Abstract
Dpb11/Cut5/TopBP1 is evolutionarily conserved and is essential for the initiation of DNA replication in eukaryotes. The Dpb11 of the budding yeast Saccharomyces cerevisiae has four BRCT domains (BRCT1 to -4). The N-terminal pair (BRCT1 and -2) and the C-terminal pair (BRCT3 and -4) bind to cyclin-dependent kinase (CDK)-phosphorylated Sld3 and Sld2, respectively. These phosphorylation-dependent interactions trigger the initiation of DNA replication. BRCT1 and -2 and BRCT3 and -4 of Dpb11 are separated by a short stretch of ∼100 amino acids. It is unknown whether this inter-BRCT region functions in DNA replication. Here, we showed that the inter-BRCT region is a GINS interaction domain that is essential for cell growth and that mutations in this domain cause replication defects in budding yeast. We found the corresponding region in the vertebrate ortholog, TopBP1, and showed that the corresponding region also interacts with GINS and is required for efficient DNA replication. We propose that the inter-BRCT region of Dpb11 is a functionally conserved GINS interaction domain that is important for the initiation of DNA replication in eukaryotes.
INTRODUCTION
Chromosomal DNA replication in eukaryotes occurs as a two-step reaction whose steps are separated temporally in the cell cycle (see reviews in references 1 and 2). In the first reaction, called licensing, the core component of the replicative helicase, Mcm2-7, is loaded onto replication origins to assemble the prereplicative complexes (pre-RCs) in the G1 phase of the cell cycle. At this point, the Mcm2-7 helicase is inactive in the pre-RC. In the following S phase, the replicative helicase is activated by the functions of two protein kinases—cyclin-dependent kinase (CDK) and Dbf4-dependent kinase (DDK)—and the replication fork is established (initiation reaction). In the budding yeast Saccharomyces cerevisiae, Sld2 and Sld3 are essential targets of CDK. CDK phosphorylation of Sld2 and Sld3 promotes a phosphodependent interaction with the tandem BRCT repeats of another replication factor called Dpb11 (3–6). These interactions promote the recruitment of GINS onto origins via the preloading complex (pre-LC), which consists of Dpb11, Sld2, DNA polymerase ε (Pol ε), and GINS. The pre-LC is an important intermediate in the subsequent formation of the active replicative helicase, which is called the CMG (Cdc45–Mcm2-7–GINS) complex, and the establishment of the replication fork (7–9). Therefore, Dpb11 is a key mediator of the formation of the new complex on replication origins and plays a crucial role in the initiation of DNA replication.
Dpb11 is evolutionarily conserved (10, 11). The functions and overall structures of Dpb11 of budding yeast and of its ortholog in fission yeast, Cut5, are similar (12, 13) (see Fig. S1 in the supplemental material). The vertebrate ortholog, TopBP1, is also a BRCT-containing protein (14), the N-terminal region of which contains BRCT1 and -2, which interacts with phosphorylated treslin, an ortholog of vertebrate Sld3 (15, 16). Although TopBP1 is also called Cut5 or Mus101, we use the designation TopBP1 here for simplicity. Vertebrate TopBP1 contains nine BRCT domains (BRCT0 to -8) (10, 17) (see Fig. S1 in the supplemental material). Although BRCT4 and -5 of TopBP1 are similar to BRCT3 and -4 of yeast Dpb11/Cut5 (18, 19), an extra BRCT (BRCT3) exists in the middle of the region located between conserved BRCT1 and -2 and BRCT4 and -5. In budding yeast, BRCT1 and -2 and BRCT3 and -4 are essential for the initiation of DNA replication. However, BRCT4 and -5 in vertebrate TopBP1, which correspond to BRCT3 and -4 of yeast Dpb11/Cut5, are dispensable for DNA replication (20). Moreover, BRCT3 itself is also required for DNA replication (20). The most likely Sld2 ortholog in vertebrates is RecQL4, which associates with TopBP1. However, it is unknown which part of TopBP1 can associate with RecQL4; moreover, RecQL4 is not required for the loading of GINS onto chromatin (21, 22). Therefore, although the Dpb11-Sld3 interaction seems to be conserved in model eukaryotes, it is not clear whether Dpb11 and TopBP1 have other conserved functions.
GINS is a heterotetrameric complex (Sld5-Psf1-Psf2-Psf3) that is loaded onto DNA in the initiation reaction to assemble the CMG complex (7, 8, 23, 24). A previous study reported that GINS interacts with Dpb11 in budding yeast (24), although the exact portion of Dpb11 required for this interaction was not identified. In this paper, we report that the inter-BRCT region located between BRCT2 and BRCT3 of Dpb11 is a GINS-associated domain and that the GINS-Dpb11 interaction is important for DNA replication and cell viability. We also found that a region located between BRCT3 and BRCT4 in vertebrate TopBP1 corresponds to the inter-BRCT region of budding yeast Dpb11 and is important for DNA replication in vertebrates.
MATERIALS AND METHODS
Strains and cell growth.
The yeast strains used are listed in Table 1. The conditions used for cell growth and synchronization were as described previously (25).
Table 1.
