Abstract
Constitutively activating mutations in receptor kinases recruit downstream effector pathways independently of upstream signaling, with consequences ranging from developmental syndromes to cancer. Classic fibrodysplasia ossificans progressiva (FOP) is a congenital syndrome resulting from highly conserved activating mutations of the glycine-serine-rich (GS) regulatory domain of ACVR1, encoding bone morphogenetic protein (BMP) type I receptor ALK2, which lead to inappropriate signaling and heterotopic ossification of soft tissues. It is unclear if constitutively active mutant ALK2 receptors (caALK2) can function independently of signaling complexes with type II receptors and ligands. We found that ablation of BmpRII and ActRIIa abrogated BMP ligand-mediated and caALK2-mediated signaling and transcription in cells and disrupted caALK2-induced heterotopic ossification in mice. Signaling via GS domain ALK2 mutants could be restored by the expression of either BMP type II receptor. The contribution of BMP type II receptors was independent of their ligand-binding or kinase function but was dependent upon an intact cytoplasmic domain. These data demonstrate that GS domain ALK2 mutants act independently of upstream signaling but may require a nonenzymatic scaffolding function provided by type II receptors to form functional, apparently ligand-independent signaling complexes. These findings define the minimal requirements for signaling of GS domain ALK2 mutants, with implications for the therapeutic targeting of their activity in disease.
INTRODUCTION
In human disease, somatic or germ line mutations may confer “constitutive” activity to mutant signaling or receptor proteins, permitting the recruitment of downstream effectors independently of upstream signaling events. So-called “constitutively activating” mutations and their gene products contribute to a broad range of clinical conditions ranging from cancer to syndromic developmental defects, including inborn disorders of metabolism, endocrine abnormalities, and sensory defects (1–3). Constitutively activating mutations, commonly referred to as “gain-of-function” mutations, can augment function in multiple ways. Constitutively active mutant receptor proteins may have true constitutive activity that is invariant with stimuli, harbor activity that is hypersensitive or amplified in response to native ligands, or have activity that can be induced with decreased specificity (4).
Fibrodysplasia ossificans progressiva (FOP) is a congenital heterotopic ossification (HO) disorder in which afflicted individuals are nearly normal at birth except for subtle skeletal malformations yet are prone to forming endochondral bone lesions in soft tissues such as skeletal muscle, ligaments, and fascia, especially following injury or inflammation (5). The classic FOP phenotype results from highly conserved activating mutations in ACVR1, encoding the bone morphogenetic protein (BMP) type I receptor kinase ALK2, due to an R206H substitution in the glycine-serine-rich (GS) regulatory domain (6, 7). While other mutations of the GS domain and other domains of the ALK2 protein may cause variant or atypical FOP, the R206H mutation accounts for at least 98% of classic FOP presentations (6). BMP signaling is typically thought to require formation of a signaling complex composed of dimeric ligands which facilitate oligomerization of BMP type II receptors (BMPRII or ACTRIIA) with type I receptors (ALK1, ALK2, ALK3, or ALK6) in tetrameric complexes. Type I receptor kinases are activated by polyphosphorylation of the GS domain by type II receptor kinases within this signaling complex. R206H and other ALK2 GS domain mutants, including FOP variant Q207E and engineered constitutively active mutant Q207D, are predicted to alter the α-helical structure of the GS domain, disrupting associations with regulatory protein FKBP12 and preventing intramolecular salt bridges which stabilize the inactive configuration (6, 8–10). An active ALK2 configuration is mimicked by GS domain mutations, rendering ALK2 kinase constitutively or promiscuously active in a manner that appears to be partly ligand independent yet, under certain conditions, hypersensitive to ligand stimulation (8, 11–14). The classic “two-kinase” model of BMP and transforming growth factor β (TGF-β) signaling suggests that the activity of ALK2 mutants might be modified by BMP ligands and complex formation with type II receptors, but the question of whether “constitutively active” ALK2 mutants (caALK2) require the participation of BMP ligands, type II receptors, or signaling complex formation to mediate their effects remains unresolved.
Since constitutively active mutant type I receptors might not require phosphorylation by type II receptor kinases but could be influenced by the formation of type II receptor and ligand signaling complexes, we tested whether or not GS domain mutant ALK2 (caALK2) receptors require the participation of BMP type II receptors to augment BMP-mediated signaling, gene expression, and differentiation. Using conditional or global type II receptor-deficient mice and their cultured tissues, we found that ligand and caALK2-mediated signaling and transcriptional activity were abrogated in compound BmpRII/ActRIIa-deficient cells and that caALK2-induced heterotopic ossification was abrogated in BmpRII/ActRIIa-deficient mice. Surprisingly, ligand-binding-deficient or kinase-deficient mutant BMP type II receptors, as well as wild-type (WT) type II receptor, could restore the activity of caALK2 in compound BmpRII/ActRIIa-deficient cells. These results demonstrate that functional caALK2 signaling complexes require type II receptors yet do not require the participation of ligands or type II receptor kinases. These results imply that disease-causing ALK2 mutations cannot effectively be circumvented by ligand inhibition or type II receptor kinase inhibition strategies. These data refine the classic “two-kinase” paradigm of BMP/TGF-β signaling with a “two-receptor” requirement for ALK2 GS domain mutants, in which so-called constitutively active type I receptors retain the need for nonenzymatic cooperation provided by type II receptors.
MATERIALS AND METHODS
Genetically modified mice.
Mice harboring Cre-loxP inducible knockout Bmpr2 alleles (Bmpr2flox/flox) were generated as previously described (15). Mice with targeted disruption of Acvr2a alleles (Acvr2a−/−) were kindly provided by En Li of the Novartis Institute of Biomedical Research, Shanghai, China (16). These mice were bred to provide compound BmpRII-conditionally deficient and ActRIIa-globally deficient (Bmpr2flox/flox:Acvr2a−/−) mice. Conditional ALK2Q207D transgenic (Tg) mice, also called CAG-Z-eGFP-caALK2 or caALK2 Tg mice, have been previously described (14, 17). All mouse experiments were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and the Harvard Medical School Institutional Animal Care and Use Committee.
PASMC isolation and cell culture.
Pulmonary artery smooth muscle cells (PASMC) were cultured from mouse pulmonary artery explants as previously described (18), using pulmonary arteries obtained from wild-type (WT), Bmpr2flox/flox, Acvr2a−/−, or compound Bmpr2flox/flox:Acvr2a−/− mice. Isolated PASMC were cultured in RPMI medium supplemented with 10% fetal calf serum (FCS), glutamine, and antibiotics (GIBCO-Invitrogen).
Ex vivo Cre-loxP-mediated recombination.
PASMC obtained from Bmpr2flox/flox or Bmpr2flox/flox:Acvr2a−/− mice were subjected to ex vivo recombination of Bmpr2flox/flox alleles using adenovirus expressing Cre (Ad.Cre) (multiplicity of infection [MOI] of 50) to yield BmpRII knockout (KO) cells or BmpRII/ActRIIa KO cells, respectively, whereas control cells were treated with Ad.GFP. Expression of Bmpr2 and Acvr2a mRNAs was measured by quantitative PCR (SYBR FAST, Kapa Biosystems, and Mastercycler realplex2; Eppendorf) as described previously (18). Following treatment with Ad.Cre, Bmpr2flox/flox cells demonstrated ≤10% residual expression of Bmpr2 mRNA compared to wild-type cells, using primers specific for exons 4 and 5 flanked by loxP sequences as previously described (18, 19).
Plasmids, adenoviruses, and siRNA.
Human C-terminally hemagglutinin (HA)-tagged wild-type ALK2 and ALK2Q207D plasmid constructs were kindly provided by Kohei Miyazono (University of Tokyo, Japan). Human HA-tagged ALK2R206H was kindly provided by Takenobu Katagiri (Saitama University, Japan). Plasmid constructs expressing the long and short isoforms of human BMPRII, along with the “kinase-dead” K230R mutant long form of human BMPRII, were generated as previously described (20, 21). Plasmids expressing N519K, D485G, C118W, and Y172X mutations in the long isoform of human BMPRII in pcDNA3 vector were generated as previously described (22, 23). Human ACTRIIA expressed in pcDNA3 was kindly provided by Yisrael Sidis (Massachusetts General Hospital, Boston). Kinase-dead mutant ACTRIIAK219R was generated via the QuikChange site-directed mutagenesis kit (Agilent Technologies). Small interfering RNAs (siRNAs) specific for Acvr2a and Acvr2b were used as previously described (18).
