Abstract
The molecular basis underlying the physiologically well-defined orexigenic function of glucocorticoid (Gc) is unclear. Brain-specific homeobox factor (Bsx) is a positive regulator of the orexigenic neuropeptide, agouti-related peptide (AgRP), in AgRP neurons of the hypothalamic arcuate nucleus. Here, we show that in response to fasting-elevated Gc levels, Gc receptor (GR) and Bsx synergize to direct activation of AgRP transcription. This synergy is dictated by unique sequence features in a novel Gc response element in AgRP (AgRP-GRE). In contrast to AgRP-GRE, Bsx suppresses transactivation directed by many conventional GREs, functioning as a gene context-dependent modulator of GR actions or a target selector for GR. Consistent with this finding, AgRP-GRE drives fasting-dependent activation of a target gene specifically in GR+ Bsx+ AgRP neurons. These results define AgRP as a common orexigenic target gene of GR and Bsx and provide an opportunity to identify their additional common targets, facilitating our understanding of the molecular basis underlying the orexigenic activity of Gc and Bsx.
INTRODUCTION
Energy balance is directed by the relationship between the amount of consumed food and energy expenditure, and both processes are regulated by the central nervous system (CNS). The hypothalamus is the main CNS structure responsible for appetite control and energy expenditure (1, 2). The peripheral hunger or satiety signals arriving at the CNS are first perceived by two populations of neurons, agouti-related peptide (AgRP) neurons and pro-opiomelanocortin (POMC) neurons, in the hypothalamic arcuate nucleus (ARC) (1, 2). Sensing the peripheral signals, AgRP neurons produce the orexigenic peptides AgRP and neuropeptide Y (NPY), and POMC neurons yield the anorexigenic peptide α-melanocyte-stimulating hormone (αMSH). AgRP and NPY increase food intake and decrease energy expenditure, while αMSH does the opposite, contributing to maintaining energy homeostasis centrally (1, 2).
Glucocorticoid (Gc) is a well-known peripheral orexigenic signal (1, 2). Fasting increases plasma levels of Gc and activates expression of NPY and AgRP in AgRP neurons (1–4). Gc replacement studies in adrenalectomized mice have also revealed that Gc triggers fasting-dependent induction of NPY/AgRP expression (4–7). These studies indicate that Gc-mediated upregulation of NPY/AgRP in the hypothalamus contributes to the orexigenic activity of Gc. However, both the molecular mechanism by which Gc controls expression of NPY/AgRP and the direct target genes of Gc in AgRP neurons remain poorly understood.
Gc primarily functions through the transcription-dependent action of Gc receptor (GR), a member of the nuclear hormone receptor superfamily (8). GR is a cytoplasmic protein that translocates to the nucleus upon binding Gc and interacts with the Gc response element (GRE) of GR target genes to regulate their expression (8). In addition to the genes with classical GREs, Gc also regulates expression of genes that contain response elements for GR-interacting transcription factors. For instance, via protein-protein interactions with the proinflammatory transcription factors AP1 and NF-κB, GR is tethered to the binding sites for AP1 and NF-κB and represses their transactivation (8, 9).
Brain-specific homeobox factor (Bsx) is a transcription factor expressed in selective regions of the CNS, including the ARC, the dorsomedial nuclei (DMH), and the lateral hypothalamic area (LHA) of the hypothalamus (10–12). Within the ARC, Bsx appears to be expressed exclusively in AgRP neurons and functions as an orexigenic transcription factor (12). Consistent with this finding, Bsx levels are increased by fasting as well as ghrelin, an orexigenic signal, and decreased by leptin, an anorexigenic signal (13). Bsx has also been suggested to link spontaneous locomotor activity and food intake, given that Bsx-null mice show impaired expression of NPY/AgRP and food-seeking locomotor behavior (12). Bsx cooperates with FoxO1 to directly activate expression of AgRP through Bsx and FoxO1 response elements in AgRP (12). However, the detailed molecular basis of Bsx action in regulating the expression of NPY/AgRP remains poorly understood.
In this report, we set out to address two specific questions. (i) Does Gc directly regulate transcription of NPY and AgRP genes in the AgRP neurons via GR? (ii) Is there any mechanism that functionally couples the peripheral orexigenic signal Gc with the orexigenic transcription factor Bsx in controlling expression of NPY/AgRP? Our results demonstrate that in AgRP neurons, the peripheral Gc signal triggers a synergistic activation of AgRP expression by GR and Bsx via a novel Gc response element in AgRP, named AgRP-GRE, identifying AgRP as the first direct and common orexigenic target gene of GR and Bsx. Interestingly, this synergy is dictated by unique sequence features in AgRP-GRE. Furthermore, we show that this sequence information can be used to identify additional common targets of GR and Bsx. Surprisingly, Bsx also represses the action of GR on many conventional GREs, suggesting that Bsx acts as a dual-function target selector for GR. Together, our results provide critical insights into the molecular mechanisms for the central orexigenic action of Gc/GR and Bsx as well as for how GR targets distinct sets of genes in different tissues (8).
MATERIALS AND METHODS
Animals.
Seven- to 10-week-old wild-type C57BL/6 mice were maintained on a normal 12-h light, 12-h dark cycle with ad libitum access to normal chow and water, unless otherwise indicated. Mice were intraperitoneally or intracerebroventricularly injected with Dex (10 mg/kg) or RU486 (50 mg/kg). All studies were approved by the Institutional Animal Care & Use Committee.
Generation of transgenic mice.
The transgenes were microinjected into single-cell-stage embryos of C57BL/6 mice by the transgenic mouse core at Baylor College of Medicine. Founder lines for each enhanced green fluorescent protein (EGFP) reporter were generated and bred into wild-type C57BL/6 mice.
ChIP.
Mouse hypothalamus was dissected and homogenized before being cross-linked with 1% formaldehyde for 15 to 20 min. The cell lysates were sonicated; immunoprecipitated with anti-GR antibody (SC-8992; Santa Cruz), anti-Bsx antibody that we have generated, or control IgG (Santa Cruz); and incubated with protein A and G agarose overnight. DNAs were eluted and reverse cross-linked at 65°C overnight. DNAs were purified with the phenol-chloroform extraction method. We also carried out chromatin immunoprecipitations (ChIPs) with P19 cells expressing either Flag-GR alone or Flag-GR and Bsx using anti-H3K4me3 (for trimethylated histone H3 lysine 4) and anti-H3Ac (for acetylated H3) antibodies (AbCam). The primers used for the subsequent PCRs are shown in Fig. S3A in the supplemental material.
Immunostaining.
Anesthetized mice were perfused transcardially with 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were removed and placed in 4% paraformaldehyde overnight, followed by incubation with 30% sucrose. Brain sections were prepared with a cryostat, incubated with antibodies against GR (SC-1004; Santa Cruz), POMC (H-029-30; Phoenix Pharmaceutical), GFP (GFP-1020; Aves), and Bsx at 4°C overnight, and monitored by 1 to 2 h of incubation with fluorescence-conjugated secondary antibodies.
In situ hybridization.
Digoxigenin-labeled antisense RNA probes were hybridized to the brain sections at 68°C. Hybridized sections were washed, incubated with anti-digoxigenin-alkaline phosphatase (AP) antibody (11093274910; Roche), and then subjected to color reaction.
Luciferase reporter assays.
HEK293 or P19 cells were maintained in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were seeded into 48-well or 10-cm plates, and transient transfections were performed using SuperFect (Qiagen) according to the manufacturer's instructions. Actin-β-galactosidase plasmid was cotransfected for normalization of the luciferase assay. Ten nanomolar Dex was used for all reporter assays.
