Abstract
Clinical therapies have traditionally been developed using two-dimensional (2D) cell culture systems, which fail to accurately capture tissue complexity. Therefore, three-dimensional (3D) cell cultures are more attractive platforms to integrate multiple cues that arise from the extracellular matrix and cells, closer to an in vivo scenario. Here we report the development of a 3D cellular model for the in vitro assessment of the outcome of oxygen- and drug-dependent therapies, exemplified by photodynamic therapy (PDT). Using a synthetic self-assembling peptide as a cellular scaffold (RAD16-I), we were able to recreate the in vivo limitation of oxygen and drug diffusion and its biological effect, which is the development of cellular resistance to therapy. For the first time, the production and decay of the cytotoxic species singlet oxygen could be observed in a 3D cell culture. Results revealed that the intrinsic mechanism of action is maintained in both systems and, hence, the dynamic mass transfer effects accounted for the major differences in efficacy between the 2D and 3D models. We propose that this methodological approach will help to improve the efficacy of future oxygen- and drug-dependent therapies such as PDT.
Introduction
Early biological-activity assessment in drug discovery has been traditionally based on two-dimensional (2D) cell cultures.1 Tissue-specific architecture, together with cell–cell and cell–extracellular matrix (ECM) interactions, are reduced when cells grow on flat and adherent substrates.1,2 Three-dimensional (3D) cultures, which basically rely on embedding cells within scaffolds resembling the ECM, are currently being developed as more advanced in vitro models for cellular organization in tissues.2 These milieus provide the third dimension, essential to integrate biomechanical, biochemical, and biophysical properties more similar to a natural microenvironment. In particular, a key advantage of 3D cultures in preclinical research is their capacity to modulate the molecular gradients that exist in living tissues for any soluble component, such as oxygen, nutrients, metabolites, and signaling molecules.3,4 In contrast, 2D cultures are characterized by artificial rich nutrition and oxygenation, being unable to reproduce in vivo mass transport limitations.1 Hence, 3D models mimic the hierarchical complexity of tissues more precisely than conventional monolayers, providing a potential bridge for the gap between 2D cultures and animal models.2,5
Our goal was to develop a 3D model to capture the mass transfer limitations occurring in vivo and, therefore, better predict the outcome of oxygen- and drug-dependent medical therapies. Up to date, in vitro models for preclinical research typically consist in 3D spheroids. They have led to major conceptual advances in understanding discrepancies in therapy effectiveness between conventional monolayer cultures and in vivo experiments.6–9 Nonetheless, spheroids are typically constituted by epithelial cells and they are limited by slow spontaneous aggregation and uncontrolled final size and shape.10,11 To avoid these drawbacks, we chose self-assembling hydrogels as cellular scaffolds. They are formed by a network of interweaving nanofibers with pore sizes of 50–200 nm, surrounding cells in a similar manner to the natural ECM and, thereby, mimicking the in vivo scenario. Moreover, they offer the ability to control the size and shape and, thus, tune oxygen and compound gradients.12,13 In particular, our 3D model was based in the self-assembling peptide hydrogel RAD16-I (commercially available as BD™PuraMatrix™), which is composed by a sequence of 16 amino acid chain AcN-(RADA)4-CONH2 (R arginine, A alanine, and D aspartic acid).14,15 RAD16-I has previously been shown to promote growth and proliferation of multiple cell types, including chondrocytes, hepatocytes, endothelial cells, osteoblasts, and neuronal cells, as well as embryonic and somatic stem cells.16–20 Moreover, this hydrogel provides a noninstructive and defined microenvironment to cells, allowing us to analyze the intrinsic effect of the 3D architecture, in contrast to the widely used natural scaffolds as collagen or BD™Matrigel™, in which cells are influenced by an heterogeneous and unidentified range of biochemical signals.21
The selected medical therapy was photodynamic therapy (PDT) as it involves the interaction of a photoactivatable drug with ambient oxygen and is therefore ideally suited to probe the mass transport effects. PDT is a successful and clinically approved therapeutic modality used for the management of neoplastic diseases, nonmalignant conditions, and skin afflictions.22–25 All of them are characterized by an abnormal cellular growth and, consequently, a poor vascular architecture in which molecular gradients of oxygen and nutrients are exacerbated. PDT is based on the administration of a photoactive dye or photosensitizer (PS), which, under aerobic conditions, is able to produce reactive oxygen species (ROS) upon exposure to per-se harmless light.22,23,26 Among the ROS produced, the electronically excited form of the dioxygen molecule, referred to as singlet oxygen (1O2), is believed to be the major mediator of primary photodynamic effects of a wide variety of PSs, playing a key role in both apoptotic and necrotic cell death.27–29 To study the oxygen–cell and PS–cell interactions in 3D cultures, time-resolved spectroscopic measurements of both the drug's and 1O2 phosphorescence were performed. Several attempts have been made to detect 1O2 phosphorescence in biological environments, cell suspensions, and even living tissue.30–32 However, in vivo detection remains technically difficult and the results are still ambiguous.33 Thus, 3D cultures not only provide a platform to simulate the PDT outcome in tissues, but also represent an intermediate stage to better understand the behavior of 1O2 in complex systems. All the experiments were carried out with the PS 5,10,15,20-tetrakis(N-methyl-4-pyridilium)-21H,23H-porphine (TMPyP), whose spectroscopic and photosensitizing properties are ideal for probing its photobehavior in cells.30,34,35 Specifically, TMPyP is water soluble and is both fluorescent and phosphorescent, essential characteristics to study mass transport effects and to report cell processes.
In this report, we describe a more predictive in vitro model for the assessment of photodynamic treatments, consisting of a 3D cellular culture that recreates the tissue complexity. Particularly, cells are exposed to molecular gradients, which cause a hampered access of oxygen and drug, distancing from the artificial situation in 2D cultures of rich oxygenation and compound accessibility.
