Abstract
Objective(s): Osteoarthritis (OA) or degenerative joint disease is the commonest form of arthritis and can lead to joint pain, decrease in joint’s range of motion, loss of function, and ultimately disability. Exercise is considered as one of the non-pharmacological treatments of OA. But the effects of exercise on knee joint cartilage remain ambiguous. The aim of the present study was to investigate the effect of a four-week moderate treadmill exercise on rats’ knee osteoarthritis.
Materials and Methods: Eighteen male Wistar rats (173 ± 1 g, 8 weeks old) were randomly divided into three groups (n = 6): Intact control, monosodium iodoacetate (MIA) only (OA), and training. The osteoarthritis model was induced by intra-articular injection of monosodium iodoacetate (MIA). Subjects followed a moderate-intensity exercise program for 28 days. Rats were killed after 28 days and histological assessment was done on their knee joints. One-way ANOVA (P<0.05) and post-hoc Tukey test was used for the statistical analysis.
Results: Histological assessment on 3 measurements of, depth ratio of lesions (P=0.001), total cartilage degeneration width (P=0.001), and significant cartilage degeneration width (P=0.001), demonstrated that moderate exercise for 4 weeks could surprisingly almost treat OA symptoms of rats’ knee joints.
Conclusion: The findings of the present study indicate that a moderate treadmill exercise program exert a beneficial influence on rats’ knee osteoarthritis.
Key Words: Exercise, Monosodium Iodoacetate, Rat’s Knee Osteoarthritis
Introduction
Osteoarthritis (OA) is considered to be the commonest form of arthritis and can result in joint pain, decrease in joint range of motion, loss of function and disability (1). It has been reported that 45 to 75% of people older than 55 years have early symptoms of OA and this is the reason why OA is regarded as a global concern (2). Among elderly, knee OA is one of five major causes of disability in developed countries and it is estimated that nearly 100000 people in the U.S. are unable to walk due to knee or hip OA (3). From 1995 to 2005, Patients diagnosed with OA have increased by 6 million and it is expected that the number of people disabled due to OA will be doubled by the year 2020 (4, 5).
Several risk factors related to OA have been studied, among which, age, joint injury, nutrition, occupation, joint deformation, genetic factors and intense exercise are more common (6,7). There have been several studies in the field of sports medicine that consider knee OA as the most important disease that can occur after injury to this joint (8-11). This emphasizes the importance of addressing long-term consequences of sports injuries.
Although its nature and symptoms are well defined and its related risk factors have been studied precisely, there is no known cure for OA (12, 13). The treatment modalities for OA include non-pharmacological and pharmacological treatments and ultimately surgery. Non-pharmacological treatments involve physiotherapy, aerobic and strength training exercises, weight loss, wearing braces and orthoses and so on (14).
Since using surgical and pharmacological treatments may be accompanied by a heavy economic burden and long-term consequences, finding effective non-surgical and non-pharmacological methods (such as exercise) is very helpful. Several studies have been conducted to investigate the effects of non-pharmacological treatments, especially exercise, as a way to treat or manage OA symptoms (15-17).
It is worth noting that some studies have determined intense exercises to have an adverse role (18-21), although most of them agree that exercise with moderate intensity is ideal (17,22-24). Exercises of low and medium impact exert elastic and compressive forces on the joint cartilage. At this magnitude, tensile forces act as potent anti-inflammatory signals and inhibit interleukin (IL)-1β, tumor necrosis factor (TNF)-α and lipopolysaccharide-induced pro-inflammatory gene transcription as observed in studies including cartilage explants and in vitro systems (25-27).
So far, studies on human models have had to evaluate the effects of treatment protocols using questionnaires or assessing some physical fitness measures. Hence, the effects of interventions on the joint’s cartilage remain ambiguous, because the researchers were unable to assess biochemical properties of the tissue in vivo (28). For this reason, there is a need to perform histopathological assessments that have to be done on animal models of OA resembling the condition in human models. According to our studies, intra-articular injection of monosodium iodoacetate (MIA) in animal models (such as rats), results in pathological changes closely resembling those seen in human OA (29). MIA injection into joints inhibits glyceraldehydes-3-phosphate dehydrogenase activity in chondrocytes, leading to disruption of glycolysis and eventual cell death (29, 30). So the aim of the present study was to examine the effects of moderate-intensity exercise on rats’ knee osteoarthritis.
