Abstract
Zinc finger protein of the cerebellum (Zic)3, a member of Gli family of transcription factors (TFs), is essential for maintaining pluripotency of embryonic stem cells (ESCs) and has been reported to activate TF Nanog in an Oct4/Sox2-independent manner. Previously, we showed that Zic3 (Z), in combination with the Yamanka factors OCT4, SOX2, and KLF4 (OSK), induces neural progenitor-like cells from human fibroblasts. However, a similar combination of TFs (OSKZ) transduced in mouse embryonic fibroblasts resulted in enhanced induced pluripotent stem cells (iPSCs) formation compared with OSK alone, but not neuroprogenitors. OSKZ-derived iPSCs are indistinguishable from mESCs in colony morphology, expression of alkaline phosphatase and pluripotency genes, and embryoid body and teratoma formation. Zic3 activates the transcription of Nanog, a key pluripotency regulator, as evidenced by a luciferase promoter assay. During the course of iPSC derivation, Zic3-mediated enhanced expression of Nanog and Tbx3, gene known to enhance iPSCs derivation, is observed. Not only does Zic3 enhance the reprogramming efficiency, but also reactivation of the endogenous Zic3 protein is essential for the generation of iPSCs, as knockdown of Zic3 during the iPSC generation with OSKM significantly reduced the number of colonies. Together, our result uncovers an important role of Zic3 in generating mouse iPSCs.
Introduction
Pluripotent stem cells (PSCs) differentiate into all somatic lineages as well as germ cells, and in principle provide unlimited specialized cells of all tissues for basic research, drug discovery, and regenerative medicine. Recent progress in the generation of induced PSCs (iPSCs) by ectopic expression of OCT4, SOX2, KLF4, and c-MYC (OSKM) has opened the door to the generation of patient-specific cells for disease modeling and regenerative medicine [1–3]. The TFs OCT4, NANOG, and SOX2 are the core regulatory players in maintaining the embryonic stem cell (ESC) fate [4]. Chromatin immunoprecipitation experiments have demonstrated that OCT4, SOX2, and KLF4, together with other ESC-specific TF such as NANOG, often co-occupy target genes, including their own promoters, hence cooperating in regulatory feedback loops to maintain self-renewal and pluripotency [5–8]. Manipulation of TFs in the regulatory network plays a pivotal role in the control of self-renewal and pluripotency of ESCs, and in some instances aids in enhanced generation of iPSCs. Using the OSKM factors, iPSCs have been derived from many different somatic cell types like stomach [9], keratinocytes [10], and lymphocytes [11] and from multiple species. However, due to use of tumorigenic factor c-MYC in the cocktail, such iPSCs are clinically not relevant. However, without c-Myc, the efficiency of iPSC generation is substantially decreased [12]. Therefore, it is of importance to find factors that can enhance the efficiency of iPSC generation in the absence of c-Myc.
We sought to identify additional, pluripotent-specific genes capable of enhancing iPSC formation in the absence of c-Myc. We tested seven factors (Table 1) all previously known to play a role in self-renewal, reversing histone modifications or inducing DNA acetylation and leading to the maintenance of pluripotency of ESCs. We transduced OSK together with one of these seven TFs in fibroblasts harboring an endogenous Pou5f1–GFP reporter to identify iPSCs. Of the tested factors, Zic3 led to the largest increase in iPSC formation. Further characterization demonstrated the cells to be authentic iPSCs. We further show that Zic3 enhances Nanog and Tbx3 expression during the course of iPSC generation. Finally, we found that shRNA-mediated knockdown of Zic3 in MEFs leads to decreased efficiency of iPSC generation. Considering all these, we conclude Zic3 to be an efficient pluripotent reprogramming factor in mouse.
Table 1.
List of Genes Screened in this Study to Check for the Enhanced iPSC Generation
| Genes | Functions | Refs |
|---|---|---|
| Khdc3l (Ecat1) | Present in human embryonic stem cells and oocytes, function unknown | Pierre et al. [19] |
| Dnmt3a | Epigenetic regulator, DNA methyl transferase | Tsumura et al. [20] |
| Lin-28 | Let-7 family microRNA regulator | Peng et al. [18] |
| Jarid2 | Recruits PRC2 in ES cells | Zhang et al. [21] |
| Kat6b (Myst4) | Epigenetic regulator, histone acetyltransferase | Sheikh et al. [15] |
| Gcn5 | Epigenetic regulator, histone acetyltransferase | Lin et al. [17] |
| Zic3 | Transcription factor, self-renewal regulator | Lim et al. [16] |
Materials and Methods
Cell culture
mESCs and Zic3-induced pluripotent stem cells (ZiPSCs) were cultured on mitomycin-treated mouse embryonic fibroblasts in mouse ESC medium (80% Dulbecco's modified Eagle medium [DMEM] high glucose, 20% FBS, 1×L-glutamine, 1×penicillin–streptomycin, 1×sodium pyruvate, 1×MEM NEAA, 1×sodium pyruvate [Invitrogen 11360], 110 μM 2-mercaptoethanol, and 50 μL mLIF [Chemicon ESG-1107]). The medium was changed every other day. Cells were passaged using 0.05% trypsin, washed, and replated at a dilution of 1:10 every 3–4 days.
