Abstract
The gold standard to assess jaundice in neonates is the serum bilirubin measurement. Blood sampling for the determination of total serum bilirubin (TSB) is painful for newborns and stressful for parents. The Bilicheck®, a new transcutaneous bilirubinometer, is considered as a more accurate measurement of bilirubin compared to the previous bilirubinometers courtesy of its advanced technology. The objective of this study was to evaluate the correlation between transcutaneous bilirubin (TcB) measurements using the Bilicheck® device and TSB in some Iranian neonates and to determine the most reliable cut-off value with the highest sensitivity and desirable specificity for bilirubin measured by the Bilicheck® on the forehead. This prospective observational study was conducted in 2011 on 560 healthy neonates with jaundice. TcB was measured using the Bilicheck® (Respironic, USA) within 30 minutes of TSB measurement via direct spectrophotometry. The results were assessed by simple linear regression analysis and receiver operative characteristic curve. There was good a correlation between TcB and TSB (r=0.969, r2=0.94), and this was not affected by sex, gestational age, postnatal age, and birth weight. TSB can be calculated through the measurement of TcB and use of the linear regression equation: TSB=-0.99+1.06TcB. Sensitivity and specificity of the Bilicheck® at the most reliable cut-off value (15 mg/dl) were 96.6% and 99%, respectively. The findings of the present study indicate that the Bilicheck® is a non-invasive, simple, easy, and reliable method for bilirubin measurement in neonatal jaundice, especially in neonates with bilirubin levels ≤15 mg/dl.
Key Words: Neonatal jaundice, Hyperbilirubinemia, Transcutaneous
Introduction
Hyperbilirubinemia has been recognized as the most common cause of readmission of healthy newborns after early hospital discharge.1 Therefore, accurate measurement of the bilirubin concentration is essential for diagnosing severe hyperbilirubinemia and guiding the clinician for timely management to prevent brain damage. Although the gold standard remains the measurement of the serum bilirubin concentration, this method is not only invasive and painful to the newborn but also stressful to parents. Moreover, depending on the location of laboratory services, there may be a delay before total serum bilirubin (TSB) results are available for management.
Over the last two decades, transcutaneous bilirubinometry has been developed as a non-invasive, safe, painless, and convenient method for the estimation of total bilirubin level but has not been widely adopted due to concerns about its accuracy. In recent years, a newer generation of transcutaneous bilirubinometers has been marketed. One of these newer transcutaneous devices is the Bilicheck® (Respironics, USA), which measures skin bilirubin using reflectance of light from the whole visible spectrum (380-760 nm). It differs from earlier models in that a multi-wavelength spectral reflectance technique allows the device to determine the optical densities attributed to bilirubin, dermal thickness, heme, and melanin in the epidermal and dermal layers in the infant skin.2 A large number of studies have reported on the significant correlation between the Bilicheck® transcutaneous bilirubin (TcB) readings and TSB in predominantly Caucasian,2,3 and Japanese neonates.4 Studies on primarily Hispanic,5 and Indian infants,6 who were more pigmented than Caucasians, showed that despite the significant correlation, TcB determinations underestimated TSB, especially in infants with relatively high TSB values. A Multiracial Malay, Chinese, and Indian infants’ study,7 showed that the Bilicheck® is not an alternative to measuring serum bilirubin in infants with hyperbilirubinemia. The only study using this device in Iran was conducted in Tabriz (North West of Iran),8 which reported that TcB had a good sensitivity, while the specificity 47.5%.
The aim of the present study was to determine the accuracy of the Bilicheck® for the measurement of total bilirubin level and to evaluate the effect of gestational age, postnatal age, or body weight on the TcB level. In addition, we sought to identify the most reliable cut-off value with the highest sensitivity and specificity for bilirubin as measured by the Bilicheck® on the forehead.
