Abstract
Poxviruses are nucleocytoplasmic large DNA viruses encompassing two subfamilies, the Chordopoxvirinae and the Entomopoxvirinae, infecting vertebrates and insects, respectively. While chordopoxvirus genomics have been widely studied, only two entomopoxvirus (EPV) genomes have been entirely sequenced. We report the genome sequences of four EPVs of the Betaentomopoxvirus genus infecting the Lepidoptera: Adoxophyes honmai EPV (AHEV), Choristoneura biennis EPV (CBEV), Choristoneura rosaceana EPV (CREV), and Mythimna separata EPV (MySEV). The genomes are 80% AT rich, are 228 to 307 kbp long, and contain 247 to 334 open reading frames (ORFs). Most genes are homologous to those of Amsacta moorei entomopoxvirus and encode several protein families repeated in tandem in terminal regions. Some genomes also encode proteins of unknown functions with similarity to those of other insect viruses. Comparative genomic analyses highlight a high colinearity among the lepidopteran EPV genomes and little gene order conservation with other poxvirus genomes. As with previously sequenced EPVs, the genomes include a relatively conserved central region flanked by inverted terminal repeats. Protein clustering identified 104 core EPV genes. Among betaentomopoxviruses, 148 core genes were found in relatively high synteny, pointing to low genomic diversity. Whole-genome and spheroidin gene phylogenetic analyses showed that the lepidopteran EPVs group closely in a monophyletic lineage, corroborating their affiliation with the Betaentomopoxvirus genus as well as a clear division of the EPVs according to the orders of insect hosts (Lepidoptera, Coleoptera, and Orthoptera). This suggests an ancient coevolution of EPVs with their insect hosts and the need to revise the current EPV taxonomy to separate orthopteran EPVs from the lepidopteran-specific betaentomopoxviruses so as to form a new genus.
INTRODUCTION
Poxviruses are large double-stranded DNA (dsDNA) viruses infecting a wide range of animals. They belong to the phylogenetically related group of viruses termed nucleocytoplasmic large DNA viruses (NCLDV) (1). They harbor linear dsDNA genomes with inverted terminal repeats (ITRs) (2). Poxvirus genomes are 130 to 375 kbp long and replicate in the cytoplasm (3). The family Poxviridae includes two subfamilies: the Chordopoxvirinae, infecting vertebrates, and the Entomopoxvirinae, infecting insects. The chordopoxviruses are classified into nine genera, including Orthopoxvirus and Avipoxvirus (4), and have been the subjects of the main body of research on poxviruses (5, 6). The entomopoxviruses (EPVs) are currently divided into three genera based on host range and virion morphology: Alphaentomopoxvirus, infecting coleopterans; Betaentomopoxvirus, infecting lepidopterans and orthopterans; and Gammaentomopoxvirus, infecting dipterans (4). However, the lack of genomic data has precluded the integration of unifying genetic criteria into this classification. That is why the orthopteran EPV Melanoplus sanguinipes entomopoxvirus was removed from the Betaentomopoxvirus genus (4) and why Diachasmimorpha entomopoxvirus, infecting both a braconid parasitic wasp and its tephritid fruit fly dipteran host, remains unclassified (7, 8). Reports of entomopoxviruses from bumblebees (9) and cockroaches (10) further show that the taxonomic biodiversity of EPV remains largely undescribed.
EPV virions are embedded within a matrix protein, termed a spheroid, forming typical oval-shaped occlusion bodies (OBs) composed mainly of the spheroidin protein (11). Spheroidin is a functional homolog of the baculovirus polyhedrin (12) in that it affords the virions some protection against inactivating environmental agents such as heat, desiccation, and UV light (12, 13). The OBs dissolve in the alkaline-reducing environment of the insect midgut with the aid of an endogenous alkaline protease and release the virions to initiate infection in columnar epithelial cells prior to systemic infection (14). Virus replication occurs principally in the fat tissue, but other tissues are also affected (15). Interestingly, while baculoviruses spread within larval tissues through the tracheal system (16), these tissues are rarely infected by EPVs. Apparently, EPVs use hemocytes to spread within susceptible tissues (11). The course of EPV infection is generally slow (13); insects can survive as long as several weeks after the initial infection and can even remain in the larval stage longer than an uninfected host (17). OBs are disseminated in the environment through regurgitation, defecation, and, ultimately, the disintegration of dead hosts (11, 18). There is also one report on transmission via parasitoids (7).