Plasmid construction, retroviral transduction, and iPSC generation
The coding region of the mouse Zic3, Gcn5, and Kat6b (Myst4) genes was amplified by reverse transcriptase–polymerase chain reaction (RT-PCR) with primers listed in Supplementary Table S1(Supplementary Data are available online at www.liebertpub.com/scd), and was cloned in pMIG-IRES-GFP plasmid (Addgene). The pMXs plasmids encoding for Oct4, Sox2, Klf4, Khdc3l (Ecat), Jarid2, Lin-28, and Dnmt3A were purchased from Addgene. pMXs and pMIG-based retroviral vectors along with the viral packaging genes gag-pol (Addgene) and VSVG (Addgene) were transfected individually into 293T cells (ATCC) using Fugene HD reagent (Roche) according to the manufacturer's directions.
iPSCs were generated as previously described [13]. Briefly, MEFs were seeded at 1.5×105 cells/well in six-well plates. Cells were transduced with retroviral vector containing supernatant for Oct3/4, Sox2, Klf4, and along with other factors (Table 1) and mixed equally at a 1:1 ratio. To achieve maximum transduction efficiency, cells were transduced twice with the viral vector supernatant. Four days post-transduction, the cells were reseeded at 1.5×105 cells per 100-mm dish and cultured with the mESC medium. Mouse iPSC colonies were picked 20–25 days post-transduction.
Alkaline phosphatase staining
Cells were washed twice with phosphate-buffered saline (PBS), fixed, and stained with alkaline phosphatase substrate as per manufacturer's instruction (Stemgent® Alkaline Phosphatase Staining Kit). After incubation with substrate for 15 min, colonies were stained purple and the image was captured using an Axiovert microscope (Zeiss).
Gene expression and real-time quantitative PCR
RNA was obtained from cells using the RNeasy microkit (Qiagen). One microgram of DNase-treated RNA was reverse transcribed using a superscript III first-strand cDNA synthesis kit (Invitrogen). cDNA was further diluted to 100, and 2 μL of cDNA was used for quantitative PCR using Sybergreen PCR kit (Invitrogen). The primer sequences are provided in Supplementary Table S1.
Immunofluorescence
Cells were fixed using 10% neutral buffered formalin (NBF) for 15 min at room temperature and rinsed twice in PBS. Permeabilization was performed for 15 min using PBS containing 0.2% Triton X-100 (PBST; Acros Organics). Nonspecific blocking was carried out with 10% normal serum corresponding to the animal source of the secondary antibody for 30 min. Primary antibodies were diluted with Dako antibody diluent, and the cells were incubated overnight at 4°C followed by incubation with secondary antibody conjugated with Alexa dyes for 1 h at room temperature. For nuclear staining, 1 μg/mL Hoechst 33258 was added along with secondary antibody incubation. The list of primary antibodies used and their dilutions can be found in Supplementary Table S2.
Flow cytometric analysis
On day 4 post-transduction, OSK and OSKZ factor-transduced cells were trypsinized and seeded onto the feeder cells at 0.15×106 cells/well in six-well plates. On day 11 post-transduction, cells were trypsinized and subjected to flow cytometric analysis to enumerate the number of GFP-expressing cells.
Luciferase assay
Twenty-four hours after seeding in 24-well plates, 293T cells were cotransfected in triplicate with 500 ng of the Nanog promoter construct, which contains 2.5 kb of 5′ promoter region of the mouse Nanog gene (plasmid 16337, addgene) [14], along with 50 ng of TK-renilla (to normalize for transfection efficiency) and combinations of Zic3, Oct4, Sox2, Klf4, and c-Myc each at 1 μg or with different concentrations of Zic3 as indicated. Total DNA content was matched with empty vector. Twenty-four hours after transfection, cells were lysed and assayed for luciferase using the dual luciferase assay system (Promega) according to the instructions of the manufacturer.
Embryoid body formation
The mESCs and ZiPSC colonies were trypsinized and plated as single-cell suspensions in low adherent plates in mESC medium without LIF for 8 days. Subsequently, the cells were cultured for another 8 days on gelatin-coated plates in mESC medium without LIF.
In vivo teratoma and tumor assay
For teratoma assay of ZiPSCs, 106 cells were detached from the feeder layer and injected subcutaneously in 6–8-week-old Rag2 γc−/− mice. Following 4–10 weeks, mice were sacrificed and the tissues were removed, fixed with formalin, and embedded with paraffin. Further sectioning and H&E staining was performed.