Generation of hypothalamic cell line stably expressing si-Bsx.
We adopted pSilencer 4.1-CMV puro vector (AM5775M; Ambion) to stably express either control short interfering RNA (siRNA) or siRNA against Bsx (si-Bsx) in the hypothalamic cell line. The mouse Bsx target sequence was AAT CTC AAC TTC ACT TCC CCT. We transfected N42 immortalized hypothalamic neurons (Cellutions Biosystems) with control siRNA- and si-Bsx-expressing vectors and selected transformants against puromycin. The selected N42 cells with si-Bsx were subsequently tested for specific downregulation of Bsx relative to the selected N42 cells with control siRNA using both reverse transcription-PCR (RT-PCR) and immunoblotting with anti-Bsx antibody (data not shown).
RESULTS
Specific expression of GR in AgRP neurons.
To test whether Gc-bound GR directly controls the transcription of NPY/AgRP in AgRP neurons, we examined the expression pattern of GR in the hypothalamus using immunohistochemical analysis. As reported previously (14), prominent GR-immunostaining signal was detected in the paraventricular nucleus (PVN) (see Fig. S8C in the supplemental material). GR was also enriched in the ARC (Fig. 1A; also see Fig. S1A in the supplemental material). Double-immunostaining analyses with Bsx, a marker for AgRP neurons (12, 13), revealed that GR is strongly coexpressed with Bsx in AgRP neurons (Fig. 1A; also see Fig. S1A). Using transgenic mice expressing GFP in POMC neurons (15), we also found that GFP+ POMC neurons displayed little to no GR expression (see Fig. S1B). Our results show a relatively specific and strong expression of GR in AgRP neurons, supporting the possible transcriptional regulation of NPY/AgRP genes by GR.
Fig 1.
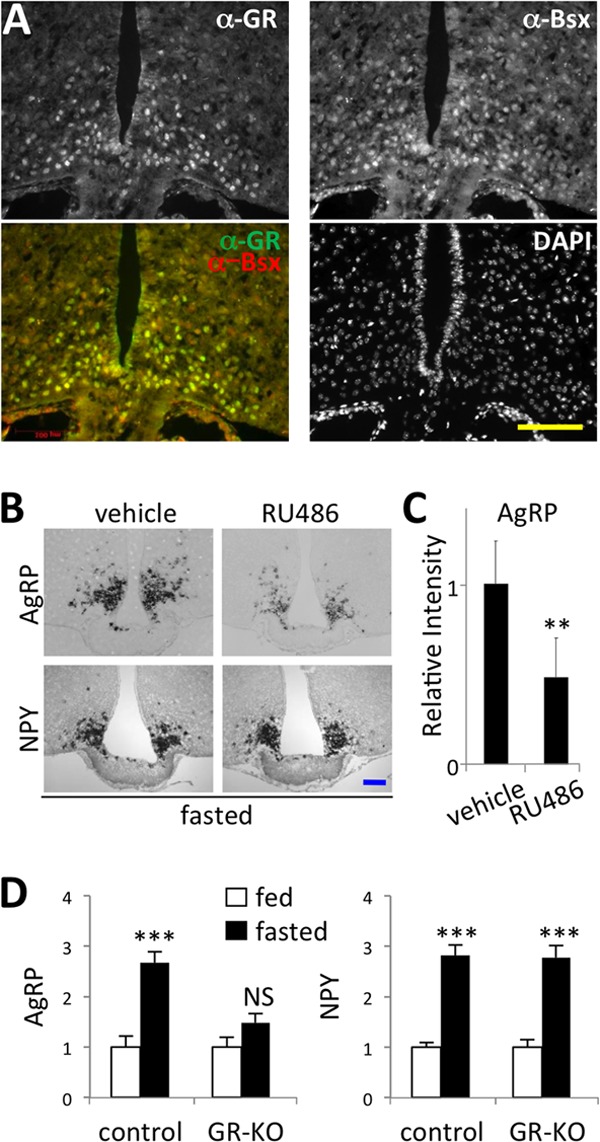
In vivo evidence for the involvement of GR in regulating expression of AgRP. (A) The coronal sections of the ARC region of mice fasted for 24 h were immunostained with antibodies against GR and Bsx. DAPI staining is included as a control for all of the nuclei in the section. The yellow scale bar indicates 100 μm. (B) In situ hybridizations for AgRP and NPY were carried out with serial coronal 12-μm sections of the ARC region of mice (n = 3 in each group) intraperitoneally injected with either vehicle or 50 mg/kg of RU486. Representative images are shown. (C) For all the images shown in panel B, the in situ signal intensity of AgRP was quantified using ImageJ and plotted against the intensity of samples injected with vehicle alone. (D) The in situ signal intensity of AgRP and NPY of serial coronal 12-μm sections of the ARC region of control (n = 3) and GRf/f:AgRP-Cre (GR-KO) mice (n = 5) was quantified using ImageJ and plotted against the intensity of fed control samples. P values of less than 0.001 and 0.0001 are denoted ** and ***, respectively (C and D).
GR is required for upregulation of AgRP by fasting.
To test whether the function of GR is necessary for inducing NPY/AgRP expression upon fasting, we employed RU486, an antagonist of GR and progesterone receptor (PR) (16). Intraperitoneal or intracerebroventricular injection of RU486 markedly suppressed fasting-mediated induction of AgRP levels compared to the injection of vehicle alone (Fig. 1B and C; also see Fig. S2 in the supplemental material). However, RU486 had no effect on NPY expression (Fig. 1B; also see Fig. S2). These results indicate that the effect of GR/PR antagonism is relatively specific with the AgRP gene, and that, based on intact NPY expression, AgRP neurons are expected to remain unmarred after acute treatment of RU486. Notably, progesterone has been shown to have no effect on expression of AgRP in hypothalamic explants (17). We also found that progesterone has no effect on the promoter activity of AgRP (see Fig. S3A in the supplemental material). These results suggest that RU486 interfered with the action of GR, not PR, in inducing AgRP expression. To further test the involvement of GR in AgRP expression, we generated mice in which GR is removed specifically from AgRP neurons by mating GRflox/flox (GRf/f) mice (18) to a knock-in line expressing Cre recombinase in AgRP neurons (19). The AgRP neuron-specific removal of GR greatly blunted the fasting-dependent induction of AgRP expression in the hypothalamus (Fig. 1D). In contrast, NPY was still robustly induced upon fasting in the GR mutant mice (Fig. 1D). These results suggest that AgRP, but not NPY, is a direct target gene of GR.
Identification of AgRP-GRE.
