Abstract
Novel 11-substituted 3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]-thiazole-2,5,10-triones 4a–i were synthesized in 75–90% yields via the hetero-Diels-Alder reaction of 5-arylidene-4-thioxo-2-thiazolidinones with 1,4-naphthoquinone. The synthesized compounds were evaluated for their antineoplastic and antimycobacterial activities. A moderate selectivity against melanoma cancer cells (GI50 (UACC-257-melanoma) = 0.22 μM) was demonstrated for 4i, whereas derivatives 4a, 4c, 4g, and 4h showed promising antimycobacterial activity at a low toxicity level.
Keywords: hetero-Diels-Alder reaction; 4-Thioxo-2-thiazolidinones; Thiopyrano[2,3-d]thiazoles; Anticancer activity; Antimycobacterial activity; Melanoma
Introduction
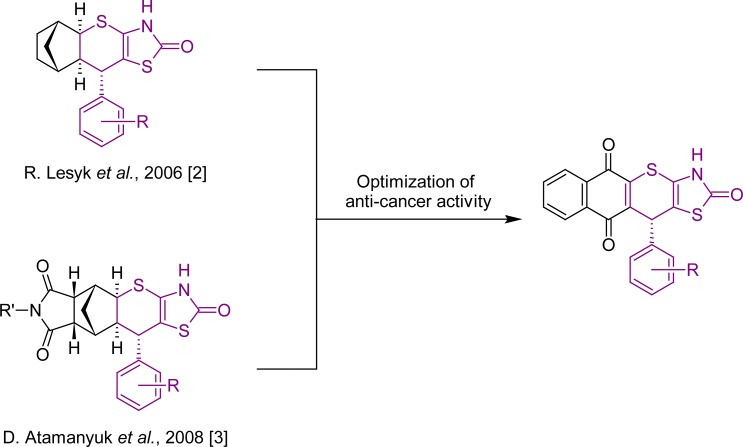
Drug resistance remains an important problem in the pharmacotherapy of cancer [1] with many medicinal chemists being involved in the search for new effective antitumor agents. The anticancer activity was shown in our earlier studies [2, 3] for norbornane-containing fused thiopyrano[2,3-d]thiazoles. Subsequently, we have decided to modify the structure of the latter compounds towards their planarization using the naphthoquinone scaffold (Sch. 1). The naphthoquinone fragment can be found both in the well-known anticancer drugs, such as doxorubicin, daunorubicin, mitoxantrone, and mitomycine C [4–6], and in the new promising antimycobacterial agents [7]. This article presents our findings on the anticancer and antimycobacterial activities of the synthesized compounds.
Sch. 1.
‘Rescaffolding’ of norbornane to the naphthoquinone moiety.
Results and Discussion
Chemistry
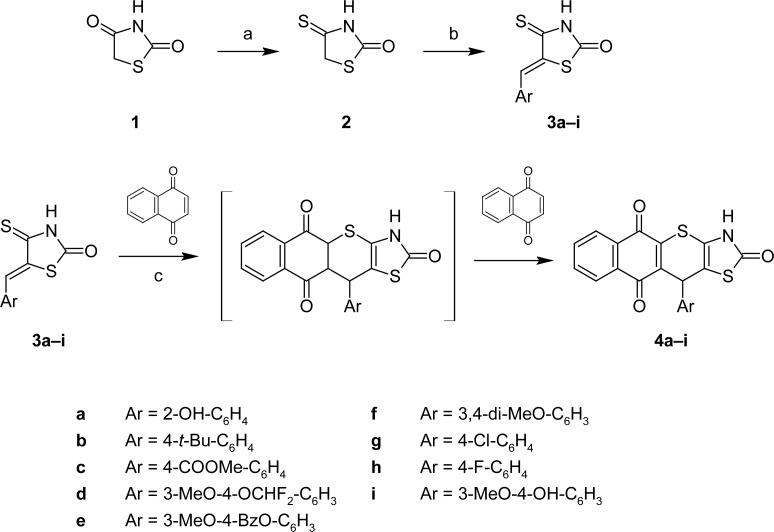
Naphthoquinones are known for their ability to participate in cycloaddition reactions due to their ring electron-deficiency. 5-Arylidene-4-thioxo-2-thiazolidinones (3a–i) were used as the heterodiene building blocks for the target compounds [2, 3, 8]. Intermediates 3a–i were prepared by the treatment of 4-thioxo-2-thiazolidinone (2) [9, 10] with appropriate aldehydes in glacial acetic acid in the presence of a catalytic amount of fused sodium acetate. The hetero-Diels-Alder reaction of 3a–i with 1,4-naphthoquinone yielded a series of novel 11-substituted 3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]thiazole-2,5,10-triones 4a–i (Sch. 2). The reaction conditions have been adapted from those described previously for the norbornane derivatives [3]. Apparently [4+2]-cycloaddition products undergo spontaneous oxidation (dehydrogenation) as a consequence of excess naphthoquinone.
Sch. 2.
Synthesis of 11-substituted benzo[6,7]thiochromeno[2,3-d]thiazole-2,5,10-triones via hetero-Diels-Alder reaction
(a) P2S5, dioxane, reflux, 5h; (b) ArCHO (1.1 eq.), AcONa (1 eq.), AcOH, 100°C; (c) 1,4-naphthoquinone (2 eq.), AcOH, hydroquinone (cat.), reflux, 1h.
The synthesized novel thiopyrano[2,3-d]thiazoles 4a–i were characterized by 1H and 13C NMR, LC-MS spectra, and elemental analyses (see Experimental section). Spontaneous in situ dehydrogenation was confirmed by the 1H NMR spectra containing a singlet peak of the 11H-proton. The latter was highly displaced in the weak magnetic field (5.40–5.75 ppm) because of the neighboring carbonyl group. This signal shift can be increased even more with an ortho-OH-substituted aryl substituent (compound 4a), and is affected most probably by the intra-molecular hydrogen bonding. The signals of naphthoquinone moiety protons and aryl substituents in position 11 were within 6.63–8.09 ppm.
