Abstract
Stem/progenitor cells and their lineage derivatives are often identified by patterns and intensity of cell clusters of differentiation presentation. However, the cell biochemical façade can prove to be elusive, transient, and subject to interlaboratory disparities. To enhance current methods of lung stem cell isolation and identification and to investigate biophysical changes, which occur during homeostasis and in response to acute lung injury, we separated cells on a discontinuous density gradient, of 1.025–1.074 g/cm3, and characterized the eluted lineages. At homeostasis, surfactant protein-C (SFTPC)-expressing cells of the alveolar type (AT)-2 lineage possessed average densities ≥1.039 g/cm3 and aquaporin-5 producing AT1 cells equilibrated at densities <1.039 g/cm3. While 0.74%±0.32% of lung cells were determined proliferating or postmitotic by BrdU nucleotide uptake, 73% of CD49f-, 72% of c-KIT-, and 61% of SCA-1-positive cells (putative alveolar progenitor lineage markers) showed densities ≤1.039 g/cm3. CD49f/EpCAMhi progenitors, as well as c-KITpos/CD45neg cells, could be enriched at the 1.039 g/cm3 interface. Following acute bleomycin-induced injury, the frequency of BrdU-incorporating cells rose to 0.92%±0.36% and density could largely explain cell-lineage distribution. Specifically, a decline in the density of mitotic/postmitotic SFTPC-positive cells to ≤1.029 g/cm3, in conjunction with an increase in CD45-positive, and proliferating CD45 and c-KIT cells in the heaviest fraction (≥1.074 g/cm3) were observed. These data attest to the generation of AT2 cells from low-density precursors and emphasize a relationship between cell density and molecular expression following injury, expanding on our current understanding of lung and progenitor cell dynamics.
Introduction
Evaluation of elemental biophysical properties can provide insight into how cell density relates to lineage. Density conservation could, in principle, also provide a template for stem cell identification, and enhance our ability to track mitosis and/or differentiation, contributing to future advancements in regenerative medicine.
As the principle organ responsible for ventilation and gas exchange in vertebrates, the lung possesses unique tissue characteristics and cell properties to withstand mechanical stretch, compression, pressure, and harmful exposure to xenobiotics and hyperoxia [1]. The complex and dynamic structure of this organ and particularly that of the delicate epithelial lining, which is prone to injury, makes epithelial stem cell characterization crucial for understanding tissue maintenance and repair. Promiscuity of reported lineage markers expressed in lung homeostasis and repair and disparate interlaboratory experimental conditions have complicated stem and amplifying cell classification [2–4]. Hence, while some concur that lung regeneration occurs through a classical multipotential stem cell, others recognize a role for facultative progenitor cells that maintain physiological functionality and convert to regional restricted progenitors [5–7]. In the distal lung, a contentious c-KIT-positive multipotential stem cell was reported in humans, while more specified epithelial progenitor cell candidates were identified by CD49f/EpCAMhi, E-Cad/Lgr6, or CD49f/CD104 immunophenotypes and/or surfactant protein-c (SFTPC) and secretoglobin, family 1A, member 1 (SCGB1A1) protein coexpression [4,8–12]. With current progress in stem cell research based largely on protein biochemistry, new approaches to tackle evolving questions of identification and differentiation are warranted.
Biophysical discrimination between cells can be accomplished by differences in density, which simplistically is defined as mass per unit volume. Isopycnic, or density gradient centrifugation/sedimentation, techniques trap cells by their tendency to equilibrate in a solution equal to its own density [13]. Historically, density gradient centrifugation was the method of choice to elaborate on processes of cell development and classification when studies in intact tissue were difficult to interpret. By this method, rat total lung cell homogenate was reported to be distributed over a density range of 1.020–1.100 g/cm3 and alveolar type (AT)-2 cells to possess densities between 1.040–1.080 g/cm3 [14,15]. While a wave of reports has indicated that stem cells of endothelial, mesenchymal, neural, hepatic, adipose, and spermatogonial origin could be isolated by density [16–21], to the best of our knowledge no study has thoroughly attempted to fractionate and track lung stem cell dynamics by density. In this report, we focus on the density of individual cell lineages and elaborate on biophysical changes, which occur during homeostasis and in response to the application of bleomycin, an inducer of epithelial injury, pulmonary inflammation, and fibroproliferation [22,23].
Materials and Methods
Mice and cell fractionation procedures
Male and female mice (C57BL/6) of age 1–2 months were purchased from the NCI (Frederik, MD) and all animal studies complied with the Institutional Animal Care and Use Committee protocols of Yeshiva University. Mice were euthanized, the thoracic cavity surgically opened and lungs perfused of blood via the pulmonary artery and harvested. The trachea and main stem bronchi were removed and after a fine mince, distal lung cells were dissociated in a collagenase/dispase mixture (Roche, Indianapolis, IN) for 1 h at 37°C. The suspension was gently inverted and filtered through a 70-μm pore size of nylon gauze (BD Biosciences, San Jose, CA). Three percent bovine calf serum in phosphate-buffered saline (PBS) was added to the resulting suspension (2 mL), which was then layered on a discontinuous isotonic synthetic, colloidal solution of polyvinylpyrrolidone-coated silica, Percoll (MP Biomedicals, Santa Ana, CA) gradient (8 mL) and centrifuged at 400 g for 17 min at 4°C in an angle rotor centrifuge (Eppendorf, San Diego, CA). Fractions were then collected and cells washed ×3 and resuspended in PBS.
Column solutions, preparation, and density measurements
A stock isotonic Percoll solution (SIPS) with a final density of 1.123 g/cm3 was prepared in a 1.5 M saline solution (v/v). This solution was then adjusted to final densities with 0.15 M saline according to the calculation:
Vy=Vi(Pi-P)/(P-Py)
Where Vy is volume of saline, Vi is volume and Pi density of SIPS, P is final density and Py is density of 0.15 M saline [24].
The refractive index was then measured using a MarkII plus ABBE refractometer (Leica Microsystems, Buffalo Grove, IL) and density was calculated using the refractive properties of Percoll [24]. The average of eight experiments was used to represent the densities of FRs one through five. The pH of the gradient was 7.7±0.03 (at 22.9°C±0.1°C) and the density of the cell suspension buffer (PBS; Invitrogen, Grand Island, NY) was ∼1.00 g/cm3.
RNA processing and quantitative real-time PCR experiments
RNA was isolated from fractionated cells by the Trizol method (Invitrogen) and purified using RNeasy columns (Qiagen, Valencia, CA). RNA quality and quantity were determined by a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA). RNA was stored at −80°C. First-strand cDNA synthesis using the SuperScript III reverse transcriptase kit (Invitrogen) was performed on 1 μg total RNA using oligo(dT)12–18 (Invitrogen). All real-time reactions were performed using an ABI-PRISM 7300 sequence detection system (Applied Biosystems, Carlsbad, CA) starting with 10 min of Taq activation at 95°C, followed by 40 cycles of primer melting (95°C, 30 s), and an annealing and extension step appropriate for each primer (58°C–60°C, 60 s). The absence of primer-dimers was verified postamplification by melting curve analysis. Except for actin, alpha-1 (Acta1) forward 5′-CGCAAATGCTTCTAGGCGCACT-3′ and reverse 5′ ACACGTCAAAAACAGGCGCCGG-3′, and telomerase (Tert) forward 5′-CATGGAGAACAAGCTGTTTGC-3′and reverse 5′-CAGGGAAGTTCACCACTGTC-3′, all primer sequences have been previously reported [25]. These include Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), CCAAT/enhancer-binding protein alpha (Cebpa), secretoglobin, family 1A, member 1 (Scgb1a1), Sftpc, Aqp5, surfactant protein B (Sftpb), and CD31. Expression Ct's were normalized to that of Gapdh and data are presented as 2−ΔΔCt.
