Abstract
This paper describes a convenient approach to the 7-aza-des-A-steroid (6,6a,7,8,9,9a-hexahydro-5H-cyclopenta[h]quinoline) scaffold starting from Grundmann’s ketone using two different pyridine annulation protocols. The biological evaluation of the pyridine and pyridinium products revealed that these compounds unexpectedly do not interfere with ergosterol and cholesterol biosynthesis. The pyridinium compound 6 showed significant antimicrobial and cytotoxic activities which are most likely due to its detergent-like structure.
Keywords: Azasteroids, Pyridine, Annulation, Antimicrobial activity, Cytotoxic activity
Introduction
Steroids are of outstanding significance for human metabolism by acting as hormones (androgens, estrogens, gestagens, glucocorticoids, mineralocorticoids) or as an essential part of cell membranes (cholesterol). In fungi and protozoa, ergosterol is an indispensible part of cell membranes, and inhibition of ergosterol biosynthesis is one of the central targets of antifungal and antiprotozoal chemotherapy. Highly active drugs have been designed in the past by introducing new structural motifs into the tetracyclic sterol scaffold by either semisynthesis starting from natural sterols, or by de novo synthesis. In glucocorticoids, introduction of halogen substituents (mostly fluorine), additional double bonds, and oxygen-containing groups (alcohols, acetals) resulted in significant enhancement of activity and selectivity [1], whereas the annulation of additional heterocyclic rings (typically at the C-2,C-3 flank) resulted in compounds with various biological 330 M. Krojer, M. Keller, and F. Bracher: activities [2, 3]. Azasteroids containing protonable nitrogen atoms at various positions of the steroid backbone or the side-chain exhibit antifungal activities, with most of them mimicking carbocationic high energy intermediates (HEI) of enzymatic steps in the post-squalene part of ergosterol biosynthesis [4], whereas abiretarone, containing a pyridine ring in place of the aliphatic side chain at C-17, is a potent inhibitor of the enzyme 17α-hydroxylase/C17,20-lyase used therapeutically in cancer therapy due to its androgen-lowering activity [5]. Recently, our group reported about highly cytotoxic 1,4-dioxo-5,10-diazasteroids [1]. 4-Azasteroids containing a lactam moiety in ring A (finasteride, dutasteride) are important inhibitors of the enzyme 5α-reductase in the treatment of benign prostatic hyperplasia and alopecia [6], and lathosterol analogues containing amide groups in the side chain are selective inhibitors of the enzyme lathosterol oxidase in cholesterol biosynthesis [7]. Steroid-derived muscle relaxants like pancuronium bromide contain quaternary piperidinium groups attached to the ring system [8].
In recent investigations we demonstrated that secosteroids, derived from Grundmann’s ketone (1), covering the rings C+D as well as the aliphatic side chain of steroids, and containing amino (A) [9] or aminopropyl substituents (B) [10], are potent inhibitors of enzymes in sterol biosynthesis, whereas the trans-dinitrile C shows moderate cytotoxicity, and its cis-isomer is inactive [11] (Fig. 1). In the androgen field, a des-A-analogue showed separation of androgenic and anabolic activities [12].
Fig. 1.
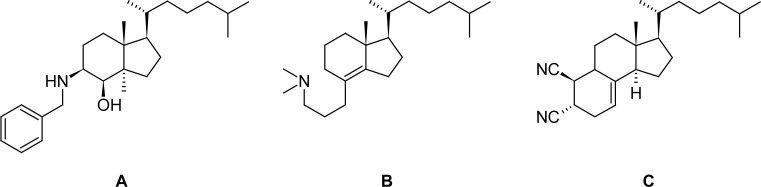
Bioactive compounds derived from Grundmann’s ketone: the aminoalcohol A[9] is a selective inhibitor of human sterol Δ8,7-isomerase, the aminopropylindene B[10] is an inhibitor of oxidosqualene cyclases from various organisms, the trans-dinitrile C[11] shows cytotoxicity.