The AgRP promoter has the binding sites of transcription factors that respond to peripheral signals, such as leptin and insulin (Fig. 2A). FoxO1, whose activity is blocked by the insulin-phosphatidylinositol 3-kinase (PI3K)-Akt pathway, binds to the AgRP promoter and upregulates AgRP levels, whereas Stat3, an effector of leptin signaling, blunts AgRP expression by inhibiting the action of FoxO1 (20–22). In addition, the 1-kb AgRP promoter has the binding sites for Bsx, a positive regulator of AgRP (12). To examine the Gc responsiveness of the AgRP promoter, we tested whether a synthetic Gc, dexamethasone (Dex), is capable of activating the AgRP promoter using luciferase reporter assays. Dex strongly activated a luciferase reporter driven by the 1-kb AgRP promoter fragment containing two Bsx, two FoxO1, and two Stat3 response elements (Fig. 2A and B). It is possible that GR indirectly controls the activity of the 1-kb AgRP promoter by associating with Bsx, FoxO1, and/or Stat3. Alternatively, GR could directly regulate the AgRP promoter by binding to GRE. To distinguish between these possibilities, we first mutated each binding site for Bsx, FoxO1, and Stat3 in the AgRP-luciferase reporter and monitored the reporter activation by Dex. This analysis revealed that the binding sites for Bsx, FoxO1, and Stat3 are dispensable for the Dex response of the AgRP promoter (see Fig. S3A in the supplemental material). To test whether recognition of GRE by GR is important for Dex-dependent activation of the AgRP promoter, we used a human GR mutant with a point mutation in the DNA binding domain (C438A) and found that this mutant GR failed to direct Dex-dependent activation of AgRP-1kb:LUC (see Fig. S3A). These results suggest that GR is recruited to the AgRP promoter via GRE rather than the binding sites for other transcription factors. Subsequent deletion analyses indeed mapped an evolutionarily conserved GRE-like motif, named AgRP-GRE, which exhibits a few nucleotide sequence variations from the consensus GRE (Fig. 2A; also see Fig. S3A). Deletion of AgRP-GRE or mutation of either half site of AgRP-GRE completely abolished the Dex response (Fig. 2C; also see Fig. S3A). Moreover, two copies of 27-mer sequence containing AgRP-GRE alone were sufficient to fully reproduce the Dex response of the luciferase reporters driven by longer promoters of AgRP (Fig. 2D). AgRP-GRE also showed specific binding to GR in gel mobility shift assays (see Fig. S4A in the supplemental material). These results demonstrate that AgRP-GRE serves as a direct binding site for GR, and it is necessary and sufficient in mediating Dex-dependent activation of the AgRP promoter.
Fig 2.
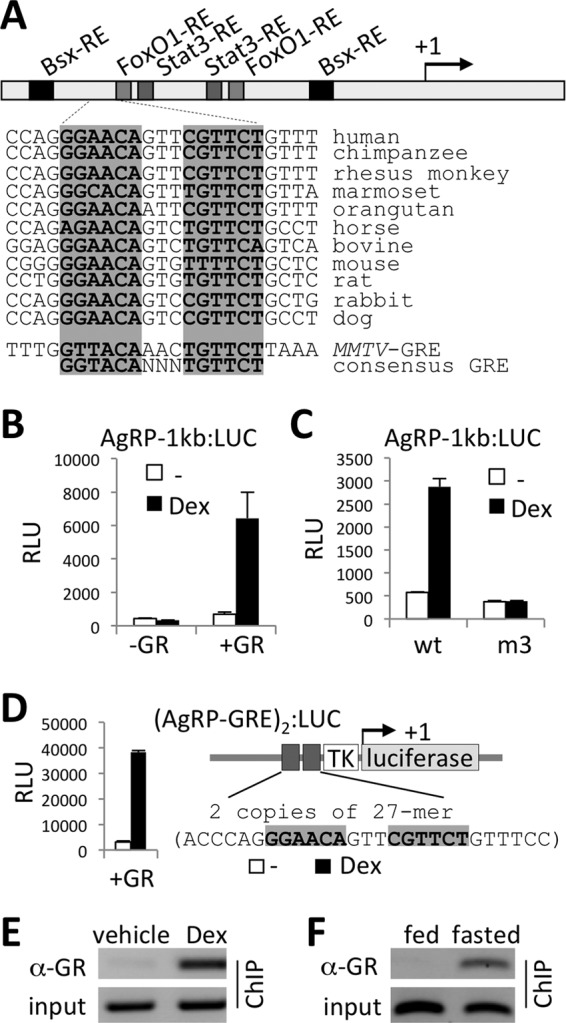
Identification of AgRP-GRE. (A) Schematic representation of the AgRP promoter. AgRP-GRE is highly conserved in mammals, as indicated. The MMTV-GRE and the consensus GRE sequences are shown for comparison. (B and C) Luciferase reporter assays with AgRP-1kb:LUC reporter (B) and its derivative with a mutation in the first half site of the AgRP-GRE (m3) (C) in HEK293 cells. (D) Luciferase reporter assays with the (AgRP-GRE)2:LUC reporter in HEK293 cells. The 27-mer sequences, as well as a schematic representation of the reporter, are shown. (E and F) ChIP for GR binding in the hypothalamus lysates of mice intraperitoneally injected with either vehicle or 10 mg/kg of Dex (E) or in the hypothalamus lysates of mice either fed or fasted for 24 h (F).
We next tested whether Dex also regulates the promoter of NPY, encoding NPY, another orexigenic neuropeptide produced in AgRP neurons. The NPY promoter has been shown to have binding sites for Bsx, FoxO1, and Stat3 (12, 20–22), as well as a GRE-like motif (23) (see Fig. S3B in the supplemental material). Dex exhibited no effect on a luciferase reporter driven by the 1-kb promoter region of NPY, which includes the putative GRE-like motif (23) (see Fig. S3B). These results, along with the minimal effect of RU486 or AgRP neuron-specific GR deletion on fasting-mediated NPY induction (Fig. 1B and D), suggest that NPY is not a direct target of GR.
Recruitment of GR to AgRP-GRE in the hypothalamus.
To further test whether peripheral Gc signal controls AgRP expression via triggering GR binding to AgRP-GRE in vivo, we performed chromatin immunoprecipitation (ChIP) assays in the hypothalamus of adult mice and monitored the recruitment of GR to the AgRP promoter. The 235-bp PCR products from our primer set encompass not only the AgRP-GRE but also the neighboring Bsx/FoxO1/Stat3 sites (see Fig. S3A in the supplemental material). Interestingly, intraperitoneal injection of Dex, but not vehicle, induced a strong GR binding to the AgRP promoter in the hypothalamus (Fig. 2E). Likewise, fasting, which increases plasma levels of Gc and activates expression of NPY/AgRP in AgRP neurons (1–4), greatly enhanced GR binding to the AgRP promoter in the hypothalamus, as shown by ChIP analyses of the hypothalamic lysates of mice fasted for 24 h or fed chow diet (Fig. 2F). These results indicate that higher levels of circulating Gc (achieved by Dex injection or fasting) triggers direct recruitment of GR to AgRP-GRE in AgRP neurons, likely contributing to a marked upregulation of AgRP upon fasting.
Gc permits Bsx to synergize with GR in activating AgRP expression.
The proximal location of the AgRP-GRE to the upstream Bsx binding site in AgRP and a common orexigenic function of Gc and Bsx (12) led us to investigate possible cross talk between Bsx and GR in upregulating AgRP expression in response to peripheral Gc signal. Although Bsx readily bound to Bsx sites in the AgRP promoter, as shown by gel mobility shift assays (see Fig. S4C in the supplemental material), it had minimal effect on activating the 1-kb AgRP promoter containing Bsx sites (Fig. 3A). Intriguingly, however, Bsx strongly synergized with GR in a Dex-dependent manner to activate the AgRP promoter (Fig. 3A). These results suggest that binding of Bsx to Bsx sites alone is insufficient to activate expression of AgRP, and that Gc plays a permissive role for Bsx to activate expression of AgRP in synergy with GR.
Fig 3.
