Abstract
Background
The recent series of reviews conducted within the Global Action Plan for Pneumonia and Diarrhoea (GAPPD) addressed epidemiology of the two deadly diseases at the global and regional level; it also estimated the effectiveness of interventions, barriers to achieving high coverage and the main implications for health policy. The aim of this paper is to provide the estimates of childhood pneumonia at the country level. This should allow national policy–makers and stakeholders to implement proposed policies in the World Health Organization (WHO) and UNICEF member countries.
Methods
We conducted a series of systematic reviews to update previous estimates of the global, regional and national burden of childhood pneumonia incidence, severe morbidity, mortality, risk factors and specific contributions of the most common pathogens: Streptococcus pneumoniae (SP), Haemophilus influenzae type B (Hib), respiratory syncytial virus (RSV) and influenza virus (flu). We distributed the global and regional–level estimates of the number of cases, severe cases and deaths from childhood pneumonia in 2010–2011 by specific countries using an epidemiological model. The model was based on the prevalence of the five main risk factors for childhood pneumonia within countries (malnutrition, low birth weight, non–exclusive breastfeeding in the first four months, solid fuel use and crowding) and risk effect sizes estimated using meta–analysis.
Findings
The incidence of community–acquired childhood pneumonia in low– and middle–income countries (LMIC) in the year 2010, using World Health Organization's definition, was about 0.22 (interquartile range (IQR) 0.11–0.51) episodes per child–year (e/cy), with 11.5% (IQR 8.0–33.0%) of cases progressing to severe episodes. This is a reduction of nearly 25% over the past decade, which is consistent with observed reductions in the prevalence of risk factors for pneumonia throughout LMIC. At the level of pneumonia incidence, RSV is the most common pathogen, present in about 29% of all episodes, followed by influenza (17%). The contribution of different pathogens varies by pneumonia severity strata, with viral etiologies becoming relatively less important and most deaths in 2010 caused by the main bacterial agents – SP (33%) and Hib (16%), accounting for vaccine use against these two pathogens.
Conclusions
In comparison to 2000, the primary epidemiological evidence contributing to the models of childhood pneumonia burden has improved only slightly; all estimates have wide uncertainty bounds. Still, there is evidence of a decreasing trend for all measures of the burden over the period 2000–2010. The estimates of pneumonia incidence, severe morbidity, mortality and etiology, although each derived from different and independent data, are internally consistent – lending credibility to the new set of estimates. Pneumonia continues to be the leading cause of both morbidity and mortality for young children beyond the neonatal period and requires ongoing strategies and progress to reduce the burden further.
Pneumonia is still the leading cause of child mortality globally [1,2]. However, an increased focus on the reduction of child mortality that arose from the United Nation's Millennium Declaration [3] and the Millennium Development Goal 4 has renewed the interest in developing more accurate estimates of the causes of child deaths. This should inform more effective health policies and track the progress of their impact. In 2001, the Child Health Epidemiology Reference Group (CHERG) – a group of independent technical experts funded by The Gates Foundation and working closely with the World Health Organization (WHO) and UNICEF– set out to systematically review and improve data collection, methods and estimates of the main causes of child deaths for 2000 [4]. Evidence from CHERG estimates – ie, that pneumonia was the leading cause of child mortality –contributed to the initiation of a number of global efforts, such as the Global Action Plan for Pneumonia (GAPP). GAPP was designed to promote the expansion and improvement in community case management, the reduction in risk factors for disease and the support for the massive roll–out of vaccination against Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae (SP) by countries through support from the GAVI Alliance [5,6]. Those efforts, alongside economic and social developments observed in many low– and middle–income countries over the past decade, have all contributed to a substantial reduction of the burden of morbidity and mortality from childhood pneumonia [7].
CHERG's work also led to several Lancet series that had a substantial impact on global, regional and national–level donors and policy–makers [7–10]. It also inspired similar efforts to address the epidemiology and provide estimates for other causes of the global burden of different diseases [11,12]. The recent series of reviews published in the Lancet and PLoS Medicine, conducted by CHERG members in collaboration with the WHO, UNICEF and USAID within the Global Action Plan for Pneumonia and Diarrhoea (GAPPD), addressed the epidemiology and the current global burden of the two leading causes of childhood death, pneumonia and diarrhea, in the year 2010–11 [13]. The series also estimated the importance of risk factors [13], effectiveness of interventions [14], barriers to achieving high coverage at the community level [15], validity of coverage measures [16–17] and main implications for health policy [7].
The recent GAPPD reviews focused at the global and regional level [13]. The aim of this paper is to supplement the Lancet's GAPPD series with further information on the underlying models and methods, to augment that already available, and thereby assure that all input data and detailed descriptions of methods are transparently presented and available in an open–access source. Additionally, this paper also provides estimates of childhood pneumonia burden at the country level to allow national policy–makers and other stakeholders to implement the proposed policies in the World Health Organization (WHO) and UNICEF member countries.
Challenges to estimation of childhood pneumonia burden
Incidence and severe morbidity. An accurate estimate of the global, regional and national burden of childhood pneumonia is very difficult to make for a number of reasons. First, the incidence of pneumonia can only be properly assessed through longitudinal community based studies [18]. Such studies are very scarce in low and middle–income countries, where the majority of the pneumonia disease burden occurs, in part because they require a major commitment from both the investigators and research funders in a low–resource setting over an extended period of time. Due to the seasonal nature of pneumonia incidence, which has various peaks in different seasons, studies measuring incidence need to be conducted over full calendar years (or multiple 12–month periods) [19]. The screening of large numbers of children needs to be active, regular and frequent (eg, no longer than 2 weeks between home visits), because recall bias leads to under–estimation especially in large families [19]. In addition to these basic methodological requirements, the most fundamental uncertainty with measuring the incidence of childhood pneumonia in a community setting comes from the choice of case definition and the accuracy of its application by the assessor who establishes the diagnosis. Since pneumonia is actually a diagnosis made on tissue pathology, there is no clinical definition that is fully accurate. In any community–based study on pneumonia incidence, the measured entity is not in fact childhood pneumonia itself, but rather the incidence of children who test positive for the chosen case definition of childhood pneumonia [20]. The case definition is based on a number of symptoms and signs; although the WHO definition of childhood pneumonia (cough or difficulty breathing and an elevated respiratory rate, defined according to the child’s age) is the most frequently used in field studies, other definitions are often encountered in the literature confounding cross study comparisons of incidence. Depending on the combination of sensitivity and specificity of the chosen case definition, the burden of “true” pneumonia in the community of children can be grossly over– or underestimated [20].
A further problem is that the clinical training of assessors differs between the studies and this often affects the application of case definitions, unless the study implementation is highly controlled. Physicians tend to use their own clinical judgment in addition to the case definition. They will be likely to provide more conservative estimates, while community health workers may over–diagnose pneumonia in a community to the level where they consider a high proportion of acute respiratory infections in a child as cases of “pneumonia” [19]. Moreover, it is important to understand for each study whether the investigators attempted to exclude cases of respiratory disease that met the clinical pneumonia case definition but were assessed in some other way as being bronchiolitis, pertussis, measles, or even asthma, malaria or neonatal sepsis.
The effect of these challenges was reflected in the first–ever attempt to estimate the incidence of childhood pneumonia, which identified only 28 studies that met the minimum quality criteria [18]. The incidence of pneumonia reported in these studies still ranged 100–fold between their minimum and maximum reported incidence rates per child–year which could reflect true heterogeneity in the burden of disease or more likely also reflects the challenges of standardizing the epidemiologic study design and its application at the field level [18]. Similar, if not greater problems are encountered with estimating the incidence of severe, life–threatening pneumonia (which requires hospital referral and treatment) in the community. This estimate cannot be based on measures of childhood pneumonia hospitalizations because parents’ care seeking behavior, access to hospitals, and medical professionals’ threshold for admission varies widely within and across geographic settings [19]. There are WHO definitions for severe pneumonia (cough and difficulty breathing with lower chest wall indrawing) and for very severe pneumonia (cough and difficulty breathing with danger signs). These definitions are useful insofar as they are applied at the community level for guiding the case management and referral of children to a hospital, hence are purposefully highly sensitive and poorly specific for truly life threatening disease. Therefore, estimates of the incidence of severe childhood pneumonia in the community are particularly rare. Moreover, great caution must be applied in making comparisons between studies or in combining data across studies to assure that only similarly designed and implemented case definitions are considered together. The best estimates of pneumonia usually come from the control arms of randomized controlled trials of vaccines. This is because severe pneumonia is usually an outcome that is being monitored over a multiple of 12 months, usually with a highly stipulated and rigorously implemented case definition. Such studies provide the best estimates of severe pneumonia in the community that we have today [19].
Mortality. Estimating mortality that results from childhood pneumonia in a community also has its significant methodological challenges. Mortality studies require similar study designs to incidence studies, although home visits do not need to be as frequent as in the former, because care–giver recall of a child death is more accurate and long–lasting than of an illness episode [21,22]. Identifying the exact cause of death can be difficult in an appreciable number of cases. The assigned cause of death is usually based on a verbal autopsy provided by a mother or another family member. These are typically based on the report of signs and symptoms around the time of death. Many of them are not specific to pneumonia, but can also be found in children with other conditions, such as sepsis and malaria. In addition, many dying children have suffered from chronic malnutrition and may have other underlying ailments, such as asthma, metabolic disorders, immunodeficient conditions (HIV), sequelae of previous injuries, chronic diarrhea, or congenital defects [23]. They may develop pneumonia in addition to an exacerbation of another ailment, or have concomitant malaria or diarrhoea. In such cases, it is challenging to assign the death of a child to a single cause through verbal autopsy. Furthermore, the clinical signs and symptoms of a pneumonia death overlap with those of other causes of death such as malaria or measles, hence misclassification errors are significant. Moreover, there are studies that focus exclusively on pneumonia as a cause of death, while others are multi–cause mortality studies, documenting the causes of all child deaths in the community. Typically, studies focused exclusively on pneumonia tend to over–estimate its contribution to overall child mortality [24]. This is because in such studies it is more likely that a number of other underlying causes or immediate causes may be misclassified as pneumonia. Therefore, multi–cause mortality studies are preferred as a source of information to single–cause studies [24].
Risk factors. In addition to estimating the incidence, severe morbidity and mortality from childhood pneumonia at the global, regional and national level, it is important to understand risk factors that contribute to the development of childhood pneumonia and that may offer clues to prevention of the disease. However, well–conducted studies of pneumonia risk factors in low resource settings are remarkably scarce. There is wide variation among risk factor studies in their focus, study design and outcome: while some explore risk factors associated with incidence of pneumonia at the community level, others focus on the risks that are associated with progression to severe disease in those who already have pneumonia [1]. A third type of study are those that are hospital–based and investigate risk factors associated with progression to death in a child receiving treatment and compare case–fatality rates among different children [1].
Another methodological challenge is that the most commonly investigated risk factors for disease or for death are commonly identified together among cases. For example, undernutrition, use of solid fuels in a household, crowding, lack of exclusive breastfeeding, low degree of maternal education, limited access to secondary care and passive care–seeking behavior are all often characteristics of poor households, where most of the deaths occur. Because of this collinearity, an assessment of the effect size of any particular risk factor in isolation from the role of others will likely lead to gross over–estimation of the true effect size [1,18,19]. Therefore, very large prospective studies are required, based on multivariable study designs, to ensure an adequate number of study participants with heterogeneity in the prevalence of risk factors and thereby allow an accurate assessment of the individual role of each risk factor. Very few such studies exist; this is a permanent research priority, because the effect sizes attributable to individual risk factors in different contexts are still poorly understood [1,18,19].
