Abstract
Primary Non Hodgkin s Lymphoma (NHL) usually arises within the lymphnodes, but 20-30% account for extra nodal sites. Oral cavity, as a primary extra nodal site for NHL, is relatively rare and diverse in presentation, response to therapy and prognosis. We report a 14 year old adolescent girl who presented with multiple gingival swellings, the most prominent one being in the right anterior maxilla. Gingival biopsy showed NHL- diffuse large B cell type. Child was completely cured with chemotherapy and now she is in complete remission and under regular follow up.
Keywords: Extra nodal, gingival, NHL, oral cavity
INTRODUCTION
Primary non-Hodgkin's lymphoma (NHL) usually arises- within the lymphnodes, but 20-30% account for extranodal sites.[1,2] Gastrointestinal tract, skin, bones, and Waldeyer's ring are the most frequently involved extranodal sites. The incidence of oral NHL is about 0.1% to 5%.[2] Oral NHL can involve the paranasal sinuses- but can also arise from within the soft tissue or bone, gingiva-, floor of the mouth, salivary gland, and cheek.[3,4] Owing to scarcity of reported cases of oral lymphomas-, the interpretation of the biological- behavior- and treatment- options involving this disease- entity becomes- difficult. Athorough clinical, histopathological-, and immunohistochemical evaluation- is essential for the diagnosis and management- of oral NHL.
CASE REPORT
A 14-year-old adolescent girl, first born to non-consanguineous parents presented with multiple gingival swellings, the most prominent one being 2 × 1 cm in the right anterior maxilla of 2 months duration [Figure 1]. Her birth, developmental, and family histories were insignificant. She presented to her dentist and initially received treatment for suspected gingivitis, but because of progressive nature of the swelling, she underwent gingival biopsy. The biopsy was reported as a lymphoproliferative disorder. The patient was then referred to our hospital for further management of the disease. A thorough clinical examination revealed no other significant lymphadenopathy. Investigations showed a normal complete haemogram, liver, and renal profile. A gingival biopsy was repeated at our institution and it showed fibrocollagenous tissue with sheets of atypical lymphoid cells showing high mitotic and apototic activity. Immunohistochemistry (IHC) showed CD45, CD20 positivity, and CD3 negative atypical lymphoid cells, favoring a diagnosis of NHL diffuse large B-cell type [Figure 2]. Subsequently, to ascertain the further extent of the disease and other organ system involvement, we proceeded with staging investigations. CSF analysis was within normal limits and cytology was negative for malignant cells. Bone marrow aspiration and biopsy done were normal. CT thorax and abdomen were also normal. However, CT PNS indicated a large homogenous soft tissue lesion in the left masticator space with erosion of the postero lateral wall of left maxillary sinus and extension of the mass lesion into the left maxillary sinus [Figure 3]. She was treated with chemotherapy according to the LMP-96 group B protocol. The post-cycle 4 PET-CT scan was done that showed complete resolution of the primary lesion with no demonstrable metabolic activity anywhere in the body. She received a total of 5 cycles of chemotherapy as per group B LMP 96 Protocol. She is on regular follow up and well for past 10 months.
Figure 1.
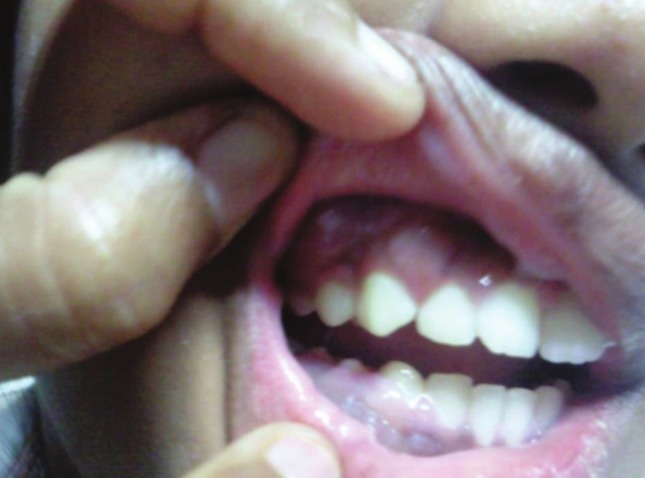
Gingival swelling
Figure 2.