Subjects and Methods
Subject
This prospective study was carried out from October to November 2011. Totally, 560 healthy neonates with Hyperbilirubinemia, who were referred to the Neonatal Ward of Nemazee Hospital affiliated to Shiraz University of Medical Sciences, were enrolled in this study. The inclusion criterion was healthy Iranian neonates with hyperbilirubinemia, and the exclusion criteria were sepsis, direct hyperbilirubinemia, major congenital anomaly, hemangioma or echymosis on the forehead, phototherapy, and blood exchange transfusion.
Protocol
After the physician ordered TSB for clinical purposes, informed consent was obtained from the parents. TcB determination was made with the BiliCheck(R) (REF B 800-13, Respironics, USA), a hand-held bilirubinometer. All the measurements were performed with the same device on the forehead by one properly trained physician. Five measurements were taken over a period of less than 2 seconds, and the average was reported as a numerical value. Blood samples for TSB measurements were collected by using the heel stick techniques. These procedures were performed within 30 minutes of TcB measurements by one physician. TSB measurements were performed by using a unistat bilirubinometer, a direct spectrophotometric device with accuracy (bias) of ±5%. All the measurements were made by the skilled personnel of the Clinical Chemistry Laboratory of the hospital. These personnel were unaware about the result of the TcB readings. In addition, for the infants who had TSB>20 mg/dl, TSB levels were determined in two milliliters of a venous sample with a diazo method in the laboratory, as part of the routine evaluation of significant hyperbilirubinemia. Basic characteristics and clinical variables such as gestational age, age at which serum bilirubin was measured (hours), sex, and birth weight were recorded.
Statistical Analysis
Analysis of the data was done using descriptive statistic analysis, simple linear regression analysis, and scatter plot technique. Receiver operative characteristic (ROC) curves were constructed to determine the best TcB cut-off value, sensitivity, and specificity. SPSS software (version 16, USA) and the medical calculator were utilized for the analysis of the data. Statistical significance was defined as a P value<0.05.
Ethical Considerations
The protocol of the study was approved by the local Ethics Committee of the university. The assignment of any medical intervention such as blood sampling for TSB determination and phototherapy was in accordance with our institution’s protocol for the management of neonatal jaundice and was not at the discretion of the investigators. TcB measurement was a non-invasive procedure and free of charge for the parents. TcB readings were not used for management, and the results were not available to any clinical staff managing the neonates. Informed consent was obtained verbally from the parents.