Here we report on the synthesis of a novel aza-des-A-steroid in which ring B of the steroid backbone is replaced by a pyridine ring. The nitrogen atom is located at a position representing C-7 of steroids, and thus we expected that this azasteroid or its quaternary N-methyl derivative might interact with ergosterol biosynthesis in fungi or cholesterol biosynthesis in human cells by mimicking one of the numerous carbocationic HEIs [10, 13] of the enzymatic steps in the post-squalene part of sterol biosynthesis.
Results and Discussion
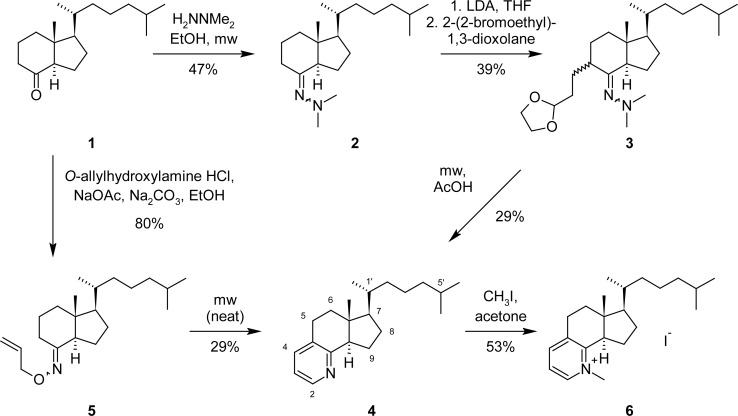
Grundmann’s ketone (1) [14] was obtained in a high yield by ozonolysis of cholecalciferol (vitamin D3), by instead using our improved workup procedure (treatment of the ozonolysis mixture with water, followed by extraction with pentane [10]). For the annulation of the pyridine ring we investigated two different approaches.
In the style of Gladiali’s pyridoannulation protocol [15], ketone 1 was first converted to N,N-dimethylhydrazone 2 by condensation with N,N-dimethylhydrazine in ethanol, and significant conversion was only obtained upon microwave irradiation. In the next step, 2 was converted to its kinetic lithium enolate with lithium diisopropylamide (LDA), followed by trapping with 2-(2-bromoethyl)-1,3-dioxolane to give regioselectively the double-masked 1,5-dicarbonyl compound 3. The absolute configuration at the newly generated stereo-center at C-5 could not be determined by NMR, but was of minor interest, since it was to be destroyed in the next step anyway. Finally, refluxing intermediate 3 in glacial acetic acid under microwave irradiation produced the annulated pyridine 4 in a 29% yield. The mechanism of this cyclization comprises in situ opening of the acetal and extrusion of dimethylamine [15]. This procedure produced the target pyridine 4 in a 5.3% overall yield in three steps from Grundmann’s ketone (1). The structure of 4 was confirmed by the typical 1H- and 13C-NMR resonances of a 2,3-disubstituted pyridine ring and fitting MS data.
Due to the poor overall yield of the approach described above, we also explored the pyridoannulation protocol of Koyama [16] involving the thermolysis of an oxime O-allyl ether. Grundmann’s ketone (1) was easily converted into the oxime ether 5 by condensation with O-allylhydroxylamine. The thermolysis of 5 to yield the target pyridine 4 turned out to be the critical step in this approach. Heating neat 5 in a sealed glass tube at 180 °C, as described in [16], did not result in the target product, and the same disappointing result was obtained upon refluxing the oxime ether in naphthalene (218 °C) or heating at 220 °C in benzene in a sealed tube for a prolonged amount of time (20 h). Finally, we found that microwave irradiation of neat 5 under air and pressure in a sealed tube led to the conversion to the annulated pyridine 4. Careful optimization of the reaction conditions led to the conclusion that high temperature (220 °C) combined with a short reaction time (5 min) gives the best yield (29%). With this protocol, pyridine 4 was obtained in a 23% overall yield in two steps from Grundmann’s ketone (1).
Due to its moderate basicity, pyridine 4 is not likely to be protonated to a significant extent under physiological conditions (pH 7.4). But a positive charge at the nitrogen would be a prerequisite for mimicking cationic HEIs in sterol biosynthesis. In order to introduce a positive charge into the molecule, we converted 4 into the N-methylpyridinium iodide 6 by reaction with methyl iodide.
