Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is an oncogenic herpesvirus and the cause of Kaposi's sarcoma, primary effusion lymphoma (PEL) and multicentric Castleman's disease. Latently infected B cells are the main reservoir of this virus in vivo, but the nature of the stimuli that lead to its reactivation in B cells is only partially understood. We established stable BJAB cell lines harboring latent KSHV by cell-free infection with recombinant virus carrying a puromycin resistance marker. Our latently infected B cell lines, termed BrK.219, can be reactivated by triggering the B cell receptor (BCR) with antibodies to surface IgM, a stimulus imitating antigen recognition. Using this B cell model system we studied the mechanisms that mediate the reactivation of KSHV in B cells following the stimulation of the BCR and could identify phosphatidylinositol 3-kinase (PI3K) and X-box binding protein 1 (XBP-1) as proteins that play an important role in the BCR-mediated reactivation of latent KSHV.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV), also referred to as human herpesvirus 8 (HHV8), and Epstein-Barr virus (EBV; HHV4) belong to the gammaherpesvirus subfamily and are associated with B cell lymphoproliferative diseases (1, 2). Although EBV is the causative agent of endemic Burkitt's lymphoma, Hodgkin's lymphoma, and posttransplant lymphoproliferative disorder (3), KSHV causes primary effusion lymphoma (PEL) (4) and multicentric Castleman's disease (5) in addition to Kaposi's sarcoma.
B cells are the main latent reservoir for both viruses in vivo (6, 7). EBV can transform and immortalize primary B lymphocytes in vitro, which results in the formation of lymphoblastoid cell lines (8). EBV-infected cell lines, derived directly from patients or generated by in vitro infection of either EBV-negative B cell lines or naive B cells, contributed significantly to the understanding of EBV biology in B cells.
In contrast, B cell lines and primary B cells are poorly permissive to infection by KSHV in vitro (9–11). Introduction of the viral DNA via electroporation into a B cell line resulted in latent persistence of KSHV (12). Only recently, KSHV- and EBV-negative BJAB cells were successfully infected with KSHV via cell-mediated transmission (13). Latently infected cells could be generated under selection (13, 14) but were poorly permissive to lytic/productive replication after stimulation with valproate, sodium butyrate, or 12-O-tetradecanoyl-phorbol-13-acetate (TPA) (13). Most of our knowledge on the lytic/productive replication of KSHV in B cells stems from work on PEL cell lines. It is possible to reactivate KSHV in these cells using chemical stimuli such as phorbol esters (e.g., TPA) or histone deacetylase inhibitors (e.g., sodium butyrate) (15–17). As for possible physiological trigger(s), reactivation of both KSHV in PEL cell lines and EBV in the EBV-positive nasopharyngeal carcinoma cell line HONE-1 and lymphoblastoid cell lines have been shown to be induced by the active form of the transcription factor X-box binding protein 1 (XBP-1) (18–21). XBP-1 is a key regulator of unfolded protein response (UPR) and is also essential for the terminal differentiation of B cells into plasma cells (49). Any perturbation that leads to the accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) initiates the removal of a 26-nucleotide intron from the XBP-1 mRNA by the ER transmembrane protein endoribonuclease inositol-requiring enzyme 1α (IRE1α) (22). This results in a transcriptional frameshift that generates the active XBP-1, which upregulates UPR genes to enhance protein folding capacity of cells. UPR activation during antibody production has been proposed to provide a link between plasma cell differentiation (23, 24) and gammaherpesviral reactivation (18, 21). Overexpression of spliced XBP-1, or its artificial induction with dithiothreitol (DTT), leads to reactivation of KSHV in PEL cells in vitro (18–21).
In the case of EBV-infected B cells, reactivation of the lytic cycle can be triggered by activating the B cell antigen receptor (BCR) by cross-linking surface immunoglobulins on the B cell surface with anti-Ig antibodies (25, 26). This, together with the involvement of plasma cell differentiation-associated cellular factors such as XBP-1, has led to the notion that triggering of the BCR on the surface of latently infected memory B cells and the ensuing plasma cell differentiation could provide the physiological stimulus for the reactivation of EBV in latently infected memory B cells (27–30). Evidence for the reactivation of murine herpesvirus 68 (MHV68) in B cells following triggering of the BCR also exists (31). Reactivation of EBV in B cells as a result of triggering the BCR involves the phosphatidylinositol 3-kinase (PI3K) pathway (28), which is also known to interact with the spliced form of XBP-1 (32, 33). Whether contact with antigen also plays a role in the reactivation of KSHV in latently infected B cells has so far not been addressed, since PEL cells lack the B cell immunoglobulin receptor on their surface (34–38).
In this study, we therefore wanted to develop an experimental system in which to study a possible role of the BCR in KSHV reactivation from latency. We established stable latent KSHV infection in an immortalized B cell line (BJAB) using a recombinant KSHV and either cell-free or cell-associated infection. Characterization of these stably infected B cell lines, named BrK.219, revealed an expression pattern of viral proteins similar to that of PEL cell lines. These cells express surface IgM and treating them with antibodies against human IgM led to a reactivation of the lytic cycle, resulting in the release of significant titers of infectious progeny. Inhibition of PI3K and XBP-1 splicing with chemical inhibitors decreased the expression of viral lytic proteins and infectious progeny production after anti-IgM treatment.
Our findings indicate that, as for EBV, the contact of latently KSHV-infected B cells with their cognate antigen might provide a trigger for viral reactivation.
MATERIALS AND METHODS
Cell culture and reagents.
HEK 293 cells and TE671 were cultured in Dulbecco's modified Eagle medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClone). Vero cells were grown in minimum essential medium (Cytogen) containing 10% FCS. The recombinant rKSHV.219 carries a constitutively expressed green fluorescent protein (GFP), a red fluorescent protein (RFP) under the control of the lytic PAN promoter, and a puromycin resistance gene (39). Vero cells stably infected with rKSHV.219 (referred to as Vero rKSHV.219) (39) were grown in the presence of 5 μg of puromycin (Sigma)/ml. A KSHV- and EBV-negative BJAB cell line (40), KSHV-positive and EBV-negative PEL cell lines (BC-3 and BCBL-1) (16, 41), and the KSHV- and EBV-double positive PEL cell line BC-1 (42) were maintained in RPMI 1640 medium (Gibco) containing 10% FCS without antibiotics. BJAB cell lines stably infected with recombinant KSHV (39) (referred to as BrK.219) were additionally treated with 4.2 μg of puromycin/ml. All cell lines were kept in a humidified incubator at 37°C and 5% CO2 and were routinely monitored for contamination with mycoplasma using a VenorGEM-Mycoplasma detection kit (Minerva-Biolabs) according to the manufacturer's guidelines.
Preparation of concentrated rKSHV.219 virus stocks in Vero cells.
Preparation of recombinant virus was performed as described previously (39). Briefly, rKSHV.219 production was induced in Vero rKSHV.219 by recombinant baculovirus expressing KSHV RTA (ORF50; replication and transcription activator) (39) and sodium butyrate (1.25 mM). After 3 days, infectious supernatants were harvested and centrifuged for 10 min at 4°C at 1,237 × g, filtered (0.45-μm pore size; Nalgene), and ultracentrifuged (L8-70 ultracentrifuge; Beckman) at 15,000 × g at 4°C for 4 to 6 h using a Beckman type 19 rotor. The viral pellets were resuspended in medium. The concentrated virus stocks were analyzed by infectivity assay.
Generation of KSHV-infected B cells by cell-mediated and cell-free transmission of rKSHV.219 in vitro.
For cell-mediated transmission, BJAB cells (40) were cocultured with Vero rKSHV.219 cells, and the lytic cycle of KSHV was induced by treatment with ectopically expressed baculoviral RTA (ORF50) (39) and sodium butyrate (1.25 mM). After 2 to 3 days, infected B cells were separated from the adherent cell lines by gently shaking the tissue culture plates and harvesting suspension cells. The lymphoblastoid cells were then resuspended in selection medium. For cell-free infection, BJAB cells were incubated with concentrated cell-free virus at a multiplicity of infection (MOI) from 1 to 10 in the presence of Polybrene (8 μg/ml). The cells were then spinoculated at 450 × g for 20 min at 32°C and incubated at 37°C for 2 to 3 days before the addition of puromycin. In both cases, the medium was changed twice a week. After 3 weeks, polyclonal BJAB cells latently infected with rKSHV.219 could be cultured and were designated as BrK.219 cells.
Infectivity assays.
To determine the titers of infectious progeny in the supernatants of reactivated KSHV-positive cells, 2.3 × 103 HEK 293 cells were seeded in each well of a 96-well plate. The next day, the cells were exposed to serial dilutions of infectious supernatants in a total volume of 100 μl. Plates were incubated at room temperature for 20 min while rocking, centrifuged at 450 × g for 20 min at 32°C, and incubated at 37°C for 3 days. GFP-expressing cells were counted, and the number of GFP-positive cells per ml of infectious supernatant was calculated and expressed in IU/ml. All samples were measured at least in duplicates. Error bars represent the standard deviations.
For titration of infectious progeny in the supernatants of reactivated KSHV-positive cells derived from experiments with the IRE1α-inhibitor IRE1i, TE671 cells (105 cells) were seeded into each well of a 12-well plate. The next day, the cells were mixed with culture supernatants, and the plates were centrifuged at 500 × g for 1 h at room temperature, followed by 37°C incubation for 2 days. The number of GFP-expressing cells was determined by flow cytometry.
STR analysis.