Materials and Methods
2D culture technique
Primary human normal dermal fibroblasts (hNDF) were kindly provided by Dr. Jesús Otero from Hospital Central de Asturias. hNDF were cultured in a humidified 5% CO2 incubator at 37°C in a fibroblast culture medium (FCM). The FCM consisted in the Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 1% penicillin–streptomycin.
3D culture technique
Self-assembling peptide scaffolds were prepared by diluting 1% (w/v) RAD16-I (BDPuraMatrix; BD Biosciences) in 25% (w/v) sucrose to obtain a final concentration of 0.6% (w/v) RAD16-I. The peptide solution was sonicated for 5 min. hNDF were harvested by trypsinization from the 2D culture flasks and suspended in 10% (w/v) sucrose to get a final concentration of 4×106 cells/mL. Equal volumes of cell suspension and liquid 0.6% (w/v) RAD16-I were mixed to obtain a final suspension of 2×106 cells/mL in 0.3% (w/v) RAD16-I. Next, 40 μL of the suspension was loaded into 30-mm-diameter cell culture inserts (Millipore), previously placed inside six-well culture dishes and wet with the FCM. The medium penetrated the insert from the bottom membrane, inducing RAD16-I gel formation through a self-assembling process. Finally, a total volume of 2 mL of the culture medium was added into the insert in consecutive small portions, favoring the leaching of the sucrose. The remaining medium in the well, rich in sucrose, was aspirated and replaced with a fresh medium. Incubation was performed at 37°C with 5% CO2 and the medium was changed every day by removing 500 μL from the well and adding 500 μL of a fresh medium into the insert.
3D culture characterization
Cellular morphology within the 3D culture was evaluated by fluorescence microscopy and field emission gun–scanning electron microscopy (FEG-SEM) analysis. For fluorescent imaging, cellular nuclei and actin filaments were stained using 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes) and phalloidin-tetramethylrhodamine B isothiocyanate (Phalloidin-TRITC; Sigma) dyes, respectively. For FEG-SEM, constructs were fixed with 5% (w/v) gluteraldehyde for 1 h and were submitted to a dehydration process, which included several immersions for 10 min in ethanolic solutions. Samples were dried using a CO2 critical point dryer. Then, constructs were sputter-coated with a gold and platinum alloy using the equipment Emitech SC7620 (60 s, 18 mA and chamber pressure 0.2 mbar) and were examined with a FEG-SEM (JEOL JSM-7100F) at 13 kV. Finally, cell viability was assessed with the calcein AM and ethidium homodimer Live/Dead fluorescent stains (Molecular Probes).
PS uptake
5,10,15,20-tetrakis(N-methyl-4-pyridyl)-21H,23H-porphine (TMPyP; Sigma) was used as PS in PDT experiments. The cellular internalization of TMPyP was determined by fluorescence spectroscopy. Cells were cultured in 2D and 3D systems during 3 days and incubated in the dark with the serum-free FCM containing 10 and 100 μM TMPyP for different times ranging from 30 min to 48 h. Cells were then washed three times with phosphate-buffered saline (PBS) and resuspended in 2% sodium dodecyl sulfate in Milli-Q water. The resulting suspensions were centrifuged at 600×g for 5 min. The extent of PS uptake was assessed by measuring the fluorescence of the supernatants and normalizing them to their protein content with bicinchoninic protein assay (Thermo Scientific). Appropriate controls were performed to ensure that signals originated from PS molecules internalized by cells.
Microscopy analysis
For 2D cell cultures, hNDF were seeded on round coverslips (25 mm diameter) and cultured toward 80%–85% confluence. For 3D cell cultures, hNDF were encapsulated in RAD16-I hydrogel and cultured during 3 days. Both cellular models were incubated in the dark with the serum-free FCM containing 100 μM TMPyP. After 24-h incubation, Hoechst stain (Molecular Probes) was added to a final concentration of 2.5 μg/mL during 30 min. Finally, cells were washed three times with PBS and replenished with the FCM. Samples were examined under two-photon excitation microscopy (Leica TCS-SP5). All images are uniformly adjusted for brightness and contrast.
Flow cytometry
Cells were cultured in 2D and 3D systems during 3 days and incubated in the dark with the serum-free FCM containing 100 μM TMPyP. After 24-h incubation, cells were washed three times with PBS. Two-dimensional cultures were trypsinized. Three-dimensional cultures were digested by incubating pronase (1 mg/mL; Roche) during 1 h and collagenase (1 mg/mL; Sigma) overnight and pipetting constructs up and down. Both collected cells were fixed with 1% p-formaldehyde during 1 h. Then, they were analyzed on BD FACSCanto II. TMPyP was measured using a 488-nm laser for excitation and 695-nm filter for detection. Cellular autofluorescence was measured using a 405-nm laser for excitation and 450-nm filter for detection.
Photodynamic treatments
For 2D cell cultures, hNDF were seeded in 48-well plates and cultured toward 80%–85% confluence. For 3D cell cultures, hNDF were encapsulated in RAD16-I hydrogel and cultured during 3 days. To select the PS working concentration, both cellular models were incubated in the dark with the serum-free FCM containing 0.5–100 μM TMPyP for 24 h. Cells were then washed three times with PBS and replenished with a fresh FCM. Cellular viability was assessed by the MTT assay 24 h after treatment. Dark cytotoxicity experiments yielded a survival cell fraction higher than 85%, demonstrating that incubation with TMPyP at the working concentrations did not induce significant cell death without irradiation. Photodynamic treatments were carried out after PS incubation in the dark. After 24-h incubation, cells were washed with PBS and replenished with a fresh FCM. Cells were then irradiated for different fluences (6 and 18 J/cm2) using a green light LED source (520–550 nm) at a fluence rate of 16 mW/cm,2 and then incubated for an additional 24 h before the MTT assay for cellular viability. To decouple oxygen and drug gradients, additional photodynamic treatments were performed in an oxygen-saturated atmosphere during illumination, by bubbling a stream of oxygen through the culture media of the 3D constructs.
