Abstract
Meniscus injury is frequently encountered in clinical practice. Current surgical therapy involving partial or complete meniscectomy relieves pain in the short-term but often leads to osteoarthritis (OA) in the long-term. Here, this study aimed to identify and characterize a novel population of meniscus-derived stem cells (MeSCs) and develop a new strategy of articular cartilage protection by intra-articular injection of these cells. The “stemness” and immune properties of MeSCs were investigated in vitro, while the efficacy of intra-articular injection of MeSCs for meniscus regeneration and OA prevention were investigated in vivo at 4, 8, and 12 weeks postsurgery. MeSCs displayed typical stem cell characteristics such as low immunogenicity and even possessed immunosuppressive function. In a rabbit meniscus injury model, transplantation of allogenous MeSCs did not elicit immunological rejection, but promoted neo-tissue formation with better-defined shape and more matured extracellular matrix. In a rabbit experimental OA model, transplantation of MeSCs further protected joint surface cartilage and maintained joint space at 12 weeks postsurgery, whereas extensive joint surface irregularities and joint space stenosis were observed in the control group. This study thus evoked a new strategy for articular cartilage protection and meniscus regeneration by intra-articular injection of MeSCs for patients undergoing meniscectomy.
Introduction
Meniscus tear is a common form of knee injury, accounting for up to 15% of all athletic injuries of the knee [1]. Due to lack of vasculature, the meniscus has poor self-healing capacity. Meniscectomy (either partial or subtotal) is currently the most common treatment modality for meniscus tear [2]. However, meniscectomy only relieves pain in the short-term but often leads to osteoarthritis (OA) in the long-term [3–5]. Hence, treatment for meniscus injury should maximally preserve and restore meniscus structure and function by repairing meniscal tears rather than just surgical excision of damaged tissue.
Over the last two decades, a number of tissue engineering strategies have emerged to replace all or part of the meniscus, with the objective of improving immediate and long-term patient outcomes [6]. Cells are an important component in tissue engineering, and should facilitate regeneration of injured meniscus through proliferation, as well as synthesis of appropriate extracellular matrix. Murphy et al. provided the first proof-of-concept evidence that local delivery of adult mesenchymal stem cells stimulates the regeneration of meniscal tissue and retards the progressive destruction normally seen in this model of OA [7]. To date, various cell types have been utilized in meniscus tissue engineering [8–10], such as adult stem cells [bone marrow mesenchymal stem cells (BMSCs) and synovium-MSCs] and tissue-specific differentiated cell types (i.e., meniscal fibrochondrocytes). However, implanted BMSCs may lead to ossification if their osteogenesis is improperly induced at ectopic site [11]. Despite that synovium-MSCs have high chondrogenic potential and potentially serve as a reservoir of stem cells in the repair response [12,13], no notable differences of regenerated meniscus in morphology between the synovium-MSCs and the BMSCs have been found in a previous in vivo study [10]. Additionally, meniscal fibrochondrocyte has also been used in meniscus tissue engineering in vitro [9]. However, its low proliferation and matrix production ability undermines successful regeneration in vivo after autologous transplantation. Therefore, a new seeding and alternative cell type is needed to facilitate the regeneration of injured meniscus.
A recent study showed that multipotent stem cells are present in the human meniscus, which are phenotypically similar to MSCs [14]. This subpopulation of meniscus-derived MSCs (MeSCs) may represent another candidate for meniscus regeneration. However, knowledge of meniscus-derived MSCs is still very much limited, and no studies to date have evaluated its effect on the regeneration of injured meniscus in vivo. Additionally, autologous MeSC transplantation is constrained by inadequate cell numbers and extended culture duration. The alternative may be to utilize an allogenous source of MeSCs for meniscus regeneration. Previous studies have shown that certain adult stem cells possess immunosuppressive properties [15–19], that is, BMSC, periodontal ligament stem cells (PDLSCs), adipose-derived stem cells (ADSCs), and umbilical cord-derived stem cells.
Based on these previous studies, we hypothesize a new strategy of articular cartilage protection through meniscus regeneration induced by intra-articular injection of MeSCs into joints subjected to meniscectomy. To test this hypothesis, the study will thus investigate (1) the isolation of a cell population from rabbit meniscus that possesses typical characteristics of stem cells; (2) the alloimmunogenicity testing of MeSCs in vitro; (3) the efficacy of intra-articular injection of MeSCs on meniscus repair in vivo within a rabbit model; and (4) the effect of injecting allogenous MeSCs on the suppression of early experimental OA within a rabbit model.
Materials and Methods
Monoclonal selection of MeSCs and colony forming unit assay
The isolation and culture protocols for rabbit MeSCs were based on previous studies [14,20]. Meniscus tissues of New Zealand white (NZW) rabbits were digested with collagenase (3 mg/mL) for 6 h. The cells from the digested tissue were cultured in Dulbecco's modified Eagle's medium (DMEM, low glucose; Gibco-BRL, Inc.) supplemented with penicillin–streptomycin and 20% (v/v) fetal bovine serum (FBS; Invitrogen, Inc.). They were seeded at a very low density (2 cells/cm2) to form colonies on a 6-cm dish. These colony-forming cells isolated from rabbit meniscus were designated as MeSCs. In this study, we use polyclonal MeSCs mixed from multiple clones. MeSCs between passages 4 and 6 were utilized for experiments. For ratio of colony formation, cells were stained with 1% (w/v) crystal violet (Sigma) in methanol for 10 min. Only colonies with diameters >2 mm were counted.
Multipotent differentiation potential of MeSCs
The multipotent differentiation potential of MeSCs toward the adipogenic, osteogenic, and chondrogenic lineages was evaluated in vitro according to established protocols [20]. For adipogenic differentiation, MeSCs were induced under the influence of 1-methyl-3-isobutylxanthine, dexamethasone, insulin, and indomethacin. For osteogenic differentiation, MeSCs were induced by treatment with β-glycerol phosphate, dexamethasone, and ascorbic acid, while for chondrogenic differentiation, MeSCs were induced by treatment with TGF-β1. Positive induction of adipogenesis, osteogenesis, and chondrogenesis was confirmed by oil red O staining, alizarin red staining (ARS), and safranin O staining, respectively.
Flow cytometry analysis of MHC-II expression by MeSCs
Rabbit MeSCs (1×106) were incubated with mouse anti-rabbit MHC class II antibody (Serotec) for 0.5 h at 4°C [21]. After washing, the cells were incubated with FITC-conjugated rabbit anti-mouse IgG (BD Pharmingen) for 45 min on ice. Subsequently, the washed samples were analyzed by with a Coulter Epics XL flow cytometer.
One-way mixed lymphocyte culture
Mixed lymphocyte culture (MLC) assay was used to evaluate the immunogenicity of rabbit MeSCs. Peripheral blood mononuclear cells (PBMCs) were collected from two different NZW rabbit donors by Ficoll-Paque Plus (1.077 g/mL; Amersham Biosciences) and cultured with lymphocyte culture medium (RPMI 1640 containing 20% [v/v] FBS and penicillin–streptomycin). PBMCs and MeSCs were exposed to 25 μg/mL mitomycin C in darkness at 37°C for 0.4 h, and then washed twice and used as stimulators (PBMC1 and MeSCs1). These exposed cells were nonproliferating due to pretreatment with mitomycin C. Untreated PBMCs were used as responders (PBMC2).