Cell line identification and control for contamination was accomplished by DNA analysis using forensic standards. DNA was extracted from cell sediments by an overnight proteinase K digestion in a Chelex in water (DNA-grade) suspension (10%) at 56°C. A total of nine short-tandem-repeat (STR) polymorphisms were analyzed using the AmpFLSTR Profiler (Applied Biosystems) multiplex PCR kit, including Amelogenin for gender determination. PCR was run on the MJ Research Thermocycler PTC-200 with the standard AmpFLSTR Profiler cycling conditions as proposed by the manufacturer. STR fragments were separated by capillary electrophoresis on an ABI Prism 310 genetic analyzer (Applied Biosystems) in POP-4 polymer; the data were processed with the ABI Prism 310 Genetic Analyzer Data Collection (v2.1) and GeneScan (v3.1) software. Allele calling was performed with the ABI Prism Genotyper (v2.5.2) software.
MTT cell viability assay.
A total of 4 × 104 cells were incubated with indicated inhibitors or control substances in each well of a 96-well plate. After 3 days of incubation the medium was removed, and 100 μl of Opti-MEM I reduced serum medium (Invitrogen) with 0.8 mM MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich] was added to each well. After 1 h of incubation at 37°C in the dark, the MTT medium was removed, and the cells were resuspended in isopropanol (100 μl/well). Finally, the absorbance of the dyed solutions was measured at an optical density at 550 nm (OD550) and normalized to OD620. Each sample was tested at least in triplicates. Graphs display the average metabolism relative to the vehicle treated sample. Error bars represent the standard deviations.
CellTitreGlo cell viability assay.
BrK.219 cells were treated with either dimethyl sulfoxide (DMSO), the IRE1α-inhibitor IRE1i, anti-IgM plus DMSO, or anti-IgM plus IRE1i. All samples were measured for cell viability at 72 h posttreatment using the CellTiter-Glo assay (Promega) according to the manufacturer's instructions.
Kinase inhibitor assay.
The toxicity of all inhibitors was tested by MTT or CellTitreGlo cell viability assay before use in BrK.219, and DMSO concentrations did not exceed 0.1%. For the PI3K experiment, 1 h prior to induction of the lytic cycle, 5 × 105 BrK.219 cells in each well of a six-well plate were treated with the PI3K inhibitors LY294002 (Biomol) and PI-103 (Calbiochem) at the indicated concentrations, an equivalent amount of DMSO (Applichem) or not treated. For the protein kinase/endoribonuclease IRE1α experiment, 1.5 × 106 BrK.219 cells in each well of a six-well plate were treated with the IRE1α-specific inhibitor (IRE1i; Chembridge) at 25 μM, an equivalent amount of DMSO or mock treated. The KSHV lytic cycle was induced by cross-linking of the BCR with anti-human IgM (μ-chain specific; 2.5 μg/ml; Southern Biotech) for 3 days. Finally, supernatants and cells were collected and processed for further analysis by infectivity assay, immunoblotting, or reverse transcription-PCR (RT-PCR).
Use of qPCR to analyze KSHV copy numbers per cell.
To determine the KSHV copy numbers per cell, TaqMan real-time quantitative PCRs (qPCR) directed against the viral open reading frame (ORF) K6 of KSHV were performed as described previously (43). A TaqMan qPCR for the cellular C-reactive protein (CRP) was used for normalization (44). Briefly, genomic DNA was extracted using the DNA blood kit (Qiagen) according to the manufacturer's instructions and eluted in 50 μl of elution buffer EB. A qPCR of KSHV was carried out in a total volume of 50 μl containing a ready-to-use master mix (QuantiTect multiplex PCR kit; Qiagen), 0.5 μM concentrations of each primer, 10 μl of DNA from the sample of interest, and 0.4 μM FAM-labeled KSHV K6 probe. Amplification was performed in the Applied Biosystems 7500 thermal cycler and visualized with ABI 7500 software. qPCR of CRP was carried out in a total volume of 20 μl containing a ready-to-use master mix (LightCycler FastStart DNA Master HybProbe; Roche), 0.3 mM MgCl2, 0.5 μM concentrations of each primer, 0.2 μM FAM-labeled CRP probe, and 5 μl of DNA from the sample of interest. Amplification was performed in the LightCycler 2.0 Instrument and analyzed with the LightCycler software. The primers (Sigma) and probes (Eurogentec) used for the quantification of KSHV and CRP had the following sequences: KSHV K6 forward (CGC CTA ATA GCT GCT GCT ACG G), HHV8 K6 reverse (TGC ATC AGC TGC CTA ACC CAG), CRP forward (CTT GAC CAG CCT CTC TCA TGC), CRP reverse (TGC AGT CTT AGA CCC CAC CC), K6 probe [5′-(6 FAM)-CAG CCA CCG CCC GTC CAA ATT C-TAMARA], and CRP probe [5′-(6 FAM)-TTT GGC CAG ACA GGT AAG GGC CAC C-TAMARA].
Immunoblotting.
For immunoblotting, cells were lysed in 1× sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 10% glycerol, 50 mM DTT, 0.01% [wt/vol] bromophenol blue) with freshly added protease inhibitor (1 mM phenylmethylsulfonyl fluoride [PMSF], 50 μM leupeptin, 100 U of aprotinin/ml, 200 μM benzamidine, 1 μM pepstatin A [in DMSO]) and protein kinase inhibitors (0.5 mM sodium orthovanadate, 0.5 mM NaF, 0.5 mM pyrophosphate, 0.5 mM sodium molybdate, and 0.5 mM β-glycerophosphate) when analysis of the phosphorylation stage of AKT kinase was intended. Samples were briefly sonicated on ice and then boiled at 100°C for 3 min.
For the detection of vIRF-3, the samples were lysed in 1% Triton, 500 mM NaCl, 2 mM EDTA, and 2 mM EGTA in phosphate-buffered saline (PBS), and protease inhibitors (1 mM PMSF), 50 μM leupeptin, 100 U of aprotinin/ml, 200 μM benzamidine, 1 μM pepstatin A [in DMSO]). The cell lysates were briefly sonicated and subsequently cleared by centrifugation at 20,000 × g at 4°C for 10 min. Proteins were separated by SDS-PAGE and electrophoretically transferred onto nitrocellulose membranes. Proteins were detected by the following primary antibodies: mouse anti-β-actin (Chemicon), rabbit anti-phospho AKT (Ser473) (193H12) (Cell Signaling Technology), rabbit anti-AKT1 (Cell Signaling Technology), anti-LANA-1 (KSHV-ORF73 [LNA-1]; Advanced Biotechnologies, Inc. [ABI]), rabbit anti-ORF50/RTA (45), mouse anti-KbZIP (Santa Cruz), mouse anti-K8.1 A/B (ABI), and rat anti-vIRF-3/K10.5 (46).
Chromatin immunoprecipitation and ChIP-on-chip analysis.
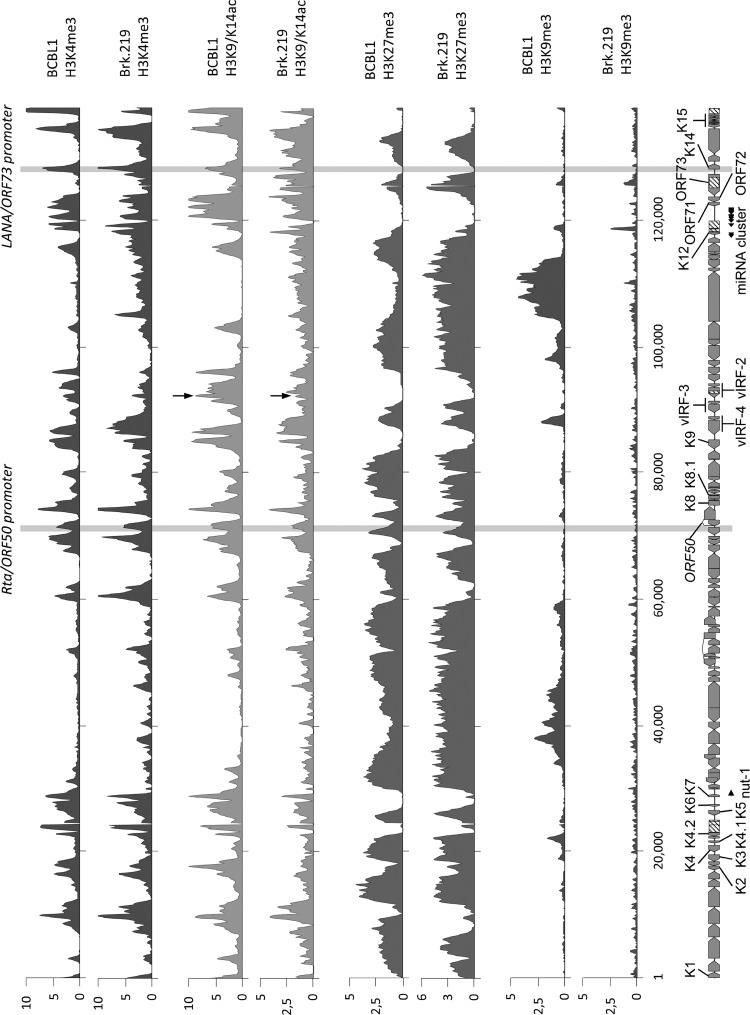
ChIP-on-chip analysis was essentially carried out as described previously (47). Briefly, chromatin was prepared from KSHV-infected BrK.219 cells and subjected to immunoprecipitation with antibodies specific for histone H3 trimethylated at lysine 4, lysine 9, or lysine 27 or acetylated at lysine 9 and/or lysine 14 (H3K4-me3, H3K9-me3, H3K27-me3, and H3K9/14-ac [Upstate/Millipore]) or with a matched isotype control antibody (IgG). The immunoprecipitated material was hybridized together with differentially labeled input DNA from the same sample to a tiled high-resolution microarray covering the complete KSHV genome (NC_009333) and normalization controls (spike-in DNA). For each sample, enrichment was calculated as the fold normalized probe fluorescence in the precipitated versus the input channels. Probes were matched to a sliding 250-bp window, shifted along the KSHV genome in 100-bp increments, and averaged values of matching probes were plotted against the window's center position. We confirmed the array data by performing quantitative real-time PCR from the immunoprecipitated material using primers specific for several loci across the genome (data not shown).