EPVs have been studied mainly because of their potential as microbial biocontrol agents. Field studies on important Asian and North American lepidopteran pests revealed that EPVs could be found in diseased larvae of the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae) (19, 20), the 2-year-cycle budworm moth, Choristoneura biennis (Lepidoptera: Tortricidae) (21), the oblique-banded leafroller moth Choristoneura rosaceana (Lepidoptera: Tortricidae), and the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae) (22). In contrast to baculoviruses, which can kill insect hosts shortly after infection and could be used in place of a chemical insecticide (23), EPVs are slow-acting pathogens and may be more appropriate for reducing the growth rate of the pest population via epizootics that affect the frequency of insect outbreaks. It has been suggested that combining fast- and slow-killing strategies could contribute to better insect pest control and diminish the need for chemical insecticides (24).
To date, only two EPV genomes have been completely sequenced: those of Melanoplus sanguinipes entomopoxvirus (MSEV) (25), infecting the North American migratory grasshopper (Orthoptera: Acrididae), and Amsacta moorei entomopoxvirus (AMEV) (26), infecting the red hairy caterpillar (Lepidoptera: Arctiidae). AMEV and MSEV have similar genome sizes of 232 kb and 236 kb, with 294 and 267 open reading frames (ORFs), respectively. However, they share little overall genome homology in terms of gene content or order. With only 106 genes in common, AMEV and MSEV share less than half of their gene content, which is the reason for the removal of MSEV from the Betaentomopoxvirus genus. However, the other orthopteran EPVs remain in the Betaentomopoxvirus genus. A single genome (AMEV) is, indeed, not sufficient to allow the proposal of unifying genomic characters for a genus. More lepidopteran EPV sequences could allow us to discriminate between several taxonomic hypotheses, as follows. (i) The genus Betaentomopoxvirus contains orthopteran and lepidopteran EPVs, and MSEV is a peculiar, divergent virus. In this case, we should not be able to find unifying characteristics for the regrouping of orthopteran viruses. (ii) Lepidopteran and orthopteran EPVs are phylogenetically interrelated. In this case, comparative genomics should show high genome structure divergence, which could encompass the diversity already observed between AMEV and MSEV. (iii) The genus Betaentomopoxvirus contains only lepidopteran EPVs, and orthopteran EPVs belong to a different genus. In this case, we expect to find unifying genomic and phylogenetic criteria excluding orthopteran EPVs from the genus Betaentomopoxvirus.
The current paucity of EPV genomic data hinders both functional and evolutionary studies. Here we present the complete genome sequences of four EPVs isolated from Lepidoptera with the aim of defining common features for betaentomopoxviruses (BetaEPVs). We sequenced EPVs isolated from Adoxophyes honmai (AHEV), Choristoneura biennis (CBEV), Choristoneura rosaceana (CREV), and Mythimna separata (MySEV). We performed genome colinearity and gene content analyses both within the BetaEPV lineage and with more distantly related poxviruses. We combined comparative genomic analyses with phylogenetic analyses in order to understand the evolution of the subfamily Entomopoxvirinae at the genomic level.
MATERIALS AND METHODS
DNA isolation and sequencing.
AHEV, CBEV, CREV, and MySEV were isolated from diseased larvae of Adoxophyes honmai (collected from a tea field in Tokyo, Japan) (19, 20), Choristoneura biennis (from the province of Ontario, Canada) (21), Choristoneura rosaceana (collected in Eastern Canada), and Mythimna separata (obtained from Fulin Sun, Chinese Center for Virus Culture Collection, Wuhan, China) (22), respectively. Viruses were propagated in their respective hosts except for CBEV, which was propagated in Choristoneura fumiferana.