Pharmacology
Anticancer activity
Synthesized fused thiopyrano[2,3-d]thiazol-2-one derivatives (4e, 4f, 4i) were evaluated for their antitumor activity (cytotoxicity) according to the US NCI protocol [11–15].
Compounds 4f and 4i were tested initially at a single concentration of 10−5 M against a full panel of 60 cancer cell lines derived from leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate, and breast cancer (Tab. 1).
Tab. 1.
Anticancer screening at a single concentration of 10−5 M against 60 cancer cell lines
| Cpd. | Mean growth % | Interval of growth % | The most sensitive cell lines | Growth % of the most sensitive cell line | Active (selected for 5-dose 60 cell lines assay) |
|---|---|---|---|---|---|
| 4f | 1.77 | −96.40 to 85.42 | SK-MEL-5 (melanoma) | −96.40 | Y |
| M14 (melanoma) | −80.36 | ||||
| MALME-3M (melanoma) | −66.70 | ||||
| 4i | 17.75 | −74.12 to 77.31 | SK-MEL-25 (melanoma) | −74.12 | Y |
| SF-295 (CNS cancer) | −50.25 | ||||
| MDA-MB-435 (breast cancer) | −48.22 |
Compounds 4f and 4i showed a considerable level of activity in the primary test and were chosen for advanced assays against the full panel (approx. 60 cell lines) at five 10-fold dilutions (100 μM, 10 μM, 1 μM, 0.1 μM, and 0.01 μM). Compound 4e was tested in the latter assays without primary pre-screening.
The full panel of individual GI50 values (μM) for each cell line is presented in Table 2 and the results of five concentrations’ screenings are summarized in Table 3. Selectivity analysis highlighted melanoma cell lines as the most sensitive targets for compounds 4f and 4i (GI50 (μM) = 1.26; 0.22 respectively). Compound 4e possessed a considerable activity level, however, the distinctive selectivity of cytotoxicity towards cancer cell lines was not observed. The most potent compound 4i showed high cytotoxic activity (GI50 < 1μM) against the following cancer cell lines: A549/ATCC and NCI-H23 (non-small cell lung cancer, GI50 (μM) = 0.65 and 0.41 respectively); SNB-75 (CNS cancer, GI50 (μM) = 0.67); LOX IMVI, SK-MEL-2, SK-MEL-5, and UACC-257 (melanoma, GI50 (μM) = 0.25, 0.98, 0.48, and 0.22 respectively); OVCAR-3 (ovarian cancer, GI50 (μM) = 0.57); UO-31 (renal cancer, GI50 (μM) = 0.57); DU-145 (prostate cancer, GI50 (μM) = 0.93); HS 578T and BT-549 (breast cancer, GI50 (μM) = 0.65 and 0.92 respectively).
Tab. 2.
Growth inhibitory concentration (GI50, μM) of compounds 4e, 4f, and 4i by cell linesa.
| Disease | 4e | 4f | 4id | Disease | 4e | 4f | 4id |
|---|---|---|---|---|---|---|---|
| Leukemia | CNS cancer | ||||||
| CCRF-CEM | 23.40 | 4.38 | 3.19 (1.84) | SF-268 | 14.10 | 4.89 | 2.53 (3.30) |
| HL-60(TB) | 15.20 | 1.98 | 2.01 (1.26) | SF-295 | 5.78 | 3.77 | 1.71 (1.14) |
| K-562 | 19.40 | 4.97 | NAc (1.98) | SF-539 | 16.70 | 3.17 | 3.26 (3.46) |
| MOLT-4 | 17.30 | 3.88 | 2.77 (3.67) | SNB-19 | 11.30 | 4.49 | 2.41 (2.85) |
| RPMI-8226 | 14.50 | 4.64 | 1.93 (1.33) | SNB-75 | 7.76 | 6.64 | 1.26 (0.67) |
| SR | 13.80 | 3.96 | 41.40 (1.96) | U251 | 15.10 | 4.27 | 1.63 (2.21) |
|
| |||||||
| NSCL cancer | Prostate cancer | ||||||
| A549/ATCC | 3.16 | 2.35 | 1.34 (0.65) | PC-3 | 17.70 | 5.41 | 1.98 (2.57) |
| EKVX | 16.30 | 4.97 | 3.24 (4.03) | DU-145 | 4.19 | 1.68 | 0.51 (0.93) |
|
|
|||||||
| HOP-62 | 14.90 | 5.87 | 0.40 (3.60) | Ovarian cancer | |||
| HOP-92 | 11.50 | 5.26 | 1.44 (2.15) | IGROV-1 | 15.40 | 3.35 | 2.48 (NT) |
| NCI-H226 | 19.20 | 5.43 | 1.74 (2.85) | OVCAR-3 | 11.20 | 3.01 | 1.03 (0.57) |
| NCI-H23 | 3.99 | 1.68 | 0.95 (0.41) | OVCAR-4 | 4.48 | 2.48 | 2.19 (1.33) |
| NCI-H322M | 10.90 | 3.78 | 2.05 (1.86) | OVCAR-5 | 32.10 | 17.70 | 10.40 (4.49) |
| NCI-H460 | 5.39 | 2.18 | 1.92 (1.66) | OVCAR-8 | 13.90 | 4.02 | 2.85 (2.05) |
| NCI-H522 | 15.90 | 7.91 | 1.96 (2.12) | SK-OV-3 | 31.70 | 13.60 | 8.28 (5.72) |
|
| |||||||
| Melanoma | Renal cancer | ||||||
| LOX IMVI | 8.03 | 1.26 | 0.16 (0.25) | 786-0 | 32.60 | 15.20 | 2.60 (5.20) |
| MALME-3M | 7.12 | 2.12 | 0.72 (1.05) | A498 | 20.50 | 4.91 | 1.95 (2.11) |
| M14 | 6.90 | 2.67 | 1.25 (1.22) | ACHN | 17.80 | 9.24 | 2.95 (1.66) |
| SK-MEL-2 | 20.30 | 4.19 | 2.21 (0.98) | CAKI-1 | 17.40 | 11.20 | 1.50 (2.92) |
| SK-MEL-28 | 12.20 | 6.22 | 1.79 (1.41) | RXF-393 | 6.98 | 2.27 | 2.52 (NT) |
