Abstract
The perinatal environment plays an important role in programming many aspects of physiology and behavior including metabolism, body weight set point, energy balance regulation and predisposition to mental health-related disorders such as anxiety, depression and attention deficit hyperactivity disorder. Maternal health and nutritional status heavily influence the early environment and have a long-term impact on critical central pathways, including the melanocortinergic, serotonergic system and dopaminergic systems. Evidence from a variety of animal models including rodents and nonhuman primates indicates that exposure to maternal high-fat diet (HFD) consumption programs offspring for increased risk of adult obesity. Hyperphagia and increased preference for fatty and sugary foods are implicated as mechanisms for the increased obesity risk. The effects of maternal HFD consumption on energy expenditure are unclear, and future studies need to address the impact of perinatal HFD exposure on this important component of energy balance regulation. Recent evidence from animal models also indicates that maternal HFD consumption increases the risk of offspring developing mental health-related disorders such as anxiety. Potential mechanisms for perinatal HFD programming of neural pathways include circulating factors, such as hormones (leptin, insulin), nutrients (fatty acids, triglycerides and glucose) and inflammatory cytokines. As maternal HFD consumption and obesity are common and rapidly increasing, we speculate that future generations will be at increased risk for both metabolic and mental health disorders. Thus, it is critical that future studies identify therapeutic strategies that are effective at preventing maternal HFD-induced malprogramming.
Key Words: Metabolic imprinting, Maternal high-fat diet, Energy balance, Energy expenditure, Obesity, Anxiety, Inflammation
Introduction to Metabolic Imprinting
Only a minority of individuals living in the obesigenic environment currently encountered in developed nations are able to maintain a healthy body weight [1]. For example, in the USA, evidence from the latest National Health and Nutrition Examination Survey (collected in 2007–2008) indicates that 68% of adults are overweight or obese [1]. Given the health risks associated with obesity (heart disease, diabetes, hypertension, cancer) [2], this has important implications for global health, and in light of the additional health-care costs associated with obesity [3], the global economy. Genome-wide association studies have identified numerous loci associated with obesity; however, their contribution to variation in body mass index and weight between individuals is estimated to be less than 2% [4], suggesting that environmental influences, such as increased availability of energy-dense food and an increasingly sedentary lifestyle, play a crucial role. Accumulating evidence from epidemiologic studies and animal models indicate that maternal health and nutritional state during pregnancy and lactation play a critical role in programming the neural circuitry that regulates energy balance and behavior in offspring, having a sustained influence on their physiology and behavior.
Metabolic imprinting refers to the programming of an offspring's future metabolic responses by a stimulus occurring during a critical developmental period. As the type and amount of available nutrition are crucial determinants of survival and reproductive success, the evolutionary purpose of metabolic imprinting is to enhance offspring survival by programming energy balance regulation so that available metabolic fuels are most efficiently utilized. Mammals are exposed to two environments during development, the intrauterine and early postnatal environments, both of which are heavily impacted by maternal diet and adiposity. Perinatal nutrition has enduring effects on many aspects of physiology and behavior including the regulation of energy balance, susceptibility to metabolic disorders [5,6], programming of body weight set point [7,8], stress response [9,10] and mental health-related behaviors [11,12]. In addition to perinatal nutrition, a number of factors associated with maternal consumption of a high-fat diet (HFD), including maternal adiposity, hyperlipidemia, lipotoxicity, glucose levels and insulin resistance, also have a long-term impact on the developing offspring and are associated with increased risk of obesity, metabolic disorders, and mental health disorders [5,11,12]. As maternal obesity is associated with consumption of a HFD in humans and a HFD is used to promote maternal obesity in most animal models, a major challenge faced by the field is the ability to disassociate the effects of the HFD from the maternal metabolic phenotype.