OBs were purified by homogenization and density gradient centrifugation using a 0.25 M sucrose-Percoll (GE Healthcare) solution (19). The purified OB suspensions were dissolved with an alkali buffer containing a reducing agent (1 M sodium carbonate and 0.4 M sodium thioglycolate). Undissolved OBs and heavy debris were pelleted by centrifugation at 900 × g for 3 min, and the supernatants were centrifuged at 20,400 × g for 10 min. Viral genomic DNA was extracted using a Puregene tissue purification kit (Qiagen). The 454 high-throughput sequencing technology was used to sequence AHEV, CBEV, and CREV in 454 single reads and MySEV in 454 paired-end reads.
Genome assembly and annotation.
The genomes were assembled de novo using Newbler, version 2.6 (27). Overlapping contigs were assembled using Geneious, version 5.5. To fill the gaps between contigs, resolve ambiguities, and position inverted terminal repeat (ITR) regions, PCR primers were designed at contig extremities, and amplicons were subjected to Sanger sequencing (28).
The annotations were performed in three steps. First, Glimmer3 (29) was used for de novo prediction of ORFs encoding more than 50 amino acids (aa) with a methionine as the start codon. Second, the protein sequences encoded by each ORF were aligned to the Viral Orthologous Clusters (VOCs) of the Viral Bioinformatics Resource Center (30, 31) and to NCBI's nonredundant protein database by using BLASTp (32) to identify functional homologies. Third, both the delimitation of ITR regions and the correction of 454 homopolymer ambiguities in coding regions were carried out manually.
Comparative genomic analyses.
Reciprocal best-hit alignments using BLASTp (32) were performed to identify orthologous proteins between the AMEV genome and the four new genomes, those of AHEV, CBEV, CREV, and MySEV. Similarly, orthologous proteins were identified between MSEV and the five BetaEPV genomes and between the vaccinia virus Western Reserve (VACV) genome and the six EPV genomes. Orthologous gene positions were retrieved on each genome and were integrated into the Circos visualization program (33). The AMEV, MSEV, and VACV genomes were set as references for the visualization of genome colinearity maps among BetaEPVs, EPVs, and poxviruses.
A clustering based on “profile hidden Markov model” alignments using the jackhmmer program of the HMMER 3 package (34) was performed on all EPV proteins to identify potentially inherited conserved genes within the EPV and BetaEPV lineages. Among these genes, we determined gene order conservation within a lineage by using the GeneSyn program (35).
Phylogenetic analyses.
A phylogenomic approach was used to position AHEV, CBEV, CREV, and MySEV within the whole-genome poxvirus phylogeny. To date, poxviruses appear to possess 49 core genes (31) that have been identified in the AHEV, CBEV, CREV, and MySEV genomes and in the genomes of representative species of all poxvirus genera. Multiple amino acid alignments were performed on the 49 poxvirus core genes, including those of AHEV, CBEV, CREV, MySEV, and 12 additional poxvirus species, by using the Clustal Omega program (36). In order to ascertain that the poxvirus core genes used for phylogenetic analyses shared the same evolutionary history and could be used as a proxy for the evolution of the virus species, we performed phylogenetic congruence tests to detect any possible conflict in phylogenetic signals between poxvirus core genes. These tests did not show any conflicting phylogenetic signal between genes (data not shown), and therefore, all the multiple amino acid alignments were concatenated prior to phylogenetic reconstruction. A maximum likelihood (ML) phylogenetic inference was performed on the concatenated multiple amino acid alignments with the substitution model and model parameters WAG+G, selected using ModelGenerator (37) under the Akaike information criterion. ML analysis was performed with the RAxML program (38), and support for nodes in ML trees was obtained from 100 bootstrap iterations.
A multiple amino acid alignment of the spheroidin gene was performed, including amino acid sequences from the AHEV, CBEV, CREV, and MySEV genomes and all the sequences available from the GenBank public database (25, 26, 39–45). An ML phylogenetic inference was performed on the multiple amino acid alignment for spheroidin with the RAxML program (38) by using the substitution model and model parameters WAG+G. The root of the tree was determined by midpoint rooting.
Nucleotide sequence accession numbers.