Singlet oxygen measurements
An appropriate number of cells were seeded in 75-cm2 flasks and were cultured to 80%–85% confluence. They were incubated in the dark with 100 μM TMPyP for 24 h. The medium was discarded and the cells were washed three times with PBS, trypsinized, and resuspended in 1.5 mL of PBS or D2O-based PBS (d-PBS) to a final concentration of 4 million cells/mL. The cell suspensions were continuously stirred during the measurements. For measurements in 3D cultures, cell constructs were prepared as described above and were cultured during 3 days. Then, they were incubated in the dark with 100 μM TMPyP for 24 h, washed three times with PBS, and carefully transferred to a 0.4-cm quartz cuvette. For D2O-based measurements, cell constructs were incubated with d-PBS for 20 min before measurements to exchange the extracellular H2O with D2O. To test the diffusion of singlet oxygen produced, an additional set of experiments was performed adding 0.75 mM bovine serum albumin (BSA) to the extracellular d-PBS. Spectroscopic measurements were carried out within the following 45 min. Control experiments were performed using the RAD16-I peptide scaffold without cells.
Statistics
All values are expressed as mean±standard deviation. Statistical differences were analyzed with GraphPad Prism 5. The unpaired Student's t-test was used to test for the significance level between two sets of measurements. The level of significance was set to p<0.05.
Additional methods
Descriptions of general spectroscopic instrumentation, quantification of gene expression by real time RT-PCR, and singlet oxygen kinetic model are available in Supplementary Methods (Supplementary Data are available online at www.liebertpub.com/tea).
Results
Engineering a 3D cellular microenvironment
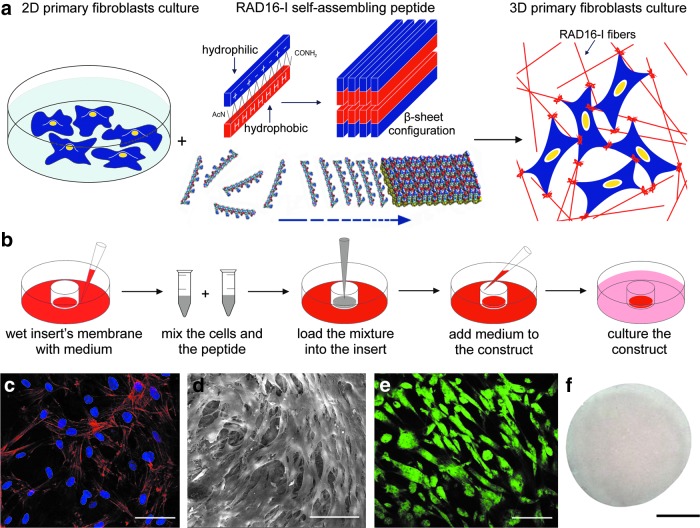
We engineered a 3D cellular model to recreate in vitro mass transfer effects. For this reason, we cultured hNDF cells within a 0.3% (w/v) RAD16-I hydrogel scaffold (Fig. 1a). Initially, liquid RAD16-I (hydrogel precursor) was mixed with a suspension of cells and loaded into a cell culture insert, prewet with the culture medium. The ionic strength and the neutral pH of the medium spontaneously induced the peptide to self-assemble into a hydrogel scaffold formed by interweaving nanofibers of 10–20-nm diameter and 50–200-nm pore size, mimicking the ECM nanofibrous architecture and porosity14,15 (Fig. 1b). We analyzed the morphology that cells exhibited when cultured in the scaffold by nuclei and actin filaments staining (Fig. 1c) and SEM (Fig 1d). Fibroblasts embedded in the 3D microenvironment spread and adopted an in vivo-like lengthened shape. Furthermore, hNDF established intercellular contacts that evolved during culture. This cellular elongation and network formation was developed due to the permissiveness microenvironment provided by the compliant 0.3% RAD16-I hydrogel. Fibroblasts remained alive during culture time, as demonstrated by calcein-AM and ethidium homodimer-1 staining (Fig. 1e). The resulting geometry of the 3D culture was a disk of ∼5 mm diameter by 0.5 mm thick, relevant dimensions for being subjected to oxygen and drug limitations within the construct, key agents in oxygen- and drug-dependent medical therapies such as PDT (Fig. 1f). All characterizations were carried out at day 3 of culture, when PDT experiments were performed.
FIG. 1.