Short hairpin RNA experiments
Zic3 silencing was performed as mentioned previously [15]. In brief, Zic3 ShRNA sequences obtained from Open biosystems (V3THS_304236, V3THS_304235) were cloned in the pTRIPZ doxycycline inducible lentiviral vector by digesting with EcoRI and XhoI and transfected into 293T cells using viral packaging plasmids pMD2.G and psPAX2 (Addgene). After 48 h, MEFs were transduced with the viral vector-containing supernatants, and puromycin (1 μg/mL) selection was performed 1 day after transduction. Cells were maintained under puromycin selection for three more days and subsequently subjected to iPSC derivation. During and after the iPSC generation, the expression of Zic3-specific shRNA was maintained by adding doxycycline (1 μg/mL) to the media for the indicated times.
Statistics
At appropriate places, results are expressed as means±SEM. For Statistical analysis Student's t test was performed and P<0.05 was considered significant.
Results
Zic3 enhances the reprogramming efficiency of MEFs
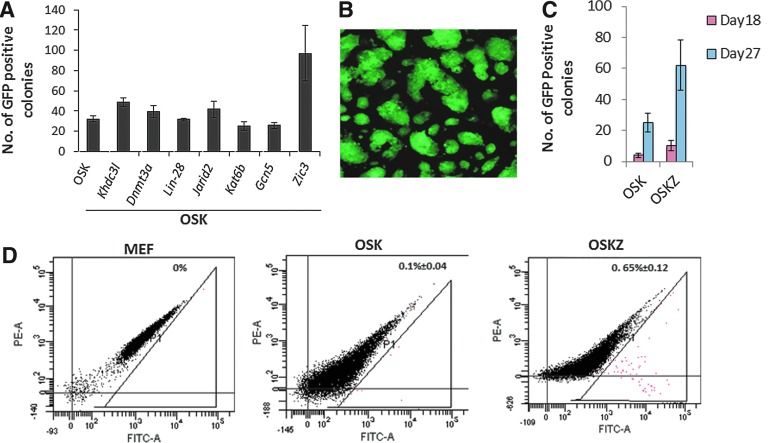
The field of iPSCs will have important impacts on many aspects of medicine. However, generation of iPSCs is inefficient, definitely when removing the tumorigenic factor c-Myc from the reprogramming factor mix [12]. In order to find alternative TFs that would increase iPSC generation in the absence of c-Myc, we screened seven TFs previously shown to play major roles in pluripotency of ESCs (Table 1) [15–21]. Among the seven factors tested in this study, retroviral transduction of Zic3 along with OSK in MEFs containing the Pou5f1-GFP reporter resulted in ∼ 2.5-fold enhanced iPSCs generation compared to OSK alone (Fig. 1A). A Pou5f1-GFP reporter construct in MEFs was used to determine the fraction of cells wherein endogenous Oct4 became activated (Fig. 1B). The number of GFP-positive colonies was determined 18 and 27 days after the first transduction day. Addition of Zic3 enhanced the reprogramming efficiency by 2.5-fold (Fig. 1C). To further confirm this observation, day 11 post-transduced Pou5f1-GFP reporter MEFs with or without Zic3 were subjected to flow cytometry analysis. There were 6 times more GFP-positive cells in cells transduced with Zic3 + OSK compared with OSK alone (Fig. 1D).
FIG. 1.
Zic3 enhances mouse-induced pluripotent stem cell (iPSC) generation. (A) The number of Oct4-GFP-positive iPSC colonies formed by transduction of OSK along with individual other TFs relative to OSK-induced iPSC colonies. (B) Immunofluorescent picture of GFP-positive OSK-Z-derived colonies. Representative example of three independent biological samples. (C) Quantification of the number of GFP-positive colonies 18 and 27 days postretroviral vector-mediated transduction of MEFs, containing the Pou5f1-GFP reporter, with OSK and OSKZ retroviruses. (D) Quantitative analysis of GFP-positive cells using FACS 11 days after transduction of Pou5f1-GFP MEFs, with either OSK or OSK+Z. Mean±SEM of n=3 experiments are shown. Color images available online at www.liebertpub.com/scd
Characterization of the OSK-Z-derived colonies
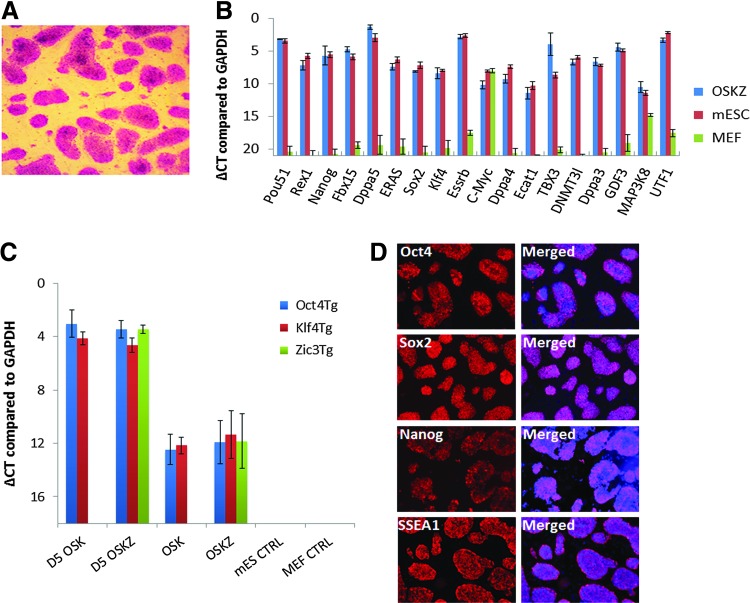
Three independent OSK-Z-derived colonies were characterized further to demonstrate that they were fully reprogrammed. Morphologically, OSK-Z-derived colonies were identical to mESCs. The OSK-Z-derived colonies stained positive for alkaline phosphatase, a marker of undifferentiated ESCs (Fig. 2A). qRT-PCR analysis showed that the expression profile of pluripotency markers, which were not expressed in the original MEFs, was similar to that of mESCs (Fig. 2B). By using transgene-specific primers, qRT-PCR analysis revealed that the transduced mouse Oct4, Sox2, Klf4, and Zic3 genes were nearly silenced (Fig. 2C). At the protein level ZiPSCs expressed Nanog, Oct4, Sox2, and SSEA1 similar to mESCs as shown by immunostaining (Fig. 2D).