Maternal Obesity Is Associated with Increased Risk of Offspring Obesity
The most common perturbation of maternal nutrition is nutritional excess and maternal obesity. In 2008, over 64% of women of child-bearing age in the USA were overweight or obese [13] and the majority consume an excess amount of calories and fat [14]. Epidemiological studies clearly indicate that maternal obesity is associated with increased incidence of obesity and metabolic syndrome in children [15]. Although this review focuses on the impact of maternal HFD consumption, it is important to note that maternal undernutrition during gestation also increases the incidence of offspring obesity; thus, the relationship between birth weight and adult adiposity is thought of as a ‘U’-shaped curve with both a very high and a very low birth weight increasing the risk of obesity in adulthood [16,17]. The relationship between maternal obesity and the development of offspring obesity appears to be independent of gestational diabetes as women who are obese, but able to maintain normal glycemia, also have heavier offspring with increased adiposity [18].
Although epidemiological studies implicate the intrauterine environment, including maternal diet and energy status, in programming offspring obesity, in these studies it is not possible to directly link maternal diet and energy status with the offspring's metabolic profile, as several other factors could contribute to the association between maternal and offspring obesity, including genetics and shared environment factors such as access to energy-dense foods and a sedentary lifestyle. Also, there is relatively limited information on normal brain development in humans, making it challenging to examine the impact of maternal diet on offspring's brain development. Furthermore, it is difficult to accurately monitor and potentially unethical to manipulate the diet of pregnant women. Thus, it is critical to use animal models to directly examine the effects of maternal overnutrition on subsequent generations and to develop effective therapeutic intervention strategies.
Animal Models of Maternal HFD Consumption/Overnutrition
Providing animals with a HFD during pregnancy and lactation is a common method of inducing maternal obesity. However, the duration of exposure to the diet (acute vs. chronic), the percent of calories from fat, and diet composition are variable across studies, hindering comparisons. The diets commonly used to promote maternal obesity are either a purified HFD with fat in place of carbohydrates as an energy source or a cafeteria diet in which animals are provided with a selection of palatable food items that have a high fat content along with their regular diet. The cafeteria diet is most effective in promoting obesity; however, as several food items are provided, it is difficult to calculate the amount and composition of the diet consumed by each animal and the diet consumed by animals in the same group is variable. Several studies have used purified diets with different sources of fat to compare the impact of maternal consumption of saturated fat [19,20,21,22], polyunsaturated fat [20,23] ω−3 [9,21,22] or ω−6 fatty acids [9,22] on offspring physiology and brain development. It is clear from these studies that the source of fat in the HFD matters. For example, a study in rodents determined that while a maternal diet high in saturated fat programs hyperphagia in offspring, exposure to a diet of equivalent percent of calories from fat as fish oil does not [21]. Thus, it is essential that future studies determine which sources of fat in a mother's diet are beneficial and which are detrimental to the developing offspring in order for physicians and nutritionists to make appropriate recommendations to expecting mothers.
Most rodent studies report that offspring exposed to a HFD during gestation and lactation have increased body weight and adiposity at weaning [24,25]. However, several studies report that maternal HFD consumption results in lighter offspring [26,27,28] which they speculate is due to HFD/obesity-induced impairments in the initiation and production of milk by obese mothers [26,29]. The differences in offspring's body weight phenotypes across studies are likely due to differences in the duration of HFD consumption and fatty acid composition of the diets. Using nonhuman primates, our group is examining the impact of chronic maternal HFD consumption on offspring body weight regulation. Briefly, infant offspring from HFD mothers are underweight at birth and display rapid catch-up growth, so that by 6 months of age they are heavier and have increased adiposity [30]. This offspring phenotype appears to be independent of whether the HFD-consuming mother is obese with insulin resistance or lean with normal sensitivity to insulin. These studies indicate that in primates, as in rodents, maternal HFD consumption, independent of maternal weight and metabolic status, predisposes offspring to increased risk of developing obesity and metabolic disorders early in life.
