Abstract
In the present investigation, some new pyrazolo[3,4-d]pyrimidin-4(5H)-one derivatives (7–12) as well as fused pyrazolo[3′,4′:4,5]pyrimido[1,2-b]pyridazin-4(1H)-one (14–16) and 7,8,9,10-tetrahydropyrazolo[3′,4′:4,5]pyrimido[1,2-b]-cinnolin-4(1H)-one (17) ring systems were synthesized using the starting compound 5-amino-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (5). The structures of the newly synthesized compounds were elucidated by IR, 1H NMR, 13C NMR, mass spectroscopy, and elemental analysis. The theoretical calculation of their lipophilicity as C log P was performed. The anti-inflammatory activity of all newly synthesized compounds was evaluated using the carrageenan-induced paw edema test in rats using indomethacin as the reference drug. Ulcer indices for the most active compounds were calculated. Seven compounds (10b, 11a–f) showed consistently good anti-inflammatory activity. In particular, 5-{[4-(4-bromophenyl)-3-(4-chlorophenyl)-1,3-thiazol-2(3H)-ylidene]amino}-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (11e) and its 3,4-bis(4-chlorophenyl) analog (11f) were found to be the most effective among the other derivatives, showing activity comparable to that of indomethacin with minimal ulcerogenic effects. Correlation of the biological data of the active compounds with their theoretically calculated C log P values revealed that lipophilicity influences the biological response.
Keywords: Pyrazolo[3,4-d]pyrimidin-4-ones; Pyrazolo[3′,4′:4,5]pyrimido[1,2-b]pyridazin-4-ones; 7,8,9,10-Tetrahydropyrazolo[3′,4′:4,5]pyrimido[1,2-b]cinnolin-4-one; C log P; Synthesis; Anti-inflammatory activity; Ulcerogenicity
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most widely used therapeutics, primarily for the treatment of pain and inflammation in arthritis for decades. NSAIDs reduce the pain and swelling associated with arthritis by blocking the metabolism of arachidonic acid by cyclooxygenase enzyme (COX) thereby the production of prostanoids (including prostaglandins, prostacyclins, and thromboxanes) [1]. Since prostanoids subserve housekeeping functions, such as gastric epithelial cytoprotection and homeostasis besides their known proinflammatory role [2], administration of NSAIDs in the long-term may lead to the development of threatening GI ulcers, bleeding, and renal disorders [3, 4]. Therefore, the discovery of new and safer anti-inflammatory drugs represents a challenging goal for such a research area [5].
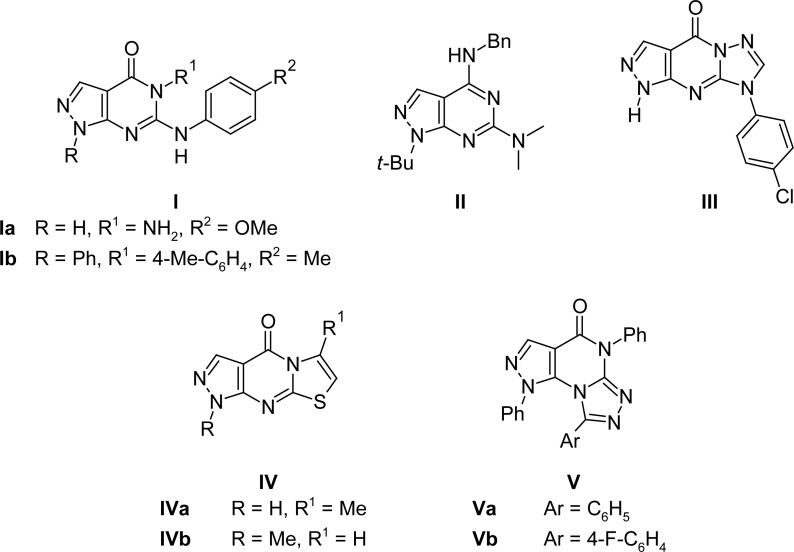
In the course of a research study devoted to the development of new classes of anti-inflammatory drugs, several pyrazolopyrimidine derivatives have been synthesized and have shown potential anti-inflammatory activity associated with remarkable systemic and gastric tolerance. These are exemplified by the variously substituted bicyclic pyrazolo[3,4-d]pyrimidine Ia,b[6, 7] and II[8] derivatives and the tricyclic pyrazolo[3,4-d]-[1,2,4]triazolo[1,5-a]pyrimidin-4-one III[6], pyrazolo[3,4-d][1,3]thiazolo[3,2-a]pyrimidin-4-one IVa,b[9], and pyrazolo[3,4-e][1,2,4]triazolo[4,3-a]pyrimidin-5-one Va,b[10, 11] derivatives (Fig. 1). Among these derivatives, compound II[8] inhibited potently cyclooxygenase-2 activity in intact cell assays (IC50 = 0.9 nM) with minor activity against cyclooxygenase-1 (IC50 = 59.6 nM).
Fig. 1.
Some selected models of pyrazolopyrimidine derivatives possessing anti-inflammatory activity.
Additionally, a large number of Schiff’s bases [12], thioureas [13], thiazolidinones [14, 15], thiazolines [16], and dioxopyrimidines [17] were reported to possess anti-inflammatory activity.
Since the combination of two pharmacophores on the same scaffold is a well-established approach to the synthesis of more potent drugs [18, 19], it is intended in the present work to incorporate the flexible straight chain azomethine and thiouredo moieties (compounds 7 and 9) as well as the rigid abovementioned heterocyclic nuclei either directly attached (compounds 8 and 12) or through imino linkage (compounds 10 and 11) at the 5-position of the pyrazolo[3,4-d]pyrimidine nucleus, hoping that these new hybrids would produce enhanced anti-inflammatory activity.
Furthermore, some heterocyclic compounds containing pyridazine [20, 21] and cinnoline [22] moieties have been recently reported as anti-inflammatory agents. This led to the synthesis of some new heterocyclic compounds containing pyridazine (compounds 14–16) and 5,6,7,8-tetrahydrocinnoline (compound 17) rings fused with a pyrazolo[3,4-d]-pyrimidine nucleus in an effort to synthesize new tricyclic and tetracyclic derivatives to investigate the effect of such a molecular variation on the anti-inflammatory activity.
The enhanced overall lipophilic characteristics of the target compounds could favor their selectivity towards the COX-2 enzyme over COX-1 leading to an increase in the GIT safety margin [23]. Therefore, the substitution pattern of the pharmacophoric groups was selected so as to impart various electronic and lipophilic properties to the molecules, which may contribute to the enhancement of the anti-inflammatory activity of the pyrazolopyrimidine nucleus. In addition to the targeted anti-inflammatory activity, it was also considered of interest to examine the ulcerogenic profiles of the newly synthesized compounds.
Results and Discussion
Chemistry
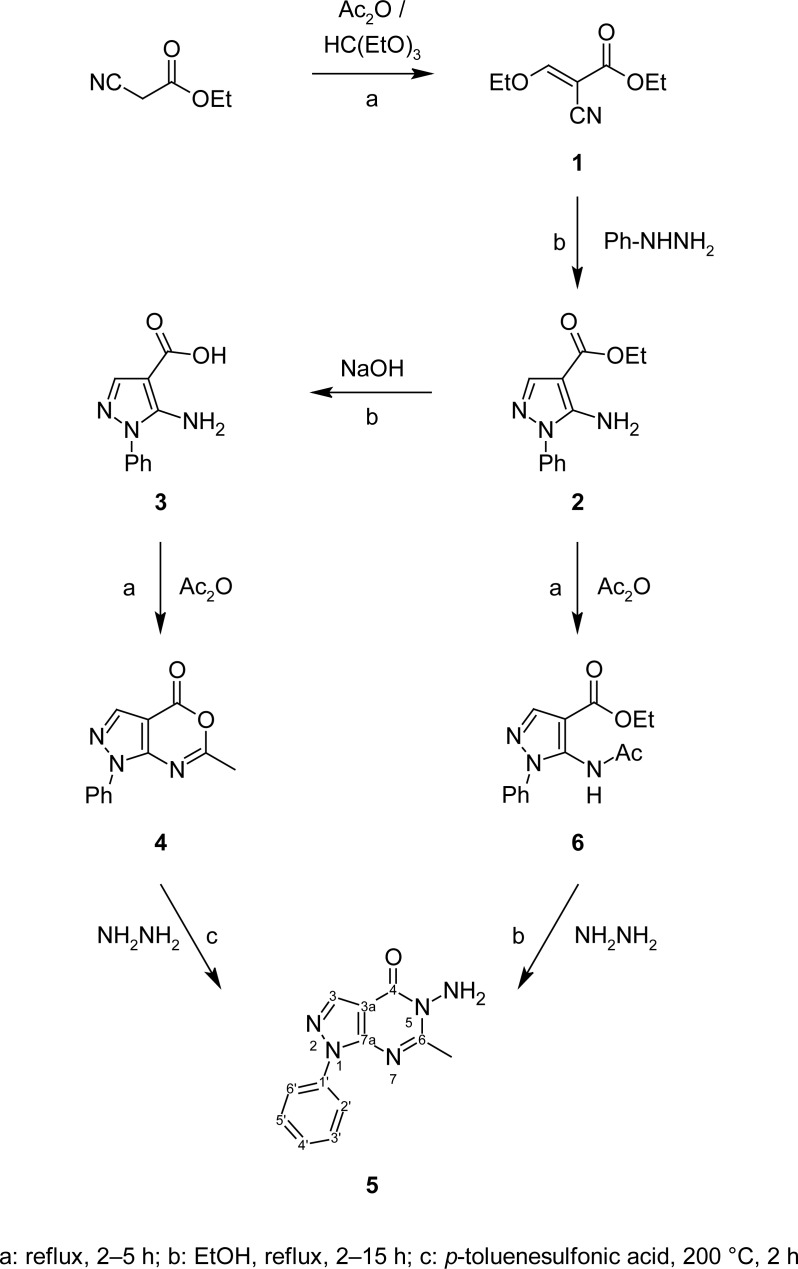
The synthetic pathways adopted for the preparation of the intermediate and target compounds are illustrated in Schemes 1, 2, and 3. The aminoester 2 was prepared following the previously reported procedure [24, 25], by reacting ethyl (ethoxymethylene)cyanoacetate (1) [26] with phenylhydrazine (Scheme 1). Hydrolysis of the aminoester 2 with alcoholic sodium hydroxide followed by neutralization afforded the corresponding carboxylic acid 3 which was then refluxed with acetic anhydride to give 6-methyl-1-phenyl-2,3-dihydropyrazolo[3,4-d][1,3]oxazin-4(1H)-one (4). Condensation of the pyrazolooxazine 4 with hydrazine hydrate afforded the key intermediate 5-amino-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (5) [27] (Method A). In an alternative route (Method B), compound 5 was prepared by acetylating the aminoester 2 with acetic anhydride followed by reacting the N-acetylated compound 6[27] with hydrazine hydrate, following the reaction conditions adopted for the synthesis of related compounds [28]. The second route was more advantageous than the first being relatively simple and giving higher reproducible yields of compound 5 (Scheme 1). The IR spectrum of compound 5 showed the NH absorption bands at 3319, 3210, 3112 cm−1 together with the amide C=O absorption band at 1695 cm−1. Its 1H NMR showed the methyl protons and CH-3 as two singlets at δ 2.58 and 8.29 ppm, respectively, beside a D2O exchangeable singlet at δ 5.73 ppm integrated for two protons assigned for the NH2 group in addition to other aromatic proton signals at their expected chemical shifts. Its 13C NMR spectrum showed a highly shielded signal due to the methyl carbon at the 6-position of the pyrazolopyrimidine nucleus at δ 23.21 ppm, and another signal due to C-3a at δ 96.64 ppm. In addition, the spectrum of 5 showed three signals due to the five methine carbons in the aromatic region at δ 122.75, 127.57, and 129.82 ppm indicating the equivalency between the two ortho and the two meta carbons of the phenyl moiety and a signal at δ 136.27 ppm for the phenyl C-1’ quaternary carbon. The spectrum also showed four signals at δ 138.50, 138.72, 159.70, and 164.96 ppm due to C-3, C-7a, C-4, and C-6, respectively.
Sch. 1.
Synthetic routes for the preparation of compound 5.
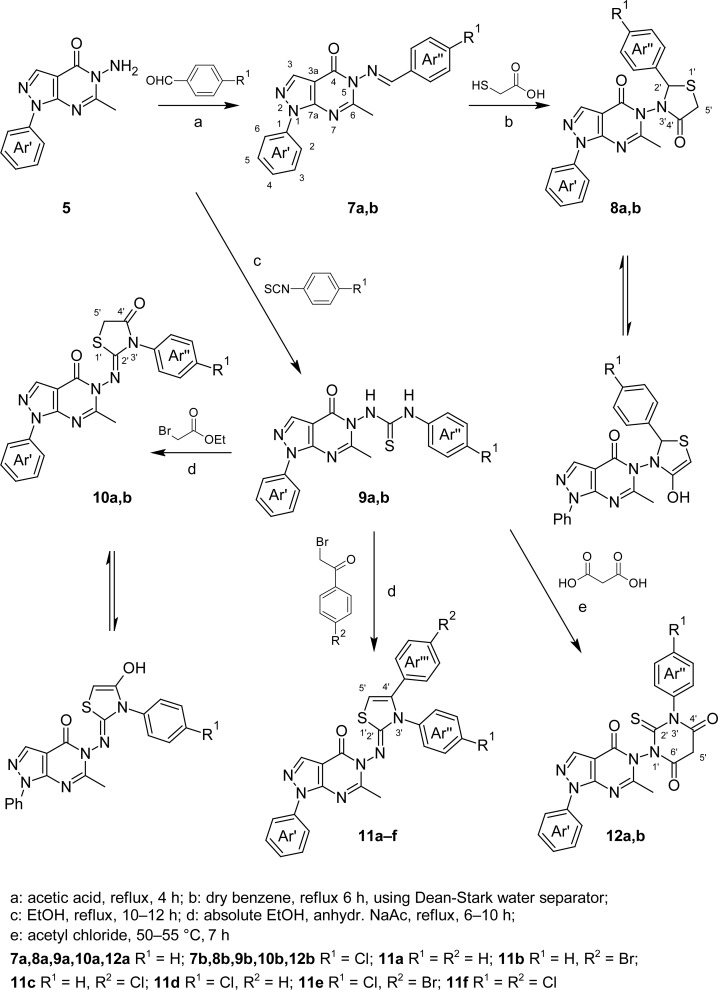
Sch. 2.