Ad.Cre and control adenovirus were purchased from Vector Laboratories. Adenoviruses expressing constitutively active human ALK2 and long and short isoforms of human BMPRII (Ad.ALK2Q207D, Ad.BMPRII-LF, and Ad.BMPRII-SF) were kindly provided by Akiko Hata (University of California at San Francisco).
Luciferase reporter assay.
Plasmids expressing wild-type and mutant forms of human ALK2, ALK3, BMPRII, and ACTRIIA, along with the BRE-Luc reporter construct and Renilla (pRL-CMV; Promega), were transfected into PASMC by nucleofection (nucleofected) using Neon (Invitrogen) or into C2C12 cells using Lipofectamine (Invitrogen) according to the manufacturers' protocols. Briefly, 106 PASMC or C2C12 cells were transfected with a total of 7 μg of DNA, incubated in RPMI with 0.5% FCS for 16 h, and treated overnight with various concentrations of recombinant BMP4 or BMP7 (R&D Systems). Recombinant BMP6 was a kind gift from Slobodan Vukicevic (University of Zagreb, Croatia). Samples were lysed and analyzed with the dual-luciferase kit (Promega) using the SpectraMax L luminometer (Molecular Devices).
ALK2Q207D-induced model of heterotopic ossification.
Inducible constitutively active ALK2Q207D transgenic mice, also called caALK2-Tg or CAG-Z-eGFP-caALK2-Tg mice (14, 17), were bred onto Bmpr2flox/flox, Acvr2a−/−, or compound Bmpr2flox/flox:Acvr2a−/− backgrounds to yield compound caALK2 transgenic, conditional BmpRII KO, ActRIIa KO, and BmpRII/ActRIIa KO mice. Recombination of conditional caALK2 and Bmpr2flox/flox alleles was achieved via a single intramuscular Ad.Cre (1 × 108 PFU) injection into the left popliteal fossa on postnatal day 7 (P7). Weekly assessments of passive hind limb mobility were performed under anesthesia (14), and heterotopic bone formation was analyzed by X-ray (MS FX In-Vivo Pro; Carestream Health), as well as by Alizarin red and alcian blue staining (24). Changes in passive range of motion (ankle flexion) were assessed by the minimum angle formed by the ankle and tibia with passive dorsoflexion under anesthesia, as previously described (14).
Immunohistochemistry.
HEK293T cells (106) were nucleofected with wild-type and mutant human BMPRII and ACTRIIA plasmids (Neon; Invitrogen). Cells were fixed with 1% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), permeabilized with methanol, and stained with antibodies specific for the myc epitope (1:100; Cell Signaling), followed by secondary antibodies tagged with Alexa Fluor 488 (Invitrogen-Molecular Probes).
Analytical ultracentrifugation (AUC).
Sedimentation velocity experiments were carried out on a Beckman Optima XL-I analytical ultracentrifuge equipped with a Ti50 rotor and cells with double-sector centerpieces. Protein samples were studied at a concentration of 7 μM buffered in 50 mM HEPES (pH 7.5), 300 mM NaCl, and 0.5 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) at 4°C, employing rotor speeds of 35,000 or 45,000 rpm. Radial absorbance scans were collected using absorbance optics at 280 nm in continuous-scan mode. Data were analyzed using the SEDFIT software package (25). The software package SEDNTERP (26) was used to convert the obtained sedimentation coefficient values to the equivalent values in water at 20°C.
Analytical gel filtration.
Size exclusion chromatography was performed on a Dionex UltiMate 3000 high-pressure liquid chromatography (HPLC) system using a Superdex S200 5/150 column and a flow rate of 0.3 ml/min. Proteins were injected at a concentration of 50 μM buffered in 50 mM HEPES (pH 7.5), 300 mM NaCl, and 0.5 mM TCEP at 4°C.
Protein expression and purification.
The kinase domains of human BMPRII (residues 189 to 517) and TGFBRII (residues 232 to 547) were subcloned into the pNIC-CH and pNIC28-Bsa4 vectors, respectively, and the resulting plasmids transformed into Escherichia coli strain BL21(DE3)R3-pRARE2 for expression. Cultures were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) overnight at 18°C and the cells harvested and lysed by ultrasonication. Recombinant proteins were purified by Ni affinity, size exclusion, and anion-exchange chromatographies. Proteins were stored at 4°C buffered in 50 mM HEPES (pH 7.4), 300 mM NaCl, 10% glycerol, 10 mM dithiothreitol (DTT), 50 mM l-arginine, and 50 mM l-glutamate. The GS and kinase domains of ALK2 (residues 172 to 499) were purified from baculovirus expression in Sf9 cells as described previously (8).
Statistical analyses.
Statistical significance was determined using the unpaired Student t test or by analysis of variance for multiple comparisons (Prism; GraphPad).
RESULTS
caALK2-induced heterotopic ossification in mice requires BMP type II receptors.
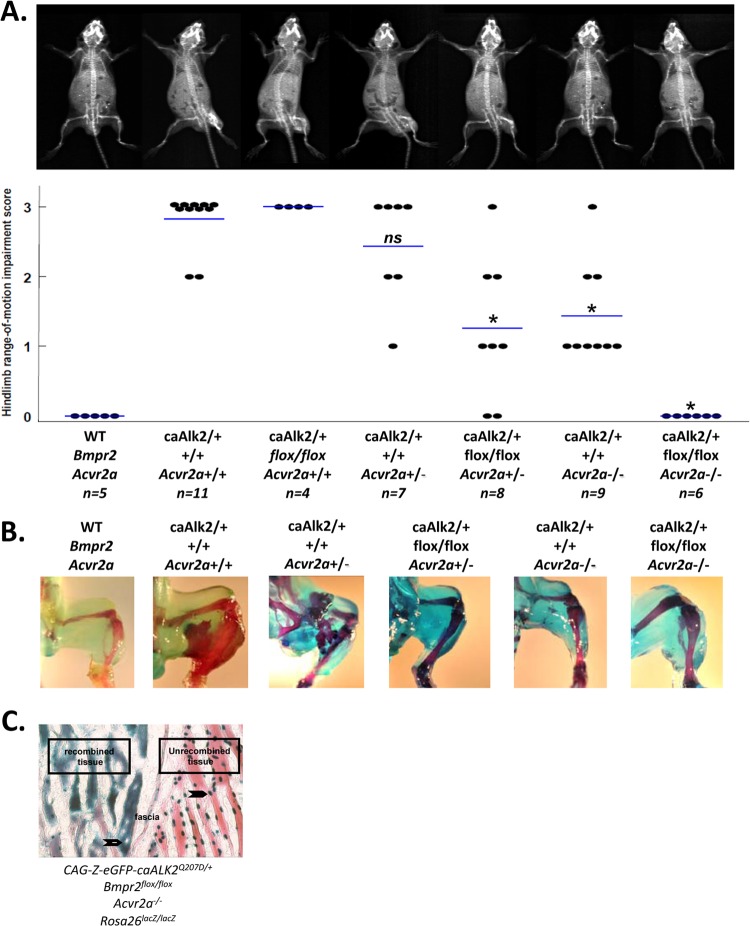
We previously found that postnatal expression of ALK2Q207D in conditional caALK2-Tg mice following intramuscular injection with Ad.Cre results in myositis and vigorous heterotopic ossification, whereas global expression of ALK2Q207D without inflammation does not induce bone (14). This model recapitulates key aspects of classic human FOP, in which heterotopic ossification (HO) associated with germ line ALK2R206H mutation is precipitated by injury or inflammation (6). Since BMP ligand-mediated signaling normally depends on formation of BMP receptor signaling complexes, we tested the requirement for type II receptors by breeding the inducible caALK2 transgene onto mice with various combinations of conditional BmpRII knockout (Bmpr2flox) or global ActRIIa knockout (Acvr2a−) alleles. By X-ray and measurement of passive range of motion, inducible caALK2 transgenic mice on a wild-type background exhibited heterotopic ossification and hind limb immobilization with complete penetrance at P30 following Ad.Cre injection at P7 (Fig. 1A). Inducible caALK2 transgenic mice also exhibited the full HO phenotype on Bmpr2flox/flox or Acvr2a+/− backgrounds. However, expression of caALK2 on a combined Bmpr2flox/flox and Acvr2a+/− background resulted in significantly diminished HO and immobility, as did expression of caALK2 on an Acvr2a−/− background. No HO or immobility was detected in caALK2-Tg mice on a compound Bmpr2flox/flox:Acvr2a−/− background. The allele-dependent effects of disrupting BmpRII and ActRIIa on caALK2-induced chondrogenesis and mineralization were confirmed by alcian blue and Alizarin red staining (Fig. 1B), with no evidence of cartilage or ossified bone appearing in caALK2-Tg:Bmpr2flox/flox:Acvr2a−/− mice. While both receptors contributed to caALK2-induced heterotopic ossification, deleting Acvr2a had a slightly greater impact than deleting Bmpr2 in this model.