Programming of Food Intake and Feeding Behavior
Rodents that experience an early environment in which they are exposed to maternal HFD consumption [31,32,33] or overnutrition [34,35,36,37] are consistently reported to be hyperphagic as adults. A number of studies have found that maternal HFD consumption plays a critical role in programming hypothalamic pathways that regulate feeding [17,38]. Early postnatal overfeeding increases the orexigenic peptides neuropeptide Y and agouti-related peptide (AGRP) in the arcuate nucleus of the hypothalamus (ARH) of juvenile rats [38]. Offspring of rat dams fed a HFD during the perinatal period also display a long-term upregulation in the expression of orexigenic peptides including galanin, enkephalin, and dynorphin in the paraventricular nucleus of the hypothalamus (PVH), and orexin and melanin-concentrating hormone in the perifornical lateral hypothalamus [32]. Exposure to HFD during gestation stimulates the proliferation of neuronal and neuroepithelial cells of the embryonic third ventricle of the hypothalamus and increases their migration to hypothalamic regions resulting in an increase in the proportion of neurons expressing orexigenic peptides [32]. Also, offspring from HFD mothers have reduced sensitivity to the anorectic effects of leptin [33]. Thus, in rodents it is postulated that perinatal exposure to overnutrition or maternal HFD consumption results in disruption of the homeostatic feedback regulation and nutrient sensing capabilities of the hypothalamic feeding circuits leading to hyperphagia. Though rodent models have significant advantages, such as a relatively short period of gestation and the ability to manipulate genetics, the critical periods for the development of energy balance regulatory systems differ between rodents and humans. In rodents, the neural pathways regulating energy balance are immature at birth and are not completely developed until the third postnatal week (mice) [39]. In contrast, in humans, nonhuman primates, pigs, and sheep, the hypothalamic circuitry that regulates feeding develops primarily prenatally [38]. Thus, models of maternal overnutrition in which the development of energy balance regulation occurs prenatally are particularly relevant. In the nonhuman primate model of maternal HFD-induced obesity, our group has determined that fetal offspring also display alterations in the development of the hypothalamic melanocortin system that may contribute to disrupted homeostatic signaling [40].
In addition to hyperphagia, there is evidence that feeding behavior and food choice are also programmed by perinatal nutrition. Overweight children are reported to have increased preference for high-fat foods which is associated with increased parental adiposity [41]. Also, children with one or two overweight parents consume a larger percentage of energy from fat than children who have two normal-weight parents [42]. In these studies it is unclear if the children's increased fat preference is due to programming as a result of perinatal HFD exposure, genetics or increased availability of high-fat food during childhood. Animal models in which offspring are exposed to a HFD during the perinatal period provide further evidence for an influence of perinatal HFD exposure on food choice. For example, adult rat offspring exposed to junk food during either gestation or lactation displayed increased preference for fatty, sugary and salty foods [21,33,43]. The source of fat in the perinatal diet influences the programming of food preference, as rat pups from mothers that consumed a HFD with lard as the main source of fat displayed increased preference for the HFD, whereas offspring exposed to maternal consumption of HFD with fish oil as the fat source do not [21]. In rodents, there is evidence that maternal HFD consumption causes perturbation in the dopamine system of adult animals in areas associated with the rewarding value of food such as the nucleus accumbens and ventral tegmental area [44]. Preliminary findings from our studies examining the impact of exposure to maternal HFD consumption on the food preference of nonhuman primate offspring also indicate that offspring display increased preference for diets with a high sugar and fat content [Sullivan and Grove, unpubl. observation]. Together, these studies provide compelling evidence that perinatal nutrition has a long-term influence on dietary preference and feeding behavior and may be an important contributing factor to the development of obesity. As differences in food preference and diet consumption will have a long-term impact on stress response and depressive behaviors of adult offspring [45], future studies examining the impact of perinatal HFD exposure on food preference should include the examination of stress response and behavioral disorders.