Etiological agents. There is a growing need to identify etiological agents that contribute to the disease development at each of the three levels of severity – episodes of community–acquired pneumonia (incidence), severe pneumonia (severe morbidity) and pneumonia deaths (mortality). This is because vaccines are now available to prevent infections with major pathogens, such and Streptococcus pneumoniae (SP), Haemophilus influenzae type b (Hib) and influenza virus (flu), while a vaccine against respiratory syncytial virus (RSV) is also being actively pursued [25–27]. However, precise estimation of the distribution of the episodes, severe episodes and deaths from childhood pneumonia by etiological agent is even more difficult than estimation of the overall disease burden itself, for a number of reasons. First and foremost, the site of infection – the lung –is generally an inaccessible organ that is in constant contact with the external environment through the naso– and oro–pharynx, which are body sites that are sampling and immunologically responding to potential pathogens. Second, the procedures needed to collect specimens from potential cases are ones that usually require a hospital facility, meaning that studies must be done in places where cases have access to a hospital facility. Such studies also require laboratory facilities that can process samples in a timely fashion and can run a multitude of tests to document presence of pathogens in a child [28]. This means that they tend to be (teaching) hospital–based and therefore do not sample across the whole range of pneumonia cases in a population. Most deaths from pneumonia occur in places where no hospital facility is available, highlighting the nearly inextricable paradox that appropriate studies cannot be done in the places where most of the death burden occurs.
Third, accepting that paradox, even in settings where studies can be done there are further issues. The choice of biological samples (specimen) in which the presence of a potential pathogen should be sought means that multiple body fluids must be collected. Ideally, for a bacterial diagnosis, samples should come from the lung tissue itself (eg, by needle aspirate), from a pleural exudate, or a blood culture sample, but this is often neither feasible nor acceptable in a professional or lay community [28]. As an alternative, analyses of collected sputum or nasopharyngeal swabs can be performed, but their contribution to understanding the etiology is complex since the pathogens identified in these locations are also commonly found among healthy children.
Fourth, the more tests performed, the more agents will be found, and statistical methods to disaggregate and associate individual pathogen contributions to etiology are lacking. This is an increasing problem with modern sensitive techniques like PCR–based tests identifying the presence of often many co–existing and potentially pathogenic agents (whose role in the disease episode is very uncertain). Finally, we don't sufficiently understand the interplay between various pathogens and how a specific time sequence (eg, a viral infection, followed by a bacterial superinfection) may act to compromise the local and/or systemic immune response to cause a serious and life–threatening episode of childhood pneumonia by a pathogen that may otherwise not cause severe disease. Even with sophisticated expansive testing a significant proportion of cases may not have an etiology associated with the case. The meaning of this has to be assessed. Some of these cases may not have pneumonia at all, while other cases may not be associated with an etiology because of statistical methods used, in spite of identification of pathogens in the upper respiratory tract; finally, some may not be assigned to a causal pathogen because of laboratory test insensitivity. There remains therefore a gap in understanding the etiological spectrum of what is clinically defined as pneumonia [25,28].
These complex issues for studying pneumonia etiology are being addressed in a large, 7 country pneumonia etiology study among children (PERCH) [25]. This study is under way and the first results are expected following the completion of the field work (in early 2014) and an analysis period.
Because of the many biologic, epidemiologic, laboratory, and statistical challenges of pneumonia etiology observational studies, the most reliable methods for estimation of the proportional contribution of different pathogens to the burden of childhood pneumonia are vaccine trials [29]. The observed reduction in the incidence of pneumonia (using various case definitions) following vaccination reveals the disease burden attributable to that specific pathogen, once the less than 100% vaccine efficacy of the product is accounted for. This approach also has its limitations, mostly insofar as a vaccine trial can only reveal the burden of one pathogen at a time. For some pathogens (such as SP), not all disease–causing strains may be included in a strain specific vaccine [30]. If the distribution of strains varies by factors that also contribute to variation in pneumonia disease burden (eg, geography, pneumonia case definition, malnutrition, HIV), then careful attention must be paid to applying the vaccine efficacy measures to the appropriate measure of pneumonia disease burden [29]. Also, vaccine–based approach may be very useful in understanding the causal contribution at the level of incidence and severe morbidity, but may be limited in their ability to inform about the pathogen contribution to mortality (which is often a rare event in vaccine trials, where enormous resources are in place that themselves reduce the risk of death). Finally, although it might be ideal to conduct vaccine trials in parallel in many geographic regions using a harmonized protocol to reveal the geographic variability in contribution of pathogens to disease, vaccine trials are not usually designed for the purpose of disease burden estimation; they are also very expensive to conduct, which limits the number of sites where they can be undertaken [31]. They are generally not sufficiently large to have acceptable statistical power to detect a mortality reduction, as there are relatively few deaths in the study population.
Moreover, after a definite proof of vaccine efficacy and effectiveness is established, there are significant ethical issues regarding the conduct of further trials if they necessitate a control arm in which children are not provided what has been shown to be a life–saving vaccine. This self–limits the accumulation of the evidence towards the importance of specific pathogens. An additional layer of complexity comes from the notion that the etiological spectrum may change markedly with increasing severity of disease: at the level of incidence of childhood pneumonia in the community, viral causes seem to be responsible for a majority of episodes. However, a proportion of these cases will result in severe and life–threatening disease. In a sub–sample of severe cases, bacterial agents seem to be over–represented. Evidence from antibiotic treatment trials, from vaccine trials, and from studies of lung puncture studies provide a firm evidence base that episodes of death from pneumonia are dominated by bacterial causes. If true, this would suggest that SP and Hib vaccine probe studies (with “proxy” endpoints of severe episodes prevented) may under–estimate the importance of these agents as a cause of death. Longitudinal studies of mortality in low–income countries that have introduced Hib and SP vaccines recently and that are achieving high vaccine coverage will likely provide confirmatory evidence of that contribution to pneumonia mortality in the coming years [25,26,31].
An overview of previous estimates
One of the earliest attempts at estimating the global burden of communicable diseases was provided by Cockburn and Assaad in the early 1970s [32]. Bulla and Hitze built on their work by specifically addressing the contribution of acute respiratory infections [33]. Almost a decade later, Leowski [34] used data from 39 countries to estimate that acute respiratory infections may have been causing about 4 million child deaths each year: 2.6 million in infants and further 1.4 million in children aged 1–4 years. In the early 1990s Garenne et al. [35] further refined these estimates using an epidemiological model that explored the association between all–cause child mortality and the proportion of deaths attributable to acute respiratory infections, showing that between 20–33% of child deaths were associated with respiratory infections [35,36].
The 21st century has seen a much larger number of efforts, mainly designed and led by CHERG and their partners, which further improved the understanding of the epidemiology and etiology of childhood pneumonia. The first estimate of global incidence of childhood pneumonia was provided by Rudan et al. [18] for 2000. In parallel, a refined estimate of childhood pneumonia mortality for the same year, based mainly on single–cause studies, was provided by Williams et al. [37]. The first estimate of pneumonia mortality from multi–cause studies was published by Black et al. in CHERG's paper on the causes of global child mortality in the year 2000 [4]. Then, estimates underwent further refinements and updates. An updated estimate of childhood pneumonia mortality for 2008 in post–neonatal children in low and middle–income countries, based on single–cause studies, was provided by Theodoratou et al. [38]. Estimates based on multi–cause studies underwent three updates: for the period 2000–2003 by Bryce et al. [39]; for 2008 by Black et al. [40]; and for 2010 by Li et al. [41].
The first comprehensive assessment of the burden of severe pneumonia according to the WHO's definition and the role of risk factors was provided by Rudan et al. [1,18]. This work was followed by the first attempt to estimate the global burden of childhood pneumonia on health systems; Nair et al. [42] used both published and unpublished information to calculate the number of hospitalizations for severe pneumonia, a number which is smaller than the estimate of cases of severe pneumonia in the community because of lack of access and/or care–seeking in many settings.
Once the “envelopes” for the burden of pneumonia incidence, severe morbidity and mortality from pneumonia in 2000 were provided, a series of efforts attempted to estimate the proportion of the burden at each level of severity that can be attributed to the main etiological agents that cause pneumonia. O'Brien et al. [43] developed the first global, regional and country estimates for the morbidity and mortality from Streptococcus pneumoniae, Watt et al. for Haemophilus influenzae type b [44], while Nair et al. generated global and regional estimates for RSV [45] and for influenza [46].
The estimates of pneumonia incidence, severe pneumonia cases, severe pneumonia hospitalizations, pneumonia mortality, and cause specific estimates are based on different and almost entirely independent sources of information, which allows for assessments of validity and consistency between the various estimates. Validation of these estimates can be approached in various ways. A few examples include: (i) an assessment of the measured proportion of all pneumonia cases that are categorized as severe; (ii) the ratio between the estimates of severe episodes and deaths, and also (iii) between all pneumonia episodes and deaths. These proportions and ratios need to largely support the observed case–fatality rates typically seen in both community–based and hospital–based data sets from individual studies. Moreover, the sum of etiology specific fractions attributed to different pathogens needs to fit within the overall burden of incidence, severe morbidity and mortality. For the Hib and pneumococcal pathogen specific estimates, they must fit within these envelopes by definition, since the methodology to estimate the absolute burden was a proportional approach – but this was not the approach for the estimation of the RSV or influenza burden. The ratios between different pathogens were also found to broadly reflect those observed in the high quality field studies or hospital–based studies further validating the estimates. Towards the end of the past decade it was notable that, regardless of all methodological challenges and uncertainty inherent to this research, all the major estimates from different sources were increasingly consistent with each other and provided a clearer global and regional picture of the burden of childhood pneumonia and its causing pathogens, albeit with wide uncertainty bounds around the point estimates [40–46]. This paper therefore brings all the estimates together and provides an update for 2010–11, in which all information is provided in a single analysis, and where country–level estimates are also be provided.
METHODS
Many steps are required to develop an internally consistent estimate of global, regional and national burden of childhood pneumonia based on best available evidence. To fully explain our approach, we developed a table (Online Supplementary Document(Online Supplementary Document)) which all input data, assumptions, methods, solutions to specific problems or dilemmas, formulae for calculation of different parameters, and the interim and final estimates are provided. In this section, we present a summary for those steps, list all sources of data and explain the rationale for each subsequent step.