The resulting azasteroid derivatives 4 and 6 were tested in an agar diffusion assay against a panel of bacteria and fungi (Tab. 1), and in an MTT assay [17] for cytotoxicity on human cells.
Tab. 1.
Antimicrobial activities determined in an agar diffusion assaya
| Compound | Bacteriab | Fungic | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| E. coli | Pseud. aer. | Staph. euq. | Strept. ent. | Yarr. lipol. | Cand. glabr. | Hyph. burt. | Asp. niger | |
| 4 | –d | – | – | – | – | – | – | – |
| 6 | 7 | – | 30 | 22 | 32 | 30 | 20 | 7 |
| Tetracycline-HCl | 30 | 25 | 42 | 25 | n.d.e | n.d. | n.d. | n.d. |
| Clotrimazole | n.d. | n.d. | n.d. | n.d. | 28 | 15 | 25 | 30 |
| Cetylpyridinium chloride | – | – | – | 10 | 8 | 9 | – | 7 |
Diameters of inhibition zones in mm, mean of 3 runs each.
Gram-negative bacteria: E. coli = Escherichia coli, Pseud. aer. = Pseudomonas aeruginosa; Gram-positive bacteria: Staph. euq. = Staphylococcus equorum, Strept. ent. = Streptococcus entericus.
Yeasts: Yarr. lipol. = Yarrowia lipolytica, Cand. glabr. = Candida glabrata; dermatophyte: Hyph. burt. = Hyphopichia burtonii; mould: Asp. niger = Aspergillus niger.
No zone of inhibition is detectable.
Not determined.
In the test for antimicrobial activity, compared to the antibiotic tetracycline and the antifungal clotrimazole, pyridine derivative 4 was found to be completely inactive. In contrast, the N-methylpyridinium salt 6 showed strong activity against Gram-positive bacteria, yeasts, and the dermatophyte Hyphopichia burtonii. This pattern of selectivity parallels the pattern of the antiseptic cetylpyridinium chloride [18], which is known to be highly active against Gram-positive bacteria and yeasts, but has a gap in its effectiveness against Gram-negative pathogens. Notably, 6 showed significantly higher antimicrobial activity than cetylpyridinium chloride.
The cytotoxicity of the compounds was determined in a MTT assay on HL-60 cells (human leukemia cell line) using the method of Mosmann [17]. The reference drug cisplatin gave an IC50 = 5 μM in this test. Pyridine 4 showed only very weak cytotoxicity (IC50 = 16 μM), whereas the pyridinium salt 6 was found to exhibit very strong cytotoxicity (IC50 = 0.8 μM).
Finally, both compounds were subjected to the assays for the detection of enzyme inhibition in the post-squalene part of cholesterol biosynthesis (on the human cell line HL-60 [19]) and ergosterol biosynthesis (model strain: Yarrowia lipolytica[20]), which have been worked out by our group previously. Both 4 and 6 were devoid of effects on sterol biosynthesis in both assays. Analysis of the sterol patterns after appropriate times of incubation showed that the sterol patterns of the cell lines were unchanged.
Conclusion
In conclusion, we worked out a convenient approach to 7-aza-des-A-steroids, which were intended to inhibit enzymes in ergosterol or cholesterol biosynthesis. Surprisingly, pyridine 4 as well as its N-methylpyridinium analogue 6 did not interfere at all with sterol biosynthesis. In a test for antimicrobial activities, the pyridine derivative 4 was inactive. In contrast, the pyridinium salt 6 showed an activity pattern quite similar to the one of cetylpyridinium chloride, but its activity was significantly higher compared to this reference drug. This allows the conclusion that the antimicrobial activity, and probably also the significant cytotoxic activity against a human cancer cell line, of 6 are most likely due to its detergent-like structure. The sterol-like partial structure of 6 might support the incorporation of this compound into cell membranes and concomitant disturbance of membrane integrity, thus leading to the observed effects.