FIG. 2.
Zic3-induced iPSCs are bona fide pluripotent cells. (A) Alkaline phosphatase expression in OSK-Z-derived colonies. Representative example of three independent biological samples. (B) Transcript levels of several pluripotency markers in OSK-Z-derived colonies showed a similar gene expression profile as that of mESCs as determined by quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR). In contrast, most pluripotency genes are not expressed in MEFs. Mean±SEM of three independent biological samples are shown. (C) Transcript levels of the transgenes in OSK iPSCs, OSK-Z-derived cells, mESCs, and MEFs were determined by qRT-PCR. (D) At the protein level OSK-Z-derived cells expressed Oct4, Sox2, Nanog, and SSEA1 as shown by immunostaining. Isotype controls were negative. Representative example of three independent biological samples. Color images available online at www.liebertpub.com/scd
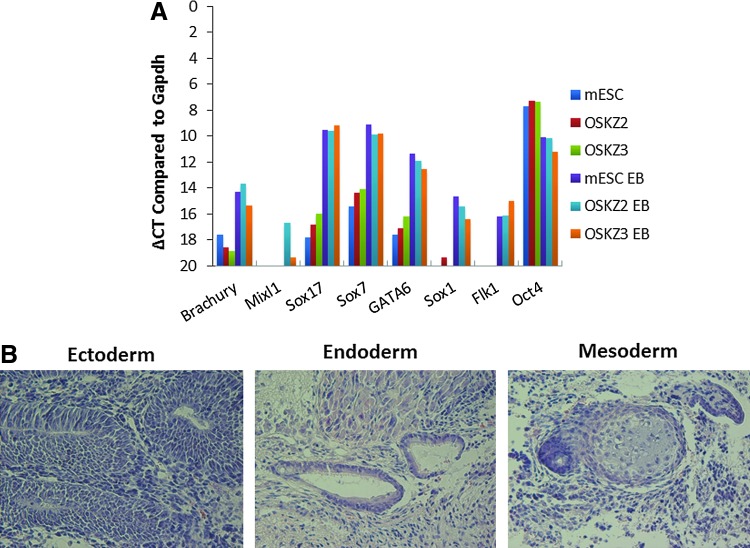
To extend the phenotypic analysis of ZiPSCs, the differentiation capacity was examined in vitro by EB formation assay and in vivo by teratoma formation. qRT-PCR after a standard EB differentiation protocol showed that OKS-Z-derived colonies could differentiate as efficiently as mESC to endoderm (Mixl1, Sox17, Sox7, and Gata6), ectoderm (Sox1), and mesoderm (Flk1, Brachyury, and Mixl1) (Fig. 3A). The expression of the pluripotency gene Oct4 decreased during EB formation (Fig. 3A). The in vivo differentiation capacity was studied by subcutaneous injection of 1 million OKS-Z-derived cells into NOD-SCID γC knockout mice. The teratomas formed exhibited cells of all three germ layers, including neuroepithelial structures (ectoderm), ciliated epithelium (endoderm), and cartilage (mesoderm) (Fig. 3B).
FIG. 3.
