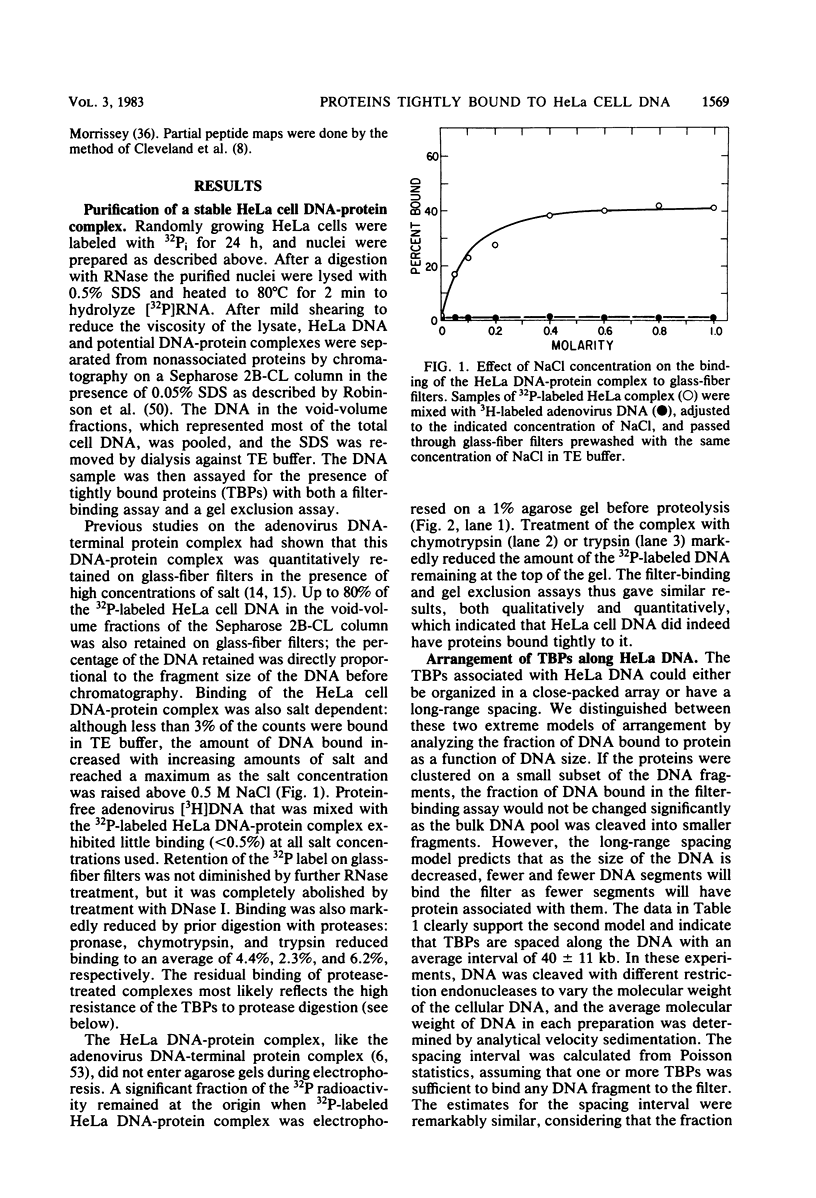
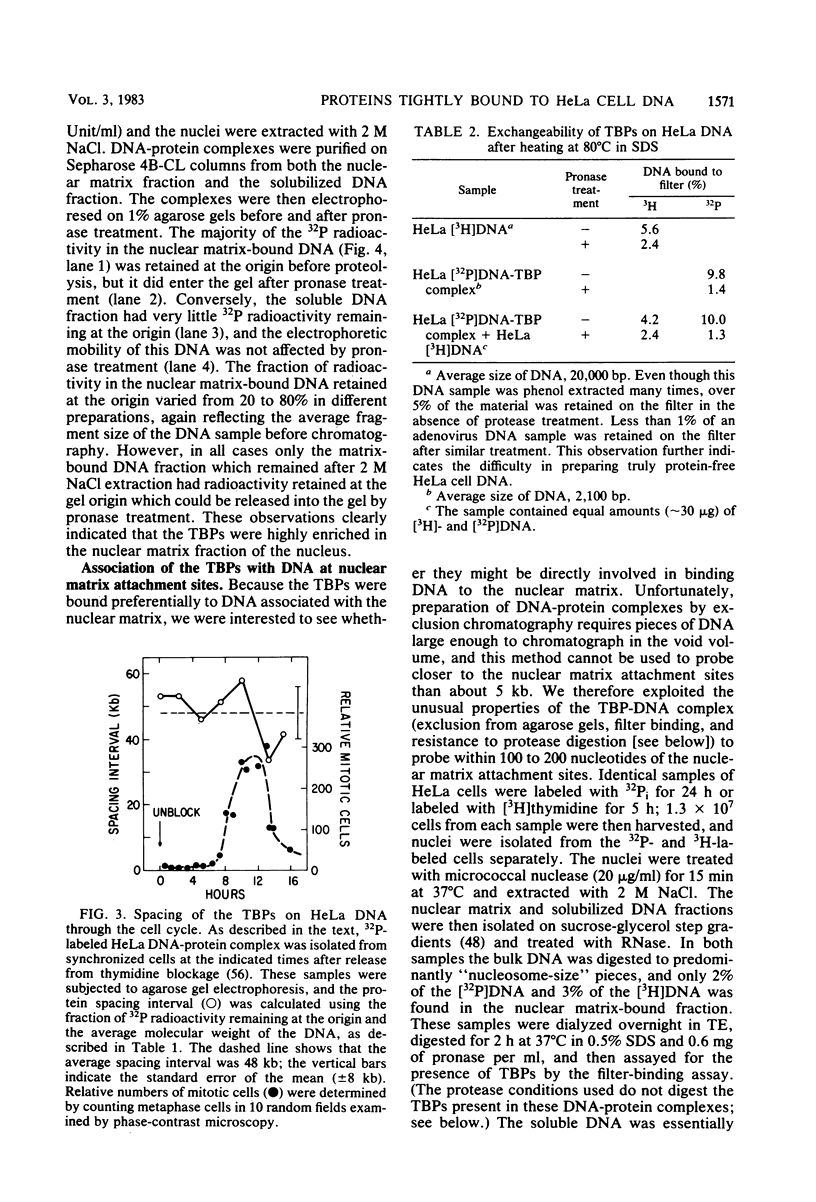
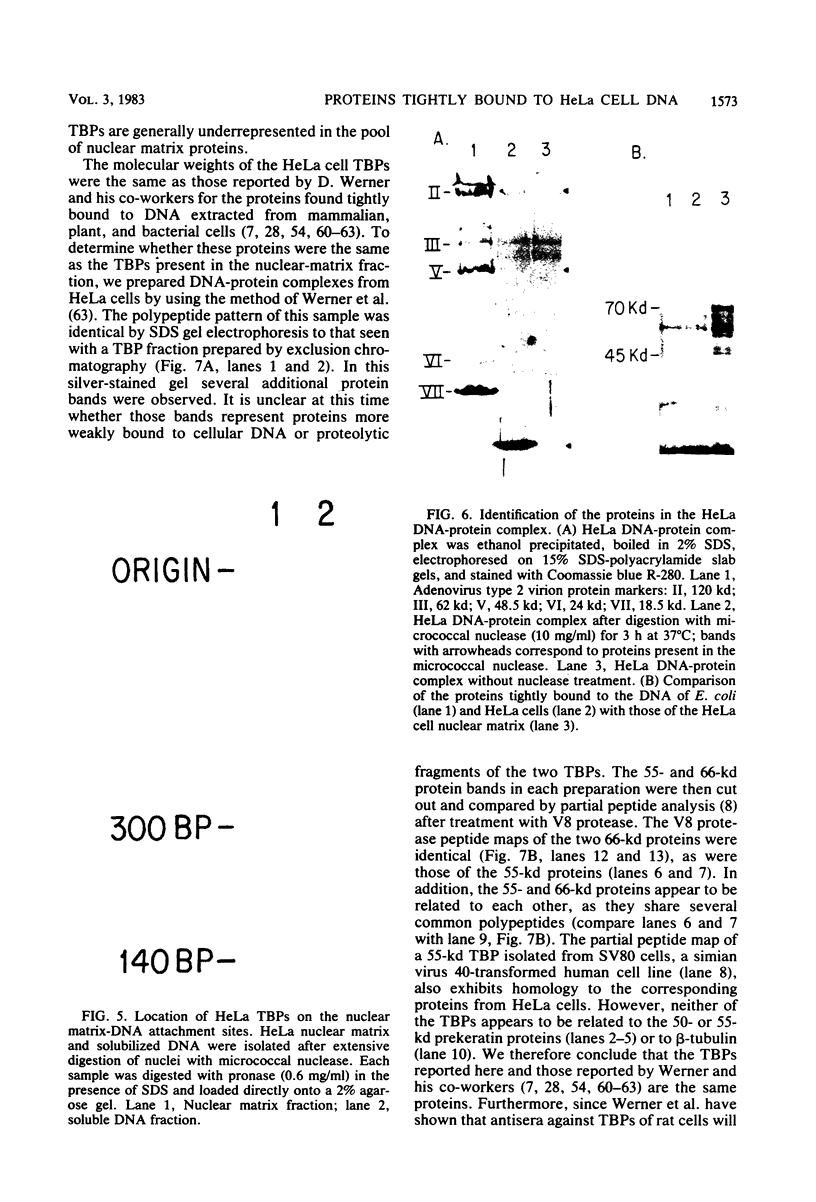
Abstract
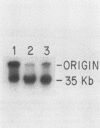
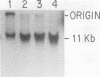
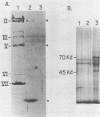
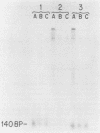
DNA-protein complexes have been isolated from HeLa cell nuclei and nuclear matrix preparations. Two proteins, 55 and 66 kilodaltons in size, remain bound to HeLa DNA after treatment at 80 degrees C in 2% sodium dodecyl sulfate and purification by exclusion chromatography on Sepharose 2B-CL in the presence of 0.3% sodium dodecyl sulfate. These proteins appear to be tightly bound but not covalently linked to the DNA, and they are distributed over the DNA with an average spacing of 40 kilobase pairs. This spacing distribution remains essentially constant throughout the cell cycle. The proteins are bound to the residual 2% of HeLa cell DNA which remains attached to the nuclear matrix after extensive nuclease digestion, a condition which reduces the average size of the DNA to approximately 150 base pairs. Our results suggest that these tightly bound proteins are involved in anchoring cellular DNA to the nuclear matrix. These tightly bound proteins are identical by partial peptide mapping to proteins found tightly bound to the DNA of mammalian, plant, and bacterial cells (D. Werner and C. Petzelt, J. Mol. Biol. 150:297-302, 1981), implying that these proteins are involved in the organization of chromosomal domains and are highly conserved in both procaryotic and eucaryotic cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph K. W. Organization of chromosomes in HeLa cells: isolation of histone-depleted nuclei and nuclear scaffolds. J Cell Sci. 1980 Apr;42:291–304. doi: 10.1242/jcs.42.1.291. [DOI] [PubMed] [Google Scholar]
- Agutter P. S., Richardson J. C. Nuclear non-chromatin proteinaceous structures: their role in the organization and function of the interphase nucleus. J Cell Sci. 1980 Aug;44:395–435. doi: 10.1242/jcs.44.1.395. [DOI] [PubMed] [Google Scholar]