Lentiviral vector preparation and transduction.
The “control” lentiviral vector (IRES-GFP alone) and the “active XBP-1” lentiviral vector (encoding XBP-1 IRES-GFP) were generated as described previously (20). Both lentiviral vectors were titrated on HEK 293T cells by flow cytometry to determine the number of GFP-expressing cells. BJAB cells were also transduced with both lentiviral vectors and analyzed by fluorescence-activated cell sorting (FACS) to ensure comparable infection levels. BrK.219 cells (2 × 105 cells) were then transduced with either the “control” or the “active XBP-1” vectors at an input equivalent to an MOI of 1 on HEK 293T cells.
RNA extraction and RT-PCR.
Total RNA was purified and reverse transcribed as previously described (69) with modification. Briefly, RNA was extracted from 2 × 106 BrK.219 cells with RNAzol (Sigma), followed by lithium precipitation. The splicing of XBP-1 mRNA was analyzed by PstI restriction as described previously (20). For the detection of the KSHV late gene ORF29, serial dilution (3 × 10-fold) of oligo(dT) (Qiagen)-primed cDNA was used for PCR amplification (Promega) using forward (5′-GCA CAC ACG TAA CTG ACC AC-3′) and reverse (5′-CAT TGG GTA CGT AGC CCA CC-3′) primers. All samples were normalized by PCR amplification of β2-microglobulin (β2M) using serially diluted cDNA with forward (5′-TGA CTT TGT CAC AGC CCA AG-3′) and reverse (5′-TCT CTG CTC CCC ACC TCT AA-3′) primers. Both sets of primers amplify across splice junctions and the sizes of the expected products were as follows: ORF29 (cDNA, 399 bp; DNA, 3,650 bp) and β2M (cDNA, 301 bp; DNA, 2,178 bp).
5′-RACE.
Total RNA was extracted using an RNeasy minikit (Qiagen), and potential residual DNA was digested by using RNase-free DNase I (Ambion) according to the manufacturer's instructions. The RNA concentration was quantified by using a NanoDrop ND-1000 UV/Vis-Spectrophotometer (Peqlab). For the reverse transcription of the total RNA and the subsequent PCR of 5′ ends, the SMARTer RACE kit (Clontech) was used according to the manufacturer's guidelines. MasterAmp Taq DNA polymerase (Epicentre) was used for the RACE (rapid amplification of cDNA ends) PCR with the gene-specific primer 5-RACE 286 (5′-TCG TAC TCT TCA CCT GGC AA-3′) under the following conditions: 10 cycles at 94°C for 30 s, 68°C for 30 s and 72°C for 180 s, 40 cycles at 94°C for 30 s, 64°C for 30 s, and 72°C for 180 s, and subsequently, 1 cycle at 72°C for 600 s. Each sample was subjected to agarose gel electrophoresis in low-melting-point agarose and ethidium bromide staining. Fragments were excised and extracted using the QIAquick gel extraction kit (Qiagen) according to the manufacturer's instructions. Recovered DNA fragments were cloned into a pGEM-T Easy Vector system (Promega), and plasmid DNA was prepared. Sequences were obtained for several clones of each fragment with M13 for primer (5′-CGC CAG GGT TTT CCC AGT CAC GAC-3′) using an ABI Prism 310 genetic analyzer.
Immunofluorescence assay.
Suspension cells were washed twice with PBS and resuspended in a low volume of PBS. Then, 2 μl of the cell suspension was plated onto SuperFrost Plus (Fisher Scientific) slides. Samples were dried, encircled with a PAP pen (G. Kisker), fixed with 3% paraformaldehyde for 10 min at room temperature, quenched immediately with 50 mM ammonium chloride solution (NH4Cl4) for 10 min at room temperature, and washed three times with PBS for 10 min each time. After blocking with 10% FCS and 1% bovine serum albumin in PBS, the samples were stained with a mouse monoclonal antibody specific for LANA-1 (NCL-HHV8-LNA [Novocastra], 1:100) and a Cy5-conjugated anti-mouse antibody (Jackson ImmunoResearch) as the secondary antibody (1:200).
RESULTS
Epigenetic patterns of latent KSHV in infected BJAB cells.
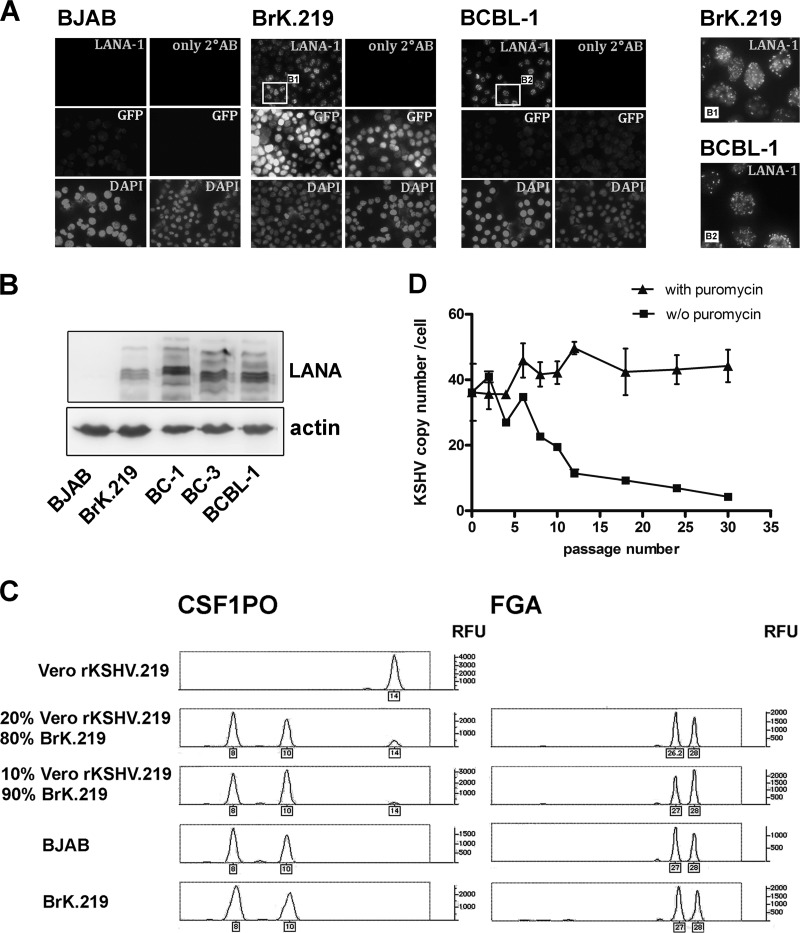
We infected the KSHV- and EBV-negative lymphoblastoid cell line BJAB with a recombinant KSHV, rKSHV.219 (39), by either cell-to-cell transmission (data not shown) or with cell-free virus (Fig. 1), with the intention of establishing a surface IgM expressing B cell line that would be stably infected with KSHV. The recombinant rKSHV.219 contains a puromycin resistance gene, a constitutively expressed GFP, and an RFP under the control of the lytic PAN promoter (39). Infection of BJAB cells with rKSHV.219 virus at an MOI of 1 to 15 in the presence of Polybrene and subsequent 3-week puromycin selection resulted in the establishment of KSHV- and GFP-positive cultures, here referred to as BrK.219 cell lines. Immunofluorescence staining for LANA (LANA-1 [latency-associated nuclear antigen 1]) in these cells showed the typical speckled nuclear pattern seen in KSHV-infected cells (Fig. 1A). Expression of LANA could also be detected by Western blotting (Fig. 1B, Fig. 3A and C, Fig. 4A and B, and Fig. 6B). We used STR analysis to confirm that the KSHV-infected cell lines generated in this manner were indeed of BJAB origin (Fig. 1C).
Fig 1.
Establishment of BrK.219 cells, LANA expression, and persistence of viral genomes. (A) Immunofluorescence staining of the latent viral nuclear antigen LANA was carried out in BrK.219 cells, the uninfected parental BJAB cell line, and the PEL cell line BCBL-1. Cells infected with rKSHV.219 express GFP constitutively. Samples were fixed, blocked, and incubated either with primary antibody against LANA, or without primary antibody, followed by a Cy5-conjugated secondary antibody. Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole). Magnified images of the boxed regions B1 and B2 are shown on the right. (B) Expression of LANA shown by immunoblotting. (C) BrK.219 cells are BJAB-derived. BrK.219 cells established by coculture with rKSHV.219-infected Vero cells as described in Materials and Methods were analyzed using STR analysis for two gene loci, CSF1PO and FGA. The result indicates that BrK.219 cells and BJAB cells share the same genetic profile but differ from Vero cells. (D) Persistence of KSHV genomes in the presence or absence of puromycin. Samples were taken twice a week, and the number of KSHV genomes was measured by qPCR and normalized to CRP (a cellular gene) copy numbers.
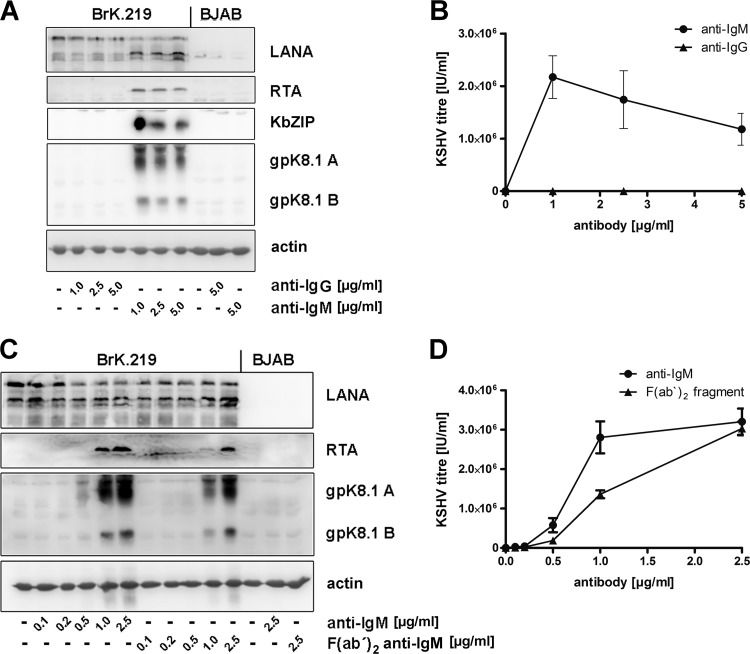
Fig 3.