Yeast strains
| Name | Genotype | Reference or source |
|---|---|---|
| W303-1a Δbar1 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 Δbar1 | Our stock |
| YST756 | W303-1a Δbar1 Δdpb11::LEU2 [YEplac195-DPB11] | This study |
| YST1296 | W303-1a Δbar1 Δsld5::LEU2 Δpsf2::LEU2 [YEplac195(URA3)-SLD5] [YEplac195 (URA3)-PSF2] | This study |
| YST1520 | W303-1a Δbar1 ura3-1::GALp-sld2-11D-MycHis9(URA3) DBF4::Tet-DBF4-MycHis9(kanMX) leu2-3,112::GALp (LEU2) | This study |
| YST1523 | W303-1a Δbar1 ura3-1::GALp-sld2-11D-MycHis9(URA3) DBF4::Tet-DBF4-MycHis9(kanMX) leu2-3,112::GALp-SD fusion (253) (LEU2) | This study |
| YST1525 | W303-1a Δbar1 ura3-1::GALp-sld2-11D-MycHis9(URA3) DBF4::Tet-DBF4-MycHis9(kanMX) leu2-3,112::GALp-SD fusion (291) (LEU2) | This study |
| YST1904 | W303-1a Δbar1 sld3-6 Δdpb11::LEU2 [YEplac195-DPB11] | This study |
| YST1908 | W303-1a Δbar1 sld3-5 Δdpb11::LEU2 [YEplac195-DPB11] | This study |
| YST1969 | W303-1a Δbar1 ura3-1::GALp-sld2-11D-MycHis9(URA3) DBF4::Tet-DBF4-MycHis9(kanMX) leu2-3,112::GALp-SD fusion (253, AAA8/9/12/13) (LEU2) | This study |
| YST1977a | W303-1a Δbar1 dpb11-AAA6 | This study |
| YST1978a | W303-1a Δbar1 dpb11-AAA6 | This study |
| YST1979 | W303-1a Δbar1 dpb11-AAA8 | This study |
| YST1981 | W303-1a Δbar1 dpb11-AAA9 | This study |
| YST1983 | W303-1a Δbar1 dpb11-AAA12 | This study |
| YST1985 | W303-1a Δbar1 dpb11-AAA13 | This study |
| YST1987b | W303-1a Δbar1 dpb11-AAA8/9/1213 | This study |
| YST1988b | W303-1a Δbar1 dpb11-AAA8/9/1213 | This study |
| YST1989b | W303-1a Δbar1 dpb11-AAA8/9/1213 | This study |
| YYK16 | W303-1a Δbar1 sld3-6 | 31 |
| YYK19 | W303-1a Δbar1 sld3-5 | 31 |
| TAT7 | MATa his3Δ200 trp1-901 leu2-3,112 ade2 LYS2::(lexAop)4-HIS3 ura3Δ::(lexAop)8-lacZ gal4 gal80 | 29 |
YST1977 and 1978 are independent isolates.
YST1987 to -89 are independent isolates.
Flow cytometry.
Flow cytometry was performed as described previously (26).
Antibodies.
Rabbit polyclonal antibodies specific to GINS and Psf3 were raised against the GINS complex and Psf3, respectively. The rabbit polyclonal antibodies specific to Sld2 and Sld3 and the mouse monoclonal antibody specific to Orc6 (SB49; a generous gift from B. Stillman) were as described previously (27, 28).
In vitro pulldown assay.
The glutathione S-transferase (GST)-fused Dpb11 and His-tagged GINS complex were expressed in Escherichia coli and purified as described previously (9). Ten picomoles of each protein and glutathione-Sepharose 4B beads (GE Healthcare Life Sciences, Pittsburgh, PA) were mixed in 100 μl of binding buffer (20 mM HEPES-NaOH [pH 7.5], 150 mM NaCl, 10% glycerol, 0.05% Tween 20, and 0.01% NP-40) and incubated for 1 h at 4°C. The beads were washed three times and then subjected to Western blotting.
Yeast two-hybrid assay.
A yeast two-hybrid assay was performed as described previously (29). LexA fusion plasmids (pBTM116 [29] and pLexA-C [Dualsystems Biotech AG, Zürich, Switzerland]) and a Gal4AD plasmid (pACT2 [29]) were used for the construction of plasmids.
Immunoprecipitation.
TAT7 yeast cells (5 × 108) harboring YEp-GINS plus the pACT2 vector encoding Gal4AD-hemagglutinin (Gal4AD-HA) (29) or the Gal4AD-HA-Dpb11 (amino acids [aa] 253 to 290) plasmid were resuspended in 400 μl of extraction buffer (50 mM HEPES-KOH [pH 7.5], 150 mM KCl, 10% glycerol, 2 mM EDTA, 0.1% Tween 20, and 0.01% Triton X-100). Subsequently, whole-cell extracts (WCEs) were prepared by bead beating. WCEs were incubated with an anti-HA antibody (16B12; Babco) for 1 h at 4°C followed by precipitation of Gal4AD-HA-tagged protein by the addition of protein G-Sepharose Fast Flow beads (GE Healthcare Life Sciences) and an additional 2 h of incubation at 4°C. The beads were washed three times and then subjected to Western blotting.
In vitro DNA replication in Xenopus egg extracts.
The in vitro DNA replication assay using Xenopus egg extracts was performed as described previously (19).
RESULTS
The inter-BRCT region of Dpb11 is required for CDK bypass replication.