The AHEV, CBEV, CREV, and MySEV genomes have been deposited in EMBL under accession numbers HF679131, HF679132, HF679133, and HF679134, respectively.
RESULTS
Features of the AHEV, CBEV, CREV, and MySEV genomes.
AHEV, CBEV, CREV, and MySEV OB particles were isolated from diseased larvae of Adoxophyes honmai, Choristoneura biennis, Choristoneura rosaceana, and Mythimna separata, respectively. The four EPV genomes were assembled in contiguous sequences ranging from 229 kb for the smallest, AHEV, to 308 kb for the largest, CBEV (Table 1). This size range is somewhat similar to that of the previously sequenced EPV genomes, AMEV and MSEV (25, 26). As expected for poxviruses (4), the genomes include a central region flanked by inverted terminal repeat (ITR) regions at the extremities. Due to the repetitive nature of the ITRs, their sequences retain a number of ambiguities. As with other EPV genomes, the nucleotide composition of the four genomes is AT rich, at approximately 80% of the total nucleotide content (Table 1).
Table 1.
General features of entomopoxvirus genomes
| Genome | Size (bp) | No. of ORFs | No. of singletons | ITR size (bp) | GC content (%) | Coding capacity (%) |
|---|---|---|---|---|---|---|
| Melanoplus sanguinipes entomopoxvirus | 236,120 | 267 | 144 | 7,201 | 18.3 | 91.6 |
| Amsacta moorei entomopoxvirus “L” | 232,392 | 294 | 73 | 9,458 | 17.8 | 95.4 |
| Adoxophyes honmai entomopoxvirus “L” | 228,750 | 247 | 27 | 5,617 | 21 | 89.8 |
| Choristoneura biennis entomopoxvirus “L” | 307,691 | 334 | 19 | 23,817 | 19.7 | 91 |
| Choristoneura rosaceana entomopoxvirus “L” | 282,895 | 296 | 11 | 13,406 | 19.5 | 90.2 |
| Mythimna separata entomopoxvirus “L” | 281,182 | 306 | 64 | 7,347 | 19.7 | 90.5 |
Genome contents.
The genome annotations predicted 247 and 334 ORFs encoding proteins of more than 50 aa for AHEV and CBEV, respectively (Table 1), with few overlaps between ORFs. This corresponds to about 90% of the genome coding capacity (Table 1). Homology searches in public databases were performed to assign a functional annotation to each ORF. Homologs could be found for approximately 80% of the ORFs and corresponded mostly to genes already found in AMEV. Overall, these conserved proteins are encoded in the central regions of genomes and are essential to virus structure and replication.
Several large gene families of unknown functions, with many members repeated in tandem, were found in EPV genomes. The N1R/p28 gene family is by far the largest, with more than 20 copies per genome and as many as 48 in CBEV. This gene family, based on the VOCs (30, 31) database, regroups the ALI, MTG, and 17K/KilA-N domain proteins previously described separately (11, 26). The tryptophan repeat and leucine-rich gene families are more modest than the N1R/p28 gene family, with copy numbers ranging from 2 to 10. Differences in genome size could be explained in part by differences in N1R/p28 gene copy numbers. Indeed, 21 gene copies represent 8% of the AHEV ORFs, while 48 copies represent 14% of the coding capacity of CBEV. The number of ORFs encoding hypothetical proteins, for which no homologs are found in the databases, was also higher in larger genomes. It is worth mentioning that a number of unknown ORFs showed similarities to proteins found in other large DNA viruses of insects, most notably to those encoded by the baculovirus antiapoptotic iap and p35 gene families. The majority of these less conserved, repeated, hypothetical, and singleton ORFs are present mostly in the terminal regions of the genomes and, remarkably, in isolated regions located right in the middle of the genome.
Genome colinearity.