Latent KSHV in BrK.219 cells is reactivated following triggering of the BCR. BrK.219 cells and their uninfected counterparts were treated with increasing amounts of antibodies against IgM or IgG for 3 days. (A) Expression of latent (LANA), immediate-early (RTA), early (KbZIP), and late (gpK8.1) proteins shown by immunoblotting. (B) Infectious KSHV titers released from antibody-stimulated BrK.219 cells, as measured on HEK 293 cells. (C) Expression of viral proteins in BrK.219 cells treated with anti-IgM and F(ab′)2 anti-IgM. (D) Infectious KSHV titers released from BrK.219 cells treated with anti-IgM or F(ab′)2 anti-IgM, as measured on HEK 293 cells.
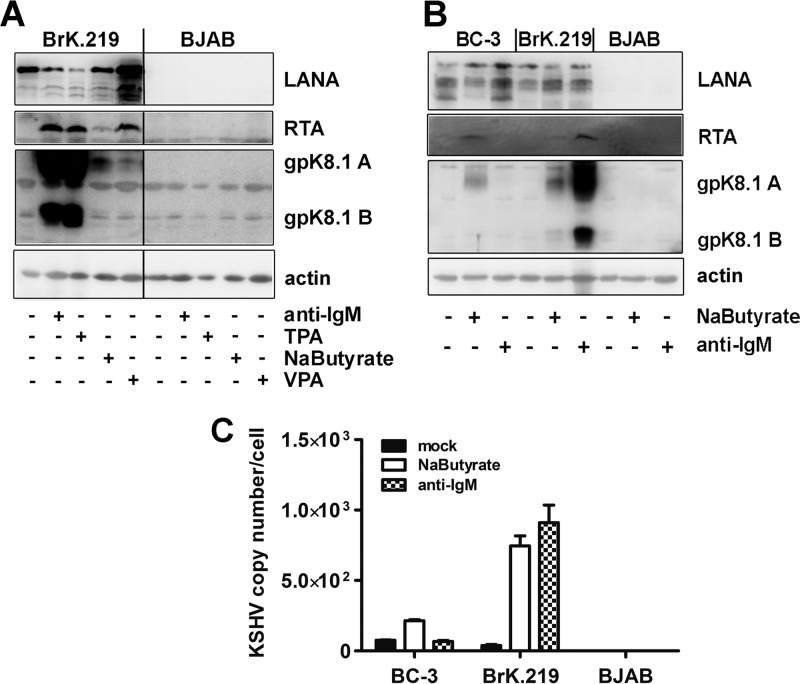
Fig 4.
Comparison of KSHV reactivation induced by antibody against IgM with other chemical stimuli in BrK.219 and in PEL cells. (A) BrK.219 cells and the uninfected parental cell line BJAB were treated with different inducers of the KSHV lytic cycle (2.5 μg of anti-IgM/ml, 50 ng of TPA/ml, 1.25 mM sodium butyrate, 1.25 mM valproic acid [VPA]) for 3 days, and the expression of viral proteins was detected by immunoblotting as described in the legend of Fig. 2. (B) The PEL cell line BC-3, BrK.219 cells, and parental BJAB cells were treated with anti-IgM or sodium butyrate, and the expression of viral proteins was monitored by immunoblotting. (C) KSHV genome copy number in induced BC-3 and BrK.219 cells, as measured by qPCR. The data shown are means of triplicates with the standard deviations.
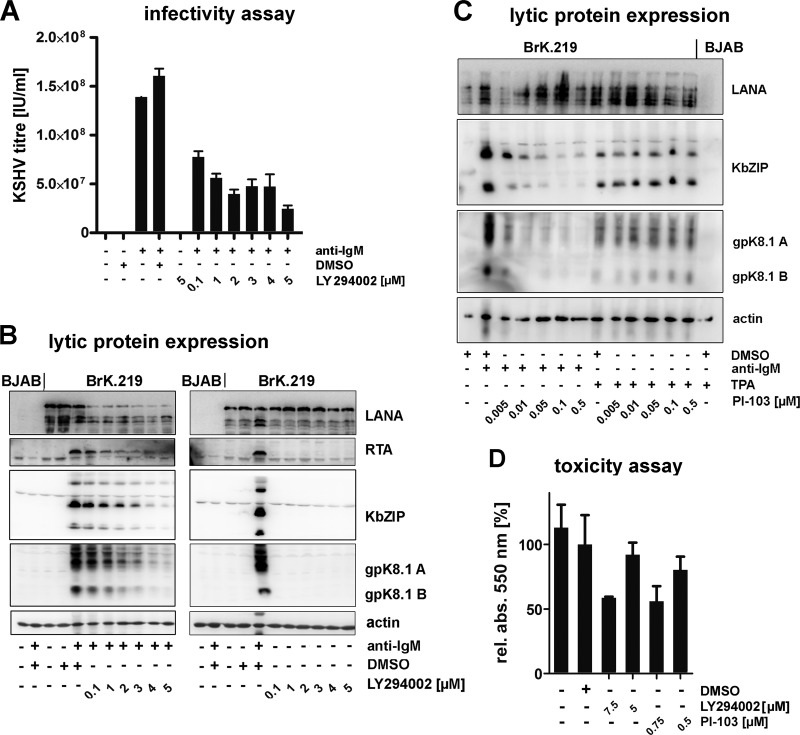
Fig 6.
PI3K is important for the reactivation of latent KSHV following triggering of the BCR. (A) LY294002 inhibits the reactivation of latent KSHV following triggering of the BCR. BJAB and BrK.219 were treated for 1 h with increasing amounts of inhibitor or vehicle control (DMSO). Next, reactivation was induced by the addition of antibodies against IgM (2.5 μg/ml). After incubation for 3 days, supernatants were titered on HEK 293 cells. The results are represented as the means of duplicate samples ± the standard deviations. (B) Cell lysates of the same experiments (left panel) and their nonreactivated control samples (right panel) were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies. (C) PI-103 inhibits KSHV reactivation induced by treatment with anti-IgM but not with TPA (2 ng/ml). (D) The metabolic activity of BrK.219 cells treated for 3 days with the inhibitor LY294002 (5 or 7.5 μM), PI-103 (0.5 or 0.75 μM) or vehicle control (DMSO) was analyzed by MTT assay. The results are presented as the means of triplicates ± the standard deviations.
Unlike PEL cell lines, which remain KSHV-positive during continued passaging, BrK.219 cells showed a rapid decline of KSHV episome number in the absence of puromycin selection (Fig. 1D). The episomal loss was also reflected in a loss of GFP expression and was not due to lytic replication, since no increase of RFP expression was observed (data not shown). Therefore, we were able to infect BJAB cells stably with recombinant KSHV, but this required continuous antibiotic selection.
It has been suggested that the stable persistence of EBV (48), as well as of KSHV (49), in newly infected cells may be linked to (so-far-unidentified) epigenetic modifications of the viral genome. Minor differences in the histone modification patterns of PEL cell lines and de novo-infected endothelial cells have been reported before (47). We therefore compared the histone modification pattern of BrK.219 cells with patterns previously reported for a PEL cell line, BCBL-1 (Fig. 2). ChIP-on-chip analysis of histone modification patterns revealed global H3K4-me3, H3K9/K14-ac, and H3K27-me3 profiles that are highly similar (Pearson correlation coefficients of 0.67, 0.49, and 0.65, respectively) to those seen in PEL cell lines (47). In contrast, although a few genomic regions exhibit H3K9 trimethylation (H3K9met) in PEL cells, this constitutive heterochromatin mark is largely absent from BrK.219 cells, as already previously reported for de novo-infected endothelial cells (Fig. 2) (47).
Fig 2.
Comparison of global histone modification patterns of KSHV genomes in BrK.219 and PEL cells. Global patterns of histone H3 trimethylated at lysine 4 (H3K4-me3), lysine 9 (H3K9-me3), or lysine 27 (H3K27-me3) or acetylated at lysine 9 and/or lysine 14 (H3K27-me3 and H3K9/14-ac). Modification patterns in BrK.219 cells were analyzed by ChIP-on-chip analysis using high-resolution KSHV tiling microarrays. Values shown on the y axis represent relative enrichment of normalized signals from the immunoprecipitated over input material. Histone modification patterns in the PEL line BCBL-1 (47) are shown for comparison. Regions highlighted in gray indicate the position of the ORF50/Rta and the ORF73/LANA promoters, both of which carry activating H3K4-me3 and H3K9/14-ac marks. In addition, an H3K19/14-ac peak, located upstream of the vIRF-3 coding region and marked by arrows, is more prominent in BCBL-1 compared to BrK.219 cells, which may reflect the lower basal expression levels of vIRF-3 during latency in BrK.219 cells. The ORF50 promoter additionally acquires repressive H3K27-me3 marks and thus exhibits the hallmarks of bivalent chromatin, which is transcriptionally silent, but “poised” for reactivation (47).
Latent KSHV reactivates upon cross-linking of the BCR.
