Abstract
How invasive and metastatic tumor cells evade anoikis induction remains unclear. We found that knockdown of RSK2 sensitizes diverse cancer cells to anoikis induction, which is mediated through phosphorylation targets including apoptosis signal-regulating kinase 1 (ASK1) and cyclic AMP (cAMP) response element-binding protein (CREB). We provide evidence to show that RSK2 inhibits ASK1 by phosphorylating S83, T1109, and T1326 through a novel mechanism in which phospho-T1109/T1326 inhibits ATP binding to ASK1, while phospho-S83 attenuates ASK1 substrate MKK6 binding. Moreover, the RSK2→CREB signaling pathway provides antianoikis protection by regulating gene expression of protein effectors that are involved in cell death regulation, including the antiapoptotic factor protein tyrosine kinase 6 (PTK6) and the proapoptotic factor inhibitor-of-growth protein 3 (ING3). PTK6 overexpression or ING3 knockdown in addition to ASK1 knockdown further rescued the increased sensitivity to anoikis induction in RSK2 knockdown cells. These data together suggest that RSK2 functions as a signal integrator to provide antianoikis protection to cancer cells in both transcription-independent and -dependent manners, in part by signaling through ASK1 and CREB, and contributes to cancer cell invasion and tumor metastasis.
INTRODUCTION
Metastasis is the most dangerous switch during tumor progression, which involves a complicated chain of events. Epithelial cells normally undergo “anoikis,” an apoptotic process, due to loss of contact with the extracellular matrix, which provides a strong physiological barrier to the development of metastasis. Resistance to anoikis is a hallmark of metastatic cancers, where cells need to survive in an anchorage-dependent environment in ascetic fluids before invading distant organs. However, the signaling mechanisms by which metastatic tumor cells become resistant to the anoikis process remain poorly understood (1, 2).
We recently reported that continued RSK2 expression contributes to the maintenance of the invasive and metastatic potential of head and neck squamous cell carcinoma (HNSCC) cells in vitro and in vivo, respectively (3). The serine/threonine kinase RSK2 is a substrate of extracellular signal-regulated kinase (ERK) and belongs to a family containing RSK1 to RSK4. RSK family members contain two distinct kinase domains, both of which are catalytically functional (reviewed in references 4 and 5). The C-terminal kinase domain is responsible for autophosphorylation at Ser386, which is critical for RSK activation, whereas the N-terminal kinase domain phosphorylates downstream substrates of RSK (6). RSK2 is involved in various cellular processes, including gene expression, the cell cycle, cell survival, and proliferation. RSK2 phosphorylates multiple signaling effectors to regulate diverse cellular functions, including gene expression by phosphorylating transcription factors such as cyclic AMP (cAMP) response element-binding protein (CREB) (7) and histone H3 (8), which remodels chromatin during mitosis and transcriptional activation; the cell cycle by phosphorylating and inhibiting Myt1 (9), a p34cdc2 inhibitory kinase; and cell survival by phosphorylating BAD (10), Bim (11), and death-associated protein kinase (DAPK) to protect cells from apoptosis.
CREB is a transcription factor whose signaling has been implicated in promoting tumor progression, stimulating growth, conferring apoptotic resistance, and supporting angiogenesis. CREB is associated with androgen-independent progression and promotes prostate cancer bone metastasis (12). In addition, expression of a dominant negative form of CREB sensitizes melanoma cells to apoptosis and inhibits their growth and metastasis (13–16). RSK2 activates CREB by phosphorylating Ser133 (17). However, there is no literature reporting a role for the RSK2→CREB pathway in anoikis regulation.
Here we demonstrate that RSK2 signaling is commonly important to protect diverse metastatic human cancer cells, including lung, breast, and head and neck cancer cells, from anoikis. Our phosphor-proteomics-based studies using the phosphoantibody microarray identified two RSK2 phosphorylation targets that are important for mediating RSK2-dependent antianoikis signals in cancer cells, including CREB and a newly identified RSK2 substrate, apoptosis signal-regulating kinase 1 (ASK1). ASK1 is a mitogen-activated protein kinase (MAPK) kinase kinase that is activated by a variety of stress-related stimuli, including oxidative stress, serum withdrawal, endoplasmic reticulum stress, and calcium influx. Activated ASK1 phosphorylates and activates two different downstream kinases, MAPK kinase kinase 4 (MKK4)/MKK7 and MKK3/MKK6, which activate c-Jun N-terminal kinase (JNK) and p38 MAPK, respectively, to induce apoptosis. ASK1 kinase activity is regulated in various ways, including phosphorylation. For example, AKT and PIM1 inhibit ASK1 by phosphorylating ASK1 S83, which keeps ASK1 inactive under unstressed conditions. Conversely, stress signals induce ASK1 S83 dephosphorylation and restore ASK1 activity (18–20). However, it remains unclear how phosphorylation at S83 inhibits ASK1.
Here we report that RSK2 promotes anoikis resistance by inhibiting ASK1 via phosphorylation at two newly identified sites, T1109 and T1326, in addition to S83. We provide evidence to show that phosphorylation at T1109/T1326 attenuates ATP binding to ASK1, whereas S83 phosphorylation inhibits ASK1 by blocking substrate MKK6 binding. Moreover, we found that RSK2 protects metastatic cancer cells from anoikis induction in both transcription-independent and -dependent manners by inhibiting ASK1 and activating CREB, respectively, in which CREB coordinates gene expression of proapoptotic effectors such as protein tyrosine kinase 6 (PTK6) and antiapoptotic effectors such as inhibitor-of-growth protein 3 (ING3).
MATERIALS AND METHODS
Reagents.
Lentiviral short hairpin RNA (shRNA) vectors targeting RSK2, ASK1, CREB, PTK6, ING3, and enhanced green fluorescent protein (eGFP) were purchased from Open Biosystems. ASK1, CREB, and ING3 small interfering RNAs (siRNAs) were purchased from Qiagen. The RSK-specific inhibitor fmk was described previously (21). SL0101 was purchased from Toronto Research Chemicals, Inc. Wortmannin and the PIM1 inhibitor quercetagetin were purchased from Santa Cruz. RSK2 constructs were previously described (22–24). An shRNA-resistant form of human RSK2 was generated by silent mutations in the shRNA-targeting region. Recombinant active human RSK2 was obtained from Invitrogen. Recombinant inactive human MKK6 was obtained from Millipore. Myelin basic protein (MBP) was obtained from Sigma. The construct for hemagglutinin (HA)-ASK1 (provided by Haian Fu) was myc tagged by PCR and subcloned into pDEST27 and a pLHCX-derived Gateway destination vector as described previously for expression in human cell lines (25). pET60-ASK1 variants were generated for bacterial recombinant protein purification. pCMV6-flag-PTK6 was obtained from Origene. Image clones for ING3 were purchased from Open Biosystems. Flag-ING3 variants were subcloned into the retroviral vector pLHCX. Various mutants were generated by using the QuikChange-XL site-directed mutagenesis kit (Stratagene).
Cell culture.
293T and SKBR3 cells were cultured in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). 212LN and A549 cells were cultured in a 1:1 mix of DMEM and Ham's F-12 medium and RPMI 1640 with 10% FBS, respectively. To generate the ASK1- and ING3-expressing cell lines, the retroviral vector pLHCX harboring ASK1 and ING3 was transfected into 212LN cells by using Lipofectamine 2000 (Invitrogen). To knock down endogenous human RSK2, CREB, ASK1, PTK6, and ING3, lentiviruses carrying shRNA were generated by transfecting 293T cells with lentiviral vectors encoding shRNA, pHRCMV8.2ΔR, and cytomegalovirus-vesicular stomatitis virus G protein (CMV-VSVG). Cells were infected with harvested lentivirus for 48 h for transient infection or were selected by 2 μg/ml puromycin for 1 week for stable selection.
Antibodies.