Fig 1.
caALK2-induced heterotopic ossification is facilitated by BMP type II receptors Acvr2a and Bmpr2 in an allele-dependent manner. (A) Ad.Cre (1 × 108 PFU) was administered to the left hindlimb on P7 to drive expression of an inducible ALK2Q207D transgene and induce viral myositis. Heterotopic ossification due to caALK2 was modulated by the presence of Bmpr2flox/flox or Acvr2a+/− alleles, as assessed by radiograph and passive ankle dorsoflexion under anesthesia at P30; range of motion is plotted, with mean values depicted by bars, and representative X-rays shown (*, P < 0.001 compared to caALK2-Tg mice on a wild-type background; ns, nonsignificant). (B) The presence of heterotopic bone and cartilage was assessed by Alizarin red and alcian blue staining, respectively. (C) Gastrocnemius muscles from a CAG-Z-eGFP-caALK2:Bmpr2flox/floxAcvr2a−/−:Rosa26LacZ/LacZ transgenic mouse were injected with Ad.Cre on P7 and stained on P12 for β-galactosidase activity (blue) and eosin (red). In tissues not undergoing recombination, expression of the unrecombined CAG-Z-eGFP-caALK2 transgene was observed as nuclear β-galactosidase activity (closed arrows, right side of fascia). Following Ad.Cre treatment, the presence of cytoplasmic β-galactosidase activity and loss of nuclear β-galactosidase activity (open arrows) reflected simultaneous recombination at the Rosa26LacZ/LacZ and CAG-Z-eGFP-caALK2 loci, suggesting that expression of caALK2 coincided with highly efficient ablation of Bmpr2.
To demonstrate efficient in vivo recombination of multiple floxed alleles following an Ad.Cre injection, β-galactosidase staining was performed on skeletal muscle sections from caALK2-Tg:Bmpr2flox/flox:Acvr2a−/−:Rosa26LacZ/LacZ mice (Fig. 1C). Unrecombined tissues demonstrated nucleus-localized β-galactosidase staining due to expression of the unrecombined caALK2 (CAG-Z-eGFP-caALK2) transgene, whereas recombined tissues demonstrated cytoplasmic β-galactosidase staining due to the recombination of Rosa26lacZ, as well as loss of nuclear β-galactosidase staining following recombination of the caALK2 transgene.
These data illustrate that a GS domain mutant ALK2 transgene requires participation of BMP type II receptors, particularly ActRIIa, for inducing heterotopic ossification in vivo.
GS domain mutant ALK2 signaling requires type II receptor.
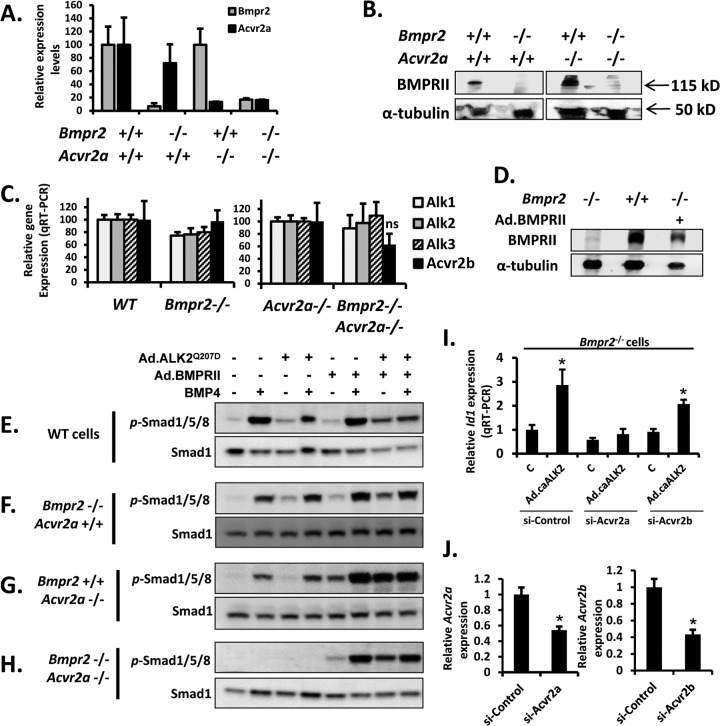
To discern the signaling defects which attenuate the development of HO in caALK2-Tg mice lacking BmpRII and/or ActRIIa, we examined ligand-mediated and ALK2Q207D-mediated signaling in wild-type (WT), Bmpr2−/− (BmpRII KO), Acvr2a−/− (ActRIIa KO), and Bmpr2−/−:Acvr2a−/− (BmpRII/ActRIIa or double KO) cells. Since the precise identity of the osteoprogenitors which drive FOP is under active investigation, PASMC, which are known to be competent for BMP/TGF-β signaling and to express a suite of BMP type II and type I receptors, including Bmpr2, Acvr2a, and Acvr2b (18), were used as a surrogate to examine signaling events. Each of these conditional or constitutive knockout cell isolates was confirmed to be depleted of Bmpr2 and/or Acvr2a mRNA by quantitative reverse transcription-PCR (RT-PCR) and to be deficient in BmpRII protein expression by immunoblotting (Fig. 2A and B). Importantly, depletion of Bmpr2 and/or Acvr2a did not exert significant effects on the levels of other BMP type II or type I receptors (Fig. 2C).
Fig 2.
BMP ligand- and caALK2-mediated phosphorylation of SMAD1/5/8 requires expression of Bmpr2 or Acvr2a. (A) Quantitative RT-PCR of wild-type, Bmpr2−/−, Acvr2a−/−, and Bmpr2−/−:Acvr2a−/− cells demonstrated depletion of Bmpr2 and Acvr2a mRNA in their respective knockout cells. (B) Immunoblotting of extracts from wild-type, Bmpr2−/−, Acvr2a−/−, and Bmpr2−/−:Acvr2a−/− cells demonstrated depletion of BmpRII protein in KO cells. (C) Quantitative RT-PCR of wild-type, Bmpr2−/−, Acvr2a−/−, and Bmpr2−/−:Acvr2a−/− cells demonstrated that loss of Bmpr2 or loss of Bmpr2 and Acvr2a did not impact the expression levels of endogenous Alk1, Alk2, Alk3, or Acvr2b, whereas Alk6 was not significantly expressed in PASMC. (D) Immunoblotting of extracts from Bmpr2−/− cells treated with Ad.BMPRII demonstrated expression of BMPRII comparable to endogenous levels in wild-type cells. (E to H) Activation of SMAD1/5/8 in pulmonary SMC isolated from wild-type, Bmpr2−/−, Acvr2a−/−, and Bmpr2−/−:Acvr2a−/− cells was assessed by immunoblotting for phosphorylated Smad1/5/8 and total Smad1 in response to BMP4 (20 ng/ml for 30 min) or transfection with adenovirus expressing ALK2Q207D (MOI of 50 for 12 h), following serum deprivation for 16 h. Both BMP4- and ALK2Q207D-induced phosphorylation of Smad1/5/8 required either Bmpr2 or Acvr2a and was abrogated in Bmpr2−/−:Acvr2a−/− cells. Ectopic expression of BMPRII (Ad.BMPRII, MOI of 50 for 12 h) rescued BMP4- and ALK2Q207D-induced phosphorylation of Smad1/5/8 in Bmpr2−/−:Acvr2a−/− cells. (I) Expression of ALK2Q207D (Ad.caALK2, MOI of 50, 12 h) induced expression of Id1 by quantitative RT-PCR in Bmpr2−/− cells. The induction of Id1 was efficiently blocked by transfection with siRNA (48 h) specific for Acvr2a but not Acvr2b or control siRNA, supporting the concept that loss of Bmpr2 and Acvr2a abolishes activity of caALK2. (J) Transfection of PASMC with siRNA specific for Acvr2a or Acvr2b for 48 h effectively reduced mRNA expression by ≥50%. Data are represented as means ± standard errors of the means (SEM) (n = 3 or 4; ns, not significantly different from control; *, P < 0.05 compared with baseline Id1 expression in control virus-treated cells [c] or cells treated with control siRNA).