Materials and Methods
Eighteen male Wistar rats (173 ± 1 g, 8 weeks old) were obtained from the Pasteur Institute (Amol, Northern Iran). The maintenance and care of the experimental rats were in accordance with the guidelines of the Ethics Committee of Guilan University. Rats were kept in individual plastic cages in a 12:12 light-dark cycle (light-on period, 6:00 AM-6:00 PM) in a controlled temperature of 22 ± 2°C and 50±5% humidity on a sawdust bedding. They were fed a standard diet in pellet forms and had access to tap water ad libitum. Body weight was recorded at regular intervals. The animals were randomly divided into three groups (n = 6): Intact control, MIA only (OA), and training.
The animals in training group were habituated on a motor-driven treadmill at a speed of 10m min-1 for 10 min/day for 1 week to reduce their stress regarding the new environment (17). After the adaptation period, a program of moderate physical training once a day, 5 days a week for 4 weeks with a speed of 18 m min-1 for 30 min/day had been performed (16). The training program started 24 hr after OA induction (17). For this purpose, the animals were anesthetized with ketamine (90 mg/kg, IP) and xylazine (20 mg/kg, IP); OA was induced by intra-articular injection of monosodium iodoacetate (Sigma-Aldrich) with a U-100 insulin needle containing 1 mg of iodoacetate diluted in 50 µl saline solution into animals’ right knee. In their left knee, 50 µl saline solution was injected (29).
On day 28, animals were killed by cervical dislocation under anesthesia. Whole knee joints were dissected, fixed in 10% formaldehyde solution in 50 cc vials and sent to a pathology laboratory. Histological procedures were done under the pathologist’s supervision. The samples decalcified with 5% formic acid, dehydrated through a descending series of ethanol with the use of an automated tissue processing apparatus. After embedding in paraffin, serial sections with a thickness of 7 µm were prepared for histological examination. Frontal and sagittal sections were prepared from tibiofemoral joints. The sections were stained with hematoxylin-eosin to observe the cellularity.
The severities of OA lesions were graded on a scale adopted from OARSI histopathology instructions. Three histopathological measures, including depth ratio of lesions (DR), total cartilage degeneration width (TDW) and significant cartilage degeneration width (SDW) were chosen for this purpose. DR is a measurement of the depth of cartilage degeneration (including areas of chondrocyte and proteoglycan loss, which may have good retention of collagenous matrix and no fibrillation) that is taken at the midpoint in each of the three zones across the tibial surface. TDW is the total width of the area of articular cartilage affected by any type of degenerative change (matrix fibrillation/loss, proteoglycan loss with or without chondrocyte death). And SDW is a measurement of the width of the tibial cartilage in which 50% or greater of the thickness (from surface to tidemark) is seriously compromised (31). Histomorphological scores in micrometers were assigned to these three measurements for statistical analysis (30).
The data were analyzed using the SPSS statistical software version 16. One-way ANOVA (P<0.05) and post-hoc Tukey test were used for the statistical analysis.
Results
Depth ratio of lesions (DR)
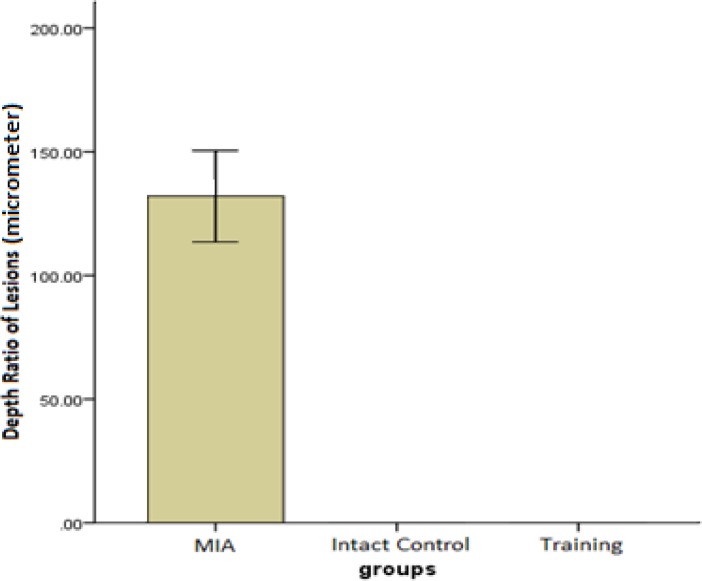
For the measurements of DR, there were significant differences between the training and MIA group (P=0.001) (Figure 1).