Antibodies against RSK2, glycogen synthase kinase 3α/β (GSK3α/β), ASK1, MBP, PIM1, and PTK6 were obtained from Santa Cruz Biotechnology. Antibodies against myc, HA, phospho-RSK (Ser380), phospho-GSK3α/β (Ser21/9), phospho-ASK1 (Ser83, Ser967, and Thr845), phospho-p38 (Thr180/Tyr182), p38, phospho-stress-activated protein kinase (phospho-SAPK)/JNK (Thr183/Tyr185), ASK1, SAPK/JNK, MKK6, CREB, AKT, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Cell Signaling Technology (CST). Antibodies against Flag, glutathione S-transferase (GST), and β-actin were obtained from Sigma. Phospho-serine/threonine antibody was obtained from Abcam. Antibody against ING3 was obtained from Proteintech.
Purification of recombinant human ASK1 proteins.
GST-fused human ASK1 proteins were purified by sonication of Escherichia coli BL21(DE3)/pLysS cells obtained from 250 ml of culture with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) induction at 25°C. Cell lysates were loaded onto a glutathione-Sepharose 4B column in phosphate-buffered saline (PBS) and eluted with elution buffer (50 mM Tris-HCl, 10 mM reduced glutathione [pH 8.0]). Proteins were desalted on a PD-10 column, and the purification efficiency was examined by Coomassie staining and Western blotting.
In vitro kinase assays.
An RSK2 kinase assay was performed to determine whether RSK2 phosphorylates ASK1. The purified recombinant GST-fused ASK1 wild type (WT) and the K709M kinase-dead mutant were incubated with recombinant active RSK2 in a solution containing 20 mM morpholinepropanesulfonic acid (MOPS), 5 mM EGTA, 1 mM dithiothreitol (DTT), 25 mM β-glycerol phosphate, 1 mM Na3VO4, and 15 mM MgCl2 along with 10 mM magnesium acetate (MgAc) and 0.1 mM ATP for 30 min at 30°C. Phosphorylation of Ser83, Ser967, or Thr845 of ASK1 was detected by the corresponding specific phosphoantibodies. To determine the kinase activity of ASK1, the kinase assay was carried out by using MKK6 or MBP as the substrate. 293T cells were transfected with GST-ASK1 variants in the presence or absence of the constitutively active RSK2 Y707A mutant for 24 h. GST-ASK1 variants in cell lysates were pulled down with a glutathione-Sepharose 4B column. The beads were washed, and kinase reactions were then initiated by adding its substrates and kinase buffer containing 40 mM MOPS (pH 7.2), 10 mM MgCl2, and 200 μM ATP for 30 min at 30°C. The reaction was stopped by adding protein-loading 6× SDS buffer. The samples were subjected to SDS-PAGE, and the status of phosphorylation of MKK6 or MBP by ASK1 was analyzed by Western blotting.
ATP-binding assays.
GST-ASK1 variants were pulled down from 293T cells coexpressed without or with the constitutively active RSK2 Y707A mutant. The beads with bound GST-ASK1 variants were washed with PBS, followed by incubation with 4 μCi [α-32P]ATP for 5 min at 30°C in ASK1 kinase buffer. The beads were then washed twice with PBS. The bead-bound ASK1 protein was eluted with 30 μl of elution buffer (50 mM Tris-HCl and 10 mM reduced glutathione [pH 8.0]) for 30 min, and radioactivity was then detected by liquid scintillation counting.
Anoikis assay.
Cells (5 × 105 per well) were cultured on 1%-agar-treated 6-well tissue culture plates for 48 to 72 h at 37°C in a 5% CO2 atmosphere. After incubation, suspended cells were harvested in complete medium and centrifuged at 1,200 rpm for 5 min. Pellets were washed with PBS, and staining with propidium iodide (PI) solution and fluorescein isothiocyanate (FITC)-conjugated annexin V was carried out according to the manufacturer's protocol (BD Pharmingen). Stained cells were analyzed by fluorescence-activated cell sorter (FACS) analysis for the apoptotic population.
Microarray data collection and analysis.
RNA was isolated by using TRIzol and a Promega SV RNA isolation kit. A quality control analysis was performed on the RNA prior to gene profiling. RNA samples were processed and hybridized onto Affymetrix human genome U133Plus2.0 chips. Raw expression values were analyzed by using DNA-Chip Analyzer software (26). Raw intensity values were normalized based on the rank-invariant probe sets, and expression values were then calculated by model-based expression (26). To confirm reproducibility, we performed hierarchical clustering from triplicates, which mostly clustered together. A set of highly variable genes was selected, with a coefficient of variation of between 0.2 and 10 and a presence call in at least 20% of all samples. The resulting 6,060 probe sets were used for hierarchical clustering.
Quantitative RNA analysis.
Total RNA was extracted from 1 ×106 cells by using RNeasy (Qiagen). Reverse transcription of total cellular RNA was done by using a first-strand cDNA synthesis kit (Amersham). Real-time detection of PTK6, ING3, and GAPDH mRNA levels was conducted with SYBR green (Bio-Rad Laboratories). mRNA levels of PTK6, ING3, and GAPDH were determined by using the following oligonucleotides as primers: PTK6 sense primer 5′-TGTGGAGTGTCTGCGTCCAATACA-3′ and antisense primer 5′-AGGCCAAGCTCTCAAGACACAAGA-3′, ING3 sense primer 5′-CAGCCTCTTCTAACAATGCCTA-3′ and antisense primer 5′-CTTCATCAAACAAAAGGACCAC-3′, and GAPDH sense primer 5′-GACATCAAGAAGGTGGTGAA-3′ and antisense primer 5′-TGTCATACCAGGAAATGAGC-3′.
RESULTS
RSK2 promotes anoikis resistance in metastatic human cancer cells.
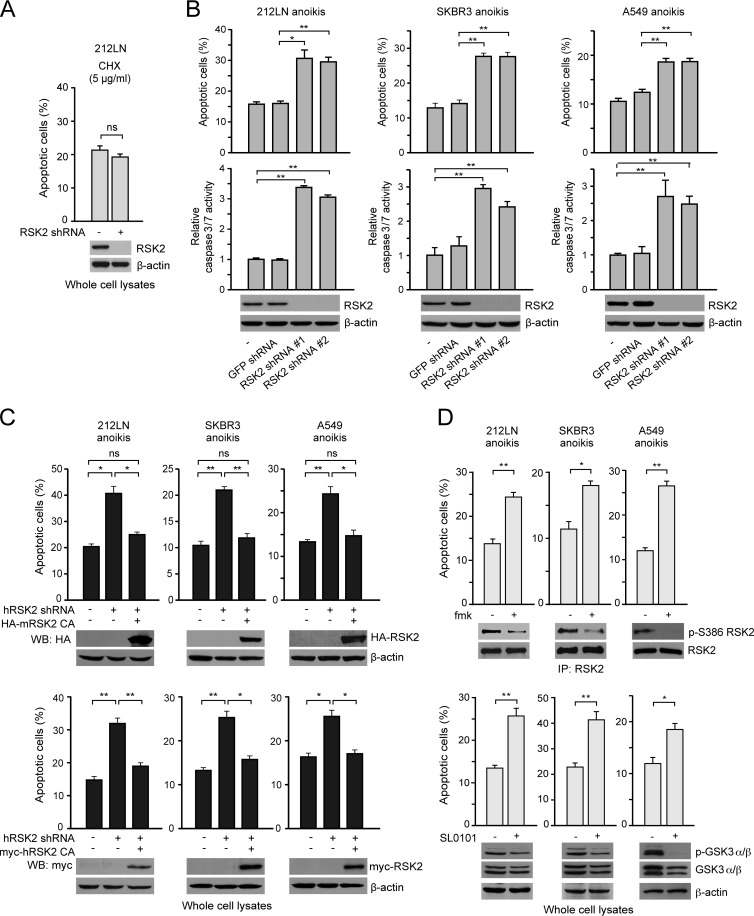
To determine whether RSK2 is important in antianoikis signaling in human cancer cells, we generated cell lines with stable RSK2 knockdown using several human metastatic cancer cells, including head and neck cancer 212LN, breast cancer SKBR3, and lung cancer A549 cells. We found that stable knockdown of RSK2 using two different shRNAs sensitizes 212LN, SKBR3, and A549 cells to detachment-induced anoikis compared to control cells harboring either an empty vector or an eGFP shRNA, as assessed by annexin V staining and caspase 3/7 activity assays (Fig. 1B), whereas we found that apoptosis induced by the control anticancer agent cycloheximide (CHX), a protein synthesis inhibitor, was not significantly affected (Fig. 1A). Moreover, knockdown of RSK2 sensitized 212LN, SKBR3, and A549 cells to anoikis induction, whereas such a phenotype was rescued by expression of murine RSK2 in a constitutively active form (RSK2 CA) (Y707A) or of an shRNA-resistant form of human RSK2 CA that is resistant to the shRNA targeting human RSK2 (Fig. 1C). In contrast, RNA interference (RNAi)-mediated RSK2 knockdown did not significantly affect detachment-induced anoikis in the nontumorigenic human epithelial cell lines HaCaT and BEAS-2B (data available upon request). We then examined whether the RSK-specific inhibitor fmk (21), which inhibits RSK2 kinase activity, could also sensitize 212LN, SKBR3, and A549 cells to anoikis. We observed that treatment with fmk effectively decreased RSK2 kinase activity, as assessed by phosphorylation at S386, an index of RSK2 activation (Fig. 1D, top). In consonance with RSK2 knockdown, fmk treatment significantly sensitized cells to anoikis induction (Fig. 1D, top). Similar results were obtained by using another RSK inhibitor, SL0101 (Fig. 1D, bottom) (27). These data together suggest that RSK2 mediates antianoikis signals in diverse metastatic cancer cells.