Development and characterization of a three-dimensional (3D) model for predicting in vitro the outcome of oxygen-dependent therapies, such as photodynamic therapy (PDT). (a) Human normal dermal fibroblasts (hNDF) were cultured on conventional monolayers. Then, cells were trypsinized and mixed with liquid RAD16-I (hydrogel precursor). Under the appropriate culture conditions (strong ionic or neutral medium), the hydrogel spontaneously self-assembled into a β-sheet configuration, forming a network of interweaving nanofibers, in which cells experienced a truly 3D microenvironment. (b) Schematic representation of the protocol for cell encapsulation in a self-assembling peptide. First, the membrane of the cell culture insert was wet with a medium. Cells were gently mixed with the solution of the self-assembling peptide. The suspension was loaded into the insert and the medium diffuses immediately through it, inducing RAD16-I gel formation through a self-assembling process. Then, washing steps were made with a culture medium. Finally, the well and the insert were filled with a culture medium. (c, d) Cellular morphology and interactions within the 3D construct were evaluated (c) by nuclei (blue) and actin filaments (red) staining and (d) scanning electron microscopy. (e) Cellular viability was assessed by calcein-AM (green) and ethidium homodimer-1 (red) staining, which indicated live and dead cells, respectively. (f ) Final geometry of the 3D disk. Scale bars (c–e) 50 μm; and (f ) 2 mm. Color images available online at www.liebertpub.com/tea
Efficiency of drug uptake is reduced in 3D cultures
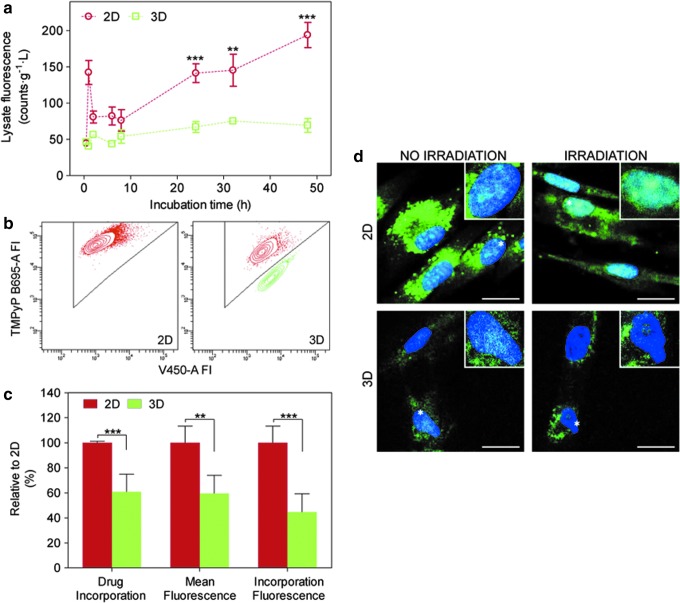
Conventional monolayer cultures are characterized by uniformly rich nutrition and oxygenation, while tissues experience mass transfer phenomena for any soluble agent. The situation is worsened in tissues originating from abnormal cellular proliferation, PDT's main therapeutic targets, as they have a primitive vascular formation that causes an inefficient delivery of oxygen and drugs. Thus, the 3D model was designed to mimic the limited drug and oxygen availability occurring in vivo. First, we characterized drug uptake by comparing cells grown in 2D and 3D cultures. Specifically, we examined the extent of TMPyP PS uptake at different incubation times, ranging from 30 min to 48 h, through fluorescence spectroscopy after lysing the cells and normalizing to the protein content. The initial working concentration of PS was 10 and 100 μM in the serum-free medium to avoid PS's aggregation (Fig. 2a and Supplementary Fig. S1). Both concentrations resulted in the same kinetics profile of TMPyP cellular incorporation, which is characterized by a significantly lower uptake in cells growing in 3D cultures relative to 2D ones. As a result, a question was raised: Do cells internalized less drug or fewer cells participated in drug uptake? To address this issue, constructs were incubated with TMPyP, digested to isolate intact cells, and then flow cytometry was performed to assess the drug content in each individual cell (Fig. 2b). Interestingly, only 60% of these cells incorporated TMPyP molecules versus 100% in 2D cultures (Fig. 2c). Moreover, the amount of TMPyP in those cells was 40% lower than in cells cultured on classic 2D cultures (Fig. 2c). Therefore, the decrease in drug uptake was related to the sum of two effects: fewer cells internalized drug and cells, which did internalize drug, and exhibited less amount of it. Then, the subcellular localization of the PS in cells coming from 2D and 3D systems was studied by confocal fluorescence microscopy (Fig. 2d). In both cultures, we found the same results, namely, TMPyP initially localized in cytoplasmic vacuoles and relocalized into the nucleus after irradiation. This behavior is consistent with literature reports36 and is confirmed by time-resolved fluorescence measurements (see below). On the other hand, the fluorescence signal intensity was lower in 3D, in agreement with the flow cytometry and cell uptake results (Fig. 2a–c).
FIG. 2.
Photosensitizer (PS) uptake profile within the 3D model. (a) Uptake of TMPyP PS by hNDF cultured in two-dimensional (2D) and 3D was determined through fluorescence emission normalized with the protein content (N=2, n=3). (b) The individual cellular content of TMPyP PS was evaluated by flow cytometry. Shown are TMPyP-positive cells (red) and -negative cells (green). FI, relative fluorescence intensity, is reported in arbitrary units (N=3, n=3). (c) Data from flow cytometry in (b) were quantified. Specifically, drug incorporation and mean fluorescence in 3D cultures were analyzed and plotted as percentages relative to 2D cultures. Moreover, total TMPyP uptake was also expressed as percentage number of positive cells multiplied by their mean fluorescence. (d) Confocal microscopy images of living hNDF in 2D and 3D after TMPyP incubation were registered before and after irradiation. All images correspond to the overlay of the TMPyP fluorescence signal (green, false color) and nuclei staining (blue) (N=2, n=2). All experiments were performed with 100 μM TMPyP. Scale bars (d) 20 μm. Error bars (a) and (c): standard deviation (SD) **p<0.01, ***p<0.001. Color images available online at www.liebertpub.com/tea
Limited oxygen and drug diffusion in 3D cultures modulate cell response to PDT
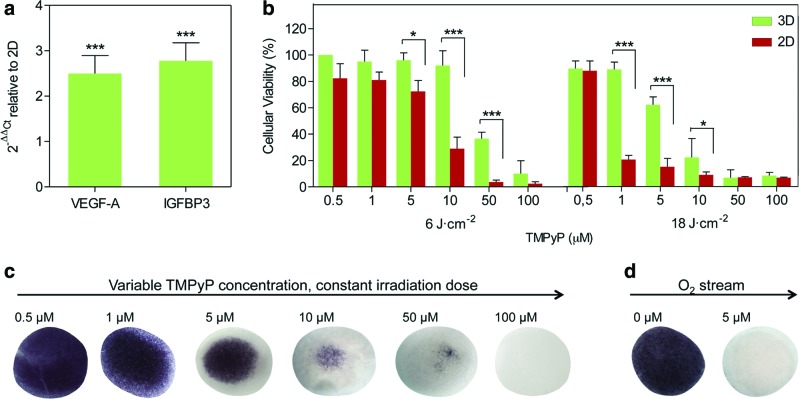
The cellular response to PDT is also governed by oxygen availability. Thus, the 3D model was engineered to in vitro recreate oxygen deficiency, capturing more accurately the in vivo situation. The oxygen level within the 3D construct was characterized by the expression of two hypoxia-responsive genes,4 specifically, the vascular endothelial growth factor and insulin-like growth factor-binding protein 3 (IGFBP3), through real-time RT-PCR (Fig. 3a). Cells grown in the 3D cultures upregulated the expression of both oxygen markers. The mRNA fold induction increased by a factor of 2–4 relative to 2D cultures, reaching a similar level than in tissues exposed to systemic hypoxia.37
FIG. 3.