Figure 1.
The mean ± SD of histological scores related to the depth ratio of lesions measured in micrometers. Higher scores show greater severity of lesions
Total cartilage degeneration width (TDW)
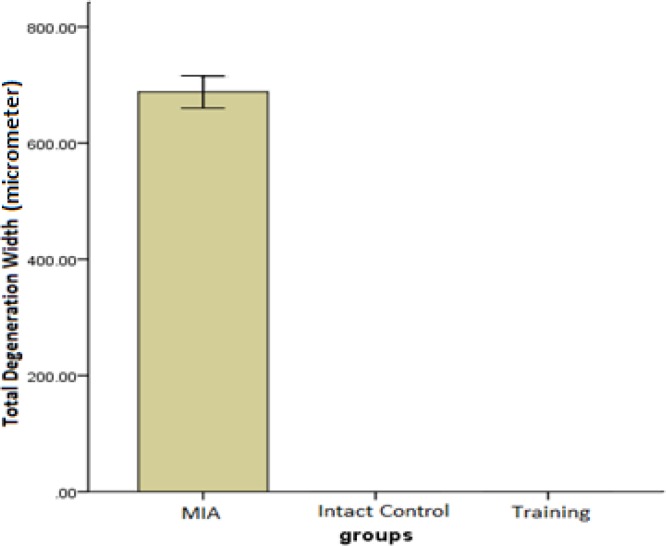
Significant differences were observed between the training and MIA group in the TDW measurements (P=0.001) (Figure 2).
Figure 2.
The mean ± SD of histological scores related to the total cartilage degeneration width measured in micrometers. Higher scores show greater severity of lesions
Significant cartilage degeneration width (SDW)