Results
According to the inclusion and exclusion criteria, 560 healthy neonates with jaundice were recruited into the study. Table 1 depicts the demographic and clinical variables of these newborns. There was no significant difference in skin color and race between these neonates, all of whom were white.
Table 1.
Basic characteristics and clinical variables of the healthy neonates with jaundice
| Basic characteristics | Clinical variables | Number (n) | Percent (%) |
|---|---|---|---|
| Sex | Male | 286 | 51.1 |
| Female | 274 | 48.9 | |
| Age when serum bilirubin was measured, hours | Age≤24 | 1 | 0.2 |
| 24<age≤48 | 27 | 4.8 | |
| 48<age≤120 | 340 | 60.7 | |
| Age>120 | 192 | 34.3 | |
| Gestational age, weeks | GA<35 | 5 | 0.9 |
| 35≤GA≤37 | 45 | 8 | |
| GA>37 | 510 | 91.1 | |
| Birth weight, gram | BW≤2500 | 32 | 5.7 |
| BW>2500 | 528 | 94.3 |
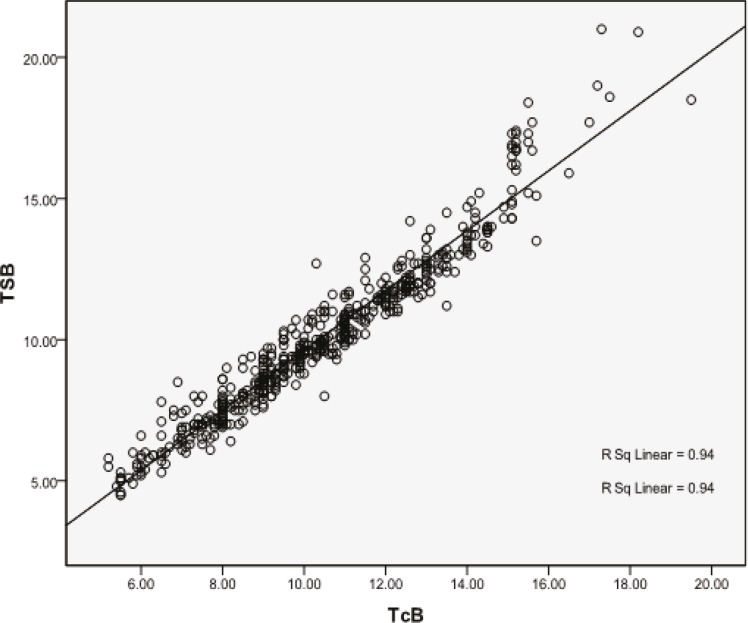
The Bilicheck® displayed a numeric value of TcB in 554 (98.9%) neonates, with a mean of 10.42 mg/dl (SD=2.58, range=5.2-19.5). In the remaining 6 (1.1%) newborns, the Bilicheck® displayed a message “very high values” only. Five of these 6 neonates had TSB levels more than 20 mg/dl by direct spectrophotometry (capillary sample) and diazo method (clot sample), and one of them had a TSB level of 18.9 mg/dl. All these newborns were excluded from the statistical analysis. There was a good correlation between TSB and TcB in all the infants (r=0.969, r2=0.94; P<0.001). The linear regression equation was TSB=-0.99+1.06 TcB. The TcB values of the infants were plotted against their TSB, with the exception of those without numeric TcB readings (figure 1). There was also a good correlation between TSB and TcB in the male (r2=0.944, P<0.001) and female (r2=0.935, P<0.001) neonates.
Figure 1.
Correlation between transcutaneous bilirubin (TcB) and total serum bilirubin (TSB
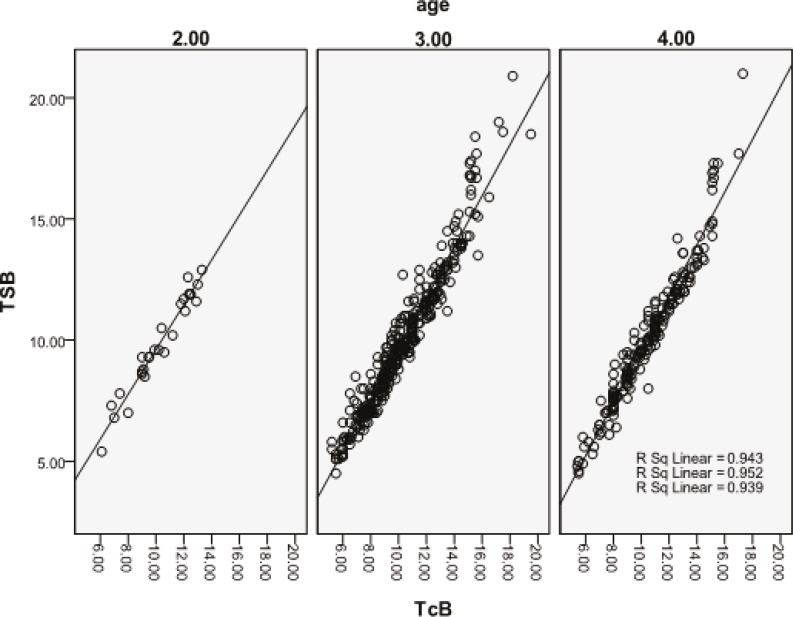
The infants were stratified into four groups based on the age at which serum bilirubin was measured (table 1). There was only one newborn in Group 1 for whom statistical analysis was impossible; however, in Groups 2, 3, and 4, there was a good correlation between TSB and TcB (figure 2). The postnatal age ranged from 21 hours to 15 days. Six newborns whose TcB showed “very high value” were in Group 4; they were excluded from the statistical analysis.