Experimental
General
Mass spectra: Hewlett Packard 5989 A, EI at 70 eV, chemical ionisation (CI) with CH4 (300 eV); NMR: Jeol GSX 400 (1H: 400 MHz, 13C: 100 MHz); melting points: Büchi Melting Point B-540 (not corrected); flash column chromatography (FCC): silica gel 60 (230–400 mesh, E. Merck, Darmstadt, Germany); microwave-assisted reactions were performed in a CEM Discovery reactor (CEM, Matthews, USA).
2-[(1R,3aR,7aR)-1-[(1R)-(1,5-Dimethylhexyl]-7a-methyloctahydro-4H-inden-4-ylidene]-1,1-dimethylhydrazine (2)
A solution of 3.00 g (11.3 mmol) Grundmann’s ketone (1) and 3.44 mL (45.4 mmol) of N,N-dimethylhydrazine in 30 mL EtOH was heated under microwave irradiation (150 W) for 4 h, then treated with 50 mL of hydrochloric acid (10%), and extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were dried over MgSO4 and the solvent was evaporated. The residue was purified by FCC (isohexane/ethyl acetate 4:1) to give 1.62 g (47%) of 2 as a pale yellow oil. 1H-NMR (CDCl3): δ (ppm) = 2.36 (s, 3H, N-CH3, 2.35 (s, 3H, N-CH3), 2.32–2.20 (m, 1H, 3a-H), 2.15–0.97 (m, 19H, 1-H, 2-H, 3-H, 5-H, 6-H, 7-H, 1’-H, 2’-H, 3’-H, 4’-H, 5’-H), 0.96 (s, 3H, 7a-CH3), 0.89–0.84 (m, 9H, 1’-CH3, 5’-CH3, 6’-H). 13C-NMR (CDCl3): δ (ppm) = 170.57 (C-4), 54.96 (C-3a), 53.40 (C-1), 46.33 (C-7a), 44.93 (C-5), 39.84 (N(CH3)2), 38.39 (C-6), 36.39 (C-7), 33.38 (C-3), 32.83 (C-1’), 27.01 (C-5’), 26.39 (C-2), 23.79 (C-2’), 22.83 (7a-CH3), 21.78 (5’-CH3), 21.49 (C-6’), 21.06 (C-3’), 19.94 (C-4’), 18.71 (1’-CH3). CI-MS m/z (rel. int.): 307 (100, M++1), 278 (18). HR-MS (EI): calcd.: 306.3035, found: 306.3002.
2-{(1R,3aR,7aR)-1-[(1R)-1,5-Dimethylhexyl]-5-[2-(1,3-dioxolan-2-yl)ethyl]-7a-methyloctahydro-4H-inden-4-ylidene}-1,1-dimethylhydrazine (3)
3.00 mL (5.28 mmol) of LDA solution (1.76 M in THF) was added to 20 mL of anhydrous THF under N2, and the mixture was cooled to 0 °C. A solution of 1.62 g (5.28 mmol) of dimethylhydrazone 2 in 30 mL anhydrous THF was added dropwise with stirring. After 2 h at 0 °C, the mixture was cooled to −78 °C, and 0.62 mL (5.28 mmol) 2-(2-bromoethyl)-1,3-dioxolane were added slowly. After stirring at −78 °C for 1 h, the mixture was allowed to warm up to room temp. and quenched with 40 mL of water. The mixture was extracted with diethyl ether (3 × 50 mL). The combined organic layers were dried over MgSO4 and the solvent was evaporated. The residue was purified by FCC (isohexane/ethyl acetate 7:3) to give 848 mg (39%) of 3 as a pale yellow oil. 1H-NMR (CDCl3): δ (ppm) = 4.83 (t, 1H, J = 4.4 Hz, 3”-H), 3.99–3.91 (m, 2H, acetal-CH2), 3.88–3.79 (m, 2H, acetal-CH2), 2.50–2.43 (m, 6H, N(CH3)2), 2.41 (dd, 1H, J = 8.5 Hz, 5.3 Hz, 3a-H), 2.31–2.19 (m, 1H, 2”-H), 2.14–2.02 (m, 1H, 2”-H), 1.95–1.83 (m, 2H, 1”-H), 1.81–1.04 (m, 17H, 1-H, 2-H, 3-H, 5-H, 6-H, 7-H, 2’-H, 3’-H, 4’-H, 5’-H), 1.02 (s, 3H, 7a-CH3), 0.93 (m, 1H, 1’-H), 0.88–0.83 (m, 9H, 1’-CH3, 5’-CH3, 6’-H). 13C-NMR (CDCl3): δ (ppm) = 174.90 (C-4), 104.23 (C-3”), 64.87 (acetal-CH2), 64.84 (acetal-CH2), 58.79 (C-3a), 54.43 (C-1), 49.15 (C-7a), 48.42 (C-5), 39.84 (N(CH3)2), 39.38 (C-6), 36.57 (C-7), 35.44 (C-3), 34.17 (C-1’), 31.38 (C-5’), 28.27 (C-2), 27.96 (C-2’), 27.54 (C-2”), 26.01 (C-1”), 24.17 (7a-CH3), 22.78 (5’-CH3), 22.51 (C-6’), 22.01 (C-3’), 21.57 (C-4’), 19.02 (1’-CH3). CI-MS m/z (rel. int.): 407 (100, M++1). HR-MS (EI): calcd.: 406.3559, found: 406.3185.