In 0.2 mL of lymphocyte culture medium, Mitomycin C–treated PBMCs (PBMC1, 1×106) or MeSCs (MeSC1, 1×106) were cocultured with responding PBMCs (PBMC2, 1×105) within the wells of a 96-well round-bottom plate at 37°C for 3 days. The evaluation system is a modification of that used in a previous study [21,22], and the results were computed by the following formula: stimulation index (PBMC2 cocultured with PBMC1, P2+P1)=(the proliferative response of PBMC1-treated groups−the proliferative response of PBMC2 untreated groups)/(the proliferative response of PBMC2 untreated groups)±standard deviation (SD); stimulation index (PBMC2 cocultured with MeSC1, P2+M1)=(the proliferative response of MeSC1-treated groups−the proliferative response of PBMC2 untreated groups)/(the proliferative response of PBMC2 untreated groups)±standard deviation (SD) [23]. PBMC1 and MeSC1 were from the same donor. All experiments were performed in triplicates. Harvesting of MeSCs and PBMC was in accordance to standard guidelines approved by the Zhejiang University Ethics Committee.
Assay of immunosuppression
The two-way MLC assay was used to test the immunosuppressive properties of MeSCs. Responding PBMCs (PBMC2, 5×104) and stimulating PBMCs (PBMC1, 5×104) were cocultured with or without stimulating MeSCs (MeSC1, 8×103) within wells of a 96-well plate at 37°C in 5% CO2 for 3 days. The percentage inhibition of allogeneic proliferation was calculated by the following formula [21,22]: percentage of maximal response (PBMC2 cocultured with PBMC1 and MeSC1, P2+P1+M1)=(the proliferative response of groups with MeSCs1)/(the proliferative response of groups without MeSCs1)×100%; percentage of maximal response (PBMC2 cocultured with PBMC1, P2+P1)=100%. PBMC1 and MeSC1 were from the same donor. All experiments were performed in triplicates.
Meniscectomy and MeSCs injection
Nine female NZW rabbits weighing 2.4–2.6 kg were utilized in this study. The rabbits were subjected to general anesthesia by administration of chloral hydrate, and their meniscus were subsequently exposed by releasing parts of the patellar ligament and joint capsule through a longitudinal incision on the anteromedial side of bilateral knee. The anterior half of medial meniscus was removed at the level of the medial collateral to create a defect, and the wound was closed in layers [10]. MeSCs (0.6×107 in 100 μL phosphate-buffered saline [PBS]) were injected into the right knee (the MeSC-treated group) at 1 and 2 weeks after meniscectomy, while the same volume of PBS was injected into the left knee (the control group) as control.
After euthanasia, three meniscus sections of rabbit from each experimental group were used for evaluation at the 4 weeks (n=3 per group), 8 weeks (n=3 per group), and 12 weeks (n=8 per group). Transmission electron microscopy (TEM) was used to assess the cell shape and collagen fibril diameter 12 weeks after meniscectomy. Treatment of animals was in accordance to standard guidelines approved by the Zhejiang University Ethics Committee.
Cell labeling and detection
The MeSCs utilized for in situ repair of meniscus were prestained with fluorescent dye DiI/CFDA (carboxyfluorescein diacetate). Briefly, the MeSCs were incubated with DiI (5 μL/mL; Sigma-Aldrich, Inc.)/CFDA(25 μL/mL; Molecular Probes, Inc.) at 37°C for 20 min, and then washed with PBS. To evaluate the survival of implanted MeSCs in the meniscus defect, a noninvasive Kodak in vivo FX small animal imaging system was used to analyze the samples at 4, 8, and 12 weeks postmeniscectomy [24]. In this fluorescent imaging system, a composite pseudocolor image represents light intensity (blue signifies least intense and red signifies most intense). The specific and control excitation wavelength of DiI and CFDA was at 550/470 nm and 470/430 nm, respectively. The fluorescent image was superimposed in real time over the white-light image of the same sample. In addition, the samples were harvested and histological sections were prepared. The positively stained cells in the histological sections of the allogenous MeSC-treated group were observed under fluorescence microscopy (IX71; Olympus, Inc.) at excitation wavelengths of 543 nm (DiI) and 470 nm (CFDA) [24], with DAPI being utilized for nuclei staining.
Histology
The cartilage surface was stained with Indian ink for macroscopical observation. Specimens were fixed, dehydrated, and embedded within paraffin blocks. Histological sections (8 μm) were prepared using a microtome, and subsequently de-paraffinized with xylene, hydrated using decreasing concentrations of ethanol, and then subjected to hematoxylin and eosin (HE) staining and safranin O staining. Histology evaluation was performed using the modified Mankin's score.
Radiographic evaluation
The X-ray photograph (A-P) of knee joint (containing femur and tibia) was captured with a Kodak-FX in vivo imaging system (Kodak, Inc.) [25]. The average distance between condyles and plateau was measured to assess the level of OA.
Immunohistochemistry
Mouse anti-rabbit monoclonal antibodies against collagen type II (Calbiochem) were used to detect the expression of collagen type II within the repaired meniscus.
Transmission electron microscopy
Tissue specimens at the 4- and 12-week time-points from the MeSC-treated group and control groups were fixed following the standard procedures for TEM to assess cell shape within the regenerated meniscus. Specimens were prefixed with 2% glutaraldehyde for 24 h and washed twice with PBS followed by postfixation treatment with 1% osmic acid for 2 h. After two washes in PBS, the specimens were dehydrated in an ethanol gradient and dried to a critical point. The specimens were then mounted and sputter-coated with gold for viewing under TEM (Quanta 10 FEI).
RNA isolation and real-time PCR
Total cellular mRNA was isolated by lysis in TRIZOL (Invitrogen, Inc.) followed by a one-step phenol chloroform–isoamyl alcohol extraction, according to the manufacturer's instructions. Real-time PCR analysis of the expression of the two genes collagen type II and biglycan (BGN) was carried out using the Brilliant SYBR Green QPCR Master Mix (TakaRa Bio, Inc.) with a Light Cycler apparatus (ABI 7900HT; Applied Biosystems, Inc.), as previously described [20]. At least three experimental replicates were performed for each real-time PCR run, and the results are presented as target gene expression normalized to GAPDH. Primer sequences used were as follows: COLLAGEN TYPE II forward 5′-ATGGACATTGGAGGGCCTGA-3′, reverse 5′-TGTTTGACACGGAGTAGCACCA-3′; BGN forward 5′-GATGGCCTGAAGCTCAA-3′, reverse 5′-GGTTTGTTGAAGAGGCTG-3′; GAPDH forward 5′-TCACCATCTTCCAGGAGCGA-3′, reverse 5′-CACAATGCCGAAGTGGTCGT-3′.