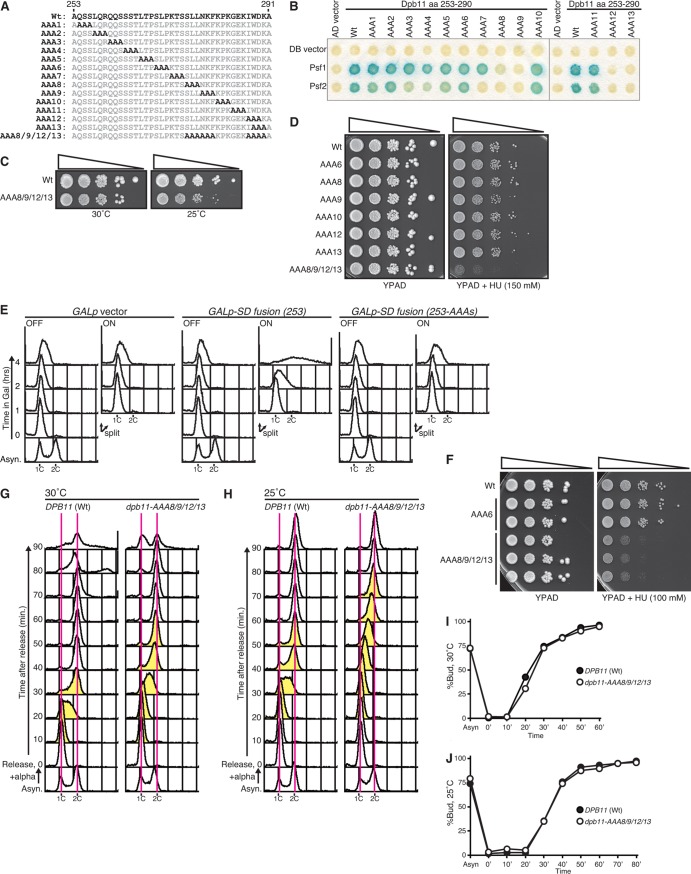
We first noticed that the region located between BRCT2 and BRCT3 of Dpb11 is important for DNA replication in budding yeast. BRCT1 and -2 and BRCT3 and -4 of Dpb11 bind to CDK-phosphorylated Sld3 and Sld2, respectively. Because Sld2 and Sld3 constitute minimal-essential targets of CDK in the initiation of DNA replication in budding yeast, simultaneous bypasses of the phosphorylation of Sld2 and Sld3 can induce DNA replication, even in the absence of CDK activity, e.g., as in G1-arrested cells (CDK bypass replication) (5, 6). One of the approaches used to induce CDK bypass replication is the combination of phosphomimetic Sld2 and the “SD fusion” protein, in which BRCT1 and -2 of Dpb11 are replaced with an unphosphorylatable Sld3, Sld3-2A (5). As shown previously, SD fusion (amino acid residue 253) induces DNA replication in G1-arrested cells (Fig. 1A and B). However, SD fusion (amino acid residue 291), which contains amino acid residues 291 to 764 of Dpb11, did not induce CDK bypass replication (SD fusion [amino acid residue 291] in Fig. 1A and B). In contrast, aa 291 to 631 of Dpb11 were sufficient for association with Sld2 (4). Consistent with the flow cytometry results, SD fusion (amino acid residue 253) cells showed severe loss of viability after the induction of the SD fusion protein (Fig. 1C), because CDK bypass replication causes genotoxic rereplication and severe loss of cell viability (25). In contrast, the expression of SD fusion (amino acid residue 291) did not affect cell viability (Fig. 1C). Therefore, CDK bypass replication was not induced in SD fusion (amino acid residue 291) cells. Because the two SD fusion proteins were expressed at similar levels after induction (Fig. 1D), these differences between SD fusion (amino acid residue 253) and SD fusion (amino acid residue 291) should be caused by the inter-BRCT region of Dpb11 (aa 253 to 290) and should indicate that the inter-BRCT region plays an important role in DNA replication.
Fig 1.
The inter-BRCT region of Dpb11 (amino acids [aa] 253 to 290) is essential for CDK bypass DNA replication. (A) Schematic drawings of the structure of Dpb11 and SD fusion proteins. The numbers placed above the structures show the positions of amino acids. The BRCT motif is shown as an open box. Sld3-2A in SD fusion is shown as a gray box. (B) YST1520 (GALp vector), YST1523 [GALp-SD fusion (253)], and YST1525 [GALp-SD fusion (291)] cells were grown in 1% yeast extract, 2% peptone, 0.004% adenine (YPA)–raffinose medium (Asynchro.) and arrested in the G1 phase using the alpha factor, and the culture was divided in two. Galactose was added to one half (ON) or the culture was left untreated (OFF), and samples were taken at the times indicated (0, 1, 2, and 5 h). The DNA contents of the samples were analyzed by flow cytometry. (C) Small aliquots of the samples described in the panel B legend were spread onto YPA-dextrose (YPAD) plates, and the viability was calculated based on the number of colonies that appeared after incubation. (D) Whole-cell extracts were prepared from the samples described in the panel B legend and were analyzed by Western blotting. Sld3 and SD fusion, Sld2, and Orc6 proteins were detected with anti-Sld3, anti-Sld2, and anti-Orc6 antibodies, respectively. The loading control (cntl.) shows the corresponding region of the membrane stained with Ponceau S. Asyn, asynchronous; endo., endogenous.
The inter-BRCT region of Dpb11 interacts with GINS.
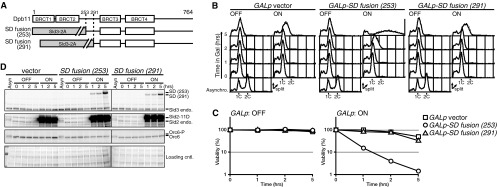
To investigate further the function of the inter-BRCT region of Dpb11 in DNA replication, we sought to identify the factor(s) that interacts with this region. Previous analyses showed that CDK-phosphorylated Sld3 and Sld2 bind to BRCT1 and -2 (aa 1 to 309) and BRCT3 and -4 (aa 291 to 631) of Dpb11, respectively (4, 6). It has also been shown that Dpb11 interacts with Psf1, which is a subunit of GINS; however, the region of Dpb11 that is required for this interaction has not been identified (24). To investigate further the interaction between Dpb11 and these proteins, we constructed a series of truncated Dpb11s (Fig. 2A) and tested their interactions with Psf1, Sld2, and Sld3 using a yeast two-hybrid assay. As expected, Sld3 and Sld2 interacted with truncated Dpb11s only when they contained BRCT1 and -2 or BRCT3 and -4, respectively. In contrast, Psf1 interacted with truncated Dpb11s if they included the inter-BRCT region (Fig. 2B). Importantly, a short stretch of amino acids located in the inter-BRCT region of Dpb11 (aa 253 to 290) was sufficient for the interaction with Psf1. Because the two-hybrid assay is performed in yeast, Psf1 may interact with Dpb11 aa 253 to 290 as a GINS complex. To test this possibility, the Dpb11 aa 253 to 290 fragment was immunoprecipitated. Because all GINS subunits were detected in the coprecipitated fraction (Fig. 2C, lane 6), we concluded that the Dpb11 aa 253 to 290 fragment interacts with the GINS complex in vivo.
Fig 2.