| SK-MEL-5 | 2.62 | 1.52 | 0.96 (0.48) | SN12C | 15.30 | 3.68 | NT (3.02) |
| UACC-257 | 11.30 | 6.13 | 1.31 (0.22) | TK-10 | 29.70 | 17.60 | 5.74 (2.16) |
| UACC-62 | 12.50 | 2.21 | 0.43 (1.59) | UO-31 | 5.62 | 10.80 | 3.33 (0.57) |
|
| |||||||
| Breast cancer | Colon cancer | ||||||
| MCF-7 | 12.90 | 2.25 | 1.07 (2.26) | COLO 205 | 10.30 | 3.37 | 2.08 (1.68) |
| NCI/ADR-RES | 22.70 | 7.07 | 8.39 (4.50) | HCC-2998 | 16.60 | NTb | 3.77 (3.24) |
| HS 578T | 4.52 | 3.71 | 0.27 (0.65) | HCT-116 | 15.60 | 3.47 | 3.36 (2.46) |
| MDA-MB-435 | 12.00 | 2.19 | 1.38 (1.48) | HCT-15 | 11.50 | 3.51 | 2.07 (1.74) |
| BT-549 | 13.40 | 2.84 | 2.74 (0.92) | HT29 | 11.20 | 3.15 | 2.35 (2.02) |
| T-47D | 16.10 | 3.42 | 2.56 (2.56) | KM12 | 16.90 | 5.99 | 2.25 (2.51) |
| MDA-MB-468 | NT | NT | NT (1.75) | SW-620 | 22.70 | 3.96 | 2.82 (3.23) |
| MDA-MB-231/ATCC | 7.81 | 3.13 | 0.86 (1.98) | ||||
Data obtained from NCI’s in vitro disease-oriented human tumor cell screen;
NT, Not Tested;
NA, not active; GI50 >100 μM;
In parentheses the data of repeated testing.
Tab. 3.
Summary of the dose-dependent assay on 60 cancer cell lines
| GI50, μM | TGI, μM | LC50, μM | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Range | Range | Range | The most sensitive cell line | GI50/TGI of the most sensitive cell line | |||||
| Cpd. | Na | (min – max) | MG_MID | (min – max) | MG_MID | (min – max) | MG_MID | ||
| 4e | 60 | 2.62 to 32.60 | 12 | 6.10 to 80.20 | 40.74 | 26.30 to 98.70 | 85.11 | SK-MEL-5 /melanoma/ | 2.62 / 6.90 |
|
| |||||||||
| 4f | 58 | 1.26 to 17.70 | 4.19 | 2.85 to 76.30 | 14.79 | 5.34 to 93.00 | 47.86 | SK-MEL-5 /melanoma/ | 1.52 / 2.85 |
|
| |||||||||
| 4ib | 58 | 0.16 to 41.40 | 1.95 | 1.09 to 50.40 | 10.96 | 4.14 to 92.40 | 42.66 | HS 578T /breast cancer/ | 0.27 / 1.09 |
| (0.22 to 5.72) | (1.7) | (1.51 to 47.10) | (8.51) | (4.93 to 86.60) | (38.02) | UACC-257 /melanoma/ | (0.22 / 1.82) | ||
N, Number of human tumor cell lines;
The results of repeated testing are in parenthesis.
The significant differences in the antineoplastic activity values of structurally similar thiopyrano[2,3-d]thiazol-2-ones 4e, 4f, and 4i encouraged us to investigate their molecular mechanisms of action. For this purpose, we have used an accessible online tool – the NCI COMPARE analysis [16].
The COMPARE analysis evaluates the similarity of the compounds’ cytotoxicity patterns with those of known anticancer standard agents and NCI synthetic compounds present in public databases [17–19]. The COMPARE analysis revealed moderate correlations at the GI50 level of the 4f pattern with pancratistatin (Pearson correlation coefficient, PCC = 0.603), didemnin B (PCC = 0.525), S-trityl-L-cysteine (PCC = 0.473), and the compound 4i pattern with trimethyltrimethylolmelamine (PCC = 0.473). The highest obtained correlation indicated certain similarity of 4f with the pro-apoptotic product, pancratistatin, which selectively influenced cancer cells. This substance is a natural compound initially extracted from Spider Lily. According to the literature data [20], the anticancer activity of pancratistatin is realized via FAS (fatty acid synthase) receptor inhibition, which launches caspase-3-mediated apoptosis. Based on the COMPARE analysis data, one could suggest that 4f could have a similar mechanism of anticancer activity to that of pancratistatin.
Additionally, it was observed that 4-OH group alkylation of 11-aryl fragment leads to the decrease in antineoplastic activity. This point can be explained by the formation of a hydrogen bond (HB) between the 4-OH group of compound 4i and some hydrogen acceptor of the biotarget. This HB could cause a 10-fold increase in GI50 in comparison with 4e and 4f.
Evaluation of antimycobacterial activity
Synthesized compounds 4a–i were evaluated in vitro for antimycobacterial activity in collaboration with the Tuberculosis Antimicrobial Acquisition and Coordinating Facility [21] using the BACTEC 460 radiometric system at a concentration 6.25 μg/mL [22–24]. The assays [25] were performed on the Mycobacterium tuberculosis H37Rv strain (ATCC 27294) with the determination of inhibition percentage and evaluation of MICs (Tab. 4).