Exposure to BMP4 ligand increased the phosphorylation of Smad1, -5, and -8 (Smad1/5/8) in WT, BmpRII KO, or ActRIIa KO cells but not BmpRII/ActRIIa KO cells (Fig. 2E to H). Expression of ALK2Q207D modestly increased steady-state levels of phosphorylated Smad1/5/8 in WT and BmpRII KO cells, had a minimal impact on ActRIIa KO cells, and had no impact on double KO cells. The inability of BmpRII/ActRIIa KO cells to recruit Smad1/5/8 phosphorylation in response to BMP4 ligand or ALK2Q207D suggested a requirement for either type II receptor for ligand or ALK2Q207D signaling. To restore BMPRII expression, BmpRII KO cells were treated with adenovirus expressing BMPRII (Fig. 2D). Ectopic expression of BMPRII in this manner enhanced basal phosphorylation of Smad1/5/8, particularly in BmpRII KO, ActRIIa KO, and double KO cells (Fig. 2E to H). Ectopic expression of BMPRII in double KO cells restored the incremental activity of BMP4 ligand or ALK2Q207D (Fig. 2H), confirming that loss of type II receptor was the critical defect in BmpRII/ActRIIa KO cells. Supporting the concept that ActRIIa and BmpRII are specifically required for caALK2 signaling, rather than an overall level of type II receptor expression, we found that siRNA targeting Acvr2a, but not Acvr2b, abrogated ALK2Q207D signaling in BmpRII KO cells (Fig. 2I and J).
Interestingly, rather than being enhanced by expression of ALK2Q207D, the activity of BMP4 was minimally impacted by the expression of ALK2Q207D in BmpRII KO or ActRIIa KO cells and was slightly decreased by expression of ALK2Q207D in wild-type cells (Fig. 2E).
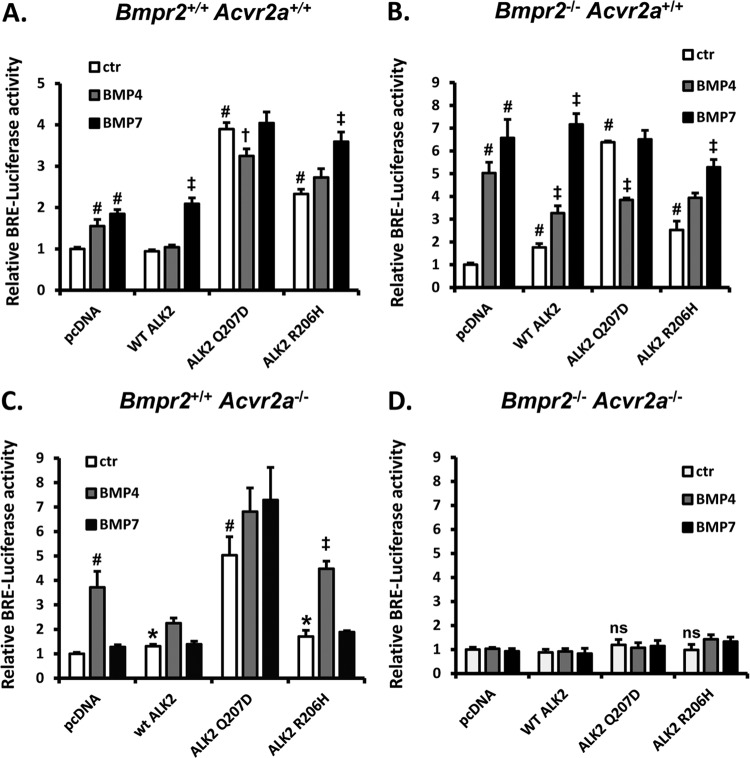
Consistent with these findings, transcriptional activity of a BMP-responsive reporter (BRE-Luc) induced by BMP ligands, ALK2Q207D, or ALK2R206H required the participation of type II receptors (Fig. 3A to D). BMP4-induced transcription was readily observed in WT, BmpRII KO, and ActRIIa KO cells, whereas BMP7 activity was diminished in ActRIIa KO cells, and activity of both ligands was abrogated in BmpRII/ActRIIa KO cells. Expression of ALK2Q207D or ALK2R206H increased basal transcriptional activity, except in BmpRII/ActRIIa KO cells (Fig. 3D). As previously observed (11), the transcriptional activity induced by overexpression of ALK2Q207D was greater than that observed with ALK2R206H, which was in turn greater than that observed with wild-type ALK2 (Fig. 3A and B).
Fig 3.
BMP ligand and GS domain mutant ALK2-mediated signaling requires expression of Bmpr2 or Acvr2a. BMP transcriptional activity was assessed by BMP-responsive element (BRE) luciferase activity in wild-type, Bmpr2−/−, Acvr2a−/−, and Bmpr2−/−:Acvr2a−/− PASMC transiently transfected with wild-type ALK2, ALK2Q207D, or ALK2R206H receptors. Following transient transfection, cells were deprived of serum for 16 h and treated with either vehicle, BMP4, or BMP7 (20 ng/ml) for 24 h. Wild-type and GS domain mutant ALK2 receptors induced BRE-luciferase expression in wild-type, Bmpr2−/−, or Acvr2a−/− cells but failed to induce BRE-luciferase activity in compound Bmpr2−/−:Acvr2a−/− cells lacking both BMP type II receptors. Data are represented as means ± SEM (n = 4; *, P < 0.05; #, P < 0.01; ns, not significant compared with baseline luciferase activity in pcDNA-transfected cells; †, P < 0.05, and ‡, P < 0.01, compared to the activity of the respective ALK2 receptor alone). Results shown are representative of 3 independent experiments.
The ectopic expression of various ALK2 transgenes modified sensitivity to BMP ligands. Overexpression of wild-type ALK2 attenuated the activity of BMP4 but not BMP7 (Fig. 3A to C), similar to previous reports (8). WT or BmpRII KO cells transfected with ALK2Q207D lost incremental responses to BMP7 and in fact demonstrated attenuated activity with BMP4. In contrast, transfection of WT, BmpRII KO, or ActRIIa KO cells with ALK2R206H resulted in preserved or decreased responses to these ligands compared to pcDNA, with BmpRII KO and ActRIIa KO cells retaining sensitivity to BMP7 and BMP4, respectively. Thus, overexpression of wild-type or GS domain ALK2 mutants generally did not amplify and in some cases diminished incremental ligand-mediated activity, particularly for BMP4 (Fig. 2E and 3A to C).
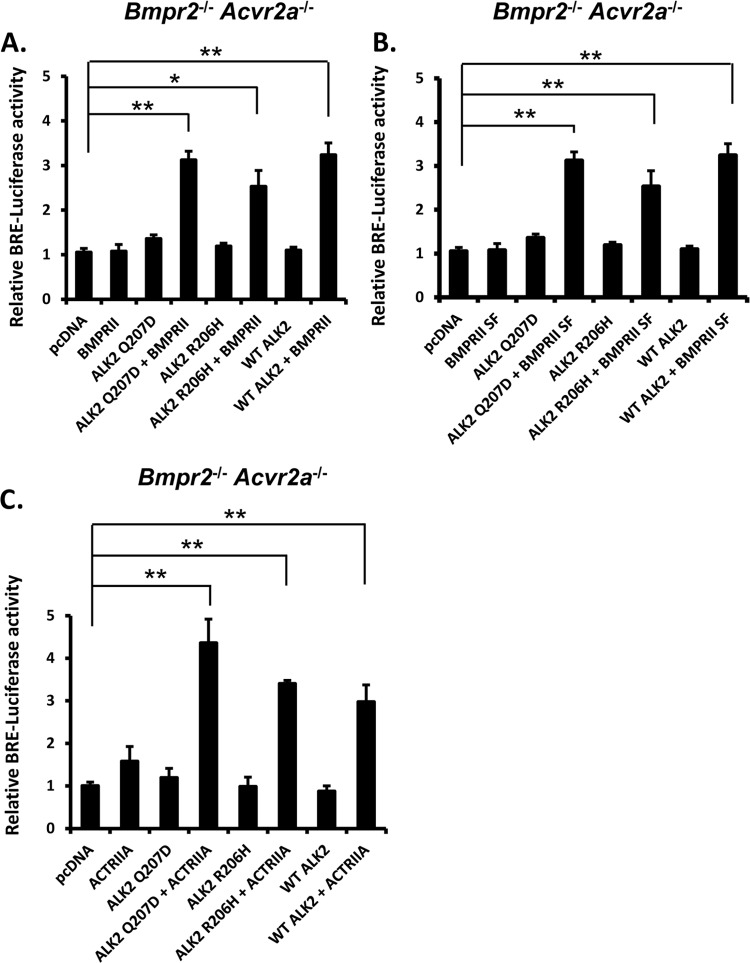
In BmpRII/ActRIIa KO cells, the transcriptional activity induced by either wild-type ALK2, ALK2R206H, or ALK2Q207D could be rescued by transfection with either BMPRII or ACTRIIA, supporting the ability of either type II receptor to facilitate mutant ALK2 receptor activity, even in the absence of exogenous ligand (Fig. 4A to C). The ability of BMPRII to rescue caALK2 signaling was not dependent on the long C-terminal domain of BMPRII, as expression of the BMPRII short isoform (BMPRII SF) also rescued wild-type and mutant ALK2 signaling (Fig. 4B).