B-cell receptor (BCR)-initiated signaling pathways regulate the development, activation and differentiation of B cells. In vivo, cross-linking of the BCR is induced by specific antigens and leads to differentiation of B cells into memory B cells or antibody-secreting plasma cells (50–52). This BCR-mediated plasma cell differentiation has been suggested to induce the reactivation of EBV and MHV68 in memory B cells (18, 53–55). In B cells latently infected with EBV reactivation was inducible in vitro by cross-linking of the BCR with antibodies to surface immunoglobulins (25, 26). Since PEL cell lines are derived from pre-plasma cells and therefore do not express the BCR, it has thus far not been possible to investigate the reactivation of KSHV in B cells as a result of BCR engagement (34–38). We investigated this question by treatment of the BrK.219 cells with soluble anti-IgM antibodies. We observed that treatment with anti-IgM antibodies induced the expression of viral lytic proteins (e.g., immediate-early RTA, early KbZIP, and late K8.1) (Fig. 3A) and virus production (Fig. 3B). This effect was specific for anti-IgM since treatment with antibodies to IgG did not induce the KSHV lytic cycle in the BrK.219 cells (Fig. 3A and B). F(ab′)2 fragments of anti-IgM antibodies were as effective as complete anti-IgM antibodies (Fig. 3C and D), indicating that an interaction of anti-immunoglobulin antibodies with Fc receptors does not contribute to the activation of the lytic cycle. Both the amount of lytic viral proteins and the number of infectious viral particles increased with increasing anti-IgM concentration in the culture and reached maximum levels at antibody concentrations between 1 and 2.5 μg/ml (Fig. 3B and D). All further experiments on BCR-mediated KSHV reactivation were carried out with the anti-IgM antibody at a concentration of 2.5 μg/ml. Thus, BCR-mediated B cell activation leads to the reactivation of recombinant KSHV.219 in our BrK.219 cell lines.
We systematically screened ∼50 BrK.219 cell lines, generated by cell-free infection as described above, for their potential to reactivate lytic KSHV replication upon stimulation with anti-IgM. Of these, 12 produced substantial infectious titers in excess of 2 × 105 IU of reactivated KSHV/ml in response to treatment with anti-IgM antibody, as assessed by an infectivity assay on HEK 293 cells (data not shown). All of the following experiments were performed with BrK.219 cell lines, in which KSHV reactivation with anti-surface immunoglobulin antibodies lead to production of more than 2 × 105 IU/ml.
Efficiency of lytic cycle reactivation by anti-IgM antibodies and other stimuli.
Several chemicals, including the phorbol ester TPA, sodium butyrate, and valproate, are known to reactivate KSHV from latency in PEL cell lines (16, 56). Therefore, we compared how efficiently antibodies to surface IgM and these chemicals activate KSHV lytic replication in BrK.219 and PEL cell lines. As shown in Fig. 4A, TPA, sodium butyrate, and valproic acid can induce the viral lytic replication cycle in BrK.219 cells. As assessed by Western blotting for the lytic glycoprotein K8.1, anti-IgM and TPA were the strongest inducers of the lytic cycle in BrK.219 (Fig. 4A). Although the three chemical compounds induced viral reactivation in both PEL cell lines and BrK.219 (Fig. 4B and C, Fig. 5B [bottom panel], and data not shown), anti-IgM antibody reactivates lytic viral protein expression only in BrK.219 cells, which express the BCR on their surfaces, but not in BCR-negative PEL cell lines (Fig. 4B and C and Fig. 5B). Cell-free virions derived from infected BJAB cells were not only able to infect HEK 293 cells, as demonstrated in infectivity assays (Fig. 3B and D), but could also infect BJAB cells, as well as other cell lines, including primary human umbilical vein endothelial cells, BCP-1, HuAR2T, Vero, and HeLa cells (data not shown).
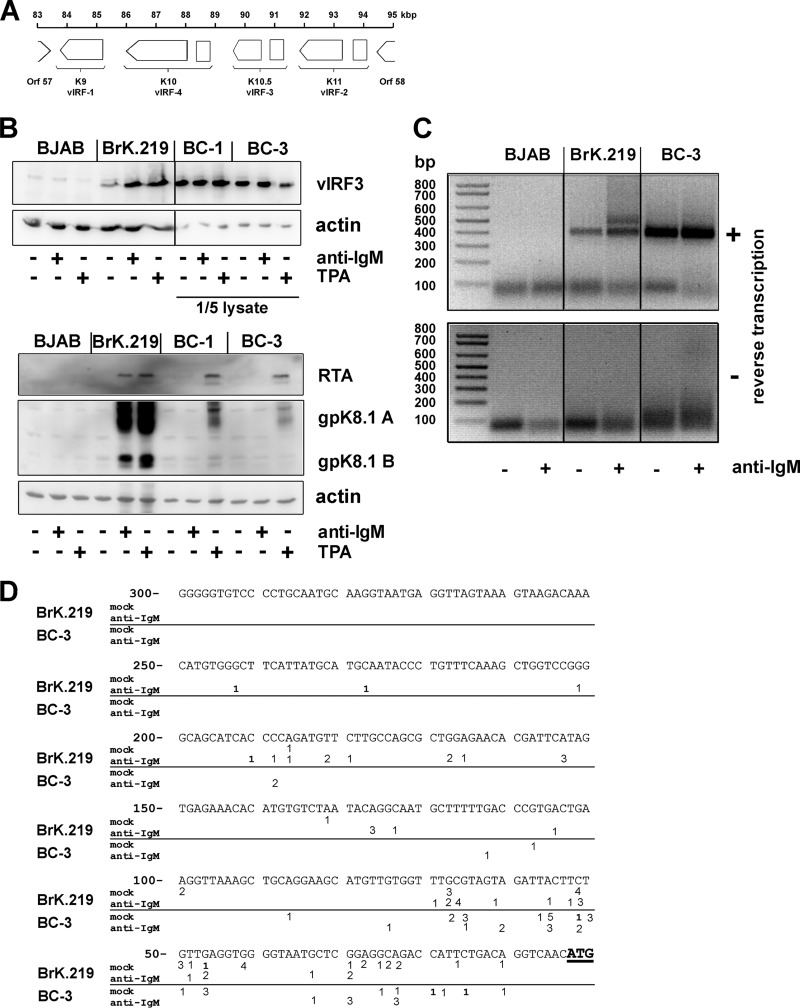
Fig 5.
vIRF-3 is an inducible gene with two 5′ start sites in BrK.219 cells. (A) Gene arrangement in the vIRF region shown between ORFs 57 and 58. (B) BJAB cells, their rKSHV.219-infected counterparts (BrK.219), and PEL cell lines (BC-3 and BC-1) were treated with either 2.5 μg of anti-IgM/ml or 25 ng of TPA/ml for 3 days or left untreated as a control. Viral protein expression was investigated by immunoblotting. For analysis of vIRF-3 expression in PEL cells, only 1/5 of the cell lysates were used. (C) 5′-RACE of vIRF-3 transcripts. BrK.219 cells, uninfected parental BJAB cells, and the PEL cell line BC-3 were treated for 3 days with anti-IgM or left untreated. Reverse transcription and PCR was performed with total RNAs. Agarose gels show the products of the 5′-RACE, which was also performed on RNA samples without reverse transcription to control for the potential amplification of contaminating DNA. (D) Location of 5′ start sites of vIRF-3 mRNAs determined by 5′-RACE. The 5′-RACE products as shown in panel C of two independent experiments were cloned, and multiple clones were sequenced to determine their 5′ ends. Numbers (ranging between 1 and 5) reflect the frequency and position of individual 5′ start sites. Whereas in BC-3 cells most transcripts originate within the first 100 bp upstream of the vIRF-3 ATG, in anti-IgM-stimulated BrK.219 cells, additional peaks of initiation are located up to further 150 bp upstream at position −101 to position −250.
Expression pattern of vIRF-3 in BrK.219 cells.
KSHV encodes four proteins with homology to the family of interferon regulatory factors (IRFs) (Fig. 5A). Expression of viral IRF-3 (vIRF-3, K10.5, or LANA-2) is restricted to KSHV-infected lymphoblastoid cells (57) and was shown to be essential for the survival of PEL cell lines (46). vIRF-3 is classified as a latent gene in PEL cells (57) since it is not induced during lytic reactivation (46). We also detected vIRF-3 expression in BrK.219 cells (Fig. 5B). However, in BrK.219 cells, in contrast to PEL cell lines, vIRF-3 protein levels increased upon reactivation of KSHV, suggesting an inducible gene expression pattern (Fig. 5B). In accord with the observation of lower basal vIRF-3 expression levels in latently infected cells, we found that a prominent histone acetylation (H3K9/K14ac) peak located ∼500 bp upstream of the vIRF-3 coding region in PEL cells was markedly reduced in noninduced BrK.219 compared to BCBL-1 cells (see the region marked with an arrow in Fig. 2).
In PEL cell lines, the latently expressed vIRF-3 transcript has variable 5′ ends, thought to result from a promoter that lacks a TATA box (58). Since all other KSHV IRFs (vIRF-1, vIRF-2, and vIRF-4) are classified as lytic genes with a well-defined transcriptional start site, we investigated whether the inducible vIRF-3 gene expression pattern in BrK.219 cells resulted from the use of an alternative transcriptional start site. We carried out a 5′-RACE experiment on vIRF-3 transcripts from BrK.219 cells reactivated with anti-IgM antibody (Fig. 5B and C). In contrast to PEL cell lines, which yielded a single broad band in 5′-RACE (only the result for BC-3 is shown in Fig. 5C), an additional slower-migrating band was detectable in the BrK.219 cell line. Sequencing of both bands revealed that the additional RACE product observed in anti-IgM-treated BrK.219 cells corresponds to multiple start sites located up to 150 bp upstream of the transcript variant predominating in BC-3 PEL cells (Fig. 5D). The results indicate the presence of additional transcript variants of vIRF-3 in anti-IgM-treated BrK.219 cells compared to latently infected PEL cells (BC-3) and mock-treated BrK.219 cells (Fig. 5C and D).
BCR-dependent reactivation of latent KSHV is mediated by PI3K.