The inter-BRCT region of Dpb11 (aa 253 to 290) is a GINS-interacting domain. (A) Schematic drawings of the Dpb11 constructs. The numbers placed above the drawings show the positions of amino acids. The BRCT motif is shown as an open box. (B) Yeast two-hybrid assay. Cells harboring the plasmids indicated were grown on SC plates lacking leucine and tryptophan, lifted with filter paper, and subjected to a β-galactosidase assay. AD, activation domain; DB, DNA binding. (C) Immunoprecipitation (IP) was performed as described in Materials and Methods. Samples were analyzed by Western blotting. Gal4AD-HA-Dpb11 aa 253 to 290 and GINS proteins were detected with anti-HA and anti-GINS antibodies, respectively. The ratio of the amounts of samples loaded on the gel was 1:10:1 (Input/IP/Sup. [supernatant]). *, nonspecific signals; Vec., vector. (D) The in vitro pulldown assay was performed as described in Materials and Methods. Samples were analyzed by Western blotting. GST-Dpb11 and GINS proteins were detected with anti-GST and anti-GINS antibodies, respectively. The ratio of the amount of samples loaded on the gel was 1:2:1 (Input/Beads ppt. [precipitates]/Sup.). (E) Low-copy-number vector (Vector) and low-copy-number DPB11 constructs (YCp-DPB11: wild-type DPB11 [Wt] and dpb11Δ253 to 290 [Δ253–290]) were introduced into YST756 (Δdpb11 [YEp–DPB11]), serially diluted, and grown on an SC plate lacking leucine and tryptophan (SC - Leu, Trp) or on an SC plate containing 5-fluoroorotic acid (5-FOA) (SC - Leu, Trp + FOA) to eliminate the YEp-DPB11 plasmid from the cells.
To determine whether Dpb11 and GINS can interact directly, we analyzed their interaction in vitro. For this purpose, Dpb11 was fused with glutathione S-transferase (GST) and purified from E. coli. The GINS complex was also purified from E. coli, and an in vitro GST pulldown assay was performed. When the GST-Dpb11 fusion included the inter-BRCT region containing aa 253 to 290, the GINS complex was coprecipitated. In contrast, GST-Dpb11 lacking aa 253 to 290 failed to pull down the GINS complex (Fig. 2D). These findings indicate that aa 253 to 290 of Dpb11 are important for the GINS-Dpb11 interaction in vitro. We named this region of Dpb11 the GINS interaction (GINI) region.
The interaction between Dpb11 and GINS is important for cellular viability.
Dpb11 lacking aa 253 to 290 (Dpb11 Δ253–290) lost the ability to interact with Psf1 and did not support cell growth (Fig. 2B and E). However, Dpb11 Δ253–290 maintained the interaction between Sld2 and Sld3, although the interaction between Dpb11 Δ253–290 and Sld3 seemed weaker than that between full-length Dpb11 and Sld3 (Fig. 2B). These results suggest that the GINS-Dpb11 interaction is important for cell viability.
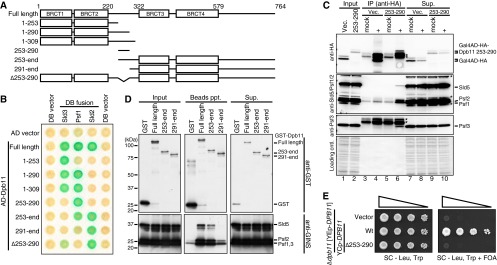
To examine further the physiological importance of the interaction between GINS and GINI of Dpb11, three consecutive amino acids in the corresponding region of Dpb11 were replaced systematically by alanines (AAA1 to -13) (Fig. 3A), and the effects of this substitution on the interaction with the Psf1 and Psf2 subunits of GINS and on cell viability were examined. The Dpb11 aa 253 to 290 fragment harboring the AAA8, AAA9, AAA12, or AAA13 mutation reduced the interaction with GINS in a two-hybrid assay (Fig. 3B). Similar results were obtained when these AAA mutations were introduced into full-length Dpb11 (see Fig. S2A in the supplemental material). In plasmid-shuffling experiments, however, all of the AAA mutants supported cell growth, although cells harboring Dpb11-AAA8/9/12/13, which simultaneously harbor all of the AAA8, AAA9, AAA12, and AAA13 substitutions, were sensitive to low temperature and to the inhibitor of DNA replication hydroxyurea (HU) (Fig. 3C and D). It is known that budding yeast cells that have defects in the replication machinery are sensitive to HU. Therefore, this result suggests that DNA replication is less efficient in Dpb11-AAA8/9/12/13 cells, although the effect of this mutation was not as severe as that observed in Dpb11 Δ253–290 (Fig. 2E).
Fig 3.
Alanine substitution mutations in the inter-BRCT region of Dpb11 cause defects in DNA replication. (A) Amino acid sequences of aa 253 to 291 of Dpb11. The mutation sites in each mutant are shown. (B) The yeast two-hybrid assay was performed as described for Fig. 2B. (C) Low-copy-number dpb11-AAA8/9/12/13 was introduced into YST756 (Δdpb11 [YEp-DPB11]) and grown on a 5-FOA-containing plate to eliminate the YEp-DPB11 plasmid from the cells. The cells grown on 5-FOA were serially diluted and grown on YPAD at the respective temperatures indicated. (D) Low-copy-number DPB11 constructs (wild-type DPB11 [Wt] and AAA mutants [AAA6 to AAA8/9/12/13]) were introduced into YST756 (Δdpb11 [YEp-DPB11]), and cells that lost YEp-DPB11 were recovered as described for panel C. These cells were serially diluted and grown on YPAD or YPAD containing 150 mM HU at 30°C. (E) YST1520 (GALp vector), YST1523 [GALp-SD fusion (253)], and YST1969 [GALp-SD fusion (253-AAAs)] cells were grown and analyzed as described for Fig. 1B. (F) Cells with wild-type DPB11 (W303-1a Δbar1 [Wt]), dpb11-AAA6 (YST1977 and YST1978 [AAA6]), and dpb11-AAA8/9/12/13 (YST1987, YST1988 and, YST1989 [AAA8/9/12/13]) were serially diluted and grown on YPAD or YPAD containing 100 mM HU at 30°C. (G) W303-1a Δbar1 [DPB11 (Wt)] and YST1987 (dpb11-AAA8/9/12/13) cells were grown in YPAD medium at 30°C (Asyn.), arrested in the G1 phase using the alpha factor, and synchronously released at 30°C. Samples were taken at every 10 min after the release (0 to 90 min). The DNA content of the samples was analyzed by flow cytometry. Flow cytometry profiles of samples under conditions of DNA replication judged from the peak position are filled in yellow. (H) The experiment was performed as described for panel G, with the exception that cells were grown at 25°C. (I and J) The proportions of budded cells of the same samples as those described for panels G and H, respectively, are shown.