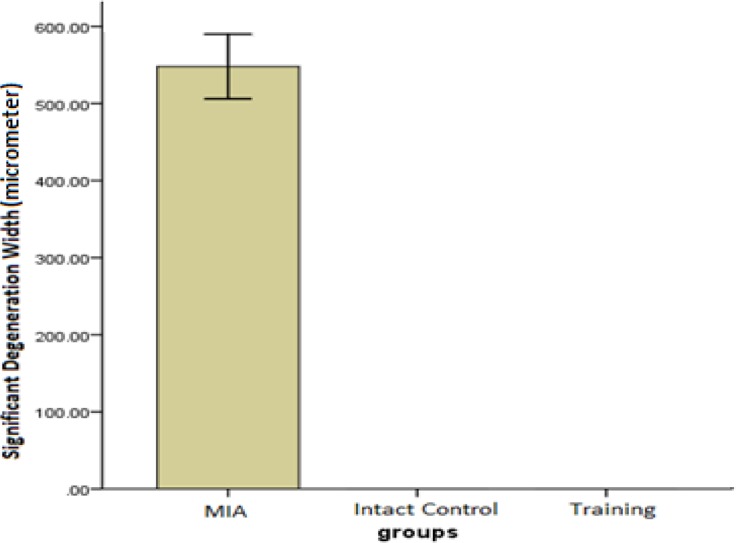
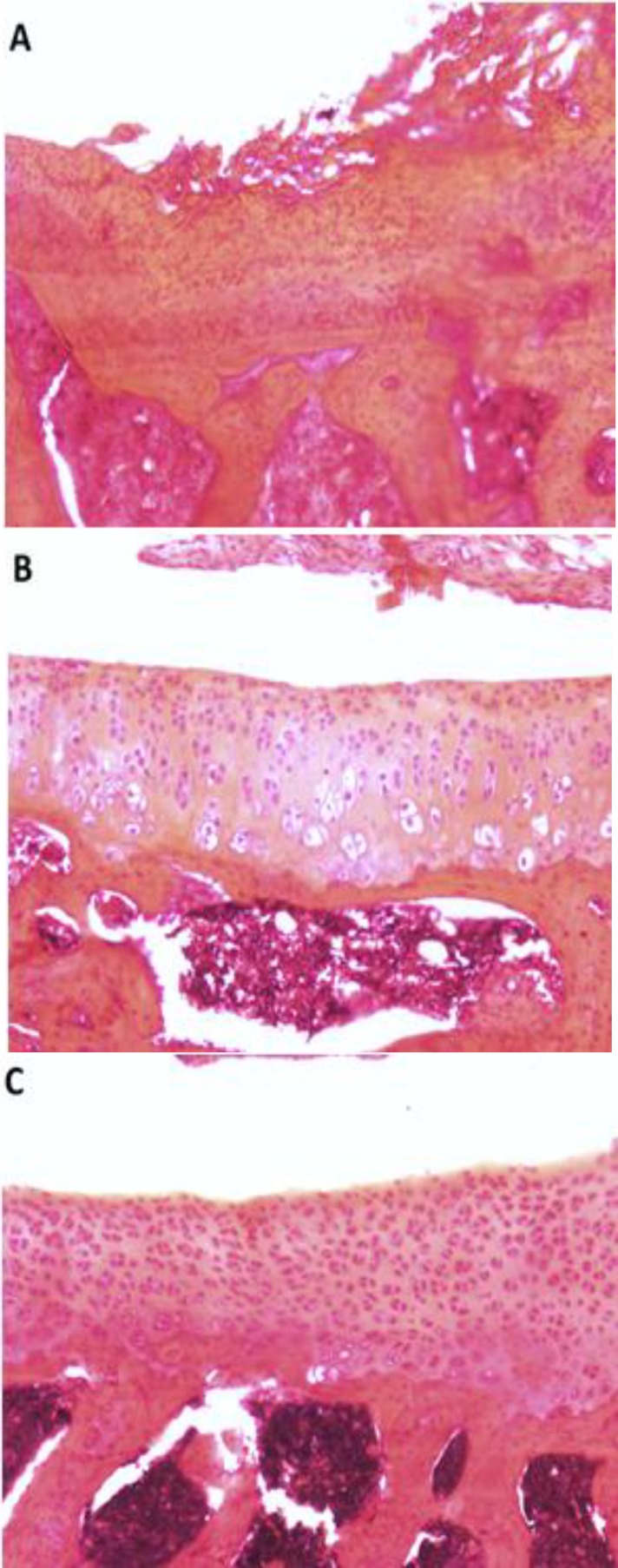
In terms of significant cartilage degeneration width, significant differences were seen between the training and MIA group (P=0.001) (Figure 3). Furthermore, photomicrographs of histomorphological changes of joint cartilage stained by Hematoxylin-Eosin for subjects in experimental groups are provided in Figure 4.
Figure 3.
The mean ± SD of histological scores related to the significant cartilage degeneration width measured in micrometers. Higher scores show greater severity of lesions.
Figure 4.