Synthetic routes for the preparation of compounds 7–12.
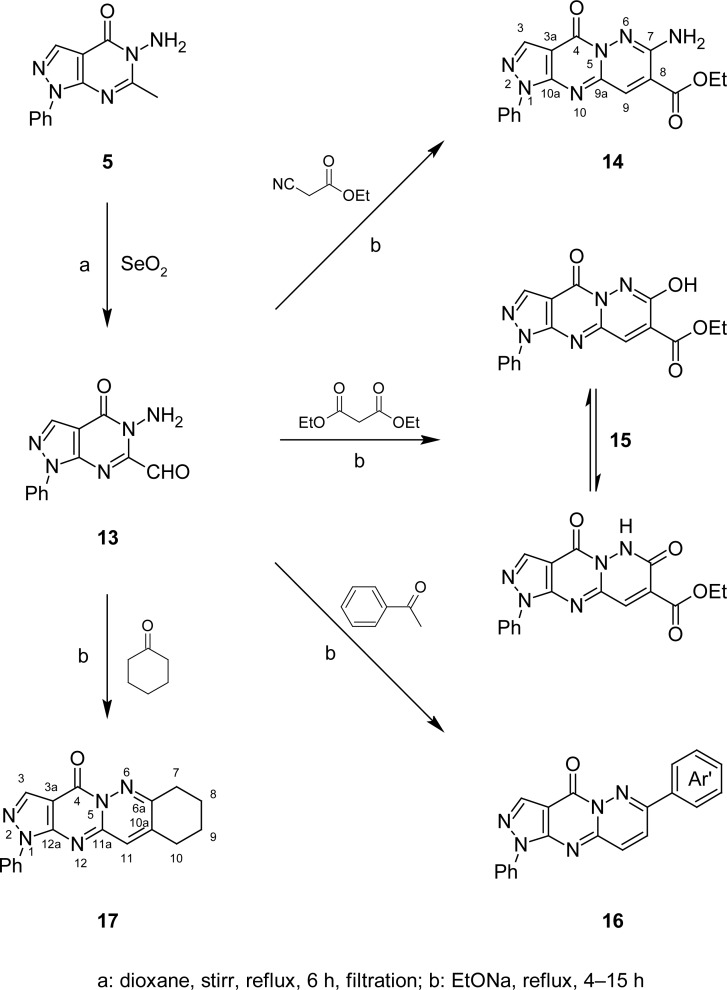
Sch. 3.
Synthetic routes for the preparation of compounds 13–17.
Condensation of 5 with benzaldehyde or p-chlorobenzaldehyde in glacial acetic acid afforded the corresponding 5-(arylideneamino)-6-methyl-1-phenyl-1,5-diyhdro-4H-pyrazolo-[3,4-d]pyrimidin-4-one derivatives 7a,b (Scheme 2). The IR spectra of compounds 7a,b showed disappearance of the absorption bands for the NH2 group present in the precursor 5. Their 1H NMR spectra revealed singlet signals resonating at δ 8.35 and 8.88 ppm, respectively, due to the N=CH proton.
Cyclocondensation of Schiff’s bases 7a,b with thioglycolic acid in refluxing dry benzene, as reported for the preparation of related compounds [29], afforded the corresponding 5-(2-aryl-4-oxo-1,3-thiazolidin-3-yl) derivatives 8a,b. The IR spectrum of 8b, as a representative example, showed a new band at 1718 cm−1 attributed to thiazolidinone C=O group. Its 1H NMR spectrum showed two doublets resonating at δ 3.56 and 3.65 ppm due to the non-equivalent, geminal methylene protons (S-CH2) of the 4-thiazolidinone ring interacting with the chiral center at position 2′. The spectra also showed a singlet resonating at δ 5.56 ppm assigned for the methine proton at the 2′-position. Furthermore, the spectrum of 8b showed a singlet at δ 5.75 ppm integrated for one proton assigned for the enol form of thiazolidinone CH-5′ in addition to a D2O exchangeable singlet at δ 9.68 ppm integrated for one proton assigned for the enolic OH proton pointing out that compound 8b exhibited a typical keto-enol tautomerism. The 13C NMR spectrum of compound 8b showed the signals corresponding to the methylene and the methine carbons C-5′ and C-2′ at δ 62.82 and 63.68 ppm, respectively, and the signal corresponding to the quaternary carbonyl carbon C-4′ at δ 170.37 ppm. The spectrum also showed two signals at δ 90.58 and 179.40 ppm due to enolic C-5′ and C-4′, respectively, confirming the keto-enol tautomerism in the thiazolidinone derivative 8b. In addition, the spectrum showed signals attributed to the carbons of the 4-chlorophenyl and phenyl substituents at their expected chemical shifts.
Treatment of 5 with phenyl or p-chlorophenyl isothiocyanate in refluxing ethanol gave 5-(arylthiocarbamoyl)amino derivatives 9a,b. The IR spectrum of compounds 9a, as a representative example, was characterized by the appearance of absorption bands at 1549, 1257, 1220, and 1045 cm−1 attributed to N–C=S function [30]. Its 1H NMR spectrum showed two deuterium exchangeable singlets due to protons of the aryl-NH and pyrazolopyrimidine-NH at δ 9.55 and 9.93 ppm. The 13C NMR spectrum of 9a showed, in addition to the signals of the 6-methylpyrazolopyrimidinone and the two phenyl group carbons at their expected chemical shifts, a highly deshielded signal at δ 181.98 ppm due to C=S.
Cyclocondensation of the thiourea derivatives 9a,b with ethyl bromoacetate, or the appropriate phenacyl bromide in refluxing absolute ethanol, in the presence of anhydrous sodium acetate gave the corresponding 5-[(3-aryl-4-oxo-1,3-thiazolidin-2-ylidene)amino] derivatives 10a,b or 5-[(3,4-diarylthiazol-2(3H)-ylidene)amino] derivatives 11a–f, respectively. The IR spectra of 10a,b and 11a–f lacked the mixed vibrational bands due to N–C=S function present in their precursors 9a,b. In addition, a new band at 1741 or 1714 cm−1 appeared in the IR spectra of 10a,b, respectively, attributed to the thiazolidinone C=O group. The 1H NMR spectra of the products 10b and 11a, as representative examples, lacked the NH protons of the parent compounds 9a,b and showed the singlet at δ 4.03 ppm integrated for two protons due to the thiazolidinone CH-5′ in compound 10b and the singlet at δ 6.21 ppm integrated for one proton due to thiazoline CH-5′ in compounds 11a. In addition, the 1H NMR spectra of 10b showed a singlet at δ 6.22 ppm integrated for one proton assigned for the enol form of thiazolidinone CH-5′ in addition to a D2O exchangeable singlet at δ 10.68 ppm integrated for one proton assigned for the enolic OH proton. This indicated that compound 10b would exhibit a typical keto-enol tautomerism. The 13C NMR spectrum of 10b revealed a shielded signal due to the methylene carbon C-5′ at δ 62.35 ppm and two deshielded signals at δ 149.08 and 168.25 ppm due to the quaternary carbons C-2′ and C-4′, respectively. In addition, the 13C NMR spectrum of 10b concluded the presence of keto-enol tautomerism as revealed from the two signals due to the enolic form of thiazolidinone C-5′ and C-4′ resonating at δ 85.88 and 181.17 ppm, respectively. The 13C NMR spectrum of the thiazoline 11a revealed a signal due to the methine carbon of the thiazole C-5′ at δ 88.34 ppm and signals characteristic for the two deshielded quaternary carbons C-4′ and C-2′ at δ 146.55 and 157.04 ppm, respectively. The 13C NMR spectra of 10b and 11a also showed signals corresponding to other carbons at their expected chemical shifts. Mass spectrum of 11c revealed its M+●+2 and M+● peaks at m/z 512 and 510, respectively, which confirmed its molecular weight. However, molecular ion peaks were not detected in the mass spectra of compounds 11a,b and 11d–f.
In addition, the thiourea derivatives 9a,b were cyclized to 1-Aryl-3-(6-methyl- 4-oxo-1-phenyl-1,4-dihydro-5H-pyrazolo[3,4-d]pyrimidin-5-yl)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione derivatives 12a,b by reaction with malonic acid in the presence of acetyl chloride following the previously reported reaction conditions [31]. The IR spectrum of 12a, as a representative example, showed two new absorption bands at 1707 and 1658 cm−1 attributed to the dioxopyrimidine C=O groups, besides the absorption band for the pyrazolopyrimidine C=O group, together with mixed vibrational bands due to N–C=S function present in their precursors 9a,b. Its 1H NMR spectrum showed the disappearance of the NH absorption bands of the precursors 9a,b and the presence of a singlet at 3.75 ppm integrated for two protons assigned for the 4,6-dioxopyrimidine CH-5′. No signal for a vinylic proton was observed in the downfield region, suggesting that compound 12a exists predominantly in the keto tautomeric form. The 13C NMR spectrum of compound 12a showed a highly shielded signal corresponding to the methylene carbon C-5′ at δ 52.49 ppm beside three signals at 151.78, 152.97, and 157.43 ppm attributed to the quaternary carbons C-4′, C-6′, and C-2′, respectively, in addition to signals due to other carbons resonated at their expected chemical shifts.
On the other hand, oxidation of the 6-methyl group in 5 with selenium dioxide in dioxane following the reported procedure [32] afforded the corresponding 5-amino-4-oxo-1-phenyl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidine-6-carbaldehyde (13) (Scheme 3). Its IR spectrum showed characteristic absorption bands at 3441, 3322, and 3188 cm−1 corresponding to NH2 together with absorption bands at 1720 and 1696 cm−1 due to CHO and pyrazolopyrimidine C=O groups, respectively. The 1H NMR spectrum of 13 displayed a D2O-exchangeable singlet at δ 4.24 ppm integrated for two protons assigned for NH2 in addition to the singlet at δ 9.53 ppm characteristic for the CH of the aldehydic group. Its 13C-NMR spectrum showed, in addition to the signals corresponding to 4-oxo-1-phenyl-1H-pyrazolo[3,4-d]pyrimidine carbons at their expected chemical shifts, a highly deshielded signal at δ 172.92 ppm corresponding to the aldehydic group carbon. Its mass spectrum showed a molecular ion peak at m/z 255 which confirmed its molecular weight. Compound 13 was used as a precursor for the synthesis of the tricyclic (14–16) and tetracyclic (17) systems following the reported reaction conditions [33]. Thus, ethyl 7-amino-4-oxo-1-phenyl-1,4-dihydropyrazolo[3′,4′:4,5]pyrimido[1,2-b]pyridazine-8-carboxylate (14) was prepared by condensing the aminoaldehyde 13 with ethyl cyanoacetate in the presence of sodium ethoxide as a catalyst. The course of the reaction is assumed to involve an intramolecular attack of the amino group on the nitrile function to furnish the target compound 14.
Likewise, ethyl 7-hydroxy-4-oxo-1-phenyl-1,4-dihydropyrazolo[3′,4′:4,5]pyrimido[1,2-b]-pyridazine-8-carboxylate (15) was obtained in good yield from 13 and diethyl malonate. The IR spectra of compounds 14 and 15 showed the ester C=O absorption band at 1727 and 1711 cm−1, respectively. Compound 14 also showed the NH2 absorption bands at 3437, 3287, and 3190 cm−1 and the amide C=O at 1678 cm−1, whereas compound 15 showed the NH and OH associated absorption bands at 3187–2936 cm−1 and the two amide C=O absorption bands at 1649 and 1622 cm−1. The 1H NMR spectra of compounds 14 and 15 showed three signals corresponding to the protons of the ethyl ester moiety and CH-9 at δ 1.31, 4.15, and 7.68 ppm in the case of compound 14, and at 1.40, 4.07, and 7.65 ppm in the case of compound 15, in addition to a D2O exchangeable singlet integrated either for two protons at δ 5.06 ppm in the case of compound 14 assigned for NH2, or a half proton at δ 9.60 ppm in the case of compounds 15 assigned for amido-NH.
In addition, the 1H NMR spectrum of 15 showed a D2O exchangeable singlet at δ 12.33 ppm integrated for a half proton attributed to an imidol-OH. This indicated that compound 15 would exhibit a typical amido-imidol tautomerism. The 13C NMR spectrum of 14 showed two high field signals for the ethyl group at δ 15.62 and 62.50 ppm, two signals at δ 129.99 and 143.87 ppm due to the methine carbons C-9 and C-3, respectively, and six signals resonating at δ 87.27, 137.97, 144.16, 145.15, 145.54, and 147.44 ppm corresponding to six quaternary carbons C-3a, C-8, C-10a, C-7, C-4, and C-9a, respectively. The most deshielded signal at δ 148.53 ppm was characterized for the quaternary O-C=O carbon. The spectrum also showed signals corresponding to the phenyl carbons at their expected chemical shifts.
Furthermore, treatment of 13 with acetophenone or cyclohexanone in the presence of sodium ethoxide afforded 1,7-diphenylpyrazolo[3′,4′:4,5]pyrimido[1,2-b]pyridazin-4(1H)-one (16) or 1-phenyl-7,8,9,10-tetrahydropyrazolo[3′,4′:4,5]pyrimido[1,2-b]cinnolin-4(1H)-one (17), respectively, in moderate yield. The 1H NMR spectra of compounds 16 and 17 lacked the NH2 signal of the precursor 13. The 1H NMR spectrum of compound 16 showed the CH-8 and CH-9 as two doublets, each integrated for one proton at δ 7.76 and 8.03 ppm, respectively. The 1H NMR spectrum of compound 17 showed the CH-8,9 and CH-7,10 as two multiplets, each integrated for two protons at δ 2.09–2.21 and 2.42–2.67 ppm, respectively, in addition to the CH-11 as a distorted singlet at δ 7.67 ppm. The spectra also showed other signals at their expected chemical shifts. The 13C NMR spectrum of 16 showed three signals at 129.68, 137.56, and 138.17 ppm due to the methine carbons C-9, C-8, and C-3, respectively, and five signals at 90.59, 138.47, 146.96, 158.47, and 164.23 attributed to five quaternary carbons C-3a, C-10a, C-7, C-4, and C-9a, respectively. In addition, the spectra showed signals attributed to carbons of the two phenyl substituents at their expected chemical shifts. Mass spectrum of 14 showed the correct molecular ion peak (M+●) which confirmed its molecular weight. MS of 15–17 did not show their molecular ion peaks.