Tab. 4.
Pre-screening of the antimycobacterial activity and acute in vivo toxicity in mice.
| Cpd. | % inhibition (at 6.25 μg/mL) | Estimation of MIC90 | LD50ip (mg/kg) |
|---|---|---|---|
| 4a | 100 | <6.25 | 800 |
| 4b | 61 | >6.25 | 650 |
| 4c | 91 | <6.25 | 180 |
| 4d | 65 | >6.25 | 710 |
| 4e | 15 | >6.25 | >1000 |
| 4f | 0 | >6.25 | 410 |
| 4g | 92 | <6.25 | >1000 |
| 4h | 96 | <6.25 | 750 |
| 4i | ND | ND | 280 |
Pre-screening allowed the identification of hits with promising antimycobacterial effects. At least 90% inhibition at 6.25 μg/mL concentration was observed for compounds 4a, 4c, 4g, and 4h. Consequently, 4a, 4g, and 4h were retested against M. tuberculosis H37Rv in a two-fold dilution from 100.00 to 0.19 μg/mL to determine the minimum inhibitory concentration (MIC) in the Microplate Alamar Blue Assay (MABA) [26]. The MIC was defined as the lowest concentration inhibiting 99% of the inoculum. Rifampin (Sigma Chemical Compound, St. Louis, MO) or isoniazid was included as a positive drug control. Compounds were tested for cytotoxicity (IC50) in VERO cells in concentrations less than or equal to 10 times the MIC. After 72 h exposure, viability was assessed on the basis of cellular conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) into a formazan product using the Promega CellTiter 96 Non-radioactive Cell Proliferation Assay [21] (Tab. 5).
Tab. 5.
MIC90 (M. tuberculosis H37Rv) and IC50 (cytotoxicity) evaluation
| Cpd. | MIC90, μg/mL | IC50, μg/mL |
|---|---|---|
| Isoniazid | 0.044 | >6 |
| Rifampin | 0.075 | >6 |
| 4a | 0.68 | 0.44 |
| 4g | 2.59 | 1.58 |
| 4h | 2.65 | 1.47 |
According to the data, compounds 4a, 4g, and 4h showed up with low MIC90 values between 0.6 and 2.7 μg/mL. However, the low ratio between their MIC90 and cytotoxicity (IC50) indicates a possible association of antimycobacterial activity with the toxicity on the mammal cells. That is why further evaluation of the acute toxicity of in vivo investigated compounds was essential to clarify their toxicological profiles.
Evaluation of acute toxicity in vivo
The synthesized compounds were evaluated for their approximate LD50 in male mice [27, 28]. The mice were kept under a constant temperature and humidity in sterile cages with water and food. The stock solutions of the compounds used in this study were prepared immediately before usage and injected intraperitoneally (ip). The LD50 values (Tab. 3) were calculated using the method described by Litchfield and Wilcoxon [27]. The results (Tab. 3) indicated that most of the tested compounds were non-toxic and well-tolerated by the experimental animals as demonstrated by their LD50 values (>500 mg/kg).
Conclusions
It was demonstrated that [4+2]-cycloaddition adducts of 5-arylidene-4-thioxo-2-thiazolidinones and 1,4-naphthoquinone undergo spontaneous in situ oxidation (dehydrogenation) due to the excess amount of 1,4-naphthoquinone. In this way, the novel 3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]thiazole-2,5,10-triones were obtained. The most potent compound, 4i, showed a high level of antineoplastic activity and moderate selectivity towards melanoma cells. The antimycobacterial activity evaluation allowed the identification of several hits with low MIC90 values and acceptable in vivo acute toxicity. The obtained results may be used for further optimization of thiopyrano[2,3-d]thiazole activity profiles. Such an optimization could be directed by in-depth studies of mammal toxicity, and pro-apoptotic and FAS-inhibiting properties to improve both the potency and safety of the studied compound series.
Experimental
Chemistry
All materials were purchased from Merck, Sigma-Aldrich, or Lancaster and were used without purification. Melting points are uncorrected and were measured in open capillary tubes on the Buchi B-545 melting point apparatus. The 1H-NMR spectra were recorded on the Varian Gemini 300 MHz, and the 13C NMR spectra on the Varian Gemini 100Hz in a DMSO-d6 or DMSO-d6+CCl4 mixture using tetramethylsilane (TMS) as an internal standard (chemical shift values are reported in ppm units, coupling constants (J) are in Hz). Abbreviations are as follows: s – singlet; d – doublet; dd – double doublet; t – triplet; m – multiplet; br – broad. The elemental analyses (C, H, and N) were performed by the Perkin-Elmer 2400 CHN analyzer and were within ±0.4% of the theoretical values. Mass spectra were obtained on the Varian1200L instrument, and LC-MS spectra on the Finnigan MAT INCOS-50. The mass spectra (ESI-MS) of the compounds showed (M−H) peaks, which is in agreement with their molecular weights.
General procedure for the preparation of 11-aryl-3,5,10,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d]thiazole-2,5,10-triones (4a–i)
A mixture of appropriate 5-arylidene-4-thioxo-2-thiazolidinone (5 mmol) and 1,4-naphthoquinone (10 mmol) was refluxed for 1 h with a catalytic amount of hydroquinone (2–3 mg) to prevent the polymerization processes in 15 ml of glacial acetic acid and was left overnight at room temperature. The precipitated crystals were filtered off, washed with methanol (5–10 ml), and recrystallized with acetic acid or DMF (10–15 ml). Substances 4a–i were isolated as dark-brown or light-brown powders, and soluble on heating in DMF and acetic acid.