Photomicrographs of Histomorphological changes of knee joint sections of subjects in each experimental group. Cellularity and surface integrity was evaluated by Hematoxylin-Eosin staining (The original magnification was ×10). A: MIA only rat; OA lesions can be seen by tibial cartilage surface clefts and decrease in cellularity. Note the pink-red sites of lesions. B: Intact control rat; there are no observable changes in cartilage surface. C: Training rat; chondrocytes can be observed in many isogenic groups, which is the indicator of cell division stimulation
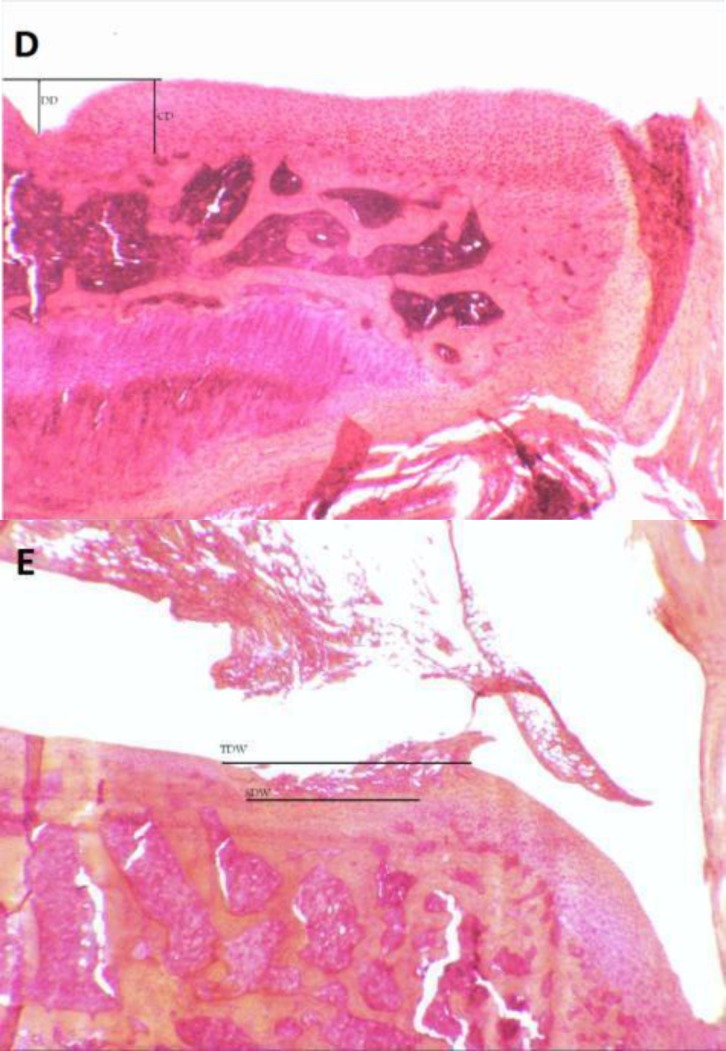
Figure 5 provide methods for assessing histopathological measures used in the present study.
Figure 5.
Method of histopathological assessment. D: Photomicrograph (The original magnification was ×3.2) showing a method for assessing depth ratio of Lesions; DD=Degeneration Depth, CD=Cartilage Depth, and DD/CD=Degeneration Depth Ratio; score 1 stands for the most severe lesion. E: Photomicrograph (The original magnification was ×3.2) showing a method for assessing Total Degeneration Width and Significant Degeneration Width; TDW= Total Degeneration Width and SDW= Significant Degeneration
Discussion
The present study is one of the few investigations that assess the effects of exercise on rats’ knee osteoarthritis. A four-week moderate intensity treadmill exercise was used as a treatment protocol. In order to induce osteoarthritic symptoms in rats’ knee joints, we used intra-articular injection of monosodium iodoacetate. This model is validated by other studies (32,33), but many caveats exist in utilizing the MIA models to represent human OA due to inherited variations, such as the biomechanical differences between two-legged and four-legged species (17).
The results of the present study show that a training protocol for 4 weeks could surprisingly almost treat OA symptoms of rats’ knee joint in 3 histological measures of depth ratio of lesions, total cartilage degeneration width, and significant cartilage degeneration width. In the training group, chondrocytes can be observed in many isogenic groups, which is the indicator of cell division stimulation (Figure. 4). Also, as presented in Figures 1, 2, and 3, training protocol used in the present study was beneficial in treating OA symptoms. From these Figures, it can be seen that the pathological score of the MIA group is nearly 130 micrometers for histological measure of depth ratio of lesions (Figure 1), 700 micrometers for total degeneration width (Figure 2), and 550 micrometers for significant degeneration width (Figure. 3). Whereas all three scores for the training group was zero and equal to healthy controls.