Fig 1.
Loss of RSK2 sensitizes diverse cancer cells to anoikis induction. (A) Stable knockdown of RSK2 by shRNA does not affect apoptosis induced by the control agent cycloheximide (CHX) compared to control cells harboring an empty vector. (B) Stable knockdown of RSK2 by two different shRNA clones sensitizes metastatic HNSCC 212LN (left), breast cancer SKBR3 (middle), and lung cancer A549 (right) cells to detachment-induced anoikis compared to control cells harboring an empty vector or a vector harboring shRNA targeting eGFP. Cells were cultured on a 1%-agar-treated dish to achieve detachment, and control cells were treated with the anticancer agent cycloheximide, an inhibitor of protein biosynthesis, to induce apoptosis. Apoptosis was assessed by FACS analysis in cells stained with FITC-conjugated annexin V and propidium iodide (top) or by caspase 3/7 activity using a Caspase-Glo 3/7 assay (bottom). (C) Overexpression of murine RSK2 (top) or shRNA-resistant human RSK2 (bottom) rescued anoikis induced by RSK2 knockdown and conferred resistance to cells from undergoing anoikis. Stable RSK2 knockdown cells were transiently transfected with murine RSK2 Y707A cDNA or shRNA-resistant human RSK2 Y707A cDNA prior to anoikis induction. (D) Treatment with small-molecule RSK inhibitors, fmk (10 μM) (top), or SL0101 (100 μM) (bottom) effectively decreases RSK2 kinase activity and sensitizes 212LN, SKBR3, and A549 cells to induction of anoikis. For fmk treatment, RSK2 activity was assessed by RSK2 immunoprecipitation (IP) and Western blotting (WB), using a specific phospho-RSK antibody that recognizes phospho-S380 (S386 for RSK2 numbering). Phosphorylation of the RSK2 substrate GSK3α/β was assessed for SL0101. P values were determined by Student's t test. All the error bars shown in the figures represent mean values ± standard deviations from three independent experiments (∗, 0.01 < P < 0.05; ∗∗, P < 0.01; ns, not significant).
RSK2 protects cancer cells from anoikis induction by inhibiting proapoptotic ASK1.
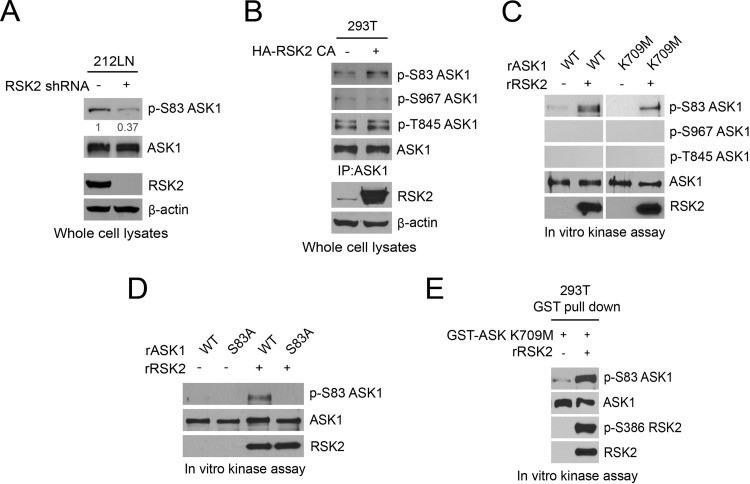
To decipher the molecular mechanism underlying RSK2-mediated antianoikis signaling, we performed a phosphor-proteomics study using the MAPK pathway phospho-antibody microarray (Full Moon BioSystems, Inc.) to profile the phosphorylation status of proteins in cells with or without stable RSK2 knockdown. We identified several antiapoptotic and proapoptotic protein factors whose phosphorylation levels decreased and increased, respectively, more than 15% in metastatic 212LN and 886LN cell lines with stable knockdown of RSK2. In particular, we found that RSK2 knockdown significantly decreased ASK1 S83 phosphorylation as well as CREB S133 phosphorylation in metastatic HNSCC cells, compared to the corresponding control cells harboring an empty vector (Fig. 2A). We decided to focus on the role of ASK1 and CREB in RSK2-mediated antianoikis signaling for the rest of the study due to their critical role in cell survival and apoptosis regulation.
Fig 2.
RSK2 inhibits ASK1 to protect metastatic cancer cells from detachment-induced anoikis. (A) Phosphoantibody microarray analysis identified novel phosphorylation targets of RSK2, whose phosphorylation states decreased in HNSCC cells when RSK2 was stably knocked down by shRNA. The signal intensities of phosphorylated proteins and the total protein levels were determined. The ratio of each protein was determined as the ratio between the percentages of phosphorylated proteins in total proteins in HNSCC-pLKO.1-RSK2 shRNA and HNSCC-pLKO.1 cells. Antiapoptotic or proapoptotic protein factors with phosphorylation status decreased or increased more than 15% in metastatic HNSCCs with RSK2 knockdown are shown. S83 phosphorylation, which inhibits ASK1, was decreased 31% in 886LN cells with stable knockdown of RSK2 compared to control cells. (B and C) Knockdown or overexpression of proapoptotic ASK1 results in reduced (B) or increased (C) sensitivity to anoikis induction in 212LN cells. (D) RNAi-mediated knockdown of ASK1 attenuates the increased sensitivity to anoikis induction in 212LN (left) and SKBR3 (right) cells with stable knockdown of RSK2. (E) Inhibition of RSK2 by a specific RSK inhibitor, fmk (6 μM), results in increased sensitivity to anoikis induction in 212LN cells, while knockdown of ASK1 rescues the phenotype of cells treated with fmk. (F) Overexpression of a constitutively active form of RSK2, the Y707A mutant, results in decreased ASK1 kinase activity. GST-tagged ASK1 was coexpressed with or without the RSK2 Y707A mutant in 293T cells. GST-ASK1 was pulled down by GST beads and used for an in vitro ASK1 kinase assay using recombinant, inactive human MKK6 protein as an exogenous substrate. ASK1 activity was assessed by the phosphorylation levels of MKK6 detected by a specific phospho-Ser/Thr antibody. (G) Western blot results show that expression of a constitutively active form of RSK2, the Y707A mutant, results in decreased activation levels of p38 (left) and JNK (right) in 212LN cells. Activation of p38 and JNK was assessed by specific antibodies recognizing phospho-p38 and phospho-SAPK/JNK, respectively. (H) Stable knockdown of RSK2 results in increased phosphorylation and activation levels of p38 and JNK in 212LN cells.