Zic3-derived iPSCs differentiate to all the lineages in vitro and in vivo. (A) qRT-PCR after a standard EB differentiation protocol showed that Zic3-induced pluripotent stem cells (ZiPSCs) could differentiate to endoderm (Mixl1, Sox17, Sox7, and Gata6), ectoderm (Sox1), and mesoderm (Flk1, Brachyury, Mixl1). OSKZ2 and OSKZ3 are respectively the second and the third clone of ZiPSCs. mESC EB, OSKZ2 EB and OSKZ3 EB are embryonic bodies derived from mESC, OSKZ2, and OSKZ3, respectively. (B) HE staining on teratomas obtained from ZiPSCs injected in NOD-SCID mice containing cells of the three different germ layers. Color images available online at www.liebertpub.com/scd
Zic3 promotes expression of Nanog and Tbx3 during the course of iPSCs generation
Previously, it was shown that Zic3 can sustain self-renewal of ESCs by enhancing Nanog promoter activity. Therefore, we investigated whether a similar enhanced expression of Nanog is also observed during the Zic3-mediated iPSCs generation and whether or not Zic3 is able to directly enhance the expression of the Nanog promoter by luciferase analysis. Therefore, 293T cells were cotransfected with the Nanog promoter construct and different combinations of Zic3, Oct4, Sox2, Klf4, and c-Myc. The Nanog promoter activity was indeed increased when Zic3 was over expressed. As shown in Fig. 4A, overexpression of Zic3 resulted in 1.5-fold enhanced luciferase activity, whereas combination of OSKZ induced a 2.5-fold increase in the Nanog promoter activity. To further confirm that the increased promoter activity is caused by Zic3, we titrated different concentrations of the Zic3 plasmid in luciferase assays. Increased Zic3 amount, keeping OSK constant, showed a linear increase in luciferase activity further confirming a role for Zic3 in enhancing Nanog expression.
FIG. 4.
Zic3 along with OSK activates the Nanog promoter. (A) Zic3 and OSK in different combination were transfected in 293T cells along with the Nanog promoter Luciferase construct. Transfection efficiency was normalized using a Renilla plasmid. (B) Different concentrations of Zic3 combined with OSK were transfected in 293T cells along with Nanog promoter Luciferase construct. Data are represented as relative luciferase activity after normalizing to transfection with the vector alone. Data shown as mean±SEM of three independent experiments. Stars indicate level of significance (*P<0.05)
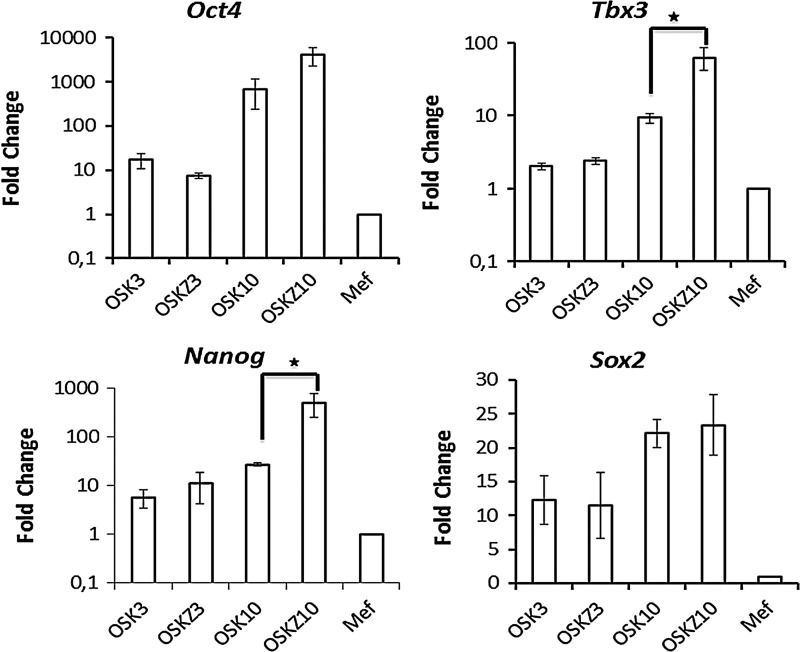
However, overexpression of Nanog along with OSK did not result in increased iPSC generation (data not shown), demonstrating that Nanog by itself cannot enhance iPSCs generation. Therefore, the upregulation of other pluripotency genes at different time points after the iPSC generation was investigated as well. qRT-PCR analysis of other pluripotency genes during OSK-Z reprogramming demonstrated that expression levels of Nanog and Tbx3 were significantly higher compared to OSK alone, 10 days post-transduction (Fig. 5).
FIG. 5.
Zic3 induces the expression of Nanog and Tbx3 during the course of iPSC derivation. Post-transduction cells were harvested on day 3 and 10 to assess expression of endogenous Oct4, Sox2, Tbx3, and Nanog by qPCR. OSK3 and OSKZ3 denote harvesting of cells on day 3, and OSK10 and OSKZ10 denote harvesting on day 10 post-transduction. Data are shown as mean±SEM of three independent experiments. Stars indicate level of significance (*P<0.05).