Fig 4.
Wild-type or GS domain mutant ALK2-mediated transcriptional activity may be facilitated by either full-length or short-isoform BMPRII or ACTRIIA. BRE-luciferase activity was restored in compound Bmpr2−/−:Acvr2a−/− cells following cotransfection of wild-type ALK2 or GS domain mutant ALK2Q207D or ALK2R206H in combination with wild-type BMPRII (A), BMPRII short isoform (SF) (B), or wild-type ACTRIIA (C). Following transfection, cells were incubated in the absence of serum for 24 h. Data are represented as means ± SEM (n = 4; *, P < 0.01; **, P < 0.001 [compared with luciferase expression in pcDNA-transfected cells]). Results shown are representative of 3 independent experiments.
These in vitro data are consistent with the requirement of either functional BmpRII or ActRIIa for the activity GS domain mutants ALK2Q207D and ALK2R206H. GS domain mutant ALK2 receptors did not render cells hypersensitive to ligands but in some cases diminished or abrogated the incremental activity of exogenous BMP ligands.
GS domain ALK2 mutants do not require BMP type II receptor kinase activity.
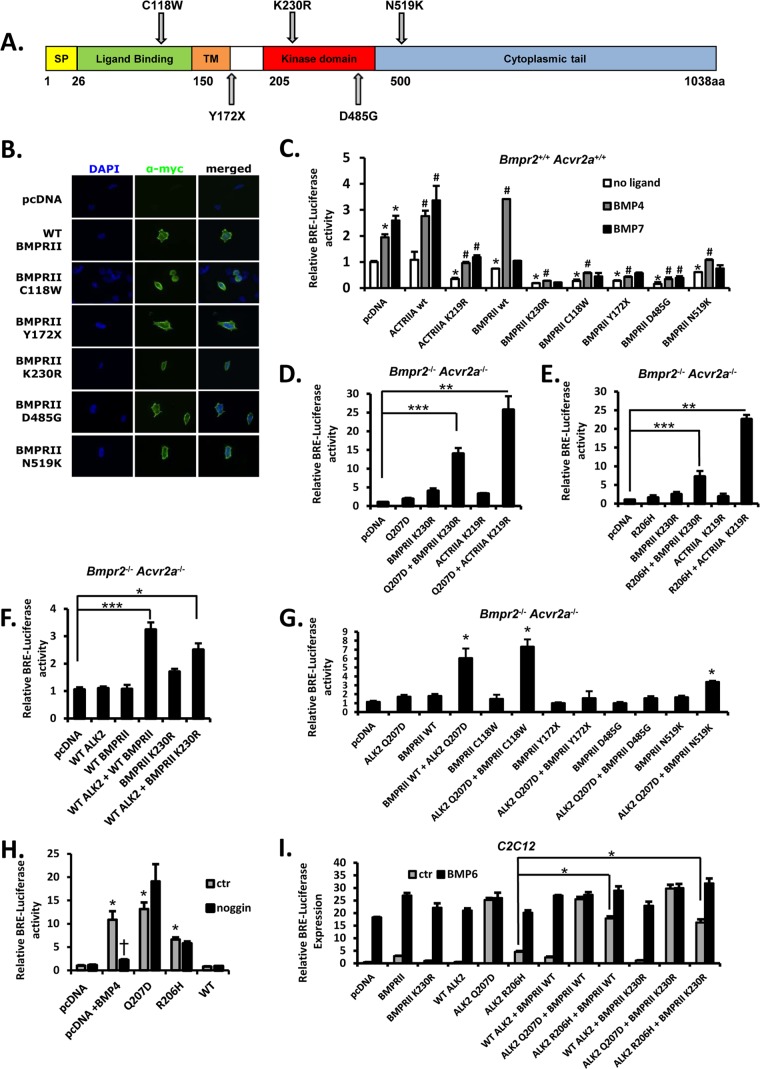
To gain insight into how the type II receptor may facilitate caALK2-mediated signaling, we tested the impact of type II receptors with mutations of various functional domains (Fig. 5A). Since type I receptors are normally activated via phosphorylation of their GS domains by type II receptor kinases, we tested the ability of kinase-defective BMPRII and ACTRIIA receptors lacking catalytic lysine residues (BMPRIIK230R and ACTRIIAK219R) to rescue caALK2 function in BmpRII/ActRIIa KO cells. These kinase-defective receptors localized to the plasma membrane in transfected HEK293T cells (Fig. 5B) and attenuated basal and BMP ligand-induced signaling in wild-type cells (Fig. 5C) consistent with dominant negative effects as previously reported (21). Interestingly, overexpression of wild-type BMPRII in wild-type cells enhanced BMP4- but attenuated BMP7-mediated responses, consistent with the preferential utilization of BmpRII by BMP4 and the use of ActRIIa by BMP7 (18). In BmpRII/ActRIIa KO cells, the function of ALK2R206H and ALK2Q207D was potently rescued by the coexpression of either BMPRIIK230R or ACTRIIAK219R (Fig. 5D and E) to a degree comparable to that of wild-type BMPRII or ACTRIIA (Fig. 4), suggesting that these GS domain ALK2 mutants do not require phosphorylation by the type II receptor for activity. ACTRIIAK219R enhanced ALK2Q207D and ALKR206H activity more efficiently than BMPRIIK230R, suggesting a greater role of ACTRIIA than BMPRII in facilitating caALK2 activity, an interpretation consistent with the observation in mice that loss of ActRIIa had greater impact than loss of BmpRII (Fig. 1A and B). Interestingly, the overexpression of wild-type ALK2 in BmpRII/ActRIIa KO cells in combination with either wild-type BMPRII or BMPRIIK230R modestly enhanced transcriptional activity (Fig. 5F), suggesting that leaky wild-type type I receptor signaling may occur independently of type II receptor kinase function under some circumstances.
Fig 5.