Input data for country–level populations and prevalence of risk factors for pneumonia incidence
Initially, we list 192 countries by World Health Organization's regional classification, with 6 main regions (the Americas (AMRO), Africa (AFRO), Eastern Mediterranean region (EMRO), European region (EURO), Western Pacific region (WPRO) and South–East Asian region (SEARO)) and further divisions by the level of development into “A”, “B”, “C”, “D” and “E” sub–regions [47]. For each country, an estimate of the population of children under the age of 5 years in 2010 was obtained from the UN's Population Division [48]. Then, the 5 most important risk factors for childhood pneumonia incidence were identified. They were selected based on consistently significant effects in multivariate study designs and previous meta–analyses [1,18]. They are: malnutrition (weight–for–age z<–2), low birth weight (≤2500 g), non–exclusive breastfeeding (in the first 4 months), solid fuel use (“yes”) and crowding (7 or more persons sharing the same household) [1,18]. The data on the prevalence of exposure to each of those 5 risk factors in each country in the year 2010(or the closest year with available data) was obtained from the recent Demographic and Health Surveys (DHS) and Multiple Indicator Cluster Surveys (MICS) [49,50]. For all countries in which data on the prevalence of exposure were not available, the prevalence was imputed based on the regional mean value, which was weighted by population size of all countries with any data. The effect size of each risk factor on pneumonia incidence was assessed through meta–analysis of the studies that reported multivariable analyses of risk factor's odds ratios (OR) in low and middle–income countries. The meta–estimates of odds ratios assigned to each risk factor were: 1.8 for malnutrition, 1.4 for low birth weight, 1.3 for non–exclusive breastfeeding, 1.8 for use of solid fuels and 2.0 for crowding. In high–income countries, where less than 2% of all cases of community–acquired pneumonia occur, we did not use the model based on risk factors but rather applied “flat” rates of incidence for “A”, “B” and “C” regions based on several high–quality studies (see Online Supplementary Document(Online Supplementary Document)), and which ranged between 0.015 and 0.060 episodes/child–year (see later). For the proportion of severe episodes in each high–income region we used one single rate which was the median of all available studies (26.7%, see later).
Computation of country–level incidence of pneumonia and severe pneumonia
In all LMIC countries, we multiplied the number of children in each country by the prevalence of exposure to each of the 5 risk factors. This provided an estimate of the absolute number of exposed children in each country who were at excess risk of developing childhood pneumonia in the year 2010. We then calculated the proportion (ie, a weighted mean) of all children in each LMIC region and country exposed to each of the 5 risk factors; then, in each country, we multiplied the proportion of children who were above, or below, the regional exposure level with the meta–estimate of the odds ratio attributable to each of the 5 risk factors.
The number of pneumonia cases in each low and middle–income country (LMIC) was calculated using a model based on the epidemiological concept of potential impact fraction [51], as follows:
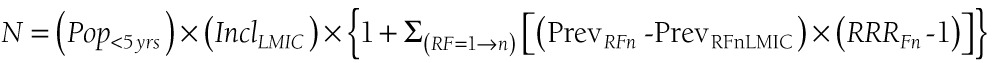 |
where N is the number of new episodes of childhood pneumonia per year in each country, Pop<5yrsis the population of children aged 0–4 years in each LMIC, IncLMIC is the estimated incidence of clinical pneumonia for all LMIC, PrevRFn is the prevalence of exposure to n–th risk factor among those under 5-year in the country of interest, PrevRFnLMIC is the prevalence of exposure to nth risk factor among under–fives in all LMIC, and RRRFn is the relative risk for developing clinical pneumonia associated with the nth risk factor (see Online Supplementary Document(Online Supplementary Document) for further details).
The incidence of pneumonia for all LMIC was derived from35 community–based studies published between1990 and 2012 (references shown in Online Supplementary Document(Online Supplementary Document)), by using the median value (0.22 episodes/child–year) and inter–quartile range (IQR) 0.11–0.51 as confidence intervals.
Although there are many possible methods to distribute the global and/or regional burden estimate among individual countries, the approach used above is our preferred solution because it is epidemiologically sound and biologically intuitive insofar as it is based on the country specific prevalence of known risk factors for pneumonia, and because it can be explained in a transparent and accessible manner. Although more complex models exist, our experience is that these sometimes result in implausibly high or low estimates for some countries, the cause of which is difficult to disentangle. This model, because of its computational simplicity and epidemiologic basis, has not suffered from this problem. The model has also been shown to distribute a known overall burden by specific countries in the absence of truly nationally representative information from many (or, in this case, from most) countries in a way which is consistent with clinical and epidemiologic knowledge.
The proportion of cases of severe pneumonia (based on the WHO definition that requires presentation of lower chest wall indrawing, and represents an indication for hospitalization) for LMIC was computed based on 9 community–based studies in LMIC that reported the proportion of severe pneumonia episodes among all pneumonia episodes (references shown in Online Supplementary Document(Online Supplementary Document)). The median value was 11.5% (IQR 8.0–33.0%). The incidence of pneumonia in high–income countries, based on a smaller number of very large, high–quality studies (references shown in Online Supplementary Document(Online Supplementary Document)), was also estimated using medians (and IQR): it was 0.015 e/cy in EUROA and AMROA regions; 0.030 e/cy in EURO Band 0.060 in EURO C. The mean of those values (for the whole HIC region), weighted by their under–five population size, was0.024 e/cy [52]. Approximately 26.7% (IQR 20.0–46.7%) of those episodes are estimated to progress to severe pneumonia, based on several studies from high–income countries (references shown in Online Supplementary Document(Online Supplementary Document)). The estimates for the number of incident and severe pneumonia episodes derived in this way did not account for the use and effect of pneumococcal conjugate vaccine (PCV) and Hib vaccination coverage in 2010 at this stage of the estimation process, so the values from this step are not considered the final pneumonia burden numbers.
Etiologic fractions of pneumonia and severe pneumonia cases
We split both the incidence and severe morbidity of childhood pneumonia by etiological agents while adjusting for the effects PCV and Hib vaccines according to country specific coverage values provided for 2010 by the UNICEF [53]. In doing so, we used the proportional contributions to all childhood pneumonia and severe childhood pneumonia from previous burden estimates on SP [43], Hib [44], RSV [45] and influenza [46] and accounted for vaccine efficacy and serotype distribution of pneumococcal disease as well as dual use of Hib and PCV where relevant. All further details are available in Online Supplementary Document(Online Supplementary Document).
Country–specific estimates of the number of deaths from childhood pneumonia
This was available for 2010 from Li et al. [41]. A more recent update was made available by the UN Inter–Agency Group for Child Mortality Estimation IGME in UNICEF's 2012 report, which we term a “2010–2011” estimate [54]. Given the important focus on child mortality, and relatively minor differences compared with the Li 2010 estimates, we elected to use the 2010–11estimates for the envelopes of pneumonia deaths by country. The same decision was made in the Lancet's series [13]. The only methodological problem with this decision is a separation of Sudan and introduction of the new country – South Sudan from 2011, but we presented our results on mortality for both Sudan nations combined, and kept it within the EMRO region, although South Sudan belongs to AFRO region in the new classification [47].
Proportional split of pneumonia deaths by etiological agent
To estimate the fraction of pneumonia deaths attributable to SP and Hib, we used the meta–analysis of the efficacy of PCV and Hib vaccines against chest X–ray confirmed pneumonia as has been described earlier (43,44), based on the assumption that the etiologic fraction of these bacteria among these particular cases approximates the etiologic fraction among the deaths. The values (33.0% for SP and 21.3% for Hib) were then adjusted by country for the use of PCV and Hib vaccine to derive the final SP and Hib proportions [43,44,53]. Since the global disease burden estimates for flu and RSV pneumonia were not able to give point estimates and confidence intervals due to lack of data we did not attempt to go beyond the published global and regional estimates for these conditions and so did not attempt to derive national–level estimates [45,46].
RESULTS
Table 1 presents our estimates for 192 countries, grouped by the WHO regions: Africa (AFRO), the Americas (AMRO), Eastern Mediterranean region (EMRO), South–East Asian region (SEARO), Western Pacific region (WPRO) and European region (EURO). Several main results emerge from the presented figures. First, the population of under–five children in the world increased from 604.9 million to 633.5 million between 2000 and 2010, but the majority of the increase was observed in low– and middle–income countries (523.3 to 547.3 million), and only a smaller share in high–income countries (81.6 to 86.2 million). Holding all else constant, an increase in total child population would increase the absolute number of pneumonia cases; however, the number of cases has decreased over the past decade, because the incidence has decreased substantially. When presenting our estimates of incidence for 2000, we reported on 28 studies published between 1960 and 2000 that suggested an estimated incidence of 0.29 (0.21–0.71) episodes per child–year globally [18]. In this most recent estimate, we used 35 studies published between 1990 and 2010 with a median incidence of 0.22 (0.11–0.51). This is a notable reduction, of nearly 25%, over a period of a decade. In high–income countries we gathered more data over the past decade, and a very rough estimate of 0.05 e/cy, based on two very large, but historic studies in the USA and the UK [55,56], was refined and replaced with the data from 9 more contemporary studies, which provide a community–based incidence of 0.015 e/cy (0.012–0.020) for HIC only (WHO's “A” regions), a more plausible estimate for the modern industrial societies.
Table 1.