We found that stable knockdown of ASK1 using two different shRNA clones in 212LN cells significantly decreased detachment-induced anoikis (Fig. 2B), while stable overexpression of myc-ASK1 sensitized 212LN cells to anoikis induction (Fig. 2C). In consonance with our observations described above (Fig. 1A and B), knockdown of RSK2 significantly sensitized cells to anoikis in 212LN and SKBR3 cells. However, knockdown of ASK1 in cells with stable knockdown of RSK2 partially rescued phenotypes due to a lack of RSK2, resulting in increased resistance to anoikis induction compared to RSK2 knockdown cells (Fig. 2D). Moreover, inhibition of RSK2 by fmk sensitized 212LN cells to anoikis induction (Fig. 2E, left), whereas ASK1 knockdown conferred resistance to fmk-sensitized anoikis induction (Fig. 2E, right). In addition, overexpression of a constitutively active form of RSK2 (Y707A) significantly decreased ASK1 kinase activity in an in vitro ASK1 kinase assay using MAPK kinase kinase 6 (MKK6) as a substrate (Fig. 2F). Furthermore, overexpression of the constitutively active RSK2 Y707A mutant decreased activation of both the p38 and JNK pathways, which are downstream signaling cascades of ASK1 (Fig. 2G, left and right, respectively). Knockdown of RSK2 resulted in increased phosphorylation of p38 and JNK in metastatic 212LN cells (Fig. 2H). These results together indicate that RSK2 inhibits ASK1 to mediate RSK2-dependent antianoikis signals in cancer cells.
RSK2 inhibits ASK1 through phosphorylation.
We next confirmed that inhibition of RSK2 by shRNA in metastatic 212LN cells decreased S83 phosphorylation levels of ASK1 by 63% (Fig. 3A). In contrast, knockdown of AKT1 resulted in only a 12% decrease in S83 phosphorylation levels of ASK1, while PIM1 knockdown did not attenuate the status of S83 phosphorylation in ASK1 (data available upon request). Moreover, treatment with the RSK inhibitor fmk decreased S83 phosphorylation levels of ASK1 by 50%, while treatment with the AKT inhibitor wortmannin decreased S83 phosphorylation levels by 25%. In contrast, treatment with the PIM1 inhibitor quercetagetin did not affect S83 phosphorylation levels of ASK1 (data available upon request). These data suggest that RSK2 is one of the predominant upstream kinases that are responsible for ASK1 S83 phosphorylation in metastatic 212LN cells.
Fig 3.
RSK2 inhibits ASK1 by phosphorylating S83. (A) Knockdown of RSK2 results in decreased phospho-S83 levels of ASK1 in 212LN cells. Phospho-S83 levels were assessed by a specific phospho-ASK1 antibody (p-S83). (B) Enforced RSK2 expression results in increased phosphorylation levels of S83 but not of S967 or T845 of ASK1. 293T cells were transiently transfected with the HA-tagged constitutively active RSK2 Y707A mutant. S83, S967, and T845 phosphorylation levels of endogenous ASK1 were determined by using specific phospho-ASK1 antibodies recognizing individual phosphorylation sites. (C) RSK2 directly phosphorylates ASK1 at S83 but not at S967 or T845. An in vitro RSK2 kinase assay was performed by using recombinant active RSK2 (rRSK2) incubated with either WT recombinant ASK1 (rASK1) or a kinase-dead mutant form, K709M, purified from E. coli. (D) RSK2 directly phosphorylates WT ASK1 at S83 but is unable to phosphorylate the S83A mutant. Purified recombinant WT ASK1 and S83A mutant proteins were used in an in vitro RSK2 kinase assay. (E) An in vitro RSK2 kinase assay was performed by using the GST-tagged ASK1 K709M mutant enriched from 293T cells by GST pulldown.
In addition, overexpression of the constitutively active form of RSK2, HA-RSK2 Y707A, in 293T cells increased S83 phosphorylation of endogenous ASK1 but did not affect the phosphorylation levels of ASK1 at S967 or T845 (Fig. 3B). Moreover, in an in vitro RSK2 kinase assay using recombinant, active RSK2 (rRSK2) and purified, wild-type (WT), recombinant ASK1 (rASK1) or a kinase-dead mutant, K709M, as the substrate, rRSK2 directly phosphorylated the WT and kinase-dead forms but not the S83A mutant of rASK1 at S83 (Fig. 3C and D). In consonance with this observation, the ASK1 kinase-dead mutants enriched from the cells were also directly phosphorylated at S83 by rRSK2 (Fig. 3E). Moreover, RSK2 interacted and colocalized with ASK1 in the cytoplasm in a phosphorylation-independent manner (data available upon request). These data suggest that ASK1 is a new substrate of RSK2.
RSK2 phosphorylates two new sites of ASK1, T1109 and T1326, which mediate an inhibitory effect on ASK1.
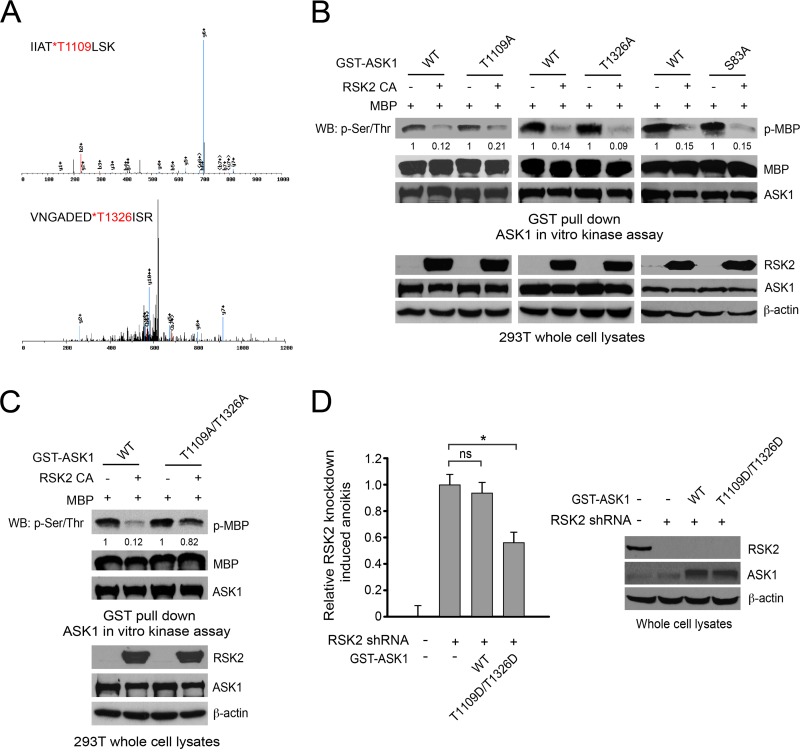
We next wondered whether RSK2 phosphorylates ASK1 at multiple sites besides S83. Thus, we performed an in vitro kinase assay using purified GST-ASK1 proteins from cells incubated with recombinant, active RSK2. The samples were applied onto SDS-PAGE gels, and the bands containing phosphorylated ASK1 proteins were excised and used for liquid chromatography-tandem mass spectrometry (LC-MS/MS). The mass spectrometry-based analysis identified two additional, new residues of ASK1 that are phosphorylated by RSK2, T1109 and T1326 (Fig. 4A). To investigate the role of T1109/T1326 phosphorylation in ASK1 activation, we performed mutational analysis to substitute T1109, T1326, or both in ASK1. We then performed an immunoprecipitation-coupled in vitro ASK1 kinase assay using distinct ASK1 variants coexpressed with the constitutively active RSK2 Y707A mutant in 293T cells, in the presence of myelin basic protein (MBP) as an exogenous substrate. Interestingly, although RSK2 similarly inhibited the ASK1 T1109A and T1326A single mutants and WT ASK1 (Fig. 4B), simultaneous substitution of both T1109 and T1326 significantly attenuated the inhibitory effect of RSK2 on ASK1 kinase activity (Fig. 4C). These results suggest that phosphorylation at both T1109 and T1326 by RSK2 is required to mediate an inhibitory effect on ASK1, while phosphorylation at either of these two sites is insufficient. Moreover, stable knockdown of RSK2 sensitizes cells to anoikis induction, whereas expression of the ASK1 phosphomimetic T1109D/T1326D mutant, but not the WT, partially rescues the increased sensitivity to anoikis induction in these cells (Fig. 4D).