Biomechanical evaluation
The compressive mechanical properties of meniscus was performed (n=5 for the MeSC-treated group and n=5 for the control group) using an Instron tension/compression system with Fast-Track software (Model 5543; Instron) as described previously [26]. Specimens were placed in PBS at room temperature for 3–4 h to equilibrate before testing. Then, they were tested using a 1-mm-diameter cylindrical indenter fitted with a 10 N maximum loading cell. The unconfined equilibrium modulus (Mpa) was determined by applying a step displacement (15% strain) and monitoring compressive force with time until equilibrium was reached. The crosshead speed used was approximately 0.06 mm/min.
Statistics
All quantitative data sets are expressed as mean±SD. Student's t-test was performed to assess whether there were statistically significant differences between data sets, and values of P<0.05 were considered to be significantly different.
Results
Identification and characterization of MeSCs
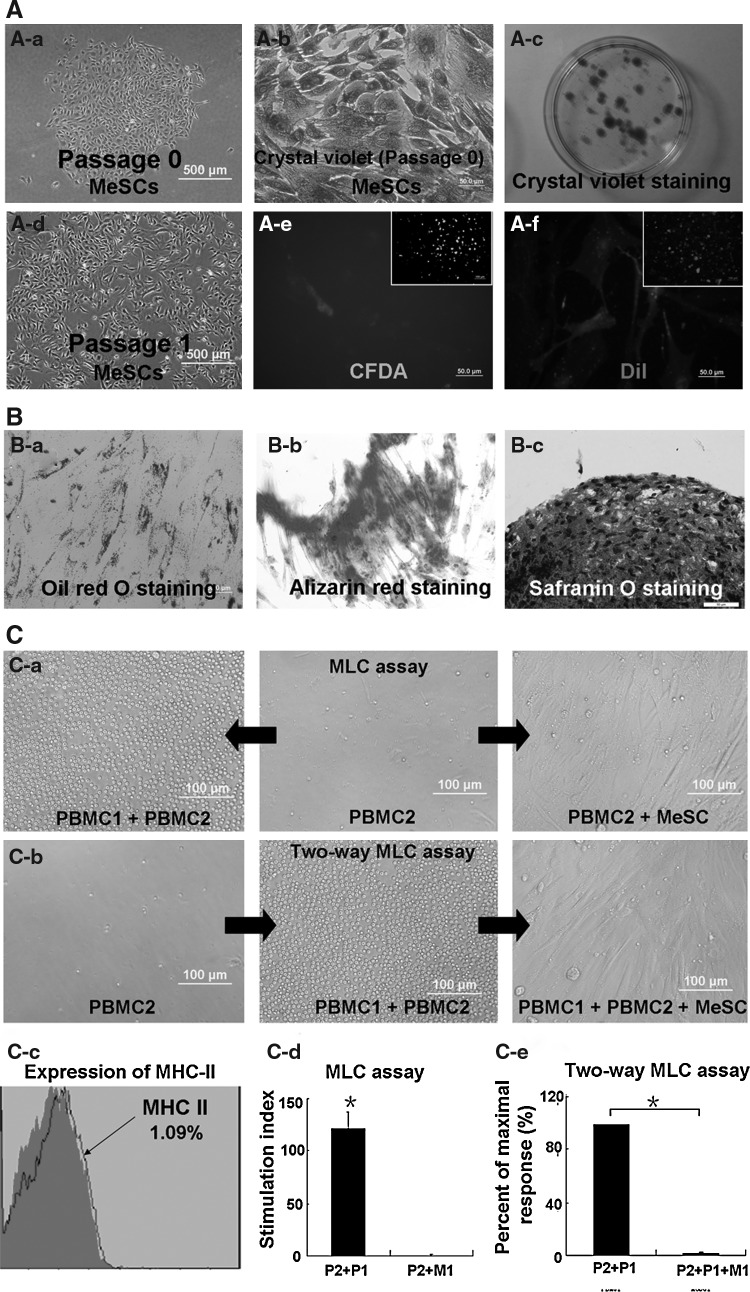
The “stemness” of rabbit MeSCs including clonogenicity (Fig. 1A) and multipotency (Fig. 1B) was characterized. Within single-cell suspension culture, some of the meniscus cells attached onto the culture plate after 4 h. About 10–12 days later, colonies formed (Fig. 1A-a) by single-meniscus-derived cells were visualized with methyl violet staining (Fig. 1A-b, A-c). The colonies formed from MeSCs were heterogeneous in size, shape, and cell density, possibly reflecting differences in cell origin from inner and outer zones of the meniscus. At P0, large polygonal and star-shaped cells were observed (Fig. 1A-a). After culturing for several passages, a homogeneous population of MSC-like cells was obtained (Fig. 1A-d). The multidifferentiation potential of MeSCs was subsequently examined (Fig. 1B). The adipogenic differentiation assay showed the accumulation of lipid droplets, which was confirmed by oil red O staining after 3 weeks of induction (Fig. 1B-a). The osteogenic differentiation assay showed that most of the MeSCs had the capacity to undergo osteogenic differentiation, as confirmed by ARS of mineralized calcium deposits (Fig. 1B-b), while the chondrogenic differentiation assay showed that micromass cultures of MeSCs were safranin O positive after 4 weeks of induction (Fig. 1B-c). Although we designated these colony-forming cells isolated from rabbit meniscus as MeSCs, more rigorous assays for stem cell properties need to be applied to define them as bona fide stem cells.
FIG. 1.
Isolation and characterization of rabbit MeSCs. Morphology of meniscus cell (A-a). Morphology of MeSCs at P0 (A-b). (A-c) shows the crystal violet staining of MeSCs, Inset of (A-c) shows the morphology of colonies formed by MeSCs. Morphology of MeSCs at P1 (A-d). (A-e) shows the CFDA-stained MeSCs. (A-f) shows the DiI-stained MeSCs. The multidifferentiation potential of MeSCs (B). Oil red O staining shows adipogenic differentiation of MeSCs (B-a). Alizarin red staining shows osteogenic differentiation of MeSCs (B-b). Safranin O staining shows chondrogenic differentiation of MeSCs (B-c). Immunogenicity and immunosuppressive properties of MeSCs in vitro (C). PBMC1 elicit the proliferation of PBMC2, while MeSC1 do not elicit allogeneic PBMC2 proliferation (C-a, C-d); MeSC1 inhibit ongoing PBMC proliferation (C-b, C-e). Flow cytometry analysis showed negative expression of MHC-II (C-c). Scale bars=50 μm (A-a A-c, A-e, A-f,; B-a, B-c), 100 μm (inset of A-e, A-f, B-b, C-a, C-b), 500 μm (A-b, A-d). *P<0.05 versus control group. MeSCs, meniscus-derived stem cells; PBMC, peripheral blood mononuclear cells; MHC-II, major histocompatibility complex class II.