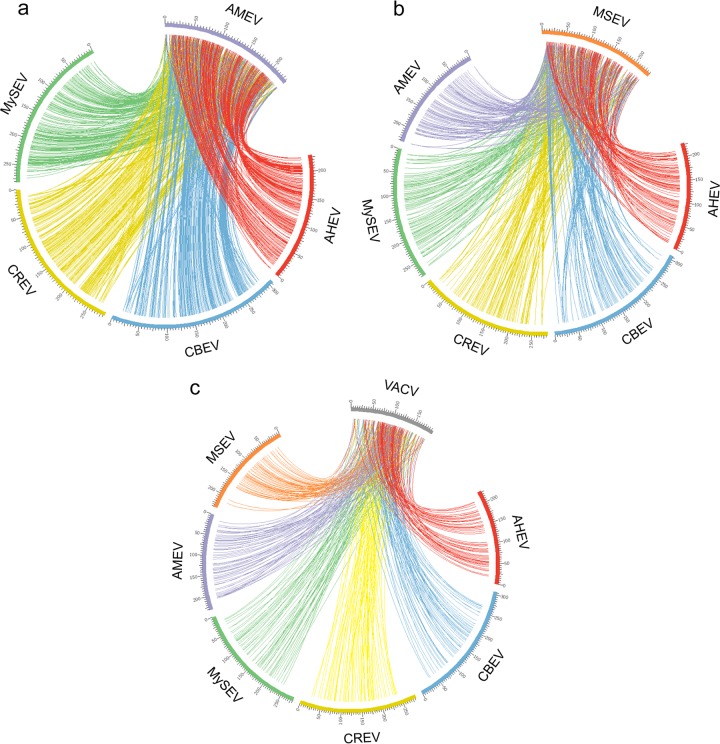
In order to compare the global genome synteny conservation among poxviruses, reciprocal best-hit alignments were performed to determine gene orthology among AMEV, MSEV, VAVC, and the four new EPVs. Genes normally have only one ortholog per genome. However, since ITRs are identical, genes located in the ITRs have two orthologs in the other genomes. We mapped the orthologous gene positions in circular colinearity maps (Fig. 1) and found high colinearity all along five lepidopteran BetaEPV genomes (Fig. 1a). Central regions are highly conserved, while extremities lose orthology and synteny conservation. This finding suggests strong gene content and order conservation within the central regions of lepidopteran BetaEPV genomes. Interestingly, AHEV had a large genomic inversion located at kbp 120 to 175. This indicates that central regions are composed of two independent parts that may undergo inversions without any apparent effect on replication.
Fig 1.
Poxvirus genome colinearity maps. The maps are based on the identification of orthologs by reciprocal best-hit analyses. The circles indicate genome colinearity conservation among lepidopteran BetaEPV genomes, with AMEV set as a reference (a), EPV genomes, with MSEV set as a reference (b), and poxvirus genomes, with VACV set as a reference (c).
Similarly, we looked for colinearity conservation among EPVs (including the orthopteran EPV MSEV) (Fig. 1b). The five lepidopteran BetaEPV genomes showed less colinearity with the MSEV genome than with each other, as illustrated by fewer connecting lines in Fig. 1b than in Fig. 1a. The loss of gene content and order conservation between the five BetaEPV genomes and the orthopteran MSEV genome indicates that MSEV is evolutionarily divergent from lepidopteran EPVs, suggesting that orthopteran and lepidopteran EPVs indeed belong to different genera.
At the Poxviridae family level, the comparison of EPV genomes to the historical chordopoxvirus model, the VACV genome (Fig. 1c), highlighted the sparseness of colinearity. The few orthologous genes are located in the central region and correspond mostly to poxvirus core genes. In summary, as the genomes become more divergent, fewer orthologs are found between genomes. However, the central regions of poxvirus genomes retained certain levels of conservation, but with many inversions and rearrangements, corroborating previous studies (26, 46).
EPV core genome.