Estimates of the number of new episodes (incidence) of community–acquired pneumonia in 2010 in children 0–4 years of age in 192 countries, shown as national–level totals (incidence, all ALRI) and by causative pathogens (SP, Hib, RSV and flu); estimates of the number of new severe episodes (according to WHO's definition) in the year 2010 that require hospitalizations, shown as national–level totals (severe episodes, all ALRI) and by causative pathogens (SP, Hib, RSV and flu); and estimates of the number of child deaths attributable to pneumonia in 2011 (mortality, all ALRI) and the proportion of deaths caused by SP and Hib
| New episodes (incidence) | New severe episodes (severe morbidity) | Deaths (mortality) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Country |
WHO Region |
Population 0–4 years |
All ALRI |
SP |
Hib |
RSV |
FLU |
All ALRI |
SP |
Hib |
RSV |
FLU |
All ALRI |
SP |
Hib |
RSV, FLU* |
|
AFRO REGION | ||||||||||||||||
| Algeria |
AfroD |
3446548 |
470713 |
34251 |
4697 |
135754 |
80351 |
53790 |
10297 |
783 |
7315 |
2251 |
2440 |
804 |
148 |
N/A |
| Angola |
AfroD |
3377576 |
856794 |
62241 |
9674 |
247099 |
146255 |
97936 |
18712 |
1613 |
13293 |
4090 |
20429 |
6733 |
1398 |
N/A |
| Benin |
AfroD |
1506408 |
424074 |
30705 |
5895 |
122303 |
72389 |
48501 |
9231 |
983 |
6558 |
2018 |
6281 |
2070 |
522 |
N/A |
| Burkina Faso |
AfroD |
2955148 |
1047365 |
76085 |
11826 |
302060 |
178785 |
119719 |
22874 |
1972 |
16250 |
5000 |
17933 |
5911 |
1227 |
N/A |
| Cameroon |
AfroD |
3054802 |
790160 |
56858 |
14815 |
227882 |
134880 |
90462 |
17094 |
2470 |
12143 |
3736 |
13341 |
4397 |
1463 |
N/A |
| Cape Verde |
AfroD |
50634 |
9874 |
691 |
395 |
2848 |
1686 |
1136 |
208 |
66 |
148 |
45 |
39 |
13 |
8 |
N/A |
| Chad |
AfroD |
2006165 |
678297 |
48155 |
19812 |
195621 |
115785 |
77827 |
14477 |
3304 |
10285 |
3164 |
14683 |
4840 |
2390 |
N/A |
| Comoros |
AfroD |
122296 |
38380 |
2769 |
645 |
11069 |
6552 |
4392 |
832 |
108 |
591 |
182 |
377 |
124 |
37 |
N/A |
| Equ. Guinea |
AfroD |
107207 |
16341 |
1144 |
654 |
4713 |
2789 |
1879 |
344 |
109 |
244 |
75 |
402 |
132 |
85 |
N/A |
| Gabon |
AfroD |
185179 |
36186 |
2579 |
943 |
10436 |
6177 |
4149 |
775 |
157 |
551 |
170 |
291 |
96 |
43 |
N/A |
| Gambia |
AfroD |
287078 |
79805 |
2667 |
802 |
23016 |
13623 |
8746 |
802 |
134 |
1338 |
412 |
987 |
171 |
56 |
N/A |
| Ghana |
AfroD |
3532887 |
795448 |
57857 |
8199 |
229407 |
135783 |
90905 |
17394 |
1367 |
12357 |
3802 |
7808 |
2573 |
490 |
N/A |
| Guinea |
AfroD |
1657883 |
546525 |
39262 |
10948 |
157618 |
93292 |
62586 |
11804 |
1826 |
8385 |
2580 |
7689 |
2534 |
895 |
N/A |
| Guin.–Bissau |
AfroD |
240350 |
75199 |
5429 |
1216 |
21687 |
12836 |
8605 |
1632 |
203 |
1159 |
357 |
1592 |
525 |
152 |
N/A |
| Liberia |
AfroD |
680701 |
212990 |
15195 |
5418 |
61426 |
36357 |
24419 |
4568 |
903 |
3245 |
999 |
1611 |
531 |
232 |
N/A |
| Madagascar |
AfroD |
3305278 |
1051407 |
76189 |
13932 |
303226 |
179475 |
120231 |
22906 |
2323 |
16272 |
5007 |
8004 |
2638 |
637 |
N/A |
| Mali |
AfroD |
2911668 |
932894 |
67350 |
15086 |
269047 |
159245 |
106745 |
20248 |
2516 |
14384 |
4426 |
23947 |
7893 |
2292 |
N/A |
| Mauritania |
AfroD |
513267 |
144982 |
10415 |
2904 |
41813 |
24748 |
16603 |
3131 |
484 |
2224 |
684 |
2099 |
692 |
244 |
N/A |
| Mauritius |
AfroD |
84433 |
13518 |
985 |
117 |
3899 |
2307 |
1544 |
296 |
20 |
210 |
65 |
20 |
7 |
1 |
N/A |
| Niger |
AfroD |
3084517 |
1127652 |
81210 |
20418 |
325215 |
192490 |
129082 |
24415 |
3405 |
17344 |
5337 |
19004 |
6264 |
2018 |
N/A |
| Nigeria |
AfroD |
26568927 |
7339761 |
513783 |
293590 |
2116787 |
1252897 |
844072 |
154465 |
48956 |
109729 |
33763 |
121201 |
39948 |
25767 |
N/A |
| S. Tome & P'e |
AfroD |
23490 |
5118 |
373 |
46 |
1476 |
874 |
585 |
112 |
8 |
80 |
25 |
79 |
26 |
4 |
N/A |
| Senegal |
AfroD |
2081483 |
591373 |
42853 |
7836 |
170552 |
100947 |
67625 |
12883 |
1307 |
9152 |
2816 |
4612 |
1520 |
367 |
N/A |
| Seychelles |
AfroD |
5623 |
862 |
63 |
7 |
248 |
147 |
98 |
19 |
1 |
13 |
4 |
2 |
1 |
0 |
N/A |
| Sierra Leone |
AfroD |
969597 |
315676 |
22866 |
4286 |
91041 |
53886 |
36101 |
6874 |
715 |
4883 |
1503 |
7262 |
2393 |
591 |
N/A |
| Togo |
AfroD |
862745 |
280487 |
20292 |
4082 |
80893 |
47879 |
32083 |
6101 |
681 |
4334 |
1333 |
3321 |
1095 |
288 |
N/A |
| Zimbabwe |
AfroD |
1692247 |
349031 |
25271 |
4852 |
100661 |
59580 |
39918 |
7598 |
809 |
5397 |
1661 |
2461 |
811 |
205 |
N/A |
| Botswana |
AfroE |
225120 |
47818 |
3347 |
1913 |
13791 |
8162 |
5499 |
1006 |
319 |
715 |
220 |
159 |
52 |
34 |
N/A |
| Burundi |
AfroE |
1184632 |
349477 |
25440 |
3373 |
100789 |
59656 |
39933 |
7648 |
562 |
5433 |
1672 |
7259 |
2393 |
428 |
N/A |
| Cen. Afr. Rep. |
AfroE |
651222 |
195417 |
13981 |
4538 |
56358 |
33358 |
22394 |
4203 |
757 |
2986 |
919 |
3911 |
1289 |
520 |
N/A |
| Congo |
AfroE |
623244 |
168619 |
12244 |
1959 |
48630 |
28783 |
19275 |
3681 |
327 |
2615 |
805 |
2001 |
659 |
141 |
N/A |
| Cote d'Ivoire |
AfroE |
2969425 |
985611 |
71421 |
13060 |
284250 |
168244 |
112707 |
21472 |
2178 |
15253 |
4693 |
11003 |
3626 |
875 |
N/A |
| D. Rep. Congo |
AfroE |
11848026 |
3671614 |
263117 |
80589 |
1058894 |
626745 |
420631 |
79104 |
13438 |
56194 |
17291 |
86897 |
28641 |
10986 |
N/A |
| Eritrea |
AfroE |
861496 |
208035 |
15163 |
1802 |
59997 |
35512 |
23766 |
4559 |
301 |
3238 |
996 |
2419 |
797 |
129 |
N/A |
| Ethiopia |
AfroE |
11931668 |
3367561 |
240540 |
82471 |
971205 |
574843 |
386005 |
72317 |
13752 |
51372 |
15807 |
37269 |
12284 |
5196 |
N/A |
| Kenya |
AfroE |
6664323 |
1645189 |
119118 |
22871 |
474473 |
280834 |
188157 |
35812 |
3814 |
25440 |
7828 |
17064 |
5624 |
1419 |
N/A |
| Lesotho |
AfroE |
274307 |
58335 |
4224 |
811 |
16824 |
9958 |
6672 |
1270 |
135 |
902 |
278 |
607 |
200 |
50 |
N/A |
| Malawi |
AfroE |
2714859 |
658512 |
47877 |
7004 |
189915 |
112408 |
75261 |
14394 |
1168 |
10225 |
3146 |
6932 |
2285 |
448 |
N/A |
| Mozambique |
AfroE |
3876419 |
1155781 |
83373 |
19438 |
333327 |
197292 |
132266 |
25065 |
3241 |
17806 |
5479 |
13167 |
4340 |
1307 |
N/A |
| Namibia |
AfroE |
286374 |
63796 |
4619 |
887 |
18399 |
10890 |
7296 |
1389 |
148 |
987 |
304 |
287 |
95 |
24 |
N/A |
| Rwanda |
AfroE |
1830654 |
397910 |
13638 |
3991 |
114757 |
67923 |
43646 |
4100 |
666 |
6659 |
2049 |
4145 |
734 |
236 |
N/A |
| South Africa |
AfroE |
5041132 |
705554 |
33436 |
14342 |
203482 |
120438 |
78749 |
10052 |
2392 |
11357 |
3494 |
5156 |
1218 |
583 |
N/A |
| Swaziland |
AfroE |
156715 |
28802 |
2091 |
344 |
8306 |
4916 |
3293 |
629 |
57 |
446 |
137 |
471 |
155 |
34 |
N/A |
| Uganda |
AfroE |
6465275 |
1745727 |
126241 |
25969 |
503468 |
297996 |
199697 |
37953 |
4330 |
26961 |
8296 |
21181 |
6981 |
1876 |
N/A |
| U. R. Tanzania |
AfroE |
8009544 |
2151379 |
156285 |
24291 |
620458 |
367240 |
245913 |
46986 |
4051 |
33378 |
10270 |
17467 |
5757 |
1195 |
N/A |
| Zambia |
AfroE |
2412190 |
576056 |
41709 |
8008 |
166135 |
98333 |
65882 |
12539 |
1335 |
8908 |
2741 |
6141 |
2024 |
511 |
N/A |
|
AMRO REGION | ||||||||||||||||
| Canada |
AmroA |
1884546 |
25275 |
866 |
271 |
13709 |
8032 |
6438 |
604 |
105 |
3774 |
755 |
27 |
5 |
2 |
N/A |
| Cuba |
AmroA |
569056 |
8208 |
598 |
79 |
4452 |
2609 |
2178 |
417 |
31 |
1140 |
228 |
63 |
21 |
4 |
N/A |
| USA |
AmroA |
21650217 |
313322 |
22733 |
3845 |
169946 |
99574 |
83169 |
15868 |
1489 |
43355 |
8671 |
799 |
263 |
59 |
N/A |
| Antigua & B'a |
AmroB |
7756 |
686 |
50 |
6 |
198 |
117 |
78 |
15 |
1 |
41 |
8 |
0 |
0 |
0 |
N/A |
| Argentina |
AmroB |
3385831 |
311588 |
22663 |
3212 |
89862 |
53188 |
35609 |
6814 |
536 |
18616 |
3723 |
952 |
314 |
60 |
N/A |
| Bahamas |
AmroB |
25507 |
2514 |
182 |
23 |
725 |
429 |
287 |
55 |
4 |
151 |
30 |
25 |
8 |
1 |
N/A |
| Barbados |
AmroB |
14562 |
1377 |
60 |
19 |
397 |
235 |
153 |
18 |
3 |
87 |
17 |
4 |
1 |
0 |
N/A |
| Belize |
AmroB |
36599 |
4795 |
349 |
46 |
1383 |
819 |
548 |
105 |
8 |
287 |
57 |
9 |
3 |
1 |
N/A |
| Brazil |
AmroB |
15156449 |
1497706 |
95518 |
14711 |
431938 |
255658 |
169535 |
28717 |
2453 |
91150 |
18230 |
3079 |
916 |
181 |
N/A |
| Chile |
AmroB |
1219437 |
88722 |
6448 |
973 |
25588 |
15145 |
10141 |
1938 |
162 |
5296 |
1059 |
145 |
48 |
10 |
N/A |
| Colombia |
AmroB |
4497661 |
488486 |
31421 |
6092 |
140879 |
83385 |
55372 |
9446 |
1016 |
29585 |
5917 |
1530 |
459 |
113 |
N/A |
| Costa Rica |
AmroB |
362979 |
37185 |
1272 |
425 |
10724 |
6348 |
4080 |
382 |
71 |
2389 |
478 |
24 |
4 |
2 |
N/A |
| Dominica |
AmroB |
5924 |
703 |
51 |
6 |
203 |
120 |
80 |
15 |
1 |
42 |
8 |
0 |
0 |
0 |
N/A |
| Dominican R. |