Immunogenicity and immunosuppressive properties of MeSCs in vitro
Previous studies showed that BMSCs are MHC-II negative and are able to suppress lymphocyte proliferation in vitro [21]. Hence in this study, the expression of MHC-II on the MeSCs surface was characterized by flow cytometry analysis and the results showed that MeSCs did not express MHC-II (Fig. 1C-c). One-way MLC assay was used to evaluate the immunogenicity of MeSCs. Allogeneic PBMC1 served as the positive control. The results demonstrated that allogeneic MeSCs (MeSC1) did not induce lymphocyte proliferation (PBMC2) unlike allogeneic PBMC (PBMC1) (Fig. 1C-a, C-d). In order to investigate the suppressive effects of MeSCs on activated lymphocytes, the two-way MLC assay was carried out. The results demonstrated that MeSCs (MeSC1) could partly inhibit the proliferation of lymphocytes in response to allogenic PBMC (PBMC2+PBMC1) (Fig. 1C-b, C-e; P<0.05).
Effects of intra-articular injection of MeSCs on meniscus repair
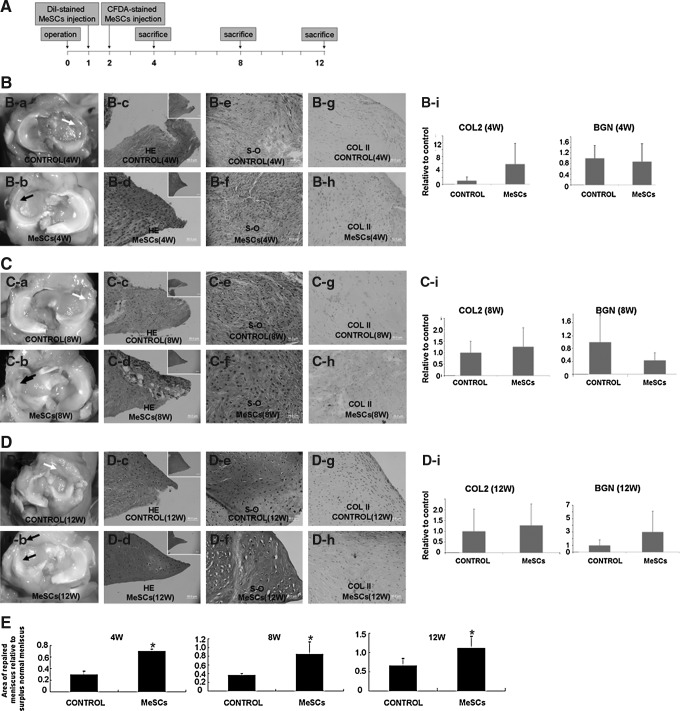
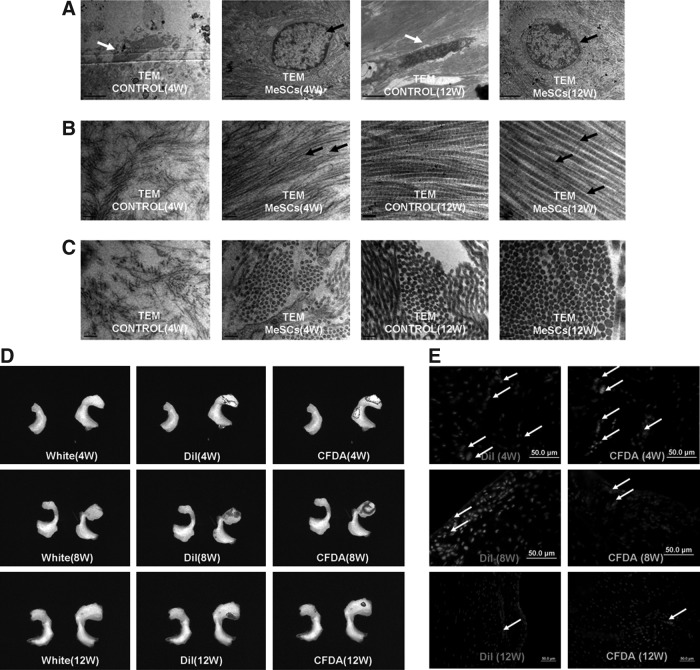
Intra-articular injection of allogenous MeSCs into the right knee was performed at 1 and 2 weeks after meniscectomy, while injection of the same volume of PBS was utilized as control. The rabbits were culled at 4, 8, and 12 weeks after meniscectomy (Fig. 2A). At 4 and 8 weeks postmeniscectomy, the gross morphology showed that the MeSC-injected group exhibited more neo-tissue formation within the anterior part of the meniscal defect compared to the control group (Fig. 2B-a, 2B-b, 2C-a, 2C-b, E; P<0.05). The HE staining of meniscus injected with MeSCs exhibited more neo-tissue formation and better-defined shape similar to normal meniscus, as compared to the control group, with little sign of any inflammation and immunological rejection (Fig. 2B-c, 2B-d, 2C-c, 2C-d). At 4 weeks postmeniscectomy, the expression levels of collagen type II in the MeSC-treated group were higher compared to the control group, while the expression levels of biglycan (BGN) were not significantly different between the two groups (Fig. 2B-i). At 12 weeks postmeniscectomy, there were significant differences between the MeSCs injection group and the control group in terms of gross morphology (Fig. 2D-a, 2D-b, E; P<0.05). Histological analysis revealed a more physiological structure in the MeSCs injection group compared to the control. At 8 and 12 weeks postmeniscectomy, safranin O staining and immunohistochemical staining showed that the MeSC-treated group had higher deposition levels of cartilage matrix (Fig. 2C-e, C-f, D-e, D-f), similar to that of normal meniscus. However, the gene expression levels of collagen type II and BGN were not significantly different between the two groups (Fig. 2C-i, D-i). TEM showed that the cell morphology in the MeSC injection group was similar to that of normal meniscus with round shape (Fig. 3A), with the collagen fibrils being more ordered (Fig. 3B) and larger (Fig. 3C) than that of the control group at both 4 and 12 weeks postmeniscectomy.
FIG. 2.
Regenerated meniscus at 4, 8, and 12 weeks postmeniscectomy. Experimental design for utilizing MeSCs for in vivo treatment of meniscus injury (A). Gross morphology and typical HE staining of control group (white arrow) and MeSC-treated group (B-a, B-d, C-a, C-d, D-a, D-d; black arrow) at 4, 8, and 12 weeks postmeniscectomy. Real-time PCR analysis of gene expression of COL2 and BGN (B-i, C-i, D-i) in vivo at 4, 8, and 12 weeks postmeniscectomy. Safranin O staining and immunohistochemical staining of control group and MeSC-treated group (B-e, B-f, C-e, C-f, D-e, D-f) at 4, 8, and 12 weeks postmeniscectomy. (E) The sequential area ratio of the repaired meniscus relative to surplus normal meniscus (n=3 for 4 and 8 weeks, n=8 for 12 weeks each group). Scale bars=50 μm (B, C, D), 200 μm (inset of B, C, D). HE, hematoxylin and eosin staining; PCR, polymerase chain reaction; COL2, collagen type II; BGN, biglycan; S-O, safranin O staining; W, weeks.