Cell culture
For growth, cells were cultured in Dulbecco's modified Eagle's media (DMEM; Invitrogen)+10% fetal calf serum (FCS; Atlanta Biologicals, Lawrenceville, GA). Mouse embryonic fibroblasts (MEFs) were isolated from the embryonic tail, grown in DMEM+FBS, and 24 h before experimentation incubated for 2 h with 10 μg/mL mytomycin-C (Santa Cruz Biotech, Santa Cruz, CA) for mitotic inactivation. For clonogenic assays, media were supplemented with penicillin/streptomycin (Invitrogen) and cells were cultivated on MEF-feeders grown on six-well plastic or glass chamber slides (Lab-Tek II CC2, Thermo Fisher Scientific). The fractionated total lung (50,000), CD49f/EpCAMhi and c-KITpos/CD45neg isolate (500) cells were resuspended in 0.5 mL media and plated at a density of 526 cells/cm2 and 714 cells/cm2, respectively. Clonal growth was analyzed after 10 days with colonies defined as ≥50 cells.
Immunofluorescence
Cells were sedimented by cytospin (Thermo Fisher Scientific), rinsed in cold PBS, fixed in 1% paraformaldehyde for 5 min, washed in PBS, and labeled with commercially available primary antibodies at 4°C overnight, which included goat or rabbit anti-SCGB1A1 (Santa Cruz Biotech), rabbit anti-Ki67 (Abcam, Cambridge, MA), and anti-prosurfactant protein C (pro-SFTPC/SFTPC; Millipore, Billerica, MA). Mouse anti-actin alpha-1 (ACTA1) and anti-β-actin (ACTB) were procured from Sigma (St. Louis, MO), and mouse anti-BrdU was purchased from eBioscience (San Diego, CA). Cells were then washed and treated with goat anti-mouse or chicken anti-goat Alexa-488 (Invitrogen) or goat anti-mouse or goat anti-rabbit Cyanine-3 (Jackson Immunoresearch Laboratories, West Grove, PA) secondaries for 45 min at room temperature, DAPI (Sigma), and covered in Fluoromount-G (Southern Biotech, Birmingham, AL) with no. 1 cover slips (Thermo Fisher Scientific). For cell BrdU experiments, cells were pulsed with 10 μM BrdU, and processed in 95% ethanol and 5% acetic acid (v/v) for 10 min, and washed ×3 in PBS, followed by a 1.5 M HCl incubation of 30 min, and an additional wash in PBS supplemented with 1% serum. Antibodies were then added and samples processed as mentioned above. Photomicrographs were captured using an Olympus IX81 microscope equipped with a XM10 cooled CCD camera head interfaced with the cellSens dimension imaging software package (Olympus, Bannockburn, IL), globally adjusted for contrast and brightness and composed using Photoshop software (Adobe systems, San Jose, CA). Confocal images were captured using an AOBS SP2 (Analytical Imaging Core, Albert Einstein College of Medicine) laser scanning microscope and Z-projections generated using the ImageJ software package. For cell length measurements, fractionated cells were incubated in cell tracker red (Invitrogen) for 0.5 h, fixed in 4% formaldehyde, washed, and rinsed in the DAPI solution. The cell and nuclear length was measured using the measurement and region of interest option of the cellSens dimension imaging software package (Olympus).
Immunoblotting
Fractionated lung cells were rinsed in cold PBS, solubilized in a boiling SDS sample buffer (8% SDS, 0.2 M Tris-HCl pH 6.8, 10 mM EDTA, 40% glycerol) supplemented with protease inhibitors (protease inhibitor cocktail; Sigma), sheared through a 20G syringe needle, and spun at 13,000 g for 30 min. Westerns were performed as previously reported [26]. In brief, protein concentrations were determined (Bio-Rad DC Protein Assay, Hercules, CA) and 70 μg of protein was loaded and electrophoresed in 10% SDS-polyacrylamide gels (Pierce, Rockford, IL), transferred onto nitrocellulose membranes (Bio-Rad) and incubated in the presence of primary antibodies at 4°C overnight. Primary antibodies included goat anti-SCGB1A1 (Santa Cruz Biotech), goat anti-AQP5 and rabbit anti-pro-SFTPC (Millipore), mouse anti-CD31 (Millipore), and mouse anti-β-actin and–ACTA1 (Sigma). Goat anti-mouse IRDye 800 CW, donkey anti-rabbit IRDye 680, or anti-goat IRDye 800 were used as secondary antibodies (Li-Cor, Lincoln, NE). Protein detection was performed using an Odyssey infrared scanner (Li-Cor). For protein densitometry, image backgrounds were subtracted, area selected, and mean signal intensity measured using the ImageJ software package (NIH). Standardized mean signal intensities were then used for statistics.
Flow cytometry and FACS analysis
Fractionated cells were washed and labeled with the following antibodies: FITC-conjugated anti-CD45R (Invitrogen), R-PE anti- SCA-1 (Ly6A/E; Invitrogen), PE/Cy5 anti- c-KIT (CD117; eBioscience), PE-anti-CD49f (eBioscience), Alexa-488-anti-EpCAM (CD326; Biolegend, San Diego, CA), biotinylated-CD31 (Santa Cruz Biotech) followed by PE/Cy5 streptavidin (Biolegend), and mouse anti-BRDU (ebioscience) followed by anti-mouse Alexa 488 (Invitrogen). Isotype controls included Alexa Fluor® 488-conjugated rat-IgG, FITC-rat IgG, R-PE-rabbit IgG (Southern Biotech, Birmingham, AL), and PE/Cy5 IgG (BD Pharmingen, San Diego, CA). Cells were analyzed on an Easycycle mini flow cytometer (Guava Technologies, Millipore), FACScan (Becton Dickinson, Franklin Lakes, NJ), or a MoFlo3 (Dako, Fort Collins, CO).
Bleomycin experiments
For injury studies, bleomycin (Novaplus, Irving, TX) was administered intraperitoneally (i.p.) at 4 units/kg body weight. Control mice were injected with an equal volume (100 μL) of sterile saline. To determine DNA synthesis, control and bleomycin-treated mice were injected with 15 mg/mL BRDU (Sigma) i.p. 1 h before sacrifice. Fractionated lung cells were then processed for flow cytometry or RT-PCR as described above.
Statistical analysis
Analysis of variance (ANOVA) and the t-test were performed for normally distributed data and the Kruskal–Wallis or Wilcoxon rank sum tests for skewed data, unless indicated otherwise. A significant difference between groups was established at P<0.05. Data are presented as average±standard error of the mean (SEM). Linear regression analysis of logarithmically transformed mean fold changes in gene expression levels from baseline was used to estimate the proportion of variance explained by the density fraction.