Although many studies have examined the impact of perinatal nutrition on food intake and food intake regulation, the number of studies examining the other component of energy balance, energy expenditure, are limited. Offspring from mothers undernourished during gestation are reported to be less active than offspring from dams fed ad libitum [46]. Offspring exposed to maternal overnutrition have also been reported to be hypoactive [31] or to have no difference in physical activity as compared to offspring exposed to a control diet [44]. The effect of maternal diet on physical activity level depends on the type of fat in the diet. Rat pups from dams fed a diet rich in polyunsaturated fat displayed increased locomotor activity when compared to offspring from dams fed a saturated fat or standard laboratory diet [20]. This study also reported increased locomotor response to stimulants in offspring from dams that consumed a saturated fat diet [20]. Currently, the impact of early exposure to HFD on metabolic rate has not been examined. Thus, a major gap in the knowledge of the field of metabolic programming is the impact of perinatal HFD exposure on the regulation of energy expenditure. Future studies are needed which examine not only the effects of perinatal exposure to HFD on average basal energy expenditure, but its impact on compensatory changes in energy expenditure in response to metabolic challenges, such as fasting, dieting and chronic consumption of a HFD.
HFD Programming of Mental Health Disorders
In addition to being associated with metabolic disorders, epidemiological data indicate that obesity is associated with increased risk of behavioral/mental health disorders, such as depression [47], anxiety [47], and attention deficit hyperactivity disorder [48]. Moreover, anxiety and depression influence the feeding behavior, food preference and physical activity level. Depression and anxiety are associated with increased craving for palatable food items [49] and decreased physical activity level [50]. Variations in mood also alter food choice, with increased preference for high-fat/high-sugar foods being reported during negative emotions [51]. Maternal nutrition has long-term implications for the offspring's risk of developing mental health disorders. Perinatal exposure to a HFD may contribute to this association by altering the development of key pathways implicated in regulating mood and behavior such as the serotonin system [52]. Recently, maternal HFD consumption has been associated with increased anxiety in rodent [12] and nonhuman primate offspring [11]. In a rat model, male adult offspring from mothers exposed to either a diet high in saturated or trans fat during gestation and lactation displayed increased anxiety and deficits in spatial learning [12]. Using a nonhuman primate model, our group recently demonstrated that perinatal exposure to a HFD causes a decrease in serotonergic tone perturbations in the serotonin system, which predisposes female offspring to increased anxiety [11]. The finding that female nonhuman primate offspring exposed to maternal HFD consumption are more sensitive to developing anxiety than male offspring is consistent with evidence in humans which suggests that females are more prone to anxiety than males and that the association between obesity and anxiety is stronger in women than men [53]. Thus, nonhuman primates appear to be an ideal model in which to examine the impact of maternal HFD consumption on the development of mental health disorders such as anxiety and depression.