FIG. 3.
The TEM, fluorescence imaging, and CCCD analysis of regenerated meniscus at 4, 8, and 12 weeks postmeniscectomy. TEM imaging of typical cells and collagen fibrils in control group (white arrow) and MeSC-treated group (A, B, C; black arrow) at 4 and 12 weeks post-meniscectomy. CCCD analysis demonstrated a positive color fluorescence signal (DiI and CFDA) at the repair site, indicating the survival of implanted MeSCs (D). Fluorescence imaging of repaired meniscus showed surviving MeSCs within the meniscus defect at 4, 8, and 12 weeks (E, white arrow) postmeniscectomy. Scale bars=50 μm (E), 2 μm (A), 0.2 μm (B, C). *P<0.05 versus control group. TEM, transmission electron microscopy.
In order to evaluate whether injected MeSCs can contribute to meniscus regeneration, the fate of MeSC was traced in vivo. The MeSCs (at passages 4 and 6) were stained with DiI (Fig. 1A-f) and CFDA (Fig. 1A-e) before being injected into the knees. A small animal fluorescent imaging system detected the signal emitted by DiI and CFDA within the regeneration site at 4, 8, and 12 weeks postmeniscectomy (Fig. 3D), and these results were confirmed by fluorescence microscopy (Fig. 3E). This indicated that the labeled MeSCs contributed to meniscus regeneration. Through image tracking and fluorescence microscopy, we found that the number of injected MeSCs decreased sharply with time. However, we could still find some live MeSCs at the repair site after 12 weeks postmeniscectomy.
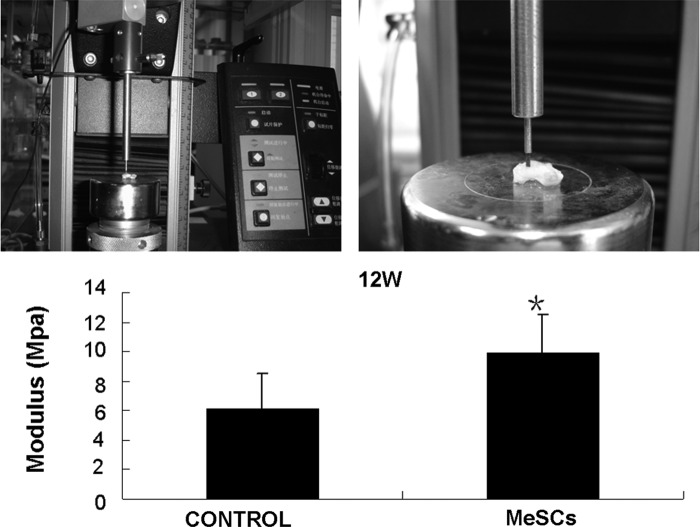
The Instron tension/compression system was used to evaluate the biomechanical properties (modulus) of our specimens (Fig. 4). All specimens were firmly attached during biomechanical testing. The values of compressive modulus were higher in the MeSC-treated group compared to the control group (9.88±2.65 vs. 6.14±2.36 Mpa, P<0.05, Fig. 4).
FIG. 4.
Biomechanical analysis of repaired tissues at 12 weeks. Statistically significant differences were found between the control group (CONTROL) and the MeSC-treated group (MeSCs) (P<0.05). *P<0.05 versus control group.
The effect of intra-articular injection of MeSCs on the suppression of OA within a rabbit model
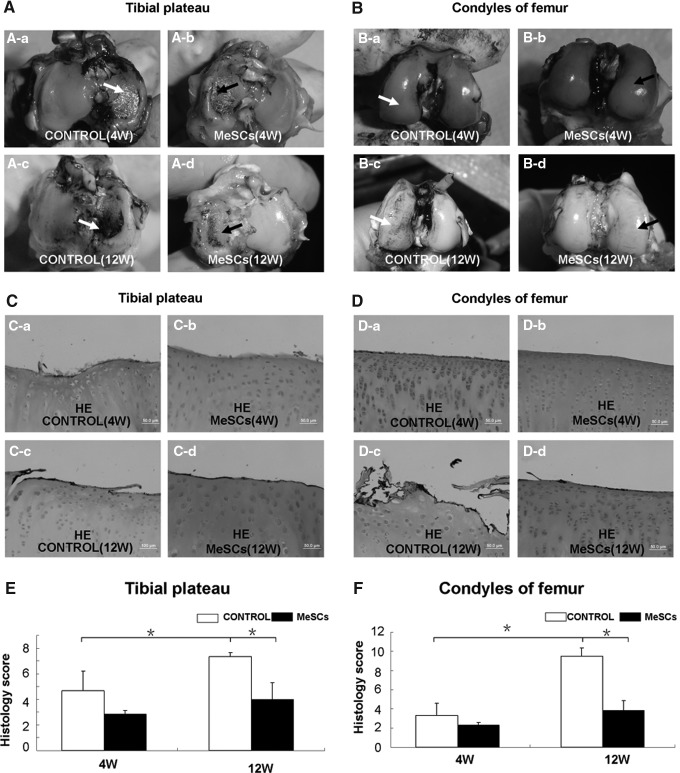
Macroscopically, the articular cartilage surface of medial tibial plateau in the rabbit model varied from complete absence of degeneration to the presence of craters in the control group at 4 weeks after meniscectomy (Fig. 5A-a), and they were more sericous at 12 weeks (Fig. 5A-c). The articular cartilage surface of medial femoral condyle was better than that of medial tibial plateau at 4 weeks after meniscectomy (Fig. 5B-a). However, we still observed fibrillation on the surface of medial femoral condyle in the control group at 12 weeks (Fig. 5B-c). By contrast, the cartilage surface seemed less affected in the MeSC-treated group both at 4 and 12 weeks postmeniscectomy (Fig. 5A-b, A-d, B-b, B-d), particularly in the surface of medial femoral condyle (Fig. 5B-b, B-d).
FIG. 5.
Joint surface evaluation of knee joint after meniscectomy and MeSCs injection. Gross morphology of the cartilage surface of tibia and femur in the control group (white arrow) and MeSC-treated group (A, B, black arrow) at 4 and 12 weeks postimplantation. The cartilage was stained with Indian ink. Typical HE staining of the cartilage of tibia and femur in the control group and MeSC-treated group (C, D) at 4 and 12 weeks postimplantation. The histological evaluation of the cartilage surface of tibia (E) and femur (F) were evaluated according to the modified Mankin's score. Scale bars=50 μm (C, D). *P<0.05 versus control group.
Microscopically, varying stages of cartilage degeneration were also observed in the control groups (4.67±1.53 vs. 7.33±0.29, P<0.05, Fig. 5C-a, C-c; 3.33±1.26 vs. 9.50±0.87, P<0.05 Fig. 5D-a, D-c). At 4 weeks postmeniscectomy, we could see no flat superficial chondrocytes on the cartilage surface of medial tibial plateau and medial femoral condyle in the control groups (Fig. 5C-a, D-a). At 12 weeks postmeniscectomy, extensive surface irregularities were seen in the control groups (Fig. 5C-c, D-c, 7.33±0.29 vs. 4.00±1.32, P<0.05, E and 9.50±0.87 vs. 3.83±1.04, P<0.05 F), particularly in the cartilage surface of medial femoral condyle (Fig. 5D-c). However, in the MeSC-treated group, only minimal damage to the superficial cell layers of the cartilage could be observed (Fig. 5C-b, C-d, D-b, D-d).