Results
Properties and distribution of lung cells eluted from a density column
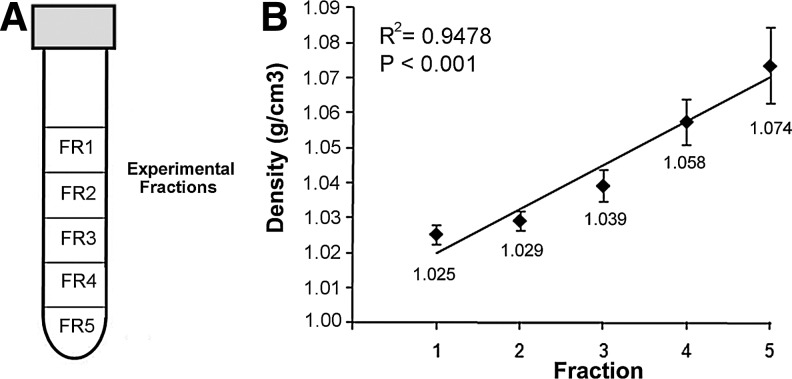
To determine an association between cell characteristics and density, the lung was harvested and total cells were enzymatically digested and subjected to isopycnic centrifugation on a gradient of five separate layers ranging from 1.00–1.07 g/cm3. As seen in Fig. 1, we detected a robust linear relationship between the fraction (FR) and calculated density (P<0.001). With regard to physical properties of total lung cells, while no differences in the cell length were observed across the fractions (immediately following sedimentation), a FR1 driven inverse relationship between the nuclear length and density equilibrium (membership in FR category) was detected (n≥4; P<0.001; Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). However, final cell equilibrium within the density gradient could best be described by the nucleus/cell (length) ratio (Table 1). Evaluating the distribution of total lung cells by density, a significant difference in the cell equilibrium was revealed, manifested as a heavier subset (FR4-FR5), denser than 1.058 g/cm3, and comprising over 60% of the total lung population, and a lighter subset (FR1-FR2) representing approximately 25% of loaded cells (n=8; P<0.001). These data indicate that nuclear properties differ among fractionated cells and that the majority of homeostatic lung cells present themselves in density fractions greater than 1.039 g/cm3.
FIG. 1.
Association between density and collected fractions. (A) Representation of the poured gradient in a tube and the respective fractions collected following sedimentation. (B) Plot establishing the relationship between density and the five fractions investigated in the study. Differences in fraction densities are statistically significant (n≥5; P<0.001). Calculated (average) densities by fraction, linear regression line, and R-squared values are shown.
Table 1.
Properties of the Studied Density Fractions and Eluted Cells from the Homeostatic Lung
| Fraction | Average density (g/cm3)a | % of total lung cellsa | Nucleus–cell ratioa | Composite cell types of the homeostatic lung |
|---|---|---|---|---|
| FR1 | 1.025±0.001 | 9.3±5.2 | 0.82 | AT1, endothelial |
| FR2 | 1.029±0.001 | 15.6±7.0 | 0.59 | AT1, endothelial |
| FR3 | 1.039±0.002 | 11.4±1.7 | 0.57 | AT2 and secretory (epithelial progenitors), myofibroblast, endothelial |
| FR4 | 1.058±0.003 | 30.3±4.7 | 0.58 | AT2, secretory, myofibroblast, endothelial |
| FR5 | 1.074±0.005 | 33.3±8.4 | 0.55 | AT2, secretory, endothelial |
Distribution of total mouse cell homogenate by density shows a majority of lung-extracted cells equilibrate at the higher densities. The nucleus to cell ratio of cells from fraction 1 significantly differs from all other fractions. Composite cell-type classification is based upon transcript and protein expression of lineage-specific markers. Average density data are also found in Fig. 1B. Nucleus and cell ratios are found in Supplementary Fig. 1. Data are presented as average±SEM. aP<0.001.
Common lung lineages can be enriched and further classified by virtue of cell density
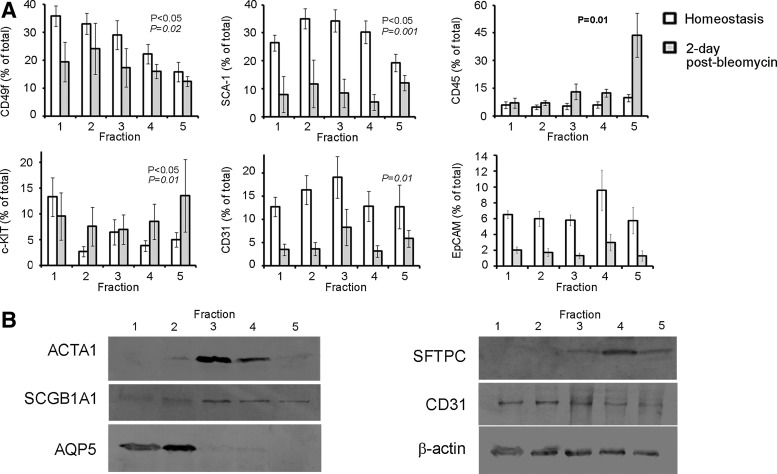
To elucidate on biophysical properties common to individual cell lineages, we fractionated lung cells by density gradient centrifugation and determined the lineage composition by flow cytometry, immunoblotting, and real-time PCR. As shown in Fig. 2, the proportion of CD49f (hematopoietic/epithelial/endothelial/progenitor cell subsets), SCA-1 (endothelial/progenitor cell subsets), and c-KIT-positive (hematopoietic as well as purported multipotential human lung stem) cells, significantly varied with density as seen by flow cytometry (n≥6; P<0.05 for all; Fig. 2A). Representative histograms depicting coresponding isotype controls for the individual markers are shown in Supplementary Fig. S2. In contrast, no significant differences in the percentage of CD31, CD45, and the epithelial cell adhesion molecule (EpCAM)-presenting cells (indicators of endothelial, hematopoietic, and epithelial lineages, respectively), were detected between the fractions.
FIG. 2.
Density distribution of common lung cell lineages. (A) Fractionated cells from sham or 2-day bleomycin-treated mice were subjected to flow cytometry and average percent of cells (±SEM) per fraction are presented. P-value representations shown: normal font—differences in the proportion of protein-expressing cells isolated between fractions from the homeostatic lung; bold font—differences in the proportion of protein-expressing cells between fractions postbleomycin treatment, and italics—overall differences in the proportion of protein-expressing cells between pre- and postbleomycin treatment regardless of fraction. No statistically significant differences were observed in the proportion of CD45-, CD31-, and EpCAM-positive cells between the density fractions in the homeostatic lung. In contrast, cells positive for c-KIT and CD49f were more prevalent in the lower fractions, while SCA-1 expressing cells equilibrated largely in the intermediate fractions. At day 2 following bleomycin treatment, only CD45-positive cells showed significant differences among the fractions equilibrating at the highest density (n≥7; P=0.01). (B) Representative western blots depicting levels of protein expression per fraction (n≥4). The fibroblastic, smooth muscle, α1-actin (ACTA1) protein signal is highest in fractions 3 and 4 (P<0.01 by densitometry). Levels of the epithelial (pro-) surfactant protein-C (SFTPC) and secretoglobin family 1A member 1 (SCGB1A1) protein bands are statistically significant between the fractions (P<0.001 for both). Levels of the AT1 epithelial protein, aquaporin-5 (AQP5) are highest in the lighter fractions (P<0.001). Bands representing levels of the endothelial CD31 protein and β-actin are also shown.
Immunoblot analysis, depicting total protein expression levels, further supported lineage-specific differences in the cell equilibrium. ACTA1 protein expression was highest in FR3 and FR4, which by protein densitometry was statistically higher than all other fractions (n=3; P<0.01; Fig. 2B). Protein levels of the epithelial SCGB1A1, representing secretory, and SFTPC, representing AT2 cells of the distal lung were found to be highest in the heavier fractions (FR4-FR5; n=4; P<0.001 for both). In contrast, deriving from AT2 cells, AT1 cells and their lineage expression of aquaporin-5 (AQP5) were demonstrated predominantly in the lighter fractions (FR1-FR2; n=4; P<0.001). Transcript expression patterns supported the protein distribution data above as determined by RT-PCR (data not shown). Next, to confirm cell viability following separation and support a relationship between protein production and respective lineages, we assessed protein expression and localization in cultivated lung cells at day 3 postfractionation. As shown in Supplementary Fig. S3, immunofluorescence of selected cells from FR5 and FR3 exhibited expected punctate SFTPC and cytoskeletal ACTA1 expression patterns reflecting that of AT2 and mesenchymal cell lineages, respectively. Although consisting of mixed cell populations, these data implicate that homeostatic lung cell protein expression and lineage can correlate with density following fractionation.