(6aR,7R,9aR)-7-[(1R)-1,5-Dimethylhexyl]-6a-methyl-6,6a,7,8,9,9a-hexahydro-5H-cyclopenta[h]quinoline (7-Aza-19-nor-des-A-cholesta-5,7,9-triene, 4)
Method 1
A solution of 848 mg (2.08 mmol) of 3 in 5 mL glacial acetic acid was refluxed under microwave irradiation (300 W) for 3 h, then neutralized carefully with saturated Na2CO3 solution, and extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were dried over MgSO4 and the solvent was evaporated. The residue was purified by FCC (isohexane/ethyl acetate 9:1) to give 182 mg (29%) of 4 as a light brown oil.
Method 2
860 mg (2.69 mmol) of the O-allyl oxime 5 was heated in a closed vessel without solvent under microwave irradiation (300 W, Tmax = 290 °C) for 5 min. The product was purified by FCC (isohexane/ethyl acetate 9:1) to give 230 mg (29%) of 4 as a light brown oil.
1H-NMR (CDCl3): δ (ppm) = 8.36 (d, 1H, J = 4.8 Hz, 2-H), 7.31 (d, 1H, J = 7.6 Hz, 4-H), 6.95 (dd, 1H, J = 7.6 Hz, 4.7 Hz, 3-H), 2.79 (t, 1H, J = 8.5 Hz, 9a-H), 2.67–2.65 (m, 2H, 5-H), 2.29–2.22 (m, 1H, 6-Ha), 1.79–1.73 (m, 2H, 8-Ha, 9-Ha), 1.49–1.32 (m, 7H, 1’-H, 7-H, 9-Hb, 8-Hb, 5’-H, 2’-H), 1.16–1.01 (m, 8H, 3’-H, 4’-H, 6-Hb, 6a-CH3), 0.94 (d, 3H, J = 6.5 Hz, 1’-CH3), 0.84 (d, 3H, J = 6.6 Hz, 6’-H), 0.82 (d, 3H, J = 6.6 Hz, 5’-CH3). 13C-NMR (CDCl3): δ (ppm) = 161.44 (C-9b), 147.26 (C-2), 135.48 (C-4), 131.67 (C-4a), 120.49 (C-3), 54.90 (C-9a), 53.27 (C-7), 42.52 (C-6a), 39.56 (C-4’), 35.71 (C-2’), 35.35 (C-8), 33.83 (C-1’), 31.73 (C-6) 28.88 (C-9), 28.06 (C-5’), 25.97 (C-5), 24.44 (C-3’), 23.40 (6a-CH3), 22.89 (C-6’), 22.64 (5’-CH3) 19.72 (1’-CH3). CI-MS m/z (rel. int.): 300 (100, M++1), 159 (12). HR-MS (EI): calcd.: 299.2613, found: 299.2608.