The possibility to reactivate latent KSHV by cross-linking of the BCR enabled us to investigate intracellular signaling cascades involved in this reactivation. Since PI3K has been reported to mediate the reactivation of EBV following BCR cross-linking (27, 28), we blocked PI3K activity using the small molecule inhibitor LY294002 prior to activating KSHV by anti-IgM stimulation (Fig. 6). LY294002 inhibited both the production of infectious KSHV (Fig. 6A) and the increased expression of immediate-early (RTA), early (KbZIP), and late (gpK8.1) viral proteins (Fig. 6B, left panel) induced by triggering BrK.219 cells with anti-IgM. Although the expression of LANA decreased in reactivated cells treated with LY294002 (Fig. 6B, left panel), this decrease was not observed in latently infected cells (Fig. 6B, right panel) and therefore not due to LY294002-induced toxicity. To exclude toxic effects, we also assessed the effects of LY294002 in a MTT cell viability assay (Fig. 6D) and found it to be nontoxic at concentrations of ≤5 μM. At a concentration of 5 μM, LY294002 effectively inhibited the phosphorylation of AKT, a target of PI3K (data not shown).
To confirm the involvement of PI3K in the BCR-mediated reactivation of KSHV in B cells, we used a second PI3K inhibitor, PI-103. PI-103 also inhibited KSHV reactivation in BrK.219 cells triggered by anti-IgM at nontoxic concentrations (Fig. 6C and D). In contrast, the reactivation of KSHV by TPA, a strong unspecific inducer of several signaling pathways, was not affected by PI-103 (Fig. 6C). Together, these results suggest that BCR-mediated reactivation of KSHV in BrK.219 cells involves the PI3K pathway.
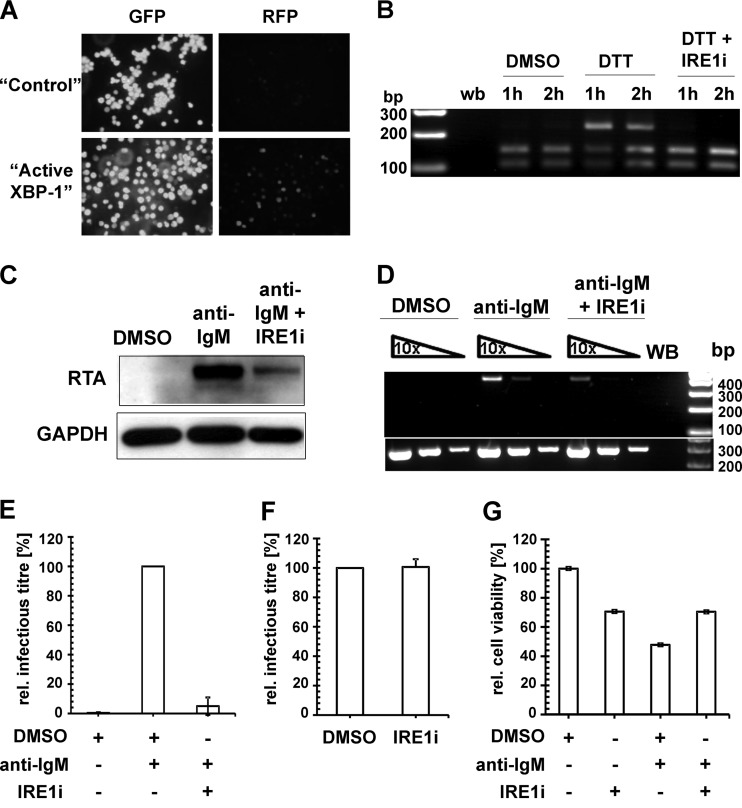
KSHV reactivation from latency following BCR activation also requires active XBP-1.
Terminal differentiation of B lymphocytes is triggered by the activation of BCR. The cross-linking of BCR on the surface of BrK.219 cells with anti-IgM antibodies induces the signaling cascade leading to the differentiation into plasma cells, which is dependent on the transcription factor XBP-1 (24). We and others have identified XBP-1 as an authentic cellular lytic switch for KSHV in PEL (20, 55). To establish whether XBP-1 can reactivate KSHV in BrK.219, we transduced BrK.219 cells with lentiviral vectors expressing either GFP or the active form of XBP-1 and observed a large fraction of cells expressing RFP in the latter population (Fig. 7A), indicating the ectopic expression of XBP-1 is sufficient to induce the lytic cycle in BrK.219 cells. To determine whether induction of KSHV lytic replication by BCR cross-linking operated through the activation of XBP-1, we validated the inhibition of XBP-1 splicing in BrK.219 cells (Fig. 7B) by an IRE1α-specific inhibitor (IRE1i) (59), and we demonstrated reduced levels of KSHV RTA protein induction (Fig. 7C) and KSHV ORF29 transcription (Fig. 7D) in BrK.219 cells in the presence of IRE1i following anti-IgM treatment. In contrast, IRE1i had a negligible effect on both RTA expression and ORF29 transcription in BrK.219 cells activated by TPA (data not shown), indicating that IRE1i did not exert a general inhibitory effect on KSHV reactivation. The inhibition of the anti-IgM mediated KSHV reactivation by IRE1i also translated into a significant reduction in virion production (Fig. 7E). Since the inhibitor IRE1i was present in the culture supernatant used for KSHV titration, it is possible that the observed reduction in KSHV titer is mediated by an unknown antiviral effect of IRE1i, thereby blocking KSHV infection of TE671 cells (used for titration). To rule out such a possibility, we mixed IRE1i with the supernatant obtained 3 days after anti-IgM treatment and showed almost identical infection levels compared to the DMSO control (Fig. 7F). In addition, BrK.219 cells treated with IRE1i in combination with anti-IgM exhibited higher levels of cell viability compared to the anti-IgM plus DMSO control (Fig. 7G), indicating that the observed reduction in KSHV titer in the presence of IRE1i is not due to cytotoxicity. Taken together, the data suggest that BCR activation triggers XBP-1 splicing, which in turn contributes to the induction of KSHV reactivation in BrK.219 cells.
Fig 7.
Lytic KSHV induction following BCR activation requires the splicing of XBP-1 mRNA. (A) Confocal images of BrK.219 cells transduced with lentiviral vectors expressing either GFP (“control”) only or the spliced form of XBP-1 (“active XBP-1”). Expression of RFP marks RTA-expressing BrK.219 cells. (B) RT-PCR analysis of the DTT-induced splicing of XBP-1 transcript in BrK.219 cells with or without treatment with IRE1α inhibitor (IRE1i; 25 μM). cDNA products derived from the spliced XBP-1 mRNA are resistant to PstI digestion (upper band, 223 bp), whereas the products for the unspliced XBP-1 mRNA are cleaved (the two lower bands, 104 and 145 bp). WB denotes water blank control. The band sizes (in bp) of the DNA ladder are as indicated. (C) Western blot analysis of KSHV RTA induction by anti-IgM antibodies (1 μg/ml) with or without IRE1i (25 μM) treatment. (D) Semiquantitative RT-PCR analysis of a KSHV late gene (ORF29) expression at 48 h posttreatment (upper panel), using serially 10-fold-diluted cDNA obtained from BrK.219 cells treated with either DMSO, anti-IgM antibodies (1 μg/ml), or anti-IgM antibodies plus IRE1i (25 μM). Input cDNA was normalized to a cellular gene (β2M lower panel). Primers for both virus and cellular genes span across intron(s), and no amplification of genomic DNA was observed under the PCR conditions used. The data from one representative experiment out of two independent experiments are shown. (E) Relative KSHV titer in supernatants from BrK.219 cells 3 days after BCR cross-linking, with or without the presence of IRE1i. Cell supernatants from BrK.219 cells treated with either DMSO, anti-IgM, or anti-IgM plus IRE1i were titrated on TE671 cells. The data are shown as the relative infectious titer where all samples were normalized to the positive control (anti-IgM) as 100% and plotted as means ± the standard deviations from three independent experiments. (F) Effect of IRE1i on the relative KSHV titer of anti-IgM induced supernatant from BrK.219 cells. The cell supernatant from BrK.219 cells induced with anti-IgM was split into two, and each half mixed with either DMSO or IRE1i; the resulting supernatants were titrated on TE671 cells. The data are shown as relative infectious titer where all samples were normalized to the positive control (anti-IgM plus DMSO) as 100% and plotted as means ± the standard deviations from three independent experiments. (G) The relative cell viability of BrK.219 cells treated with either DMSO, IRE1i, anti-IgM plus DMSO, or anti-IgM plus IRE1i is plotted as the percent concentration of ATP (proportional to the number of live cells) normalized to the DMSO control as 100%. The data (means ± the standard deviations) from one representative experiment out of two independent experiments are shown.
DISCUSSION
Many lymphotropic viruses, such as EBV, KSHV, or the related murine herpesvirus MHV68, use B cells as their latent reservoir. In latently infected B cells, initiation of plasma cell differentiation may cause viral reactivation, as has been demonstrated in vitro for EBV and MHV68 in a model of B cell activation that involves cross-linking of the B cell receptor (BCR) with anti-immunoglobulins (25, 26, 31).
De novo KSHV infection occurs in CD19-positive B cells (60), especially in IgM(λ) B cells (61). The commonly used model system for KSHV latency, the PEL cells, are (post)germinal center (GC)-derived B cells, displaying only a few markers of B cell differentiation (62, 63). They lack the BCR on their surface but typically express the postgerminal center B cell marker CD138 (syndecan-1), suggesting that they are in a pre-plasma cell-like stage (34–38). In contrast, the human germinal center-like BJAB lymphoma cells (64), used to establish our system, are mature B cells with the expression of IgM (65) and CD19 on their surface. Consequently, KSHV-infected BJAB cells might serve as a model to investigate KSHV effects in mature B cells that respond to triggering of their surface IgM receptor by cognate antigen. Here, we show for the first time that BCR activation also triggers KSHV reactivation and that this involves a PI3K-dependent signaling pathway and the active XBP-1.