Proliferating lineages of the homeostatic lung can be separated by cell density
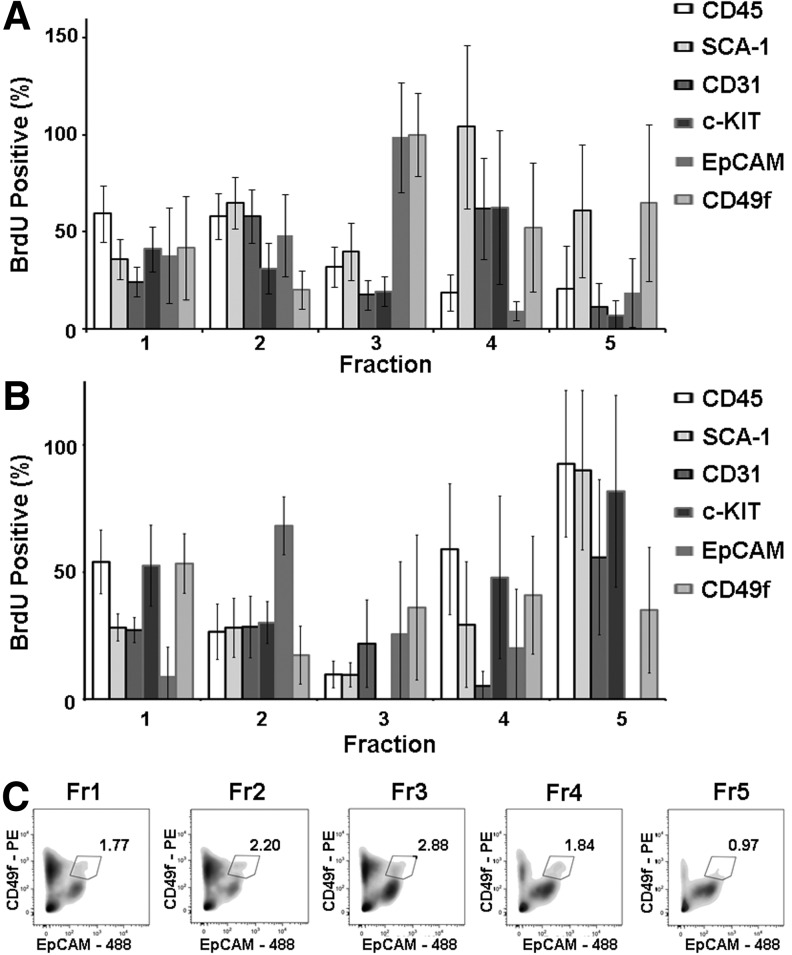
To clarify if adult lung proliferative populations convey unique biophysical properties conducive to elution within specific density fractions, we pulsed mice with the thymidine analogue 5-bromo-2′-deoxyuridine (BrdU), a putative tool for identifying cellular DNA synthesis, and after an hour, sacrificed the mice and harvested the lungs. In the healthy lung, the percent of mitotic/postmitotic BrdU-incorporating cells was 0.74%±0.32% as detected by flow cytometry (n=9). Although statistically nonsignificant, when separated by density, over half of these cells were positioned in FR3-FR5 (Supplementary Table S1). To determine if mitotically active cells belonged to common stem cell lineages, using flow cytometry, we combined BrdU incorporation with presentation of CD45, c-Kit, CD49f, EpCAM, SCA-1, and CD31 cell surface markers. As seen in Fig. 3A, although variability in protein expression existed, BrdU-incorporating CD45-positive cells comprised over 50% of the lighter fractions (FR1 and FR2), to possibly correspond with a previously reported free-floating anchorage-independent progenitor cell population [25]. Meanwhile, in FR4, proliferating cells were largely characterized by SCA-1 and CD31 and c-KIT protein presentation suggesting equilibrium of fibroblastic, endothelial, and/or (human) multilineage stem cells, respectively [10]. FR4 also uniquely and consistently displayed mRNA expression of telomerase reverse transcriptase (Tert; data not shown), which had been shown to catalyze the replacement of shortening telomeres of mitotic cells. Next, to distinguish between cKIT-positive hematopoetic and the purported c-KITpos/CD45neg stem cells, we analyzed fractionated cell presentation of c-KIT and CD45 epitopes. While c-KITpos/CD45neg cells were found distributed throughout the fractions, the largest proportion equilibrated in FR3 with this immunophenotype representing 7.6%±0.28% of the fraction (n=4; representative flow plot seen in Supplementary Fig. S4). In fact, 93.0%±7.1% of c-KIT expressing cells in FR3 were c-KITpos/CD45neg. Nevertheless, BrdU-positive cycling cells of FR3 were primarily characterized by CD49f or EpCAM expression (n≥5; Fig. 3A). As CD49f and EpCAMhi double-positive cells were reported to serve as multipotent bronchial and alveolar epithelial cell progenitors [8], we quantified and isolated these cells by FACS. Distributed throughout the fractions, a peak in CD49f/EpCAMhi cell equilibrium was detected at 1.039g/cm3 (FR3), where an average of 2.2%±1.2% of cells exhibited this immunophenotype (Fig. 3C). Differences in the distribution of CD49f/EpCAMhi cells among the density fractions were found to be statistically significant (n=4; P=0.03). Taken together, these data confirm the separation of proliferating lung cells by density and suggest the utilization of density fractionation for progenitor cell enrichment and identification.
FIG. 3.
Density properties of lung progenitor cell lineages. Sham or 2-day postbleomycin-treated mice were injected with BrdU, euthanized, and lungs harvested after 1 h. Cells were then dissociated, colabeled for BrdU and CD45, SCA-1, c-KIT, CD31, EpCAM, or CD49f, and processed for flow cytometry. (A) In untreated mice, BrdU-incorporating cells of FR3, primarily belonged to EpCAM and CD49f cell lineages. In contrast, BrdU-incorporating cells of fraction FR4 were SCA-1-positive (n≥5). (B) Bleomycin treatment diminished the presence of BrdU-incorporating EpCAM and CD49f putative progenitor cells in fraction 3 introducing, instead, proliferating CD45, SCA-1, and c-KIT cell lineages to fraction 5 (n≥4). Data are presented as average±SEM. (C) Representative flow cytometry analysis of CD49f and EpCAM expression demonstrating the gating and percentage of sorted CD49f and EpCAMhi cells from density fractions.