(1R,3aR,7aR)-1-[(1R)-1,5-Dimethylhexyl]-7a-methyloctahydro-4H-inden-4-one O-allyloxime (5)
3.03 g (11.5 mmol) of Grundmann’s ketone (1), 2.15 g (19.6 mmol) of O-allylhydroxylamine hydrochloride, 1.34 g (22.6 mmol) of sodium acetate, and 2.40 g (22.6 mmol) of Na2CO3 were dispersed in 25 mL ethanol. This mixture was refluxed for 6 h, stirred for another 12 h at ambient temp., and then evaporated to dryness. The residue was taken up in 60 mL CH2Cl2 and washed with 3 × 50 mL hydrochloric acid (1.5%). The organic layer was dried over MgSO4 and the solvent was evaporated. The residue was purified by FCC (isohexane/ethyl acetate 47:3) to give 2.93 g (80%) of 5 as a colourless oil. 1H-NMR (CDCl3): δ (ppm) = 6.02–5.95 (m, 1H, 2”-H), 5.29–5.15 (m, 2H, 3”-H), 4.52 (m, 2H, 1”-H), 2.30–2.25 (m, 1H, 3a-H), 2.06–1.99 (m, 1H, 1-H), 1.88–1.82 (m, 1H, 3-H), 1.62–1.46 (m, 9 H, 6-H, 5-H, 3-H, 3’-H, 2’-H), 1.33–1.24 (m, 5H, 2-H, 4’-H, 1’-H), 1.12–1.09 (m, 3H, 7-H, 5’-H), 0.96 (s, 3H, 7a-CH3), 0.89–0.85 (m, 9 H, 1’-CH3, 5’-CH3, 6’-H). 13C-NMR (CDCl3): δ (ppm) = 160.63 (C-4), 134.72 (C-2”), 116.91 (C-3”), 74.19 (C-1”), 56.08 (C-3a), 52.68 (C-1), 46.59 (C-7a), 39.54 (C-5), 37.64 (C-6), 36.17 (C-7), 35.16 (C-3), 34.04 (C-1’), 28.03 (C-5’), 24.85 (C-2), 23.90 (C-2’), 23.13 (7a-CH3), 23.08 (5’-CH3), 22.65 (C-6’), 22.08 (C-3’), 19.65 (C-4’), 18.90 (1’-CH3). CI-MS m/z (rel. int.): 320 (100, M++1), 262 (50). HR-MS (EI): calcd.: 319.2875, found: 319.2867.
(6aR,7R,9aR)-7-[(1R)-1,5-Dimethylhexyl]-1,6a-dimethyl-6,6a,7,8,9,9a-hexahydro-5H-cyclopenta[h]quinolinium iodide (6)
126 mg (0.42 mmol) of pyridine 5 were dissolved in a minimal quantity of acetone and treated with 0.65 ml (1.04 mmol) of methyl iodide (caution! highly toxic agent!). The mixture was stirred in a well-ventilated hood for 16 h at ambient temp., then the precipitate was collected by filtration and recrystallized from ethanol to give 99 mg (53%) of 6 as white crystals. M.p. 218 °C. 1H-NMR (CDCl3): δ (ppm) = 9.43 (d, 1H, J = 5.9 Hz, 2-H), 8.17 (d, 1H, J = 7.8 Hz, 4-H), 7.83 (t, 1H, J = 7.0 Hz, 3-H), 4.56 (s, 3H, 1-CH3), 3.21 (t, 1H, J = 9.4 Hz, 9a-H), 2.89 (m, 2H, 5-H), 2.48–2.38 (m, 1H, 6-Ha), 2.08 (m, 1H, 8-Ha), 2.00–1.90 (m, 1H, 9-Ha), 1.67–1.35 (m, 7H, 1’-H, 7-H, 9-Hb, 8-Hb, 5’-H, 2’-H), 1.23–1.10 (m, 8H, 3’-H, 4’-H, 6-Hb, 6a-CH3), 0.99 (d, 3H, J = 6.0 Hz, 1’-CH3), 0.86 (d, 6H, J = 6.5 Hz, 5’-CH3, 6’-H). 13C-NMR (CDCl3): δ (ppm) = 157.70 (C-9b), 144.96 (C-2), 143.61 (C-4), 138.13 (C-4a), 123.65 (C-3), 51.10 (C-7), 48.58 (C-9a), 45.76 (1-CH3), 43.42 (C-6a), 38.35 (C-4’), 34.39 (C-2’), 33.34 (C-8), 31.91 (C-1’), 30.83 (C-6), 27.00 (C-5’), 26.87 (C-9), 25.07 (C-5), 24.16 (6a-CH3), 23.32 (C-3’), 21.77 (C-6’), 21.53 (5’-CH3), 18.81 (1’-CH3). CI-MS m/z (rel. int.): 314 (M+, cation, 54), 300 (100). HR-MS (EI): calcd.: 313.2769, found: 313.2726.