- Bekhor I., Mirell C. J. Simple isolation of DNA hydrophobically complexed with presumed gene regulatory proteins (M3). Biochemistry. 1979 Feb 20;18(4):609–616. doi: 10.1021/bi00571a010. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976 Nov;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Bowen B. C. DNA fragments associated with chromosome scaffolds. Nucleic Acids Res. 1981 Oct 10;9(19):5093–5108. doi: 10.1093/nar/9.19.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capesius I., Krauth W., Werner D. Proteinase K-resistant and alkali-stably bound proteins in higher plant DNA. FEBS Lett. 1980 Feb 11;110(2):184–186. doi: 10.1016/0014-5793(80)80068-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Comings D. E., Okada T. A. Nuclear proteins. III. The fibrillar nature of the nuclear matrix. Exp Cell Res. 1976 Dec;103(2):341–360. doi: 10.1016/0014-4827(76)90271-8. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A., Jost E. Characterization of nuclear structures containing superhelical DNA. J Cell Sci. 1976 Nov;22(2):303–324. doi: 10.1242/jcs.22.2.303. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Mapping sequences in loops of nuclear DNA by their progressive detachment from the nuclear cage. Nucleic Acids Res. 1980 Jul 11;8(13):2895–2906. doi: 10.1093/nar/8.13.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R., Lang J., Hayday A., Lania L., Fried M., Chiswell D. J., Wyke J. A. Active viral genes in transformed cells lie close to the nuclear cage. EMBO J. 1982;1(4):447–452. doi: 10.1002/j.1460-2075.1982.tb01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs D. H., Pearson G. D. Filter-binding assay for covalent DNA-protein complexes: adenovirus DNA-terminal protein complex. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5291–5295. doi: 10.1073/pnas.75.11.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs D. H., Robinson A. J., Bodnar J. W., Jones C. J., Pearson G. D. Detection of covalent DNA-protein complexes: the adenovirus DNA-terminal protein complex and HeLa DNA-protein complexes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):741–753. doi: 10.1101/sqb.1979.043.01.082. [DOI] [PubMed] [Google Scholar]
- Dijkwel P. A., Mullenders L. H., Wanka F. Analysis of the attachment of replicating DNA to a nuclear matrix in mammalian interphase nuclei. Nucleic Acids Res. 1979 Jan;6(1):219–230. doi: 10.1093/nar/6.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman C. W. Bidirectional chromosome replication: some topological considerations. J Theor Biol. 1974 Jan;43(1):187–195. doi: 10.1016/s0022-5193(74)80052-4. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B., Anderson C. W. Adenovirus type 2 terminal protein: purification and comparison of tryptic peptides with known adenovirus-coded proteins. J Virol. 