Mechanism by Which Maternal HFD Programs Physiology and Behavior
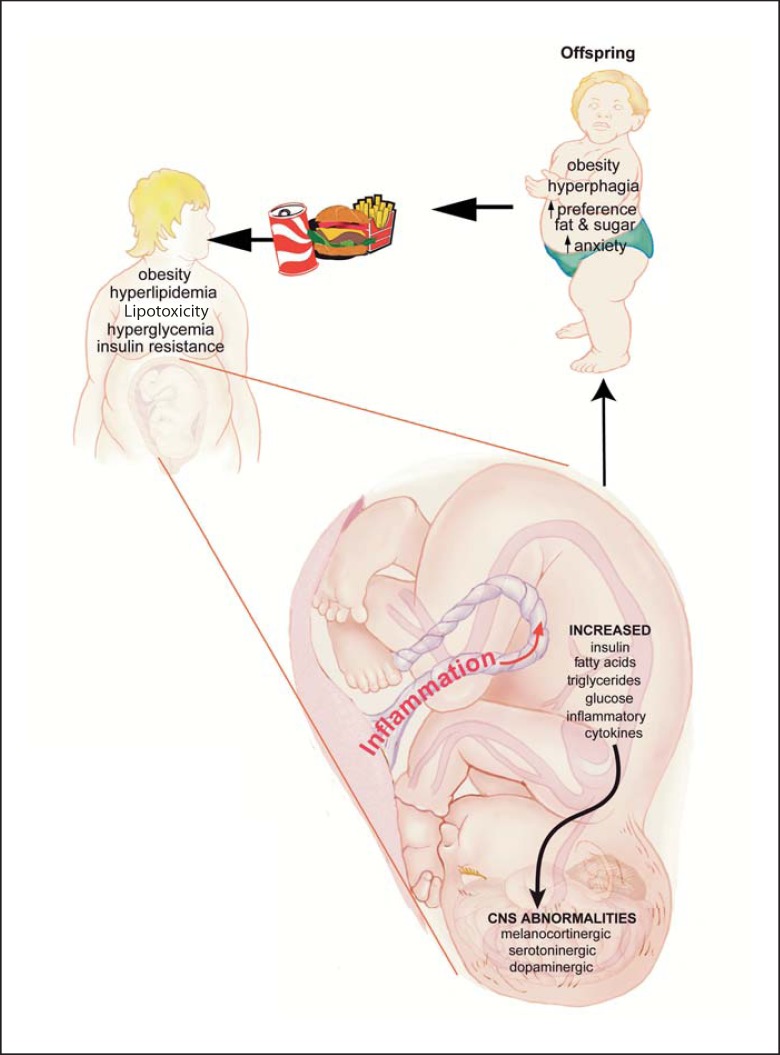
Although there is clear evidence from studies in both rodents and nonhuman primates that maternal HFD consumption leads to increased risk of obesity and metabolic diseases, the mechanisms responsible are largely unknown. We have very limited information on the impact of maternal HFD consumption on the brain and the complex neural circuitry that regulates physiology and behavior. Recent evidence indicates that circulating factors such as hormones (leptin, insulin), nutrients (fatty acids, triglycerides and glucose) and inflammatory cytokines play important roles (fig. 1). Maternal obesity and diabetes result in maternal hyperglycemia [54], and as glucose can readily pass through the blood-placenta barrier, it is transferred to the fetus. However, the elevated insulin levels associated with maternal obesity do not cross the placenta [15]; thus, the fetal pancreas must secrete increased levels of insulin to respond to the maternal hyperglycemia. This fetal hyperinsulinemia is postulated to be involved in the programming of obesity and diabetes in the developing offspring [55]. Administering insulin to rats during the last term of gestation produces obesity in the offspring [6,56] and administering insulin to the hypothalamus of rat pups during the time that projects from the ARH to the PVH results in elevations in body weight, insulin level, impaired glucose tolerance, and increased vulnerability to diabetes [57]. Insulin is an important growth factor in the central nervous system [55]; thus, it is postulated that early exposure to hyperinsulinemia alters the development of the brain circuitry regulating energy balance and behavior.
Fig. 1.
Maternal obesity and HFD consumption have an enduring impact on the developing fetal brain and the juvenile offspring. A fetus from a HFD-consuming mother experiences a fetal environment in which levels of glucose, insulin, fatty acids, triglycerides and inflammatory cytokines are elevated, leading to changes in the critical neural circuitry for the regulation of physiology and behavior.
The hyperleptinemia that offspring from obese mothers experience during development is also implicated in metabolic imprinting. There is substantial evidence in rodents that postnatal leptin is a critical factor in the development of neural pathways in the hypothalamus [39,58]. Human studies report that leptin is elevated in obese and diabetic mothers [59,60] and lower in infants that experienced intrauterine growth restriction at term [61]. However, in human and nonhuman primate gestation, circulating leptin levels do not rise until after hypothalamic development is mostly complete [61,62]. Though critical for brain development in rodents, there is limited evidence for leptin's role in the development of primate brains [63].