AmroB |
1054063 |
121820 |
8813 |
1773 |
35133 |
20795 |
13934 |
2650 |
296 |
7239 |
1448 |
587 |
193 |
51 |
N/A |
| El Salvador |
AmroB |
616802 |
72388 |
3616 |
829 |
20877 |
12357 |
8079 |
1087 |
138 |
4515 |
903 |
221 |
54 |
15 |
N/A |
| Grenada |
AmroB |
9687 |
1021 |
74 |
10 |
295 |
174 |
117 |
22 |
2 |
61 |
12 |
0 |
0 |
0 |
N/A |
| Guyana |
AmroB |
64818 |
7186 |
523 |
72 |
2072 |
1227 |
821 |
157 |
12 |
429 |
86 |
19 |
6 |
1 |
N/A |
| Honduras |
AmroB |
966002 |
184407 |
13435 |
1658 |
53183 |
31478 |
21068 |
4039 |
277 |
11036 |
2207 |
478 |
157 |
26 |
N/A |
| Jamaica |
AmroB |
246543 |
31065 |
2264 |
269 |
8959 |
5303 |
3549 |
681 |
45 |
1860 |
372 |
104 |
34 |
6 |
N/A |
| Mexico |
AmroB |
11094854 |
1110027 |
40375 |
11872 |
320132 |
189482 |
122060 |
12138 |
1980 |
71108 |
14222 |
4069 |
759 |
248 |
N/A |
| Panama |
AmroB |
345142 |
38834 |
2112 |
415 |
11200 |
6629 |
4354 |
635 |
69 |
2404 |
481 |
128 |
33 |
8 |
N/A |
| Paraguay |
AmroB |
740282 |
139661 |
10141 |
1623 |
40278 |
23840 |
15965 |
3049 |
271 |
8330 |
1666 |
368 |
121 |
26 |
N/A |
| St. Kitts & N's |
AmroB |
4582 |
441 |
32 |
4 |
127 |
75 |
50 |
10 |
1 |
26 |
5 |
0 |
0 |
0 |
N/A |
| Saint Lucia |
AmroB |
15115 |
1492 |
109 |
14 |
430 |
255 |
170 |
33 |
2 |
89 |
18 |
0 |
0 |
0 |
N/A |
| St. Vinc. & G's |
AmroB |
9254 |
967 |
70 |
8 |
279 |
165 |
110 |
21 |
1 |
58 |
12 |
1 |
0 |
0 |
N/A |
| Suriname |
AmroB |
47543 |
6578 |
477 |
85 |
1897 |
1123 |
752 |
143 |
14 |
392 |
78 |
20 |
7 |
2 |
N/A |
| Trinidad & Tobago |
AmroB |
95484 |
9784 |
710 |
114 |
2822 |
1670 |
1118 |
214 |
19 |
584 |
117 |
38 |
13 |
3 |
N/A |
| Uruguay |
AmroB |
246446 |
17570 |
647 |
188 |
5067 |
2999 |
1933 |
194 |
31 |
1125 |
225 |
53 |
10 |
3 |
N/A |
| Venezuela |
AmroB |
2926202 |
308502 |
22291 |
4789 |
88972 |
52661 |
35295 |
6702 |
799 |
18310 |
3662 |
927 |
305 |
85 |
N/A |
| Bolivia |
AmroD |
1234922 |
137114 |
9915 |
2040 |
39544 |
23405 |
15685 |
2981 |
340 |
8145 |
1629 |
1909 |
629 |
169 |
N/A |
| Ecuador |
AmroD |
1469919 |
163860 |
10901 |
1437 |
47257 |
27971 |
18596 |
3277 |
240 |
9934 |
1987 |
712 |
219 |
38 |
N/A |
| Guatemala |
AmroD |
2167408 |
481781 |
35042 |
4966 |
138946 |
82240 |
55058 |
10535 |
828 |
28785 |
5757 |
2012 |
663 |
126 |
N/A |
| Haiti |
AmroD |
1237203 |
345081 |
24156 |
13803 |
99521 |
58905 |
39684 |
7262 |
2302 |
19842 |
3968 |
4090 |
1348 |
870 |
N/A |
| Nicaragua |
AmroD |
677569 |
141434 |
10304 |
1272 |
40790 |
24143 |
16159 |
3098 |
212 |
8464 |
1693 |
503 |
166 |
28 |
N/A |
| Peru |
AmroD |
2909336 |
313170 |
12566 |
3545 |
90318 |
53458 |
34584 |
3778 |
591 |
19904 |
3981 |
1040 |
211 |
67 |
N/A |
|
EMRO REGION | ||||||||||||||||
| Bahrain |
EmroB |
93006 |
9763 |
327 |
91 |
2816 |
1667 |
1070 |
98 |
15 |
227 |
101 |
5 |
1 |
0 |
N/A |
| Cyprus |
EmroB |
63553 |
7253 |
528 |
70 |
2092 |
1238 |
829 |
159 |
12 |
156 |
69 |
1 |
0 |
0 |
N/A |
| Iran (Isl. Rep.) |
EmroB |
6149331 |
729564 |
51069 |
29183 |
210406 |
124537 |
83900 |
15354 |
4866 |
15102 |
6712 |
4168 |
1374 |
886 |
N/A |
| Jordan |
EmroB |
816013 |
87843 |
6400 |
790 |
25334 |
14995 |
10036 |
1924 |
132 |
1893 |
841 |
268 |
88 |
15 |
N/A |
| Kuwait |
EmroB |
281414 |
29357 |
994 |
284 |
8467 |
5011 |
3218 |
299 |
47 |
681 |
303 |
38 |
7 |
2 |
N/A |
| Lebanon |
EmroB |
321684 |
35518 |
2569 |
517 |
10243 |
6063 |
4063 |
773 |
86 |
760 |
338 |
49 |
16 |
4 |
N/A |
| Libyan A. J. |
EmroB |
715540 |
80748 |
5883 |
726 |
23288 |
13784 |
9225 |
1769 |
121 |
1740 |
773 |
60 |
20 |
3 |
N/A |
| Oman |
EmroB |
281883 |
32111 |
1074 |
300 |
9261 |
5481 |
3518 |
323 |
50 |
746 |
332 |
25 |
4 |
1 |
N/A |
| Qatar |
EmroB |
90524 |
9669 |
331 |
97 |
2788 |
1650 |
1061 |
100 |
16 |
224 |
100 |
4 |
1 |
0 |
N/A |
| Saudi Arabia |
EmroB |
3145187 |
337985 |
11445 |
3273 |
97475 |
57694 |
37052 |
3441 |
546 |
7842 |
3485 |
372 |
65 |
20 |
N/A |
| Syrian A. R. |
EmroB |
2493561 |
280849 |
20309 |
4178 |
80997 |
47941 |
32127 |
6106 |
697 |
6006 |
2669 |
572 |
189 |
51 |
N/A |
| Tunisia |
EmroB |
868231 |
99837 |
6989 |
3993 |
28793 |
17042 |
11481 |
2101 |
666 |
2067 |
919 |
209 |
69 |
44 |
N/A |
| U. A. Emir. |
EmroB |
420630 |
46752 |
1660 |
517 |
13483 |
7981 |
5137 |
499 |
86 |
1079 |
480 |
14 |
3 |
1 |
N/A |
| Afghanistan |
EmroD |
5545968 |
2040302 |
146694 |
39565 |
588423 |
348280 |
233617 |
44102 |
6598 |
43379 |
19280 |
30913 |
10189 |
3494 |
N/A |
| Djibouti |
EmroD |
113169 |
24926 |
1808 |
306 |
7189 |
4255 |
2850 |
544 |
51 |
535 |
238 |
446 |
147 |
33 |
N/A |
| Egypt |
EmroD |
9008118 |
680363 |
47625 |
27215 |
196217 |
116138 |
78242 |
14318 |
4538 |
14084 |
6259 |
4765 |
1570 |
1013 |
N/A |
| Iraq |
EmroD |
5188175 |
893131 |
62519 |
35725 |
257579 |
152457 |
102710 |
18796 |
5957 |
18488 |
8217 |
7568 |
2494 |
1609 |
N/A |
| Morocco |
EmroD |
3021924 |
385554 |
27959 |
3343 |
111194 |
65814 |
44029 |
8406 |
557 |
8316 |
3696 |
3103 |
1019 |
165 |
N/A |
| Pakistan |
EmroD |
21418111 |
6728235 |
487755 |
86960 |
1940423 |
1148510 |
769337 |
146640 |
14501 |
144236 |
64105 |
64853 |
21376 |
5039 |
N/A |
| Somalia |
EmroD |
1667479 |
650669 |
45547 |
26027 |
187653 |
111069 |
74827 |
13693 |
4340 |
13469 |
5986 |
18089 |
5962 |
3846 |
N/A |
| Sudan |
EmroD |
6391368 |
2061300 |
148754 |
34001 |
594479 |
351864 |
235876 |
44722 |
5670 |
43989 |
19550 |
26894 |
8864 |
3681 |
N/A |
| Yemen |
EmroD |
4057096 |
1150463 |
83436 |
14494 |
331793 |
196384 |
131540 |
25084 |
2417 |
24673 |
10966 |
15193 |
5008 |
1152 |
N/A |
|
SEARO REGION | ||||||||||||||||
| Indonesia |
SearoB |
21578876 |
3918360 |
274285 |
156734 |
1130055 |
668864 |
450611 |
82462 |
26135 |
99135 |
22531 |
19147 |
6311 |
4071 |
N/A |
| Sri Lanka |
SearoB |
1892699 |
433688 |
31610 |
3757 |
125076 |
74030 |
49545 |
9503 |
626 |
11425 |
2597 |
298 |
98 |
16 |
N/A |
| Thailand |
SearoB |
4360687 |
648021 |
45361 |
25921 |
186889 |
110617 |
74522 |
13638 |
4322 |
16395 |
3726 |
903 |
298 |
192 |
N/A |
| Timor Leste |
SearoB |
192839 |
67370 |
4716 |
2695 |
19429 |
11500 |
7748 |
1418 |
449 |
1704 |
387 |
489 |
161 |
104 |
N/A |
| Bangladesh |
SearoD |
14707333 |
4484527 |
326317 |
44752 |
1293338 |
765509 |
512461 |
98105 |
7462 |
117940 |
26805 |
18310 |
6035 |
1114 |
N/A |
| Bhutan |
SearoD |
70891 |
12773 |
894 |
511 |
3684 |
2180 |
1469 |
269 |
85 |
323 |
73 |
152 |
50 |
32 |
N/A |
| DPR of Korea |
SearoD |
1704446 |
393494 |
27545 |
15740 |
113484 |
67169 |
45252 |
8281 |
2625 |
9955 |
2263 |
1744 |
575 |
371 |
N/A |
| India |
SearoD |
127960004 |
35361230 |
2475286 |
1414449 |
10198179 |
6036162 |
4066541 |
744177 |
235859 |
894639 |
203327 |
388144 |
127932 |
82519 |
N/A |
| Maldives |
SearoD |
25984 |
4061 |
284 |
162 |
1171 |
693 |
467 |
85 |
27 |
103 |
23 |
6 |
2 |
1 |
N/A |
| Myanmar |
SearoD |
3956305 |
1213300 |
84931 |
48532 |
349916 |
207110 |
139530 |
25534 |
8093 |
30697 |
6976 |
9129 |
3009 |
1941 |
N/A |
| Nepal |
SearoD |
3506023 |
832451 |
58272 |
33298 |
240079 |
142099 |
95732 |
17519 |
5552 |
21061 |
4787 |
5501 |
1813 |
1170 |
N/A |
|
WPRO REGION | ||||||||||||||||
| Australia |
WproA |
1457527 |
32776 |
1204 |
385 |
17778 |
10416 |
8374 |
841 |
149 |
2724 |
1654 |
38 |
7 |
3 |
N/A |
| Brunei D'lam |
WproA |
37385 |
899 |
65 |
9 |
488 |
286 |
239 |
46 |
3 |
70 |
42 |
3 |
1 |
0 |
N/A |
| Japan |
WproA |
5430793 |
135770 |
9504 |
5431 |
73642 |
43148 |
36251 |
6634 |
2103 |
10150 |
6163 |
231 |
76 |
49 |
N/A |
| New Zealand |
WproA |
311974 |
7036 |
264 |
90 |
3816 |
2236 |
1800 |
184 |
35 |
583 |
354 |
31 |
6 |
2 |
N/A |
| Singapore |
WproA |
230550 |
5764 |
403 |
231 |
3126 |
1832 |
1539 |
282 |
89 |
431 |
262 |
9 |
3 |
2 |
N/A |
| Cambodia |
WproB |
1491690 |
373583 |
27150 |
4096 |
107741 |
63771 |
42699 |
8162 |
683 |
12489 |
7583 |
2101 |
693 |
140 |
N/A |
| China |
WproB |
81595595 |
6488544 |
454198 |
259542 |
1871296 |
1107594 |
746183 |
136551 |
43279 |
208931 |
126851 |
43089 |
14202 |
9161 |
N/A |
| Cook Islands |
WproB |
2096 |
210 |
15 |
2 |
61 |
36 |
24 |
5 |
0 |
7 |
4 |
0 |
0 |
0 |
N/A |
| Fiji |
WproB |
89552 |
14426 |
1051 |
125 |
4161 |
2463 |
1648 |
316 |
21 |
484 |
294 |
30 |
10 |
2 |
N/A |
| Kiribati |
WproB |
9948 |
1625 |
118 |
18 |
469 |
277 |
186 |
35 |
3 |
54 |
33 |
19 |
6 |
1 |
N/A |
| Lao Peop's DR |
WproB |
682861 |
212441 |
15325 |
3573 |
61268 |
36264 |
24312 |
4607 |
596 |
7049 |
4280 |
1076 |
355 |
107 |
N/A |
| Malaysia |
WproB |
2828151 |
285716 |
20781 |
2945 |
82400 |
48772 |
32652 |
6248 |
491 |
9559 |
5804 |
199 |
66 |
12 |
N/A |
| Marshall Isl. |
WproB |
5400 |
934 |
59 |
10 |
269 |
159 |
106 |
18 |
2 |
32 |