Physicochemical studies
Since lipophilicity is a significant physicochemical property that determines distribution, bioavailability, metabolic activity, and elimination, the corresponding theoretical C log P values in n-octanol buffer were calculated [34].
Biological evaluation
Anti-inflammatory activity
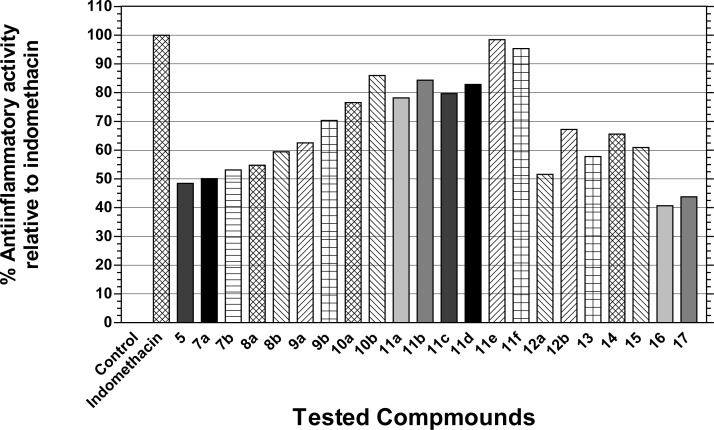
The in vivo anti-inflammatory effects of the newly synthesized pyrazolopyrimidine derivatives were assessed by utilizing the functional model of carrageenan-induced rat paw edema [35] using indomethacin as the reference drug. Mean changes and the percent increase in paw edema weight of animals pretreated with the tested compounds after 4 h from the induction of inflammation were measured, together with the percent inhibition of induced rat paw edema by the tested compounds. The anti-inflammatory activity (relative potency) of the tested compounds relative to that of indomethacin was also calculated (Table 1) (Fig. 2).
Tab. 1.
Results of anti-inflammatory activity of the tested compounds against carrageenan-induced rat paw edema in rats; Calculated lipophilicity C log P values.
| Comp. No. | Mean increase in edema weight ± SEMa,b | Mean % increase in edema weight ± SEM | %Inhibition of Paw edema from control group | % Activity relative to indo-methacine | C log P |
|---|---|---|---|---|---|
| Control | 1.57 ± 0.033 | 20.84 ± 0.237 | – | – | – |
| Indomethacin | 0.50 ± 0.026* | 6.37 ± 0.366* | 68.15 | 100.0 | – |
| 5 | 1.05 ± 0.089* | 19.82 ± 1.600 | 33.12 | 48.60 | 1.36 |
| 7a | 1.03 ± 0.033* | 15.32 ± 0.515* | 34.39 | 50.46 | 2.15 |
| 7b | 1.00 ± 0.063* | 15.19 ± 1.400* | 36.31 | 53.28 | 2.87 |
| 8a | 0.98 ± 0.017* | 12.48 ± 0.376* | 37.58 | 55.14 | 3.14 |
| 8b | 0.93 ± 0.042* | 12.98 ± 0.702* | 40.76 | 59.81 | 3.85 |
| 9a | 0.90 ± 0.058* | 12.71 ± 0.748* | 42.68 | 62.63 | 2.09 |
| 9b | 0.82 ± 0.048* | 15.71 ± 1.035* | 47.77 | 70.10 | 2.80 |
| 10a | 0.75 ± 0.022* | 9.50 ± 0.326* | 52.23 | 76.64 | 2.19 |
| 10b | 0.65 ± 0.022* | 8.31 ± 0.430* | 58.60 | 85.99 | 2.91 |
| 11a | 0.73 ± 0.021* | 10.54 ± 0.468* | 53.50 | 78.50 | 6.65 |
| 11b | 0.67 ± 0.049* | 8.42 ± 0.482* | 57.32 | 84.11 | 7.52 |
| 11c | 0.72 ± 0.031* | 10.76 ± 0.457* | 54.14 | 79.44 | 7.37 |
| 11d | 0.68 ± 0.065* | 8.73 ± 0.868* | 56.69 | 83.18 | 7.37 |
| 11e | 0.52 ± 0.031* | 8.74 ± 0.929* | 66.88 | 98.14 | 8.23 |
| 11f | 0.55 ± 0.043* | 9.29 ± 0.961* | 64.97 | 95.33 | 8.08 |
| 12a | 1.02 ± 0.060* | 13.56 ± 0.795* | 35.03 | 51.40 | 1.77 |
| 12b | 0.85 ± 0.043* | 19.33 ± 0.905 | 45.86 | 67.29 | 2.48 |
| 13 | 0.95 ± 0.043* | 15.10 ± 1.066* | 39.49 | 57.95 | 1.40 |
| 14 | 0.87 ± 0.076* | 11.12 ± 1.138* | 44.59 | 65.43 | 1.68 |
| 15 | 0.92 ± 0.060* | 16.35 ± 1.016* | 41.40 | 60.75 | 1.93 |
| 16 | 1.13 ± 0.067* | 14.33 ± 0.980* | 28.03 | 41.13 | 2.78 |
| 17 | 1.10 ± 0.052* | 15.77 ± 0.756 * | 29.94 | 43.93 | 2.26 |
SEM denoted the standard error of the mean.
Number of animals N = 6 rats.
* Significant difference from control group using Dunnett’s test; p< 0.001.
Fig. 2.
The Anti-inflammatory Effect of the Tested compounds.
Carrageenan-induced inflammation is a non-specific inflammation resulting from a complex of diverse mediators [36]. It is believed to be biphasic [35], the early phase (1–2 h) involves the release of histamine, serotonin, and bradykinin, and the late phase (3–4 h) is due to the release of prostaglandin-like substances [37–39]. Accordingly, a decrease in the second phase may be attributed to inhibition of cyclooxygenase [39]. This explains the weak inhibitory effect of NSAIDs, such as indomethacin, in the early phase, in contrast to their strong inhibition in the late phase [40]. Therefore, the carrageenan-induced rat paw edema model is conventional, sensitive, and accepted for the screening of newer anti-inflammatory agents [41].
Results listed in Table 1 revealed that the pyrazolopyrimidine derivatives bearing thiazolidinone (10a,b) and thiazoline (11a–f) moieties exhibited good anti-inflammatory activity (52.23–66.88% edema reduction), while the rest of the tested compounds exhibited moderate activity (28.03–47.77% edema reduction). The reference drug indomethacin induced a 68.15% edema reduction at an equivalent dose as compared to the control group.
Regarding the effect of the electronic nature of the para-substituent on the activity, results revealed that among the thiazolidinone derivatives 10a,b, compound 10b with the electron withdrawing chloro group exhibited higher activity (58.60% edema reduction) than the unsubstituted compound 10a (52.23% edema reduction), corresponding to 85.99% and 76.64% of indomethacin activity, respectively. This relationship between the electronic nature of the substituent and activity holds true in most of the other series.
Among the thiazoline derivatives 11a–c, having a phenyl ring at 3′-position, compound 11a (4′-phenyl) and compound 11c (4′-p-chlorophenyl) were nearly equipotent and displayed the same anti-inflammatory activity (53.50% and 54.14% edema reduction, respectively) corresponding to 78.50% and 79.44% of indomethacin activity, respectively, whereas the 4′-p-bromophenyl analog 11b was slightly more active (57.32% edema reduction) showing 84.11% of indomethacin activity.
Replacing the phenyl ring at the 3′-position of the thiazoline nucleus in 11a–c with the p-chlorophenyl moiety in compounds 11d–f led, in most cases, to higher percentages of edema reduction. While compound 11d (4′-phenyl) exhibited considerable anti-inflammatory activity (56.69% edema reduction) corresponding to 83.18% of indomethacin activity, its 4′-p-bromophenyl analog 11e and 4′-p-chlorophenyl analog 11f exhibited the most potent anti-inflammatory activities among the tested compounds with 66.88% and 64.97% edema reduction corresponding to 98.14% and 95.33% of indomethacin activity, respectively. The good anti-inflammatory activity of compounds 11e and 11f could be attributed to the presence of a second electron withdrawing bromo or chloro group, respectively, at the para-position of the phenyl ring present at the 4′-position of the thiazoline moiety. Furthermore, since lipophilicity is a significant physicochemical property for anti-inflammatory drugs which determines their distribution in the body and their ability to cross membranes and enter cells [42], the higher lipophilicity value (C log P) of 11e and 11f (C log P = 8.23 and 8.08, respectively) supports high activity. Concerning the thiazoline derivatives 11c and 11d, lipophilicity does not seem to influence their biological response since C log P of 11c = C log P of 11d = 7.37. It is interesting that the potency slightly increased when a p-chlorophenyl moiety was incorporated into the 3′-position of the thiazoline nucleus in 11d (56.69%), rather than at the 4′-position in 11c (54.14%).
The presence of an imino linkage separating the rigid heterocyclic ring structures from the pyrazolopyrimidine nucleus in compounds 10a,b and 11a–f seems to favorably affect their biological responses in comparison to the corresponding thiazolidinone derivatives 8a,b and dioxopyrimidinethione derivatives 12a,b lacking such a spacer. Compounds 8a,b and 12a,b exhibited moderate anti-inflammatory activity with 37.58%, 40.76%, 35.03%, and 45.86% edema reduction corresponding to 55.14%, 59.81%, 51.40%, and 67.29% of indomethacin activity, respectively. Furthermore, moderate anti-inflammatory activity corresponding to 48.60%, 50.46%, 53.28%, 62.63%, and 70.10% of indomethacin activity was displayed by the 6-methylpyrazolopyrimidine derivatives substituted at the 5-position with an amino (5), an arylidene amino (7a,b), or an arylthiocarbamoylamino (9a,b) function, respectively.
Among the tricyclic pyrazolo[3′,4′:4,5]pyrimido[1,2-b]pyridazin-4(1H)-one 14–16, the 7-NH2, 8-COOC2H5 derivative 14 and its 7-OH analog 15 caused 44.59% and 41.40% edema reduction retaining better activity comparable to that of the starting compound 13 (39.49% edema reduction). In contrast, the 7-phenyl analog 16 showed the least anti-inflammatory activity among the tested compounds, with 28.05% edema reduction which is equal to 41.13% of indomethacin activity.
Replacement of the isolated 7-phenyl ring in compound 16 by a cyclohexyl ring fused to the 7- and 8-positions of the pyrazolo[3′,4′:4,5]pyrimido[1,2-b]pyridazin-4(1H)-one nucleus imparted only slightly higher anti-inflammatory activity for the tetracyclic analog 17 (29.94% edema reduction) comparable to that of compound 16 (28.03% edema reduction).
Results also indicated the correlation between the percent edema reduction by the synthesized bicyclic derivatives 5–13 and lipophilicity theoretically calculated C log P values. Higher activity corresponds to a high C log P value (Table 1). On the other hand, no clear relationship between percent edema reduction and C log P values emerged for the tricyclic 14–16 and tetracyclic 17 derivatives (Table 1).
Gastric ulcerogenic activity
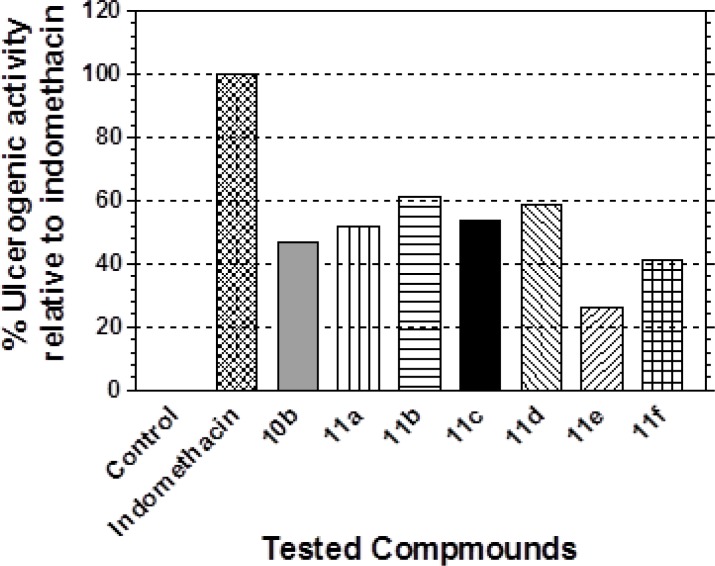
Compounds 10b and 11a–f exhibiting moderate to potent anti-inflammatory profiles in the pre-mentioned animal models were evaluated for their ulcerogenic potential in rats (Table 2) according to Meshali’s method [43]. The ulcer index was calculated according to Robert’s method [44].
Tab. 2.