The oxygen gradient and photodynamic treatment response of the 3D culture. (a) Expression levels of oxygen-responsive genes, in particular, the vascular endothelial growth factor (VEGF) and insulin-like growth factor-binding protein 3 (IGFBP3), were determined through real-time RT-PCR. Ct values relative to the ribosomal unit 18S were obtained and reported as fold increase (ΔΔCt) with respect to 2D cultures (N=2, n=3). (b) Human fibroblasts cultured on 2D and 3D were subjected to PDT, using different concentrations of TMPyP, PS, and light doses. The resulting cellular viability was assessed by MTT assay and compared between both culture models to study the effect of the drug and oxygen gradient (N=2, n=3). (c) MTT assay showed the survival pattern within the 3D construct during the photosensitization experiments. (d) The oxygen gradient was decoupled from the drug gradient by circulating an oxygen stream during PDT and the survival pattern was also evaluated by MTT assay. Error bars (a) and (b): SD. *p<0.05, **p<0.01, ***p<0.001. Color images available online at www.liebertpub.com/tea
To determine the effects of limited oxygen and TMPyP diffusion on the cellular response to PDT, TMPyP-mediated photosensitization experiments in the 2D and 3D models were performed, under the same conditions of drug incubation and light irradiation (Fig. 3b and Supplementary Fig. S2). Cellular viability was assessed by the MTT assay 24 h after treatment (Fig. 3b, c). Three-dimensional cultures were found to be more resistant to PDT than traditional 2D monolayers, exhibiting a radial survival pattern in which the core of the construct maintained a larger percentage of living cells. We further decoupled the oxygen and TMPyP mass transport effects by circulating an oxygen stream during irradiation (Fig. 3d). Complete cell death was now observed under conditions in which cell viability had been 80% in the absence of oxygen flow. Control of samples exposed to oxygen stream and irradiation was performed and no change in cellular viability was observed. This data indicate that the high cellular survival observed under static conditions was due to the low oxygen concentration in the core of the construct, which created a protective microenvironment for cells.
Photophysical and singlet oxygen measurements in 3D cultures confirm hampered drug and oxygen availability
Mechanistic insight into PDT enabled us to elucidate whether mass transfer limitations affected the intrinsic mechanism of action of the therapy. The information could be extracted from singlet oxygen (1O2) and TMPyP time-resolved phosphorescence detection. In particular, when a photoactivatable drug absorbs a photon, it accesses a short-lived electronically excited state (1PS), which then either returns to its ground state emitting light (fluorescence) and heat or undergoes intersystem crossing to form a longer lived triplet excited state (3PS) (Fig. 4a). Such 3PS can in turn release its energy by emission of light (phosphorescence) or can transfer it to nearby oxygen molecules to form 1O2. Thus, probing the photoprocesses of the drug and the phosphorescence of 1O2 provides direct evidence about the drug's microenvironment and its accessibility to oxygen, and also about the details of 1O2 production and the fate of this ROS.
FIG. 4.
Photophysical and singlet oxygen measurements. (a) A modified Jablonski diagram of excited states and the interaction with oxygen. (b, c) Normalized steady-state (b) and time-resolved (c) fluorescence emission spectra of TMPyP previously incorporated in the RAD16-I scaffold without cells, hNDF 2D cultures, and hNDF 3D cultures (N=5, n=2). (d) Time-resolved singlet oxygen phosphorescence at 1275 nm from the RAD16-I scaffold without cells, hNDF 2D cultures, and hNDF 3D cultures incubated with TMPyP (N=5, n=2). (e, f ) TMPyP triplet (e) and singlet oxygen (f ) phosphorescence lifetimes in H2O, D2O, and D2O with 0.77 mM BSA containing the RAD16-I scaffold without cells, hNDF 2D cultures, and hNDF 3D cultures (N=5, n=2). Error bars (e) and (f ): SD. Color images available online at www.liebertpub.com/tea
The fluorescence emission spectra of TMPyP, both in 2D and 3D, showed two well-resolved bands, in contrast to the structure-less broad band typically observed in water and in the peptide scaffold (Fig. 4b). This indicates that TMPyP is internalized by the cells in both cultures and is hardly retained by the nanofiber scaffold. The multiexponential decay kinetics (lifetimes 1.5, 5.7, and 12 ns), as opposed to the monoexponential decay found in RAD16-I aqueous-like environments (lifetime ca. 5 ns), confirms the cell internalization and indicates that TMPyP localizes in multiple subcellular sites in both cultures30 (Fig. 4c). Based on literature data, the 1.5 and 12 ns components were assigned to TMPyP bound to nuclear DNA38,39 and the 5.7 ns to molecules localized in the lysosomes.35
The triplet-state kinetics was likewise derived from time-resolved phosphorescence measurements. As in the case of fluorescence, biexponential behavior was found in both types of cell cultures (Fig. 4d, e), in contrast to the monoexponential decay in aqueous environments and in the peptide scaffold. The longer lived component, with similar lifetime in the 2D and 3D cultures, was assigned to molecules bound to nuclear DNA in agreement with literature results.35,40 The long lifetime is indicative of poor oxygen accessibility to TMPyP in this case. The shorter lived component was assigned to molecules in the lysosomes for consistency with the fluorescence results. Interestingly, such a lysosomal population, which is responsible for photodynamic damage, had a longer lifetime in 3D cultures than in 2D ones (7±1 μs vs. 4±1 μs, respectively), indicating that, on average, TMPyP is less accessible to oxygen in the 3D cultures and therefore it produces 1O2 with lower efficiency. This conclusion is consistent with the different cellular response to PDT observed in both types of cultures and with the enhancing effect of an oxygen stream on cell death (Fig. 3).