Most of the research conducted on KSHV reactivation in B cells has thus far been carried out in cell lines established from PEL samples. These lines are stably infected with KSHV, and the viral lytic replication cycle can be induced with chemical compounds such as TPA, sodium butyrate, and valproate and/or the overexpression of the viral lytic cycle activator RTA (16, 56, 66, 67). However, owing to their pre-plasma cell differentiation stage (34–38) and consequent absence of surface IgM, the role of the BCR cannot be studied in PEL cells.
We therefore generated BJAB cell lines stably infected with recombinant KSHV.219 using either a cell-free infection protocol (see Materials and Methods) or cell-cell transmission (data not shown). The KSHV genome copy number in the derived BrK.219 cell lines is comparable to PEL cell lines (Fig. 4C), but the stable persistence of viral episomes requires continuous selection with puromycin (Fig. 1D). This resembles what has been observed with other cell lines that were experimentally infected with KSHV. It has been suggested that epigenetic modifications of the latent viral genome may be responsible for successful persistence in the absence of a selection marker (49), but the nature of such modifications is still unknown. Similar to de novo-infected endothelial cells (47), KSHV episomes in latently infected BrK.219 cells adopt epigenetic profiles that are akin to those observed in PEL lines. Notably, activation-associated histone modifications are not simply excluded from lytic gene regions during latency. Rather, latent episomes acquire abundant H3K27-me3, a facultative heterochromatin mark that mediates transcriptional repression by polycomb group (PcG) proteins. A number of loci, including the ORF50/Rta promoter, become bivalently modified (i.e., they carry both activating H3K4-me3 and repressive H3K27-me3 marks) and hence are repressed but remain “poised” for rapid reactivation. Interestingly, although a few regions exhibit H3K9 trimethylation (H3K9-me3) in PEL cells, this constitutive heterochromatin mark is largely absent from de novo-infected BrK.219 and endothelial cells (Fig. 2) (47). These data suggest that H3K9-me3 is not acquired during primary latency establishment or within the first few weeks of a de novo infection but rather may progressively replace H3K27-me3 during long-term latent infection at a select number of loci, potentially leading to further stabilization of viral latency.
Treatment of BrK.219 cells with anti-IgM, but not anti-IgG results in a strong reactivation of KSHV, as demonstrated by the expression of immediate-early (RTA), early (KbZIP), or late (gpK8.1) viral proteins and the production of significant titers of infectious KSHV (Fig. 3 and 4). The amount of infectious progeny produced after stimulation with anti-IgM ranges from 2 × 105 to 106 IU/ml in 12 of ∼50 independently established KSHV-infected B cell lines (data not shown). Reactivation of BrK.219 cells is twice or even three times more efficient than reactivation of the PEL cell lines BC-1 and BC-3, measured by the KSHV copy numbers per cell (data not shown) and by the expression of viral lytic proteins (Fig. 5B, lower panel). However, reactivation of our most potent KSHV production cell line, Vero rKSHV.219, yielded KSHV copy numbers per cell that were up to 15 to 20 times those found in reactivated BrK.219 (data not shown). When examining the expression of viral proteins in BrK.219 cell lines, we noticed that vIRF-3/K10.5/LANA-2, a constitutively expressed latent protein in PEL cells (57, 68), is more strongly expressed after activation of the lytic replication cycle in BrK.219 cells. This is accompanied by the expression of a vIRF-3/K10.5 transcript with an extended 5′-untranslated region (Fig. 5). Similar to the latent vIRF-3 transcript in PEL cells, where expression is controlled by a promoter lacking a TATA box and does not initiate at a single, well-defined transcriptional start site (58), the “lytic” vIRF-3/K10.5 transcript has multiple start sites located ∼100 bp upstream of the start sites most commonly used by the latent transcript in PEL cells (Fig. 5D). The inducible gene expression pattern in BrK.219 cells is accompanied by a reduced H3K9/K14 acetylation upstream of the vIRF-3 gene (Fig. 2). Since all other KSHV vIRFs show increased expression after activation of the lytic replication cycle (68–72), and since vIRF-3 expression has been shown to be crucial for the survival of PEL cells (46), we speculate that the “default” expression pattern of vIRF-3 in B cells may be similar to BrK.219 cells (i.e., may be inducible) but that its expression has been fixed in a constitutive manner in PEL cells by increased H3K9/K14 acetylation to allow their survival and expansion as a monoclonal B cell population. In this, the constitutive expression of vIRF-3 in PEL cells would resemble the EBV latent Wp promoter-driven expression of the lytic viral bcl-2 homologue, BHRF1, which has been shown to result from alternative splicing and contribute to the survival of Burkitt's lymphoma cells (73).
It is well established that BCR stimulation/B cell activation leads to plasma cell differentiation. Several studies on both EBV and KSHV focused on cellular proteins involved in plasma cell differentiation to understand their role in both inhibition and initiation of viral reactivation (74). A recent report showed that EBV latent membrane protein 1 (LMP-1) downregulates the B-lymphocyte-induced maturation protein 1 (BLIMP-1) expression in germinal center (GC) B cells and thereby inhibits their differentiation into plasma cells and subsequently the reactivation of EBV (75). In the case of MHV68, it was shown that the viral protein M2 can directly induce the expression of cellular proteins for plasma cell differentiation and thus viral reactivation (54). In vivo BCR-mediated initiation of plasma cell differentiation depends on IRE-1 and XBP-1, both of which are important mediators of BCR signaling (23, 24). Ectopic expression of the active XBP-1 has previously been demonstrated to promote KSHV reactivation in PEL cell lines (20, 55).
Consistent with these reports, we show that XBP-1 is also required for the reactivation of KSHV in BCR-positive B cells following BCR stimulation. It is possible that PI3K and XBP-1 act in a common pathway, since recent publications demonstrate an interaction of the regulatory PI3K subunits with the active XBP-1 (32, 33). This promotes the unfolded protein response (32, 33), leading to the initiation of plasma cell differentiation in primary B cells and, in the case of BrK.219 cells, lytic replication of KSHV.
Based on these findings, we established an infection model system that allows investigation of the entire process of KSHV reactivation starting from BCR cross-linking, continuing through signaling cascades involving partners such as PI3K and XBP-1, and going all the way to infectious virion production.
ACKNOWLEDGMENTS
We thank Christian Michaelis and Jessica Rückert for their support with DNA preparations, Sabine Hübner for qPCR to analyze CRP and KSHV copy numbers per cell, Sandra Flucht and Jenny Witthuhn for sequencing, and Martin Mynarek for the critical revision of the manuscript. We also thank Jeffrey Vieira and Patricia O'Hearn for the rKSHV.219 construct. The vIRF-3 antibody was a generous gift from Elisabeth Kremmer and Frank Neipel.
This study was supported by the Collaborative Research Center 900 of the Deutsche Forschungsgemeinschaft and the European Union Integrated Project INCA (LSHC-CT-2005-018704).
Footnotes
Published ahead of print 15 May 2013
REFERENCES
- 1. Cesarman E. 2011. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 305:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor GS, Blackbourn DJ. 2011. Infectious agents in human cancers: lessons in immunity and immunomodulation from gammaherpesviruses EBV and KSHV. Cancer Lett. 305:263–278 [DOI] [PubMed] [Google Scholar]
- 3. Saha A, Robertson ES. 2011. Epstein-Barr virus-associated B-cell lymphomas: pathogenesis and clinical outcomes. Clin. Cancer Res. 17:3056–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186–1191 [DOI] [PubMed] [Google Scholar]
- 5. Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280 [PubMed] [Google Scholar]
- 6. Nealy MS, Coleman CB, Li H, Tibbetts SA. 2010. Use of a virus-encoded enzymatic marker reveals that a stable fraction of memory B cells expresses latency-associated nuclear antigen throughout chronic gammaherpesvirus infection. J. Virol. 84:7523–7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorley-Lawson DA. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. 1:75–82 [DOI] [PubMed] [Google Scholar]
- 8. Pope JH, Scott W, Moss DJ. 1973. Human lymphoid cell transformation by Epstein-Barr virus. Nat. New Biol. 246:140–141 [DOI] [PubMed] [Google Scholar]
- 9. Bechtel JT, Liang Y, Hvidding J, Ganem D. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474–6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blackbourn DJ, Lennette E, Klencke B, Moses A, Chandran B, Weinstein M, Glogau RG, Witte MH, Way DL, Kutzkey T, Herndier B, Levy JA. 2000. The restricted cellular host range of human herpesvirus 8. AIDS 14:1123–1133 [DOI] [PubMed] [Google Scholar]
- 11. Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182–5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Lagunoff M. 2005. Establishment and maintenance of Kaposi's sarcoma-associated herpesvirus latency in B cells. J. Virol. 79:14383–14391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Myoung J, Ganem D. 2011. Infection of lymphoblastoid cell lines by Kaposi's sarcoma-associated herpesvirus: critical role of cell-associated virus. J. Virol. 85:9767–9777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho H, Kang H. 2012. KSHV infection of B-cell lymphoma using a modified KSHV BAC36 and coculturing system. J. Microbiol. (Seoul) 50:285–292 [DOI] [PubMed] [Google Scholar]
- 15. Luka J, Kallin B, Klein G. 1979. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology 94:228–231 [DOI] [PubMed] [Google Scholar]
- 16. Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342–346 [DOI] [PubMed] [Google Scholar]
- 17. zur Hausen H, O'Neill FJ, Freese UK, Hecker E. 1978. Persisting oncogenic herpesvirus induced by the tumor promoter TPA. Nature 272:373–375 [DOI] [PubMed] [Google Scholar]