1979 Sep;31(3):823–835. doi: 10.1128/jvi.31.3.823-835.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig M. Organization of mammalian chromosomal DNA: supercoiled and folded circular DNA subunits from interphase cell nuclei. Acta Biol Med Ger. 1978;37(3):421–432. [PubMed] [Google Scholar]
- Hodge L. D., Mancini P., Davis F. M., Heywood P. Nuclear matrix of HeLa S3 cells. Polypeptide composition during adenovirus infection and in phases of the cell cycle. J Cell Biol. 1977 Jan;72(1):194–208. doi: 10.1083/jcb.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T., Nakane M., Anzai K., Ando T. Supercoiled DNA folded by non-histone proteins in cultured mammalian cells. Nature. 1975 Dec 4;258(5534):445–447. doi: 10.1038/258445a0. [DOI] [PubMed] [Google Scholar]
- Igó-Kemenes T., Zachau H. G. Domains in chromatin structure. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):109–118. doi: 10.1101/sqb.1978.042.01.012. [DOI] [PubMed] [Google Scholar]
- Jeppesen P. G., Bankier A. T. A partial characterization of DNA fragments protected from nuclease degradation in histone depleted metaphase chromosomes of the Chinese hamster. Nucleic Acids Res. 1979 Sep 11;7(1):49–67. doi: 10.1093/nar/7.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauth W., Werner D. Analysis of the most tightly bound proteins in eukaryotic DNA. Biochim Biophys Acta. 1979 Oct 25;564(3):390–401. doi: 10.1016/0005-2787(79)90030-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Non-histone proteins and long-range organization of HeLa interphase DNA. J Mol Biol. 1982 Apr 5;156(2):325–344. doi: 10.1016/0022-2836(82)90332-1. [DOI] [PubMed] [Google Scholar]
- Marsden M. P., Laemmli U. K. Metaphase chromosome structure: evidence for a radial loop model. Cell. 1979 Aug;17(4):849–858. doi: 10.1016/0092-8674(79)90325-8. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto L. H. Enrichment of satellite DNA on the nuclear matrix of bovine cells. Nature. 1981 Dec 3;294(5840):481–482. doi: 10.1038/294481a0. [DOI] [PubMed] [Google Scholar]
- McCready S. J., Godwin J., Mason D. W., Brazell I. A., Cook P. R. DNA is replicated at the nuclear cage. J Cell Sci. 1980 Dec;46:365–386. doi: 10.1242/jcs.46.1.365. [DOI] [PubMed] [Google Scholar]