Figure 2.
Correlation between transcutaneous bilirubin (TCB) and total serum Bilirubin (TSB) in the different postnatal age (hours) groups. Group 2 (24<age£48): n=27, r2=0.0.943; P<0.001; Group 3 (48<age£120): n=340, r2=0.952; P<0.001; and Group 4 (age>120): n=186, r2=0.939; P<0.001
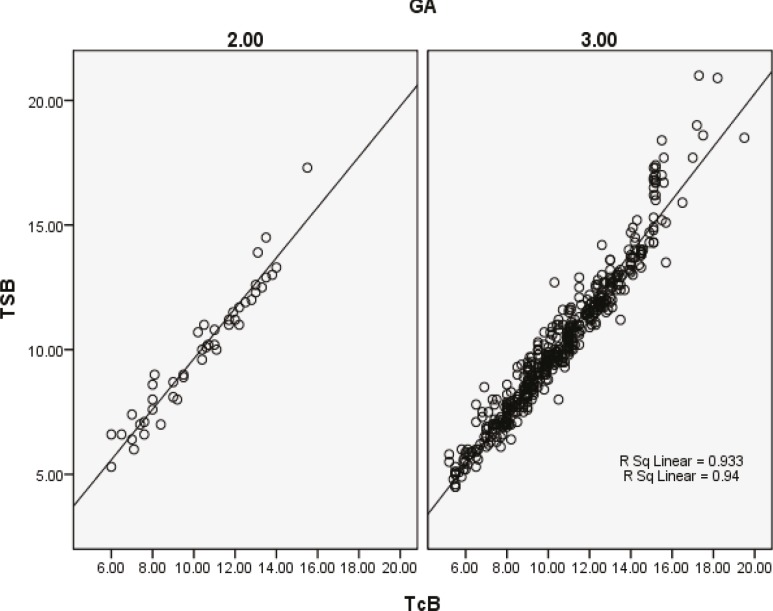
There was good correlation between TcB and TSB in gestational age 35≤gestational age≤37 and >37 weeks (figure 3).There were only 5 newborns in Group 1 (gestational age<35 weeks) for whom statistical analysis was impossible. The correlation coefficients between TcB and TSB in birth weight ≤500 gr and >2500 gr were r=0.963 and r=0.956, respectively with a P<0.001 in both groups.
Figure 3.
Correlation between transcutaneous bilirubin (TcB) and total serum bilirubin (TSB) in different gestational ages. Group 2 (35£gestational age£37): n=45, r2=0.933; P<0,001 and Group 3 (gestational age>37): n=504, r2=0.94; P<0.001