Although the dpb11-AAA8, -AAA9, -AAA12, or -AAA13 mutation alone did not yield growth defects in the plasmid-shuffling assay performed in the wild-type background, each led to a growth defect when introduced in the temperature-sensitive alleles of the SLD3 background, sld3-5 and sld3-6, even at the permissive temperature (see Fig. S2B and C in the supplemental material [+Vector in the right panels]). Sld3 is required for the recruitment of GINS to origins via its phosphorylation-dependent interaction with Dpb11 (5, 6). It is known that Sld3 also interacts with GINS (24). However, none of the mutations were suppressed by high-copy-number GINS or DPB11 (see Fig. S2D in the supplemental material). The capability of these sld3-ts mutants to interact with Dpb11 was not affected by the dpb11-AAA mutation (see Fig. S2E in the supplemental material). Therefore, we surmised that the synthetic growth defect caused by sld3-ts and the dpb11-AAA8, -AAA9, -AAA12, or -AAA13 mutation was caused by the reduced Dpb11-GINS interaction. Thus, the Dpb11-GINS interaction plays some role in cellular viability.
The Dpb11-AAA8/9/12/13 mutant shows replication defects.
The results mentioned above suggest that the Dpb11-GINS interaction is important for the initiation of DNA replication. To examine further the role of the Dpb11-GINS interaction in the initiation of DNA replication, we constructed the mutant version of the SD fusion (amino acid residue 253) protein, SD fusion (253-AAAs), which harbors the AAA8/9/12/13 mutation in the Dpb11 portion. In contrast with the wild-type SD fusion (amino acid residue 253), which induced DNA replication in G1-arrested cells, SD fusion (253-AAAs) did not induce DNA replication (Fig. 3E).
To test further the capability of Dpb11-AAA8/9/12/13 to induce DNA replication under the normal conditions, a sole copy of genomic DPB11 was replaced with the dpb11-8/9/12/13 mutant, because dpb11-8/9/12/13 was viable in the plasmid-shuffling assay (Fig. 3D). As expected, the integrated dpb11-8/9/12/13 supported cell growth, and cells showed HU sensitivity (Fig. 3F, AAA8/9/12/13). To monitor the initiation and progression of DNA replication, the dpb11-8/9/12/13 cells were arrested in G1 using the alpha factor and released synchronously. Synchronous culture revealed that DNA replication was delayed in the dpb11-8/9/12/13 mutant (Fig. 3G). In wild-type DPB11 cells, DNA replication was initiated between 10 and 20 min after release, and bulk DNA replication was finished by 40 min. In contrast, in dpb11-8/9/12/13 cells, although DNA replication was initiated between 10 and 20 min, the progression of DNA replication was much slower than that of the wild type, and bulk DNA replication was finished by 50 min. The difference was obvious at around 20 to 40 min (Fig. 3G). The phenotype was stronger at 25°C than it was at 30°C (Fig. 3H) and was observed only for the dpb11-8/9/12/13 mutant and not for any mutants harboring a single AAA substitution (see Fig. S3A in the supplemental material). This S-phase delay was not caused by poor synchronization because the results with respect to the appearance of buds, which indicates the commitment to the new cell cycle after release, were similar in the wild-type DPB11 and dpb11-8/9/12/13 cells at both temperatures (Fig. 3I and J). Therefore, these results suggest that the Dpb11-GINS interaction is important for efficient DNA replication.
If the loss or weakening of the Dpb11-GINS interaction is responsible for inefficient DNA replication in the dpb11-8/9/12/13 mutant, increased dosage of GINS should suppress these defects. This was the case. The introduction of the high-copy-number GINS plasmid into SD fusion (253-AAAs) cells enabled the initiation of DNA replication when they were arrested in the G1 phase (Fig. 4A). The slow DNA replication and HU sensitivity observed in dpb11-8/9/12/13 cells were also suppressed by the introduction of the GINS plasmid (Fig. 4B to D). Moreover, the introduction of the high-copy-number GINS plasmid into sld3-ts dpb11-AAA cells suppressed the growth defect (see Fig. S2B and C in the supplemental material [+GINS in the right panels]). Therefore, we concluded that the replication defects observed in dpb11-8/9/12/13 cells were caused by the loss or weakening of the Dpb11-GINS interaction.
Fig 4.
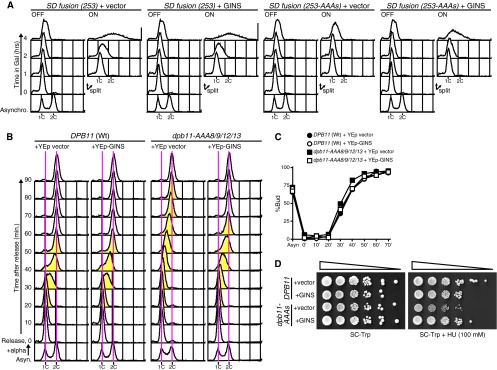
Replication defects caused by alanine substitution mutations in the inter-BRCT region of Dpb11 can be suppressed by high-copy-number GINS. (A) YST1523 [SD fusion (253)] and YST1969 [SD fusion (253-AAAs)] cells harboring the high-copy-number vector plasmid (+ vector) or high-copy-number GINS plasmid (+ GINS) were grown and analyzed as described for Fig. 2E. The expression level of GINS was also examined by Western blotting (see Fig. S3B in the supplemental material). (B) W303-1a Δbar1 [DPB11 (Wt)] and YST1987 (dpb11-AAA8/9/12/13) cells harboring the high-copy-number vector plasmid (+ YEp vector) or high-copy-number GINS plasmid (+ YEp-GINS) were grown and analyzed as described for Fig. 2H. The expression level of GINS was also examined by Western blotting (see Fig. S3C in the supplemental material). (C) Proportions of budded cells of the samples were determined as described in the panel B legend. (D) W303-1a Δbar1 (DPB11) and YST1987 (dpb11-AAAs) cells harboring the high-copy-number vector plasmid (+vector) or high-copy-number GINS plasmid (+GINS) were serially diluted and grown on SC lacking tryptophan (SC-Trp) or SC lacking tryptophan containing 100 mM HU (SC-Trp + HU) at 30°C.