Ulcerogenic effects of compounds 10b and 11a–f in comparison with indomethacin.
| Comp. No. | % Incidence/10 | Average No. of ulcer | Average severity | Ulcer index | % Ulcerogenicity relative to indomethacin |
|---|---|---|---|---|---|
| Control | – | – | – | Nil | – |
| Indomethacin | 10 | 4.4 | 2.86 | 17.26 | 100.00 |
| 10b | 6 | 0.8 | 1.25 | 8.05 | 46.63 |
| 11a | 6 | 1.6 | 1.38 | 8.98 | 52.02 |
| 11b | 8 | 1.2 | 1.33 | 10.53 | 61.00 |
| 11c | 6 | 1.6 | 1.63 | 9.23 | 53.47 |
| 11d | 8 | 1.0 | 1.20 | 10.20 | 59.09 |
| 11e | 2 | 0.8 | 1.75 | 4.55 | 26.36 |
| 11f | 4 | 1.6 | 1.5 | 7.10 | 41.13 |
Results (Table 2) (Fig. 3) indicated that compounds 10b, 11a, 11c, and 11f at the oral doses of 10 mg/kg body weight exhibited little gastric ulcerogenic effects, about 41–53% that of indomethacin. Also, they showed better gastrointestinal safety profiles (40–60% ulceration) in the population of test animals when compared to indomethacin, which was found to cause 100% ulceration under the same experimental conditions. In addition, the thiazoline derivative 11e proved to have very little ulcerogenic effects with the lowest ulcer index corresponding to 26% of indomethacin’s and a superior gastrointestinal safety profile (20% ulceration) in the population of test animals. On the other hand, it should be pointed out that compounds 11b and 11d exhibited the highest ulcer index (10.53 and 10.20, respectively) and produced ulceration in 80% of the experimental animals, but their ulcerogenic effect is still much less than that produced by indomethacin (about 61% and 59% that of indomethacin, respectively). These results revealed the advantageously better gastric tolerance of the tested compounds compared to indomethacin.
Fig. 3.
The Ulcerogenic Effect of the Tested compounds.
Experimental
Chemistry
Melting points were determined in open glass capillaries on a Stuart melting point apparatus and were uncorrected. The IR spectra were recorded, for potassium bromide discs, ν (cm−1), on the Perkin Elmer 1430 spectrophotometer. The 1H NMR spectra were determined on Jeol (500 MHz) at the microanalytical unit, Faculty of Science, Alexandria University, using DMSO-d6 as a solvent and tetramethylsilane (TMS) as internal standard. The chemical shifts are given in ppm δ values. Splitting patterns were designated as follows: s, singlet; d, doublet; t, triplet; and m, multiplet. The 13C NMR spectra were determined on Jeol (125 MHz), Faculty of Science, Alexandria University, using TMS as internal standard. Mass spectra were run on a Finnigan mass spectrometer model SSQ/7000 (70 ev), Faculty of Science, Cairo University. Microanalyses were performed at the microanalytical unit, Faculty of Science, Cairo University and at the microanalytical unit, Central lab., Faculty of Pharmacy, Alexandria University. The results of the microanalysis were within ±0.4% of the calculated values. For column chromatography, silica gel (60–200 mesh size), Adwic Laboratory Chemicals, Cairo, Egypt was used. Follow-up of the reactions and checking the homogeneity of the compounds were done by the ascending TLC run on silica gel G (Merck 60)-coated glass plates visualized by iodine vapors. Preparative TLC was performed on 20×20 cm plates coated with 30 g silica gel 60 GF 254 for TLC, Adwic Laboratory Chemicals. A duo-UV lamp (λ 254 nm), Desaga, Heidelberg, Germany was used to find the location of the spots.
Compounds 1[26], 2[24, 25], 3[27], 4[27], and 6[27] were prepared according to the reported procedures.
5-Amino-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (5)
Method A: By reaction of hydrazine hydrate with pyrazolooxazine (4):
As previously described by Pathak et al.[27], compound 5 was prepared in 27% yield (ethanol–chloroform); m.p. 214–215°C, (reported yield 30%, m.p. 215–217°C).
Method B: By reaction of hydrazine hydrate with the N-acetylated aminoester (6):
A mixture of 6 (2.73 g, 10 mmol) and hydrazine hydrate 99% (10 ml) in ethanol (25 ml) was heated under reflux for 6 h during which the product was partially crystallized out. The reaction mixture was cooled, and the separated product was filtered, washed with ethanol, dried, and crystallized from ethanol-chloroform mixture to yield 2.01 g (86%) of 5 as white crystals, mp: 214–216 °C; IR (KBr, ν, cm−1): 3319, 3210, 3112 (NH), 1695 (C=O), 1607, 1567 (C=N), 1536, 1510 (C=C), 1404, 1323 (C–N lactam); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.58 (s, 3H, CH3), 5.73 (s, 2H, NH2, D2O exchangeable), 7.34 (t, 1H, J = 7.65 Hz, CH-4), 7.48 (t, 2H, J = 7.65 Hz, CH-3,5), 7.53 (d, 2H, J = 7.65 Hz, CH-2,6), 8.29 (s, 1H, CH-3); 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 23.21 (CH3), 96.64 (C-3a), 122.75 (C-2,6), 127.57 (C-4), 129.82 (C-3,5), 136.27 (C-1), 138.50 (C-3), 138.72 (C-7a), 159.70 (C-4), 164.96 (C-6).
The products obtained from both methods were identical as revealed by mp, mixed mp, and superimposability of the IR and 1H NMR spectra. However, method B was superior to method A concerning the yield of the prepared compound.
General procedure for the synthesis of 5-(arylideneamino)-6-methyl-1-phenyl-1,5-diyhdro-4H-pyrazolo[3,4-d]pyrimidin-4-ones (7a,b)
A mixture of 5 (0.24 g, 1 mmol) and benzaldehyde or 4-chlorobenzaldehyde (1 mmol) in glacial acetic acid (5 ml) was heated under reflux for 4h. The reaction mixture was cooled and poured onto crushed ice. The product obtained was filtered, washed with H2O, dried, and crystallized from the appropriate solvent.
5-(Benzylideneamino)-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (7a)
White powder (88%, EtOH); mp: 184–186 °C; IR (KBr, ν cm−1): 1697 (C=O), 1613,1572 (C=N), 1536, 1492 (C=C), 1421, 1314 (C–N lactam); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.46 (s, 3H, CH3), 7.36–7.56 (m, 8H, Ar CH), 7.67 (d, 2H, J = 6.8 Hz, Ar” CH-2,6), 8.05 (s, 1H, CH-3), 8.35 (s, 1H, N=CH); EI-MS m/z (%): 329 [M+●] (absent), 305 (31.6) [M+● – C2H3, + 3H], 226 (0.3) [M+● – C6H5-CH=N, + 1H], 185 (100) [M+● – C6H5-CH=N, – CH3-C≡N, + 1H]. Anal. Calcd. for C19H15N5O (329.36): C, 69.29; H, 4.59; N, 21.26. Found: C, 69.03; H, 4.57; N, 21.19.
5-[(4-Chlorobenzylidene)amino]-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]-pyrimidin-4-one (7b)
Yellowish white powder (85%, EtOH/CHCl3); mp: 195–196 °C; IR (KBr, ν, cm−1): 1698 (C=O), 1610, 1569 (C=N), 1534, 1491 (C=C), 1422, 1315 (C–N lactam), 896 (C–Cl); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.52 (s, 3H, CH3), 7.38 (d, 2H, J = 6.8 Hz, Ar” CH-2,6), 7.51 (t, 1H, J = 7.6 Hz, Ar’ CH-4), 7.54–7.64 (m, 4H, Ar’ CH-3,5 + Ar” CH-3,5), 7.69 (d, 2H, J = 7.6 Hz, Ar’ CH-2,6), 8.36 (s, 1H, CH-3), 8.88 (s, 1H, N=CH); EI-MS m/z (%):365 (6.80) [M+● + 2], 363 (21.15) [M+●], 315 (28.85) [M+● – Cl, – CH3, +2H], 226 (23.08) [M+● – 4-Cl-C6H4-CH=N, + 1H], 199 (23.08) [M+● – 4-Cl-C6H4-CH=N, – C2H3, + 1H], 184 (100) [M+● – 4-Cl-C6H4-CH=N, – CH3-C≡N]. Anal. Calcd. for C19H14ClN5O (363.80): C, 62.73; H, 3.88; N, 19.25. Found: C, 62.60; H, 3.87; N, 19.18.
General procedure for the synthesis of 6-Methyl-5-(2-aryl-4-oxo-1,3-thiazolidin-3-yl)-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-ones (8a,b)
To a well-stirred suspension of the appropriate Schiff’s base 7a or 7b (1 mmol) in dry benzene (50 ml), mercaptoacetic acid (0.11 g, 1.2 mmol) was added. The reaction mixture was refluxed with stirring for 6 h with simultaneous azeotropic removal of water using a Dean–Stark water separator. After cooling, the solution obtained was washed with a saturated solution of sodium hydrogen carbonate (2 × 50 ml), then with water (2 × 50 ml), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure and the product was crystallized from ethanol.
6-Methyl-5-(4-oxo-2-phenyl-1,3-thiazolidin-3-yl)-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]-pyrimidin-4-one (8a)
Pale yellow needles (74%); mp: 179–181 °C; IR (KBr, ν, cm−1): 1720 (C=O), 1659 (C=O), 1610, 1522, 1491 (C=N, C=C), 1314 (C–N lactam), 1286, 1177, 1088 (C–S–C); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.46 (s, 3H, CH3), 3.75 (d, 1H, J = 16.0 Hz, CH-5′), 3.88 (d, 1H, J = 16.0 Hz, CH-5′), 5.84 (s, 1H, CH-2′), 6.39 (s, 1 H, CH-5′, enol form), 7.35 (t, 1H, J = 7.6 Hz, Ar” CH-4), 7.37 (t, 1H, J = 7.6 Hz, Ar’ CH-4), 7.43-7.48 (m, 8H, Ar CH), 7.80 (s, 1H, CH-3), 10.10 (s, 1 H, enolic OH, D2O exchangeable); EI-MS m/z (%): 403 [M+●] (absent), 379 (1.0) [M+● – C2H3, + 3H], 199 (32.8) [M+● – C2H3, – 4-oxo-2-phenyl-1,3-thiazolidine, + 1H], 185 (100) [M+● – 4-oxo-2-phenyl-1,3-thiazolidine, – CH3-C≡N, + 1H]. Anal. Calcd. for C21H17N5O2S (403.46): C, 62.52; H, 4.25; N, 17.36. Found: C, 62.75; H, 4.25; N, 17.43.
5-[2-(4-Chlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (8b)
Pale yellow amorphous powder (78%); mp: 191–195 °C; IR (KBr, ν, cm−1): 1718 (C=O), 1654 (C=O), 1611, 1559, 1491 (C=N, C=C), 1422, 1314 (C–N lactam), 1282, 1086 (C–S–C), 826 (C–Cl); 1H NMR (500 MHz, CDCl3) δ (ppm): 2.57 (s, 3H, CH3), 3.56 (d, 1H, J = 16.0 Hz, CH-5′), 3.65 (d, 1H, J = 16.0 Hz, CH-5′), 5.56 (s, 1H, CH-2′), 5.75 (s, 1H, CH-5′, enol form), 7.17 (d, 2H, J = 8.4 Hz, Ar” CH-2,6), 7.24 (d, 2H, J = 8.4 Hz, Ar” CH-3,5), 7.29–7.36 (m, 3H, Ar’ CH-3,4,5), 7.41 (d, 2H, J = 7.6 Hz, Ar’ CH-2,6), 7.68 (s, 1H, CH-3), 9.68 (s, 1H, enolic OH, D2O exchangeable); 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 22.73 (CH3), 62.82 (C-5′), 63.68 (C-2′), 90.58 (C-5′, enol form), 95.52 (C-3a), 124.08 (Ar’ C-2,6), 128.65 (Ar’ C-4), 129.48 (Ar” C-3,5), 129.61 (Ar’ C-3,5), 130.0 (Ar” C-2,6), 131.05 (Ar” C-4), 135.09 (Ar” C-1), 136.41 (Ar’ C-1), 137.47 (C-3), 138.52 (C-7a), 149.58 (C-4), 163.52 (C-6), 170.37 (C-4′), 179.40 (C-4′, enol form); EI-MS m/z (%): 439 [M+● + 2], 437 [M+●] (absent), 415 (4.2) and 413 (13.3) [M+● – C2H3, + 3H], 341 (3.7) and 339 (10.8) [M+● – SCH2, – C=O, – C2H3, + 3H], 226 (8.1) [M+● – 2-(4-chlorophenyl)-4-oxo-1,3-thiazolidine, + 1H], 186 (100) [M+● – 2-(4-chlorophenyl)-4-oxo-1,3-thiazolidine, –CH3-C≡N, + 2H]. Anal. Calcd. for C21H16ClN5O2S (437.90): C, 57.60; H, 3.68; N, 15.99. Found: C, 57.83; H, 3.69; N, 16.04.
General procedure for the synthesis of 1-Aryl-6-methyl-5-(arylthiocarbamoyl)- amino-1,5-diyhdro-4H-pyrazolo[3,4-d]pyrimidin-4-one (9a,b)
A solution of 5 (2.41 g, 10 mmol) in ethanol (25 ml) was treated with the selected isothiocyanate derivative (10 mmol) and heated under reflux for 10–12 h. The reaction mixture was concentrated under vacuum and left to cool to room temperature. The deposited product was filtered and crystallized from ethanol.
1-(6-Methyl-4-oxo-1-phenyl-1,4-dihydro-5H-pyrazolo[3,4-d]pyrimidin-5-yl)-3-phenylthiourea (9a)
Pale yellow amorphous powder (76%); mp: 145–150 °C; IR (KBr, ν, cm−1): 3404, 3303, 3203 (NH), 1644 (C=O), 1615, 1496 br (C=N, C=C, δ NH), 1355 (C–N lactam), 1549, 1257, 1220, 1045 (NCS amide I, II, III and IV mixed vibrational bands, respectively); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.47 (s, 3H, CH3), 7.12 (t, 1H, J = 6.85 Hz, Ar” CH-4), 7.29 (t, 1H, J = 7.65 Hz, Ar’ CH-4), 7.37 (t, 2H, J = 6.85 Hz, Ar” CH-3,5), 7.44 (t. dist., 2H, Ar’ CH-3,5), 7.51 (d, 2H, J = 6.85 Hz, Ar” CH-2,6), 7.53 (d, 2H, J = 7.65 Hz, Ar’ CH-2,6), 7.95 (s, 1H, CH-3), 9.55 (s, 1H, NH-C6H5, D2O exchangeable), 9.93 (s, 1H, pyrazolopyrimidine-NH, D2O exchangeable); 13C NMR (125 MHz, DMSO-d6) δ (ppm): 19.50 (CH3), 96.28 (C-3a), 123.81 (Ar’ C-2,6), 126.37 (Ar” C-4), 127.85 (Ar’ C-4), 128.43 (Ar” C-2,6), 130.01 (Ar” C3,5 and Ar’ C-3,5), 138.39 (Ar” CH-1), 138.52 (Ar’ C-1), 139.50 (C-3), 139.85 (C-7a), 150.12 (C-4), 164.51 (C-6), 181.98 (C=S); EI-MS m/z (%): 376 [M+●] (absent), 318 (1.9) [M+● – S, – C2H3, + 1H], 302 (0.8) [M+● – S, – CH3-C≡N, – H], 287 (0.6) [M+● – C6H5-NH, + 3H], 216 (10.1) [M+● – C6H5-NH-CS, - C2H3, + 3H], 186 (69.3) [M+● – C6H5-NH-CS-NH, – CH3-C≡N, + 2H]. Anal. Calcd. for C19H16N6OS (376.43): C, 60.62; H, 4.28; N, 22.33. Found: C, 60.39; H, 4.27; N, 22.26.