- McParland R. H., Brown L. R., Pearson G. D. Cleavage of lambda DNA by a site-specific endonuclease from Serratia marcescens. J Virol. 1976 Sep;19(3):1006–1011. doi: 10.1128/jvi.19.3.1006-1011.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nakane M., Ide T., Anzai K., Ohara S., Andoh T. Supercoiled DNA folded by nonhistone proteins in cultured mouse carcinoma cells. J Biochem. 1978 Jul;84(1):145–157. doi: 10.1093/oxfordjournals.jbchem.a132103. [DOI] [PubMed] [Google Scholar]
- Nelkin B. D., Pardoll D. M., Vogelstein B. Localization of SV40 genes within supercoiled loop domains. Nucleic Acids Res. 1980 Dec 11;8(23):5623–5633. doi: 10.1093/nar/8.23.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G. L., Bekhor I. Enrichment of selected active human gene sequences in the placental deoxyribonucleic acid fraction associated with tightly bound nonhistone chromosomal proteins. Biochemistry. 1981 Jun 9;20(12):3568–3578. doi: 10.1021/bi00515a041. [DOI] [PubMed] [Google Scholar]
- Okada T. A., Comings D. E. Higher order structure of chromosomes. Chromosoma. 1979 Apr 5;72(1):1–14. doi: 10.1007/BF00286426. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B. Sequence analysis of nuclear matrix associated DNA from rat liver. Exp Cell Res. 1980 Aug;128(2):466–470. doi: 10.1016/0014-4827(80)90083-x. [DOI] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Structure and properties of the bacterial nucleoid. Cell. 1982 Oct;30(3):667–669. doi: 10.1016/0092-8674(82)90269-0. [DOI] [PubMed] [Google Scholar]
- Plagens U. Effect of salt-treatment on manually isolated polytene chromosomes from Chironomus tentans. Chromosoma. 1978 Aug 21;68(1):1–19. doi: 10.1007/BF00330369. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Chernokhvostov V. V., Roodyn A. V., Zbarsky I. B., Georgiev G. P. Proteins tightly bound to DNA in the regions of DNA attachment to the skeletal structures of interphase nuclei and metaphase chromosomes. Cell. 1981 Nov;27(1 Pt 2):65–73. doi: 10.1016/0092-8674(81)90361-5. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Mantieva V. L., Georgiev G. P. The similarity of DNA sequences remaining bound to scaffold upon nuclease treatment of interphase nuclei and metaphase chromosomes. Nucleic Acids Res. 1979 Nov 24;7(6):1713–1735. doi: 10.1093/nar/7.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. E., Keller J. M. The polypeptide composition and ultrastructure of nuclear ghosts isolated from mammalian cells. Biochim Biophys Acta. 1976 Oct 22;444(3):899–911. doi: 10.1016/0304-4165(76)90336-6. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bodnar J. W., Coombs D. H., Pearson G. D. Replicating adenovirus 2 DNA molecules contain terminal protein. Virology. 1979 Jul 15;96(1):143–158. doi: 10.1016/0042-6822(79)90180-6. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Spiess E., Neuer B., Werner D. Isolation and visualisation of alkali stable protein/DNA complexes. Biochem Biophys Res Commun. 1982 Jan 29;104(2):548–556. doi: 10.1016/0006-291x(82)90672-6. [DOI] [PubMed] [Google Scholar]
- Tatchell K., Van Holde K. E. Reconstitution of chromatin core particles. Biochemistry. 1977 Nov 29;16(24):5295–5303. doi: 10.1021/bi00643a021. [DOI] [PubMed] [Google Scholar]
- Thilly W. G. Maintenance of perpetual synchrony in HeLa S3 culture: theoretical and empirical approaches. Methods Cell Biol. 1976;14:273–285. doi: 10.1016/s0091-679x(08)60489-6. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Wanka F., Mullenders L. H., Bekers A. G., Pennings L. J., Aelen J. M., Eygensteyn J. Association of nuclear DNA with a rapidly sedimenting structure. Biochem Biophys Res Commun. 1977 Jan 24;74(2):739–747. doi: 10.1016/0006-291x(77)90364-3. [DOI] [PubMed] [Google Scholar]
- Warren A. C., Cook P. R. Supercoiling of DNA and nuclear conformation during the cell-cycle. J Cell Sci. 1978 Apr;30:211–226. doi: 10.1242/jcs.30.1.211. [DOI] [PubMed] [Google Scholar]
- Werner D., Hadjiolov D., Neuer B. Protease inducible alkali lability of DNA from proliferating and non-proliferating cells. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1047–1054. doi: 10.1016/0006-291x(81)91929-x. [DOI] [PubMed] [Google Scholar]
- Werner D., Krauth W., Hershey H. V. Internucleotide protein linkers in Ehrlich ascites cell DNA. Biochim Biophys Acta. 1980 Jul 29;608(2):243–258. doi: 10.1016/0005-2787(80)90170-7. [DOI] [PubMed] [Google Scholar]
- Werner D., Petzelt C. Alkali-stably bound proteins in eukaryotic and prokaryotic DNAs show common characteristics. J Mol Biol. 1981 Aug 5;150(2):297–302. doi: 10.1016/0022-2836(81)90453-8. [DOI] [PubMed] [Google Scholar]
- Werner D., Zimmermann H. P., Rauterberg E., Spalinger J. Antibodies to the most tightly bound proteins in eukaryotic DNA. Exp Cell Res. 1981 May;133(1):149–157. doi: 10.1016/0014-4827(81)90365-7. [DOI] [PubMed] [Google Scholar]