11-(2-Hydroxyphenyl)-3, 11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]thiazole-2,5,10-trione (4a)
Yield 92%, mp > 240°C. 1H NMR (DMSO-d6) δ: 5.75 (s, 1H, ArCH), 6.65 (t, 1H, J = 7.6 Hz, arom.), 6.82 (d, 1H, J = 8.1 Hz, arom.), 7.03 (t, 1H, J = 7.6 Hz, arom.), 7.10 (d, 1H, J = 7.6 Hz) (4H, arom.); 7.86 (m, 2H, arom.), 7.91 (m, 1H, arom.), 8.06 (d, J = 3.1 Hz, arom.), 9.96 (s, 1H, OH), 11.75 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 34.18, 108.64, 115.37, 115.93, 120.26, 127.09, 127.40, 128.60, 129.23, 129.69, 131.80, 132.05, 134.83, 135.79, 136.16, 144.78, 154.27, 171.69, 180.38. LC-MS: m/z 392.0 (M-1). Anal. Calcd. for C20H11NO4S2 (393.44): C, 61.06; H, 2.82; N, 3.56. Found: C, 61.30; H, 2.95; N, 3.45.
11-(4-tert-Butylphenyl)-3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]thiazole-2,5,10-trione (4b)
Yield 82%, mp 228–230°C. 1H NMR (DMSO-d6) δ: 1.21 (s, 9H, C(CH3)3), 5.49 (s, 1H, ArCH), 7.27 (d, J = 8.4 Hz, 2H, arom.), 7.32 (d, J = 8.4 Hz, 2H, arom.), 7.86 (m, 2H, arom.), 7.96 (m, 1H, arom.), 8.04 (m, 1H, arom.), 11.93 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 31.52, 31.59, 34.74, 108.91, 116.37, 126.41, 127.11, 127.48, 128.01, 131.68, 131.92, 134.90, 135.81, 136.29, 139.84, 143.19, 150.63, 171.42, 180.51, 181.42. LC-MS: m/z 432.4 (M-1). Anal. Calcd. for C24H19NO3S2 (433.55): C, 66.49; H, 4.42; N, 3.23. Found: C, 66.40; H, 4.50; N, 3.05.
Methyl 4-(2,5,10-trioxo-3,5,10,11-tetrahydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]thiazol-11-yl)benzoate (4c)
Yield 80%, mp 217–219°C. 1H NMR (DMSO-d6) δ: 3.80 (s, 3H, COOCH3), 5.59 (s, 1H, ArCH), 7.51 (d, J = 8.3 Hz, 2H, arom.), 7.83 (m, 2H, arom.), 7.87 (d, J = 8.3 Hz, 2H, arom.), 7.91 (m, 1H, arom.), 8.02 (m, 1H, arom.), 11.97 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 31.30, 36.34, 52.94, 108.11, 116.73, 127.10, 127.44, 128.85, 129.53, 130.52, 131.91, 134.95, 135.52, 135.86, 143.97, 148.04, 163.13, 166.72, 171.38, 181.37. LC-MS: m/z 434.0 (M-1). Anal. Calcd. for C22H13NO5S2 (435.48): C, 60.68; H, 3.01; N, 3.22. Found: C, 60.55; H, 3.20; N, 3.05.
11-[4-(Difluoromethoxy)-3-methoxyphenyl]-3,11-dihydro-2H-benzo[6,7]thio-chromeno[2,3-d][1,3]thiazole-2,5,10-trione (4d)
Yield 87%, mp 223–225°C. 1H NMR (DMSO-d6) δ: 3.79 (s, 1H, OCHF2), 3.81 (s, 3H, OCH3), 5.48 (s, 1H, ArCH), 6.93 (d, J = 8.3 Hz, 1H, arom.), 7.08 (d, J = 8.3 Hz, 1H, arom.), 7.17 (s, 1H, arom.), 7.86 (m, 2H, arom.), 7.96 (m, 1H, arom.), 8.05 (m, 1H, arom.), 11.93 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 56.53, 108.68, 113.30, 116.46, 117.39, 120.45, 122.20, 127.08, 127.46, 128.36, 129.24, 131.79, 131.97, 134.89, 135.80, 139.53, 141.57, 143.65, 151.48, 171.47, 180.54, 181.41. LC-MS: m/z 472.0 (M-1). Anal. Calcd. for C22H13F2NO5S2 (473.47): C, 55.81; H, 2.77; N, 2.96. Found: C, 55.95; H, 2.85; N, 2.80.
11-[4-(Benzyloxy)-3-methoxyphenyl]-3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d]-[1,3]thiazole-2,5,10-trione (4e)
Yield 76%, mp >240°C. 1H NMR (DMSO-d6) δ: 3.74 (s, 3H, OCH3), 4.99 (s, 2H, CH2), 5.43 (s, 1H, ArCH), 6.82 (d, J = 8.4 Hz, 1H, arom.), 6.92 (d, J = 8.4 Hz, 1H, arom.), 6.97 (s, 1H, arom.), 7.29 (t, J = 7.2 Hz, 1H, arom.), 7.36 (m, 4H, arom.), 7.82 (m, 2H, arom.), 7.93 (m, 1H, arom.), 8.01 (m, 1H, arom.), 11.90 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 56.18, 70.45, 109.14, 112.38, 114.25, 116.19, 120.39, 127.41, 128.46, 128.56, 128.92, 129.14, 131.65, 131.93, 134.81, 135.79, 136.17, 137.80, 142.88, 148.05, 149.88, 171.49, 180.54, 181.43. LC-MS: m/z 512.0 (M-1). Anal. Calcd. for C28H19NO5S2 (513.59): C, 65.48; H, 3.73; N, 2.73. Found: C, 65.60; H, 3.90; N, 2.60.