Zic3 is required for reprogramming of MEFs
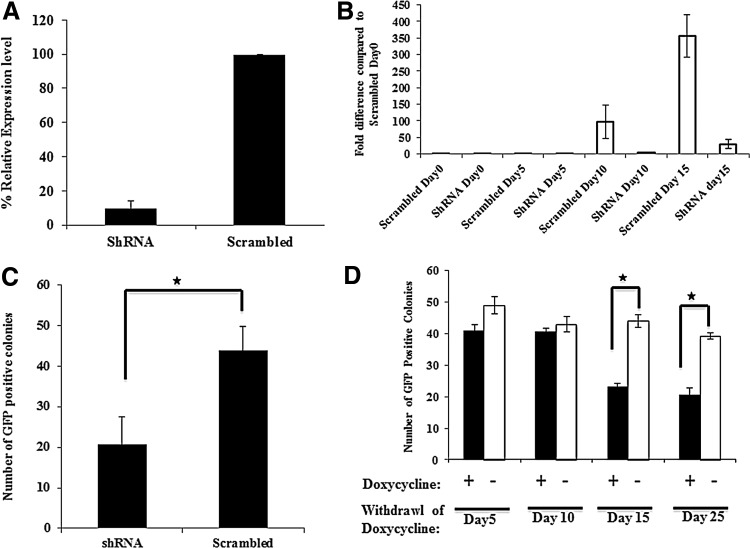
These studies thus suggest that Zic3 plays an important role in reprogramming. Therefore, we tested if endogenous Zic3 is required for reprogramming of MEFs. Therefore, Zic3 was silenced by shRNA before the transduction of Pou5f1-GFP MEFs with OSKM retroviruses. The Zic3 shRNA knockdown in mESCs reduced the expression of Zic3 about 93% compared to mESCs transfected with a scrambled shRNA as determined by qRT-PCR (Fig. 6A). Previous studies and results from the present study demonstrated that Zic3 directly enhances the expression from the Nanog promoter. To demonstrate the functional significance of Zic3, we assessed Nanog expression in cells transduced with an anti-Zic3-shRNA during the course of reprogramming. Reduced Zic3 expression resulted in decreased expression of Nanog compared to the nonsilencing control (Fig. 6B). Further, looking at the role of endogenous Zic3 showed that depletion of Zic3 during reprogramming decreased the number of colonies about twofold compared to those transduced with scrambled shRNA (Fig. 6C). Thus, endogenous Zic3 is also required for reprogramming of MEFs.
FIG. 6.
shRNA-mediated Zic3 silencing reduces generation of iPSCs. (A) Endogenous Zic3 was efficiently silenced by shRNA in mESC as demonstrated by qRT-PCR. (B) Nanog expression was analyzed by qRT-PCR 0, 5, 10, and 15 days post-transduction of an anti-Zic3 shRNA or a nonsilencing control shRNA. Data are shown as fold induction compared to nonsilencing control at day 0. (C) Quantification of the number of GFP-positive colonies 27 days after retroviral vector-mediated transduction with OSKM vectors of MEFs, containing the Pou5f1-GFP reporter and initially transduced with shRNA against Zic3 or with scrambled shRNA. (D) Quantification of the number of GFP-positive colonies 27 days after retroviral vector-mediated transduction with OSKM vectors of MEFs, containing the Pou5f1-GFP reporter and initially transduced with shRNA against Zic3 or with scrambled shRNA. Doxycycline treatment was stopped after 5, 10, 15, and 25 days, respectively. Stars indicate level of significance (*P<0.05). Data are represented as mean±SEM of three independent experiments.
To further substantiate the role Zic3 in reprogramming, we tested at which time during reprogramming Zic3 is required. Endogenous Zic3 was inhibited by Zic3-specific shRNA for different time periods by exposing the cells to doxycycline for 5, 10, 15, or 25 days. GFP-positive colonies were counted on day 27 post-transduction. There was no difference in the number of GFP-positive colonies when Zic3 expression was inhibited for the initial 5 or 10 days. However, there was a ∼2.3-fold decrease in GFP-positive colonies when expression of Zic3 was inhibited for 15 or 25 days of reprogramming, indicating that Zic3 plays a major role during the later part of reprogramming (Fig. 6D). Altogether, our results demonstrate that Zic3 not only enhances iPSCs generation from MEFs, but it is also required for iPSC generation using classical reprogramming factors.
Discussion
The generation of iPSC using the cocktail of TFs Oct4, Sox2, Klf4, and c-Myc is a highly reproducible but an inefficient process. Within this cocktail the proto-oncogene c-Myc enhances the efficiency of iPSC generation by OSK, but it also increases the tumorigenicity of the resulting iPSCs [12]. We therefore screened for alternative TFs that could enhance the reprogramming efficiency by OSK. We here demonstrate that Zic3 can enhance the reprogramming efficiency of MEFs. The iPSCs generated with OSK and Zic3 were similar to mESC in morphology, pluripotency gene and protein expression, and in vivo and in vitro differentiation ability. Therefore, OSK-Z-derived cells are reprogrammed into bona fide PSCs. This is in line with studies from other groups who identified alternative TFs such as Zinc finger protein 296 (Zfp296), which can enhance the reprogramming efficiency by two fold along with OSKM [22] and the Gli-like TF Glis1 along with OSK [23].
ZIC3, a member of the GLI superfamily of TFs, has been implicated in human congenital anomalies [24] and is a marker for brain tumors (medulloblastoma and meningioma) [25]. Zic3 is required for the maintenance of pluripotency in mouse (m) and human (h) ESC by directly controlling expression of Nanog [15, 26]. Our study also demonstrated that overexpression of Zic3 directly modulates the expression of Nanog. RNA interference-mediated suppression of Zic3 in ESCs induces expression of several endodermal lineage genes, indicating that it prevents endodermal differentiation whilst possessing neuroectoderm differentiation-enhancing abilities and the ability to control mesoderm development [15]. Zic3 is also an immediate early gene induced by FGF signaling during neural specification [27]. Further, Zic3 appears to play a role in the maintenance of the neural progenitor cell (NPC) fate by preventing neuronal differentiation [28]. These studies demonstrate thus that Zic3 plays important roles in both maintenance of ESC pluripotency as well as commitment to and maintenance of neuroprogenitor cells.