Radiological analyses were conducted at 12 weeks postmeniscectomy, to compare knee joint degeneration between the control group (Fig. 6A) and MeSC-treated group (Fig. 6B). Radiographs demonstrated that the injection of MeSCs inhibited the progression of experimental OA, as evidenced by undamaged joint surface cartilage and normal joint space width (Fig. 6C; P<0.05).
FIG. 6.
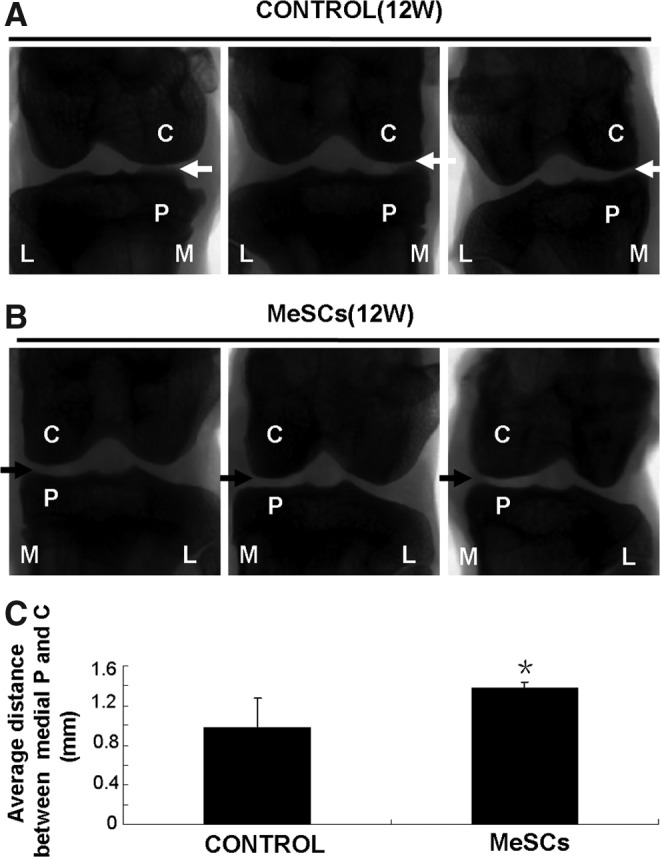
Radiographic evaluation of knee joint after meniscectomy and MeSCs injection. X-ray photo of control group (A, white arrow) and MeSC-treated group (B, black arrow). (C) Average distance between medial knee joint. C, condyles of femur; P, tibial plateau; M, medial of knee joint; L, lateral of knee joint. *P<0.05 versus control group.
Discussion
Our results demonstrated that (1) a unique cell subpopulation with the typical characteristics of mesenchymal stem cells (designated MeSCs) were successfully isolated and identified within rabbit meniscus tissue; (2) MLC indicates that MeSCs are nonimmunogenic and are able to suppress the immune response in vitro; (3) intra-articular injection of allogenous MeSCs promoted the regeneration of meniscus in vivo without eliciting obcious immunological rejection; and (4) intra-articular injection of allogenous MeSCs effectively protected joint surface and maintained joint space width in an experimental OA model (rabbit knee joint). Collectively, these results suggest that intra-articular injection of allogenous MeSCs hold much promise for clinical applications in meniscus regeneration and OA prevention in the future.
A number of studies have focused on the use of adult stem cells as a cell source for meniscus repair including bone marrow-derived and synovium-derived MSCs [10]. However, meniscus repair with these cell types are suboptimal. A recent study suggests that multipotent stem cells are present in the human meniscus [14]. Sekiya et al. demonstrated that compared to adult stem cells derived from other connective tissues, MeSCs had stronger and more stable expression of proline arginine-rich end leucine-rich repeat protein (PRELP), a connective tissue glycoprotein of the leucine-rich repeat family regulating collagen fibril growth in various connective tissues [14,27,28]. Consistent with the study of Sekiya et al., we have previously found that MeSCs displayed higher expression of collagen type II than adult stem cells derived from bone marrow and synovium (unpublished data). These data thus suggest that MeSCs can be a promising cell source for meniscus regeneration in vivo. In this study, our results demonstrated that intra-articular transplantation of MeSCs enhanced meniscus regeneration with improved physiological and structural properties of neo-tissue.
Although autologous transplantation of adult stem cells represents an ideal strategy for tissue regeneration, limited cell numbers hinder its clinical application. Allogenous transplantation of stem cells offers an alternative approach for the regeneration of injured tissue. However, stem cells from different tissues may exhibit different immune properties. For example, BMSCs have been reported to be nonimmunogenic and immunosuppressive [21,22]. Various adult tissue-derived stem cells, such as ADSCs, tendon stem/progenitor cells (TSPCs), and PDLSCs, are known to exhibit analogous immune properties of BMSCs [15,17,23,29,30]. However, telencephalon tissue-derived neuronal stem cells have been reported to be rejected upon transplantation [31]. Therefore, it is necessary to rigorously characterize the immune properties of MeSCs. Our immunological assays indicated that, similar to BMSCs, MeSCs are negative for MHC-II expression. This implies that MeSCs lack the ability to present alloantigen directly to recipient CD4+T cells [21]. In addition, MeSCs did not stimulate but instead actively suppressed allogenous PBMCs. It indicated that even with the assistance of APC, MeSCs could not activate allogeneic PBMCs to elicit an immune response in vitro. This results suggests that allogenous MeSCs can be clinically utilized for meniscus regeneration.
Our in vivo studies utilizing allogenous MeSCs showed that allogenous MeSCs injection did not elicit immune rejection. In addition, we found that allogenous MeSC-treated groups exhibited higher intensity of safranin O staining with the presence of round meniscal cells, thus indicating that the injected MeSCs play an important role in modulating the healing processes of meniscus tissue. The allogenous MeSC-treated group also displayed better results in TEM and real-time PCR analysis of meniscus-related gene expression, without eliciting any inflammation and immunological rejection. Nevertheless, the mechanism of immunosuppression by MeSCs in vivo was not fully explored, which is a limitation of this study. Several different mechanisms of BMSC-mediated immunosuppression were proposed. Hoffman and colleagues hypothesized that allogenous MSCs could integrate into bone marrow to influence early immune cell growth [22], as well as migration into the thymus to play a role in T cell selection [32]. Blancher and colleagues found that ADSCs effected immune suppression through release of soluble factors, such as IL-10 and TGF-beta [33]. Hence, further studies are required to elucidate the exact mechanism of MeSC-mediated immunosuppression.