The kinetics of 1O2 decay likewise reflects the localization, mobility, and reactivity of this ROS (Fig. 4d, f, Supplementary Fig. S3 and Supplementary Table S1). Advantage was taken of the well-known deuterium isotope effect on the 1O2 lifetime (τΔ)41 to reveal differences in its kinetics in both cell cultures; τΔ in H2O is 3.3 μs, whereas in D2O, it increases by ca. 20-fold (68 μs).42 Thus, for cultures incubated with d-PBS, we measured τΔ=67±2 μs in 2D, but 56±5 μs in 3D. It is worth noting that the lifetime in the 3D nanofiber scaffold without cells was also 67±2 μs, which ruled out any significant quenching effect by the peptide RAD16-I. The similarity of the 2D value with that in D2O indicates that a substantial fraction of 1O2 was able to escape from the cells, as previously observed in many laboratories.30–32 Therefore, the decrease of 1O2 lifetime in the 3D cultures could be assigned to 1O2 quenching outside the cells by the ECM proteins. This was confirmed by studying the effect of externally added proteins such as BSA.43 Thus, in the presence of BSA, the lifetime of 1O2 decreased further down to 47±2 μs in 2D and to 40±5 μs in 3D cell cultures (Fig. 4f).
Discussion
We report a 3D culture model in which cells are exposed to mass transfer phenomena, distancing from the artificial situation of rich oxygenation and nutrition present in 2D monolayers. In this study, we have used the self-assembling peptide RAD16-I as a cellular scaffold, in which the high cell density, the produced ECM, and the construct dimensions spontaneously create oxygen and drug deficiencies. Such are the situations encountered in PDT, whose outcome is critically dependent on the supply of both oxygen and the PS.
Skin afflictions, such as nonmelanoma skin cancer, actinic keratosis, keloid disease, psoriasis, acne, or rosacea, are one of the main therapeutic targets for PDT.44–46 For this reason, fibroblasts were selected as the cell source. Moreover, given that fibroblasts are one of the principal constituents of the tumor stroma,47 this 3D model can be further used to examine the therapeutical effects on cancer-associated fibroblasts in coculture with epithelial tumoral cells.
There are other examples of 3D models in PDT, but they are mainly based on spheroids48–50 and natural biomaterials such as collagen or Matrigel.8,51,52 Cellular and oxygenation properties of spheroids are totally dependent on the culture technique.50 On the other hand, natural scaffolds represent a good alternative because they provide the spectrum of chemical and physical cues but, at the same time, this signal mixture hampers the isolation of effects of specific factors, such as mass transfer phenomena. Thus, we chose a model based on the synthetic RAD16-I scaffold where specific-signaling motives are absent.
Consistently with previous works,8,49 our results demonstrate that cells respond differently to PDT in 2D versus 3D microenvironments, as the photodynamic effect observed in 3D cultures is dramatically lower. To go deeper into these results, mechanistic studies were performed. In this regard, the proposed 3D model is a good candidate to address these questions in a controlled way. First of all, mass transport limitations of the 3D system were revealed by flow cytometry and uptake studies. The PS is probably not able to reach the cells buried in the most inner part of the construct. In addition, overexpression of hypoxia genes indicated a deficient oxygen supply throughout the construct. The situation would be aggravated in the actual PDT treatments due to oxygen consumption. In fact, total death was only observed using a 20-fold higher TMPyP concentration or when a continuous flow of oxygen was maintained during the PDT treatments, despite the demonstrated presence of free-drug and/or hypoxic cells, which could not undergo photodynamic treatment. This apparent paradox leads us to formulate the hypothesis of a death-signaling cascade that could be triggered to break the protective microenvironment created by oxygen and drug limitations, inducing neighboring cell death.53,54 These results merit further in-depth studies to determine the nature of these phenomena. Whenever this protective barrier was not exceeded, 3D cultures yielded a heterogeneous population of cells in which a niche more resistant to the photodynamic treatments was localized at the central core of the construct. Thus, our 3D model aptly reproduces one of the most important factors limiting the efficacy of PDT in clinical practice.
An additional mechanistic insight is provided by spectroscopic and photophysical measurements. The production of the cytotoxic 1O2 upon photoexcitation of TMPyP was unambiguously demonstrated in both 2D and 3D systems by observation of its phosphorescence at 1275 nm. This is the first experimental observation of 1O2 phosphorescence in a 3D cell culture system. The slower rate of 1O2 production in the 3D systems confirmed the deficient oxygen supply. Likewise, the faster decay of 1O2 revealed a quenching effect of the secreted ECM proteins. On the other hand, the subcellular localization of TMPyP remained the same in both types of cultures as revealed by fluorescence kinetics and microscopy. Taken together, these results indicate that the major differences between 2D and 3D cultures stem from dynamic mass transfer effects rather than intrinsic mechanism of action of PDT. As such, our 3D model may pave the way for the development of more versatile platforms for better in vitro drug testing and therapy assessment.
Supplementary Material
Acknowledgments
This work was supported by a grant of the Spanish Ministry of Economy and Competitiveness (CTQ2010-20870-C03-01). M.A.-R. and M.G.-D. thank the Comissionat per a Universitats i Recerca del Departament d'Innovació, Universitats i Empresa de la Generalitat de Catalunya i del Fons Social Europeu for a predoctoral fellowship.