Obesity has recently been described as a state of chronic inflammation. Increased adiposity is associated with elevations in peripheral markers of inflammation, such as C-reactive protein, interleukin-6, interleukin-1β, and tumor necrosis factor-α [64]. These inflammatory markers are associated with increased risk of cardiovascular disease, heart disease, insulin resistance, type 2 diabetes mellitus and hypertension [64]. The association between obesity and increased inflammatory cytokines has been confirmed in pregnant women such that obese pregnant women have increased levels of inflammatory cytokines which lead to endothelial dysfunction [65]. Exposure of the developing fetus to increased circulating cytokines has been proposed as a potential mechanism by which maternal HFD consumption impacts brain development. The development of many neural systems that are critical in regulating energy balance, such as the melanocortinergic system, the serotonergic system, and the dopaminergic system, is sensitive to circulating cytokine levels [64]. Also, rodent studies report that AGRP [66] and pro-opiomelanocortin [67] neurons in the ARH are directly impacted by cytokines. A recent study by Bilbo and Tsang [12] reported that offspring from mothers consuming a saturated fat diet had increased microglial activation in the hippocampus at birth that persisted into adulthood. We have recently observed that fetuses from nonhuman primates consuming a HFD have increased circulating and hypothalamic cytokines [40]. We postulate that exposure to increased inflammatory cytokines leads to the perturbations in the melanocortin [40] and serotonin system observed in fetal offspring [11]. Given the large number of neurotransmitter systems that are influenced by inflammation, future research is needed to examine the impact of maternal HFD-induced inflammation on each critical regulator of physiology and behavior.
In summary, many common brain regions and neurotransmitters regulate energy balance, stress response and mental health disorders including the melanocortinergic system, serotoninergic system and dopaminergic system (fig. 1). Thus, it is not surprising that maternal HFD consumption has a long-term impact on metabolic and behavioral regulation. As maternal HFD consumption and obesity are commonplace and rapidly increasing, we speculate that future generations will be at increased risk for both metabolic and mental health disorders. Given the prevalence of maternal obesity, future studies need to identify therapeutic strategies that are effective at preventing maternal HFD-induced malprogramming.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman P. Health risks associated with overweight and obesity. Obes Rev. 2007;8(suppl 1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Bachman KH. Obesity, weight management, and health care costs: a primer. Dis Manag. 2007;10:129–137. doi: 10.1089/dis.2007.103643. [DOI] [PubMed] [Google Scholar]
- 4.Loos RJ. Recent progress in the genetics of common obesity. Br J Clin Pharmacol. 2009;68:811–829. doi: 10.1111/j.1365-2125.2009.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol E. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 6.Jones AP, Dayries M. Maternal hormone manipulations and the development of obesity in rats. Physiol Behav. 1990;47:1107–1110. doi: 10.1016/0031-9384(90)90359-c. [DOI] [PubMed] [Google Scholar]
- 7.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 8.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 9.D’Asti E, Long H, Tremblay-Mercier J, Grajzer M, Cunnane SC, Di Marzo V, Walker CD. Maternal dietary fat determines metabolic profile and the magnitude of endocannabinoid inhibition of the stress response in neonatal rat offspring. Endocrinology. 2010;151:1685–1694. doi: 10.1210/en.2009-1092. [DOI] [PubMed] [Google Scholar]
- 10.Augustyniak RA, Singh K, Zeldes D, Singh M, Rossi NF. Maternal protein restriction leads to hyper-responsiveness to stress and salt-sensitive hypertension in male offspring. Am J Physiol. 2010;298:R1375–R1382. doi: 10.1152/ajpregu.00848.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30:3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 13.King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–291. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- 14.Alberti-Fidanza A, Parizkova J, Fruttini D. Relationship between mothers’ and newborns’ nutritional and blood lipid variables. Eur J Clin Nutr. 1995;49:289–298. [PubMed] [Google Scholar]