1-(4-Chlorophenyl)-3-(6-methyl-4-oxo-1-phenyl-1,4-dihydro-5H-pyrazolo[3,4-d]pyrimidin-5-yl)thiourea (9b)
Yellow microcrystalline powder (88%); mp: 157–160 °C; IR (KBr, ν, cm−1): 3318, 3184 (NH), 1672 (C=O), 1627, 1492 (C=N, C=C), 1546 (δ NH), 1306 (C–N lactam), 1598, 1258, 1214, 1048 (NCS amide I, II, III and IV mixed vibrational bands, respectively), 877 (C–Cl); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.59 (s, 3H, CH3), 7.30 (d, 2H, J = 7.6 Hz, Ar” CH-2,6), 7.36 (t, 1H, J = 6.9 Hz, Ar’ CH-4), 7.43 (d, 2H, J = 7.6 Hz, Ar” CH-3,5), 7.47 (t, 2H, J = 6.9 Hz, Ar’ CH-3,5), 7.50 (d, 2H, J = 6.9 Hz, Ar’ CH-2,6), 7.94 (s, 1H, CH-3), 9.70 (s, 1H, NH-4-Cl-C6H4, D2O exchangeable), 9.97 (s, 1H, pyrazolopyrimidine-NH, D2O exchangeable); EI-MS m/z (%): 412 (5.7) [M+● + 2], 410 (21.2) [M+●], 362 (15.25) [M+● – Cl, – CH3, + 2H], 328 (15.25) [M+● – Cl, – CH3, – H2S, + 2H], 315 (23.73) [M+● – Cl, – C2H3, – H2S, + H], 239 (47.46) [M+● – 4-Cl-C6H4-N=C=S, – 2H], 226 (23.71) [M+● – 4-Cl-C6H4-NH-CS-NH, + 1H], 184 (45.76) [M+● – 4-Cl-C6H4-NH-CS-NH, – CH3-C≡N]. Anal. Calcd. for C19H15ClN6OS (410.88): C, 55.54; H, 3.68; N, 20.45. Found: C, 55.76; H, 3.69; N, 20.53.
General procedure for the synthesis of 5-[3-Aryl-4-oxo-1,3-thiazolidin-2-ylidene)-amino]-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-ones (10a,b)
A solution of the appropriate thiourea derivative 9a or 9b (1 mmol) in absolute ethanol (20 ml) was treated with ethyl bromoacetate (0.167 g, 1 mmol) and anhydrous sodium acetate (0.082 g, 1 mmol). The reaction mixture was heated under reflux for 6–8 h, the solvent was evaporated under reduced pressure, and the residue was extracted with chloroform (3×20 ml). The chloroformic layer was washed with water, dried (anhydrous Na2SO4), and evaporated under vacuum. The products 10a and 10b were purified by preparative TLC using C6H6: EtOAc (8:2, v/v) as developing solvent.
6-Methyl-5-[(4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene)amino]-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (10a)
White needles (82%, EtOH/CHCl3); mp: 264–267 °C; IR (KBr, ν, cm−1): 1741 (C=O), 1654 (C=O), 1618, 1595 (C=N), 1559, 1493 (C=C), 1411, 1310 (C–N lactam), 1275, 1071 (C– S–C); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.46 (s, 3H, CH3), 4.16 (s, 2H, CH-5′), 6.45 (s, 1 H, CH-5′, enol form), 6.86 (d, 2H, J = 7.6 Hz, Ar” CH-2,6), 7.10 (t, 1H, J = 7.6 Hz, Ar” CH-4), 7.33 (t, 2H, J = 7.6 Hz, Ar” CH-3,5), 7.41–7.52 (m, 3H, Ar’ CH-3,4,5), 7.57 (d, 2H, J = 8.4 Hz, Ar’ CH-2,6), 8.03 (s, 1H, CH-3), 10.75 (s, 1 H, enolic OH, D2O exchangeable); EI-MS m/z (%): 416 [M+●] (absent), 392 (18.4) [M+● – C2H3, + 3H], 300 (1.2) [M+● – C6H5-NCO, + 3H], 211 (1.2) [M+● – 4-oxo-3-phenyl-1,3-thiazolidin-2-ylideneamino, – CH3, + 1H], 186 (100) [M+● – 4-oxo-3-phenyl-1,3-thiazolidin-2-ylideneamino, – CH3-C≡N, + 2H]. Anal. Calcd. for C21H16N6O2S (416.46): C, 60.56; H, 3.87; N, 20.18. Found: C, 60.35; H, 3.86; N, 20.11.
5-{[3-(4-Chlorophenyl)-4-oxo-1,3-thiazolidin-2-ylidene]amino}-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (10b)
Pale yellow needles (87%, EtOH); mp: 264–267 °C; IR (KBr, ν, cm−1): 1714 (C=O), 1651 (C=O), 1613, 1589 (C=N), 1522, 1494 (C=C), 1411, 1311 (C–N lactam), 1246, 1078 (C– S–C), 820 (C–Cl); 1H NMR (500 MHz, CDCl3) δ (ppm): 2.45 (s, 3H, CH3), 4.03 (s, 2H, CH-5′), 6.22 (s, 1 H, CH-5′, enol form), 7.37 (d, 2H, J = 8.4 Hz, Ar” CH-2,6), 7.40 (t, 1H, J = 7.6 Hz, Ar’ CH-4), 7.52 (t, 2H, J = 7.6 Hz, Ar’ CH-3,5), 7.53 (d, 2H, J = 7.6 Hz, Ar’ CH-2,6), 7.58 (d, 2H, J = 8.4 Hz, Ar” CH-3,5), 7.73 (s, 1H, CH-3), 10.68 (s, 1 H, enolic OH, D2O exchangeable); 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 16.96 (CH3), 62.35 (C-5′), 85.88 (C-5′, enol form), 95.47 (C-3a), 123.45 (Ar’ C-2,6), 123.80 (Ar” C-2,6), 129.37 (Ar’ C-4), 130.19 (Ar” C-4), 131.82 (Ar” C-3,5), 132.23 (Ar” C-1), 132.64 (Ar’ C-3,5), 133.26 (Ar’ C-1), 136.43 (C-3), 138.06 (C-7a), 149.08 (C-2′), 154.10 (C-4), 162.32 (C-6), 168.25 (C-4′), 181.17 (C-4′, enol form); EI-MS m/z (%): 452 [M+● + 2], 450 [M+●] (absent), 354 (23.6) and 352 (64.0) [M+● – C=O, – SCH2, – C2H3, + 3H], 297 (6.0) [M+● – 4-Cl-C6H4, – C2H3, – OH, + 2H], 226 (18.4) [M+● – 3-(4-chlorophenyl)-4-oxo-1,3-thiazolidin-2-ylideneamino, + H], 184 (100) [M+● – 3-(4-chlorophenyl)-4-oxo-1,3-thiazolidin-2-ylideneamino, – CH3-C≡N]. Anal. Calcd. for C21H15ClN6O2S (450.90): C, 55.94; H, 3.35; N, 18.64. Found: C, 56.14; H, 3.36; N, 18.71.
General procedure for the synthesis of 5-[3,4-Diaryl-1,3-thiazol-2(3H)-ylidene)-amino]-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-ones (11a–f)
A solution of the appropriate thiourea derivative 9a or 9b (1 mmol) in absolute ethanol (20 ml) was treated with the selected phenacyl bromide (1 mmol) and anhydrous sodium acetate (0.082 g, 1 mmol). The reaction mixture was heated under reflux for 8–10 h, partially concentrated and left to cool overnight. The separated product was filtered, washed with aqueous ethanol, dried, and crystallized from ethanol.
5-[(3,4-Diphenyl-1,3-thiazol-2(3H)-ylidene)amino]-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (11a)
White amorphous powder (80%); mp: 258–260 °C; IR (KBr, ν, cm−1): 1616 (C=O), 1590, 1497 (C=N, C=C), 1315 (C–N lactam), 1248, 1178, 1075 (C–S–C); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.46 (s, 3H, CH3), 6.21 (s, 1H, CH-5′), 6.95 (t, 1H, J = 6.8 Hz, Ar” CH-4), 7.31 (d, 2H, J = 6.8 Hz, Ar” CH-2,6), 7.38–7.41 (m, 3H, J = 7.6 Hz, Ar”‘ CH-3,4,5), 7.53 (t, 1H, J = 7.6 Hz, Ar’ CH-4), 7.56–7.59 (m, 8H, Ar CH), 7.73 (s, 1H, CH-3); 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 17.64 (CH3), 88.34 (C-5’), 95.29 (C-3a), 118.24 (Ar” C-4), 118.67 (Ar’ C-4), 123.01 (Ar”‘ C-4), 123.78 (Ar” C-2,6), 124.17 (Ar’ C-2,6), 128.83 (Ar”‘ C-2,6), 129.29 (Ar”‘ C-1), 129.48 (Ar’ C-1), 129.66 (Ar”‘ C-3,5), 130.01 (Ar’ C-3,5), 134.29 (Ar” C-1), 136.92 (C-3), 137.14 (C-7a), 137.40 (Ar” C-3,5), 146.55 (C-4’), 157.04 (C-2′), 161.17 (C-4), 164.70 (C-6); EI-MS m/z (%): 476 [M+●] (absent), 461 (12.4) [M+● – CH3], 318 (100) [M+● – C6H5, – C2HS, – C2H3, + 3H], 184 (54.7) [M+● – 3,4-diphenylthiazol-2(3H)-ylideneamino, – CH3-C≡N]. Anal. Calcd. for C27H20N6OS (476.55): C, 68.05; H, 4.23; N, 17.64. Found: C, 67.82; H, 4.23; N, 17.57.
5-{[4-(4-Bromophenyl)-3-phenyl-1,3-thiazol-2(3H)-ylidene]amino}-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (11b)
Yellow microcrystalline powder (88%); mp: 248–252 °C; IR (KBr, ν, cm−1): 1621 (C=O), 1599, 1508 (C=N, C=C), 1398, 1314 (C–N lactam), 1253, 1063 (C–S–C), 693 (C–Br); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.58 (s, 3H, CH3), 5.73 (s, 1H, CH-5′), 7.31 (d, 2H, J = 6.9 Hz, Ar” CH-2,6), 7.35 (d, 2H, J = 7.6 Hz, Ar”‘ CH-2,6), 7.38–7.40 (m, 3H, Ar” CH-3,4,5), 7.50 (d, 2H, J = 7.6 Hz, Ar”‘ CH-3,5), 7.53 (t. dist., 1H, Ar’ CH-4), 7.63 (t, 2H, J = 8.4 Hz, Ar’ CH-3,5), 8.02 (d, 2H, J = 8.4 Hz, Ar’ CH-2,6), 8.29 (s, 1H, CH-3); EI-MS m/z (%): 557 [M+● + 2], 555 [M+●] (absent), 503 (3.8), 501 (4.1) [M+● – SCH, - CH3, + 6H], 318 (20.9) [M+● – 4-Br-C6H4, - C2HS, - C2H3, + 3H], 261 (5.3) [M+● – 4-Br-C6H4, - C6H5-N, - C2H, - C2H3, + 5H], 184 (44.08) [M+● – 4-(4-bromophenyl)-3-phenylthiazol-2(3H)-ylideneamino, - CH3-C≡N]. Anal. Calcd. for C27H19BrN6OS. ½ H2O (564.46): C, 57.45; H, 3.57; N, 14.88. Found: C, 57.56; H, 3.58; N, 14.94.
5-{[4-(4-Chlorophenyl)-3-phenyl-1,3-thiazol-2(3H)-ylidene]amino}-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (11c)
Pale yellow needles (82%); mp: 258–259 °C; IR (KBr, ν, cm−1): 1618 (C=O), 1588, 1498 (C=N, C=C), 1418, 1317 (C–N lactam), 1248, 1073 (C–S–C), 862 (C–Cl); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.46 (s, 3H, CH3), 6.19 (s, 1H, CH-5′), 6.94 (d, 2H, J = 6.8 Hz, Ar” CH-2,6), 6.95 (d, 2H, J = 7.6 Hz, Ar”‘ CH-2,6), 7.29–7.32 (m, 3H, Ar” CH-3,4,5), 7.38 (d, 2H, J = 7.6 Hz, Ar”‘ CH-3,5), 7.50–7.54 (m, 3H, Ar’ CH-3,4,5), 7.57 (d, 2H, J = 6.9 Hz, Ar’ CH-2,6), 7.74 (s, 1H, CH-3); EI-MS m/z (%): 512 (0.39) [M+● + 2], 510 (1.09) [M+●], 487 (0.48) and 485 (1.41) [M+● – C2H3, +2H], 378 (1.09) [M+● – 4-Cl-C6H4, – C2H, + 4H], 318 (24.35) [M+● – 4-Cl-C6H4, – C2HS, – C2H3, + 3H], 261 (4.02) [M+● – 4-Cl-C6H4, – C2H, – C6H5-N, – C2H3, + 5H], 184 (33.71) [M+● – 4-(4-chlorophenyl)-3-phenylthiazol-2(3H)-ylideneamino, – CH3-C≡N]. Anal. Calcd. for C27H19ClN6OS (511.00): C, 63.46; H, 3.75; N, 16.45. Found: C, 63.52; H, 3.76; N, 16.51.