We previously demonstrated that forced expression of Zic3 together with OCT4, SOX2, and KLF4 in human fibroblasts results in the formation of neuroprogenitor cells, called Zic3-induced cells (ZiC), and this without prior establishment of an iPSC phenotype [13]. This phenotype was maintained through many passages and was dependent on Zic3. Zic3-induced cell lines formed neuroendocrine tumors after subcutaneous injection in mice without evidence of mesoderm or endoderm formation. Zic3-induced cells could be differentiated into cells of the astrocyte, oligodendrocyte, and motor neuron lineage in vitro and formed glial as well as neural cells in vivo after injection in the striatum of postnatal adult mice. However, in the present report, we demonstrate that overexpression of Zic3 along with Oct4, Sox2, and Klf4 in mouse fibroblasts does not lead to the formation of neuroprogenitor cells, but instead it enhances the formation of iPSCs. It is still unclear why Zic3 overexpression together with OSK enhances iPSC generation in MEFs, while it induces the generation of neuroprogenitor cells in human fibroblasts [13]. One possible explanation might be that the Zic3 transgene remains expressed in human fibroblasts, while it is silenced in MEFs, as continuous expression of the Zic3 transgene can promote neuroectoderm differentiation. Silencing of Zic3 in the human neuroprogenitor cells was associated with a 60%–80% decrease in expression levels for the neuroectodermal genes PAX6, OTX1, and OTX2 without concomitant increase in endodermal or mesodermal gene expression. Thus, the continuous expression of Zic3 in the human cells is indeed essential for the neuroprogenitor fate. However, why the Zic3 transgene remains expressed in the human fibroblast, but is silenced in the mouse fibroblast is still unclear.
Analysis of the genes expressed by Zic3 overexpression during iPS generation demonstrated that expression of Nanog and Tbx3 are greatly enhanced (Fig. 5). These data are consistent with previously reported data, wherein Zic3 was shown to enhance Nanog activity in mESCs [26]. However, enhanced expression of Nanog may not be the sole reason for enhanced iPSC generation as overexpression of Nanog along with OSK did not result in increased iPSC generation (data not shown). Tbx3, the other gene that is enhanced by Zic3, was previously reported to sustain pluripotency, and enhance the reprogramming efficiency and quality of derived iPSCs [29]. Expression of Tbx3 is directly regulated by binding of Nanog and Tcf3 to its promoter. Further experiments have to be performed to dissect the downstream effector genes responsible for the enhanced generation of iPSCs by Zic3.
Zic3 overexpression not only enhances the generation of iPSCs, but reactivation of endogenous Zic3 is also required for the generation of iPSCs. Indeed after silencing of Zic3 significantly fewer colonies were obtained upon retroviral transduction with OSKM. This is not surprising, as Zic3 is required for the maintenance of PSCs [15].
In conclusion, we here demonstrate that combined overexpression of Zic3 along with OSK in mouse fibroblasts enhanced the reprogramming efficiency about two- to threefold. Furthermore, Zic3 is also required for the generation of iPSCs since shRNA-mediated knockdown of endogenous Zic3 during iPSC generation negatively affected with the reprogramming efficiency.
Supplementary Material
Acknowledgments
We would like to thank Dr. Valerie Roobrouck and Dr. Yemiao Chen (Katholieke Universiteit, Leuven) for critical review of all the data. J.D. is a postdoctoral fellow of the “Fonds voor Wetenschappelijk Onderzoek Vlaanderen” (FWO). This work was supported in part by grants from FWO (G.0832.11N and G.0667.07), IUAPVII-07 and grants from Katholieke Universiteit Leuven (EIW-B4855-EF/05/11, ETH-C1900-PF and EME-C2161-GOA/11/012) to C.M.V.
Author Disclosure Statement
No competing interest exists and we have nothing to disclose.