Clonogenic adult lung epithelial cells predominantly equilibrate around 1.039 gr/cm3
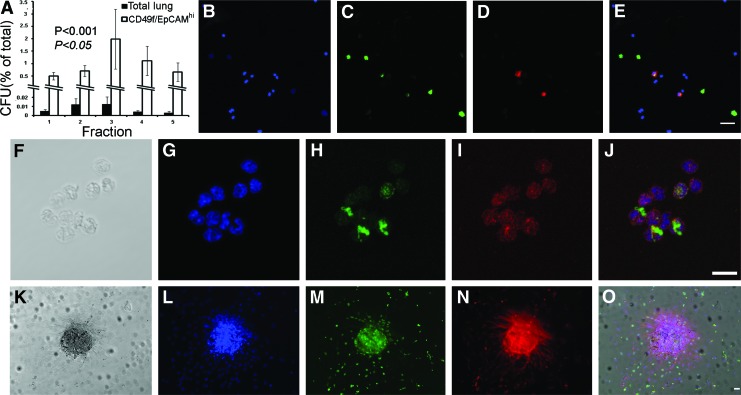
To determine if the density of cells with clonogenic potential corresponds with those in synthesis, we performed colony-forming assays on fractionated lung cells (Materials and Methods). As seen in Fig. 4A, the greatest amount of colony-forming units (CFUs) of total lung cells was demonstrated in FR3 with 0.013%±0.008% of the cells displaying a colony-forming activity. In fact, cells isolated from FR2 and FR3 were fourfold more likely to possess clonogenic properties than in any other fraction (n≥4; P<0.001). Also observed in this figure, sorted CD49f/EpCAMhi cells displayed higher levels of CFUs compared to total lung (n=4; P<0.01) with, once again, cells from FR3 demonstrating the highest proportion of clonogenic properties, 2.0%±1.2% (P>0.05). Comparable levels of the CFU activity for sorted c-KITpos/CD45neg cells were observed (data not shown). To validate progenitor cell cycling, we labeled cells for the Ki-67 protein, which is expressed in active phases of the cell cycle [27]. As demonstrated by immunofluorescent microscopy, fractions 3–5 predominantly displayed Ki-67 localization with BrdU, with a representative labeling of cells from FR4 seen in Fig. 4B–E. Prevalence of mitotic cells in these fractions was also shown to correspond with reported multipotential epithelial progenitor cells that coexpress SFTPC and SCGB1A1 (n=4; Fig. 4F–J). A representative colony expressing SFTPC and incorporating BrdU is seen in Fig. 4K–O (n=4). These data shed light on inherent density characteristics of clonogenic lung progenitor cells and indicate that epithelial progenitors may transition between density states as they progress through the cell cycle or differentiation.
FIG. 4.
The clonogenic activity of cells isolated from the homeostatic lung. (A) Total lung and sorted CD49f/EpCAMhi cells were cultivated on mitotic-inactivated mouse embryonic fibroblasts (MEFs) and colony-forming units (CFUs) were assessed at day 10 (n=4 for all experiments). The lung cell CFU activity was statistically higher in fractions (FRs) 2 and 3 (P<0.001; normal font). CD49f/EpCAMhi cell clonogenicity was significantly higher than total lung cells in all fractions (P<0.01; not shown), with FR3 displaying the greatest CFU capacity (P<0.05; italics). (B–E) Control mice were injected with BrdU, and after 1 h euthanized. Lungs were harvested and cells dissociated, separated by density, and processed for immunofluorescence. Micrographs of FR4 cells labeled for (B) DAPI nuclear stain (blue); (C) Ki-67 (green); (D) BrdU (red); and (E) overlay. Scale bar=50 micron. (F) Transparent image of a cluster of putative epithelial (progenitor and more differentiated) cells isolated from FR3 and labeled for (G) DAPI (blue); (H) SCGB1A1 (green); (I) SFTPC (red); and (J) overlay. Scale bar=20 micron. (K–O) Clonogenicity of SFTPC-positive cells. Micrographs of fraction 4 cells incubated for an additional 3 days, treated with BrdU for 1 h, and prepared for immunolabeling. (K) Transparent light; (L) DAPI nuclear stain (blue); (M) BrdU (green); (N) SFTPC (red); and (O) overlay. Scale bar=50 micron. Color images available online at www.liebertpub.com/scd
The relationship between cell density and gene expression increases following acute lung injury
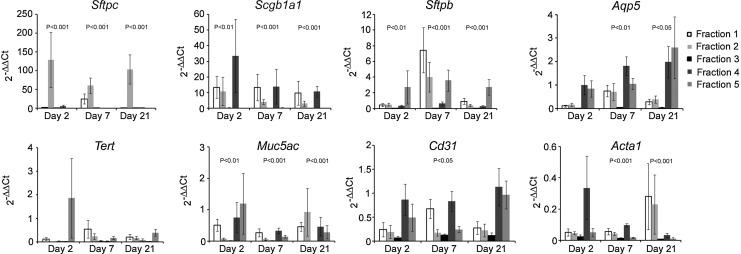
To determine changes in epithelial cell properties following acute injury, we monitored lineage-specific transcript expression in mice treated with bleomycin. As seen in Fig. 5, normalized RT-PCR gene expression was significantly affected by bleomycin injury as early as day 2 as demonstrated by 2−ΔΔCT values of the epithelial Sftpc, Sftpb, Scgb1a1, and Muc5ac genes (n=7 for each gene per day). Importantly, the overall 2-day change in gene expression for the Muc5ac, Scgb1a1, Sftpb, and Sftpc genes significantly differed across the density fractions (P≤0.008 by the Kruskal–Wallis test). Notably, at this day, an average 128-fold increase in Sftpc expression was detected in FR2 compared to the lowest increase in FR3 (overall P=0.0002 by the Kruskal–Wallis test), with the density explaining 70% of the variation in the mean fold change in Sftpc gene expression (Supplementary Fig. S5). Accordingly, the fraction alone could explain 53%, 42%, and 36% (R2 regression values) of the variation in change in Sftpb, Scgb1a1, and Muc5ac gene expression, respectively. Specifically, Sftpc levels in FR2 and FR4 showed significant differences in mean fold gene expression to FR3 (P<0.001 and P=0.03, respectively). By day 7, changes in transcription from baseline differed significantly across the density fractions for all genes except Tert (P≤0.05 by the Kruskal–Wallis test). At this time point, the fraction explained 45% of the variation in the mean fold change in Sftpc gene expression with the lowest mean fold change detected in FR3 and the highest in FR1 and FR2 (overall P=0.004 by the Kruskal–Wallis test). The most significant contribution of the density to these changes was for Sftpb, Scgb1a1, and Acta1 explaining 68%, 55%, and 62% of the mean fold change in gene expression, respectively. At day 21, overall changes in gene expression from baseline significantly differed across the density fractions for all genes except Tert and Cd31 (P≤0.05 by the Kruskal–Wallis test). Specifically, 74% of the variation in the mean fold change in Sftpc gene expression could be explained by fraction (overall P=0.002 by the Kruskal–Wallis test), with the lowest mean fold change detected in FR3. Furthermore, density explained 73%, 54%, 45%, and 45% of the variation in Sftpb, Scgb1a1, Muc5ac, and Acta1 21-day mean fold changes in expression, respectively. Highest increases in Sftpc expression observed in FR2 combined with variances in gene expression significantly explained by cell density, indicate that lighter cells of the lung regenerate the AT2 epithelial population following bleomycin-induced injury.
FIG. 5.
Changes in cell densities correspond with alterations of gene expression following bleomycin-induced injury. Mice were treated with bleomycin and at respective days, lungs were harvested, cells dissociated and fractionated, total mRNA was isolated, reverse transcribed, and RT-PCR was performed. Data are presented as 2−ΔΔCt, normalized to control-untreated mice (average±SEM; n=7 for each gene). Bleomycin treatment initially affected epithelial cell-lineage gene expression at day 2, significantly influencing endothelial and fibroblastic mRNA expression after one week. P-values represent differences in gene expression between the fractions for each time point.