Agar diffusion assay
The assay was performed as described in detail previously [21] using the microorganisms listed in Table 1. Paper discs (6 mm diameter) were impregnated with 30 μg of each test substance or the reference drugs. The diameters of the zones of inhibition were measured manually. The experiments were performed in triplicate. The antibiotic tetracycline-HCl and the antifungal clotrimazole were used as reference drugs.
MTT assay
The assay originally worked out by Mosmann [17] was performed as described in detail previously [21] using HL-60 cells. The experiments were performed in triplicate. Cisplatin was used as the reference drug.
Screening for inhibition of cholesterol biosynthesis
The qualitative assay was performed as described in detail previously [19] using HL-60 cells.
Screening for inhibition of ergosterol biosynthesis
The qualitative assay was performed as described in detail recently [20] using the yeast Yarrowia lipolytica as the test strain.
Sch. 1.
Two approaches to the target compounds 4 and 6.
Acknowledgments
We thank Christoph Müller and Martina Stadler for performing the screenings.
Footnotes
This article is available from: http://dx.doi.org/10.3797/scipharm.1303-03
Authors’ Statement
Competing Interests
The authors declare no conflict of interest.
References
- [1].Mayer CD, Bracher F. Cytotoxic ring A-modified steroid analogues derived from Grundmann’s ketone. Eur J Med Chem. 2011;46:3227–3236. doi: 10.1016/j.ejmech.2011.04.036. http://dx.doi.org/10.1016/j.ejmech.2011.04.036. [DOI] [PubMed] [Google Scholar]
- [2].Singh H, Paul JD, Yadav MR, Kumar M. Heterosteroids and drug research. Prog Med Chem. 1991;28:233–300. doi: 10.1016/s0079-6468(08)70366-7. http://dx.doi.org/10.1016/S0079-6468(0870366-7) [DOI] [PubMed] [Google Scholar]
- [3].Barthakur MG, Gogoi S, Dutta M, Boruah RC. A facile three-component solid-phase synthesis of steroidal A-ring fused pyrimidines under microwave irradiation. Steroids. 2009;74:730–734. doi: 10.1016/j.steroids.2009.03.006. http://dx.doi.org/10.1016/j.steroids.2009.03.006. [DOI] [PubMed] [Google Scholar]
- [4].Burbiel J, Bracher F. Azasteroids as antifungals. Steroids. 200368:587–594. doi: 10.1016/s0039-128x(03)00080-1. http://dx.doi.org/10.1016/S0039-128X(03)00080-1. [DOI] [PubMed] [Google Scholar]
- [5].Shah S, Ryan CJ. Abiraterone acetate. Drugs Fut. 2009;34:873–880. http://dx.doi.org/10.1358/dof.2009.34.11.1441113. [Google Scholar]
- [6].Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M. An overview on 5α-reductase inhibitors. Steriods. 2010;75:109–153. doi: 10.1016/j.steroids.2009.10.005. http://dx.doi.org/10.1016/j.steroids.2009.10.005. [DOI] [PubMed] [Google Scholar]
- [7].Giera M, Renard D, Plössl F, Bracher F. Lathosterol side chain amides - A new class of human lathosterol oxidase inhibitors. Steroids. 200873:299–308. doi: 10.1016/j.steroids.2007.10.015. http://dx.doi.org/10.1016/j.steroids.2007.10.015. [DOI] [PubMed] [Google Scholar]
- [8].Roizen MF, Feeley TW. Pancuronium bromide. Ann Intern Med. 1978;88:64–68. doi: 10.7326/0003-4819-88-1-64. http://dx.doi.org/10.7326/0003-4819-88-1-64. [DOI] [PubMed] [Google Scholar]