Fig 4.
T1109 and T1326 are identified as new RSK2 phosphorylation sites that, when phosphorylated, inhibit ASK1. (A) Mass spectrometry spectra of phosphothreonine peptide fragments of ASK1 containing T1109 and T1326. GST-ASK1 K709M protein purified from 293T cells was incubated with recombinant active RSK2. Bands from SDS-PAGE gels were excised and applied for LC-MS/MS. (B and C) Substitution of either T1109, T1326, or S83 did not affect RSK2-dependent attenuation of ASK1 kinase activity (B), whereas the T1109A/T1326A double mutant showed resistance to RSK2-dependent inhibition (C). Distinct GST-tagged ASK1 variants were coexpressed in the presence or absence of a constitutively active form of RSK2, the Y707A mutant, in 293T cells. At 24 h posttransfection, cells were harvested, and GST-ASK1 proteins were pulled down by GST beads. An in vitro ASK1 kinase assay was performed by using myelin basic protein as a substrate. (D) Expression of the ASK1 T1109D/T1326D mutant in a dominant negative form results in less proapoptotic activity that with WT ASK1 in cells with RSK2 knockdown. RSK2 knockdown cells were transiently transfected with ASK1 variants. An anoikis assay was performed at 48 h posttransfection.
Phosphorylation at T1109/T1326 and S83 inhibits ASK1 by attenuating ATP and substrate MKK6 binding, respectively.
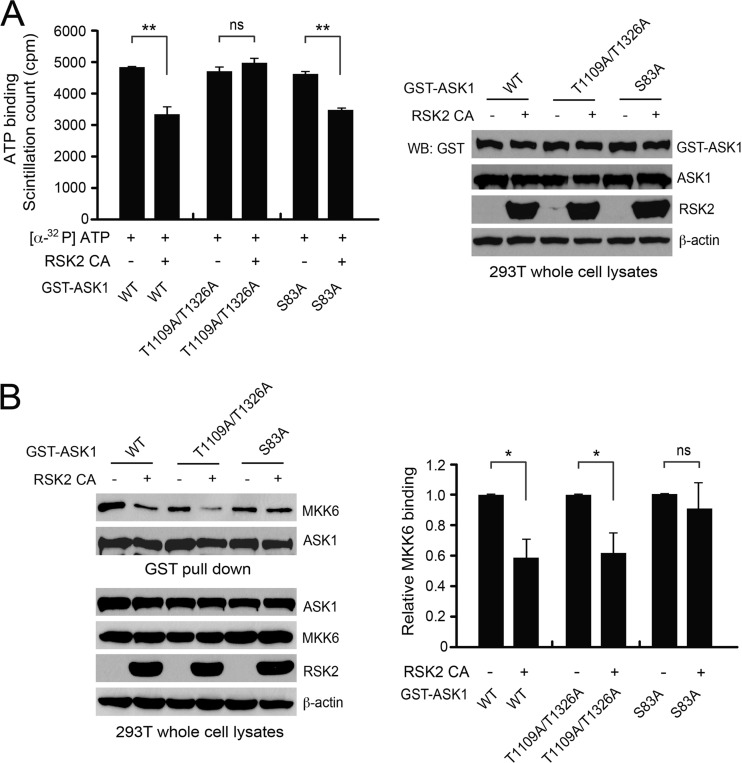
We next explored the molecular mechanisms underlying S83 and T1109/T1326 phosphorylation-mediated inhibition of ASK1. We found that coexpression of RSK2 led to decreased [α-32P]ATP binding to WT ASK1 and the S83A mutant, whereas substitution of T1109/T1326 abolished the RSK2-dependent attenuation of ATP binding to ASK1 (Fig. 5A). In contrast, coexpression of RSK2 significantly attenuated ASK1 substrate MKK6 binding to WT ASK1 and the T1109A/T1326A mutant, while substitution of S83 reversed the attenuated MKK6 binding to ASK1 in the presence of overexpressed RSK2 (Fig. 5B). These results suggest novel molecular mechanisms by which RSK2-mediated S83 and T1109/T1326 phosphorylation inhibits ASK1.
Fig 5.
Phosphorylation at T1109/T1326 and S83 inhibits ASK1 by attenuating ATP and substrate binding, respectively. (A, left) Substitution of T1109/T1326, but not S83, reversed the decreased ATP binding to ASK1 in the presence of RSK2 CA (constitutively active Y707A mutant form). GST-ASK1 variants expressed in the presence or absence of RSK2 CA were enriched from 293T cell lysates by GST pulldown, followed by incubation with [α-32P]ATP. Unbound [α-32P]ATP was washed away, and bound [α-32P]ATP on ASK1 was measured with a scintillation counter. (Right) Western blot results show expression levels of GST-ASK1 and RSK2 in 293T cells. (B) Mutation at S83, but not at T1109/T1326, results in an increase of MKK6 binding to ASK1 in the presence of RSK2 CA. GST-ASK1 variants were pulled down from cells coexpressed with or without RSK2 CA. Bound endogenous MKK6 was detected by immunoblotting. (Left) Representative immunoblotting result. (Right) Relative intensity of the MKK6 bands from three different experiments, which are normalized to values for the control samples without RSK2 expression.
The RSK2→CREB pathway protects cancer cells from anoikis in part by upregulating gene expression of the antiapoptotic factor PTK6 and downregulating gene expression of the proapoptotic factor ING3.
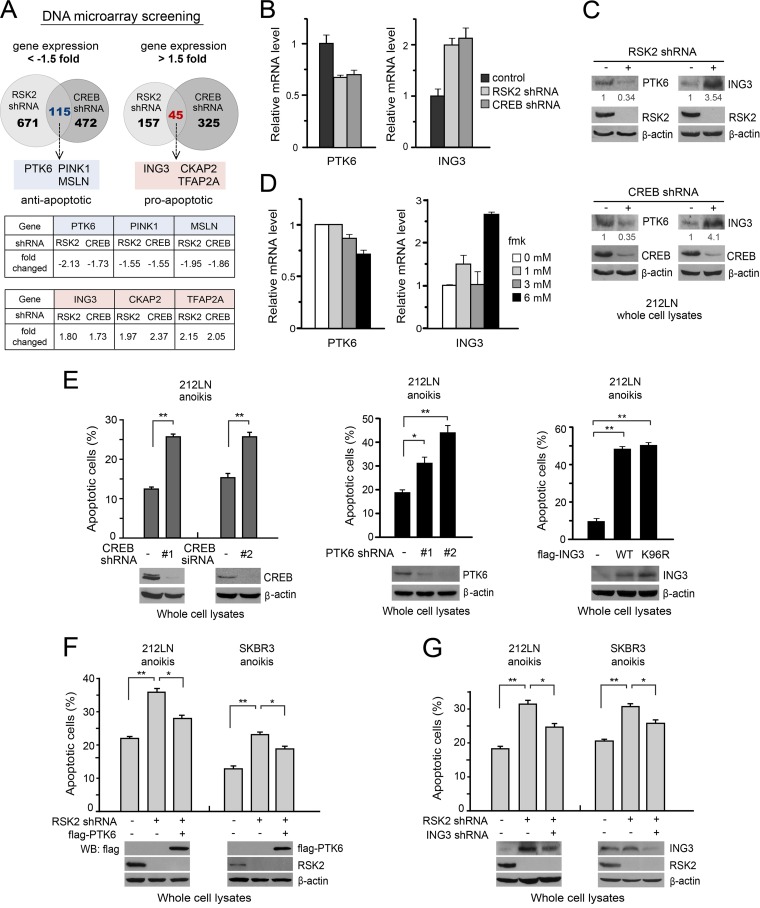
RSK2 activates the transcription factor CREB by phosphorylating S133 (17). We previously showed that RSK2 promotes HNSCC cell invasion at least in part through phosphorylation and activation of the downstream substrate CREB (3). To explore the molecular mechanism underlying RSK2-CREB-mediated antianoikis signaling, we performed DNA microarray analysis using metastatic 212LN cells with stable knockdown of RSK2 or CREB. We identified 115 and 45 genes that are commonly downregulated and upregulated, respectively, more than 1.5-fold in 212LN cells with stable knockdown of individual RSK2 knockdown and CREB knockdown (Fig. 6A, top). Among these genes, we found that several antiapoptotic protein factors, including protein tyrosine kinase 6 (PTK6), PTEN-induced putative kinase 1 (PINK1), and mesothelin (MSLN), are transcriptionally downregulated. Conversely, we found that several proapoptotic effectors, including inhibitor-of-growth protein 3 (ING3), cytoskeleton-associated protein 2 (CKAP2), and transcription factor AP-2 alpha (TFAP2A), are transcriptionally upregulated. All of them are novel transcription targets of RSK2→CREB signaling (Fig. 6A, bottom).