References
- 1.Okita K. Ichisaka T. Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Boyer LA. Lee TI. Cole MF. Johnstone SE. Levine SS. Zucker JP. Guenther MG. Kumar RM. Murray HL, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L. Daley GQ. Molecular basis of pluripotency. Hum Mol Genet. 2008;17:R23–R27. doi: 10.1093/hmg/ddn050. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J. Ng HH. TGFbeta and SMADs talk to NANOG in human embryonic stem cells. Cell Stem Cell. 2008;3:127–128. doi: 10.1016/j.stem.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Kim J. Chu J. Shen X. Wang J. Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loh YH. Wu Q. Chew JL. Vega VB. Zhang W. Chen X. Bourque G. George J. Leong B, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 9.Aoi T. Yae K. Nakagawa M. Ichisaka T. Okita K. Takahashi K. Chiba T. Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 10.Aasen T. Raya A. Barrero MJ. Garreta E. Consiglio A. Gonzalez F. Vassena R. Bilic J. Pekarik V, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 11.Seki T. Yuasa S. Oda M. Egashira T. Yae K. Kusumoto D. Nakata H. Tohyama S. Hashimoto H, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M. Koyanagi M. Tanabe K. Takahashi K. Ichisaka T. Aoi T. Okita K. Mochiduki Y. Takizawa N. Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A. Declercq J. Eggermont K. Agirre X. Prosper F. Verfaillie CM. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J Mol Cell Biol. 2012;4:252–255. doi: 10.1093/jmcb/mjs015. [DOI] [PubMed] [Google Scholar]
- 14.Gu PL. Le Menuet D. Chung ACK. Mancini MA. Wheeler DA. Cooney AJ. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol Cell Biol. 2005;25:8507–8519. doi: 10.1128/MCB.25.19.8507-8519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 15.Sheikh BN. Dixon MP. Thomas T. Voss AK. Querkopf is a key marker of self-renewal and multipotency of adult neural stem cells. J Cell Sci. 2012;125:295–309. doi: 10.1242/jcs.077271. [DOI] [PubMed] [Google Scholar]
- 16.Lim LS. Loh YH. Zhang WW. Li YX. Chen X. Wang YN. Bakre M. Ng HH. Stanton LW. Zic3 is required for maintenance of pluripotency in embryonic stem cells. Mol Biol Cell. 2007;18:1348–1358. doi: 10.1091/mbc.E06-07-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin W. Srajer G. Evrard YA. Phan HM. Furuta Y. Dent SYR. Developmental potential of Gcn5(-/-) embryonic stem cells in vivo and in vitro. Dev Dynam. 2007;236:1547–1557. doi: 10.1002/dvdy.21160. [DOI] [PubMed] [Google Scholar]
- 18.Peng SP. Chen LL. Lei XX. Yang L. Lin HF. Carmichael GG. Huang YQ. Genome-wide studies reveal that lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. 2011;29:496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- 19.Pierre A. Gautier M. Callebaut I. Bontoux M. Jeanpierre E. Pontarotti P. Monget P. Atypical structure and phylogenomic evolution of the new eutherian oocyte- and embryo-expressed KHDC1/DPPA5/ECAT1/OOEP gene family. Genomics. 2007;90:583–594. doi: 10.1016/j.ygeno.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Tsumura A. Hayakawa T. Kumaki Y. Takebayashi S. Sakaue M. Matsuoka C. Shimotohno K. Ishikawa F. Li E, et al. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells. 2006;11:805–814. doi: 10.1111/j.1365-2443.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z. Jones A. Sun CW. Li C. Chang CW. Joo HY. Dai QA. Mysliwiec MR. Wu LC, et al. PRC2 Complexes with JARID2, MTF2, and esPRC2p48 in ES Cells to Modulate ES Cell Pluripotency and Somatic Cell Reprograming. Stem Cells. 2011;29:229–240. doi: 10.1002/stem.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischedick G. Klein DC. Wu G. Esch D. Hoing S. Han DW. Reinhardt P. Hergarten K. Tapia N. Scholer HR. Sterneckert JL. Zfp296 is a novel, pluripotent-specific reprogramming factor. Plos One. 2012;7:e34645. doi: 10.1371/journal.pone.0034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maekawa M. Yamaguchi K. Nakamura T. Shibukawa R. Kodanaka I. Ichisaka T. Kawamura Y. Mochizuki H. Goshima N. Yamanaka S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474:225–229. doi: 10.1038/nature10106. [DOI] [PubMed] [Google Scholar]
- 24.Ware SM. Peng JL. Zhu LR. Fernbach S. Colicos S. Casey B. Towbin J. Belmont JW. Identification and functional analysis of ZIC3 mutations in heterotaxy and related congenital heart defects. Am J Hum Genet. 2004;74:93–105. doi: 10.1086/380998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aruga J. Nozaki Y. Hatayama M. Odaka YS. Yokota N. Expression of ZIC family genes in meningiomas and other brain tumors. Bmc Cancer. 2010;10:79. doi: 10.1186/1471-2407-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim LS. Hong FH. Kunarso G. Stanton LW. The pluripotency regulator Zic3 Is a direct activator of the nanog promoter in ESCs. Stem Cells. 2010;28:1961–1969. doi: 10.1002/stem.527. [DOI] [PubMed] [Google Scholar]
- 27.Marchal L. Luxardi G. Thome V. Kodjabachian L. BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc Natl Acad Sci USA. 2009;106:17437–17442. doi: 10.1073/pnas.0906352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue T. Ota M. Ogawa M. Mikoshiba K. Aruga J. Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J Neurosci. 2007;27:5461–5473. doi: 10.1523/JNEUROSCI.4046-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J. Yuan P. Yang H. Zhang J. Soh BS. Li P. Lim SL. Cao S. Tay J, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.