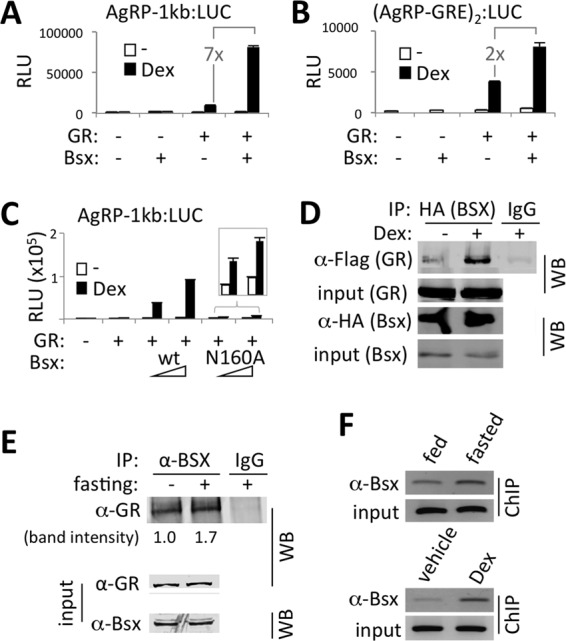
Synergy of GR and Bsx in activating expression of AgRP. (A to C) Luciferase reporter assays with AgRP-1kb:LUC reporter (A and C) and (AgRP-GRE)2:LUC reporter (B) in HEK293 cells transfected with expression vectors as indicated. Bsx-N160A is a DNA binding-defective mutant form of Bsx (C). (D) Anti-HA antibody-coimmunoprecipitated HA-tagged Bsx and Flag-tagged GR from HEK293 cells transfected with expression vectors for HA-Bsx and Flag-GR. Immunoprecipitation with IgG was carried out as a negative control. IP, immunoprecipitation; WB, Western blotting. (E) Coimmunoprecipitation of endogenous GR and Bsx in the hypothalamus lysates of mice either fed or fasted for 24 h. Association of GR and Bsx observed in fed mice was further enhanced in fasted mice by approximately 1.7-fold. (F) ChIP for Bsx binding in the hypothalamus lysates of mice either fed or fasted for 24 h (top) or of mice intraperitoneally injected with either vehicle or 10 mg/kg of Dex (lower).
To test whether the synergy between Bsx and Dex requires Bsx binding sites in the AgRP promoter, we utilized the (AgRP-GRE)2:LUC reporter, which has AgRP-GRE but no Bsx binding sites. The stimulatory effect of Bsx on GR transactivation was significantly weakened in this reporter, although the Dex-dependent synergy between GR and Bsx was still observed; i.e., ∼7-fold enhancement over GR alone with AgRP-1kb:LUC versus ∼2-fold enhancement with (AgRP-GRE)2:LUC (Fig. 3A and B). Consistent with this finding, a DNA binding-defective mutant Bsx, which failed to bind to Bsx sites in the AgRP promoter region (see Fig. S4C in the supplemental material), was much less potent in synergizing with GR than wild-type Bsx (Fig. 3C). These results suggest that AgRP-GRE alone is capable of launching a synergistic activation of AgRP promoter by GR and Bsx and that the Dex-dependent synergy between GR and Bsx becomes much more robust when both GR and Bsx bound to GRE and Bsx binding sites, respectively, in the AgRP promoter. Our results also establish that the AgRP promoter functions as a central sensor for the fasting-elevated peripheral Gc levels in inducing AgRP expression.
Gc-activated GR facilitates Bsx recruitment to the AgRP promoter in the ARC.
To understand the molecular basis underlying Dex-dependent transcriptional synergy between GR and Bsx on the AgRP promoter, we considered the possibility that Dex promotes the association between GR and Bsx and controls the recruitment of the GR/Bsx complex to the AgRP promoter. Interestingly, Bsx was coimmunoprecipitated with GR in a Dex-stimulated manner in HEK293T cells transfected with Flag-tagged GR and HA-tagged Bsx (Fig. 3D), indicating that Gc signal facilitates the association between GR and Bsx in cells. Importantly, we also observed coimmunoprecipitation of endogenous GR and Bsx in the mouse hypothalamus, which is further enhanced upon fasting (Fig. 3E).
To investigate whether Bsx recruitment to the AgRP promoter is enhanced by peripheral signals that activate GR in the AgRP neurons, such as fasting or Dex injection, in vivo, we performed ChIP experiments with the mouse hypothalamic lysates and the primer set used for the GR recruitment study described above (Fig. 2E and F). Fasting substantially enhanced the recruitment of Bsx to the AgRP promoter in the hypothalamus (Fig. 3F, upper). Given that fasting increases Bsx levels in the ARC (12, 13), the enhanced binding of Bsx upon fasting could be due simply to the increased protein levels of Bsx. Notably, while we observed an increase in Bsx levels in the hypothalamic ARC region upon fasting in our immunostaining results (see Fig. S5 in the supplemental material), we failed to see a significant difference in our immunoblotting results with the whole hypothalamic lysates (Fig. 3E). This is likely due to the fact that fasting affects Bsx levels only in the ARC (i.e., AgRP neurons) but not in other hypothalamic neurons expressing Bsx (13). Importantly, Bsx binding to the AgRP promoter was also enhanced only 2 h after intraperitoneal injection of Dex (Fig. 3F, lower). We also found that Bsx levels in mice with AgRP neuron-specific deletion of GR were similar to those in control mice (data not shown), suggesting that Dex injection, unlike fasting (13), would not induce the expression of Bsx in AgRP neurons. Overall, these results suggest that the peripheral Gc signal facilitates Bsx recruitment to the AgRP promoter.
Together, our results suggest that the gain of protein-protein interactions between Bsx- and Gc-bound GR contribute to the fasting-enhanced binding of Bsx to the AgRP promoter. Furthermore, our results support a model in which the peripheral Gc signal triggers an assembly of a transcriptionally active complex composed of Bsx and GR on the AgRP promoter, which leads to a robust induction of AgRP.
Opposite action of Bsx on AgRP-GRE and conventional GREs.
To test whether Gc-dependent synergy between Bsx and GR is a universal feature of all GR target genes, we monitored the effect of Bsx on multiple GRE-dependent reporters. Surprisingly, Bsx blunted Dex transactivation of a reporter driven by the well-characterized classical GREs defined in mouse mammary tumor virus (MMTV:LUC) (8) (Fig. 4A) as well as in other known GR target genes (see Fig. S6 in the supplemental material). These results point to the intriguing possibility that Bsx functions as a binding site-dependent dual regulator of Gc/GR target genes within the AgRP neurons; i.e., as an activator of the AgRP-GRE-like motif and, at the same time, an inhibitor of other GREs. This would allow Bsx to effectively limit the spectrum of activated GR target genes in AgRP neurons upon arrival of peripheral Gc signal by strongly activating a group of orexigenic genes with AgRP-GRE-like motifs while inhibiting unwanted GR target genes with conventional GREs.
Fig 4.
