proteins. Adv Mater. 2011;23:4279. doi: 10.1002/adma.201100060. [DOI] [PMC free article] [PubMed] [Google Scholar]