Another limitation of our in vivo study is that rabbit meniscus could heal spontaneously after 12 weeks as in the rat model [10]. Buschmann and colleagues compared human meniscus with rabbit and sheep meniscus, and found that the main structural features, including cellular distribution, vascularity, and collagen structure, are rather similar in human and sheep but different in rabbits [34]. Murphy and colleagues found that the spontaneous healing of meniscus is also limited in the caprine model, which is similar to the human clinical situation [7]. Hence, in order to demonstrate the clinical efficacy of intra-articular injection of MeSCs for meniscus repair, larger animal models such as sheep, dog, and pig [35] are required for further studies.
In our in vivo study, it was demonstrated that intra-articular injection of MeSCs can delay or reduce the progression of OA induced by meniscectomy. However, the mechanism of OA suppression remains unknown. Some researchers have hypothesized that the neo-meniscal tissue in the MeSC-treated groups is associated with protection against degenerative changes linked to OA after meniscal injury [14]. For example, Sekiya and colleagues demonstrated that synovial stem cells inhibited the fibrillation of cartilage surface at 12 weeks postsurgery [10]. Injection of BMSCs also reduced the degree of cartilage degeneration, osteophyte formation, and subchondral sclerosis [7,36–38]. MSCs may have a direct role in cartilage protection by direct remodeling of the articular cartilage surface or by acting to preserve subchondral or trabecular bone based on the relationship between early bone changes and the development of OA [39–41]. However, Sekiya and colleagues found that only a small portion of the cells adhered to the cartilage defect after injection [42]. In our study, we also could not detect any live injected MeSCs on the cartilage surface by image tracking (data not shown). These results suggest that other mechanisms may be involved. Many studies showed that cartilage degradation and pro-inflammatory pathways of OA are induced by the up-regulation of inflammatory cytokines [43–45]. Inflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor (TNFα) [46], have been demonstrated to be upregulated after meniscus injury. Furthermore, an animal model of meniscus injury in rabbits showed that the elevated expression of IL-1 will last for at least 14 days [47], and upregulated inflammatory cytokines will in turn increase matrix metalloproteinase activity [48,49], proteoglycan release [50], as well as suppress collagen synthesis [51]. Pennesi and colleagues found that MSCs could prevent the occurrence of severe, irreversible damage to the bone and cartilage by downregulating the expression of inflammatory cytokines [16]. In our in vitro study, we also demonstrated that allogeneic MeSCs not only decreased lymphocyte proliferation compared to allogeneic PBMC, but also partly inhibited the proliferation of lymphocytes in response to allogeneic PBMC. These results suggest that injected allogenous MeSCs may suppress OA progression by enhancing meniscus regeneration, as well as by inhibiting inflammatory cytokines.
Conclusions
Our study demonstrated that MeSCs can be isolated from meniscus and that these cells posses immunosuppressive properties. Intra-articular injection of MeSCs promoted meniscus regeneration, protected joint surface cartilage, and maintained joint space width. These findings suggest a new strategy of articular cartilage protection through meniscus regeneration induced by intra-articular injection of MeSCs for patients undergoing meniscectomy.
Acknowledgments
This work was supported by NSFC grants (81201396, 81271970, 81125014, 81071461, J1103603, 31000436), National Basic Research Program of China (973 program; 2012CB966604), the National High Technology Research and Development Program of China (2012AA020503), Zhejiang Province grants (Z2100086, Y2100095, LY12H06006), NCET-08-0487, and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents. The authors thank Wang Li for the TEM imaging, and Song Xinghui and Zhang Wei for their expert technical assistance.
Author Disclosure Statement
The authors of this article have no potential conflict of interest.
Reference
- 1.Majewski M. Susanne H. Klaus S. Epidemiology of athletic knee injuries: a 10-year study. Knee. 2006;13:184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.van TTG. Hannink G. Buma P. Meniscus replacement using synthetic materials. Clin Sports Med. 2009;28:143–156. doi: 10.1016/j.csm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Roos H. Lauren M. Adalberth T. Roos EM. Jonsson K. Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Englund M. Guermazi A. Roemer FW. Yang M. Zhang Y. Nevitt MC. Lynch JA. Lewis CE. Torner J. Felson DT. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST Study. Ann Rheum Dis. 2010;69:1796–1802. doi: 10.1136/ard.2009.121681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang A. Moisio K. Chmiel JS. Eckstein F. Guermazi A. Almagor O. Cahue S. Wirth W. Prasad P. Sharma L. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Ann Rheum Dis. 2011;70:74–79. doi: 10.1136/ard.2010.130278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buma P. Ramrattan NN. van TTG. Veth RP. Tissue engineering of the meniscus. Biomaterials. 2004;25:1523–1532. doi: 10.1016/s0142-9612(03)00499-x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy JM. Fink DJ. Hunziker EB. Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Baker BM. Nathan AS. Huffman GR. Mauck RL. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthritis Cartilage. 2009;17:336–345. doi: 10.1016/j.joca.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horie M. Sekiya I. Muneta T. Ichinose S. Matsumoto K. Saito H. Murakami T. Kobayashi E. Intra-articular Injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27:878–887. doi: 10.1634/stemcells.2008-0616. [DOI] [PubMed] [Google Scholar]
- 11.Harris MT. Butler DL. Boivin GP. Florer JB. Schantz EJ. Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi Y. Sekiya I. Yagishita K. Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki T. Muneta T. Sakaguchi Y. Nimura A. Yokoyama A. Koga H. Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 14.Segawa Y. Muneta T. Makino H. Nimura A. Mochizuki T. Ju YJ. Ezura Y. Umezawa A. Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh K. Zvonic S. Garrett S. Mitchell JB. Floyd ZE. Hammill L. Kloster A. Di HY. Ting JP, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 16.Augello A. Tasso R. Negrini SM. Cancedda R. Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 17.Wada N. Menicanin D. Shi S. Bartold PM. Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667–676. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- 18.Sun Z. Han Q. Zhu Y. Li Z. Chen B. Liao L. Bian C. Li J. Shao C. Zhao RC. NANOG has a role in mesenchymal stem cells' immunomodulatory effect. Stem Cells Dev. 2011;20:1521–1528. doi: 10.1089/scd.2010.0366. [DOI] [PubMed] [Google Scholar]
- 19.Kita K. Gauglitz GG. Phan TT. Herndon DN. Jeschke MG. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 2010;19:491–502. doi: 10.1089/scd.2009.0192. [DOI] [PubMed] [Google Scholar]