11-(3,4-Dimethoxyphenyl)-3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]thiazole-2,5,10-trione (4f)
Yield 80%, mp >240°C. 1H NMR (DMSO-d6) δ: 3.72 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 5.40 (s, 1H, ArCH), 6.73 (d, J = 8.0 Hz, 1H, arom.), 6.81 (d, J = 8.0 Hz, 1H, arom.), 6.85 (s, 1H, arom.), 7.77 (m, 2H, arom.), 8.03 (d, J = 8.2 Hz, 1H, arom.), 8.07 (d, J = 8.2 Hz, 1H, arom.), 11.72 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 56.03, 109.19, 112.07, 112.69, 116.13, 120.43, 127.04, 127.42, 131.66, 131.96, 134.84, 135.44, 135.79, 136.23, 142.80, 148.98, 149.57, 171.49, 180.55, 181.46. LC-MS: m/z 436.0 (M-1). Anal. Calcd. for C22H15NO5S2 (437.50): C, 60.40; H, 3.46; N, 3.20. Found: C, 60.35; H, 3.50; N, 3.10.
11-(4-Chlorophenyl)-3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]thiazole-2,5,10-trione (4g)
Yield 82%, mp 223–225°C. 1H NMR (DMSO-d6) δ: 5.48 (s, 1H, ArCH), 7.24 (d, J = 8.5 Hz, 2H, arom.), 7.31 (d, J = 8.5 Hz, 2H, arom.), 7.80 (m, 2H, arom.), 7.98 (d, J = 7.7 Hz, 2H, arom.), 8.03 (d, J = 7.7 Hz, 2H, arom.), 11.81 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 108.37, 116.56, 126.99, 127.35, 129.47, 130.25, 131.62, 131.82, 132.83, 134.81, 135.57, 135.72, 141.78, 143.52, 171.23, 180.27, 181.16. LC-MS: m/z 410.0, 412.0 (M-1, Cl). Anal. Calcd. for C20H10ClNO3S2 (411.89): C, 58.32; H, 2.45; N, 3.40. Found: C, 58.20; H, 2.70; N, 3.30.
11-(4-Fluorophenyl)-3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d][1,3]thiazole-2,5,10-trione (4h)
Yield 75%, mp >250°C. 1H NMR (DMSO-d6) δ: 5.50 (s, 1H, ArCH), 7.00 (t, J = 8.5 Hz, 2H, arom.), 7.35 (dd, J = 8.5 Hz, 2H, arom.), 7.81 (m, 2H, arom.), 7.99 (d, J = 7.2 Hz, 2H, arom.), 8.05 (d, J = 7.2 Hz, 2H, arom.), 11.81 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 108.74, 116.23, 116.46, 127.08, 127.44, 130.42, 130.50, 131.72, 131.93, 134.91, 135.83, 135.94, 139.20, 143.44, 171.43, 180.50, 181.40. LC-MS: m/z 394.0 (M-1). Anal. Calcd. for C20H10FNO3S2 (395.43): C, 60.75; H, 2.55; N, 3.54. Found: C, 60.80; H, 2.70; N, 3.50.
11-(4-Hydroxy-3-methoxyphenyl)-3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d]-[1,3]thiazole-2,5,10-trione (4i)
Yield 63%, mp 208–210°C. 1H NMR (DMSO-d6) δ: 3.72 (s, 3H, OCH3), 5.37 (s, 1H, ArCH), 6.73 (d, J = 8.0 Hz, 1H, arom.), 6.81 (d, J = 8.0 Hz, 1H, arom.), 6.85 (s, 1H, arom.), 7.84 (m, 2H, arom.), 7.94 (d, J = 5.8 Hz, 1H, arom.), 8.01 (d, J = 5.8 Hz, 1H, arom.), 9.00 (s, 1H, OH), 11.88 (s, 1H, NH). 13C NMR (DMSO-d6) δ: 56.29, 109.34, 112.47, 116.34, 117.04, 120.68, 126.94, 127.33, 131.87, 133.86, 134.71, 135.68, 136.28, 142.46, 146.77, 148.77, 171.32, 180.36, 181.29. LC-MS: m/z 422.0 (M-1). Anal. Calcd. for C21H13NO5S2 (423.47): C, 59.56; H, 3.09; N, 3.31. Found: C, 59.40; H, 2.95; N, 3.40.
Biological screening
In vitro anticancer screening
In vitro anticancer screening assays were performed on cancer cell lines derived from leukemia (CCRF-CEM, HL-60(TB), K-562, MOLT-4, RPMI-8226), non-small cell lung cancer (A549/ATCC, EKVX, HOP-62, HOP-92, NCI-H226, NCI-H23, NCI-H322M, NCI-H460, NCI-H522), colon cancer (COLO 205, HCT-116, HCT-15, HT29, KM12, SW-620), CNS cancer (SF-268, SF-295, SF-539, SNB-19, SNB-75, U251), melanoma (LOX IMVI, MALME-3M, M14, SK-MEL-2, SK-MEL-28, SK-MEL-5, UACC-257, UACC-62), ovarian cancer (IGROV-1, OVCAR-3, OVCAR-4, OVCAR-5, SK-OV-3), renal cancer (786-0, A498, ACHN, CAKI-1, RXF-393, SN12C, TK-10, UO-31), prostate cancer (PC-3, DU-145), and breast cancer (MCF-7, NCI/ADR-RES, MDA-MB-231/ATCC, HS 578T, MDA-MB-435, BT-549, T-47D) according to the NCI procedure. The screening was performed as a two-stage process, beginning with the evaluation of all compounds against the 60 cell lines at a single dose of 10 μM. The compounds that demonstrated significant growth inhibition were evaluated on the 60 cell panel at five concentrations.