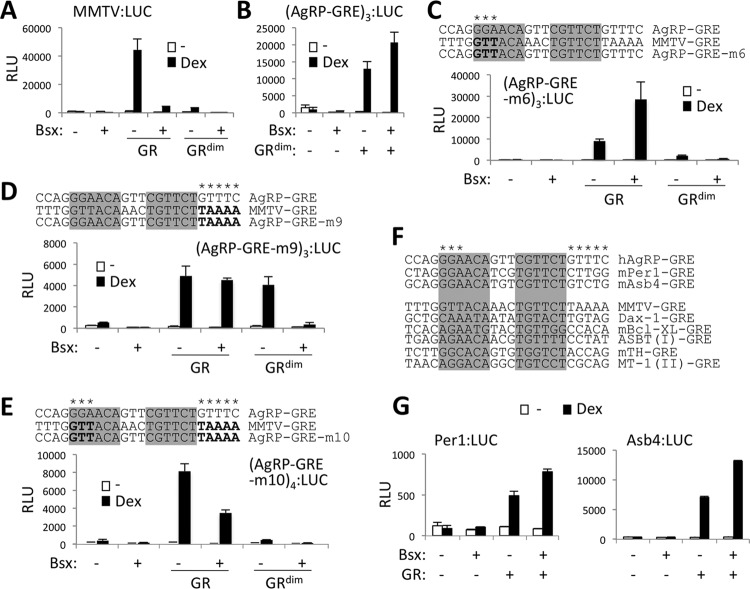
AgRP-GRE as a prototypic tool to find additional targets of GR and Bsx. (A to E and G) Luciferase reporter assays with MMTV:LUC (A), (AgRP-GRE)3:LUC (B), (AgRP-GRE-m6)3:LUC (C), (AgRP-GRE-m9)3:LUC (D), and (AgRP-GRE-m10)4:LUC (E) in HEK293 cells and Per1:LUC and Asb4:LUC (G) reporters in P19 cells transfected with the indicated expression vectors. Sequences of each hybrid GRE are shown (C to E). (F) Sequences of AgRP-GRE, Per1-GRE, and Asb4-GRE as well as of MMTV-GRE and other known GREs that are negatively regulated by Bsx are shown. The unique sequences in AgRP-GRE and AgRP-GRE-like motifs are indicated with an asterisk.
In our effort to understand the molecular mechanisms by which Bsx evokes the opposite effects on Dex-dependent GR transactivation in AgRP-GRE and conventional GREs, we employed a dimerization-defective mutant GR (GR-A458T; named GRdim) (24). As expected, GRdim was inefficient in transactivating conventional GREs, such as MMTV-GRE (Fig. 4A). Surprisingly, it fully supported GR transactivation of AgRP-GRE; furthermore, it synergized with Bsx (Fig. 4B). These results prompted us to ask whether the dual function of Bsx is dictated by the sequences in GREs and their flanking regions. A unique sequence feature in AgRP-GRE that is not shared by conventional GREs may be responsible for the unexpected responsiveness of AgRP-GRE to GRdim as well as for the synergistic activation of AgRP-GRE by Bsx and GR. To identify the critical sequences in AgRP-GRE, we generated a series of five hybrid constructs in which some sequences of AgRP-GRE were swapped for those of MMTV-GRE (see Fig. S3C in the supplemental material), and we tested their responsiveness to GRdim and Bsx. AgRP-GRE-m6, in which the well-conserved GGA residues in the first half site of AgRP-GRE were replaced by the sequences in the comparable positions in MMTV-GRE, was not activated by GRdim, like MMTV-GRE, while this mutant behaved like AgRP-GRE against wild-type GR (Fig. 4C). In AgRP-GRE-m9, in which the sequences downstream of the second half site were changed to those in MMTV-GRE, Bsx did not synergize with GR; moreover, it suppressed transactivation by GRdim (Fig. 4D). To further test the roles of GGA and GTTTC in AgRP-GRE, we created a new mutant (m10) in which both motifs were replaced by the sequences in the comparable positions in MMTV-GRE (Fig. 4E). This mutant showed the properties of both m6 and m9 mutants; i.e., m10 was similar to m6 for the diminished ability to respond to GRdim, while m10 was not able to support the synergy of GR and Bsx, like m9 (Fig. 4E). Moreover, like MMTV-GRE, transactivation of m10 by wild-type GR was suppressed by Bsx (Fig. 4E). Overall, m10 behaved like MMTV-GRE. Together, our mutational analyses revealed that the sequences in the first half site and the region flanking the second half site in AgRP-GRE play critical roles for the two unique properties of AgRP-GRE (i.e., the synergy with Bsx and the full responsiveness to GRdim).
Identification of AgRP-GRE-like GREs.
To identify additional common target genes of GR and Bsx in AgRP neurons, we searched for genomic GREs containing sequences similar to the first half site (GGA) and the flanking region of the second half site (GTTTC) of AgRP-GRE. Our initial bioinformatics analysis identified approximately 20 AgRP-GRE-like sequences that are conserved in both human and mouse and located within 10 kb of gene-coding regions, including the previously reported GRE in Per1 (25, 26) and a new AgRP-GRE-like motif that we identified in Asb4 (Fig. 4F). Notably, Per1 has been demonstrated to be expressed in the ARC in a manner commensurate with Gc levels (27–30), and Asb4 has been found to be induced in AgRP neurons by fasting (31).
To test whether Per1 and Asb4 are common targets of GR and Bsx that are regulated via AgRP-GRE-like motifs, we made luciferase reporters driven by genomic regions encompassing their GRE motifs. Both reporters were synergistically activated by Dex and Bsx (Fig. 4G). These results suggest that AgRP-GRE-like motifs direct upregulation of Per1 and Asb4 in the ARC and also support the validity of our approach to use AgRP-GRE as a prototypic GRE to further identify common targets of GR and Bsx in AgRP neurons.
Bsx as an in vivo target selector of GR and Gc-triggered assembly of active chromatin on the AgRP promoter.
To test whether Bsx acts as a target selector for GR-regulated genes in vivo, we established N42 immortalized hypothalamic neurons (Cellutions Biosystems) that stably express either control siRNA or siRNA against Bsx (si-Bsx). In N42 cells expressing control siRNA, AgRP expression was induced in a Dex-dependent manner, but Dex failed to induce AgRP expression in N42 cells expressing si-Bsx (Fig. 5A). In contrast, Dex-induced expression of Sgk1, whose expression is directed by a conventional GRE (thus, it is negatively regulated by Bsx), was significantly improved in N42 cells expressing si-Bsx relative to N42 cells with control siRNA. These results confirm our luciferase reporter assays and demonstrate that Bsx is indeed an in vivo target selector of GR.
Fig 5.
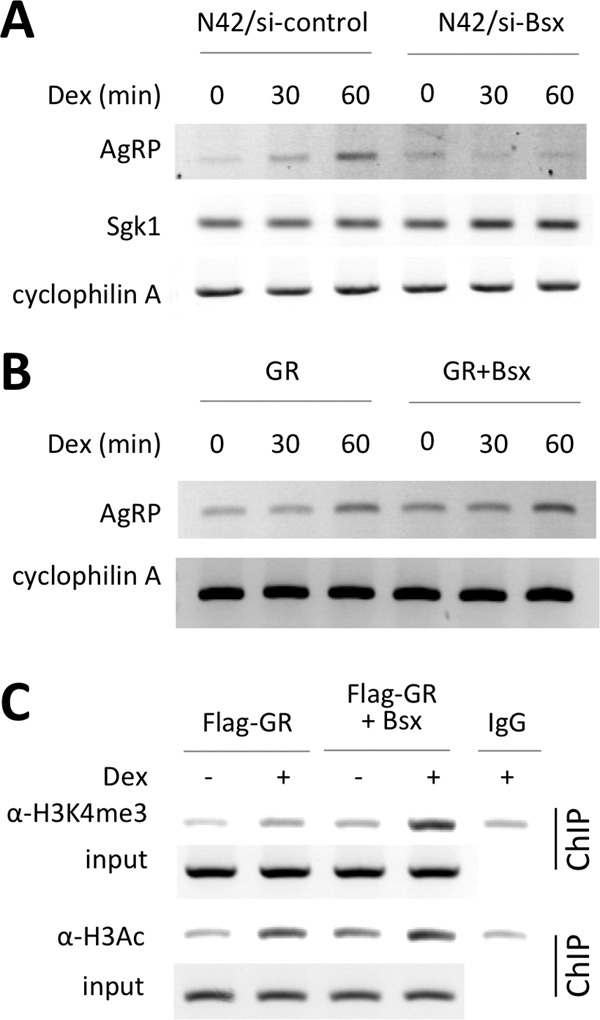
Bsx as an in vivo target selector of GR- and Gc-triggered assembly of active chromatin on the AgRP promoter. (A) Dex-dependent expression of AgRP and Sgk1, which are positively and negatively regulated by Bsx, respectively, were compared between N42 cells expressing either control siRNA or si-Bsx using RT-PCR. (B and C) Dex-dependent expression of AgRP (B) and formation of active chromatin on the AgRP promoter (C) were compared between P19 cells expressing either GR alone or both GR and Bsx using RT-PCR and ChIP, respectively. H3K4me3 and H3Ac indicate two open chromatin marks, trimethylated histone H3 lysine 4 and acetylated H3, respectively (C).