19 |
5 |
2 |
0 |
N/A |
| Micronesia |
WproB |
13237 |
2620 |
118 |
50 |
756 |
447 |
292 |
35 |
8 |
91 |
55 |
23 |
5 |
2 |
N/A |
| Mongolia |
WproB |
296799 |
60292 |
4389 |
582 |
17388 |
10292 |
6889 |
1320 |
97 |
2019 |
1226 |
332 |
109 |
20 |
N/A |
| Nauru |
WproB |
1025 |
97 |
7 |
1 |
28 |
16 |
11 |
2 |
0 |
3 |
2 |
1 |
0 |
0 |
N/A |
| Niue |
WproB |
152 |
15 |
1 |
0 |
4 |
3 |
2 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
N/A |
| Palau |
WproB |
2046 |
211 |
12 |
4 |
61 |
36 |
24 |
3 |
1 |
7 |
4 |
0 |
0 |
0 |
N/A |
| Papua N. G. |
WproB |
962437 |
166267 |
11905 |
3755 |
47951 |
28382 |
19051 |
3579 |
626 |
5476 |
3325 |
2038 |
672 |
264 |
N/A |
| Philippines |
WproB |
11254421 |
2428448 |
170059 |
96399 |
700364 |
414536 |
279254 |
51127 |
16075 |
78227 |
47495 |
8974 |
2958 |
1896 |
N/A |
| R. of Korea |
WproB |
2371820 |
249811 |
17487 |
9992 |
72045 |
42643 |
28728 |
5257 |
1666 |
8044 |
4884 |
56 |
18 |
12 |
N/A |
| Samoa |
WproB |
22338 |
3377 |
245 |
43 |
974 |
576 |
386 |
74 |
7 |
113 |
68 |
7 |
2 |
1 |
N/A |
| Solomon Isl. |
WproB |
79962 |
19101 |
1381 |
290 |
5509 |
3261 |
2185 |
415 |
48 |
635 |
386 |
59 |
20 |
5 |
N/A |
| Tonga |
WproB |
13792 |
2223 |
162 |
19 |
641 |
379 |
254 |
49 |
3 |
75 |
45 |
4 |
1 |
0 |
N/A |
| Tuvalu |
WproB |
1015 |
126 |
9 |
2 |
36 |
22 |
14 |
3 |
0 |
4 |
3 |
0 |
0 |
0 |
N/A |
| Vanuatu |
WproB |
33152 |
8344 |
584 |
334 |
2406 |
1424 |
960 |
176 |
56 |
269 |
163 |
9 |
3 |
2 |
N/A |
| Viet Nam |
WproB |
7185862 |
1728193 |
124101 |
35174 |
498411 |
295003 |
197920 |
37310 |
5865 |
57086 |
34660 |
3553 |
1171 |
420 |
N/A |
|
EURO REGION | ||||||||||||||||
| Andorra |
EuroA |
4001 |
58 |
4 |
1 |
31 |
18 |
15 |
3 |
0 |
9 |
2 |
0 |
0 |
0 |
N/A |
| Austria |
EuroA |
386431 |
5604 |
406 |
78 |
3040 |
1781 |
1488 |
283 |
30 |
913 |
186 |
5 |
2 |
0 |
N/A |
| Belgium |
EuroA |
616259 |
8882 |
647 |
80 |
4817 |
2823 |
2356 |
452 |
31 |
1456 |
296 |
7 |
2 |
0 |
N/A |
| Croatia |
EuroA |
389100 |
5610 |
409 |
52 |
3043 |
1783 |
1488 |
285 |
20 |
919 |
187 |
8 |
2 |
0 |
N/A |
| Czech Rep. |
EuroA |
547804 |
7892 |
575 |
68 |
4280 |
2508 |
2093 |
401 |
26 |
1294 |
263 |
23 |
7 |
1 |
N/A |
| Denmark |
EuroA |
326007 |
4413 |
168 |
55 |
2394 |
1402 |
1129 |
117 |
21 |
770 |
157 |
5 |
1 |
0 |
N/A |
| Estonia |
EuroA |
78229 |
1129 |
82 |
12 |
613 |
359 |
300 |
57 |
5 |
185 |
38 |
2 |
1 |
0 |
N/A |
| Finland |
EuroA |
299477 |
4314 |
314 |
37 |
2340 |
1371 |
1144 |
219 |
14 |
708 |
144 |
7 |
2 |
0 |
N/A |
| France |
EuroA |
3974436 |
53589 |
2019 |
534 |
29067 |
17031 |
13698 |
1409 |
207 |
9391 |
1910 |
61 |
12 |
3 |
N/A |
| Germany |
EuroA |
3466740 |
49718 |
3450 |
516 |
26967 |
15800 |
13146 |
2408 |
200 |
8192 |
1666 |
67 |
21 |
4 |
N/A |
| Greece |
EuroA |
586137 |
8500 |
615 |
118 |
4610 |
2701 |
2257 |
430 |
46 |
1385 |
282 |
35 |
12 |
3 |
N/A |
| Hungary |
EuroA |
490804 |
7071 |
515 |
61 |
3835 |
2247 |
1875 |
360 |
24 |
1160 |
236 |
27 |
9 |
1 |
N/A |
| Iceland |
EuroA |
23511 |
339 |
25 |
3 |
184 |
108 |
90 |
17 |
1 |
56 |
11 |
0 |
0 |
0 |
N/A |
| Ireland |
EuroA |
358318 |
5011 |
282 |
53 |
2718 |
1592 |
1307 |
197 |
21 |
847 |
172 |
4 |
1 |
0 |
N/A |
| Israel |
EuroA |
735243 |
10618 |
772 |
113 |
5759 |
3375 |
2818 |
539 |
44 |
1737 |
353 |
13 |
4 |
1 |
N/A |
| Italy |
EuroA |
2901653 |
41871 |
3047 |
418 |
22711 |
13307 |
11109 |
2127 |
162 |
6856 |
1395 |
30 |
10 |
2 |
N/A |
| Luxembourg |
EuroA |
28783 |
389 |
15 |
4 |
211 |
124 |
100 |
11 |
1 |
68 |
14 |
0 |
0 |
0 |
N/A |
| Malta |
EuroA |
19130 |
278 |
20 |
4 |
151 |
88 |
74 |
14 |
2 |
45 |
9 |
0 |
0 |
0 |
N/A |
| Monaco |
EuroA |
2001 |
29 |
2 |
0 |
16 |
9 |
8 |
1 |
0 |
5 |
1 |
0 |
0 |
0 |
N/A |
| Netherlands |
EuroA |
934218 |
12528 |
435 |
126 |
6795 |
3981 |
3192 |
303 |
49 |
2208 |
449 |
18 |
3 |
1 |
N/A |
| Norway |
EuroA |
303047 |
4085 |
150 |
45 |
2216 |
1298 |
1044 |
105 |
17 |
716 |
146 |
3 |
1 |
0 |
N/A |
| Poland |
EuroA |
1933388 |
27852 |
2030 |
241 |
15107 |
8851 |
7388 |
1417 |
93 |
4568 |
929 |
126 |
41 |
7 |
N/A |
| Portugal |
EuroA |
516604 |
7448 |
542 |
69 |
4040 |
2367 |
1976 |
379 |
27 |
1221 |
248 |
3 |
1 |
0 |
N/A |
| San Marino |
EuroA |
1401 |
20 |
1 |
0 |
11 |
6 |
5 |
1 |
0 |
3 |
1 |
0 |
0 |
0 |
N/A |
| Serbia & Montenegro |
EuroA |
604144 |
8747 |
634 |
110 |
4744 |
2780 |
2322 |
443 |
43 |
1428 |
290 |
30 |
10 |
2 |
N/A |
| Slovakia |
EuroA |
275895 |
3688 |
123 |
34 |
2000 |
1172 |
938 |
86 |
13 |
652 |
133 |
35 |
6 |
2 |
N/A |
| Slovenia |
EuroA |
99368 |
1433 |
104 |
14 |
777 |
455 |
380 |
73 |
5 |
235 |
48 |
2 |
1 |
0 |
N/A |
| Spain |
EuroA |
2521375 |
36353 |
2647 |
339 |
19718 |
11553 |
9644 |
1848 |
131 |
5958 |
1212 |
50 |
16 |
3 |
N/A |
| Sweden |
EuroA |
557426 |
7682 |
382 |
72 |
4167 |
2441 |
1989 |
266 |
28 |
1317 |
268 |
10 |
2 |
1 |
N/A |
| Switzerland |
EuroA |
376228 |
5431 |
395 |
56 |
2946 |
1726 |
1441 |
276 |
22 |
889 |
181 |
3 |
1 |
0 |
N/A |
| UK |
EuroA |
3765820 |
50844 |
1913 |
560 |
27578 |
16158 |
13000 |
1335 |
217 |
8898 |
1810 |
165 |
32 |
10 |
N/A |
| Albania |
EuroB |
207681 |
6230 |
436 |
249 |
3379 |
1980 |
1664 |
304 |
96 |
981 |
200 |
66 |
22 |
14 |
N/A |
| Bosnia & Herzegovina |
EuroB |
164958 |
4784 |
346 |
67 |
2595 |
1520 |
1270 |
242 |
26 |
780 |
159 |
24 |
8 |
2 |
N/A |
| Bulgaria |
EuroB |
373095 |
10245 |
470 |
122 |
5557 |
3256 |
2643 |
328 |
47 |
1763 |
359 |
219 |
50 |
15 |
N/A |
| Georgia |
EuroB |
256459 |
7488 |
539 |
143 |
4061 |
2380 |
1990 |
376 |
55 |
1212 |
247 |
108 |
36 |
12 |
N/A |
| Romania |
EuroB |
1079244 |
32377 |
2266 |
1295 |
17561 |
10290 |
8645 |
1582 |
501 |
5100 |
1037 |
807 |
266 |
172 |
N/A |
| FYR Macedonia |
EuroB |
111863 |
3236 |
235 |
39 |
1755 |
1029 |
859 |
164 |
15 |
529 |
108 |
10 |
3 |
1 |
N/A |
| Turkey |
EuroB |
6412702 |
172393 |
6203 |
1724 |
93506 |
54786 |
43984 |
4330 |
667 |
30306 |
6164 |
2212 |
408 |
126 |
N/A |
| Armenia |
EuroB |
226376 |
6661 |
475 |
167 |
3613 |
2117 |
1773 |
332 |
65 |
1070 |
218 |
80 |
26 |
11 |
N/A |
| Azerbaijan |
EuroB |
795163 |
23855 |
1670 |
954 |
12939 |
7581 |
6369 |
1166 |
369 |
3758 |
764 |
1448 |
477 |
308 |
N/A |
| Kyrgyzstan |
EuroB |
595111 |
17168 |
1250 |
166 |
9312 |
5456 |
4555 |
872 |
64 |
2812 |
572 |
599 |
197 |
35 |
N/A |
| Tajikistan |
EuroB |
870519 |
25144 |
1828 |
267 |
13638 |
7991 |
6672 |
1276 |
104 |
4114 |
837 |
2097 |
691 |
136 |
N/A |
| Turkmenistan |
EuroB |
505844 |
14823 |
1062 |
325 |
8040 |
4711 |
3943 |
741 |
126 |
2391 |
486 |
824 |
271 |
104 |
N/A |
| Uzbekistan |
EuroB |
2737750 |
82133 |
5749 |
3285 |
44549 |
26102 |
21929 |
4013 |
1272 |
12938 |
2632 |
4970 |
1638 |
1057 |
N/A |
| Belarus |
EuroC |
514996 |
30900 |
2163 |
1236 |
16760 |
9820 |
8250 |
1510 |
479 |
4868 |
990 |
51 |
17 |
11 |
N/A |
| Kazakhstan |
EuroC |
1640953 |
94676 |
6892 |
914 |
51352 |
30088 |
25117 |
4811 |
354 |
15510 |
3155 |
1408 |
464 |
83 |
N/A |
| Latvia |
EuroC |
115275 |
6673 |
484 |
82 |
3619 |
2121 |
1771 |
338 |
32 |
1090 |
222 |
19 |
6 |
1 |
N/A |
| Lithuania |
EuroC |
166177 |
9592 |
698 |
96 |
5203 |
3048 |
2545 |
487 |
37 |
1571 |
319 |
19 |
6 |
1 |
N/A |
| R. of Moldova |
EuroC |
214693 |
12557 |
902 |
256 |
6811 |
3991 |
3339 |
629 |
99 |
2029 |
413 |
161 |
53 |
19 |
N/A |
| Russian Federation |
EuroC |
8117113 |
487027 |
34092 |
19481 |
264163 |
154777 |
130036 |
23797 |
7542 |
76721 |
15604 |
1618 |
533 |
344 |
N/A |
| Ukraine | EuroC | 2376293 | 139669 | 9980 | 3376 | 75756 | 44387 | 37167 | 6966 | 1307 | 22460 | 4568 | 629 | 207 | 87 | N/A |
ALRI – acute lower respiratory infection, SP – Streptococcus pneumoniae, Hib – Haemophilus influenzae type B, RSV – respiratory syncytial virus, FLU – influenza virus
*For viral etiologies, N/A indicates that estimates are not available at the national level at this point, due to very little available information and high degree of uncertainty of regional and global estimates.
The 2000 estimate of the proportion of pneumonia episodes that are severe was 8.6% (7.0–13.0%), and was based on 6 studies, all of them from LMIC [18]. The estimate for 2010 is based on 9 studies and brings the estimate for LMIC upward, to 11.5% (8.0–33.0%). In HIC for 2000, we did not have an evidence–based estimate for the proportion of pneumonia episodes in the community that develop into severe cases. In this current analysis, we found 9 more recent studies from HIC that show a much higher estimate of the proportion – 26.7% (20.0–46.7%). However, many of them come from hospital–based studies, where more severe episodes are likely to be clustered, and a lower threshold for severity is generally applied. Still, an increasing trend in the proportion of severe episodes in LMICs seems consistent with a higher proportion expected in HICs. Nevertheless, in an effort not to overestimate the severe pneumonia burden we elected to use the proportion of pneumonia episodes developing into severe disease from the LMIC in all HIC also.