5-{[3-(4-Chlorophenyl)-4-phenyl-1,3-thiazol-2(3H)-ylidene]amino}-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (11d)
White crystals (91%); mp: 267–269 °C; IR (KBr, ν, cm−1): 1613 (C=O), 1590, 1496 (C=N, C=C), 1375 (C–N lactam), 1247, 1095 (C–S–C), 852 (C–Cl); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.75 (s, 3H, CH3), 6.21 (s, 1H, CH-5′), 7.37 (d, 2H, J = 7.65 Hz, Ar” CH-2,6), 7.38 (d, 2H, J = 7.65 Hz, Ar” CH-3,5), 7.40 (t, 1H, J = 7.65 Hz, Ar”‘ CH-4), 7.53 (t, 1H, J = 7.65 Hz, Ar’ CH-4), 7.56–7.60 (m, 8H, Ar CH), 7.73 (s, 1H, CH-3); EI-MS m/z (%): 512 [M+● + 2], 510 [M+●] (absent), 354 (25.50) and 352 (66.30) [M+● – C6H5, – C2HS, – C2H3, + 3H], 297 (8.90) [M+● – C6H5, – 4-Cl-C6H4, – C2H3, + 2H], 226 (18.10) [M+● – 3-(4-chlorophenyl)-4-phenylthiazol-2(3H)-ylideneamino, +1H], 184 (100) [M+● – 3-(4-chlorophenyl)-4-phenylthiazol-2(3H)-ylideneamino, – CH3-C≡N]. Anal. Calcd. for C27H19ClN6OS (511.00): C, 63.46; H, 3.75; N, 16.45. Found: C, 63.31; H, 3.74; N, 16.41.
5-{[4-(4-Bromophenyl)-3-(4-chlorophenyl)-1,3-thiazol-2(3H)-ylidene]amino}-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (11e)
White microcrystalline powder (84%); mp: 261–263 °C; IR (KBr, ν, cm−1): 1610 (C=O), 1589, 1495 (C=N, C=C), 1412, 1314 (C–N lactam), 1248, 1148, 1099 (C–S–C), 870 (C– Cl), 695 (C–Br); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.59 (s, 3H, CH3), 6.22 (s, 1H, CH-5′), 7.33 (d, 2H, J = 8.45 Hz, Ar” CH-2,6), 7.36 (d, 2H, J = 8.45 Hz, Ar” CH-3,5), 7.39 (d, 2H, J = 8.45 Hz, Ar”‘ CH-2,6), 7.41 (d, 2H, J = 8.45 Hz, Ar”‘ CH-3,5), 7.50 (m, 3H, Ar’ CH-3,4,5), 7.54 (d, 2H, J = 8.45 Hz, Ar’ CH-2,6), 8.01 (s, 1H, CH-3H); EI-MS m/z (%): 593 [M+● + 4], 591 [M+● + 2], 589 [M+●] (absent), 397 (4.7) and 395 (11.6) [M+● – 4-Br-C6H4, – CH3-C≡N, + 3H], 354 (22.2) and 352 (60.2) [M+● – 4-Br-C6H4, – C2HS, – C2H3, + 2H], 297 (4.1) [M+● – 4-Br-C6H4, – 4-Cl-C6H4, – C2H3 + 2H], 226 (14.1) [M+● – 4-(4-bromophenyl)-3-(4-chlorophenyl)thiazol-2(3H)-ylideneamino, +1H], 184 (100) [M+● – 4-(4-bromophenyl)-3-(4-chlorophenyl)thiazol-2(3H)-ylideneamino, – CH3-C≡N]. Anal. Calcd. for C27H18BrClN6OS (589.89): C, 54.97; H, 3.08; N, 14.25. Found: C, 54.76; H, 3.07; N, 14.19.
5-{[3,4-Bis(4-chlorophenyl)-1,3-thiazol-2(3H)-ylidene]amino}-6-methyl-1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (11f)
Pale yellow crystals (86%); mp: 272–275 °C; IR (KBr, ν, cm−1):1613 (C=O), 1589, 1495 (C=N, C=C), 1412, 1311 (C–N lactam), 1247, 1096, 1078 (C–S–C), 851 (C–Cl); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.46 (s, 3H, CH3), 6.21 (s, 1H, CH-5′), 7.37 (d, 2H, J = 8.0 Hz, Ar” CH-2,6), 7.40 (d, 2H, J = 6.8 Hz, Ar”‘ CH-3,5), 7.52 (t, 1H, J = 7.6 Hz, Ar’ CH-4), 7.54–7.59 (m, 8H, Ar CH), 7.73 (s, 1H, CH-3); EI-MS m/z (%): 549 [M+● + 4], 547 [M+● + 2], 545 [M+●] (absent), 354 (17.6) and 352 (51.8) [M+● – 4-Cl-C6H4, – C2HS, – C2H3, + 2H], 297 (2.4) [M+● – 2 × 4-Cl-C6H4, – C2H3 + 2H], 226 (15.0) [M+● – 3,4-bis(4-chlorophenyl)thiazol-2(3H)-ylideneamino, +1H], 198 (2.7) [M+● – 3,4-bis(4-chlorophenyl)thiazol-2(3H)-ylideneamino, – C2H3], 184 (100) [M+● – 3,4-bis(4-chlorophenyl)thiazol-2(3H)-ylideneamino, – CH3-C≡N]. Anal. Calcd. for C27H18Cl2N6OS (545.44): C, 59.45; H, 3.33; N, 15.41. Found: C, 59.32; H, 3.33; N, 15.39.
General procedure for the synthesis of 1-Aryl-3-(6-methyl-4-oxo-1-phenyl-1,4-dihydro-5H-pyrazolo[3,4-d]pyrimidin-5-yl)-2-thioxodihydropyrimidine-4,6(1H,5H)-diones (12a,b)
A mixture of the appropriate thiourea derivative 9a or 9b (15 mmol), malonic acid (2.08 g, 20 mmol), and acetyl chloride (10 ml) was heated for 7 h at 50–55 °C on a water bath, cooled, then poured into ice-cold water (50 ml). The separated product was filtered, washed with water, dried, and purified by preparative TLC using C6H6 : EtOAc : CHCl3 (5:1:5, v/v/v) as developing solvent.
1-(6-Methyl-4-oxo-1-phenyl-1,4-dihydro-5H-pyrazolo[3,4-d]pyrimidin-5-yl)-3-phenyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (12a)
Yellow crystals (78%, EtOH/CHCl3); mp: 242–244 °C; IR (KBr, ν, cm−1):1707, 1658 (2 C=O, 4,6-dioxopyrimidine), 1634 (C=O, amide), 1597, 1497 (C=N, C=C), 1402, 1310 (C–N lactam), 1579, 1268, 1164, 1024 (N−C=S amide I, II, III and IV mixed vibrational bands, respectively); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.25 (s, 3H, CH3), 3.75 (s, 2H, CH-5′), 7.36–7.51 (m, 10 H, Ar CH), 8.03 (s, 1H, CH-3); 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 21.66 (CH3), 52.49 (C-5′), 105.87 (C-3a), 121.92 (Ar’ C-2,6), 122.28 (Ar” C-2,6), 123.44 (Ar” C-4), 124.24 (Ar’ C-4), 128.51 (Ar” C-3,5), 129.40 (Ar’ C-3,5), 136.45 (Ar” C-1), 136.99 (Ar’ C-1), 137.33 (C-7a), 138.09 (C-3), 145.16 (C-4), 149.64 (C-6), 151.78 (C-4′), 152.97 (C-6′), 157.43 (C-2′); EI-MS m/z (%): 444 [M+●] (absent), 312 (8.2) [M+● – C6H5-N=C=O, – CH2, + 1H], 308 (100) [M+● – C6H5-N=C=S, – 1H], 273 (30.4) [M+● – C6H5-N=C=O, – CH2-C=O, – CH3, + 5H], 144 (10.1) [M+● – 2,3-dihydro-3-phenyl-2-thioxo-4,6(1H,5H)-dioxopyrimidine, – CH3-C≡N, – N=C=O, + 2H]. Anal. Calcd. for C22H16N6O3S (444.47): C, 59.45; H, 3.63; N, 18.91. Found: C, 59.32; H, 3.63; N, 18.87.
1-(4-Chlorophenyl)-3-(6-methyl-4-oxo-1-phenyl-1,4-dihydro-5H-pyrazolo[3,4-d]pyrimidin-5-yl)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (12b)
Pale yellow amorphous powder (81%. EtOH); mp: 198–201 °C; IR (KBr) ν (cm−1): 1701, 1664 (2 C=O, 4,6-dioxopyrimidine), 1640 (C=O, amide), 1578, 1494 (C=N, C=C), 1402, 1317 (C–N lactam), 1544, 1237, 1170, 1018 (N−C=S amide I, II, III and IV mixed vibrational bands, respectively), 833 (C−Cl); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.05 (s, 3H, CH3), 3.00 (s, 2H, CH-5′), 7.45–7.51 (m, 9H, Ar CH), 7.90 (s, 1H, CH-3); EI-MS m/z (%): 480 [M+● + 2], 478 [M+●] (absent), 360 (15.8) [M+● – 4-Cl-C6H4-N-, + 7H], 296 (11.6) [M+● – 4-Cl-C6H4-N=C=S, – O, + 3H], 267 (42.1) [M+● – 4-Cl-C6H4-N=C=S, – CH2-C=O], 241 (22.1) [M+● – 4-Cl-C6H4-N=C=S, – CH2-C=O, – C=O, + 2H], 226 (27.4) [M+● – 2,3-dihydro-3-(4-chlorophenyl)-2-thioxo-4,6(1H,5H)-dioxopyrimidine, + 1H], 186 (52.6) [M+● – 2,3-dihydro-3-(4-chlorophenyl)-2-thioxo-4,6(1H,5H)-dioxopyrimidine, – CH3-C≡N, + 2H]. Anal. Calcd. for C22H15ClN6O3S (478.91): C, 55.17; H, 3.16; N, 17.55. Found: C, 54.94; H, 3.15; N, 17.49.
5-Amino-4-oxo-1-phenyl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidine-6-carbaldehyde (13)
To a hot solution of 5 (2.41 g, 10 mmol) in dioxane (50 ml), powdered selenium dioxide (2.20 g, 20 mmol) was added portion-wise while stirring. After complete addition, the reaction mixture was refluxed with stirring for 6 h. The precipitated selenium was removed by filtration. The filtrate was evaporated under reduced pressure and the dark yellow residue was treated with cold water (50 ml). The product was extracted with chloroform (3×20 ml), washed with water, dried (anhydrous Na2SO4), and evaporated to dryness under reduced pressure. The residue was purified by chromatography on a column of silica-gel. Elution with a mixture of C6H6 : EtOAc : CHCl3 (5:1:5, v/v/v) yielded 13 as a pale yellow crystals (1.78 g, 69.8%); mp: 145–146°C; IR (KBr, ν, cm−1): 3441, 3322, 3188 (NH), 1720 (HC=O), 1696 (C=O), 1616, 1597 (C=N), 1535, 1500 (C=C), 1550 (δ NH), 1411, 1316 (C–N lactam); 1H NMR (500 MHz, CDCl3) δ (ppm): 4.24 (s, 2H, NH2, D2O exchangeable), 7.21 (t, 1H, J = 6.2 Hz, Ph CH-4), 7.32 (d, 2H, J = 6.2 Hz, Ph CH-3,5), 7.37 (d, 2H, J = 6.2 Hz, Ph CH-2,6), 8.48 (s, 1H, CH-3), 9.53 (s, 1H, CHO); 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 95.92 (C-3a), 123.65 (Ph C-2,6), 127.98 (Ph C-4), 129.18 (Ph C-3,5), 138.02 (C-3), 138.97 (C-7a), 144.51 (Ph C-1), 157.39 (C-4), 159.10 (C-6), 172.92 (CHO); EI-MS m/z (%): 255 (52.5) [M+●], 226 (12.3) [M+● – CHO], 203 (11.5) [M+● – CHO, – C≡N, + 3H], 185 (9.2) [M+● – CHO, – C≡N, – NH2, + 1H], 173 (19.6) [M+● – CHO, – C≡N, – N2H2, + 3H]. Anal. Calcd. for C12H9N5O2 (255.23): C, 56.47; H, 3.55; N, 27.44. Found: C, 56.33; H, 3.56; N, 27.34.
Ethyl 7-amino-4-oxo-1-phenyl-1,4-dihydropyrazolo[3′,4′:4,5]pyrimido[1,2-b]-pyridazine-8-carboxylate (14)
A mixture of 13 (0.306 g, 1.2 mmol) and ethyl cyanoacetate (0.271 g, 2.4 mmol) in sodium ethoxide solution (20 mg of sodium dissolved in 20 ml of absolute EtOH) was heated under reflux for 6 h. After cooling, the reaction mixture was neutralized with 10% acetic acid (pH 6–7), partially concentrated under reduced pressure, and poured onto crushed ice. The precipitated product was filtered, washed with water, air dried, and crystallized from ethanol to yield 14 as a pale yellow amorphous powder (0.328 g, 78.1%); mp: 155– 156 °C; IR (KBr, ν, cm−1): 3437, 3287, 3190 (NH), 1727 (C=O, ester), 1678 (C=O, amide), 1620, 1599, 1501 (C=N, C=C), 1534, (δ NH), 1401, 1317 (C–N lactam), 1280, 1020 (C–O–C); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 1.31 (t, 3H, J = 6.9 Hz, CH2CH3), 4.15 (q, 2H, J = 6.9 Hz, CH2CH3), 5.06 (s, 2H, NH2, D2O exchangeable), 7.24 (t, 1H, J = 8.4 Hz, Ph CH-4), 7.45 (t, 2H, J = 8.4 Hz, Ph CH-3,5), 7.52 (d, J = 8.4 Hz, Ph CH-2,6), 7.68 (s, 1H, CH-9), 8.29 (s, 1H, CH-3); 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 15.62 (CH2CH3), 62.50 (CH2CH3), 87.27 (C-3a), 123.73 (Ph C-2,6), 124.07 (Ph C-4), 129.59 (Ph C-3,5), 129.99 (C-9), 137.97 (C-8), 138.38 (Ph C-1), 143.87 (C-3), 144.16 (C-10a), 145.15 (C-7), 145.54 (C-4), 147.44 (C-9a), 148.53 (C=O); EI-MS m/z (%): 350 (10.42) [M+●], 298 (25.0) [M+● – NH2-C≡N, – CH3, +5H], 293 (10.42) [M+● – NH2, C2H5O, + 4H], 269 (20.83) [M+● – NH2-C≡N, – C2H5O, + 6H], 211 (12.5) [M+● – NH2-C≡N, – C2H5, – CO2, – C2H, +1H], 198 (28.1) [M+● – NH2-C≡N, – C2H5, – CO2, – C3H], 184 (12.5) [M+● – NH2-C≡N, – C2H5, – CO2, – C3HN]. Anal. Calcd. for C17H14N6O3 (350.33): C, 58.28; H, 4.03; N, 23.99. Found: C, 58.46; H, 4.04; N, 24.07.