P19 cells expressed a low level of GR but no Bsx (data not shown). P19 cells weakly supported Dex-induced expression of AgRP even after transient expression of additional GR (Fig. 5B). However, Dex-dependent AgRP expression was significantly enhanced in P19 cells upon transient expression of both GR and Bsx (Fig. 5B). Based on these results, we examined our model for an assembly of a transcriptionally active complex composed of Bsx and GR on the AgRP promoter. Consistent with this idea, Dex was able to establish higher levels of two open chromatin marks, trimethylated histone H3 lysine 4 (H3K4me3) and acetylated H3 (H3Ac), in P19 cells expressing both GR and Bsx relative to P19 cells expressing GR alone (Fig. 5C). These results, together with our results shown in Fig. 2 and 3, demonstrate that the peripheral Gc signal recruits Bsx and GR and triggers formation of a transcriptionally active enhanceosome on the AgRP promoter.
AgRP-GRE directs fasting-induced gene expression to AgRP neurons.
Our data strongly suggest that AgRP-GRE can function as a central sensor for the fasting-elevated peripheral Gc levels in triggering transactivation of AgRP in animals. To further test this idea in vivo, we made two enhanced GFP (EGFP) (32) reporters, which are driven by the CMV minimal promoter linked to either the AgRP 1-kb promoter fragment or seven copies of the 27-mer sequence containing AgRP-GRE alone (Fig. 6A; also see Fig. S7A in the supplemental material). The reporter cassettes were flanked by insulators (33). Both reporters were activated weakly by Dex, and this activation was further facilitated by Bsx in a Dex-dependent manner in HEK293 cells (see Fig. S7B). To investigate the in vivo response of the AgRP 1-kb promoter and AgRP-GRE to fasting, we made transgenic mouse lines for both GFP reporters and analyzed the pattern of GFP expression in adult mice with or without 24 h of fasting. The AgRP 1-kb promoter directed GFP expression weakly yet specifically in the ARC but not in other brain areas (Fig. 6B to D; also see Fig. S8 in the supplemental material). Intriguingly, fasting led to a significant upregulation of GFP specifically in the ARC (Fig. 6B). Closer examination of GFP+ cells revealed that GFP is expressed in AgRP neurons expressing Bsx and GR (Fig. 6C and D) but not in POMC neurons (see Fig. S8A). In addition, GFP was expressed neither in the DMH, which expresses Bsx but not GR, nor in the PVN, which expresses GR but not Bsx (see Fig. S8B and C). Remarkably, the transgenic mouse line with the (AgRP-GRE)7:EGFP reporter also exhibited fasting-directed GFP induction in the Bsx+ AgRP neurons, although the overall GFP expression level was lower than that in the transgenic mice with the AgRP 1-kb promoter (see Fig. S7C and D), indicating that AgRP-GRE is capable of mediating fasting-triggered upregulation of the AgRP gene. Our results suggest that (i) AgRP-GRE alone is sufficient to recapitulate the endogenous fasting responsiveness of AgRP expression in AgRP neurons and that (ii) for a robust fasting-dependent induction of AgRP, binding sites for both GR and Bsx, as well as a peripheral Gc signal, are required.
Fig 6.
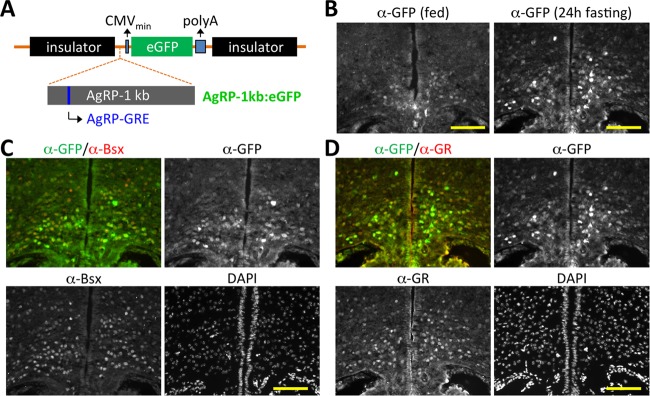
AgRP neuronal expression of GFP directed by 1-kb AgRP promoter in transgenic mice. (A) Schematic representation of an EGFP reporter flanked by two insulators and driven by a minimal CMV promoter fused to the 1-kb AgRP promoter. (B to D) The coronal sections of the ARC region of transgenic mice with the 1-kb AgRP promoter linked to EGFP, either fed (B) or fasted for 24 h (B to D), were immunostained with antibodies against GFP, Bsx, and GR. Yellow scale bars, 100 μm.
DISCUSSION
Gc is a physiologically well-defined peripheral orexigenic cue (1, 2). However, the underlying molecular mechanism for the appetite-stimulating action of Gc has been unclear. In this study, we showed that the gene encoding the orexigenic neuropeptide AgRP is a direct target of GR in AgRP neurons. Furthermore, we found that specific sequences in AgRP-GRE mediate the synergistic induction of the AgRP gene by Bsx and GR in response to Gc signal, which sets AgRP-GRE apart from conventional GREs. The unique feature of AgRP-GRE allowed us to identify other putative common target genes of GR and Bsx in AgRP neurons (Fig. 7A), as exemplified by our discovery of functional AgRP-GRE-like motifs in two genes, Per1 and Asb4, that are expressed in ARC neurons (Fig. 4F and G). Future genome-wide searches for AgRP-GRE-like motifs (e.g., beyond the 10-kb limit that we initially used in this study), coupled with further dissection of critical nucleotides in AgRP-GRE, are expected to uncover additional, key target genes of GR/Bsx in AgRP neurons that play important roles in central Gc actions. Studies of these genes would facilitate our understanding of the orexigenic function of the Gc signal and Bsx in AgRP neurons. For instance, Per1, as a putative target of Gc and Bsx, might link circadian rhythm, which is tightly coupled to food intake (34), to the control of energy balance by AgRP neurons. Asb4, another putative target of Gc and Bsx, could play a role in the cross talk between insulin and Gc signals in the CNS, considering that it mediates degradation of insulin receptor substrate 4 in the hypothalamic AgRP/POMC neurons (35).
Fig 7.