Author Contributions
C.E.S. and S.N. designed research; M.A.-R., M.G.-D. and M.B. performed research; M.A.-R. and M.G.-D. analyzed data; and M.A.-R., M.G.-D., C.E.S., and S.N. wrote the article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Yamada K.M. Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Griffith L.G. Swartz M.A. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 3.Minchinton A.I. Tannock I.F. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 4.Derda R. Laromaine A. Mammoto A. Tang S.K.Y. Mammoto T. Ingber D.E. Whitesides G.M. Paper-supported 3D cell culture for tissue-based bioassays. Proc Natl Acad Sci U S A. 2009;106:18457. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pampaloni F. Reynaud E.G. Stelzer E.H. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 6.Shaw K.R. Wrobel C.N. Brugge J.S. Use of three-dimensional basement membrane cultures to model oncogene-induced changes in mammary epithelial morphogenesis. J Mammary Gland Biol Neoplasia. 2004;9:297. doi: 10.1007/s10911-004-1402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celli J.P. Rizvi I. Evans C.L. Abu-Yousif A.O. Hasan T. Quantitative imaging reveals heterogeneous growth dynamics and treatment-dependent residual tumor distributions in a three-dimensional ovarian cancer model. J Biomed Opt. 2010;15:051603. doi: 10.1117/1.3483903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi I. Celli J.P. Evans C.L. Abu-Yousif A.O. Muzikansky A. Pogue B.W. Finkelstein D. Hasan T. Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res. 2010;70:9319. doi: 10.1158/0008-5472.CAN-10-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein O.J. Bhayana B. Park Y.J. Evans C.L. In vitro optimization of EtNBS-PDT against hypoxic tumor environments with a tiered, high-content, 3D model optical screening platform. Mol Pharm. 2012;9:3171. doi: 10.1021/mp300262x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrecht D.R. Underhill G.H. Wassermann T.B. Sah R.L. Bhatia S.N. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods. 2006;3:369. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 11.Mehta G. Hsiao A.Y. Ingram M. Luker G.D. Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 2012;164:192. doi: 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semino C.E. Can we build artificial stem cell compartments? J Biomed Biotechnol. 2003;2003:164. doi: 10.1155/S1110724303208019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S. Holmes T. Lockshin C. Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci U S A. 1993;90:3334. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S. Holmes T.C. DiPersio C.M. Hynes R.O. Su X. Rich A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials. 1995;16:1385. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- 16.Holmes T.C. de Lacalle S. Su X. Liu G. Rich A. Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci U S A. 2000;97:6728. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kisiday J. Jin M. Kurz B. Hung H. Semino C. Zhang S. Grodzinsky A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garreta E. Genové E. Borrós S. Semino C.E. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12:2215. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 19.Genové E. Schmitmeier S. Sala A. Borrós S. Bader A. Griffith L.G. Semino C.E. Functionalized self-assembling peptide hydrogel enhance maintenance of hepatocyte activity in vitro. J Cell Mol Med. 2009;13:3387. doi: 10.1111/j.1582-4934.2009.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dégano I.R. Quintana L. Vilalta M. Horna D. Rubio N. Borrós S. Semino C. Blanco J. The effect of self-assembling peptide nanofiber scaffolds on mouse embryonic fibroblast implantation and proliferation. Biomaterials. 2008;30:1156. doi: 10.1016/j.biomaterials.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Lee J. Cuddihy M.J. Kotov N.A. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14:61. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 22.Agostinis P. Berg K. Cengel K.A. Foster T.H. Girotti A.W. Gollnick S.O. Hahn S.M. Hamblin M.R. Juzeniene A. Kessel D. Korbelik M. Moan J. Mroz P. Nowis D. Piette J. Wilson B.C. Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolmans D.E.J.G. Fukumura D. Jain R.K. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y. Baron E.D. Photodynamic therapy: current evidence and applications in dermatology. Semin Cutan Med Surg. 2011;30:199. doi: 10.1016/j.sder.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Babilas P. Schreml S. Landthaler M. Szeimies R.M. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26:118. doi: 10.1111/j.1600-0781.2010.00507.x. [DOI] [PubMed] [Google Scholar]
- 26.Dougherty T.J. Gomer C.J. Henderson B.W. Jori G. Kessel D. Korbelik M. Moan J. Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weishaupt K.R. Gomer C.J. Dougherty T.J. Identification of singlet oxygen as the cytotoxic agent in photo-inactivation of the murine tumor. Cancer Res. 1976;36:2326. [PubMed] [Google Scholar]
- 28.Redmond R.W. Kochevar I.E. Spatially resolved cellular responses to singlet oxygen. Photochem Photobiol. 2006;82:1178. doi: 10.1562/2006-04-14-IR-874. [DOI] [PubMed] [Google Scholar]