- 20.Yin Z. Chen X. Chen JL. Shen WL. Hieu NTM. Gao L. Ouyang HW. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163–2175. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 21.Liu H. Kemeny DM. Heng BC. Ouyang HW. Melendez AJ. Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 22.Bartholomew A. Sturgeon C. Siatskas M. Ferrer K. McIntosh K. Patil S. Hardy W. Devine S. Ucker D, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 23.Shen W. Chen J. Yin Z. Chen X. Liu H. Heng BC. Chen W. Ouyang HW. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair. Cell Transplant. 2012;21:943–958. doi: 10.3727/096368911X627453. [DOI] [PubMed] [Google Scholar]
- 24.Chen X. Song XH. Yin Z. Zou XH. Wang LL. Hu H. Cao T. Zheng M. Ouyang HW. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27:1276–1287. doi: 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 25.Zou XH. Cai HX. Yin Z. Chen X. Jiang YZ. Hu H. Ouyang HW. A novel strategy incorporated the power of mesenchymal stem cells to allografts for segmental bone tissue engineering. Cell Transplant. 2009;18:433–441. doi: 10.3727/096368909788809839. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y. Chen LK. Zhu DC. Zhang GR. Guo C. Qi YY. Ouyang HW. The inductive effect of bone morphogenetic protein-4 on chondral-lineage differentiation and in situ cartilage repair. Tissue Eng Part A. 2010;16:1621–1632. doi: 10.1089/ten.TEA.2009.0681. [DOI] [PubMed] [Google Scholar]
- 27.Ezura Y. Chakravarti S. Oldberg A. Chervoneva I. Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ameye L. Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–16R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 29.Saldanha-Araujo F. Ferreira FI. Palma PV. Araujo AG. Queiroz RH. Covas DT. Zago MA. Panepucci RA. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7:66–74. doi: 10.1016/j.scr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Pelekanos RA. Li J. Gongora M. Chandrakanthan V. Scown J. Suhaimi N. Brooke G. Christensen ME. Doan T, et al. Comprehensive transcriptome and immunophenotype analysis of renal and cardiac MSC-like populations supports strong congruence with bone marrow MSC despite maintenance of distinct identities. Stem Cell Res. 2012;8:58–73. doi: 10.1016/j.scr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Lee EM. Kim JY. Cho BR. Chung WK. Yoon BW. Kim SU. Lee BC. Hwang WS. Moon SY. Lee JS. Ahn C. Down-regulation of MHC class I expression in human neuronal stem cells using viral stealth mechanism. Biochem Biophys Res Commun. 2005;326:825–835. doi: 10.1016/j.bbrc.2004.11.106. [DOI] [PubMed] [Google Scholar]
- 32.Devine SM. Cobbs C. Jennings M. Bartholomew A. Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 33.Puissant B. Barreau C. Bourin P. Clavel C. Corre J. Bousquet C. Taureau C. Cousin B. Abbal M, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 34.Chevrier A. Nelea M. Hurtig MB. Hoemann CD. Buschmann MD. Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J Orthop Res. 2009;27:1197–1203. doi: 10.1002/jor.20869. [DOI] [PubMed] [Google Scholar]
- 35.Peretti GM. Gill TJ. Xu JW. Randolph MA. Morse KR. Zaleske DJ. Cell-based therapy for meniscal repair: a large animal study. Am J Sports Med. 2004;32:146–158. doi: 10.1177/0095399703258790. [DOI] [PubMed] [Google Scholar]
- 36.Tuan RS. Stemming cartilage degeneration: adult mesenchymal stem cells as a cell source for articular cartilage tissue engineering. Arthritis Rheum. 2006;54:3075–3078. doi: 10.1002/art.22148. [DOI] [PubMed] [Google Scholar]
- 37.McIlwraith CW. Frisbie DD. Rodkey WG. Kisiday JD. Werpy NM. Kawcak CE. Steadman JR. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27:1552–1561. doi: 10.1016/j.arthro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Al FH. Nor HBM. Chen HC. Aminuddin BS. Ruszymah BH. The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 2012;47:458–464. doi: 10.1016/j.exger.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Li B. Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res. 1997;12:641–651. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez MA. Gonzalez-Rey E. Rico L. Buscher D. Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 41.Park MJ. Park HS. Cho ML. Oh HJ. Cho YG. Min SY. Chung BH. Lee JW. Kim HY. Cho SG. Transforming growth factor beta-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum. 2011;63:1668–1680. doi: 10.1002/art.30326. [DOI] [PubMed] [Google Scholar]
- 42.Koga H. Shimaya M. Muneta T. Nimura A. Morito T. Hayashi M. Suzuki S. Ju YJ. Mochizuki T. Sekiya I. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10:R84. doi: 10.1186/ar2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai SM. Shan ZZ. Nishioka K. Yudoh K. Implication of interleukin 18 in production of matrix metalloproteinases in articular chondrocytes in arthritis: direct effect on chondrocytes may not be pivotal. Ann Rheum Dis. 2005;64:735–742. doi: 10.1136/ard.2004.026088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tahiri K. Korwin-Zmijowska C. Richette P. Heraud F. Chevalier X. Savouret JF. Corvol MT. Natural chondroitin sulphates increase aggregation of proteoglycan complexes and decrease ADAMTS-5 expression in interleukin 1 beta-treated chondrocytes. Ann Rheum Dis. 2008;67:696–702. doi: 10.1136/ard.2007.078600. [DOI] [PubMed] [Google Scholar]
- 45.Lamacchia C. Rodriguez E. Palmer G. Vigne S. Martin P. Talabot-Ayer D. Seemayer CA. Gabay C. Articular inflammation is controlled by myeloid cell-derived interleukin 1 receptor antagonist during the acute phase of arthritis in mice. Ann Rheum Dis. 2012;71:281–287. doi: 10.1136/annrheumdis-2011-200429. [DOI] [PubMed] [Google Scholar]
- 46.Wehling N. Palmer GD. Pilapil C. Liu F. Wells JW. Muller PE. Evans CH. Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60:801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochi M. Uchio Y. Okuda K. Shu N. Yamaguchi H. Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17:724–731. doi: 10.1053/jars.2001.23583. [DOI] [PubMed] [Google Scholar]
- 48.Flood S. Parri R. Williams A. Duance V. Mason D. Modulation of interleukin-6 and matrix metalloproteinase 2 expression in human fibroblast-like synoviocytes by functional ionotropic glutamate receptors. Arthritis Rheum. 2007;56:2523–2534. doi: 10.1002/art.22829. [DOI] [PubMed] [Google Scholar]
- 49.Hot A. Zrioual S. Lenief V. Miossec P. IL-17 and tumour necrosis factor alpha combination induces a HIF-1alpha-dependent invasive phenotype in synoviocytes. Ann Rheum Dis. 2012;71:1393–1401. doi: 10.1136/annrheumdis-2011-200867. [DOI] [PubMed] [Google Scholar]
- 50.de Loo FA v. Arntz OJ. van EFH. van LPL. den v Berg WB. Reduced cartilage proteoglycan loss during zymosan-induced gonarthritis in NOS2-deficient mice and in anti-interleukin-1-treated wild-type mice with unabated joint inflammation. Arthritis Rheum. 1998;41:634–646. doi: 10.1002/1529-0131(199804)41:4<634::AID-ART10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Cao M. Stefanovic-Racic M. Georgescu HI. Miller LA. Evans CH. Generation of nitric oxide by lapine meniscal cells and its effect on matrix metabolism: stimulation of collagen production by arginine. J Orthop Res. 1998;16:104–111. doi: 10.1002/jor.1100160118. [DOI] [PubMed] [Google Scholar]