Ethyl 4,7-dioxo-1-phenyl-1,4,6,7-tetrahydropyrazolo[3′,4′:4,5]pyrimido[1,2-b]-pyridazine-8-carboxylate (15)
A mixture of 13 (0.383 g, 1.5 mmol) and diethyl malonate (0.32 g, 2.0 mmol) in sodium ethoxide solution (20 mg of sodium dissolved in 20 ml of absolute EtOH) was heated under reflux for 15 h. The reaction mixture was evaporated under reduced pressure. The resulting residue was dissolved in ice-cold water (10 ml) and acidified (pH 5–6) with acetic acid (10%). The precipitated product was filtered, washed with water, air dried, and crystallized from ethanol to give 15 as pale yellow crystals (0.44 g, 83.5%); mp: 172–173 °C; IR (KBr, ν, cm−1): 3187, 3066, 2936 (NH or OH associated), 1711 (C=O, ester), 1649, 1622 (2 x C=O, amide), 1596, 1498 (C=N, C=C), 1545 (δ NH), 1396, 1103 (C–N lactam), 1236, 1023 (C–O–C); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 1.40 (t, 3H, J = 6.9 Hz, CH2CH3), 4.07 (q. dist., 2H, CH2CH3), 7.35–7.51 (m, 5H, Ph CH), 7.65 (s, 1H, CH-9), 8.07 (s, 1H, CH-3), 9.60 (s, ½ H, amide NH, D2O exchangeable), 12.33 (s, ½ H, imidol OH, D2O exchangeable); EI-MS m/z (%): 351 [M+●] (absent), 337 (0.66) [M+● – CH3, + 1H], 327 (1.11) [M+● – COH, + 5H], 263 (0.27) [M+● – C2H5, – CO2, – OH, + 2H], 226 (2.34) [M+● – C2H5, – CO2, – OH, – C2N, + 3H], 198 (35.57) [M+● – C2H5, – CO2, – OH, – C2N, – C2H], 184 (47.39) [M+● – C2H5, – CO2, – OH, – C2N, – C2HN]. Anal. Calcd. for C17H13N5O4 (351.32): C, 58.12; H, 3.73; N, 19.93. Found: C, 57.94; H, 3.72; N, 19.87.
1,7-Diphenylpyrazolo[3′,4′:4,5]pyrimido[1,2-b]pyridazin-4(1H)-one (16)
A mixture of 13 (0.383 g, 1.5 mmol) and acetophenone (0.360 g, 3.0 mmol) in sodium ethoxide solution (20 mg of sodium dissolved in 20 ml of absolute EtOH) was heated under reflux for 8 h. After cooling, the reaction mixture was neutralized (pH 6–7) with acetic acid (10%), partially evaporated under reduced pressure, and poured onto crushed ice. The precipitated product was filtered, washed with water, air dried and crystallized from ethanol to yield 16 as a yellowish brown microcrystalline powder (0.37 g, 72.7%); mp: 118–119 °C; IR (KBr, ν, cm−1): 1662 (C=O), 1618, 1598 (C=N), 1532, 1499 (C=C), 1403, 1310 (C–N lactam); 1H NMR (500 MHz, CDCl3) δ (ppm): 7.35–7.55 (m, 10H, 2 × C6H5 CH), 7.76 (d, 1H, J = 6.9 Hz, CH-8), 8.03 (d, 1H, J = 6.9 Hz, CH-9), 8.16 (s, 1H, CH-3); 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 90.59 (C-3a), 121.80 (Ph C-2,6), 123.02 (Ph C-4), 126.96 (Ar’ C-3,5), 127.86 (Ar’ C-2,6), 128.47 (Ph C-3,5), 129.68 (C-9), 130.29 (Ar’ C-4), 131.50 (Ar’ C-1), 137.26 (Ph C-1), 137.56 (C-8), 138.17 (C-3), 138.47 (C-10a), 146.96 (C-7), 158.47 (C-4), 164.23 (C-9a); EI-MS m/z (%): 339 [M+●] (absent), 317 (8.46) [M+● – C2H2, + 4H], 226 (3.96) [M+● – C6H5, – C2HN, + 3H], 211 (1.54) [M+● – C6H5, – C3H2N, + H], 184 (69.08) [M+● – C6H5, – 2 × C2HN]. Anal. Calcd. for C20H13N5O (339.35): C, 70.79; H, 3.86; N, 20.64. Found: C, 70.58; H, 3.85; N, 20.57.
1-Phenyl-7,8,9,10-tetrahydropyrazolo[3′,4′:4,5]pyrimido[1,2-b]cinnolin-4(1H)-one (17)
A mixture of 13 (0.383 g, 1.5 mmol) and cyclohexanone (0.168 g, 2.0 mmol) in sodium ethoxide solution (20 mg of sodium dissolved in 20 ml of absolute EtOH) was heated under reflux for 4 h. After cooling, the reaction mixture was neutralized (pH 6–7) with acetic acid (10%), partially evaporated under reduced pressure, and poured onto crushed ice. The precipitated product was filtered, washed with water, air dried, and crystallized from ethanol to give 17 as a yellow powder (0.31 g, 65.1%); mp: 142–145 °C; IR (KBr, ν, cm−1): 1700 (C=O), 1598, 1500 (C=N, C=C), 1403, 1312 (C–N lactam); 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.09–2.21 (m, 4H, CH-8,9), 2.42–2.67 (m, 4H, CH-7,10), 7.34–7.52 (m, 5H, Ph CH), 7.67 (s. dist., 1H, CH-11), 8.00 (s, 1H, CH-3); EI-MS m/z (%): 317 [M+●] (absent), 313 (13.1) [M+● – 4H], 287 (8.6) [M+● – C2H4, – 2H], 255 (14.6) [M+● – C5H8, + 6H], 237 (16.6) [M+● – C4H8, – C≡N, + 2H], 226 (15.3) [M+● – C5H8, – C≡N, + 3H], 184 (22.8) [M+● – C5H8, – C≡N, – C2HN]. Anal. Calcd. for C18H15N5O (317.34): C, 68.13; H, 4.76; N, 22.07. Found: C, 68.27; H, 4.76; N, 22.16.
Physicochemical studies
Determination of lipophilicity as C log P
Lipophilicity was theoretically calculated as C log P values in an octanol–water-buffer by the ClogP program of Biobyte. [34].
Biological evaluation
Anti-inflammatory activity
The in vivo carrageenan-induced rat paw edema model of inflammation was used to evaluate the anti-inflammatory properties of the newly synthesized compounds according to the previously reported Winter et al. method [35].
Adult male albino rats weighing 100–120 g (obtained from the animal house of the department of Physiology, Faculty of Medicine, Alexandria University) were used throughout the work. Animals were randomly divided into groups of six rats each. They were kept in an animal house under standard conditions of light and temperature with free access to food and water ad libitum and allowed to be accustomed to their environment for two days before testing.
Indomethacin, used as a reference standard (Khahira for Pharmaceutical and Chemical Industry, Cairo, Egypt), and the test compounds were dissolved in DMSO and were injected intraperitoneally at a dose level of 10 mg/kg body weight. Control animals, on the other hand, were intraperitoneally injected with appropriate volume of DMSO only. After one hour of the above treatment, an inflammatory edema in the right hind paw of all animals was induced by subcutaneous injection of 0.05 ml of freshly prepared solution of 1% carrageenan (λ-Carrageenan Sigma–Aldrich Corp. St. Louis, MO, USA) in sterile normal saline (0.9%), into the subplantar tissue of the paw. An equal volume of saline was injected to the left hind paw and served as internal control for the degree of inflammation in the right hind paw.
Four hours after carrageenan injection, animals were decapitated and both the right and left hind paws were excised at a standard point and weighed. The difference in weight from edema between right and left paws was taken as a measure of the degree of inflammation for each animal. The mean increase in weight of the carrageenan-injected paw over the other paw in each group was measured and the percent increase of edema was calculated. The anti-inflammatory efficacy of the tested compounds was assessed by comparing the change in paw weight in the treated animals with that in control animals (treated with DMSO only) and expressed as a percent inhibition of the edema (percent protection against inflammation), calculated according to the following equation:
where Wt is the mean increase in paw weight in rats treated with the test compounds and Wc is the mean increase in paw weight in the control group. The anti-inflammatory activity of the test compounds relative to that of indomethacin was also determined (Table 2).
Gastric ulcerogenic effect
The ulcerogenic effect of the most active compounds 10b and 11a–f as well as indomethacin, used as the reference standard, was evaluated according to Meshali’s method [43]. Adult male albino rats weighing between 100 and 120 g were used. Animals were divided into groups of five animals each. Rats were fasted 20 h before drug administration. Water was given ad libitum. The control group received the vehicle (1% gum acacia) orally. Other groups received indomethacin or test compounds orally in a dose of 10 mg/kg body weight suspended in 1% gum acacia. Rats were fasted for 2 h, allowed to feed for 2 h, then fasted for another 20 h. Rats were given another two doses on the second and third days. On the fourth day, rats were sacrificed by diethyl ether; the stomach of each rat was removed, opened along the greater curvature, and rinsed with cold 0.9% saline. The stomach was stretched, by pins, on a corkboard and inspected with a 3x magnifying lens for any evidence of mucosal damage. The ulcer index was calculated according to Robert’s method [44]. The number of mucosal damage (red spots) was counted and their severity (ulcerogenic severity) was graded from 0 to 4 according to the following score assignment:
| Score | Score | ||
|---|---|---|---|
| Normal (No injury) | 0 | Slight injury | 3 |
| Latent small red spot | 1 | Severe injury | 4 |
| Wide red spot | 2 |
The following figures were calculated:
–– % Incidence/10 = [number of rats showing ulcer of any grade divided by total number of rats in the group × 100]/10.
–– Average number of ulcers: number of ulcers in the group/total number of rats in the group.
-
–– Average severity: Σ [each ulcer multiplied by its score of severity]/number of ulcers in the group.
Ulcer index = the sum of the 3 figures.
Results are tabulated in Table 2.
Statistics
In the anti-inflammatory study, data obtained were expressed as the mean ± standard error (SEM). Statistical significance was determined by comparing the values of the test compounds and the standard with those obtained from the control group of animals using One-way ANOVA with Dunnett’s post-test using the GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com). The difference in results was considered significant if P < 0.001. Results are presented in Table 1.
Conclusions
In the present study, various substituted pyrazolopyrimidines and pyridazine- as well as tetrahydrocinnoline-fused pyrazolopyrimidines were synthesized and screened for anti-inflammatory and ulcerogenic potential. From the preliminary anti-inflammatory screening results, it could be revealed that the pyrazolopyrimidine derivatives bearing the thiazolidinone (10b) and thiazoline (11a–f) moieties exhibited good anti-inflammatory activity while the rest of the tested compounds exhibited moderate activity. However, none of the newly synthesized compounds were found to be superior over the reference drug.
Taking the results of the ulcerogenicity study of the most active compounds (10b and 11a–f) into consideration, it could be claimed that compounds 11e and 11f presented a promising anti-inflammatory profile with high anti-edematous activity comparable to indomethacin. They caused the highest inhibition of carrageenan-induced paw edema, among the tested compounds with minimal ulcerogenic effects and a good safety margin. In addition, compounds 10b and 11a–d achieved the advantage of being much less ulcerogenic despite their slightly lower anti-inflammatory activity as compared to indomethacin. The structure and biological activity relationship of the most active compounds showed that the presence of an electron withdrawing bromo or chloro group at the para-position of the phenyl ring attached either to the 3-position of the thiazolidinone ring (10b) or to the 3 and 4-positions of the thiazoline ring (11a–f), as well as the presence of an imino linkage separating the rigid 5-membered heterocyclic ring structures from the 5-position of the pyrazolopyrimidine nucleus, seem to be responsible for good biological activity in comparison to compounds 8a and 8b lacking such a spacer. Furthermore, results showed that the better biological activity profile of the most active derivatives correlates with the lipophilicity theoretically calculated as the C log P values which might be attributed to better distribution and bioavailability of these derivatives.
Since the GI problems due to NSAIDs continue to be the major impediment to their use in therapeutics, the novel properties of the new anti-inflammatory derivatives prove them to be useful lead molecules for the development of better NSAIDs with a greatly improved therapeutic index.
However, further investigation is needed in order to gain insight into the mechanism of action of the examined compounds.
Acknowledgments
The author would like to thank Dr. C. Hansch and Biobyte Corp. 201, West 4th Street, Suite 204, Claremont, CA 91711, USA for free access to the C-QSAR program. Thanks is also extended to Prof. Essam El-Shamy, Department of Physiology, Faculty of Medicine, University of Alexandria, AR Egypt, for facilities, help, and support during the anti-inflammatory screening and the ulcerogenicity study.
Footnotes
This article is available from: http://dx.doi.org/10.3797/scipharm.1211-21
Author’s Statements
Competing Interests
The author declares no conflict of interest.
Animal Rights
All experiments involving animals were performed in accordance with international laws and policies for the Care and Use of Laboratory Animals and the applied protocols were approved by the Institutional Ethical Committee (Animal Care and Use Committee (ACUC), Faculty of Pharmacy, Alexandria University) (ACUC Project Number: 19).