Fig 6.
RSK2 protects cells from anoikis induction, in part by signaling through CREB to upregulate the antiapoptotic factor PTK6 and downregulate the proapoptotic factor ING3. (A) DNA microarray analysis identifies novel transcription targets of the RSK2→CREB signaling pathway. (Top) Diagram showing overlapping transcription targets that are either downregulated (115 genes) (left) or upregulated (45 genes) (right) from 212LN cells with individual expression of RSK2 shRNA and CREB shRNA, compared to control cells harboring an empty lentiviral vector. (Middle) Representative, novel RSK2→CREB transcription targets that are downregulated in RSK2 and CREB knockdown cells, including the antiapoptotic factors PTK6, PINK1, and MSLN. (Bottom) Representative, novel RSK2→CREB transcription targets that are upregulated in RSK2 and CREB knockdown cells, including the proapoptotic factors ING3, CKAP2, and TFAP2A. mRNA level changes (fold) in RSK2 and CREB knockdown cells are shown in the bottom rows of the middle and bottom panels, compared to control cells harboring an empty vector. (B) Real-time reverse transcription-PCR results show decreased mRNA levels of the antiapoptotic factor PTK6 (left) and increased mRNA levels of the proapoptotic factor ING3 (right) in 212LN cells with stable knockdown of RSK2 or CREB, compared to control cells with an empty vector. (C) Western blot results show decreased and increased protein levels of PTK6 and ING3, respectively, in 212LN cells with stable knockdown of RSK2 (top) or CREB (bottom). (D) Real-time reverse transcription-PCR results show decreased and increased mRNA levels of PTK6 (left) and ING3 (right), respectively, in 212LN cells treated with increasing concentrations of the RSK inhibitor fmk for 24 h. (E) Knockdown of CREB (left) or PTK6 (middle) or overexpression of ING3 (right) results in increased sensitivity to anoikis induction in cancer cells. (F) Expression of Flag-tagged PTK6 significantly attenuated the increased sensitivity to anoikis induction in 212LN and SKBR3 cells with stable knockdown of RSK2. (G) Knockdown of ING3 results in a significant attenuation of the increased sensitivity to anoikis induction in 212LN and SKBR3 cells with RSK2 knockdown. Apoptotic cell death was assessed by annexin V staining.
We decided to focus on the role of PTK6 and ING3 in RSK2→CREB antianoikis signaling, due to their important function in regulation of cell survival and apoptosis. PTK6 (also known as Brk) belongs to the Src family and is frequently overexpressed in a variety of tumor types. PTK6 expression enhances survival of breast and ovarian cancer cells deprived of matrix attachment, probably by regulating IGF-1R expression and phosphorylation, while knockdown of PTK6 induces apoptosis in these cells (28–31). ING3 is a member of the ING tumor suppressor family proteins and is involved in apoptosis, cell growth, and cancer progression, including the regulation of invasion and metastasis (32, 33). ING3 expression is remarkably reduced in HNSCCs and melanomas (34–36), which may promote UV-induced apoptosis through the Fas/caspase-8 pathway in melanoma cells. The interruption of ING3 degradation in cancer cells enhances apoptosis (37). We performed real-time quantitative PCR and Western blotting and confirmed that stable knockdown of RSK2 or CREB in 212LN cells decreased gene and protein expression levels of the antiapoptotic factor PTK6 (Fig. 6B, left, and C) and increased expression levels of the proapoptotic factor ING3 (Fig. 6B, right, and C). Moreover, inhibition of RSK2 kinase activity by the RSK-specific inhibitor fmk also decreased mRNA levels of the antiapoptotic factor PTK6 and increased the mRNA level of the proapoptotic factor ING3 (Fig. 6D).
We found that knockdown of CREB or PTK6, or overexpression of WT ING3 or the K96R constitutively active mutant, sensitizes cancer cells to detachment-induced anoikis (Fig. 6E). In addition, overexpression of PTK6 in RSK2 knockdown cells significantly reduced the increased sensitivity to anoikis induction in 212LN and SKBR3 cells due to loss of RSK2 (Fig. 6F), while knockdown of ING3 in RSK2 knockdown 212LN and SKBR3 cells partially rescued the increased sensitivity to detachment-induced anoikis due to RSK2 deficiency (Fig. 6G). These data together suggest that, in addition to RSK2-ASK1 antianoikis signaling, RSK2 also provides antianoikis protection to metastatic cancer cells in a transcription-dependent way by activating CREB to regulate the gene expression of PTK6 and ING3.
RSK2 provides antianoikis signaling via ASK1 and CREB in transcription-independent and -dependent manners, respectively.
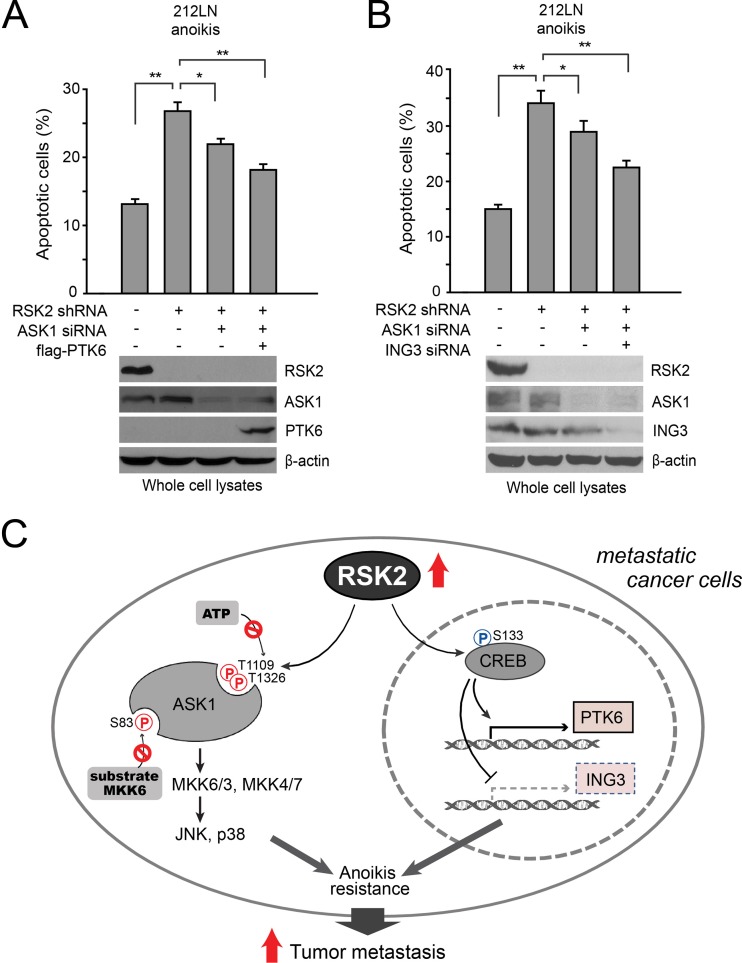
We next determined whether RSK2 coordinates ASK1 and PTK6/ING3 to provide an antianoikis advantage to metastatic cancer cells. Indeed, overexpression of PTK6 or knockdown of ING3 in addition to ASK1 knockdown enhanced attenuated anoikis induction in cells with stable knockdown of RSK2, compared to cells with only ASK1 knockdown (Fig. 7A and B, respectively). These data together suggest that RSK2 functions as a signal integrator to provide antianoikis protection to cancer cells in both transcription-independent and -dependent manners, in part by signaling through ASK1 and CREB, respectively, conferring a prosurvival and prometastatic advantage to human cancers (Fig. 7C).