Human tumor cell lines of the cancer screening panel were grown in RPMI 1640 medium containing 5% fetal bovine serum and 2 mM L-glutamine. For the typical screening experiment, cells were inoculated into 96 well microtiter plates in 100 μL of media. After 24 h, two plates of each cell line were fixed in situ with TCA, to represent a measurement of the cell population at the time of drug addition. Experimental drugs were solubilized in dimethyl sulfoxide at a 400-fold final test concentration and stored frozen. At the time of drug addition, an aliquot of frozen concentrate was thawed and diluted to two-fold of the desired test concentration with complete medium supplemented with 50 μg/ml gentamicin. Additional four, 10-fold, or ½ log serial dilutions were made to provide a total of five drug concentrations plus the control. Three dose-response parameters were calculated for each experimental agent. GI50 represents the drug concentration resulting in a 50% reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation. TGI represents the drug concentration resulting in total growth inhibition. The LC50, the concentration of the drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning, indicates a net loss of cells following treatment.
Antimycobacterial screening
In vitro evaluation of antimycobacterial activity against Mycobacterium tuberculosis H37Rv
The primary screen was conducted at 6.25 μg/mL (or molar equivalent of the highest molecular weight compound in a series of congeners) against M. tuberculosis H37Rv (ATCC 27294) in BACTEC 12B medium using the Microplate Alamar Blue Assay (MABA) [21]. Compounds exhibiting fluorescence were tested in the BACTEC 460-radiometric system [23]. Compounds effecting <90% inhibition in the primary screen (MIC > 6.25 μg/mL) were not evaluated further.
BACTEC radiometric method of suspectibility testing
The inocula for suspectibility testing were either from a positive BACTEC isolation vial with a growth index (GI) of 500 and more or from the suspension of organisms isolated earlier on the conventional medium. The culture was well-mixed and 0.1 mL positive BACTEC culture was added to each of the vials containing the test drugs. The drug vials were supplemented by rifampicin (0.25 μg/mL). A control vial was inoculated with a 1:100 microdilution of the culture. A suspension equivalent to the McFarland no.1 standard was prepared in the same manner as a BACTEC positive vial when growth from a solid medium was used. Each vial was tested immediately with BACTEC to provide CO2 in the headspace. The vials were incubated at 37ºC and tested daily with a BACTEC instrument. When the GI in the control read was at least 30, the increase in GI (ΔGI) from the previous day in the control was compared with that in the drug vial. The following formula was used to interpret the results:
If a clear suspectibility pattern (the difference in ΔGI of the control and the drug) was not seen at the time the control ΔGI was 30, the vials were read for one or two additional days to establish a definite pattern of ΔGI differences.
Median lethal dose (LD50) evaluation
The median lethal dose (LD50), the dose of the selected compounds that causes 50% mortality in mice, was determined from dose–response curves with at least four doses by the method of Litchfield and Wilcoxon [27].
Acknowledgments
We thank Dr. V. L. Narayanan from the Drug Synthesis and Chemistry Branch, National Cancer Institute, Bethesda, MD, USA, for the in vitro evaluation of anticancer activity. Antimycobacterial data were provided by the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) through a research and development contract with the US National Institute of Allergy and Infectious Diseases.
Footnotes
This article is available from: http://dx.doi.org/10.3797/scipharm.1301-13
Authors’ Statement
Competing Interests
The authors declare no conflict of interest.
References
- [1].Wall AM, Abraham DJ, editors. Burger's Medicinal Chemistry and Drug Discovery. 6th ed. Vol. 5. Wiley-Interscience; 2003. Drug Resistance in Cancer Chemotherapy; pp. 281–291. Chemotherapeutic agents. http://dx.doi.org/10.1002/0471266949. [Google Scholar]
- [2].Lesyk R, Zimenkovsky B, Atamanyuk D, Jensen F, Kiec-Kononowicz K, Gzella A. Anticancer thiopyrano[2,3-d][1,3]thiazol-2-ones with norbornane moiety. Synthesis, cytotoxicity, physico-chemical properties, and computational studies. Bioorg Med Chem. 2006;14:5230–5240. doi: 10.1016/j.bmc.2006.03.053. http://dx.doi.org/10.1016/j.bmc.2006.03.053. [DOI] [PubMed] [Google Scholar]
- [3].Atamanyuk D, Zimenkovsky B, Lesyk R. Synthesis and anticancer activity of novel thiopyrano[2,3-d]thiazole-based compounds containing norbornane moiety. J Sulfur Chem. 2008;29:151–162. http://dx.doi.org/10.1080/17415990801911723. [Google Scholar]
- [4].Donawho CK, Shoemaker AR, Palma JP, Triggle D, Taylor J, editors. Comprehensive Medicinal Chemistry II. Elsevier; 2006. Principles of Chemotherapy and Pharmacology; pp. 33–53. http://dx.doi.org/10.1016/B0-08-045044-X/00203-0. [Google Scholar]
- [5].Perkins WE, Schroeder RL, Carrano RA, Imondi AR. Myocardial effects of mitoxantrone and doxorubicin in the mouse and guinea pig. Cancer Treat Rep. 1984:841–847. http://www.ncbi.nlm.nih.gov/pubmed/6733698. [PubMed] [Google Scholar]
- [6].Selassie CD, Hansch C, Khwaja TA. Structure-activity relationships of antineoplastic agents in multidrug resistance. J Med Chem. 1990;33:1914–1919. doi: 10.1021/jm00169a014. http://dx.doi.org/10.1021/jm00169a014. [DOI] [PubMed] [Google Scholar]