Roles of various type II receptor functional domains in GS domain mutant ALK2 signaling. (A) Schematic illustration of BMPRII mutants used in our study, and corresponding functional domains. SP, signal peptide; TM, transmembrane domain. (B) Cellular localization of wild-type and mutant BMPRII receptors. HEK293T cells were transiently transfected with N-terminally myc-tagged BMPRII plasmids encoding wild-type or mutant receptor proteins and visualized by immunofluorescence with labeled anti-myc antibody (green) and nuclear counterstain (DAPI, blue). (C) Effects of kinase-dead and mutant type II receptors upon basal and ligand-induced BMP-mediated transcription were evaluated in wild-type PASMC via BRE-luciferase activity. Following transfection, cells were deprived of serum and then treated with BMP4 or BMP7 (20 ng/ml) or no ligand. BMP4- and BMP7-induced BRE-luciferase activity was preserved in cells transfected with wild-type ACTRIIA or BMPRII, whereas ACTRIIA and BMPRII mutants with defective kinase function (KD), defective ligand binding (C118W), or truncated or misfolded cytoplasmic domains (Y172X, D485G, and N519K, respectively) attenuated basal and BMP ligand-induced BRE-luciferase activity, suggesting dominant negative effects. Data are represented as mean ± SEM (n = 4; *, P < 0.01 compared with pcDNA control; #, P < 0.02 versus baseline for each wild-type or mutant type II receptor). (D and E) Compound knockout Bmpr2−/−:Acvr2a−/− PASMC were cotransfected with GS domain mutant ALK2Q207D (D) or ALK2R206H (E) or wild-type ALK2 (F) alone or in combination with kinase-deficient BMP type II receptor BMPRIIK230R or ACTRIIAK219R. Following transfection, cells were deprived of serum and luciferase activity assayed, demonstrating that type II receptor kinase activity was not required for the rescue of GS domain mutant and wild-type ALK2 activity in double knockout cells. Data are represented as mean ± SEM (n = 4; *, P < 0.05; **, P < 0.01; ***, P < 0.001 [compared with luciferase activity in pcDNA-transfected cells]). Results shown are representative of 3 independent experiments. (G) Compound knockout Bmpr2−/−:Acvr2a−/− PASMC were cotransfected with ALK2Q207D and various BMPRII mutants. Ligand-binding-defective mutant BMPRIIC118W restored caALK2-mediated BRE-luciferase activity, whereas BMPRII lacking the cytoplasmic segment (BMPRIIY172X) and BMPRII with completely or partially misfolded kinase domains (BMPRIID485G and BMPRIIN519K) were completely or partially defective in rescuing caALK2 function, respectively. Data are mean ± SEM (n = 10; *, P < 0.001 [compared with baseline luciferase expression in pcDNA-transfected cells]). (H) Transfection of wild-type cells with GS domain mutant ALK2Q207D or ALK2R206H, but not wild-type ALK2, or the addition of BMP4 ligand (40 ng/ml) enhanced BRE-luciferase activity. Coincubation with noggin (1,000 ng/ml for 24 h) starting 1 h after transfection inhibited the activity of exogenous BMP4 but did not inhibit the activity of ALK2Q207D or ALK2R206H. Data are mean ± SEM (n = 10; *, P < 0.001 compared with baseline luciferase expression in-pcDNA transfected cells; †, P = 0.02 compared to the activity of BMP4). (I) The effects of overexpressing wild-type and kinase-deficient type II receptor upon GS domain mutant, wild-type ALK2, and ligand-mediated activity in C2C12 cells were evaluated via BRE-luciferase activity in the absence of serum and presence or absence of BMP6 (30 ng/ml, 6 h). Wild-type and kinase-deficient BMPRII could enhance the activity of ALK2R206H but not ALK2Q207D under these conditions. Data are represented as mean ± SEM (n = 6 [unstimulated] and n = 3 [stimulated]; *, P < 0.001).
GS domain ALK2 mutants do not require BMP type II receptor ligand binding activity.
Pathological missense mutations of the BMPRII ligand-binding domain, which are associated with heritable forms of pulmonary arterial hypertension (HPAH), can result from substitutions of cysteine resides which permit proper folding of the extracellular ligand-binding domain (23). Ligand-binding-defective BMPRII proteins may serve as hypofunctional or dominant negative alleles, having markedly decreased sensitivity to exogenous ligands and diminished ability to initiate downstream signaling, although they retain the capacity to bind and phosphorylate type I receptors (22, 27). We examined the ligand-binding-deficient mutant BMPRIIC118W (Fig. 5A), which, consistent with previous reports of severely impaired ligand binding and ligand-mediated signaling (22, 23), markedly suppressed basal and ligand-mediated signaling when expressed in wild-type cells (Fig. 5C). Both surface and perinuclear expression of BMPRIIC118W was observed in HEK293T cells (Fig. 5B), consistent with prior observations of partitioning between the endoplasmic reticulum and the surface membrane (23).
Despite partial membrane localization and dominant negative effects in wild-type cells, coexpression of BMPR2C118W potently rescued the transcriptional activity of ALK2Q207D in BmpRII/ActRIIa KO cells to levels comparable to that of wild-type BMPRII (Fig. 5G). The potent rescue via BMPR2C118W demonstrates that ALK2 GS domain mutants do not require type II receptor ligand-binding function and suggests ligand-independent function of ALK2Q207D. Consistent with this notion of ligand-independent function, the incremental transcriptional activity of ALK2R206H or ALK2Q207D mutants, in contrast to BMP4 ligand, was not susceptible to inhibition by high levels of the exogenous inhibitor noggin (Fig. 5H).
The type II receptor cytoplasmic domain is required for caALK2 function.
A C-to-G transversion in exon 4 of human BMPR2, reported in HPAH, results in a prematurely truncated protein, BMPRIIY172X (Fig. 5A), which contains complete ligand-binding and transmembrane domains but lacks the entire kinase and C-terminal domains (28). BMPRIIY172X was at least partially expressed on the surface of HEK293T cells (Fig. 5B) and suppressed basal and BMP ligand-induced BRE-Luc activity in wild-type cells (Fig. 5C), consistent with a dominant negative effect. However, in contrast to kinase-deficient mutants BMPRIIK230R and ACTRIIK219R, BMPRIIY172X did not rescue the activity of ALK2Q207D in BmpRII/ActRIIa KO cells (Fig. 5G), suggesting that cytoplasmic domains of type II receptors are required for facilitating the activity of caALK2, despite type II receptor kinase function being dispensable.
The BMPRIID485G mutant receptor associated with HPAH features an aspartate substitution in the kinase domain (Fig. 5A). BMPRIID485G retains localization to the plasma membrane and the ability to bind BMP4, but it has a defective kinase domain like in BMPRIIK230R. Unlike BMPRIIK230R, however, BMPRIID485G is predicted to have a misfolded kinase domain which prevents effective interactions with BMP type I receptors (22, 23). BMPRIID485G was detected in the plasma membrane of HEK293T cells (Fig. 5B) but severely attenuated basal and BMP4-induced BRE-Luc activity in wild-type cells, consistent with a dominant negative effect (Fig. 5C). Unlike BMPRIIK230R, BMPRIID485G did not rescue caALK2 activity in BmpRII/ActRIIa KO cells, supporting the need for effective interactions of type I and type II receptor kinase domains in caALK2-mediated signaling (Fig. 5G).
The BMPRIIN519K mutant receptor associated with HPAH (Fig. 5A) has previously been shown to localize to the surface membrane in human PASMC and to activate BRE-Luc in normal mouse mammary gland epithelial cells, although its ability to bind ALK3 in HeLa cells and phosphorylate ALK6 in an in vitro kinase assay is impaired (22, 23). The N519K substitution occurs on the C-terminal boundary of the BMPRII kinase domain and is thought to have a partial destabilizing effect on BMPRII kinase and cytoplasmic domain folding. BMPRIIN519K was expressed in the plasma membrane of HEK293T cells and attenuated basal and ligand-mediated signaling in wild-type cells (Fig. 5B and C). Consistent with its known impaired function, BMPRIIN519K rescued caALK2 function in BmpRII/ActRIIa KO cells but with substantially lower efficiency than wild-type BMPRII (Fig. 5G).
The diminished or absent complementation of caALK2 by BMPRIIY172X, BMPRIID485G, and BMPRIIN519K underscored the importance of interactions between type I and type II receptor cytoplasmic domains, while the potent complementation of caALK2 by BMPRIIK230R and ACTRIIAK219R demonstrated dispensability of type II receptor kinase activity. The effective complementation of caALK2 by ligand-binding mutant BMPRIIC118W, the inability of an excess of noggin to attenuate caALK2 function in the absence of exogenous ligand, and the antagonistic relationship between ligand- and caALK2-mediated signaling support the concept that the contribution of the type II receptor to the activity of caALK2 mutants is ligand independent.
Intrinsic affinity of BMP type I and II receptor kinase domains.