The GINI region is important for DNA replication in vertebrates.
Dpb11 is an evolutionarily conserved protein. Its vertebrate ortholog TopBP1 is also required for DNA replication (18, 19). Therefore, we wondered if the GINI region exists in vertebrate TopBP1. BRCT1 and -2 and BRCT3 and -4 of yeast Dpb11/Cut5 show the highest similarity to BRCT1 and -2 and BRCT4 and -5 of TopBP1, respectively (see Fig. S1 in the supplemental material). The N-terminal region containing BRCT1 and -2 interacts with phosphorylated treslin, which is an ortholog of Sld3 (15, 16). The region located between BRCT2 and BRCT4 in TopBP1 (aa 284 to 535) is longer than that of Dpb11/Cut5 and contains one extra BRCT domain, BRCT3 (Fig. 5A; see also Fig. S1 in the supplemental material). In addition, the overall similarity of the region to the inter-BRCT region of yeast Dpb11 is not high. However, this region seems to be important for DNA replication in vertebrates. Because the minimal fragment of TopBP1 (TopBP1 Δ531–1485) necessary to induce efficient DNA replication in Xenopus egg extracts contains not only the treslin-interacting region, BRCT0 to -2, but also most of this inter-BRCT region, aa 284 to 535 (20), we expected that the region located between BRCT2 and BRCT4 in TopBP1 would include the GINI region. Thus, we examined whether the region can specifically interact with GINS. In a yeast two-hybrid assay, the Psf3 subunit of Xenopus GINS, Xl Psf3, interacted with the region located between BRCT2 and BRCT4 of TopBP1 (aa 284 to 530), as well as with full-length and aa 1 to 530 of TopBP1, only when the other three GINS subunits (Sld5, Psf1, and Psf2) were expressed simultaneously (Fig. 5A and B). This indicates that Xenopus GINS interacts with the region located between BRCT2 and BRCT4 of TopBP1 as a complex.
Fig 5.
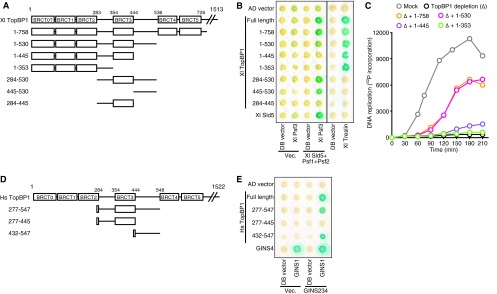
The inter-BRCT region located between BRCT3 and BRCT4 of TopBP1 associates with GINS and is important for DNA replication in vertebrates. (A) Schematic drawings of the Xenopus TopBP1 constructs used as described for panels B and C. The numbers placed above the drawings show the positions of amino acids. The BRCT motif is shown as an open box. (B) The yeast two-hybrid assay was performed as described for Fig. 2B. (C) The in vitro replication assay was performed as described in Materials and Methods. (D) Schematic drawings of the human TopBP1 constructs used as described for panel E. The numbers placed above the drawings show the positions of amino acids. The BRCT motif is shown as an open box. (E) The yeast two-hybrid assay was performed as described in the panel B legend.
To dissect the region located between BRCT2 and BRCT4, we generated truncated constructs of this region and examined their capability to interact with GINS (Fig. 5A). The region located between BRCT3 and BRCT4 of TopBP1 (aa 445 to 530), but not the regions located between BRCT2 and BRCT3 plus BRCT3 (aa 284 to 445), interacted with GINS (Fig. 5B). This indicates that the region located between BRCT3 and BRCT4 of TopBP1 is the GINI region in vertebrate TopBP1. Interestingly, multiple alignment of amino acid sequences of the inter-BRCT region of Dpb11, Cut5, and human and Xenopus TopBP1s also suggested that the region located between BRCT3 and BRCT4 of TopBP1, but not the regions located between BRCT2 and BRCT3 or BRCT3, is the GINI region in vertebrate TopBP1, although the amino acid sequences are not very similar (see Fig. S4 in the supplemental material).
To investigate the function of the GINI region of TopBP1 (the region located between BRCT3 and BRCT4) in DNA replication in vertebrates, we investigated whether the existence of this inter-BRCT region contributes to the ability of TopBP1 to induce DNA replication in Xenopus egg extracts (Fig. 5C). We used the TopBP1 N-terminal fragment containing BRCT0 to -5 (aa 1 to 758) as a positive control (Fig. 5A and C), because both the TopBP1 aa 1 to 758 and full-length TopBP1 efficiently restore TopBP1 function in TopBP1-depleted extracts (30). The TopBP1 N-terminal fragment containing BRCT0 to -3 (aa 1 to 530) induced DNA replication at a level that was similar to that induced by the TopBP1 aa 1 to 758 fragment (Fig. 5C). However, DNA replication was severely reduced for the BRCT0 to -3 fragment (aa 1 to 445), which lacks the GINI region located between BRCT3 and BRCT4 (aa 446 to 530) (Fig. 5C). This difference in DNA replication was not caused by the capability to associate with treslin, because all constructs harboring BRCT0 to -2 interacted similarly with treslin (Fig. 5B). Therefore, the GINI region located between BRCT3 and BRCT4 (aa 445 to 530) of TopBP1 is important for the efficient in vitro DNA replication in Xenopus egg extracts, as was observed in the case of the GINI region of Dpb11 in budding yeast.
TopBP1 is highly conserved in vertebrates. For example, the amino acid sequences of Xenopus and human TopBP1s are 62% identical. However, the inter-BRCT region located between BRCT3 and BRCT4 (aa 445 to 536 in Xenopus and aa 445 to 547 in humans) is less conserved (36% identity). Therefore, we investigated the interaction between the regions located between BRCT3 and BRCT4 of human TopBP1 and GINS. The human GINS includes four proteins, GINS1 to -4, which correspond to Psf1, Psf2, Psf3, and Sld5 of yeast GINS, respectively. In a yeast two-hybrid assay, human GINS1 interacted with the region located between BRCT2 and BRCT4 (aa 277 to 547), the region located between BRCT3 and BRCT4 (aa 432 to 547), and full-length TopBP1, but not with the region located between BRCT2 and BRCT3 plus BRCT3 (aa 277 to 445), only when the GINS2 to -4 subunits were expressed simultaneously (Fig. 5D and E), as in the case of the Xenopus TopBP1-GINS interaction. Thus, we concluded that the region located between BRCT3 and BRCT4 is the GINI region in vertebrate TopBP1.