- [7].Janin YL. Antituberculosis drugs: ten years of research. Bioorg Med Chem. 2007;15:2479–2513. doi: 10.1016/j.bmc.2007.01.030. http://dx.doi.org/10.1016/j.bmc.2007.01.030. [DOI] [PubMed] [Google Scholar]
- [8].Kaminskyy D, Vasylenko O, Atamanyuk D, Gzella A, Lesyk R. Isorhodanine and thiorhodanine motifs in the synthesis of fused thiopyrano[2,3-d][1,3]thiazoles. Synlett. 2011;10:1385–1388. http://dx.doi.org/10.1055/s-0030-1260765. [Google Scholar]
- [9].Murugan R, Anbazhagan S, Narayanan S. Synthesis and in vivo antidiabetic activity of novel dispiropyrrolidines through [3+2] cycloaddition reactions with thiazolidinedione and rhodanine derivatives. Eur J Med Chem. 2009;44:3272–3279. doi: 10.1016/j.ejmech.2009.03.035. http://dx.doi.org/10.1016/j.ejmech.2009.03.035. [DOI] [PubMed] [Google Scholar]
- [10].Komaritsa I, Baranov S, Grishuk A. 4-Thiazolidines, derivatives and analogs - V. arylidene derivatives of isorhodanine. Chem Heterocycl Compd. 1967;3:533–534. http://dx.doi.org/10.1007/BF00481594. [Google Scholar]
- [11].Boyd MR, Paull KD. Some practical considerations and applications of the national cancer institutein vitro anticancer drug discovery screen. Drug Dev Res. 1995;34:91–109. http://dx.doi.org/10.1002/ddr.430340203. [Google Scholar]
- [12].Teicher BA, editor. Cancer Drug Discovery and Development. Vol. 2. Totowa, NJ, USA: Humana Press; 1997. The NCI In Vitro Anticancer Drug Discovery Screen; pp. 23–43. [Google Scholar]
- [13].Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Nat Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. http://dx.doi.org/10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- [14].Monks A, Scudiero DA, Johnson GS, Paull KD, Sausville EA. Mini-review. The NCI anti-cancer drug screen: a smart screen to identify effectors of novel targets. Anti-Cancer Drug Des. 1997;12:533–541. http://www.ncbi.nlm.nih.gov/pubmed/9365500. [PubMed] [Google Scholar]
- [15].Shoemaker RH, Scudiero DA, Melillo G, Currens MJ, Monks AP, Rabow AA, Covell DG, Sausville EA. Application of High-Throughput, Molecular-Targeted Screening to Anticancer Drug Discovery. Curr Top Med Chem. 2002;2:229–246. doi: 10.2174/1568026023394317. http://dx.doi.org/10.2174/1568026023394317. [DOI] [PubMed] [Google Scholar]
- [16]. DTP Compare. http://dtp.nci.nih.gov/docs/compare/compare.html.
- [17].Paull KD, Lin CM, Malspeis L, Hamel E. Identification of novel antimitotic agents acting at the tubulin level by computer-assisted evaluation of differential cytotoxicity data. Cancer Res. 1992;52:3892–3900. http://www.ncbi.nlm.nih.gov/pubmed/1617665. [PubMed] [Google Scholar]
- [18].Paull KD, Shoemaker RH, Hodes L, Monks A, Scudiero D, Rubinstein L, Plowman J, Boyd MR. Display and Analysis of Patterns of Differential Activity of Drugs Against Human Tumor Cell Lines: Development of Mean Graph and COMPARE Algorithm. J Natl Cancer Inst. 1989;81:1088–1092. doi: 10.1093/jnci/81.14.1088. http://dx.doi.org/10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
- [19].Zaharevitz DW, Holbeck SL, Bowerman C, Svetlik PA. COMPARE: a web accessible tool for investigating mechanisms of cell growth inhibition. J Mol Graphics Modell. 2002;20:297–303. doi: 10.1016/s1093-3263(01)00126-7. http://dx.doi.org/10.1016/S1093-3263(01)00126-7. [DOI] [PubMed] [Google Scholar]
- [20].Kekre N, Griffin C, McNulty J, Pandey S. Pancratistatin causes early activation of caspase-3 and the flipping of phosphatidyl serine followed by rapid apoptosis specifically in human lymphoma cells. Cancer Chemother Pharmacol. 2005;56:29–38. doi: 10.1007/s00280-004-0941-8. http://dx.doi.org/10.1007/s00280-004-0941-8. [DOI] [PubMed] [Google Scholar]
- [21].Orme IM. Search for New Drugs for Treatment of Tuberculosis. Antimicrob Agents Chemother. 2001;45:1943–1946. doi: 10.1128/AAC.45.7.1943-1946.2001. http://dx.doi.org/10.1128/AAC.45.7.1943-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Collins L, Franzblau SG. Microplate Alamar Blue Assay versus BACTEC 460 System for High-Throughput Screening of Compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. http://www.ncbi.nlm.nih.gov/pubmed/9145860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Heifets L. Qualitative and quantitative drug-susceptibility tests in mycobacteriology. Am Rev Respir Dis. 1988;137:1217–1222. doi: 10.1164/ajrccm/137.5.1217. http://www.ncbi.nlm.nih.gov/pubmed/3195815. [DOI] [PubMed] [Google Scholar]
- [24].Gruppo V, Johnson CM, Marietta KS, Scherman H, Zink EE, Crick DC, Adams LB, Orme IM, Lenaerts AJ. Rapid Microbiologic and Pharmacologic Evaluation of Experimental Compounds against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2006;50:1245–1250. doi: 10.1128/AAC.50.4.1245-1250.2006. http://dx.doi.org/10.1128/AAC.50.4.1245-1250.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. Rapid, Low-Technology MIC Determination with Clinical Mycobacterium tuberculosis Isolates by Using the Microplate Alamar Blue Assay. J Clin Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. http://www.ncbi.nlm.nih.gov/pubmed/9466742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Tuberculosis Antimicrobial Acquisition & Coordinating Facility. Sponsored by the NIAID of the US National Institutes of Health. http://www.taacf.org.
- [27].Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. http://www.ncbi.nlm.nih.gov/pubmed/18152921. [PubMed] [Google Scholar]
- [28].Smith WG, Ellis GP, West GB, editors. Progress in Medicinal Chemistry. Butterworths; 1961. Pharmacological screening tests; pp. 1–33. [Google Scholar]