Fig 7.
RSK2 mediates antianoikis signals through both ASK1 and CREB transcription targets. (A and B) Knockdown of RSK2 results in increased sensitivity to anoikis induction, and knockdown of ASK1 significantly rescues this phenotype, while simultaneous knockdown of ASK1 and overexpression of PTK6 (A) or knockdown of ING3 (B) results in a further rescue effect. Stable RSK2 knockdown cells were transiently cotransfected with ASK1 siRNA and Flag-PTK6 or ING3 siRNA for 24 h, prior to transfer onto an agar-treated plate and culture for 48 h. Apoptotic cell death was assessed by annexin V staining. (C) Proposed model of RSK2-mediated antianoikis signaling in metastatic cancer cells. RSK2 is a signal integrator in metastatic cells, which phosphorylates and regulates multiple protein factors in both acute and chronic ways to provide antianoikis signals.
DISCUSSION
Our finding that RSK2 commonly provides antianoikis signals to protect diverse metastatic cancer cells provides new insights into an understanding of the proinvasive and prometastatic role of RSK2. RSK2 functions as a signal integrator, which regulates a network of signaling effectors to mediate antianoikis signals in both transcription-independent and -dependent manners. We identified the proapoptotic factor ASK1 as a novel phosphorylation target of RSK2. Although S83 was identified as an AKT and PIM1 phosphorylation site, RSK2 appears to act as one of the predominant upstream kinases that phosphorylate ASK1 at S83 in 212LN cells. In addition to phosphorylation at the inhibitory S83 site, RSK2 also inhibits ASK1 by phosphorylating two newly identified sites, T1109 and T1326. This is supported by the observation that the phosphomimetic T1109D/T1326D mutant functions as a dominant negative form of ASK1 that, when overexpressed, results in decreased sensitivity to anoikis induction in cells with stable knockdown of RSK2.
Our studies also for the first time revealed novel molecular mechanisms underlying Ser/Thr phosphorylation-dependent inhibition of ASK1, in which phosphorylation at both T1109 and T1326 exclusively attenuates the ability of ASK1 to bind to ATP, whereas phosphorylation at S83 blocks substrate MKK6 binding to ASK1. One explanation for the impact of these phosphorylation events is that they could be compatible with specific regulated states of ASK1 potentially impacting ATP binding; alternatively, phosphorylation could impact protein-protein interaction sites and potentially regulate ASK1 in this manner. Together, these results suggest that RSK2 mediates antianoikis signals, at least in part, by inhibiting ASK1 through phosphorylation at multiple sites. This may provide a regulatory window for cancer cells to promptly respond to anoikis induction, at least in part by RSK2-dependent phosphorylation and inhibition of proapoptotic ASK1, thereby providing antianoikis protection to cancer cells. In accordance with this concept, we observed that knockdown of ASK1 resulted in a partial rescue of the increased sensitivity to anoikis induction in cells upon stable knockdown of RSK2. This finding suggests that additional RSK2 targets besides ASK1 participate in RSK2-dependent antianoikis signaling. Such targets should include other protein factors, similar to ASK1, whose phosphorylation levels are regulated by RSK2 to confer antianoikis protection in a transcription-independent manner.
In addition, RSK2 regulates antianoikis signaling by altering gene expression of protein effectors involved in regulation of cell survival and apoptosis. In particular, our DNA microarray-based studies identified a spectrum of RSK2→CREB transcription targets that are accountable for RSK2-dependent antianoikis protection. These targets include the antiapoptotic factor PTK6 and the proapoptotic factor ING3, which are newly identified transcription targets of RSK2 and CREB. Knockdown of RSK2 or CREB decreases the PTK6 gene expression level, which correlates with increased cell sensitivity to anoikis induction in metastatic cancer cells. Overexpression of PTK6 partially rescues the increased sensitivity to anoikis induction in cancer cells with knockdown of RSK2. Our findings are consistent with recent observations in which PTK6 was suggested to promote anchorage-independent proliferation in breast cancer cells transformed by IGF-1R (31). This warrants future studies to examine whether RSK2 signaling is involved in IGF-1R-dependent activation of PTK6 to provide antianoikis signaling in cancer cells. In addition to PTK6, we also identified the proapoptotic factor ING3 as another novel RSK2→CREB transcription target. Our gain-of-function and loss-of-function studies demonstrated that RSK2 also, in part, signals through CREB to downregulate ING3 to protect cancer cells from anoikis.
Our studies demonstrate that the simultaneous alteration of the RSK2 phosphorylation target ASK1 and the RSK2-CREB transcription target PTK6 or ING3 results in an enhanced effect on the rescue of increased sensitivity to anoikis in cells with RSK2 knockdown. Our studies suggest that a complicated antianoikis signaling network of RSK2 exists in cancer cells that contribute to the cellular response to anoikis. Such a network consists of a spectrum of phosphorylation and transcription targets of RSK2, which may provide acute and chronic mediation of RSK2-dependent antianoikis signals in metastatic cancer cells, respectively. Thus, this study showcases the complexity of the cellular process, demonstrating that the response to anoikis induction and regulation may involve diverse protein factors in different signaling pathways, such that the interaction, cross talk, and coordination of various RSK2 downstream effectors may exert regulatory functions to control cellular sensitivity to detachment-induced anoikis. Such a mechanism can be explored for anticancer therapies.
ACKNOWLEDGMENTS
This work was supported in part by American Cancer Society grant RSG-11-081-01 (S.K.), an NIH/NCI SPORE in Head and Neck Cancer (grant P50CA128613) career development program award (S.K.), a Robbins scholar award (S.K.). T.J.B. is funded by the NIH. J.T. acknowledges support from the NIH (grant GM071434).
S.K. is an American Cancer Society basic research scholar and a Robbins scholar. Z.G.C., S.K., F.R.K., H.F., and D.M.S. are Georgia Cancer Coalition scholars.
