Abstract
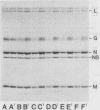
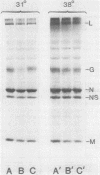
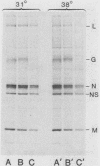
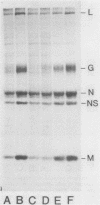
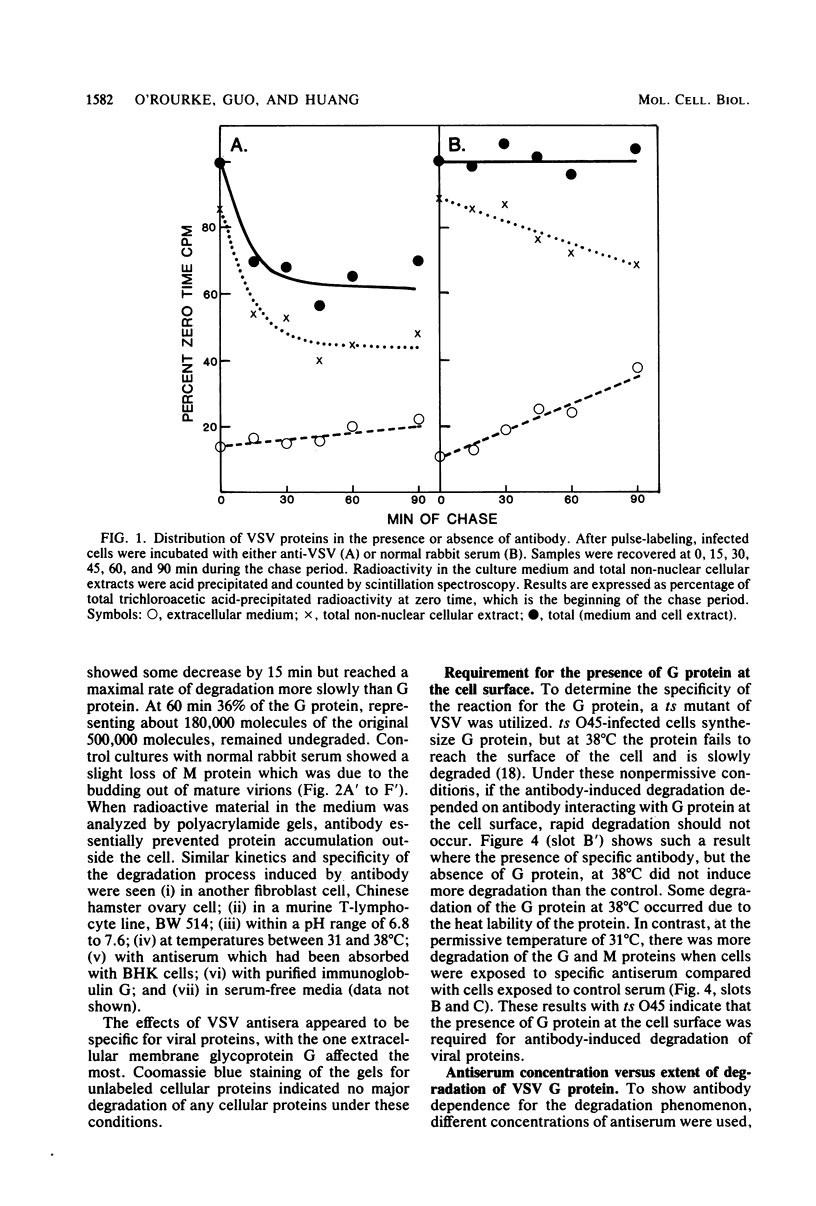
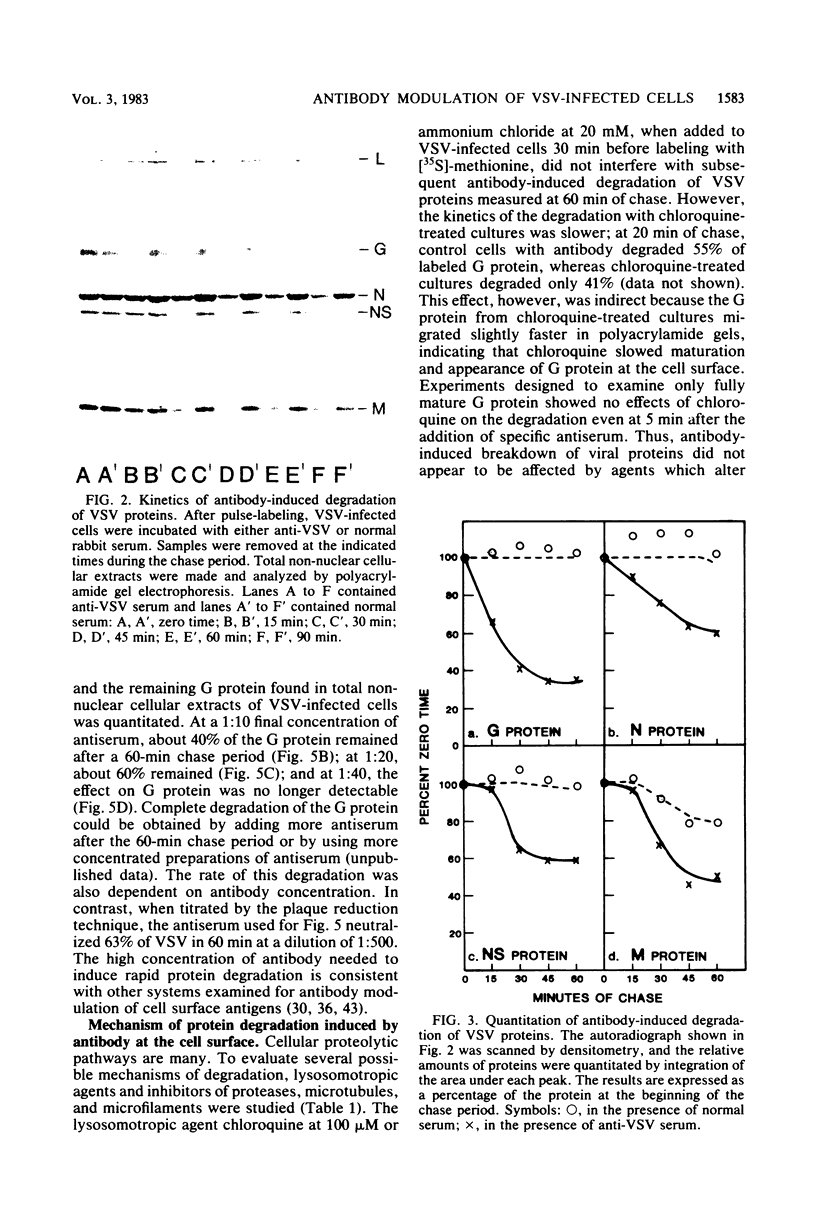
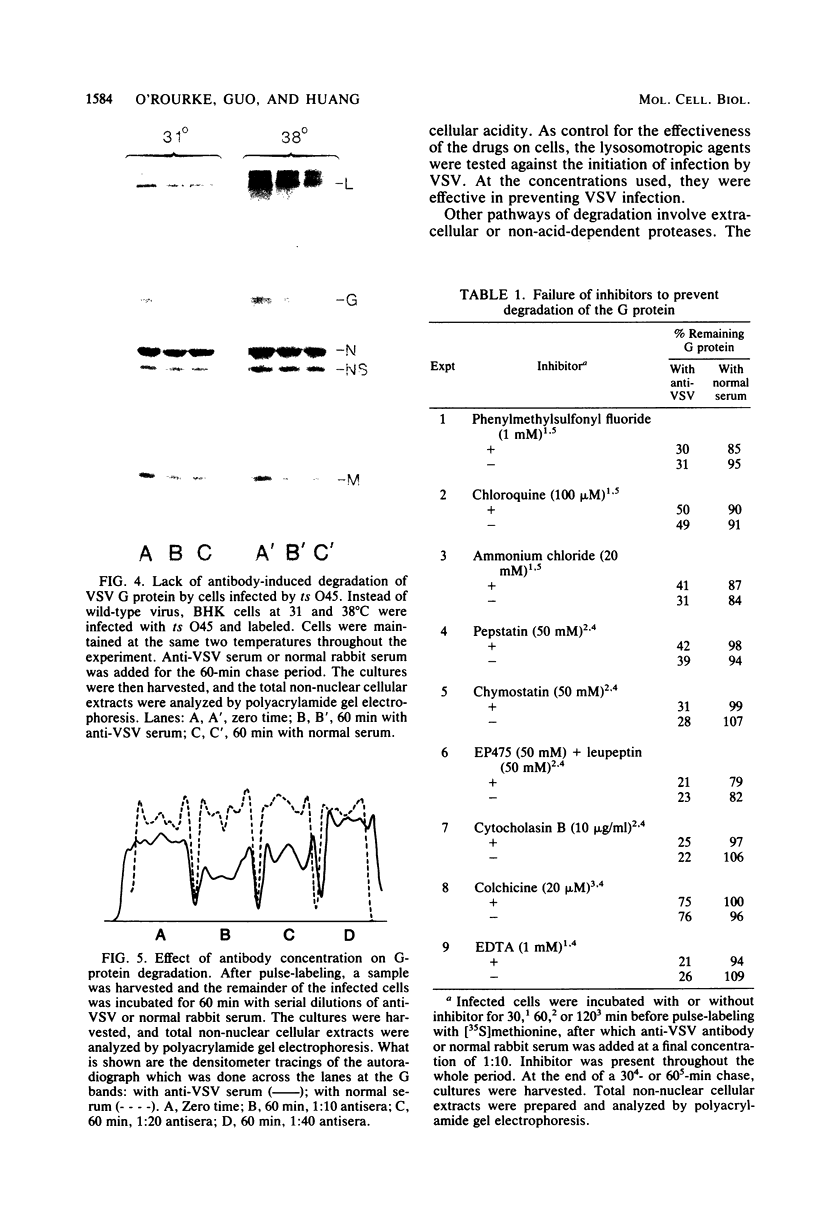
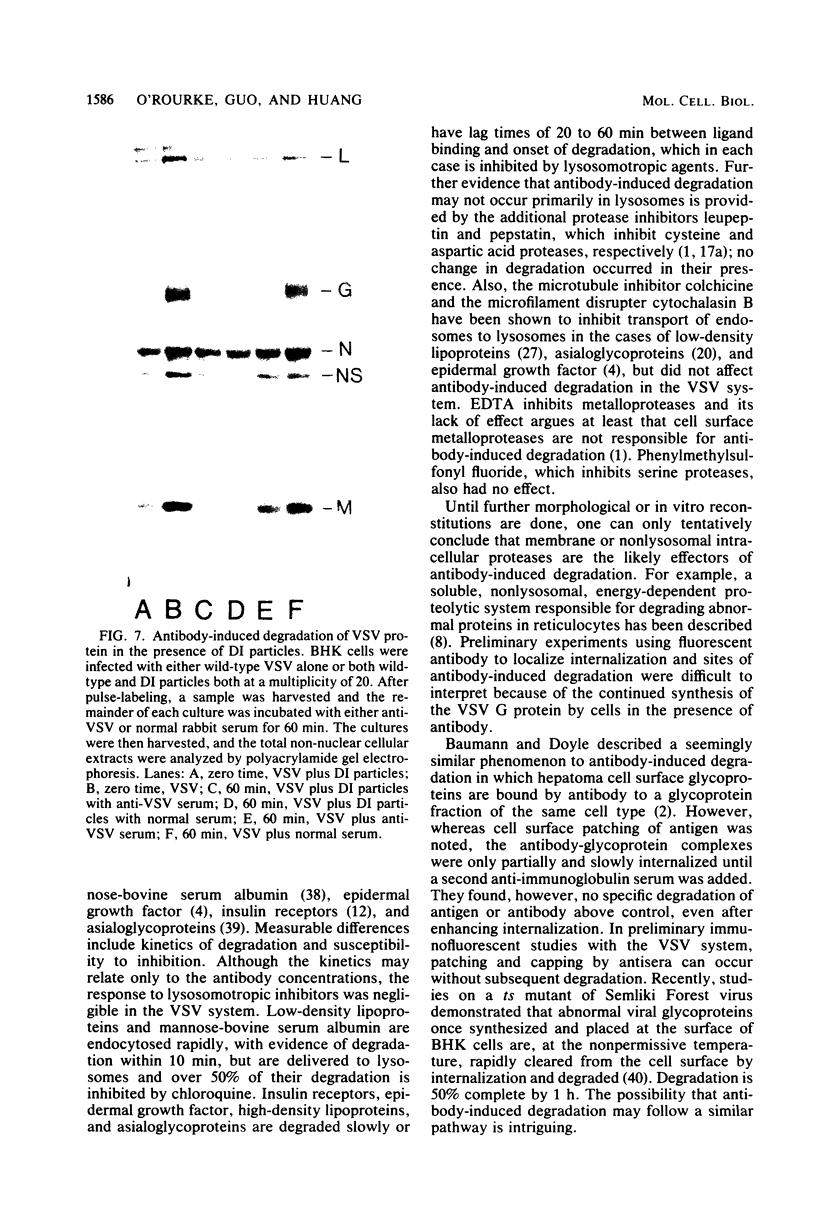
When vesicular stomatitis virus-infected baby hamster kidney cells were treated with rabbit anti-vesicular stomatitis virus serum, there was a loss of the viral glycoprotein G into acid-soluble products. This degradation occurred within minutes at 37 degrees C and required the presence of G protein at the cell surface. The degree of degradation depended on antiserum concentration. The antiserum, also, prevented maturation of extracellular virions and induced partial degradation of the intracellular viral proteins, without affecting host proteins. The degradation could not be prevented by the presence of lysosomotropic agents, protease inhibitors, colchicine, or cytochalasin B. Similar kinetics and specificity of degradation was obtained with cells infected with vesicular stomatitis virus mutants that were less cytopathic. These results characterize a model system for studying the parameters and consequences of antigenic modulation as well as for studying the fate of viral antigens during persistent infections.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Doyle D. Metabolic fate of cell surface glycoproteins during immunoglobulin-induced internalization. Cell. 1980 Oct;21(3):897–907. doi: 10.1016/0092-8674(80)90453-5. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Stockert E., Old L. J. Modification of the antigenic structure of the cell membrane by thymus-leukemia (TL) antibody. Proc Natl Acad Sci U S A. 1967 Sep;58(3):954–957. doi: 10.1073/pnas.58.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Friedkin M., Rozengurt E. Colchicine inhibits epidermal growth factor degradation in 3T3 cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):480–484. doi: 10.1073/pnas.77.1.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Willingham M. C., Pastan I. alpha 2-macroglobulin adsorbed to colloidal gold: a new probe in the study of receptor-mediated endocytosis. J Cell Biol. 1981 Apr;89(1):29–34. doi: 10.1083/jcb.89.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. Review article initial stages in infection with animal viruses. J Gen Virol. 1982 Mar;59(Pt 1):1–22. doi: 10.1099/0022-1317-59-1-1. [DOI] [PubMed] [Google Scholar]
- Edidin M., Weiss A. Antigen cap formation in cultured fibroblasts: a reflection of membrane fluidity and of cell motility. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2456–2459. doi: 10.1073/pnas.69.9.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Huang A. S. Mapping temperature-sensitive mutants of vesicular stomatitis virus by RNA heteroduplex formation. J Gen Virol. 1981 Nov;57(Pt 1):103–117. doi: 10.1099/0022-1317-57-1-103. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P. Synthesis and maturation of glycoproteins of enveloped animal viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):26–39. doi: 10.1093/clinids/2.1.26. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Green A., Olefsky J. M. Evidence for insulin-induced internalization and degradation of insulin receptors in rat adipocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):427–431. doi: 10.1073/pnas.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Immunologic injury in measles virus infection. II. Suppression of immune injury through antigenic modulation. J Exp Med. 1975 Oct 1;142(4):864–876. doi: 10.1084/jem.142.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O., Tolleshaug H., Berg T. The effects of colchicine and cytochalasin B on uptake and degradation of asialo-glycoproteins in isolated rat hepatocytes. Exp Cell Res. 1979 Aug;122(1):159–167. doi: 10.1016/0014-4827(79)90570-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Joseph B. S., Oldstone M. B. Antibody-induced capping of measles virus antigens on plasma membrane studied by electron microscopy. J Virol. 1975 May;15(5):1248–1255. doi: 10.1128/jvi.15.5.1248-1255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R. Antibody-induced changes in expression of the H-2 antigen. Eur J Immunol. 1974 Nov;4(11):732–739. doi: 10.1002/eji.1830041106. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Stockert E., Boyse E. A., Kim J. H. Antigenic modulation. Loss of TL antigen from cells exposed to TL antibody. Study of the phenomenon in vitro. J Exp Med. 1968 Mar 1;127(3):523–539. doi: 10.1084/jem.127.