References
- [1].Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. http://www.ncbi.nlm.nih.gov/pubmed/5284360. [DOI] [PubMed] [Google Scholar]
- [2].Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. http://www.ncbi.nlm.nih.gov/pubmed/9737710. [PubMed] [Google Scholar]
- [3].Peskar BM. On the synthesis of prostaglandins by human gastric mucosa and its modification by drugs. Biochem Biophys Acta. 1977;487:307–314. doi: 10.1016/0005-2760(77)90007-8. http://dx.doi.org/10.1016/0005-2760(77)90007-8. [DOI] [PubMed] [Google Scholar]
- [4].Allison MC, Howatson AG, Torrance CJ, Lee FD, Russel RI. Gastrointestinal damage associated with the use of nonsteroidal anti-inflammatory drugs. N Engl J Med. 1992;327:749–754. doi: 10.1056/NEJM199209103271101. http://dx.doi.org/10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- [5].Van JR, Botting RM. New insights into the mode of action of antiinflammatory drugs. Inflamm Res. 1992;44:1–10. doi: 10.1007/BF01630479. http://dx.doi.org/10.1007/BF01630479. [DOI] [PubMed] [Google Scholar]
- [6].Russo F, Guccione S, Romeo G, Scolaro LM, Pucci S, Caruso A, Cutuli V, Amico-Roxas M. Synthesis and pharmacological properties of pyrazolotriazolopyrimidine derivatives. Eur J Med Chem. 1992;27:73–80. http://dx.doi.org/10.1016/0223-5234(92)90064-8. [Google Scholar]
- [7].Shishoo CJ, Pathak US, Rathod IS, Jain KS, Nargund LG, Taranalli AD, Patel H, Shirsath VS. Synthesis and pharmacological evaluation of some novel 5-aryl-6-arylamino-1-phenylpyrazolo- [3,4-d]pyrimidin-4(5H) -ones as analgesic and antiinflammatory agents. Indian J Chem. 1999;38:684–695. [Google Scholar]
- [8].Devesa I, Alcaraz MJ, Riguera R, Ferrándiz ML. A new pyrazolo pyrimidine derivative inhibitor of cyclooxygenase-2 with anti-angiogenic activity. Eur J Pharmacol. 2004;488:225–230. doi: 10.1016/j.ejphar.2004.02.015. http://dx.doi.org/10.1016/j.ejphar.2004.02.015. [DOI] [PubMed] [Google Scholar]
- [9].Russo F, Guccione S, Romeo G, Barretta GU, Pucci S, Caruso A, Amico-Roxas M, Cutuli V. Pyrazolothiazolopyrimidine derivatives as a novel class of anti-inflammatory or antinociceptive agents: synthesis, structural characterization and pharmacological evaluation. Eur J Med Chem. 1993;28:363–376. http://dx.doi.org/10.1016/0223-5234(93)90123-V. [Google Scholar]
- [10].El-Kerdawy MM, El-Ashmawy MB, Shehata IA, Barghash AEM, El-Bendary ER, El-Kashef HA. Fused pyrimidines synthesis and anti-inflammatory testing of certain novel imidazo[1,2-c]quinazoline, pyrazolo[3,4-d]triazolo[3,4-b]pyrimidine and pyrimido[2,1-a]phthalazine derivatives. Saudi Pharm J. 1997;5:46–51. [Google Scholar]
- [11].Shaaban MA, Abou Sier AH, El-Ansary AK, Kadry HH, Abd El-Latif HA. Synthesis and pharmacological activities of certain pyrazolotriazolopyrimidine and pyrazolotriazepine derivatives. Bull Fac Pharm Cairo Univ. 2001;39:67–77. [Google Scholar]
- [12].Sondhi SM, Dinodia M, Jain S, Kumar A. Synthesis of biologically active novel bis Schiff bases, bis hydrazone and bis guanidine derivatives. Indian J Chem. 2009;48:1128–1136. [Google Scholar]
- [13].Keche AP, Hatnapure GD, Tale RH, Rodge AH, Birajdar SS, Kamble VM. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg Med Chem Lett. 2010;22:3445–3448. doi: 10.1016/j.bmcl.2012.03.092. http://dx.doi.org/10.1016/j.bmcl.2012.03.092. [DOI] [PubMed] [Google Scholar]
- [14].Ottanà R, Maccari R, Barreca ML, Bruno G, Rotondo A, Rossi A, Chiricosta G, Di Paola R, Sautebin L, Cuzzocrea S, Vigorita MG. 5-Arylidene-2-imino-4-thiazolidinones: Design and synthesis of novel anti-inflammatory agents. Bioorg Med Chem. 2005;13:4243–4252. doi: 10.1016/j.bmc.2005.04.058. http://dx.doi.org/10.1016/j.bmc.2005.04.058. [DOI] [PubMed] [Google Scholar]
- [15].Kumar A, Rajput CS. Synthesis and anti-inflammatory activity of newer quinazolin-4-one derivatives. Eur J Med Chem. 2009;44:83–90. doi: 10.1016/j.ejmech.2008.03.018. http://dx.doi.org/10.1016/j.ejmech.2008.03.018. [DOI] [PubMed] [Google Scholar]
- [16].Sondhi SM, Singh J, Kumar A, Jamal H, Gupta PP. Synthesis of amidine and amide derivatives and their evaluation for anti-inflammatory and analgesic activities. Eur J Med Chem. 2009;44:1010–1015. doi: 10.1016/j.ejmech.2008.06.029. http://dx.doi.org/10.1016/j.ejmech.2008.06.029. [DOI] [PubMed] [Google Scholar]
- [17].Bekhit AA, El-Sayed OA, Aboulmagd E, Park JY. Tetrazolo[1,5-a]quinoline as a potential promising new scaffold for the synthesis of novel anti-inflammatory and antibacterial agents. Eur J Med Chem. 2004;39:249–255. doi: 10.1016/j.ejmech.2003.12.005. http://dx.doi.org/10.1016/j.ejmech.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [18].Pillai AD, Rathod PD, Franklin PX, Patel M, Nivsarkar M, Vasu KK, Padh H, Sudarsanam V. Novel drug designing approach for dual inhibitors as anti-inflammatory agents: implication of pyridine template. Biochem Biophys Res Commun. 2003;301:183–187. doi: 10.1016/s0006-291x(02)02996-0. http://dx.doi.org/10.1016/S0006-291X(02)02996-0. [DOI] [PubMed] [Google Scholar]
- [19].Venkatachalam SR, Salaskar A, Chattopadhyay A, Barik A, Mishra B, Gangabhagirathic R, Priyadarsini KI. Synthesis, pulse radiolysis, and in vitro radioprotection studies of melatoninolipoamide, a novel conjugate of melatonin and a-lipoic acid. Bioorg Med Chem. 2006;14:6414–6419. doi: 10.1016/j.bmc.2006.05.042. http://dx.doi.org/10.1016/j.bmc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- [20].Abouzid KAM, Khalil NA, Ahmed EM, Abd El-Latif HA, El-Araby ME. Structure-based molecular design, synthesis, and in vivo anti-inflammatory activity of pyridazinone derivatives as nonclassic COX-2 inhibitors. Med Chem Res. 2010;19:629–642. http://dx.doi.org/10.1007/s00044-009-9218-4. [Google Scholar]
- [21].Refaat HM, Khalil OM, Kadry HH. Synthesis and anti-inflammatory activity of certain piperazinylthienyl pyridazine derivatives. Arch Pharm Res. 2007;30:803–811. doi: 10.1007/BF02978828. http://dx.doi.org/10.1007/BF02978828. [DOI] [PubMed] [Google Scholar]
- [22].Tonk RK, Bawa S, Chawla G, Deora GS, Kumar S, Rathore V, Mulakayala N, Rajaram A, Kalle AM, Afzal O. Synthesis and pharmacological evaluation of pyrazolo[4,3-c]cinnoline derivatives as potential anti-inflammatory and antibacterial agents. Eur J Med Chem. 2010;57:176–184. doi: 10.1016/j.ejmech.2012.08.045. http://dx.doi.org/10.1016/j.ejmech.2012.08.045. [DOI] [PubMed] [Google Scholar]
- [23].Bekhit AA, Ashour HMA, Abdel Ghany YS, Bekhit AEA, Baraka A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur J Med Chem. 2008;43:456–463. doi: 10.1016/j.ejmech.2007.03.030. http://dx.doi.org/10.1016/j.ejmech.2007.03.030. [DOI] [PubMed] [Google Scholar]
- [24].Schmidt P, Druey J. Heilmittelchemische untersuchungen in der heterocyclischen reihe. XIV. Pyrazolo[3,4-d]pyrimidine. Helv Chim Acta. 1956;39:986–991. http://dx.doi.org/10.1002/hlca.19560390345. [Google Scholar]
- [25].Peet NP, Lentz NL, Sunder S, Dudley MW, Ogden AML. Conformationally restrained, chiral (phenylisopropyl) amino-substituted pyrazolo [3,4-d]pyrimidines and purines with selectivity for adenosine A1 and A2 receptors. J Med Chem. 1992;35:3263–3269. doi: 10.1021/jm00095a024. http://dx.doi.org/10.1021/jm00095a024. [DOI] [PubMed] [Google Scholar]
- [26].Cuvigny T, Normant H. Synthèse de composés γ-éthyléniques à partir des magnésiens vinyliques. Bull Soc Chim France. 1961:2423–2433. [Google Scholar]
- [27].Pathak US, Gandhi S, Rathod IS. Synthesis of some pyrazolo[3,4-d]pyrimidines. Indian J Chem. 1994;33:734–737. [Google Scholar]
- [28].Perissin M, Favre M, Lau-Duc C, Huguet F, Gaultier C, Narcisse G. Synthesis and pharmacological activities of some substituted thienopyrimidine-4-ones. Eur J Med Chem. 1988;23:453–456. [Google Scholar]
- [29].Küçükgüzel ŞG, Oruç EE, Rollas S, Şahin F, Özbek A. Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4 oxadiazoles and some related compounds. Eur J Med Chem. 2002;37:197–206. doi: 10.1016/s0223-5234(01)01326-5. http://dx.doi.org/10.1016/S0223-5234(01)01326-5. [DOI] [PubMed] [Google Scholar]
- [30].Omar AMME, AboulWafa OM. Novel estradiol-17α-1,2,4-triazoline derivatives. Synthesis and in vitro anabolic-catabolic properties and binding affinity to steroid receptors. J Heterocycl Chem. 1984;21:1419–1423. http://dx.doi.org/10.1002/jhet.5570210539. [Google Scholar]
- [31].Srivastava VK, Satsangi RK, Shankar K, Kishor K. Some new 5-substituted benzylidene-1-phenyl-3-(2′-pyridinobutan-4′-yl)- thiobarbituric acids as psychopharmacological agents. Pharmazie. 1981;36:252–253. [PubMed] [Google Scholar]
- [32].Rathelot P, Vanelle P, Gasquet M, Delmas F, Crozet MP, Timon-David P, Maldonado J. Synthesis of novel functionalized 5-nitroisoquinolines and evaluation of in vitro antimalarial activity. Eur J Med Chem. 1995;30:503–508. http://dx.doi.org/10.1016/0223-5234(96)88261-4. [Google Scholar]
- [33].Perandones F, Soto JL. Synthesis of pyrido[2,3-d]pyrimidines from aminopyrimidinecarbaldehydes. J Heterocycl Chem. 1998;35:413–419. http://dx.doi.org/10.1002/jhet.5570350226. [Google Scholar]
- [34].C-QSAR program Biobyte Corp . 201 West 4th Street, Suite 204, Claremont, CA: [Google Scholar]
- [35].Winter CA, Risely EA, Nuss GM. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. http://www.ncbi.nlm.nih.gov/pubmed/14001233. [DOI] [PubMed] [Google Scholar]
- [36].Shen TY. Nonsteroidal antiinflammatory agents. In: Wolf ME, editor. Burger’s Medicinal Chemistry. New York: John Wiley and Sons; 1980. pp. 1217–1219. [Google Scholar]
- [37].Di Rosa M, Willoughby DA. Screens for anti-inflammatory drugs. J Pharm Pharmacol. 1971;23:297–298. doi: 10.1111/j.2042-7158.1971.tb08661.x. http://dx.doi.org/10.1111/j.2042-7158.1971.tb08661.x. [DOI] [PubMed] [Google Scholar]
- [38].Tsurumi K, Kyuki K, Niwa M, Kokuba S, Fujimura H. Pharmacological investigations of the new anti-inflammatory agent 2-(10,11-dihydro-10-oxodibenzo[b,f]thiepin-2-yl)propionic acid. Arzneimittelforschung. 1986;36:1796–1803. http://www.ncbi.nlm.nih.gov/pubmed/3566840. [PubMed] [Google Scholar]
- [39].Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol. 1996;303:217–220. doi: 10.1016/0014-2999(96)00140-9. http://dx.doi.org/10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- [40].Gavalas A, Kourounakis L, Litina D, Kourounakis P. Anti-inflammatory and immuno-modulating effects of the novel agent γ-(2-aminoethylamino)-2-butyrothienone. Inhibitory effects on mouse paw oedema. Arzneimittelforschung. 1991;41:423–426. http://www.ncbi.nlm.nih.gov/pubmed/1859517. [PubMed] [Google Scholar]
- [41].Winter CA. In: Nonsteroidal antiinflammatory drugs. Gattini S, Dukes MNG, editors. Amsterdam: Excepta Medica; 1965. p. 190. [Google Scholar]
- [42].Brooks PM, Day RO. Nonsteroidal antiinflammatory drugs-differences and Similarities. N Engl J Med. 1991;324:1716–1725. doi: 10.1056/NEJM199106133242407. http://dx.doi.org/10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- [43].Meshali M, Al-Sabbagh H, Foda A. Effect of encapsulation of flufenamic acid with acrylic resins on Its bioavailability and gastric ulcerogenic activity in rats. Acta Pharm Technol. 1983;29:217–219. [Google Scholar]
- [44].Robert A, Nezamis JE, Philip JP. Effect of prostaglandin E1 on gastric secretion and ulcer formation in the rat. Gastroenterology. 1968;55:481–487. http://www.ncbi.nlm.nih.gov/pubmed/5698627. [PubMed] [Google Scholar]