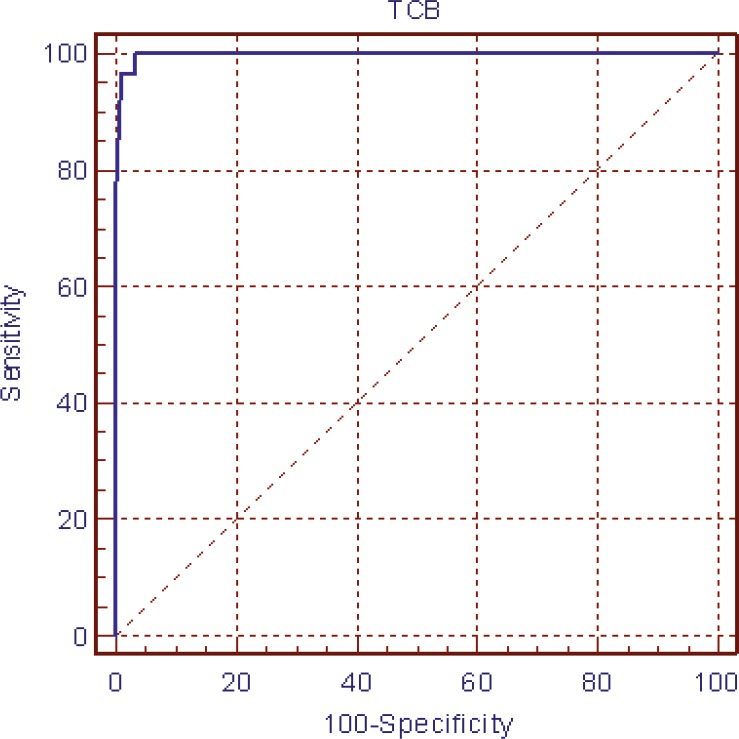
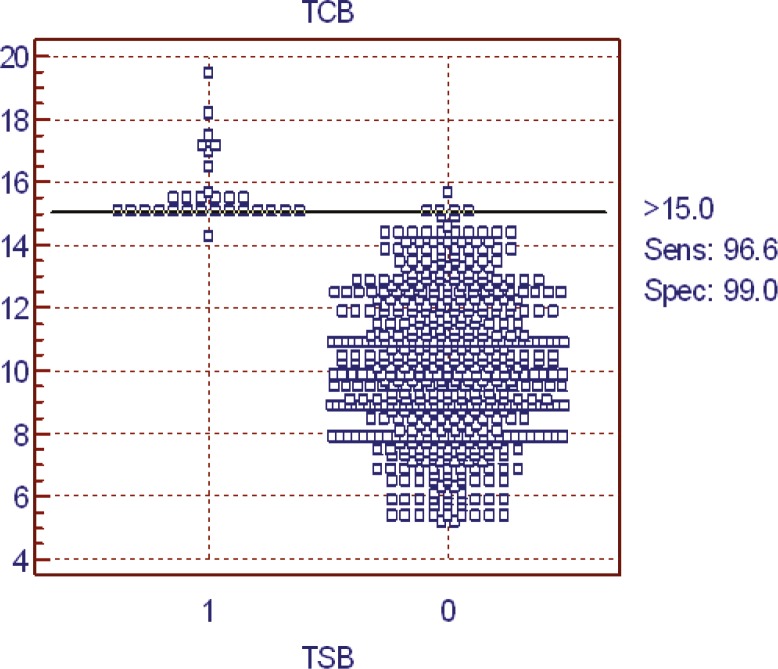
The ROC curves determined the TcB cut-off value of 15 mg/dl with highest sensitivity and specificity (96.6% and 99%, respectively, illustrated in figures 4 and 5). The PPV and NPV of TcB at the TcB cut-off values of 15 were 95.7% and 99.8%, respectively. In the bilirubin levels ≤15 mg/dl, TcB-TSB was 0.45±0.03 mg/dl, whereas in the bilirubin levels >15 mg/dl, TcB-TSB was -1.18±0.66 mg/dl.
Figure 4.
Receiver operator characteristic curve for the Bilicheck® to detect serum bilirubin>15 mg/dl. (Area under the ROC curve=0.997 and 95% confidence interval=0.987 to 1.000.)
Figure 5.
The sensitivity and specificity of TcB at TcB cut-off value >15 mg/dl. (0: TcB≤15 mg/dl, 1: TcB>15 mg/dl
The mean (±SD) difference between the TSB and TcB was -0.35 0.24 mg/dl (P>0.05).