- 18. Bhende PM, Dickerson SJ, Sun X, Feng WH, Kenney SC. 2007. X-box-binding protein 1 activates lytic Epstein-Barr virus gene expression in combination with protein kinase D. J. Virol. 81:7363–7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalton-Griffin L, Wilson SJ, Kellam P. 2009. X-box binding protein 1 contributes to induction of the Kaposi's sarcoma-associated herpesvirus lytic cycle under hypoxic conditions. J. Virol. 83:7202–7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson SJ, Tsao EH, Webb BL, Ye H, Dalton-Griffin L, Tsantoulas C, Gale CV, Du MQ, Whitehouse A, Kellam P. 2007. X box binding protein XBP-1s transactivates the Kaposi's sarcoma-associated herpesvirus (KSHV) ORF50 promoter, linking plasma cell differentiation to KSHV reactivation from latency. J. Virol. 81:13578–13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu F, Feng J, Harada JN, Chanda SK, Kenney SC, Sun R. 2007. B cell terminal differentiation factor XBP-1 induces reactivation of Kaposi's sarcoma-associated herpesvirus. FEBS Lett. 581:3485–3488 [DOI] [PubMed] [Google Scholar]
- 22. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96 [DOI] [PubMed] [Google Scholar]
- 23. Iwakoshi NN, Lee AH, Glimcher LH. 2003. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol. Rev. 194:29–38 [DOI] [PubMed] [Google Scholar]
- 24. Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. 2001. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412:300–307 [DOI] [PubMed] [Google Scholar]
- 25. Takada K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27–32 [DOI] [PubMed] [Google Scholar]
- 26. Tovey MG, Lenoir G, Begon-Lours J. 1978. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature 276:270–272 [DOI] [PubMed] [Google Scholar]
- 27. Goswami R, Gershburg S, Satorius A, Gershburg E. 2012. Protein kinase inhibitors that inhibit induction of lytic program and replication of Epstein-Barr virus. Antivir. Res. 96:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwakiri D, Takada K. 2004. Phosphatidylinositol 3-kinase is a determinant of responsiveness to B cell antigen receptor-mediated Epstein-Barr virus activation. J. Immunol. 172:1561–1566 [DOI] [PubMed] [Google Scholar]
- 29. Limon JJ, Fruman DA. 2012. Akt and mTOR in B cell activation and differentiation. Front. Immunol. 3:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pauls SD, Lafarge ST, Landego I, Zhang T, Marshall AJ. 2012. The phosphoinositide 3-kinase signaling pathway in normal and malignant B cells: activation mechanisms, regulation, and impact on cellular functions. Front. Immunol. 3:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moser JM, Upton JW, Gray KS, Speck SH. 2005. Ex vivo stimulation of B cells latently infected with gammaherpesvirus 68 triggers reactivation from latency. J. Virol. 79:5227–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. 2010. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 16:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. 2010. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat. Med. 16:438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carbone A, Gaidano G. 1997. HHV-8-positive body-cavity-based lymphoma: a novel lymphoma entity. Br. J. Haematol. 97:515–522 [DOI] [PubMed] [Google Scholar]
- 35. Gaidano G, Carbone A. 2001. Primary effusion lymphoma: a liquid phase lymphoma of fluid-filled body cavities. Adv. Cancer Res. 80:115–146 [DOI] [PubMed] [Google Scholar]
- 36. Gaidano G, Gloghini A, Gattei V, Rossi MF, Cilia AM, Godeas C, Degan M, Perin T, Canzonieri V, Aldinucci D, Saglio G, Carbone A, Pinto A. 1997. Association of Kaposi's sarcoma-associated herpesvirus-positive primary effusion lymphoma with expression of the CD138/syndecan-1 antigen. Blood 90:4894–4900 [PubMed] [Google Scholar]
- 37. Jenner RG, Maillard K, Cattini N, Weiss RA, Boshoff C, Wooster R, Kellam P. 2003. Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphoma has a plasma cell gene expression profile. Proc. Natl. Acad. Sci. U. S. A. 100:10399–10404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Connell FP, Pinkus JL, Pinkus GS. 2004. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am. J. Clin. Pathol. 121:254–263 [DOI] [PubMed] [Google Scholar]
- 39. Vieira J, O'Hearn PM. 2004. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325:225–240 [DOI] [PubMed] [Google Scholar]
- 40. Menezes J, Leibold W, Klein G, Clements G. 1975. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine 22:276–284 [PubMed] [Google Scholar]
- 41. Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648–2654 [PubMed] [Google Scholar]
- 42. Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708–2714 [PubMed] [Google Scholar]
- 43. Dedicoat M, Newton R, Alkharsah KR, Sheldon J, Szabados I, Ndlovu B, Page T, Casabonne D, Gilks CF, Cassol SA, Whitby D, Schulz TF. 2004. Mother-to-child transmission of human herpesvirus-8 in South Africa. J. Infect. Dis. 190:1068–1075 [DOI] [PubMed] [Google Scholar]
- 44. Wandinger K, Jabs W, Siekhaus A, Bubel S, Trillenberg P, Wagner H, Wessel K, Kirchner H, Hennig H. 2000. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology 55:178–184 [DOI] [PubMed] [Google Scholar]
- 45. Lukac DM, Kirshner JR, Ganem D. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348–9361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wies E, Mori Y, Hahn A, Kremmer E, Sturzl M, Fleckenstein B, Neipel F. 2008. The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood 111:320–327 [DOI] [PubMed] [Google Scholar]
- 47. Gunther T, Grundhoff A. 2010. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS Pathog. 6:e1000935. 10.1371/journal.ppat.1000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leight ER, Sugden B. 2001. Establishment of an oriP replicon is dependent upon an infrequent, epigenetic event. Mol. Cell. Biol. 21:4149–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grundhoff A, Ganem D. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Invest. 113:124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamel KM, Liarski VM, Clark MR. 2012. Germinal center B cells. Autoimmunity 45:333–347 [DOI] [PubMed] [Google Scholar]
- 51. Shlomchik MJ, Weisel F. 2012. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 247:52–63 [DOI] [PubMed] [Google Scholar]
- 52. Yoshida T, Mei H, Dorner T, Hiepe F, Radbruch A, Fillatreau S, Hoyer BF. 2010. Memory B and memory plasma cells. Immunol. Rev. 237:117–139 [DOI] [PubMed] [Google Scholar]
- 53. Laichalk LL, Thorley-Lawson DA. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liang X, Collins CM, Mendel JB, Iwakoshi NN, Speck SH. 2009. Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog. 5:e1000677. 10.1371/journal.ppat.1000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun CC, Thorley-Lawson DA. 2007. Plasma cell-specific transcription factor XBP-1s binds to and transactivates the Epstein-Barr virus BZLF1 promoter. J. Virol. 81:13566–13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shaw RN, Arbiser JL, Offermann MK. 2000. Valproic acid induces human herpesvirus 8 lytic gene expression in BCBL-1 cells. AIDS (London) 14:899–902 [DOI] [PubMed] [Google Scholar]
- 57. Rivas C, Thlick AE, Parravicini C, Moore PS, Chang Y. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cunningham C, Barnard S, Blackbourn DJ, Davison AJ. 2003. Transcription mapping of human herpesvirus 8 genes encoding viral interferon regulatory factors. J. Gen. Virol. 84:1471–1483 [DOI] [PubMed] [Google Scholar]
- 59. Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, Harding HP. 2012. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. U. S. A. 109:E869–E878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. 2008. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J. Virol. 82:4793–4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hassman LM, Ellison TJ, Kedes DH. 2010. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. J. Clin. Invest. 121:752–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hamoudi R, Diss TC, Oksenhendler E, Pan L, Carbone A, Ascoli V, Boshoff C, Isaacson P, Du MQ. 2004. Distinct cellular origins of primary effusion lymphoma with and without EBV infection. Leukemia Res. 28:333–338 [DOI] [PubMed] [Google Scholar]
- 63. Matolcsy A, Nador RG, Cesarman E, Knowles DM. 1998. Immunoglobulin VH gene mutational analysis suggests that primary effusion lymphomas derive from different stages of B cell maturation. Am. J. Pathol. 153:1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wei F, Zaprazna K, Wang J, Atchison ML. 2009. PU. 1 can recruit BCL6 to DNA to repress gene expression in germinal center B cells. Mol. Cell. Biol. 29:4612–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Singer PA, Williamson AR. 1980. Cell surface immunoglobulin mu and gamma chains of human lymphoid cells are of higher apparent molecular weight than their secreted counterparts. Eur. J. Immunol. 10:180–186 [DOI] [PubMed] [Google Scholar]
- 66. Lukac DM, Renne R, Kirshner JR, Ganem D. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF50 transactivator, a homolog of the EBV R protein. Virology 252:304–312 [DOI] [PubMed] [Google Scholar]
- 67. Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. U. S. A. 95:10866–10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fakhari FD, Dittmer DP. 2002. Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 76:6213–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jenner RG, Alba MM, Boshoff C, Kellam P. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moore PS, Boshoff C, Weiss RA, Chang Y. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739–1744 [DOI] [PubMed] [Google Scholar]
- 71. Paulose-Murphy M, Ha NK, Xiang C, Chen Y, Gillim L, Yarchoan R, Meltzer P, Bittner M, Trent J, Zeichner S. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kelly GL, Long HM, Stylianou J, Thomas WA, Leese A, Bell AI, Bornkamm GW, Mautner J, Rickinson AB, Rowe M. 2009. An Epstein-Barr virus antiapoptotic protein constitutively expressed in transformed cells and implicated in Burkitt lymphomagenesis: the Wp/BHRF1 link. PLoS Pathog. 5:e1000341. 10.1371/journal.ppat.1000341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kurosaki T. 2011. Regulation of BCR signaling. Mol. Immunol. 48:1287–1291 [DOI] [PubMed] [Google Scholar]
- 75. Vrzalikova K, Vockerodt M, Leonard S, Bell A, Wei W, Schrader A, Wright KL, Kube D, Rowe M, Woodman CB, Murray PG. 2011. Downregulation of BLIMP1α by the EBV oncogene, LMP-1, disrupts the plasma cell differentiation program and prevents viral replication in B cells: implications for the pathogenesis of EBV-associated B-cell lymphomas. Blood 117:5907–5917 [DOI] [PMC free article] [PubMed] [Google Scholar]