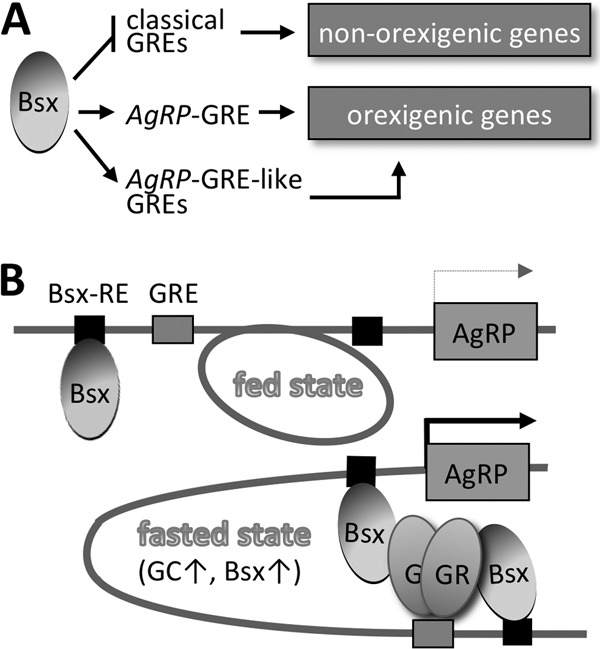
Roles of Bsx in AgRP neurons. (A) Dual function of Bsx segregates GREs into two groups, positive targets, such as AgRP-GRE, and negative targets. The positive targets can be associated with critical orexigenic genes in AgRP neurons. (B) The working model for the synergistic transactivation of the AgRP gene by GR and Bsx. Fasting increases nuclear GR due to increased Gc levels. Both GR and Bsx likely are required to form a transcriptionally active enhanceosome at the AgRP promoter. Protein-protein interactions between GR and Bsx, as well as binding sites for both GR and Bsx, likely play critical roles in the synergy.
GR is known to target distinct sets of genes in different tissues (8). Our results suggest that Bsx determines the scope of GR target genes in AgRP neurons through its GRE sequence-dependent dual function that activates some GREs (e.g., AgRP-GRE, Per1-GRE, and Asb4-GRE) while simultaneously suppressing other GREs (Fig. 7A). For instance, Bsx inhibits the GRE in tyrosine hydroxylase (TH) (Fig. 4F; also see Fig. S6 in the supplemental material), which was recently shown to be downregulated in the ARC (36). Active silencing of TH, a marker of dopaminergic neurons, by Bsx may be important for the function of AgRP neurons, in which synaptic release of GABA is required for normal regulation of energy balance (19). Overall, the dual function of Bsx would pose a distinct advantage in surviving food restriction, which increases levels of both Gc (i.e., increased levels of activated, nuclear GR) and Bsx, by allowing GR to upregulate a specific set of targets while keeping unnecessary GRE-containing genes silent in AgRP neurons (Fig. 7A). In other tissues or cell types, factors similar to Bsx may also act as target selectors by activating only a subset of GR target genes while actively suppressing other targets of GR.
Our results revealed that AgRP-GRE serves as a focal regulatory point that responds to fasting or peripheral Gc signal by dictating a synergy of Gc-bound GR and Bsx (Fig. 7B). First, both Bsx and GR are required for activating expression of AgRP in vivo, as evidenced by our finding that the 1-kb AgRP promoter drives the target gene expression to AgRP neurons, which express both GR and Bsx, but not to other cell types expressing either GR or Bsx alone (Fig. 6; also see Fig. S8 in the supplemental material). Second, Gc-activated GR is a permissive factor for Bsx in upregulating AgRP expression, given that Bsx alone failed to activate the 1-kb AgRP promoter construct containing the Bsx sites in the absence of Dex (Fig. 3A). It is notable that while AgRP-GRE was sufficient to direct the synergy of GR and Bsx in reporter assays, the synergy between GR and Bsx was more robust in the 1-kb AgRP reporter containing AgRP-GRE and Bsx binding sites than in AgRP-GRE alone (Fig. 3A and B). Consistent with this, the DNA binding-defective mutant Bsx was not as efficient as wild-type Bsx in enhancing GR activity in the 1-kb AgRP reporter (Fig. 3C). These results suggest that Bsx binding to its cognitive sites also contributes to a strong activation of AgRP. It is possible that, under physiological fasting conditions, levels of Gc and Bsx are not high enough to enable the synergy through AgRP-GRE alone; thus, both AgRP-GRE and Bsx sites are required for maximal synergy. Third, our results suggest that Gc-activated GR binding to AgRP-GRE is a prerequisite for efficient or stable Bsx binding to AgRP-GRE or an assembly of enhanceosomes consisting of GR and Bsx on AgRP-GRE/Bsx binding sites (Fig. 7B). Although we observed a strong Dex-dependent enhancement in recruitment of both GR and Bsx to the AgRP promoter region with 10 mg/kg of Dex (Fig. 2E and 3F), we observed only a weak increase in GR recruitment and no increase in Bsx recruitment with 2.5 mg/kg of Dex (data not shown). These results suggest that GR binding to AgRP-GRE with sufficient affinity is required for enhanced binding of Bsx. These results, coupled with the inability of Bsx alone to activate the 1-kb AgRP promoter construct containing the Bsx sites (Fig. 3A), provide compelling evidence supporting that AgRP-GRE, together with the Bsx binding sites, senses the rise in Gc levels and Bsx levels upon fasting by triggering formation of an enhanceosome consisting of GR and Bsx, thereby leading to the upregulation of AgRP (Fig. 7B).
Given that Gc induces expression of both NPY and AgRP (4–7), our results that GR directly regulates expression of AgRP, but probably not NPY, were unexpected. Neither RU486 injection nor AgRP neuron-specific deletion of GR blocked fasting-dependent induction of NPY (Fig. 1B to D; also see Fig. S2 in the supplemental material). Dex failed to activate the NPY promoter (see Fig. S3B). It is possible that Gc regulates expression of NPY using a different mechanism that does not involve GR. Alternatively, inhibition of Gc/GR action alone may not be sufficient to shut down fasting-mediated induction of NPY expression due to a redundant pathway that is also activated by fasting. Further investigation is warranted to clarify this issue.
In summary, we found that GR and Bsx synergistically activate expression of AgRP via AgRP-GRE. Moreover, our dissection of AgRP-GRE defined a new class of GREs that dictates a positive action of Bsx. Future identification and characterization of more genes with AgRP-GRE-like motifs would provide critical insights into the molecular understanding of the central orexigenic action of Gc/GR and Bsx in the AgRP neurons.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (DK064678 to J.W.L. and NS054941 to S.K.L.); March of Dimes and Christopher Reeve Foundation (S.K.L.); Research Institute of Pharmaceutical Sciences, Research Settlement Fund for the new faculty of SNU, POSCO TJ Park Science Fellowship, Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology (2012R1A1A1001749), and National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1220120) (S.L.); and the World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (R31-10105) (J.W.L. and K.Y.C.).
We thank Dan Marks and Roger Cone for help with intracerebroventricular injection and reagents and Karen Thiebes for critical reading of the manuscript.
Footnotes
Published ahead of print 13 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00094-13.