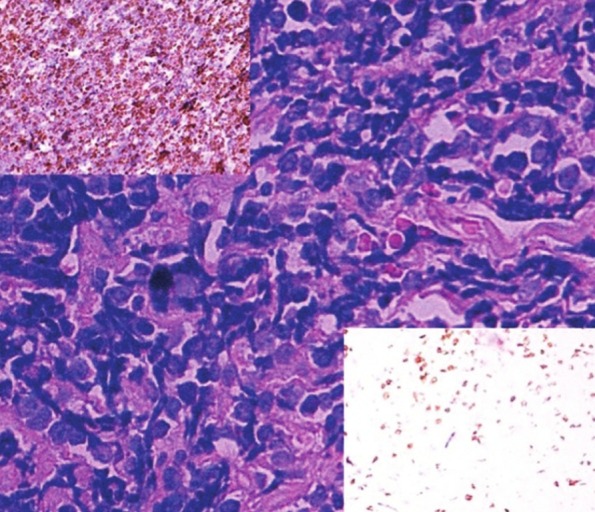
Sheets of atypical lymphoid cells with hyperchromatic nuclei and scant cytoplasm (H and E, × 400). The upper insert shows increased number of CD 20 positive cells (IHC × 100). Only a few scattered cells in the background are positive for CD 3 in the lower insert (IHC × 100). This staining pattern is diagnostic of NHL – Diffuse large B-cell type
Figure 3.
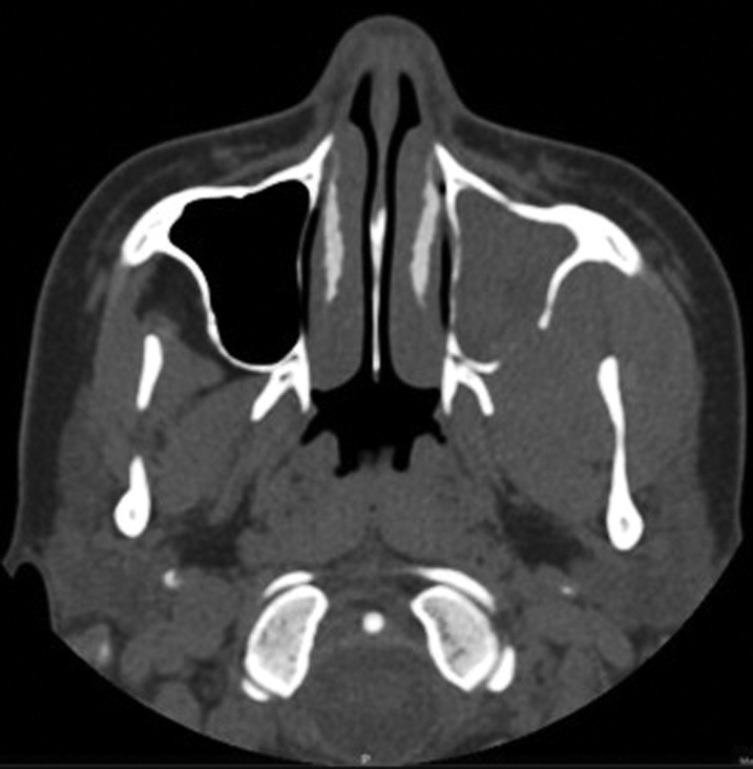
CT scan – Paranasal sinuses: a large homogenous soft tissue lesion in the left masticator space with erosion of the postero lateral wall of left maxillary sinus and extension of the mass lesion into the left maxillary sinus
DISCUSSION
Lymphoma is the third most common neoplasm of the head and neck; the first two being squamous cell carcinoma and salivary gland neoplasm.[5] About 20-30% of NHL arise from extranodal sites.[1,2] It may present in a variety of ways, occasionally providing a diagnostic dilemma owing to the protean manifestations of its presentation. The disease may present with nasal obstruction, rhinorrhea, hypoacousia, and cranial nerve palsies. Most lesions occur in the Waldeyer's ring and occurrence in the oral cavity is rare. Our patient had multiple gingival swellings, with most prominent swelling being in the right anterior maxilla. Secondary organ involvement along with the primary in the oral cavity is generally observed,[6] but it was not seen in our patient.