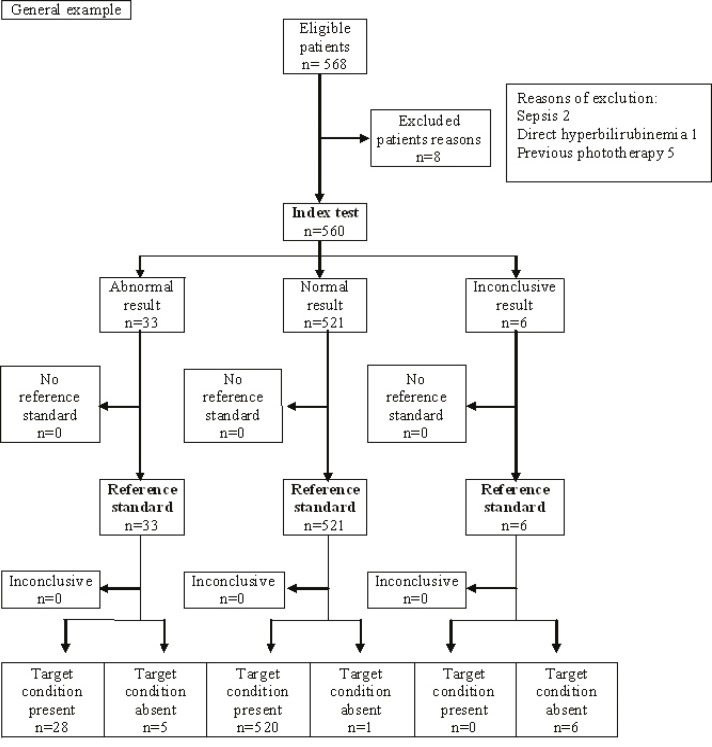
The STARD flow diagram used for check of accuracy of index test(Transcutaneous measurement) with standard reference test(spectrophotometry) that showed in figure 6.
Figure 6.
STARD flow diagram. Index test: TcB measurement by Bilicheck machine; Reference standard: TSB measurement by specterphotometry
Discussion
Neonatal jaundice, due to its potential for producing permanent encephalopathy, persists as a challenge for physicians. Painless evaluation of neonatal jaundice is highly desirable. The present study assessed a new transcutaneous bilirubinometer, the Bilicheck® in Iranian neonates with jaundice. Our results demonstrated a statistically significant correlation between the Bilicheck® readings and TSB levels. The correlation coefficient in this study (r=0.969) was comparable to those obtained by Rubaltelli et al. (r=0.89),2 Ebbsen et al. (r=0.88),3 Roberston et al. (r=0.937),9 and Janjindamai et al. (r=0.950).10 The mean difference between the two measurements in our study was small (0.35%); the Bilicheck® readings can, therefore, be employed as an alternative to TSB measurements in the range within which the Bilicheck® showed a numeric value. We had only a small group of neonates (1.1%), for whom the Bilicheck® displayed the message of “very high values”, and we excluded their TSB in the statistical analysis owing to the absence of a numerical value for TcB. In the Boo et al. study,7 the Bilicheck® machine was not able to provide a reading in10.5% of the neonates.
The factors that interfere with the accuracy of transcutaneous bilirubin measurements are believed to be race, gestational age, and body weight.2 The Bilicheck® has the theoretical advantage of isolating the light absorption of bilirubin from other factors such as hemoglobin, melanin, and dermal thickness.2 Our results confirmed that the TcB derived from the Bilicheck® is not affected by birth weight, gestational age, and postnatal age. These findings are similar to the observations of Ebbesen et al.3 and Hosseini et al.8 Ebbesen et al.3 reported that female infants admitted in Neonatal Intensive Care Unit (NICU) had TcB levels on the forehead greater than their male counterparts (P=0.003), whereas our TcB readings were not affected by sex.
The most important feature of a screening tool for neonatal jaundice is its capability to detect significant hyperbilirubinemia with 100% sensitivity. Missing a case of severe hyperbilirubinemia with resultant kernicterus is totally unacceptable. The device must also have a desirable level of specificity because over-diagnosis leads to unnecessary admissions and work up. For minimizing the problem of under- and over-diagnosis, we assessed the sensitivity, specificity, PPV, and NPV of TcB at different TcB cut-off values by the ROC curve. The maximum sensitivity, specificity, and especially NPV were at the TSB cut-off value of 15 mg/dl. In the bilirubin levels ≤15 mg/dl, TcB-TSB was 0.45±0.03 mg/d, which good and acceptable; however, in the bilirubin levels >15 mg/dl, TcB-TSB was -1.18 0.66 mg/dl. It indicates a tendency to underestimate TSB levels in infants with higher bilirubin levels. The Bilicheck® is, therefore, a reliable screening tool for hyperbilirubinemia with bilirubin levels ≤15mg/dl; this finding chimes in with those of the Samanta S et al.11 and Ho EY et al.12 studies.