The beneficial effects of this exercise program may be explained by its ability to suppress the signal transduction pathways of pro-inflammatory/catabolic mediators, while stimulating anabolic pathways. Mechanical strain of low magnitude inhibits inflammation by suppressing IL‐1β and TNF‐α‐induced transcription of multiple pro-inflammatory mediators involved in cartilage degradation. This also results in the up-regulation of proteoglycan and collagen synthesis that is drastically inhibited in inflamed joints (34).
These findings were also observed in a dose-response study by Galois et al (16), in which moderate exercise had positive effects on osteoarthritic joints of rats, even though intense exercise had converse effects. It seems that intensity, frequency and duration of aerobic exercise modulates chondrocyte response in some form, as seen in this study. The researchers suggested that the positive effects of moderate exercise could be related to a reduced level of chondrocyte apoptosis through anti-apoptotic capacities of stress-induced Hsp70 overexpression.
Also another study that has been conducted by Cifuentes et al (17) indicated that a moderate exercise program could protect chondrocytes and increase defense mechanism against oxidative stress. They showed that this effect could be the result of increased activity levels of anti-oxidant enzymes such as superoxide dismutase (SOD) and myeloperoxidase (MPO).
The current treatments for osteoarthritis reduce pain and inflammation but have no significant effect on disease progression. In fact, effective treatments that would reverse the disease have not been developed (35), even though a limited number of studies have suggested that some anti-arthritic agents have the ability to arrest the pathologic process in humans and animals (36,37). Further research is required to establish the efficacy of such treatments.
The mechanisms of OA are multifactorial and in order to treat this disease it is essential to find a way that not only can protects the cartilage against degenerative damage by stimulating its intrinsic repair capacity of chondroprotection, but also neutralizes the inflammatory/destructive potential of the mediators involved in oxidative-inflammatory stress as reactive oxygen species (ROS), nitric oxide (NO), proteolytic enzymes (such as metalloproteinases) and inflammatory cytokines (such as IL-1β) (38). The results of our study suggest that aerobic exercise with moderate intensity can successfully accomplish this goal.
Cartilage, in fact, is an avascular tissue, and chondrocyte metabolism depends on diffusion and convection of synovial fluid for nutrition. Cyclic loading induced by physiological and overuse activities produces deformations, pressure gradients and fluid flows within the tissue. Mechanical stress has a direct effect on chondrocyte metabolism and can, under certain conditions, induce anti-apoptotic factors. Furthermore, compressive, tensile, and shear forces of appropriate/low physiological intensity also promote the up-regulation of proteoglycans and collagen synthesis, which are drastically inhibited in inflamed joints (38). Additionally, exercise promotes important changes in anti-oxidant enzyme activities, reducing oxidative damage and increasing tissue resistance against free radicals (39). Some studies have shown increases in SOD, Glutathione peroxidase and Catalase activities after aerobic exercise training in young rats (40, 41).
Conclusion
Regarding the findings of the present study, it can be concluded that a moderate exercise program can significantly exert beneficial effects on osteoarthritic joints of rats. However, this claim has to be investigated further. One of the mechanisms for observing such effects may be the age of the subjects (8 weeks). Probably at this age, cartilage is more capable of repairing itself and thus, older rats were needed to induce more acceptable results. Therefore, we suggest that further studies be administered on examining the effects of this exercise protocol on older rats or by using other OA induction models (such as ACL or MCL transection and meniscectomy methods) and also with other exercise modalities such as aquatic exercises with different intensity, frequency and duration on both human and animal models.
Acknowledgment
The present study has been conducted at the personal expense of the authors.