The signs and symptoms suggestive of lymphoma in the head and neck region are the presence of numbness, tooth mobility, swelling, unexplained dental pain, or ill-defined lytic osseous changes.[7] Other differential diagnosis includes a dental abscess, periodontal infection or benign reactive hyperplasia. Our patient also was initially treated for suspected gingivitis but as the swelling was progressive, she underwent an incisional biopsy that rendered a diagnosis of diffuse large B-cell lymphoma.
Lymphoma presenting as gingival swelling is reported in literature mainly in adults;[8,9,10,11] Fukuda et al.[12] reviewed 20 cases of malignant lymphoma of the oral cavity, 17 of which originated in the soft tissues and three involved the jaw bones. Epstein et al.[13] reviewed 361 cases and reported seven gingival presentations
CT of the head and neck, chest, abdomen, and pelvis are the mainstay of staging oropharyngeal extranodal lymphomas. All baseline investigations like complete blood counts along with bone marrow biopsy are equally mandatory. Concurrent PET with 18F-fluorodeoxyglucose (FDG) is a useful method for staging and assessment of therapeutic response. Immunohistochemistry is useful for distinguishing cell types and thus aids in differential diagnosis. In our patient, the tumour cells were positive for CD45, CD20, and negative for CD3, thus rendering a diagnosis of diffuse large B-cell NHL.
The etiology of diffuse large B-cell lymphoma remains unknown. They may originate de novo or with underlying immunodeficiencies which is a significant risk factor and diffuse large B-cell lymphoma in the setting of immunodeficiency is more often Epstein-Barr virus-positive than sporadic type.[14] No evidence of immunodeficiency state was found in our patient. Her HIV and EBV serology were negative.
The standard treatment comprises of a combination of chemotherapy with cyclophosphamide, methotrexate, doxorubicin, vincristine, cytarabine arabinoside, and prednisone. Our patient attained complete remission after 4 cycles of chemotherapy based on LMP 96 GROUP B PROTOCOL and received a total of 5 cycles. At present, she is in complete remission and is under regular follow-up, 16 month post-diagnosis and 10 months after the last cycle of chemotherapy.
Fluorine 18-fluorodeoxyglucose (FDG)-PET (Positron Emission Tomography) PET-CT is emerging as a powerful imaging modality for diagnosis, staging, treatment, and monitoring of lymphoma patients. In 15% of the patients, there is a change in staging of the disease when PET scans are used compared to conventional CT scans. PET is very useful in detecting extranodal involvement in 15-20% of newly diagnosed lymphoma patients. New targets for therapy will emerge as the molecular pathogenesis of the malignant lymphomas are elucidated using gene expression arrays. This information will also be valuable for developing more sensitive diagnostic tools, measurement of submicroscopic disease, and early response to therapy and for identifying new prognostic subgroups.[15] The addition of monoclonal antibodies directed to CD20 antigen (Rituximab) to the existing CHOP chemotherapy in adults showed improved initial response and decrease in remissions. Similar approaches are being explored in childhood B-cell lymphomas. Antibodies to CD30 antigen and small molecule inhibitors like ALK inhibitors are currently in early phase clinical trials. Radioimmunoconjugates and drug conjugates to more specifically deliver cytotoxic therapy have considerable promise in the treatment of lymphomas. In near future, targeted therapy will replace the dose-intensive chemotherapy protocols in children, thereby minimizing the complications and also be valuable for children with advanced NHL. Newer treatment for NHL should be biology driven focusing on specific molecule or pathway targets.[15] The role of oral maxillo facial surgeon in the treatment of NHL is mostly limited to diagnostic biopsies and treatment of complications of therapy. There is no role for performing major tumor resection or debulking surgery in children with NHL.