The present study has several limitations. First, the sample size of the preterm infants with gestational age <35 was too small to allow the study to evaluate the confounding effects due to prematurity. Second, the sample size of a postnatal age ≤24 hours was too small to evaluate the reliability of this device on the first day of life. Third, serum bilirubin was measured using direct spectrophotometry rather than the ideal method of high performance liquid chromatography (HPLC). Finally, since this study was done on newborns from the Iranian city of Shiraz, these results cannot be generalized to populations with a more mixed ethnicity.
It is deserving of note that no attempt was made in the current study to evaluate the use of the Bilicheck® in sick and preterm infants. Our study included all neonates who were well enough to be discharged after birth and returned to hospital from their home. Further studies must be conducted to evaluate the reliability of the Bilicheck® in the NICU and small preterm infants. Today, TcB levels are also recommended to identify infants at risk of developing hyperbilirubinemia.13,14 Future studies can identify the reliability of this device for predischarge bilirubin determination during the first postnatal hours. HPLC measurements of the serum bilirubin are generally taken to be the gold standard, although this method is not widely utilized in routine clinical practice. In a multi-center study, Rubatelli et al.2 found that the correlation between the Bilicheck® and HPLC was similar to that between HPLC and standard laboratory methods (direct spectrophotometry and diazo method). In the Kaynak-Turkmen M study,15 there was also a good correlation between TcB and HPLC. In fact, we drew upon direct spectrophotometry and diazo method routinely to measure serum bilirubin; clinical decision-making is based on these results, providing the standard against any new method.
Conclusion
The findings of the present study indicate that the Bilicheck® is a reliable screening tool for hyperbilirubinemia in healthy-term and near-term newborns, especially with bilirubin levels ≤15mg/dl after the second day of life. In neonates with TSB>15 mg/dl, this device can underestimate the level of bilirubin and may affect clinical decision-making.
Acknowledgment
The present article was extracted from the thesis written by Dr Kiyani Rad and was financially supported by Shiraz University of Medical Sciences’ grant number 89-5499. The authors would like to thank Dr. Nasrin Shokrpour for editorial assistance, and Mr. Mehrab Sayadi for statistical consultation.
Conflict of Interest: None declared.
References
- 1.Mercier CE, Barry SE, Paul K, Delaney TV, Horbar JD, Wasserman RC, et al. Improving newborn preventive services at the birth hospitalization: a collaborative, hospital-based quality-improvement project. Pediatrics. 2007;120:481–8. doi: 10.1542/peds.2007-0233. doi: 10.1542/peds.2007-0233. PubMed PMID: 17766519. [DOI] [PubMed] [Google Scholar]
- 2.Rubaltelli FF, Gourley GR, Loskamp N, Modi N, Roth-Kleiner M, Sender A, et al. Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics. 2001;107:1264–71. doi: 10.1542/peds.107.6.1264. doi: 10.1542/peds.107.6.1264. PubMed PMID: 11389241. [DOI] [PubMed] [Google Scholar]