References
- 1.Jastan Marani M, Purfarzi F, Moharramzad Y. The effect of combination of glucosamine and chlorochin compared with glucosamine in the treatment of knee osteoarthritis. J Ardabil Univ Med Sci. 2009;9:157–163. [Google Scholar]
- 2.Nicholson S, Dickman K, Maradiegue A. Reducing premature osteoarthritis in the adolescent through appropriate screening. J Pediatr Nurs. 2009;24:69–74. doi: 10.1016/j.pedn.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Rajaee A, Alirezaee A, Velaee N. The Comparison between effects of glucosamine and MSM in the treatment of knee osteoarthritis. J Shahid Beheshti Univ Med Sci. 2009;5:283–287. [Google Scholar]
- 4.Bitton R. The Economic Burden of Osteoarthritis. Am J Manag Care. 2009;15:230–235. [PubMed] [Google Scholar]
- 5.Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology. 2005;44:1531–1537. doi: 10.1093/rheumatology/kei049. [DOI] [PubMed] [Google Scholar]
- 6.Bronner F, Farach-Carson M. Bone and Osteoarthritis. Springer; 2007. [Google Scholar]
- 7.Darrow M. The Knee Sourcebook. 1st ed. McGraw-Hill; 2002. [Google Scholar]
- 8.Klug M, Shrier I, McBain K, Shultz R, Meeuwisse WH, Garza D, et al. The Prevention of Sport Injury: An Anlaysis of 12000 Published Manuscripts. Clin J Sport Med . 2010;20:407–412. doi: 10.1097/JSM.0b013e3181f4a99c. [DOI] [PubMed] [Google Scholar]
- 9.Hede A, Hejgaard N, Sandberg H, Jacobsen K. Sports injuries of the knee ligaments: a prospective stress radiographic study. Br J Sports Med. 1985;19:8–10. doi: 10.1136/bjsm.19.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikkelsson O, Nupponen H, Kaprio J, Kautiainen H, Mikkelsson M, Kujala M. Adolescent flexibility, endurance strength, and physical activity as predictors of adult tension neck, low back pain, and knee injury: a 25 year follow up study. Br J Sports Med. 2006;40:107–113. doi: 10.1136/bjsm.2004.017350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med . 2005;39:212–216. doi: 10.1136/bjsm.2004.011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadipur M, Mozaffari R, Safavi M. The effect of pomegranate juice in protecting knee joint cartilage in experimental model of osteoarthritis. Med J Islam Azad Univ. 2007;17:199–203. [Google Scholar]
- 13.TM N, Heesch KC, Brown WJ. Efficacy of a progressive walking program and glucosamine sulphate supplementation on osteoarthritic symptoms of the hip and knee: a feasibility trial. Arthritis Res Ther. 2010;12:25–37. doi: 10.1186/ar2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Moskowitz RW. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Clyman B. Exercise in the Treatment of Osteoarthritis. Current Rheumatol Reports. 2001;3:520–523. doi: 10.1007/s11926-001-0067-5. [DOI] [PubMed] [Google Scholar]
- 16.Galois L, Etienne S, Grossin L, Watrin-Pinzano A, Cournil-Henrionnet C, Loeuille D, et al. Dose–response relationship for exercise on severity of experimental osteoarthritis in rats: a pilot study. OsteoArthritis Cartilage. 2004;12:779–786. doi: 10.1016/j.joca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Cifuentes D, Rocha LG, Silva LA, Brito AC, Rueff-Barroso CR, Porto LC, et al. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthritis Cartilage. 2010;18:1088–1095. doi: 10.1016/j.joca.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Saxon L, Finch C, Bass S. Sports participation, sports injuries and osteoarthritis: implications for prevention. Sports Med. 1999;28:123–135. doi: 10.2165/00007256-199928020-00005. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Park JA, Yang SH, Kim KY, Kim BK, Lee EY, et- al. Evaluation of osteoarthritis induced by treadmill-running exercise using the modiWed Mankin and the new OARSI assessment system. Springer-Verlag; 2010. [DOI] [PubMed] [Google Scholar]