Acute bleomycin injury alters cell lineage and density patterns of the lung
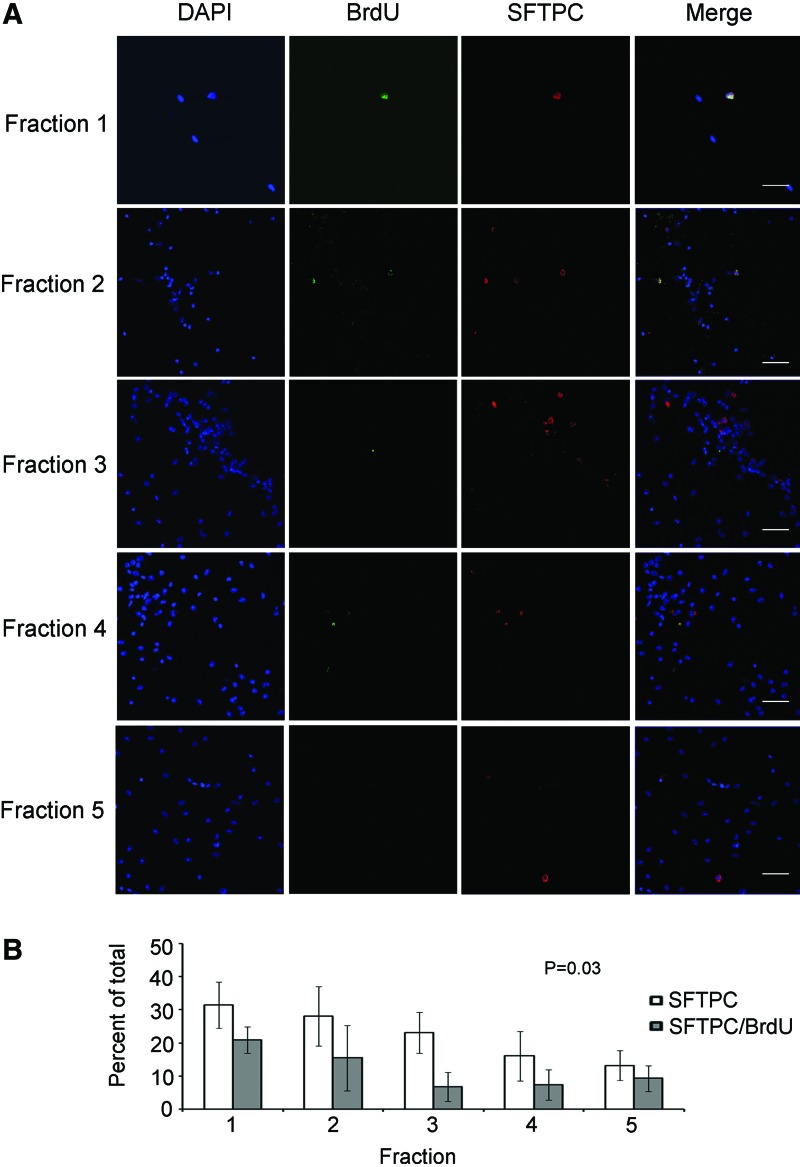
To determine changes in cell-lineage densities that occur during lung injury, we performed cytometry on day 2 postbleomycin-treated mice. This time point was chosen due to reported progenitor cell recruitment into the cycle [28] and our detection of changes in Sftpc expression. At day 2, the proportion of all lineage cells significantly changed from baseline (P=0.003 by the Wilcoxin test), with a decline in the percentage of CD49f, SCA-1, and CD31-positive cells in the lighter density fractions concomitant with increased percentages of CD45 and c-KIT expressing cells in the heavier fractions (FR4 and FR5; n≥4; Fig. 2A). These changes could be interpreted as an influx of denser blood leukocytes into the damaged tissue. Application of BrdU to day 2 postbleomycin-treated mice demonstrated an elevated frequency of DNA-synthesizing cells, which rose to 0.92%±0.36% (from 0.74%±0.32% in the homeostatic lung), manifested as an approximate twofold increase in cells of FR3 and FR4 (Supplementary Table S1). Together with over half of the eluted cells expressing CD45 in FR4, elevated CD45, SCA-1, and c-KIT presentation on proliferating cells of FR5, reveal a considerable (bleomycin-induced) change in progenitor demographics of the heavier fractions, plausibly representing a shift toward hematopoietic and/or fibroblastic lineages (n≥5; Fig. 3B). Finally, we determined SFTPC expression in cells of day 2 bleomycin-treated mice by immunofluorescence and following quantification established a shift in the proportion of SFTPC-positive cells to the lighter densities (n=4; Fig. 6A, B). Specifically, the proportion of SFTPC expressing cells and their incorporation of BrdU at this time point were higher in the lighter (FR1 and FR2; >15%) than the heavier fractions (FR4 and FR5; ≤9%). These differences were statistically significant (P=0.03). Selected SFTPC-positive/BrdU-incorporating cells eluted from FR2 can be seen in Supplementary Fig. S6. These results correlate with elevated Sftpc transcript expression data, which indicate that cells eluted from lighter fractions participate in AT2 epithelium regeneration in the bleomycin-exposed lung. Taken together, an influx of high-density hematopoietic cells in conjunction with the presence of low-density proliferating epithelial cells works to repopulate the lung following acute injury.
FIG. 6.
Low-density mouse SFTPC progenitors are activated following acute lung injury. Mice were injected with BrdU 2 days postbleomycin treatment, after 1 h euthanized, lungs harvested, cells dissociated and separated by density, colabeled for BrdU and SFTPC and processed for immunofluorescence. (A) Representative micrographs from fractions 1–5. From left to right: DAPI nuclear stain (blue); BrdU (green); SFTPC (red); and merged images. Scale bar=50 micron. (B) Quantitative analysis of SFTPCpos epithelial and mitotic/postmitotic (BrdU-incorporating) SFTPC-positive cells within the fractions (n=4). FRs 1 and 2 display the largest ratio of SFTPC-expressing and BrdU-incorporating SFTPC-positive cells. Differences in the proportion of SFTPCpos mitotic/postmitotic cells between the fractions are statistically significant (n=4; P=0.03). Color images available online at www.liebertpub.com/scd
Discussion
Advances in understanding regenerative cell dynamics can be made by methodologies that shed light on cell properties, particularly in the complex biomechanical environment of the lung. By application of total lung cells to a density gradient, we establish that lineage-defined cells can be subdivided into heavier and lighter subsets, and that after acute injury, a strong relationship between the lung cell density and lineage prevails. We also reveal that mitotic/postmitotic cells are quite common in the lung and that distinct progenitor subsets can be enriched within specific density fractions. Finally, our results discriminate between two classes of proliferating epithelial cells, which can be distinguished by their mode of activation and density.
These findings extend prior reports that demonstrate lung and stem cell separation using a density gradient [14,16–21,29]. Moreover, this study expands on principle concepts of cell biology by utilizing a combination of factors, which can more accurately classify states of homeostasis, proliferation, and injury [8,9,30,31].
Complementary to current biochemical methods of cell recognition, biophysical cell separation may be used to further elaborate on the transition between cycling and quiescent cell states, enhancing the current understanding of pulmonary stem cell division [32,33]. As the nuclear density of diploid cells was reported to be greater than 1.286 g/cm3 [29], which is significantly higher than the cell density overall; changes in the proportion of genetic material (nuclear density), in a given volume, might substantially influence cell equilibrium in the fractions, more than water (70% of cell composition), protein and polysaccharide molecule, and lipid content [13,14].