- 15.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 16.Levin BE. Synergy of nature and nurture in the development of childhood obesity. Int J Obes (Lond) 2009;33(suppl 1):S53–S56. doi: 10.1038/ijo.2009.18. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan EL, Grove KL. Metabolic imprinting in obesity. Forum Nutr. 2010;63:186–194. doi: 10.1159/000264406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 2006. [DOI] [PubMed]
- 19.Abel EL, Reddy PP. Prenatal high saturated fat diet modifies behavioral effects of prenatal alcohol exposure in rats. Alcohol. 1997;14:25–29. doi: 10.1016/s0741-8329(96)00081-x. [DOI] [PubMed] [Google Scholar]
- 20.Brenneman DE, Rutledge CO. Effect of dietary lipid on locomotor activity and response to psychomotor stimulants. Psychopharmacology (Berl) 1982;76:260–264. doi: 10.1007/BF00432557. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima Y. Fish-oil high-fat diet intake of dams after day 5 of pregnancy and during lactation guards against excessive fat consumption of their weaning pups. J Nutr Sci Vitaminol (Tokyo) 2008;54:46–53. doi: 10.3177/jnsv.54.46. [DOI] [PubMed] [Google Scholar]
- 22.Wainwright PE, Huang YS, Bulman-Fleming B, Levesque S, McCutcheon D. The effects of dietary fatty acid composition combined with environmental enrichment on brain and behavior in mice. Behav Brain Res. 1994;60:125–136. doi: 10.1016/0166-4328(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 23.Raygada M, Cho E, Hilakivi-Clarke L. High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings’ aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase C activity. J Nutr. 1998;128:2505–2511. doi: 10.1093/jn/128.12.2505. [DOI] [PubMed] [Google Scholar]
- 24.Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav. 1995;57:681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- 25.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- 26.Ferezou-Viala J, Roy AF, Serougne C, Gripois D, Parquet M, Bailleux V, Gertler A, Delplanque B, Djiane J, Riottot M, Taouis M. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol. 2007;293:R1056–R1062. doi: 10.1152/ajpregu.00117.2007. [DOI] [PubMed] [Google Scholar]
- 27.Rolls BJ, Rowe EA. Pregnancy and lactation in the obese rat: effects on maternal and pup weights. Physiol Behav. 1982;28:393–400. doi: 10.1016/0031-9384(82)90130-5. [DOI] [PubMed] [Google Scholar]
- 28.Rolls BJ, Rowe EA, Fahrbach SE, Agius L, Williamson DH. Obesity and high-energy diets reduce survival and growth rates of rat pups. Proc Nutr Soc. 1980;39:51a. [PubMed] [Google Scholar]
- 29.Rasmussen KM, Hilson JA, Kjolhede CL. Obesity may impair lactogenesis II. J Nutr. 2001;131:3009s–3011s. doi: 10.1093/jn/131.11.3009S. [DOI] [PubMed] [Google Scholar]
- 30.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 32.Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker CD, Naef L, D’Asti E, Long H, Xu Z, Moreau A, Azeddine B. Perinatal maternal fat intake affects metabolism and hippocampal function in the offspring: a potential role for leptin. Ann NY Acad Sci. 2008;1144:189–202. doi: 10.1196/annals.1418.023. [DOI] [PubMed] [Google Scholar]
- 34.Oscai LB, McGarr JA. Evidence that the amount of food consumed in early life fixes appetite in the rat. Am J Physiol. 1978;235:R141–R144. doi: 10.1152/ajpregu.1978.235.3.R141. [DOI] [PubMed] [Google Scholar]
- 35.Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–158. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- 36.Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dorner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999;11:541–546. doi: 10.1046/j.1365-2826.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- 37.Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dorner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome X-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999;836:146–155. doi: 10.1016/s0006-8993(99)01662-5. [DOI] [PubMed] [Google Scholar]
- 38.Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS. Development of metabolic systems. Physiol Behav. 2005;86:646–660. doi: 10.1016/j.physbeh.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 39.Djiane J, Attig L. Role of Leptin during perinatal metabolic programming and obesity. J Physiol Pharmacol. 2008;59(suppl 1):55–63. [PubMed] [Google Scholar]
- 40.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151:1622–1632. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher JO, Birch LL. Fat preferences and fat consumption of 3- to 5-year-old children are related to parental adiposity. J Am Diet Assoc. 1995;95:759–764. doi: 10.1016/S0002-8223(95)00212-X. [DOI] [PubMed] [Google Scholar]