- 20.Kujala UM, Kaprio J, Sarna S. Osteoarthritis of weight bearing joints of lower limbs in former elite male athletes. Br Med J. 1994:308. doi: 10.1136/bmj.308.6923.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Vignon E, Valat JP, Rossignol M, Avouac B, Rozenberg S, Thoumie P, et al. Osteoarthritis of the knee and hip and activity: a systematic international review and synthesis (OASIS) Joint Bone Spine. 2006;73:442–455. doi: 10.1016/j.jbspin.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Sutton AJ, Muir KR, Mockett S, Fentem P. A case-control study to investigate the relation between low and moderate levels of physical activity and osteoarthritis of the knee using data collected as part of the Allied Dunbar National Fitness Survey. Ann Rheum Dis. 2001;60:756–64. doi: 10.1136/ard.60.8.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazeem A, Olubayo A, Ganiyu A. Plasma Nitric Oxide and Acute Phase Proteins after Moderate and Prolonged Exercises. Iran J Basic Med Sci. 2012;15:602–607. [PMC free article] [PubMed] [Google Scholar]
- 25.Fehrenbacher A, Steck E, Rickert M, Roth W, Richter W. Rapid regulation of collagen but not metalloproteinase 1, 3, 13, 14 and tissue inhibitor of metalloproteinase 1, 2, 3 expression in response to mechanical loading of cartilage explants in vitro. Arch Biochem Biophys. 2003;410:39–47. doi: 10.1016/s0003-9861(02)00658-6. [DOI] [PubMed] [Google Scholar]
- 26.Mio K, Saito S, Tomatsu T, Toyama Y. Intermittent compressive strain may reduce aggrecanase expression in cartilage: a study of chondrocytes in agarose gel. Clin Orthop Relat Res. 2005;433:225–32. doi: 10.1097/01.blo.0000150466.30696.c6. [DOI] [PubMed] [Google Scholar]
- 27.Park S, Hung CT, Ateshian GA. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage. 2004;12:65–73. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Roos EM, Dahlberg Leif. Positive Effects of Moderate Exercise on Glycosaminoglycan Content in Knee Cartilage: A Four-Month, Randomized, Controlled Trial in Patients at Risk of Osteoarthritis. Arthritis Rheumatism. 2005;52:3507–3514. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 29.Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett . 2004;370:236–240. doi: 10.1016/j.neulet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Hadipur M, Mozaffari R. Protective effects of nutrients on articular cartilage of rats and its histological assessment. J Daneshvar Pezeshki. 2008;15:88–94. [Google Scholar]
- 31.Grewin N, Bendele M, Glasson S, Carlson S. The OARSI histopathology initiative- recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18:524–534. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Schuelert Niklas, McDougall Jason. Grading of monosodium iodoacetate-induced steoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett. 2009;465:184–188. doi: 10.1016/j.neulet.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 33.Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-Iodoacetate-Induced Histologic Changes in Subchondral Bone and Articular Cartilage of Rat Femorotibial Joints: An Animal Model of Osteoarthritis. Toxicol Pathol. 2003;31:619–624. doi: 10.1080/01926230390241800. [DOI] [PubMed] [Google Scholar]
- 34.Roman-Blas JA, Jimenez SA. NF-kB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis: Review. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Lay E, Samiric T, Handley CJ, Ilic MZ. Short- and long-term exposure of articular cartilage to curcumin or quercetin inhibits aggrecan loss. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh P. The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Semin Arthritis Rheum. 1999;28:211–67. doi: 10.1016/s0049-0172(99)80021-3. [DOI] [PubMed] [Google Scholar]
- 37.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 38.Knobloch TJ, Madhavan S, Nam J, Agarwal Jr S, Agarwal S. Regulation of chondrocytic gene expression by biomechanical signals. Crit Rev Eukaryot Gene Expr. 2008;18:139–150. doi: 10.1615/critreveukargeneexpr.v18.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinho RA, Andrades ME, Oliveira MR, Pirola AC, Zago MS, Silveira PCL, et al. Imbalance in SOD/CAT activities in rat skeletal muscles submitted to treadmill training exercise. Cell Biol Int. 2006;30:848–853. doi: 10.1016/j.cellbi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Meydani M, Evans W, Handelman G, Fielding RA, Meydani SN, Fiatarone MA, et al. Antioxidant response to exercise-induced oxidative stress and protection by vitamin E. Ann N Y Acad Sci. 1992;669:363–374. doi: 10.1111/j.1749-6632.1992.tb17124.x. [DOI] [PubMed] [Google Scholar]
- 41.Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, et al. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol. 1994;266:375–380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]