- 29.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumors. J Photochem Photobiol B. 1997;39:1. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez-Banzo A. Sagrista M.L. Mora M. Nonell S. Kinetics of singlet oxygen photosensitization in human skin fibroblasts. Free Radic Biol Med. 2008;44:1926. doi: 10.1016/j.freeradbiomed.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Hackbarth S. Schlothauer J. Preuss A. Roeder B. New insights to primary photodynamic effects - Singlet oxygen kinetics in living cells. J Photochem Photobiol B. 2010;98:173. doi: 10.1016/j.jphotobiol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Skovsen E. Snyder J.W. Lambert J.D.C. Ogilby P.R. Lifetime and diffusion of singlet oxygen in a cell. J Phys Chem B. 2005;109:8570. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- 33.Kanofsky J.R. Measurement of singlet-oxygen in vivo: progress and pitfalls. Photochem Photobiol. 2011;87:14. doi: 10.1111/j.1751-1097.2010.00855.x. [DOI] [PubMed] [Google Scholar]
- 34.Maisch T. Spannberger F. Regensburger J. Felgentrager A. Baumler W. Fast and effective: intense pulse light photodynamic inactivation of bacteria. J Ind Microbiol Biotechnol. 2012;39:1013. doi: 10.1007/s10295-012-1103-3. [DOI] [PubMed] [Google Scholar]
- 35.Snyder J.W. Lambert J.D.C. Ogilby P.R. 5,10,15,20-tetrakis(N-methyl-4-pyridyl)-21H,23H-porphine (TMPyP) as a sensitizer for singlet oxygen imaging in cells: characterizing the irradiation-dependent behavior of TMPyP in a single cell. Photochem Photobiol. 2006;82:177. doi: 10.1562/2005-05-30-RA-553. [DOI] [PubMed] [Google Scholar]
- 36.Patito I.A. Rothmann C. Malik Z. Nuclear transport of photosensitizers during photosensitization and oxidative stress. Biol Cell. 2001;93:285. doi: 10.1016/s0248-4900(01)01118-2. [DOI] [PubMed] [Google Scholar]
- 37.Marti H.H. Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci U S A. 1998;95:15809. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chirvony V.S. Primary photoprocesses in cationic 5,10,15,20-meso-tetrakis(4-N-methylpyridiniumyl) porphyrin and its transition metal complexes bound with nucleic acids. J Porphyrins Phthalocyanines. 2003;7:766. [Google Scholar]
- 39.Paoli V.M.D. Paoli S.H.D. Borissevitch L.E. Tedesco A.C. Fluorescence lifetime and quantum yield of TMPyPH2 associated with micelles and DNA. J All Comp. 2002;344:27. [Google Scholar]
- 40.Snyder J.W. Skovsen E. Lambert J.D.C. Poulsen L. Ogilby P.R. Optical detection of singlet oxygen from single cells. Phys Chem Chem Phys. 2006;8:4280. doi: 10.1039/b609070m. [DOI] [PubMed] [Google Scholar]
- 41.Schweitzer C. Schmidt R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem Rev. 2003;103:1685. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson F. Helman W.P. Ross A.B. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J Phys Chem Ref Data. 1995;24:663. [Google Scholar]
- 43.Snyder J.W. Skovsen E. Lambert J.D.C. Ogilby P.R. Subcellular, time- resolved studies of singlet oxygen in single cells. J Am Chem Soc. 2005;127:14558. doi: 10.1021/ja055342p. [DOI] [PubMed] [Google Scholar]
- 44.Chiu L.L. Sun C.H. Yeh A.T. Torkian B. Karamzadeh A. Tromberg B. Wong B.J. Photodynamic therapy on keloid fibroblasts in tissue-engineered keratinocyte-fibroblast co-culture. Lasers Surg Med. 2005;37:231. doi: 10.1002/lsm.20213. [DOI] [PubMed] [Google Scholar]
- 45.Byun J.Y. Lee G.Y. Choi H.Y. Myung K.B. Choi Y.W. The expressions of TGF-beta(1) and IL-10 in cultured fibroblasts after ALA-IPL photodynamic treatment. Ann Dermatol. 2011;23:19. doi: 10.5021/ad.2011.23.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendoza J. Sebastian A. Allan E. Allan D. Mandal P. Alonso-Rasgado T. Bayat A. Differential cytotoxic response in keloid fibroblasts exposed to photodynamic therapy is dependent on photosensitiser precursor, fluence and location of fibroblasts within the lesion. Arch Dermatol Res. 2012;304:549. doi: 10.1007/s00403-012-1264-y. [DOI] [PubMed] [Google Scholar]
- 47.Bhowmick N.A. Neilson E.G. Moses H.L. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bigelow C.E. Mitra S. Knuechel R. Foster T.H. ALA- and ALA-hexylester-induced protoporphyrin IX fluorescence and distribution in multicell tumour spheroids. Br J Cancer. 2001;85:727. doi: 10.1054/bjoc.2001.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huygens A. Huyghe D. Bormans G. Verbruggen A. Kamuhabwa A.R. Roskams T. de Witte P.A.M. Accumulation and photocytotoxicity of hypericin and analogs in two- and three-dimensional cultures of transitional cell carcinoma cells. Photochem Photobiol. 2003;78:607. doi: 10.1562/0031-8655(2003)078<0607:aapoha>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Madsen S.J. Sun C.H. Tromberg B.J. Cristini V. De Magalhaes N. Hirschberg H. Multicell tumor spheroids in photodynamic therapy. Lasers Surg Med. 2006;38:555. doi: 10.1002/lsm.20350. [DOI] [PubMed] [Google Scholar]
- 51.Wright K.E. Liniker E. Loizidou M. Moore C. Macrobert A.J. Phillips J.B. Peripheral neural cell sensitivity to mTHPC-mediated photodynamic therapy in a 3D in vitro model. Br J Cancer. 2009;101:658. doi: 10.1038/sj.bjc.6605197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans C.L. Abu-Yousif A.O. Park Y.J. Klein O.J. Celli J.P. Rizvi I. Zheng X. Hasan T. Killing hypoxic cell populations in a 3D tumor model with EtNBS-PDT. PLoS One. 2011;6:e23434. doi: 10.1371/journal.pone.0023434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubio N. Fleury S.P. Redmond R.W. Spatial and temporal dynamics of in vitro photodynamic cell killing: extracellular hydrogen peroxide mediates neighbouring cell death. Photochem Photobiol Sci. 2009;8:457. doi: 10.1039/b815343d. [DOI] [PubMed] [Google Scholar]
- 54.Feine I. Pinkas I. Salomon Y. Scherz A. Local oxidative stress expansion through endothelial cells—a key role for gap junction intercellular communication. PLoS One. 2012;7:e41633. doi: 10.1371/journal.pone.0041633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.