- 42.Eck LH, Klesges RC, Hanson CL, Slawson D. Children at familial risk for obesity: an examination of dietary intake, physical activity and weight status. Int J Obes Relat Metab Disord. 1992;16:71–78. [PubMed] [Google Scholar]
- 43.Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98:843–851. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- 44.Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD. Maternal high-fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology (Berl) 2008;197:83–94. doi: 10.1007/s00213-007-1008-4. [DOI] [PubMed] [Google Scholar]
- 45.Maniam J, Morris MJ. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 2010;35:717–728. doi: 10.1016/j.psyneuen.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol. 2003;285:R271–R273. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- 47.Rofey DL, Kolko RP, Iosif AM, Silk JS, Bost JE, Feng W, Szigethy EM, Noll RB, Ryan ND, Dahl RE. A longitudinal study of childhood depression and anxiety in relation to weight gain. Child Psychiatry Hum Dev. 2009;40:517–526. doi: 10.1007/s10578-009-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122:E1–E6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 49.Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Teychenne M, Ball K, Salmon J. Physical activity, sedentary behavior and depression among disadvantaged women. Health Educ Res. 2010;25:632–644. doi: 10.1093/her/cyq008. [DOI] [PubMed] [Google Scholar]
- 51.Macht M. How emotions affect eating: a five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Kiyohara C, Yoshimasu K. Molecular epidemiology of major depressive disorder. Environ Health Prev Med. 2009;14:71–87. doi: 10.1007/s12199-008-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desai RA, Manley M, Desai MM, Potenza MN. Gender differences in the association between body mass index and psychopathology. CNS Spectr. 2009;14:372–383. doi: 10.1017/s1092852900023026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leung TW, Lao TT. Placental size and large-for-gestational-age infants in women with abnormal glucose tolerance in pregnancy. Diabet Med. 2000;17:48–52. doi: 10.1046/j.1464-5491.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 55.Simerly RB. Hypothalamic substrates of metabolic imprinting. Physiol Behav. 2008;94:79–89. doi: 10.1016/j.physbeh.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones AP, Pothos EN, Rada P, Olster DH, Hoebel BG. Maternal hormonal manipulations in rats cause obesity and increase medial hypothalamic norepinephrine release in male offspring. Brain Res Dev Brain Res. 1995;88:127–131. doi: 10.1016/0165-3806(95)00078-r. [DOI] [PubMed] [Google Scholar]
- 57.Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Lifelong enhanced diabetes susceptibility and obesity after temporary intrahypothalamic hyperinsulinism during brain organization. Exp Clin Endocrinol. 1992;99:91–95. doi: 10.1055/s-0029-1211143. [DOI] [PubMed] [Google Scholar]
- 58.Bouret SG. Development of hypothalamic neural networks controlling appetite. Forum Nutr. 2010;63:84–93. doi: 10.1159/000264396. [DOI] [PubMed] [Google Scholar]
- 59.Lepercq J, Hauguel DE, Mouzon S, Timsit J, Catalano PM. Fetal macrosomia and maternal weight gain during pregnancy. Diabetes Metab. 2002;28:323–328. [PubMed] [Google Scholar]
- 60.Hauguel DE Mouzon S, Shafrir E. Carbohydrate and fat metabolism and related hormonal regulation in normal and diabetic placenta. Placenta. 2001;22:619–627. doi: 10.1053/plac.2001.0698. [DOI] [PubMed] [Google Scholar]
- 61.Davidowa H, Plagemann A. Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. Neuroreport. 2000;11:2795–2798. doi: 10.1097/00001756-200008210-00037. [DOI] [PubMed] [Google Scholar]
- 62.Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143:975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 63.Grayson BE, Kievit P, Smith MS, Grove KL. Critical determinants of hypothalamic appetitive neuropeptide development and expression: species considerations. Front Neuroendocrinol. 2010;31:16–31. doi: 10.1016/j.yfrne.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 65.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007;92:969–975. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- 66.Scarlett JM, Zhu X, Enriori PJ, Bowe DD, Batra AK, Levasseur PR, Grant WF, Meguid MM, Cowley MA, Marks DL. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology. 2008;149:4837–4845. doi: 10.1210/en.2007-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL. Regulation of central melanocortin signaling by interleukin-1β. Endocrinology. 2007;148:4217–4225. doi: 10.1210/en.2007-0017. [DOI] [PubMed] [Google Scholar]