REFERENCES
- 1. Fehm HL, Born J, Peters A. 2004. Glucocorticoids and melanocortins in the regulation of body weight in humans. Horm. Metab. Res. 36: 360– 364 [DOI] [PubMed] [Google Scholar]
- 2. Coppola A, Diano S. 2007. Hormonal regulation of the arcuate nucleus melanocortin system. Front. Biosci. 12: 3519– 3530 [DOI] [PubMed] [Google Scholar]
- 3. Jeanrenaud B, Rohner-Jeanrenaud F. 2000. CNS-periphery relationships and body weight homeostasis: influence of the glucocorticoid status. Int. J. Obes. Relat. Metab. Disord. 24(Suppl. 2):S74–S76 [DOI] [PubMed] [Google Scholar]
- 4. Makimura H, Mizuno TM, Isoda F, Beasley J, Silverstein JH, Mobbs CV. 2003. Role of glucocorticoids in mediating effects of fasting and diabetes on hypothalamic gene expression. BMC Physiol. 3: 5. 10.1186/1472-6793-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savontaus E, Conwell IM, Wardlaw SL. 2002. Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res. 958:130– 138 [DOI] [PubMed] [Google Scholar]
- 6. Dhillo WS, Small CJ, Gardiner JV, Bewick GA, Whitworth EJ, Jethwa PH, Seal LJ, Ghatei MA, Hinson JP, Bloom SR. 2003. Agouti-related protein has an inhibitory paracrine role in the rat adrenal gland. Biochem. Biophys. Res. Commun. 301: 102– 107 [DOI] [PubMed] [Google Scholar]
- 7. Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, Ozaki N, Nagasaki H, Oiso Y. 2008. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphate-activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology 149: 4544– 4553 [DOI] [PubMed] [Google Scholar]
- 8. Stahn C, Löwenberg M, Hommes DW, Buttgereit F. 2007. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol. Cell. Endocrinol. 275: 71– 78 [DOI] [PubMed] [Google Scholar]
- 9. Adcock IM, Caramori G. 2001. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 79: 376– 384 [DOI] [PubMed] [Google Scholar]
- 10. Cremona M, Colombo E, Andreazzoli M, Cossu G, Broccoli V. 2004. Bsx, an evolutionary conserved brain specific homeobox gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus. Gene Expr. Patterns 4: 47– 51 [DOI] [PubMed] [Google Scholar]
- 11. McArthur T, Ohtoshi A. 2007. A brain-specific homeobox gene, Bsx, is essential for proper postnatal growth and nursing. Mol. Cell. Biol. 27: 5120– 5127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sakkou M, Wiedmer P, Anlag K, Hamm A, Seuntjens E, Ettwiller L, Tschöp MH, Treier M. 2007. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 5: 450– 463 [DOI] [PubMed] [Google Scholar]
- 13. Nogueiras R, López M, Lage R, Perez-Tilve D, Pfluger P, Mendieta-Zerón H, Sakkou M, Wiedmer P, Benoit SC, Datta R, Dong JZ, Culler M, Sleeman M, Vidal-Puig A, Horvath T, Treier M, Diéguez C, Tschöp MH. 2008. Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology 149: 3009– 3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ceccatelli S, Cintra A, Hökfelt T, Fuxe K, Wikström AC, Gustafsson JA. 1989. Coexistence of glucocorticoid receptor-like immunoreactivity with neuropeptides in the hypothalamic paraventricular nucleus. Exp. Brain Res. 78: 33– 42 [DOI] [PubMed] [Google Scholar]
- 15. Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. 2001. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 25(Suppl. 5):S63–S67 [DOI] [PubMed] [Google Scholar]
- 16. Baulieu EE. 1988. RU486 (an anti-steroid hormone) receptor structure and heat shock protein mol. wt 90,000 (hsp 90). Hum. Reprod. 3: 541– 547 [DOI] [PubMed] [Google Scholar]
- 17. Brewer JA. 2003. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat. Med. 9: 1318– 1322 [DOI] [PubMed] [Google Scholar]
- 18. Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. 1999. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23: 99– 103 [DOI] [PubMed] [Google Scholar]
- 19. Tong Q, Ye C-P, Jones JE, Elmquist JK, Lowell BB. 2008. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11: 998– 1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. 2006. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med. 12: 534– 540 [DOI] [PubMed] [Google Scholar]
- 21. Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. 2006. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat. Neurosci. 9: 901– 906 [DOI] [PubMed] [Google Scholar]
- 22. Muraoka O, Xu B, Tsurumaki T, Akira S, Yamaguchi T, Higuchi H. 2003. Leptin-induced transactivation of NPY gene promoter mediated by JAK1, JAK2 and STAT3 in the neural cell lines. Neurochem. Int. 42: 591– 601 [DOI] [PubMed] [Google Scholar]
- 23. Zhang K, Rao F, Pablo Miramontes-Gonzalez J, Hightower CM, Vaught B, Chen Y, Greenwood TA, Schork AJ, Wang L, Mahata M, Stridsberg M, Khandrika S, Biswas N, Fung MM, Waalen J, Middelberg RP, Heath AC, Montgomery GW, Martin NG, Whitfield JB, Baker DG, Schork NJ, Nievergelt CM, O'Connor DT. 2012. Neuropeptide Y (NPY): genetic variation in the human promoter alters glucocorticoid signaling, yielding increased NPY secretion and stress responses. J. Am. Coll. Cardiol. 60: 1678– 1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G. 1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93: 531– 541 [DOI] [PubMed] [Google Scholar]
- 25. So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. 2009. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc. Natl. Acad. Sci. U. S. A. 106: 17582– 17587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. 2005. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J. Biol. Chem. 280: 42036– 42043 [DOI] [PubMed] [Google Scholar]
- 27. Matsui D, Takekida S, Okamura H. 2005. Molecular oscillation of Per1 and Per2 genes in the rodent brain: an in situ hybridization and molecular biological study. Kobe J. Med. Sci. 51: 85– 93 [PubMed] [Google Scholar]
- 28. Feillet CA, Mendoza J, Albrecht U, Pévet P, Challet E. 2008. Forebrain oscillators ticking with different clock hands. Mol. Cell Neurosci. 37: 209– 221 [DOI] [PubMed] [Google Scholar]
- 29. Minana-Solis MC, Angeles-Castellanos M, Feillet C, Pevet P, Challet E, Escobar C. 2009. Differential effects of a restricted feeding schedule on clock-gene expression in the hypothalamus of the rat. Chronobiol. Int. 26: 808– 820 [DOI] [PubMed] [Google Scholar]
- 30. Shieh KR, Yang SC, Lu XY, Akil H, Watson SJ. 2005. Diurnal rhythmic expression of the rhythm-related genes, rPeriod1, rPeriod2, and rClock, in the rat brain. J. Biomed. Sci. 12: 209– 217 [DOI] [PubMed] [Google Scholar]
- 31. Li JY, Kuick R, Thompson RC, Misek DE, Lai YM, Liu YQ, Chai BX, Hanash SM, Gantz I. 2005. Arcuate nucleus transcriptome profiling identifies ankyrin repeat and suppressor of cytokine signalling box-containing protein 4 as a gene regulated by fasting in central nervous system feeding circuits. J. Neuroendocrinol. 17: 394– 404 [DOI] [PubMed] [Google Scholar]
- 32. Hechler D, Nitsch R, Hendrix S. 2006. Green-fluorescent-protein-expressing mice as models for the study of axonal growth and regeneration in vitro. Brain Res. Rev. 52: 160– 169 [DOI] [PubMed] [Google Scholar]
- 33. Reddi PP, Urekar CJ, Abhyankar MM, Ranpura SA. 2007. Role of an insulator in testis-specific gene transcription. Ann. N. Y. Acad. Sci. 1120: 95– 103 [DOI] [PubMed] [Google Scholar]
- 34. Sahar S, Sassone-Corsi P. 2012. Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol. Metab. 23: 1– 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li JY, Chai B, Zhang W, Wu X, Zhang C, Fritze D, Xia Z, Patterson C, Mulholland MW. 2011. Ankyrin repeat and SOCS box containing protein 4 (Asb-4) colocalizes with insulin receptor substrate 4 (IRS4) in the hypothalamic neurons and mediates IRS4 degradation. BMC Neurosci. 12: 95. 10.1186/1471-2202-12-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jovanovic Z, Tung YC, Lam BY, O'Rahilly S, Yeo GS. 2010. Identification of the global transcriptomic response of the hypothalamic arcuate nucleus to fasting and leptin. J. Neuroendocrinol. 22:915– 925 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.