An analysis of the prevalence of exposure to the 5 main risk factors in the year 2010 in comparison to 2000 shows that the prevalence of malnutrition declined in all LMICs from 26.9% to 21.9%, low birth weight from 15.9% to 8.8%, non–exclusive breastfeeding from 64.4% to 52.6% and solid fuel use from 65.5% to 52.2% [49,50]. The exposure to all of those risk factors fell by 20–30%, which provides plausibility to the finding that our estimate of the incidence of pneumonia fell by 25% between 2000 and 2010 in LMICs. We could not perform a similar comparison with the crowding risk factor, because of the change of the definition of crowding from “5 or more” to “7 or more” residents in the same household between surveys done in 2000 and 2010.
This study also exposed rather dramatic changes in the importance of different etiological agents along the spectrum of pneumonia episode severity. At the level of all incident episodes in a community, RSV is the most common pathogen, present in about 28.8% of all episodes, followed by influenza (17.0%), while SP (adjusted for vaccine use of both Hib and PCV) is estimated to account for only 6.9% of cases and Hib (adjusted for vaccine use) in 2.8% of cases. However, at the level of severe episodes, RSV's contribution decreased to 22.6% and influenza to 7.0%, while SP rose to 18.3% and Hib to 4.1%. Bacterial etiologies become even more important in the subgroup of the children who eventually die of the disease, with the dominant causes being SP (32.7%) and Hib (15.7%). Again, both of these estimates account for Hib and PCV vaccine use in 2010.
DISCUSSION
Although there is seemingly more evidence used in this study than there was available in the previous studies of global childhood pneumonia morbidity [1,18], the increase in evidence is only slight: 35 studies in 1990–2010 to estimate global pneumonia incidence, in comparison to 28 studies for the period 1960–2000 [18]. This also means that the studies published between 1990 and 2000 were used to produce both estimates. However, the most recent studies (those published after 2000) are consistently showing a substantially lower incidence of community–based pneumonia than was the case historically, which implies that the burden of morbidity is steadily decreasing. This also suggests that the estimates presented in this paper maybe more closely related to the situation in the year 2000 rather than 2010, because we used the information from the previous two decades in the context of scarcity of information, and the true morbidity figures for 2010 are likely to be even smaller, ie, less than 0.20 e/cy. In addition, the decreasing trend is quite consistent with apparent improvements in risk factor prevalence, as recorded by DHS and MICS [49,50].
With more evidence, the proportion of pneumonia cases that are severe has been revised upward. For HIC, most of these estimates were relevant to children hospitalized for pneumonia, thus clustering the most severe cases, while most studies in LMICs are community–based and encompass a full spectrum of severity. Still, it appears that the increase in the proportion of severe episodes in the LMICs is a valid trend, given the high proportion in HICs (which may reflect increased proportion of parents seeking care and lower threshold for hospitalization). This finding may seem paradoxical (ie, that the proportion of pneumonia that is severe in nature is higher in high income settings that LMIC) and may be explained by a propensity to hospitalize children in HIC or by a faster reduction in pneumonia incidence at the community level than in the incidence of severe pneumonia episodes. We could speculate that improved social, economic and lifestyle conditions in many LMICs over the past decade have a rather major effect on pneumonia incidence in the whole population, while the cases that progress into severe episodes are still clustering in the areas with persistent poverty which are not really enjoying the benefits of economic growth, so it is more difficult to reduce them. If this is true, then the proportional contribution of severe episodes to all pneumonia episodes in the community is set to continue increasing over time, although both the cases of pneumonia in the community and severe cases are being reduced – but the former is being reduced at a faster pace than the latter.
It is reassuring that the etiological estimates for SP, Hib, RSV and influenza, which were based on entirely different data sets from those that were used for the estimates of pneumonia incidence, severe morbidity and mortality, and which were also conducted independently of each other, are all “fitting” into the envelopes of pneumonia incidence, severe morbidity and pneumonia deaths at the global, regional and (for SP and Hib) also at the national level [37–46]. At the level of pneumonia incidence, there is likely a multitude of etiological causes that contribute to pneumonia in the community. Therefore, at the global level, the four major pathogens combined explain about 55% of all episodes, according to our computations, but this grows to considerably more among pneumonia deaths [43–46]. The modeling effort does not reveal whether the unattributed fractions are likely from these four pathogens or from other pathogens. As an example, at the level of severe episodes, it appears that the four pathogens explain nearly 95% of all episodes in Europe and 83% in the Americas, but only 48% in Eastern Mediterranean region and as little as 39% in sub–Saharan Africa. A large ongoing project funded by The Gates Foundation – “The Pneumonia Etiology Research for Child Health (PERCH)” study – will try to explain etiology of childhood pneumonia better at the global level [25]. It is a 7–site study in LMICs, coordinated by Johns Hopkins University, to determine the etiology, or causes, of pneumonia, and the first results are expected in the latter part of 2014 [25].
All burden of disease estimates must cope with the issue of uncertainty in the data and the estimates. All the estimates of childhood pneumonia at either global, regional or (especially) national levels are inherently uncertain, for the many reasons mentioned in the beginning of this paper. The evidence to population models remains limited, and the case definitions used across studies are not all the same, yet the estimates are rather robust. What makes them plausible, if not certain, is that they are internally consistent: mutually independent cause–specific etiology estimates “fit” into the “envelopes” of total cases, severe cases and deaths; in addition, case–fatality rates between incident cases and deaths, and severe episodes and deaths, based on our model, resemble those observed in real data. This is all reassuring, but it also needs to be noted that the estimates of uncertainty (presented in Online Supplementary Document(Online Supplementary Document)) are still probably (substantially) under–estimated. This is because each and every parameter derived from a previous parameter (eg, proportion of all acute lower respiratory infection (ALRI) cases that are SP) has its own uncertainty, as do the estimates of vaccine effectiveness, risk factor odds ratios, rates of vaccine coverage, and all other parameters in the model. We typically expressed only uncertainty related to each specific parameter, without adding the uncertainty of all the previous parameters from which the new estimate has been derived. This makes the whole table in the Online Supplementary Document(Online Supplementary Document) seem more precise than it actually may be, given that the uncertainties are really large, and only the consistency between different estimates in the much bigger picture is what gives it some credibility with the current amount of evidence. CHERG aims to continue identifying new sources of published and unpublished good quality data and updating these estimates regularly with this new information and so increase the quality of its estimates towards the Millennium Development Goal 4 target in 2015 and well beyond, until preventable childhood diseases are adequately controlled and responded to everywhere in the world [57,58].