- [9].König M, Müller C, Bracher F. Stereoselective synthesis of a new class of potent and selective inhibitors of human Δ8,7-sterol isomerase. Bioorg Med Chem. 2013;21:1925–1943. doi: 10.1016/j.bmc.2013.01.041. http://dx.doi.org/10.1016/j.bmc.2013.01.041. [DOI] [PubMed] [Google Scholar]
- [10].Lange S, Keller M, Müller C, Oliaro-Bosso S, Balliano G, Bracher F. Aminopropylindenes derived from Grundmann’s ketone as a novel chemotype of oxidosqualene cyclase inhibitors. Eur J Med Chem. 2013;63:758–764. doi: 10.1016/j.ejmech.2013.03.002. http://dx.doi.org/10.1016/j.ejmech.2013.03.002. [DOI] [PubMed] [Google Scholar]
- [11].Mayer CD, Allmendinger L, Bracher F. Synthesis of novel steroid analogues containing nitrile and disulfide moieties via palladium-catalyzed cross-coupling reactions. Tetrahedron. 2012;68:1810–1818. http://dx.doi.org/10.1016/j.tet.2011.11.076. [Google Scholar]
- [12].Zanati G, Wolff ME. Synthesis of an androgenic-anabolic nonsteroid. J Med Chem. 1973;16:90–91. doi: 10.1021/jm00259a028. http://dx.doi.org/10.1021/jm00259a028. [DOI] [PubMed] [Google Scholar]
- [13].Burden RS, Cooke DT, Carter GA. Inhibitors of sterol biosynthesis and growth in plants and fungi. Phytochemistry. 1989;28:1791–1804. http://dx.doi.org/10.1016/S0031-9422(00)97862-2. [Google Scholar]
- [14].Windaus A, Grundmann W. Über die Konstitution des Vitamins D2. II. Liebigs Ann Chem. 1936;524:295–299. http://dx.doi.org/10.1002/jlac.19365240116. [Google Scholar]
- [15].Chelucci G, Gladiali S, Marchetti M. A three-step pyridoannelation of carbonyl compounds. J Heterocycl Chem. 1988;25:1761–1765. http://dx.doi.org/10.1002/jhet.5570250630. [Google Scholar]
- [16].Koyama J, Sugita T, Suzuta Y, Irie H. Thermolysis of oxime O-allyl ethers: A new method for pyridine synthesis. Chem Pharm Bull. 1983;31:2601–2606. http://dx.doi.org/10.1248/cpb.31.2601. [Google Scholar]
- [17].Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. http://dx.doi.org/10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- [18].Pitten FA, Kramer A. Efficacy of cetylpyridinium chloride used as oropharyngeal antiseptic. Arzneimittelforschung. 2001;51:588–595. doi: 10.1055/s-0031-1300084. http://dx.doi.org/10.1055/s-0031-1300084. [DOI] [PubMed] [Google Scholar]
- [19].Giera M, Plössl F, Bracher F. Fast and easy in vitro screening assay for cholesterol biosynthesis inhibitors in the post squalene pathway. Steroids. 200772:633–642. doi: 10.1016/j.steroids.2007.04.005. http://dx.doi.org/10.1016/j.steroids.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [20].Müller C, Staudacher V, Krauß J, Giera M, Bracher F. A convenient cellular assay for the identification of the molecular target of ergosterol biosynthesis inhibitors and quantification of their effects on total ergosterol biosynthesis. Steroids. 2013;78:483–493. doi: 10.1016/j.steroids.2013.02.006. http://dx.doi.org/10.1016/j.steroids.2013.02.006. [DOI] [PubMed] [Google Scholar]
- [21].Wollein U, Bracher F. The gramine route to pyrido[4,3-b]indol-3-ones – identification of a new cytotoxic lead. Sci Pharm. 2011;79:21–30. doi: 10.3797/scipharm.1011-11. http://dx.doi.org/10.3797/scipharm.1011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]