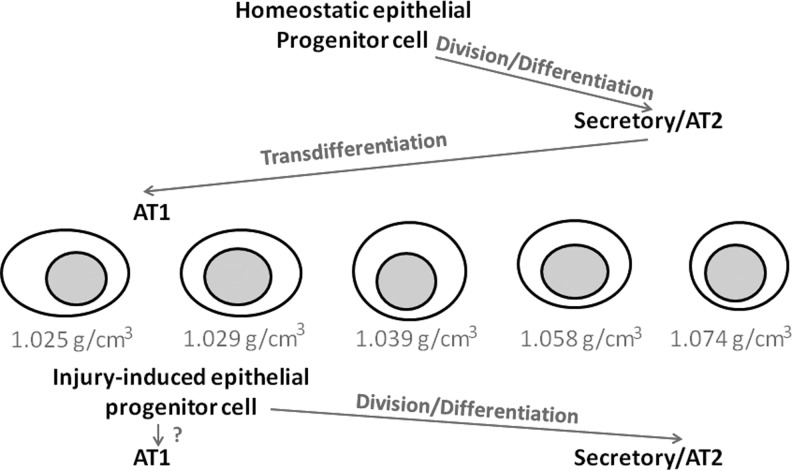
Our data demonstrate that by application of total lung cells to a density gradient, the distal epithelium can be subdivided into lighter AT1 and heavier AT2 cell types (Table 1). Furthermore, at homeostasis, over 73% of the CD49f, 72% of the c-KIT, and 61% of the SCA-1 expressing cells, often cited for stem cell characteristics equilibrated in fractions lighter than 1.039 g/cm3. Accordingly, the highest frequency of clonogenic CD49f/EpCAMhi and c-KITpos/CD45neg purported epithelial progenitors eluted at 1.039 g/cm3, as compared to the SFTPC/SCGB1A1 (double-positive) precursor and committed SFTPC and SCGB1A1 (single positive) cells that equilibrated at densities equal to, or greater than 1.039 g/cm3. These findings suggest that epithelial progenitor cycling and commitment is accompanied by an increase in cell density and that terminally differentiated AT2 and secretory (daughter) cell types might maintain a relatively stable mass. In contrast, in a process reported to take over a week in vitro [34], we reveal that the well-reported AT2-derived transdifferentiation into AT1 subsets involves a decrease in cell density. We speculate that expression of the AQP5 water channel by AT2 cells can play a pivotal role in AT1 commitment reducing the cell density through an increase in the cellular water content and hypertrophy [35–38]. Aquaporin 5 expression was previously reported to play an important role in lung water transport and physiology [39].
Differing from the homeostatic state, the process of transdifferentiation may increase during lung damage and repair [40]. To determine dynamic changes in cell densities after lung injury, we applied the glycopeptide antibiotic bleomycin, which has been shown to induce symptoms of acute lung epithelial injury, pulmonary inflammation, and fibroproliferation [22,23]. In this study, we detected an increase in cell proliferation and a widespread change in lung epithelial transcript expression. Moreover, we observed that membership within a density fraction explained a large proportion of the variation in gene expression across the gene with an elevation in high-density CD45, SCA-1, and c-KIT-positive cell types, and an upsurge in the proportion of low-density proliferating SFTPC expressing cells. An increase in low-density SFTPC expressing cells following bleomycin-mediated damage can be explained by de-novo activation of discrete injury-induced (low-density) epithelial progenitors and a block in their cell cycle progression (where an increase in cell density may occur). These findings are supported by others that report bleomycin disruption of SFTPCpos progenitor colony-forming growth through a DNA damage mediated G2/M cell cycle checkpoint [41,42]. Speculatively, the presence of low-density, injury-induced epithelial progenitors could assist remaining facultative stem cells in repopulating the AT2 epithelium, while bypassing a two-step mechanism for AT2-mediated AT1 regeneration. AT1 cells are the most abundant epithelial type of the alveolus and are crucial in maintaining the lung barrier (Fig. 7). At the same time, high-density SCA-1, c-KIT, and CD45 cell proliferation on the background of an acutely injured lung could elaborate on a rapid influx of hematopoietic/leukocyte inflammatory subsets and/or the expansion of previously reported SCA-1pos fibroblast progenitors [43]. Taken together, biophysical cell sorting may expand on our understanding of organ regeneration and elucidate some controversies of epithelial progenitor identification—a possible product of multiple, yet common biochemical approaches [8,44].
FIG. 7.
Proposed paths of epithelial maturation in homeostatic and injured lungs. In homeostasis, while dividing epithelial cells increase in density from an average density of 1.039 g/cm3 to differentiate into AT2 and secretory cells, transdifferentiation to AT1 cells involves a drop in density. In contrast, bleomycin-induced injury activates progenitors with a density ≤1.029 g/cm3. Activation of a lower density mitotic epithelial cell could assist AT2 repopulation and accelerate AT1 barrier regeneration.
These findings should be interpreted in the context of the study design. While disparities between cell isolates, spin column preparations and parameters (pH, and osmotic pressure), and/or a moderate sample size for each experiment could explain observed variation within some data points, these were not enough to offset the findings of this study. Furthermore, while purification of lineage-defined subpopulations in these mixed cell fractions was unattainable, detection of subsets with disparate biophysical properties, such as cells undergoing division or transitions, was clearly within reach. Additionally, as stem cells are reportedly quiescent with impermeable membranes, the probability of injury resulting from density gradient centrifugation is expected to be low [45–47]. Therefore, cell separation by density can be used as a tool to capture static and dynamic information and to enrich for selective differentiated and progenitor cell types. Finally, while we investigated multiple potential and reported candidate stem cell types (CD49f/EpCAMhi, c-KITpos/CD45neg, as well as SPCpos/SCGB1A1pos), we could not specify if lung regeneration is powered by multiple unipotential lineage-specific precursor cells, or multipotential cells that upon differentiation beget several lineages. We speculate that both exist and that, compared to unipotential cells, multipotential stem cells might endure upon division and commitment not only large changes in molecular expression, but also in cell density. Consequently, while lung epithelia derive from stem cells at 1.039 g/cm3 in homeostasis, lower density epithelial SFTPC-positive cells (<1.039 g/cm3) function together with higher density c-KIT-positive cells [10], which we now found to exist, in injured murine lungs. Future studies using alternate cell separation techniques, narrower gradient densities, as well as cell-lineage tracing, could help further define novel progenitor cell subsets and their mechanistics of development.