Acknowledgements
Shamim Qazi is a staff member of the World Health Organization. The expressed views and opinions do not necessarily express the policies of the World Health Organization.
Funding: This work was supported by the Bill and Melinda Gates Foundation.
Ethical approval: Not required.
Authorship declaration: All co–authors designed and conducted the study and contributed to the writing of the paper.
Additional Material
REFERENCES
- 1.Rudan I, Boschi–Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Millennium Development Goals. Available from: http://www.un.org/millenniumgoals/ Accessed: 1 April 2013.
- 4.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 5.Greenwood B. A global action plan for the prevention and control of pneumonia. Bull World Health Organ. 2008;86:322–2A. doi: 10.2471/BLT.08.053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lob–Levyt J. Contribution of the GAVI Alliance to improving health and reducing poverty. Philos Trans R Soc Lond B Biol Sci. 2011;366:2743–7. doi: 10.1098/rstb.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra M, Mason E, Borrazzo J, Campbell H, Rudan I, Liu L, et al. Ending of preventable deaths from pneumonia and diarrhoea: an achievable goal. Lancet. 2013;381:1499–506. doi: 10.1016/S0140-6736(13)60319-0. [DOI] [PubMed] [Google Scholar]
- 8.Bryce J, El Arifeen S, Bhutta ZA, Black RE, Claeson M, Gillespie D, et al. Getting it right for children: a review of UNICEF joint health and nutrition strategy for 2006–15. Lancet. 2006;368:817–9. doi: 10.1016/S0140-6736(06)69299-4. [DOI] [PubMed] [Google Scholar]
- 9.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 10.Walley J, Lawn JE, Tinker A, de Francisco A, Chopra M, Rudan I, et al. Primary health care: making Alma–Ata a reality. Lancet. 2008;372:1001–7. doi: 10.1016/S0140-6736(08)61409-9. [DOI] [PubMed] [Google Scholar]
- 11.Feachem RG, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, et al. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–78. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marais BJ, Raviglione MC, Donald PR, Harries AD, Kritski AL, Graham SM, et al. Scale–up of services and research priorities for diagnosis, management, and control of tuberculosis: a call to action. Lancet. 2010;375:2179–91. doi: 10.1016/S0140-6736(10)60554-5. [DOI] [PubMed] [Google Scholar]
- 13.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–16. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhutta ZA, Das JK, Walker N, Rizvi A, Campbell H, Rudan I, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381:1417–29. doi: 10.1016/S0140-6736(13)60648-0. [DOI] [PubMed] [Google Scholar]
- 15.Gill CJ, Young M, Schroder K, Carvajal–Velez L, McNabb M, Aboubaker S, et al. Bottlenecks, barriers, and solutions: results from multicountry consultations focused on reduction of childhood pneumonia and diarrhoea deaths. Lancet. 2013;381:1487–98. doi: 10.1016/S0140-6736(13)60314-1. [DOI] [PubMed] [Google Scholar]
- 16.Campbell H, El Arifeen S, Hazir T, O'Kelly J, Bryce J, Rudan I, et al. Measuring coverage in MNCH: Challenges in monitoring the proportion of young children with pneumonia who receive antibiotic treatment. PLoS Med. 2013;10:e1001421. doi: 10.1371/journal.pmed.1001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazir T, Begum K, El Arifeen S, Khan AM, Huque MH, Kazmi N, et al. Measuring coverage in MNCH: A prospective validation study in Pakistan and Bangladesh on measuring correct treatment of childhood pneumonia. PLoS Med. 2013;10:e1001422. doi: 10.1371/journal.pmed.1001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudan I, Tomaskovic L, Boschi–Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 19.Lanata CF, Rudan I, Boschi–Pinto C, Tomaskovic L, Cherian T, Weber M, et al. Methodological and quality issues in epidemiological studies of acute lower respiratory infections in children in developing countries. Int J Epidemiol. 2004;33:1362–72. doi: 10.1093/ije/dyh229. [DOI] [PubMed] [Google Scholar]
- 20.Campbell H, Biloglav Z, Rudan I. Reducing bias from test misclassification in burden of disease studies: use of test to actual positive ratio––new test parameter. Croat Med J. 2008;49:402–14. doi: 10.3325/cmj.2008.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez L, Reyes H, Tome P, Ridaura C, Flores S, Guiscafre H. Validation of the verbal autopsy method to ascertain acute respiratory infection as cause of death. Indian J Pediatr. 1998;65:579–84. doi: 10.1007/BF02730899. [DOI] [PubMed] [Google Scholar]
- 22.Byass P, Chandramohan D, Clark SJ, D'Ambruoso L, Fottrell E, Graham WJ, et al. Strengthening standardised interpretation of verbal autopsy data: the new InterVA–4 tool. Glob Health Action. 2012;5:1–8. doi: 10.3402/gha.v5i0.19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker N, Fischer–Walker C, Bryce J, Bahl R, Cousens S. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol. 2010;39(Suppl 1):i21–31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris SS, Tomaskovic L, Black RE. Predicting the distribution of under–five deaths by cause in countries without adequate vital registration systems. Int J Epidemiol. 2003;32:1041–51. doi: 10.1093/ije/dyg241. [DOI] [PubMed] [Google Scholar]
- 25.Levine OS, O'Brien KL, Deloria–Knoll M, Murdoch DR, Feikin DR, DeLuca AN, et al. The Pneumonia etiology research for child health project: a 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54(Suppl 2):S93–101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adegbola RA. Childhood pneumonia as a global health priority and the strategic interest of the Bill and Melinda Gates Foundation. Clin Infect Dis. 2012;54(Suppl 2):S89–92. doi: 10.1093/cid/cir1051. [DOI] [PubMed] [Google Scholar]
- 27.Shaw CA, Ciarlet M, Cooper BW, Dionigi L, Keith P, O'Brien KB, et al. The path to an RSV vaccine. Curr Opin Virol. 2013pii:S1879-6257(13)00063-1 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.McIntosh K. Community–acquired pneumonia in children. N Engl J Med. 2002;346:429–37. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 29.Theodoratou E, Johnson S, Jhass A, Madhi SA, Clark A, Boschi–Pinto C, et al. The effect of Haemophilus influenzae type b and pneumococcal conjugate vaccines on childhood pneumonia incidence, severe morbidity and mortality. Int J Epidemiol. 2010;39(Suppl 1):i172–85. doi: 10.1093/ije/dyq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster J, Theodoratou E, Nair H, Seong AC, Zgaga L, Huda T, et al. An evaluation of emerging vaccines for childhood pneumococcal pneumonia. BMC Public Health. 2011;11(Suppl 3):S26. doi: 10.1186/1471-2458-11-S3-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson HL, Deloria–Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockburn WC, Assaad F. Some observations on the communicable diseases as public health problems. Bull World Health Organ. 1973;49:1–12. [PMC free article] [PubMed] [Google Scholar]
- 33.Bulla A, Hitze KL. Acute respiratory infections: a review. Bull World Health Organ. 1978;56:481–98. [PMC free article] [PubMed] [Google Scholar]
- 34.Leowski J. Mortality from acute respiratory infections in children under 5 years of age: global estimates. World Health Stat Q. 1986;39:138–44. [PubMed] [Google Scholar]
- 35.Garenne M, Ronsmans C, Campbell H. The magnitude of mortality from acute respiratory infections in children under 5 years in developing countries. World Health Stat Q. 1992;45:180–91. [PubMed] [Google Scholar]
- 36.World Bank. Investing in health: the world development report. Washington, DC: World Bank; 1993. [Google Scholar]
- 37.Williams BG, Gouws E, Boschi–Pinto C, Bryce J, Dye C. Estimates of world–wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/S1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 38.Theodoratou E, Zhang JS, Kolcic I, Davis AM, Bhopal S, Nair H, et al. Estimating pneumonia deaths of post–neonatal children in countries of low or no death certification in 2008. PLoS ONE. 2011;6:e25095. doi: 10.1371/journal.pone.0025095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bryce J, Boschi–Pinto C, Shibuya K, Black RE, Child Health WHO, Epidemiology Research Group WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 40.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 42.Nair H, Simőes EA, Rudan I, Gessner BD, Azziz–Baumgartner E, Zhang JS, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–90. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria–Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 44.Watt JP, Wolfson LJ, O’Brien KL, Henkle E, Deloria–Knoll M, McCall N, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–11. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 45.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta–analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta–analysis. Lancet. 2011;378:1917–30. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. Definition of region groupings. Available from: http://www.who.int/healthinfo/global_burden_disease/definition_regions/en/ Accessed: 1 April 2013.
- 48.United Nations. World population prospects: population database. United Nations Population Division; 2010. Available from: http://esa.un.org/unpp Accessed: 1 April 2013.
- 49.Macro ORC. Demographic and Health Survey. Calverton, MD: ORC Macro. Available from: http://www.measuredhs.com Accessed: 1 April 2013.
- 50.UNICEF. Multiple Indicators Cluster Survey (MICS). New York, NY: UNICEF. Available from: http://www.unicef.org/statistics/index_24302.html Accessed: 1 April 2013.
- 51.Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. New York: Lippincott, Williams & Wilkins Publishers; 1998. [Google Scholar]
- 52.Madhi SA, De Wals P, Grijalva CG, Grimwood K, Grossman R, Ishiwada N, et al. The burden of childhood pneumonia in the developed world: a review of the literature. Pediatr Infect Dis J. 2013;32:e119–27. doi: 10.1097/INF.0b013e3182784b26. [DOI] [PubMed] [Google Scholar]
- 53.UNICEF. Immunization. Statistics. Available from: http://www.unicef.org/immunization/index_statistics.html Accessed: 1 April 2013.
- 54.UN Inter–agency Group for Child Mortality Estimation. Levels and trends in child mortality. New York: United Nations International Children’s Emergency Fund, 2012. [Google Scholar]
- 55.Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 56.Anderson HR. Respiratory disease in childhood. Br Med Bull. 1986;42:167–71. doi: 10.1093/oxfordjournals.bmb.a072116. [DOI] [PubMed] [Google Scholar]
- 57.Rudan I, El Arifeen S, Black RE, Campbell H. Childhood pneumonia and diarrhoea: setting our priorities right. Lancet Infect Dis. 2007;7:56–61. doi: 10.1016/S1473-3099(06)70687-9. [DOI] [PubMed] [Google Scholar]
- 58.Rudan I, Lawn J, Cousens S, Rowe AK, Boschi–Pinto C, Tomaskovic L, et al. Gaps in policy–relevant information on burden of disease in children: a systematic review. Lancet. 2005;365:2031–40. doi: 10.1016/S0140-6736(05)66697-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