In conclusion, primary extranodal lymphoma should be considered as a rare differential diagnosis for the gingival swelling when common causes are ruled out. Dentists, pediatricians, and oral maxillofacial surgeons, who treat these children, should be aware of this rare possibility, as correct diagnosis is very essential for appropriate treatment at an early stage of disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Clark RM, Fitzpatrick PJ, Gospodarowitcz MK. Extranodal malignant lymphomas of the head and neck. J Otolaryngol. 1983;12:239–45. [PubMed] [Google Scholar]
- 2.Spatafore CM, Keyes G, Skidmore AE. Lymphoma: An unusual presentation. J Endod. 1989;15:438–41. doi: 10.1016/S0099-2399(89)80179-7. [DOI] [PubMed] [Google Scholar]
- 3.Ratech H, Burke JS, Blayney DW, Sheibani K, Rappaport H. A clinicopathologic study of malignant lymphomas of the nose, paranasal sinuses, and hard palate, including cases of lethal midline granuloma. Cancer. 1989;64:2525–31. doi: 10.1002/1097-0142(19891215)64:12<2525::aid-cncr2820641220>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Wong DS, Fuller LM, Butler JJ, Shullenberg CC. Extranodal non-Hodgkin's lymphomas of the head and neck. Am J Roetgenol Radium Ther Nucl Med. 1975;123:471–81. doi: 10.2214/ajr.123.3.471. [DOI] [PubMed] [Google Scholar]
- 5.Griffin TJ, Hurst PS, Swanson J. Non Hodgkin's lymphoma: Case involving four third molar extraction sites. Oral Surg Oral Med Oral Pathol. 1988;65:671–4. doi: 10.1016/0030-4220(88)90006-0. [DOI] [PubMed] [Google Scholar]
- 6.Takashashu H, Tezuka F, Fujita S, Okabe H. Primary extranodal malignant non-Hodgkin's lymphoma of the oral region: Analysis of 11 autopsy cases. J Oral Pathol. 1987;16:242–50. doi: 10.1111/j.1600-0714.1987.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 7.Angiero F, Stefani M, Crippa R. Primary non-Hodgkin's lymphoma of the mandibular gingiva with maxillary gingival recurrence. Oral Oncol Extra. 2006;42:123–8. [Google Scholar]
- 8.Gould AR, Alpert B. Painless swelling of the anterior maxillary gingival. J Oral Maxillofac Surg. 1987;45:785–8. doi: 10.1016/0278-2391(87)90203-5. [DOI] [PubMed] [Google Scholar]
- 9.Park YW. Non-Hodgkin's lymphoma of the anterior maxillary gingival. Otolaryngol Head Neck Surg. 1998;119:146. doi: 10.1016/S0194-5998(98)70188-3. [DOI] [PubMed] [Google Scholar]
- 10.Yasumoto M, Shibuya H, Fukuda H, Takeda M, Mukai T, Korenaga T. Malignant lymphoma of the gingiva: MR evaluation. AJNR Am J Neuroradiol. 1998;19:723–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Raut A, Huryn J, Pollack A, Zlotolow I. Unusual gingival presentation of posttransplantation lymphoproliferative disorder: A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:436–41. doi: 10.1067/moe.2000.107446. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda Y, Ishida T, Fujimoto M, Ueda T, Aozasa K. Malignant lymphoma of the oral cavity: Clinicopathologic analysis of 20 cases. J Oral Pathol. 1987;16:8–12. doi: 10.1111/j.1600-0714.1987.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JB, Epstein JD, Le ND, Gorsky M. Characteristics of oral and paraoral malignant lymphoma: A population based review of 361 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:519–25. doi: 10.1067/moe.2001.116062. [DOI] [PubMed] [Google Scholar]
- 14.Duarte EC, Silveira-Júnior JB, Gomez RS, Paes RP, Lacerda JC, Mesquita RA. Plasmablastic lymphoma of oral mucosal type: A case report. Oral Oncology Extra. 2005;41:121–4. [Google Scholar]
- 15.Gross TG, Perkins SL. Principles and practice of Pediatric oncology. 6th ed. United States: Lippincott Williams and Wilkins; 2011. Malignant non-Hodgkin lymphomas in children; pp. 663–82. [Google Scholar]