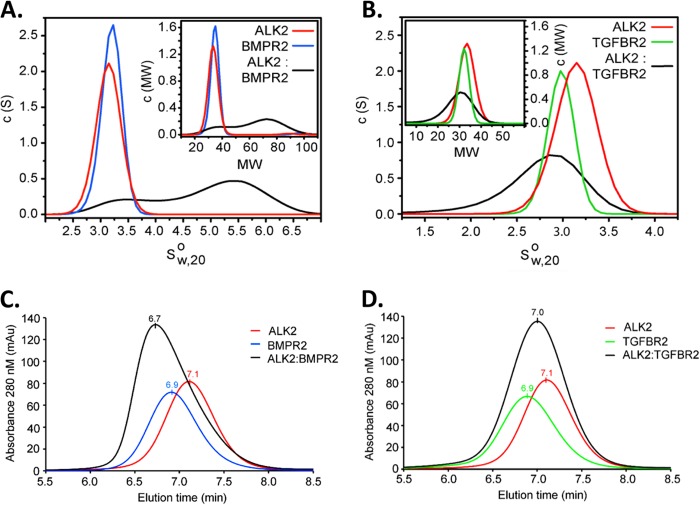
We tested whether or not BMP type II and type I receptor cytoplasmic domains might have intrinsic affinity, to examine the concept that caALK2 might have the potential to form signaling complexes without ligand-induced assembly. We expressed the recombinant kinase domain of wild-type BMPRII and the GS and kinase domains of ALK2 and assessed their ability to form a complex in solution by analytical ultracentrifugation (AUC). In isolation both recombinant proteins were monomeric, but upon mixing a clear shift to higher sedimentation coefficients was observed, indicating that a 1:1 BMPRII-ALK2 heterodimer was formed in solution (Fig. 6A). A control experiment mixing ALK2 with the TGFBRII kinase domain showed no shift by AUC, further demonstrating that intracellular assembly was both constitutive and specific (Fig. 6B). Similar results were obtained using analytical gel filtration (Fig. 6C and D), with the elution of a large earlier peak with the combination of BMPRII and ALK2 but not TGFβRII and ALK2. The observation that cognate type II receptor and ALK2 kinase domains assemble spontaneously in solution supports the concept that oligomerization of BMP type II and type I receptor complexes might occur independently of ligand to enable the signaling of caALK2.
Fig 6.
BMPRII and ALK2 cytoplasmic domains assemble spontaneously in solution. (A) BMP receptor kinase domains specifically assemble in the absence of ligand, based upon analytical ultracentrifugation (AUC) sedimentation velocities of ALK2 and BMPRII cytoplasmic domains in solution. Differential sedimentation coefficient distributions [c(s)] are plotted versus the apparent sedimentation coefficient corrected to water at 20°C (s20°,w0) (main graphs), together with the differential molecular weight distribution [c(M)] versus molecular weight (MW; in thousands) (insets). (B) In contrast, no association between ALK2 and TGFβRII is observed, based upon AUC sedimentation velocity of these cytoplasmic domains when combined in solution. (C and D) By size exclusion chromatography, ALK2 assembles with BMPRII (C) but not TGFβRII (D) based on a small shift to an earlier elution time seen only with BMPRII. Peak elution times are labeled, with a flow rate of 0.3 ml/min.
DISCUSSION
In this study, we examined the minimal requirements for activity of GS domain ALK2 mutants thought to be constitutively active based on their ability to initiate signaling in the absence of upstream signaling. In contrast to constitutively activating mutations affecting other kinases, e.g., members of the epidermal growth factor receptor (EGFR) pathway, in which activating mutations may permit homodimerization and signaling independently of ligand (29), the activity of caALK2 appears to require hetero-oligomerization with its cognate type II receptors, either BMPRII or ACTRIIA, in order to phosphorylate Smad effectors. Also in contrast to other mutant receptor kinases exhibiting ligand hypersensitivity, we found that ligand- and caALK2-mediated signaling were not synergistic and were sometimes antagonistic in the case of BMP4 and caALK2 in WT and BmpRII KO cells (Fig. 2E and 3A and B). A similar antagonistic relationship between BMP4- and ALK2R206H-mediated signaling observed in Drosophila (12) was attributed to the lack of affinity of BMP4 for ALK2 (30), and this was possibly magnified in our system by forced utilization of ActRIIa, which is also known to be less efficient for BMP4 signaling than BmpRII (18). The lack of synergy with ligand-mediated signaling and the ability of GS domain ALK2 mutants to cooperate with a ligand-binding-defective type II receptor provide evidence for ligand-independent function. While type I and type II receptor cytoplasmic domain interactions appear to be essential for caALK2 function, type II receptor kinase function is dispensable, suggesting that GS domain ALK2 mutants may not require phosphorylation to be active. Kinase-defective type II receptors ACTRIIAK219R and BMPRIIK230R, despite exhibiting their expected dominant negative effects in wild-type cells (Fig. 5C), were also found to enhance wild-type ALK2 signaling modestly in double KO cells (Fig. 5F), suggesting that type II-type I complex formation is sufficient to initiate leaky signal transduction in the absence of GS domain mutations when receptors are overexpressed.
In our studies, ActRIIb did not appear to contribute significantly to caALK2-mediated signaling, as the siRNA-mediated knockdown of Acvr2b failed to attenuate caALK2 signaling in BmpRII KO cells (Fig. 2I and J). In contrast, siRNA against Acvr2a abrogated caALK2 signaling in these cells, supporting our other observations that ActRIIa and BmpRII together account for the type II receptor requirement of GS domain ALK2 mutants.
The contention that GS domain ALK2 mutant proteins may function independently of ligand is limited by the possibility that autocrine, paracrine, or intracellular (e.g., endosomal) BMP signaling occurs in the absence of exogenous ligand. The failure of high concentrations of noggin to attenuate caALK2 signaling diminishes the possibility of autocrine or paracrine extracellular signaling but would not exclude autocrine intracellular signaling. However, the prior observation that the ligand-binding function of ALK2R206H is dispensable for its activity (12), taken with the current finding that type II receptor ligand-binding function is dispensable for caALK2 activity, greatly diminishes the possibility of autocrine signaling.
The observation that kinase-deficient type II receptor BMPRIIK230R or ACTRIIAK219R could augment caALK2 signaling was not restricted to type II receptor-deficient cells, as BMPRIIK230R could also potentiate activity of ALK2R206H when coexpressed in unmodified C2C12 cells in the absence of exogenous ligand (Fig. 5I), supporting further the concept that type II receptors facilitate caALK2 signaling independently of their kinase and ligand-binding function. ALK2Q207D activity was not potentiated by ligands in wild-type PASMC, nor was it enhanced by the coexpression of BMPRII in C2C12 cells (Fig. 3A and 5I), suggesting that ALK2Q207D signaling may be maximal under these assay conditions and not limited by the availability of endogenous type II receptors. While C2C12 cells transfected with ALK2R206H could be further stimulated with ligand, incremental signaling was additive compared to each treatment alone rather than exhibiting signal amplification.
A recent study reported that signaling of a human ALK2R206H transgene in Drosophila requires the activity of type II receptor orthologue, Punt, based on its disruption following inducible RNAi-mediated suppression of Punt (12). The findings in Drosophila are consistent with the present data, which further define the minimal signaling requirements of GS domain ALK2 mutants using a type II receptor-deficient mammalian system and in a transgenic mouse model of FOP mated with type II receptor-deficient strains to demonstrate allele-dependent effects of type II receptor expression. Importantly, we show that ActRIIa is the dominant type II receptor providing cosignaling function for caALK2 in vitro and in vivo, consistent with the known potent signaling of ActRIIa-ALK2 complexes (18). In the Drosophila study, when a series of Ala substitutions were introduced in place of three Ser residues in the ALK2R206H GS domain, receptor activity was abolished (12), a result that was interpreted to suggest that phosphorylation may be required for ALK2R206H activity. In contrast, we found that ALK2 GS domain mutants could be rescued in BmpRII/ActRIIa KO cells by kinase-deficient BMPRII or ACTRIIA, suggesting that phosphorylation of caALK2 is dispensable. The discrepancy could potentially be reconciled by an alternate interpretation that replacement of the three GS domain Ser residues with Ala might offset the perturbation of the α-helical GS domain structure normally engendered by R206H mutations, providing stability to the inactive state that can no longer be overcome by phosphorylation (8, 9), resulting in a constitutively inactive protein. The latter interpretation would not require any assumptions about whether caALK2 activity necessitates or is modulated by GS domain phosphorylation. Alternately, autophosphorylation of type I receptors might occur when assembled in the context of a heteromeric signaling complex, potentiating their own activity in the presence of GS domain-activating mutations.
The requirement for the type II receptor despite constitutive enzymatic activity of the type I kinase may reflect structural requirements of the signaling complex for the phosphorylation of SMAD substrates. The ability of type I receptor to dock and/or phosphorylate SMAD substrates may be impaired as a result of dysfunctional cytoplasmic interactions of type II and type I receptors, as suggested by the failure of BMPRIID485G and BMPRIIY172X to support caALK2 activity. Detailed structural studies of the type II and type I receptor cytoplasmic domains complexed with SMAD could reveal critical features of this interaction.