Our data indicate that cell density can be related to lineage and that by exploitation of biophysical properties one can address aspects of cell behavior. Separation of cell populations by equilibrium sedimentation can be used in combination with other strategies affording a far more accurate approach to studies of tissue dynamics. Understanding cell density properties and structure, in addition to insights into function, will enhance current strategies of cell identification and isolation, required tenets in regenerative lung therapies.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Joseph Alexander Foundation. We would like to thank Peter Tepler, Moshe Miller, Daniel Green, Yitzchok Abed, and Dr. Chen-Yu Jiao for data collection and technical support, Drs. Jinghang Zhang, Lydia Tesfa, and Olisambu Uche from the Einstein Flow Cytometry Core Facility (supported by: NCI P30 CA013330), and Dr. Simon Spivack for helpful discussions.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Williams L. Peter Y. Adult stem cells of the lung in organ dysfunction. J Organ Dysfunction. 2007;3:31–35. [Google Scholar]
- 2.Kotton DN. Fabian AJ. Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raiser DM. Kim CF. Commentary: Sca-1 and cells of the lung: a matter of different sorts. Stem Cells. 2009;27:606–611. doi: 10.1002/stem.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stripp BR. Hogan BL. Thannickal VJ. Lung stem cells: looking beyond the hype. Nat Med. 2011;17:788–789. doi: 10.1038/nm0711-788. [DOI] [PubMed] [Google Scholar]
- 5.Smith MK. Koch PJ. Reynolds SD. Direct and indirect roles for B-Catenin in facultative basal progenitor cell differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;302:580–594. doi: 10.1152/ajplung.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flozak AS. Lam AP. Russell S. Jain M. Peled ON. Sheppard KA. Beri R. Mutlu GM. Budinger GR. Gottardi CJ. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem. 2009;285:3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stripp BR. Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc. 2008;5:328–333. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQualter JL. Yuen K. Williams B. Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman HA. Li X. Alexander JP. Brumwell A. Lorizio W. Tan K. Sonnenberg A. Wei Y. Vu TH. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajstura J. Rota M. Hall SR. Hosoda T. D'Amario D. Sanada F. Zheng H. Ogorek B. Rondon-Clavo C, et al. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Oeztuerk-Winder F. Guinot A. Ochalek A. Ventura JJ. Regulation of human lung alveolar multipotent cells by a novel p38alpha MAPK/miR-17–92 axis. EMBO J. 2012;31:3431–3441. doi: 10.1038/emboj.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anversa P. Kajstura J. Leri A. Loscalzo J. Tissue-specific adult stem cells in the human lung. Nat Med. 2011;17:1038–1039. doi: 10.1038/nm.2463. [DOI] [PubMed] [Google Scholar]
- 13.Alberts B, editor. Molecular Biology of the Cell. Garland Science; New York, NY: 2008. [Google Scholar]
- 14.Hoffman L. Scheintaub H. Bienkowski R. Smith DG. Density distribution of rat lung cells. Chest. 1975;67:34S–36S. doi: 10.1378/chest.67.2_supplement.34s. [DOI] [PubMed] [Google Scholar]
- 15.Mason R. Williams MC. Clements JA. Isolation and identification of type 2 alveolar epithelial cells. Chest. 1975;67:36S–37S. doi: 10.1378/chest.67.2_supplement.36s. [DOI] [PubMed] [Google Scholar]
- 16.Burnham EL. Taylor WR. Quyyumi AA. Rojas M. Brigham KL. Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–860. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 17.Nowak K. Rafat N. Belle S. Weiss C. Hanusch C. Hohenberger P. Beck G. Circulating endothelial progenitor cells are increased in human lung cancer and correlate with stage of disease. Eur J Cardiothorac Surg. 2009;37:758–763. doi: 10.1016/j.ejcts.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Liu S. Tang Z. Xiong T. Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Z. Feng M. Activation, isolation, identification and culture of hepatic stem cells from porcine liver tissues. Cell Prolif. 2011;44:558–566. doi: 10.1111/j.1365-2184.2011.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Che X. Guo J. Wang B. Bai Y. Rapid isolation of muscle-derived stem cells by discontinuous Percoll density gradient centrifugation. In Vitro Cell Dev Biol Anim. 2011;47:454–458. doi: 10.1007/s11626-011-9433-4. [DOI] [PubMed] [Google Scholar]
- 21.Jeon MS. Yi TG. Lim HJ. Moon SH. Lee MH. Kang JS. Kim CS. Lee DH. Song SU. Characterization of mouse clonal mesenchymal stem cell lines established by subfractionation culturing method. World J Stem Cells. 2011;3:70–82. doi: 10.4252/wjsc.v3.i8.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huot AE. Gundel RM. Hacker MP. Effect of erythrocytes on alveolar macrophage cytostatic activity induced by bleomycin lung damage in rats. Cancer Res. 1990;50:2351–2355. [PubMed] [Google Scholar]
- 23.Redente EF. Jacobsen KM. Solomon JJ. Lara AR. Faubel S. Keith RC. Henson PM. Downey GP. Riches DW. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L510–L518. doi: 10.1152/ajplung.00122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharpe PT. Methods of Cell Separation. Elsevier Science Publishing Company, Inc.; New York: 1988. [Google Scholar]
- 25.Peter Y. Sen N. Levantini E. Keller S. Ingenito EP. Ciner A. Sackstein R. Shapiro SD. CD45/CD11b positive subsets of adult lung anchorage-independent cells harness epithelial stem cells in culture. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.553. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter Y. Comellas A. Levantini E. Ingenito EP. Shapiro SD. Epidermal growth factor receptor and claudin-2 participate in A549 permeability and remodeling: implications for non-small cell lung cancer tumor colonization. Mol Carcinog. 2009;48:488–497. doi: 10.1002/mc.20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholzen T. Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Doupe DP. Alcolea MP. Roshan A. Zhang G. Klein AM. Simons BD. Jones PH. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher RF. Holbrook DJ., Jr. Irvin JL. Density gradient isolation of rat liver nuclei with high DNA content. J Cell Biol. 1963;17:231–236. doi: 10.1083/jcb.17.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teisanu RM. Chen H. Matsumoto K. McQualter JL. Potts E. Foster WM. Bertoncello I. Stripp BR. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2010;44:794–803. doi: 10.1165/rcmb.2010-0098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohle SJ. Anandaiah A. Fabian AJ. Fine A. Kotton DN. Maintenance and repair of the lung endothelium does not involve contributions from marrow-derived endothelial precursor cells. Am J Respir Cell Mol Biol. 2012;47:11–19. doi: 10.1165/rcmb.2011-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQualter JL. Bertoncello I. Concise review: Deconstructing the lung to reveal its regenerative potential. Stem Cells. 2012;30:811–816. doi: 10.1002/stem.1055. [DOI] [PubMed] [Google Scholar]
- 33.Borok Z. Whitsett JA. Bitterman PB. Thannickal VJ. Kotton DN. Reynolds SD. Krasnow MA. Bianchi DW. Morrisey EE, et al. Cell plasticity in lung injury and repair: report from an NHLBI workshop, April 19–20, 2010. Proc Am Thorac Soc. 2011;8:215–222. doi: 10.1513/pats.201012-067CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demaio L. Tseng W. Balverde Z. Alvarez JR. Kim KJ. Kelley DG. Senior RM. Crandall ED. Borok Z. Characterization of mouse alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1051–L1058. doi: 10.1152/ajplung.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma T. Fukuda N. Song Y. Matthay MA. Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobbs LG. Gonzalez R. Matthay MA. Carter EP. Allen L. Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci U S A. 1998;95:2991–2996. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter EP. Umenishi F. Matthay MA. Verkman AS. Developmental changes in water permeability across the alveolar barrier in perinatal rabbit lung. J Clin Invest. 1997;100:1071–1078. doi: 10.1172/JCI119617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umenishi F. Carter EP. Yang B. Oliver B. Matthay MA. Verkman AS. Sharp increase in rat lung water channel expression in the perinatal period. Am J Respir Cell Mol Biol. 1996;15:673–679. doi: 10.1165/ajrcmb.15.5.8918374. [DOI] [PubMed] [Google Scholar]
- 39.Verkman AS. Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol. 2007;159:324–330. doi: 10.1016/j.resp.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park KS. Wells JM. Zorn AM. Wert SE. Laubach VE. Fernandez LG. Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H. Matsumoto K. Brockway BL. Rackley CR. Liang J. Lee JH. Jiang D. Noble PW. Randell SH. Kim CF. Stripp BR. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waltmire CN. Alberts DS. Dorr RT. Sequence-dependent cytotoxicity of combination chemotherapy using paclitaxel, carboplatin and bleomycin in human lung and ovarian cancer. Anticancer Drugs. 2001;12:595–602. doi: 10.1097/00001813-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 43.McQualter JL. Brouard N. Williams B. Baird BN. Sims-Lucas S. Yuen K. Nilsson SK. Simmons PJ. Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 44.Kim CF. Jackson EL. Woolfenden AE. Lawrence S. Babar I. Vogel S. Crowley D. Bronson RT. Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Hackett TL. Shaheen F. Johnson A. Wadsworth S. Pechkovsky DV. Jacoby DB. Kicic A. Stick SM. Knight DA. Characterization of side population cells from human airway epithelium. Stem Cells. 2008;26:2576–2585. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds SD. Shen H. Reynolds PR. Betsuyaku T. Pilewski JM. Gambelli F. Di Giuseppe M. Ortiz LA. Stripp BR. Molecular and functional properties of lung SP cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L972–L983. doi: 10.1152/ajplung.00090.2006. [DOI] [PubMed] [Google Scholar]
- 47.Summer R. Kotton DN. Sun X. Fitzsimmons K. Fine A. Translational physiology: origin and phenotype of lung side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L477–L483. doi: 10.1152/ajplung.00020.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.