Footnotes
Published ahead of print 22 April 2013
REFERENCES
- 1. Gupta GP, Massague J. 2006. Cancer metastasis: building a framework. Cell 127:679–695 [DOI] [PubMed] [Google Scholar]
- 2. Fidler IJ. 2003. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3:453–458 [DOI] [PubMed] [Google Scholar]
- 3. Kang S, Elf S, Lythgoe K, Hitosugi T, Taunton J, Zhou W, Xiong L, Wang D, Muller S, Fan S, Sun SY, Marcus AI, Gu TL, Polakiewicz RD, Chen GZ, Khuri FR, Shin DM, Chen J. 2010. p90 ribosomal S6 kinase 2 promotes invasion and metastasis of human head and neck squamous cell carcinoma cells. J. Clin. Invest. 120:1165–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blenis J. 1993. Signal transduction via the MAP kinases: proceed at your own RSK. Proc. Natl. Acad. Sci. U. S. A. 90:5889–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frodin M, Gammeltoft S. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 151:65–77 [DOI] [PubMed] [Google Scholar]
- 6. Fisher TL, Blenis J. 1996. Evidence for two catalytically active kinase domains in pp90rsk. Mol. Cell. Biol. 16:1212–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buck M, Poli V, Hunter T, Chojkier M. 2001. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell 8:807–816 [DOI] [PubMed] [Google Scholar]
- 8. He Z, Ma WY, Liu G, Zhang Y, Bode AM, Dong Z. 2003. Arsenite-induced phosphorylation of histone H3 at serine 10 is mediated by Akt1, extracellular signal-regulated kinase 2, and p90 ribosomal S6 kinase 2 but not mitogen- and stress-activated protein kinase 1. J. Biol. Chem. 278:10588–10593 [DOI] [PubMed] [Google Scholar]
- 9. Palmer A, Gavin AC, Nebreda AR. 1998. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 17:5037–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimamura A, Ballif BA, Richards SA, Blenis J. 2000. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr. Biol. 10:127–135 [DOI] [PubMed] [Google Scholar]
- 11. Dehan E, Bassermann F, Guardavaccaro D, Vasiliver-Shamis G, Cohen M, Lowes KN, Dustin M, Huang DC, Taunton J, Pagano M. 2009. betaTrCP- and Rsk1/2-mediated degradation of BimEL inhibits apoptosis. Mol. Cell 33:109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu D, Zhau HE, Huang WC, Iqbal S, Habib FK, Sartor O, Cvitanovic L, Marshall FF, Xu Z, Chung LW. 2007. cAMP-responsive element-binding protein regulates vascular endothelial growth factor expression: implication in human prostate cancer bone metastasis. Oncogene 26:5070–5077 [DOI] [PubMed] [Google Scholar]
- 13. Jean D, Bar-Eli M. 2000. Regulation of tumor growth and metastasis of human melanoma by the CREB transcription factor family. Mol. Cell. Biochem. 212:19–28 [PubMed] [Google Scholar]
- 14. Jean D, Tellez C, Huang S, Davis DW, Bruns CJ, McConkey DJ, Hinrichs SH, Bar-Eli M. 2000. Inhibition of tumor growth and metastasis of human melanoma by intracellular anti-ATF-1 single chain Fv fragment. Oncogene 19:2721–2730 [DOI] [PubMed] [Google Scholar]
- 15. Jean D, Harbison M, McConkey DJ, Ronai Z, Bar-Eli M. 1998. CREB and its associated proteins act as survival factors for human melanoma cells. J. Biol. Chem. 273:24884–24890 [DOI] [PubMed] [Google Scholar]
- 16. Xie S, Price JE, Luca M, Jean D, Ronai Z, Bar-Eli M. 1997. Dominant-negative CREB inhibits tumor growth and metastasis of human melanoma cells. Oncogene 15:2069–2075 [DOI] [PubMed] [Google Scholar]
- 17. Xing J, Ginty DD, Greenberg ME. 1996. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273:959–963 [DOI] [PubMed] [Google Scholar]
- 18. Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. 2001. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. 2005. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene 24:3954–3963 [DOI] [PubMed] [Google Scholar]
- 20. Gu JJ, Wang Z, Reeves R, Magnuson NS. 2009. PIM1 phosphorylates and negatively regulates ASK1-mediated apoptosis. Oncogene 28:4261–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen MS, Zhang C, Shokat KM, Taunton J. 2005. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science 308:1318–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang S, Dong S, Gu TL, Guo A, Cohen MS, Lonial S, Khoury HJ, Fabbro D, Gilliland DG, Bergsagel PL, Taunton J, Polakiewicz RD, Chen J. 2007. FGFR3 activates RSK2 to mediate hematopoietic transformation through tyrosine phosphorylation of RSK2 and activation of the MEK/ERK pathway. Cancer Cell 12:201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang S, Dong S, Guo A, Ruan H, Lonial S, Khoury HJ, Gu TL, Chen J. 2008. Epidermal growth factor stimulates RSK2 activation through activation of the MEK/ERK pathway and Src-dependent tyrosine phosphorylation of RSK2 at Tyr-529. J. Biol. Chem. 283:4652–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang S, Elf S, Dong S, Hitosugi T, Lythgoe K, Guo A, Ruan H, Lonial S, Khoury HJ, Williams IR, Lee BH, Roesel JL, Karsenty G, Hanauer A, Taunton J, Boggon TJ, Gu TL, Chen J. 2009. Fibroblast growth factor receptor 3 associates with and tyrosine phosphorylates p90 RSK2, leading to RSK2 activation that mediates hematopoietic transformation. Mol. Cell. Biol. 29:2105–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, Ge Q, Gu TL, Polakiewicz RD, Roesel JL, Chen GZ, Boggon TJ, Lonial S, Fu H, Khuri FR, Kang S, Chen J. 2011. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol. Cell 44:864–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li C, Wong WH. 2001. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. U. S. A. 98:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith JA, Poteet-Smith CE, Xu Y, Errington TM, Hecht SM, Lannigan DA. 2005. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 65:1027–1034 [PubMed] [Google Scholar]
- 28. Aubele M, Vidojkovic S, Braselmann H, Ritterswurden D, Auer G, Atkinson MJ, Tapio S, Hofler H, Rauser S, Bartlett JM. 2009. Overexpression of PTK6 (breast tumor kinase) protein—a prognostic factor for long-term breast cancer survival—is not due to gene amplification. Virchows Arch. 455:117–123 [DOI] [PubMed] [Google Scholar]
- 29. Xiang B, Chatti K, Qiu H, Lakshmi B, Krasnitz A, Hicks J, Yu M, Miller WT, Muthuswamy SK. 2008. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc. Natl. Acad. Sci. U. S. A. 105:12463–12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmandt RE, Bennett M, Clifford S, Thornton A, Jiang F, Broaddus RR, Sun CC, Lu KH, Sood AK, Gershenson DM. 2006. The BRK tyrosine kinase is expressed in high-grade serous carcinoma of the ovary. Cancer Biol. Ther. 5:1136–1141 [DOI] [PubMed] [Google Scholar]
- 31. Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, Zou L, Yao J, Lu Y, Epstein CB, Natesan S, Richardson AL, Polyak K, Mills GB, Hahn WC, Brugge JS. 2010. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS One 5:e11729 doi:10.1371/journal.pone.0011729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ythier D, Larrieu D, Brambilla C, Brambilla E, Pedeux R. 2008. The new tumor suppressor genes ING: genomic structure and status in cancer. Int. J. Cancer 123:1483–1490 [DOI] [PubMed] [Google Scholar]
- 33. Campos EI, Chin MY, Kuo WH, Li G. 2004. Biological functions of the ING family tumor suppressors. Cell. Mol. Life Sci. 61:2597–2613 [DOI] [PubMed] [Google Scholar]
- 34. Gunduz M, Ouchida M, Fukushima K, Ito S, Jitsumori Y, Nakashima T, Nagai N, Nishizaki K, Shimizu K. 2002. Allelic loss and reduced expression of the ING3, a candidate tumor suppressor gene at 7q31, in human head and neck cancers. Oncogene 21:4462–4470 [DOI] [PubMed] [Google Scholar]
- 35. Richards HW, Medrano EE. 2009. Epigenetic marks in melanoma. Pigment Cell Melanoma Res. 22:14–29 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Dai DL, Martinka M, Li G. 2007. Prognostic significance of nuclear ING3 expression in human cutaneous melanoma. Clin. Cancer Res. 13:4111–4116 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Li G. 2006. ING3 promotes UV-induced apoptosis via Fas/caspase-8 pathway in melanoma cells. J. Biol. Chem. 281:11887–11893 [DOI] [PubMed] [Google Scholar]