Given the submaximal activity of ALK2R206H observed in this and other studies, it remains possible that ligands still enhance the interaction and assembly of type II-type I signaling complexes (perhaps from heterodimer to heterotetramer), particularly since endogenous levels of receptor expression are considerably lower than with transient transfection. While we did not observe amplification or hypersensitivity of ligands despite overexpression of caALK2 mutants, biological responses might require signaling thresholds accomplished by the additive effects of ligand-mediated and basal ALK2R206H signaling.
The ability of FOP-causing mutant ALK2 proteins to recruit signaling independently of ligand or type II receptor kinase function has several theoretical and practical implications. “Preformed complexes” of BMP type I and type II receptors, i.e., hetero-oligomeric complexes formed in the absence of ligand, have previously been detected by coimmunoprecipitation, as well as on the surface of live cells by immunofluorescence copatching (20). The consequences of BMP2 ligand-mediated signaling have been found to depend on whether signaling results from the nucleation of receptors by ligand or the binding of preformed complexes, with canonical BMP-Smad signaling occurring via the latter (21). Our data demonstrating the requirement for type I and type II cytoplasmic domain interactions but dispensability of ligand-mediated activation support the notion that basal activity of GS domain mutant ALK2 receptors arises from preformed complexes. The observation that type II receptors are required for caALK2-induced heterotopic ossification in mice suggests that signaling via preformed complexes occurs in vivo and may contribute to the pathophysiology of FOP. Therefore, strategies which specifically disrupt oligomerization of caALK2 with type II receptors could be used to inhibit signaling and its consequences, while on the other hand, strategies which aim to intercept ligands or prevent ligand-mediated complex assembly might have a more limited impact on FOP. While cell-permeative molecules which selectively inhibit ALK2 receptor kinase activity might be used to inhibit heterotopic ossification in FOP (14), the dispensability of type II receptor kinase function in our model demonstrates that selective type II receptor kinase inhibitors would fail to arrest the activity of GS domain ALK2 mutants. Complementary strategies for attenuating caALK2-mediated signaling could interfere with type II and type I cytoplasmic domain interactions, potentially at the level of assembly or docking with SMAD substrates, acting by either allosteric or competitive mechanisms to disrupt this essential intermolecular association.
ACKNOWLEDGMENTS
We thank K. Bloch, P. ten Dijke, M. Jose-Goumans, and T. Michel for helpful discussions.
We declare that we have no conflicts of interest.
This work was supported by an Actelion Entelligence Young Investigator Award (J.B.), the U.S. National Institutes of Health (grants HL079943 and AR057374 to P.B.Y., grant DE020843 to Y.M., and grant HL007604 to J.B.), the Pulmonary Hypertension Association (P.B.Y), the Leducq Foundation Transatlantic Network of Excellence (N.W.M. and P.B.Y.), the Howard Hughes Medical Institute (P.B.Y.), and the Deutsche Forschungsgemeinschaft-funded Berlin-Brandenburg School for Regenerative Therapies (P.K.). P.F. is supported by a Wellcome Trust Career Development Fellowship (095751/Z/11/Z). The Structural Genomics Consortium is a not-for-profit, public-private partnership that receives funds from AbbVie, Boehringer Ingelheim, the Canada Foundation for Innovation, the Canadian Institutes for Health Research, Genome Canada, GlaxoSmithKline, Janssen, Lilly Canada, the Novartis Research Foundation, the Ontario Ministry of Economic Development and Innovation, Pfizer, Takeda, and the Wellcome Trust (092809/Z/10/Z).
Footnotes
Published ahead of print 9 April 2013
REFERENCES
- 1. Di Nicolantonio F, Bardelli A. 2006. Kinase mutations in cancer: chinks in the enemy's armour? Curr. Opin. Oncol. 18:69–76 [DOI] [PubMed] [Google Scholar]
- 2. Parma J, Duprez L, Van Sande J, Paschke R, Tonacchera M, Dumont J, Vassart G. 1994. Constitutively active receptors as a disease-causing mechanism. Mol. Cell. Endocrinol. 100:159–162 [DOI] [PubMed] [Google Scholar]
- 3. Tao YX. 2008. Constitutive activation of G protein-coupled receptors and diseases: insights into mechanisms of activation and therapeutics. Pharmacol. Ther. 120:129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Refetoff S, Dumont J, Vassart G. 2001. Thyroid disorders, p 4029–4075 In Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B. (ed), The metabolic and molecular bases of inherited disease, 8th ed McGraw-Hill, New York, NY [Google Scholar]
- 5. Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. 2008. Fibrodysplasia ossificans progressiva. Best Pract. Res. Clin. Rheumatol. 22:191–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. 2009. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum. Mutat. 30:379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Brown MA, Kaplan FS. 2006. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 38:525–527 [DOI] [PubMed] [Google Scholar]
- 8. Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN. 2012. Structure of the BMP receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J. Biol. Chem. 287:36990–36998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Groppe JC, Shore EM, Kaplan FS. 2007. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin. Orthop. Relat. Res. 462:87–92 [DOI] [PubMed] [Google Scholar]
- 10. Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. 1998. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 273:25628–25636 [DOI] [PubMed] [Google Scholar]
- 11. Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T. 2009. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J. Biol. Chem. 284:7149–7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le VQ, Wharton KA. 2012. Hyperactive BMP signaling induced by ALK2(R206H) requires type II receptor function in a Drosophila model for classic fibrodysplasia ossificans progressiva. Dev. Dyn. 241:200–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, Shore EM. 2009. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J. Clin. Invest. 119:3462–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. 2008. BMP type I receptor inhibition reduces heterotopic ossification. Nat. Med. 14:1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beppu H, Lei H, Bloch KD, Li E. 2005. Generation of a floxed allele of the mouse BMP type II receptor gene. Genesis 41:133–137 [DOI] [PubMed] [Google Scholar]
- 16. Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. 1999. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev. Biol. 213:157–169 [DOI] [PubMed] [Google Scholar]
- 17. Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y. 2006. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis 44:159–167 [DOI] [PubMed] [Google Scholar]
- 18. Yu PB, Beppu H, Kawai N, Li E, Bloch KD. 2005. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J. Biol. Chem. 280:24443–24450 [DOI] [PubMed] [Google Scholar]
- 19. Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. 2008. Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J. Biol. Chem. 283:3877–3888 [DOI] [PubMed] [Google Scholar]
- 20. Gilboa L, Nohe A, Geissendorfer T, Sebald W, Henis YI, Knaus P. 2000. Bone morphogenetic protein receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors. Mol. Biol. Cell 11:1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. 2002. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 277:5330–5338 [DOI] [PubMed] [Google Scholar]
- 22. Nasim MT, Ghouri A, Patel B, James V, Rudarakanchana N, Morrell NW, Trembath RC. 2008. Stoichiometric imbalance in the receptor complex contributes to dysfunctional BMPR-II mediated signalling in pulmonary arterial hypertension. Hum. Mol. Genet. 17:1683–1694 [DOI] [PubMed] [Google Scholar]
- 23. Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. 2002. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum. Mol. Genet. 11:1517–1525 [DOI] [PubMed] [Google Scholar]
- 24. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764 [DOI] [PubMed] [Google Scholar]
- 25. Schuck P. 2000. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78:1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laue TM, Shah BD, Ridgeway TM, Pelletier SL. 1992. Analytical ultracentrifugation in biochemistry and polymer science. Royal Society of Chemistry, Cambridge, United Kingdom [Google Scholar]
- 27. Delot EC, Bahamonde ME, Zhao M, Lyons KM. 2003. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development 130:209–220 [DOI] [PubMed] [Google Scholar]
- 28. Cogan JD, Pauciulo MW, Batchman AP, Prince MA, Robbins IM, Hedges LK, Stanton KC, Wheeler LA, Phillips JA, III, Loyd JE, Nichols WC. 2006. High frequency of BMPR2 exonic deletions/duplications in familial pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 174:590–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gotoh N. 2011. Somatic mutations of the EGF receptor and their signal transducers affect the efficacy of EGF receptor-specific tyrosine kinase inhibitors. Int. J. Clin. Exp. Pathol. 4:403–409 [PMC free article] [PubMed] [Google Scholar]
- 30. ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. 1994. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 269:16985–16988 [PubMed] [Google Scholar]