DISCUSSION
Dpb11 plays a crucial role in the initiation of DNA replication in budding yeast. This study showed that, in addition to the phosphopeptide-binding activity of BRCTs, the inter-BRCT region of Dpb11 is important for efficient DNA replication via its interaction with GINS; as mentioned above, we named this region GINI (for GINS interaction region). Although the BRCT pair requires phosphorylation for the interaction with its binding partner, it seems that this GINI-GINS interaction does not require such posttranslational modification (Fig. 2D). Such a notion was also supported by the observation that GINS lacking CDK phosphorylation sites does not affect cell growth (see Fig. S2F in the supplemental material). A previous in vitro assay performed under conditions that were more stringent did not detect the interaction between Dpb11 and GINS alone. However, the association of GINS with Dpb11 was enhanced by the coexistence of Pol ε and phosphorylated Sld2 (9). Moreover, Dpb11 coimmunoprecipitated with Psf1 even in the G1 phase only when cells were treated with cross-linker. However, such interaction was greatly enhanced in S-phase cells (9). These results suggest that pre-LC assembly enhances the Dpb11-GINS interaction in vitro and in vivo. Thus, it is likely that Pol ε and phosphorylated Sld2 stabilize the association between GINS and Dpb11 and that this might render the Dpb11-GINS interaction cell cycle dependent (see Fig. S5 in the supplemental material).
Multiple protein-protein interactions in a complex stabilize the complex. As described above, the GINS-Dpb11 interaction in the pre-LC is stabilized by other components of the same complex. We speculate that the GINS-Dpb11 interaction also contributes to stabilization of the pre-LC and preinitiation complex (pre-IC), which leads to the efficient assembly of the active replicative helicase, the CMG complex, in the initiation of DNA replication (see Fig. S5 in the supplemental material). In the pre-IC, GINS interacts with Sld3 (24). Therefore, the Sld3-GINS interaction may further stabilize the Dpb11-GINS interaction and, consequently, the pre-IC when the active CMG complex assembles (see Fig. S5 in the supplemental material). This hypothesis is supported by the fact that the temperature-sensitive mutants of SLD3, sld3-5 and sld3-6, are synthetically lethal with the dpb11-AAA mutants that lost the interaction with GINS, respectively (see Fig. S2B and C in the supplemental material). The sld3-5 mutation reduces the interaction between Sld3 and Cdc45 (31), and the sld3-6 mutation reduces the level of the Sld3 protein because of a lack of interaction between Sld3 and Sld7 (32). Consequently, both mutations might reduce the level of the pre-IC containing Cdc45-Sld3-Sld7 at origins and, eventually, the association between origins and GINS, even at permissive temperatures. Thus, we surmise that the combination of the sld3 and dpb11-AAA mutations reduces the recruitment of GINS severely, although a single mutation alone might not reduce the recruitment to the same extent (see Fig. S5 in the supplemental material). This idea was also supported by the fact that the high copy number of GINS suppressed the synthetic lethality of sld3-ts dpb11-AAA cells (see Fig. S2B and C in the supplemental material).
Our results showed that the Dpb11-GINS interaction was also observed between the vertebrate Dpb11 ortholog, TopBP1, and GINS and is important for efficient DNA replication. The inter-BRCT region located between BRCT3 and BRCT4 in vertebrate TopBP1 interacted with the GINS complex and was required for efficient DNA replication (Fig. 5). These findings suggest that GINI works similarly in vertebrate TopBP1 and in budding yeast, although the conservation of the amino acid sequence between yeast GINI and vertebrate GINI was not high (see Fig. S4 in the supplemental material). Many protein-protein interactions might be required for the proper assembly of replication proteins on replication origins to activate the replicative helicase. We propose that the Dpb11/TopBP1-GINS interaction is one such interaction. Moreover, an organism-specific fine-tuning might exist. BRCT4 and -5 in TopBP1 may be one such example. In contrast to yeast Dpb11/Cut5, BRCT4 and -5 of TopBP1, which are most similar to the C-terminal BRCT pair in Dpb11/Cut5, are dispensable for efficient DNA replication in Xenopus egg extracts (Fig. 5), as reported previously by Kumagai et al. (20). The Sld3-Cut5 interaction in fission yeast is another example, because the phosphorylation of Sld3 is important, but not essential, for the initiation of DNA replication (33, 34). Nonetheless, most of the protein-protein interactions seem to be required for the initiation of DNA replication, and the overall process of activation of the replicative helicase is conserved among various organisms.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Yagura, S. Endo, H. Oizumi, and Y. Tanaka for technical help and discussions.
This study was partly supported by grants to Y. Kubota and S. Tanaka from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
Published ahead of print 6 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00431-13.