- 3.Ebbesen F, Rasmussen LM, Wimberley PD. A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatr. 2002;91:203–11. doi: 10.1080/080352502317285225. doi: 10.1111/j.1651-2227.2002.tb01696.x. PubMed PMID: 11952010. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Yamada D, Itakura Y, Ogawa Y. Clinical evaluation of a new device for transcutaneous bilirubin measurement in Japanese infants. J Saitama Med Sch. 2002;29:245–9. [Google Scholar]
- 5.Engle WD, Jackson GL, Sendelbach D, Manning D, Frawley WH. Assessment of a transcutaneous device in the evaluation of neonatal hyperbilirubinemia in a primarily Hispanic population. Pediatrics. 2002;110:61–7. doi: 10.1542/peds.110.1.61. doi: 10.1542/peds.110.1.61. PubMed PMID: 12093947. [DOI] [PubMed] [Google Scholar]
- 6.Lodha R, Deorari AK, Jatana V, Paul VK. Non-invasive estimation of total serum bilirubin by multi-wavelength spectral reflectance in neonates. Indian Pediatr. 2000;37:771–5. PubMed PMID: 10906811. [PubMed] [Google Scholar]
- 7.Boo NY, Ishak S. Prediction of severe hyperbilirubinaemia using the Bilicheck transcutaneous bilirubinometer. J Paediatr Child Health. 2007;43:297–302. doi: 10.1111/j.1440-1754.2007.01062.x. doi: 10.1111/j.1440-1754.2007.01062.x. PubMed PMID: 17444833. [DOI] [PubMed] [Google Scholar]
- 8.Hoseini MB, Heidarzadeh M, Maleki MJ. Validity of Transcutaneus Bilirubinometry in Measurement of Total Serum Bilirubin level of Neonates. Medical Journal o Tabriz University of Medical Sciences and Health Services. 2010;32:45–9. [Google Scholar]
- 9.Robertson A, Kazmierczak S, Vos P. Improved transcutaneous bilirubinometry: comparison of SpectR(X) BiliCheck and Minolta Jaundice Meter JM-102 for estimating total serum bilirubin in a normal newborn population. J Perinatol. 2002;22:12–4. doi: 10.1038/sj.jp.7210592. doi: 10.1038/sj.jp.7210592. PubMed PMID: 11840236. [DOI] [PubMed] [Google Scholar]
- 10.Janjindamai W, Tansantiwong T. Accuracy of transcutaneous bilirubinometer estimates using BiliCheck in Thai neonates. J Med Assoc Thai. 2005;88:187–90. PubMed PMID: 15962669. [PubMed] [Google Scholar]
- 11.Samanta S, Tan M, Kissack C, Nayak S, Chittick R, Yoxall CW. The value of Bilicheck as a screening tool for neonatal jaundice in term and near-term babies. Acta Paediatr. 2004;93:1486–90. doi: 10.1080/08035250410033042. doi: 10.1111/j.1651-2227.2004.tb02634.x. PubMed PMID: 15513577. [DOI] [PubMed] [Google Scholar]
- 12.Ho EY, Lee SY, Chow CB, Chung JW. BiliCheck transcutaneous bilirubinometer: a screening tool for neonatal jaundice in the Chinese population. Hong Kong Med J. 2006;12:99–102. PubMed PMID: 16603775. [PubMed] [Google Scholar]
- 13.Campbell DM, Danayan KC, McGovern V, Cheema S, Stade B, Sgro M. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141–5. doi: 10.1093/pch/16.3.141. PubMed PMID: 22379376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickremasinghe AC, Karon BS, Saenger AK, Cook WJ. Effect of universal neonatal transcutaneous bilirubin screening on blood draws for bilirubin analysis and phototherapy usage. J Perinatol. 2012;32:851–5. doi: 10.1038/jp.2012.10. doi: 10.1038/jp.2012.10. PubMed PMID: 22343396. [DOI] [PubMed] [Google Scholar]
- 15.Kaynak-Türkmen M, Aydoğdu SA, Gökbulut C, Yenisey C, Söz O, Cetinkaya-Cakmak B. Transcutaneous measurement of bilirubin in Turkish newborns: comparison with total serum bilirubin. Turk J Pediatr. 2011;53:67–74. PubMed PMID: 21534342. [PubMed] [Google Scholar]