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ose L., Røken I., Norum K. R., Berg T. The effect of ammonia, chloroquine, leupeptin, colchicine and cytochalasin B on degradation of high density lipoproteins in isolated rat hepatocytes. Exp Cell Res. 1980 Nov;130(1):127–135. doi: 10.1016/0014-4827(80)90049-x. [DOI] [PubMed] [Google Scholar]
- Ostlund R. E., Jr, Pfleger B., Schonfeld G. Role of microtubules in low density lipoprotein processing by cultured cells. J Clin Invest. 1979 Jan;63(1):75–84. doi: 10.1172/JCI109281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E. L., Perlman S. M., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. VI. Correlation of defective particle RNA synthesis with standard RNA replication. J Mol Biol. 1974 May 5;85(1):127–136. doi: 10.1016/0022-2836(74)90133-8. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–250. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Oldstone M. B. The formation and fate of virus antigen-antibody complexes. J Immunol. 1977 Jan;118(1):316–322. [PubMed] [Google Scholar]
- Pesando J. M., Ritz J., Lazarus H., Tomaselli K. J., Schlossman S. F. Fate of a common acute lymphoblastic leukemia antigen during modulation by monoclonal antibody. J Immunol. 1981 Feb;126(2):540–544. [PubMed] [Google Scholar]
- Rao D. D., Huang A. S. RNA synthesis of vesicular stomatitis virus. X. Transcription and replication by defective interfering particles. J Virol. 1980 Dec;36(3):756–765. doi: 10.1128/jvi.36.3.756-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Davie J. M., Rosenstreich D. L., Cehrs K. U. Antibody-mediated internalization of B lymphocyte surface membrane immunoglobulin. Exp Cell Res. 1973 Oct;81(2):317–329. doi: 10.1016/0014-4827(73)90521-1. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W., Cremona P., Leonard C., Stremmel P. Antigenic modulation as a mechanism for tumor escape from immune destruction: identification of modulation-positive and modulation-negative mouse lymphomas with xenoantisera to murine leukemia virus gp70. J Immunol. 1980 Oct;125(4):1715–1723. [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Nilsson M., Norum K. R. Uptake and degradation of 125I-labelled asialo-fetuin by isolated rat hepatocytes. Biochim Biophys Acta. 1977 Aug 25;499(1):73–84. doi: 10.1016/0304-4165(77)90230-6. [DOI] [PubMed] [Google Scholar]
- Ukkonen P., Saraste J., Korpela K., Pesonen M., Käriäinen L. Temperature-dependent internalization of virus glycoproteins in cells infected with a mutant of Semliki Forest virus. EMBO J. 1982;1(2):191–196. doi: 10.1002/j.1460-2075.1982.tb01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Heine J. W., Goldstein G., Schnaitman C. A. Use of antiviral-antiferritin hybrid antibody for localization of viral antigen in plasma membrane. J Virol. 1971 Feb;7(2):274–277. doi: 10.1128/jvi.7.2.274-277.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A., Cohen E. P. Studies on the effect of specific antisera on the metabolism of cellular antigens. II. The synthesis and degradation of TL antigens of mouse cells in the presence of TL antiserum. J Immunol. 1974 Apr;112(4):1296–1307. [PubMed] [Google Scholar]