REFERENCES
- 1. Tanaka S, Araki H. 2010. Regulation of the initiation step of DNA replication by cyclin-dependent kinases. Chromosoma 119:565–574 [DOI] [PubMed] [Google Scholar]
- 2. Labib K. 2010. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev. 24:1208–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Masumoto H, Muramatsu S, Kamimura Y, Araki H. 2002. S-Cdk-dependent phosphorylation of Sld2 essential for chromosomal DNA replication in budding yeast. Nature 415:651–655 [DOI] [PubMed] [Google Scholar]
- 4. Tak YS, Tanaka Y, Endo S, Kamimura Y, Araki H. 2006. A CDK-catalysed regulatory phosphorylation for formation of the DNA replication complex Sld2-Dpb11. EMBO J. 25:1987–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zegerman P, Diffley JF. 2007. Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature 445:281–285 [DOI] [PubMed] [Google Scholar]
- 6. Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H. 2007. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature 445:328–332 [DOI] [PubMed] [Google Scholar]
- 7. Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, van Deursen F, Edmondson RD, Labib K. 2006. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 8:358–366 [DOI] [PubMed] [Google Scholar]
- 8. Moyer SE, Lewis PW, Botchan MR. 2006. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc. Natl. Acad. Sci. U. S. A. 103:10236–10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muramatsu S, Hirai K, Tak YS, Kamimura Y, Araki H. 2010. CDK-dependent complex formation between replication proteins Dpb11, Sld2, Pol (epsilon), and GINS in budding yeast. Genes Dev. 24:602–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia V, Furuya K, Carr AM. 2005. Identification and functional analysis of TopBP1 and its homologs. DNA Repair (Amst) 4:1227–1239 [DOI] [PubMed] [Google Scholar]
- 11. Tanaka S, Tak YS, Araki H. 2007. The role of CDK in the initiation step of DNA replication in eukaryotes. Cell Div. 2:16 doi:10.1186/1747-1028-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saka Y, Fantes P, Sutani T, McInerny C, Creanor J, Yanagida M. 1994. Fission yeast cut5 links nuclear chromatin and M phase regulator in the replication checkpoint control. EMBO J. 13:5319–5329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Araki H, Leem SH, Phongdara A, Sugino A. 1995. Dpb11, which interacts with DNA polymerase II(epsilon) in Saccharomyces cerevisiae, has a dual role in S-phase progression and at a cell cycle checkpoint. Proc. Natl. Acad. Sci. U. S. A. 92:11791–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mäkiniemi M, Hillukkala T, Tuusa J, Reini K, Vaara M, Huang D, Pospiech H, Majuri I, Westerling T, Makela TP, Syvaoja JE. 2001. BRCT domain-containing protein TopBP1 functions in DNA replication and damage response. J. Biol. Chem. 276:30399–30406 [DOI] [PubMed] [Google Scholar]
- 15. Kumagai A, Shevchenko A, Dunphy WG. 2011. Direct regulation of Treslin by cyclin-dependent kinase is essential for the onset of DNA replication. J. Cell Biol. 193:995–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boos D, Sanchez-Pulido L, Rappas M, Pearl LH, Oliver AW, Ponting CP, Diffley JF. 2011. Regulation of DNA replication through Sld3-Dpb11 interaction is conserved from yeast to humans. Curr. Biol. 21:1152–1157 [DOI] [PubMed] [Google Scholar]
- 17. Rappas M, Oliver AW, Pearl LH. 2011. Structure and function of the Rad9-binding region of the DNA-damage checkpoint adaptor TopBP1. Nucleic Acids Res. 39:313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Hatten RA, Tutter AV, Holway AH, Khederian AM, Walter JC, Michael WM. 2002. The Xenopus Xmus101 protein is required for the recruitment of Cdc45 to origins of DNA replication. J. Cell Biol. 159:541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashimoto Y, Takisawa H. 2003. Xenopus Cut5 is essential for a CDK-dependent process in the initiation of DNA replication. EMBO J. 22:2526–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumagai A, Shevchenko A, Dunphy WG. 2010. Treslin collaborates with TopBP1 in triggering the initiation of DNA replication. Cell 140:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuno K, Kumano M, Kubota Y, Hashimoto Y, Takisawa H. 2006. The N-terminal noncatalytic region of Xenopus RecQ4 is required for chromatin binding of DNA polymerase alpha in the initiation of DNA replication. Mol. Cell. Biol. 26:4843–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sangrithi MN, Bernal JA, Madine M, Philpott A, Lee J, Dunphy WG, Venkitaraman AR. 2005. Initiation of DNA replication requires the RECQL4 protein mutated in Rothmund-Thomson syndrome. Cell 121:887–898 [DOI] [PubMed] [Google Scholar]
- 23. Kubota Y, Takase Y, Komori Y, Hashimoto Y, Arata T, Kamimura Y, Araki H, Takisawa H. 2003. A novel ring-like complex of Xenopus proteins essential for the initiation of DNA replication. Genes Dev. 17:1141–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takayama Y, Kamimura Y, Okawa M, Muramatsu S, Sugino A, Araki H. 2003. GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev. 17:1153–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanaka S, Araki H. 2011. Multiple regulatory mechanisms to inhibit untimely initiation of DNA replication are important for stable genome maintenance. PLoS Genet. 7:e1002136 doi:10.1371/journal.pgen.1002136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanaka S, Diffley JF. 2002. Interdependent nuclear accumulation of budding yeast Cdt1 and Mcm2-7 during G1 phase. Nat. Cell Biol. 4:198–207 [DOI] [PubMed] [Google Scholar]
- 27. Tanaka S, Nakato R, Katou Y, Shirahige K, Araki H. 2011. Origin association of sld3, sld7, and cdc45 proteins is a key step for determination of origin-firing timing. Curr. Biol. 21:2055–2063 [DOI] [PubMed] [Google Scholar]
- 28. Liang C, Stillman B. 1997. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev. 11:3375–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartel PL, Fields S. 1995. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 254:241–263 [DOI] [PubMed] [Google Scholar]
- 30. Hashimoto Y, Tsujimura T, Sugino A, Takisawa H. 2006. The phosphorylated C-terminal domain of Xenopus Cut5 directly mediates ATR-dependent activation of Chk1. Genes Cells 11:993–1007 [DOI] [PubMed] [Google Scholar]
- 31. Kamimura Y, Tak YS, Sugino A, Araki H. 2001. Sld3, which interacts with Cdc45 (Sld4), functions for chromosomal DNA replication in Saccharomyces cerevisiae. EMBO J. 20:2097–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanaka T, Umemori T, Endo S, Muramatsu S, Kanemaki M, Kamimura Y, Obuse C, Araki H. 2011. Sld7, an Sld3-associated protein required for efficient chromosomal DNA replication in budding yeast. EMBO J. 30:2019–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakajima R, Masukata H. 2002. SpSld3 is required for loading and maintenance of SpCdc45 on chromatin in DNA replication in fission yeast. Mol. Biol. Cell 13:1462–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fukuura M, Nagao K, Obuse C, Takahashi TS, Nakagawa T, Masukata H. 2011. CDK promotes interactions of Sld3 and Drc1 with Cut5 for initiation of DNA replication in fission yeast. Mol. Biol. Cell 22:2620–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.