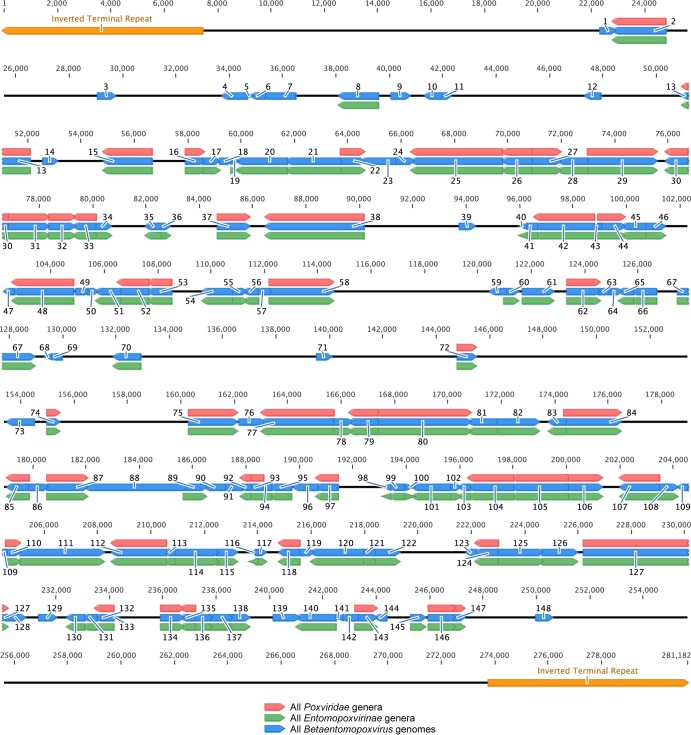
Protein clustering was performed on all EPV proteins to identify core genes for the Entomopoxvirinae subfamily and the Betaentomopoxvirus genus. This analysis grouped together ORFs sharing homologous domains. The size of the cluster corresponded to the number of times a particular homolog group was found in the genomes. Clusters representing gene families, such as the N1R/p28 gene family, contained more than a hundred genes. It was not possible to assign orthology between gene copies for such large clusters. They were, therefore, removed from the analyses, and we concentrated on genes present only once per genome. Core genes were defined as single-copy-number genes in the genomes of all members of a particular group. We determined that 104 genes are conserved in all EPV genomes and 148 in all BetaEPV genomes (Fig. 2 and Table 2). The 104 EPV core genes include the 49 poxvirus core genes (31) and 55 EPV-specific genes. Among these 55 genes, we identified the spheroidin, DNA photolyase, ubiquitin, putative thioredoxin, protein tyrosine phosphatase 2, protein phosphatase 1B, protein phosphatase 2C, lipase, and Ca2+ binding protein (BP) genes, as well as 46 ORFs of unknown function initially identified in the genome of AMEV. The 44 supplementary ORFs defining the BetaEPV core genes include those encoding the Cu/Zn superoxide dismutase, thymidine kinase, and a second poly(A) polymerase small subunit VP39, as well as 41 genes of unknown function. Although not included as core genes, the N1R/p28, leucine-rich, and tryptophan repeat gene families are present in all EPV genomes.
Fig 2.
Localization of the 148 betaentomopoxvirus core genes in the genome of MySEV. Red, green, and blue arrows represent genes conserved in all poxvirus genera, in all EPV genera, and in all BetaEPV genomes, respectively. Orange arrows represent ITRs. MySEV noncore genes are not displayed. Genes are numbered as in Table 2.
Table 2.
Betaentomopoxvirus core genome
| No. | Predicted function or similaritya | ORF no. in the genome of: |
Cluster | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AHEV | AMEV | CBEV | CREV | MySEV | MSEV | VACV | |||
| 1 | Cu/Zn superoxide dismutase | AHEV230 | AMEV255 | CBEV291 | CREV257 | MySEV026 | B1 | ||
| 2 | Metalloprotease (Cop-G1L) | AHEV231 | AMEV256 | CBEV292 | CREV258 | MySEV027 | MSEV056 | VACV078 | B1 |
| 3 | Thymidine kinase | AHEV022 | AMEV016 | CBEV044 | CREV024 | MySEV035 | |||
| 4 | Unknown; similar to AMEV004 | AHEV024 | AMEV004 | CBEV065 | CREV045 | MySEV039 | |||
| 5 | Unknown; similar to AMEV017 | AHEV025 | AMEV017 | CBEV046 | CREV026 | MySEV040 | B2 | ||
| 6 | Unknown; similar to AMEV018 | AHEV026 | AMEV018 | CBEV047 | CREV027 | MySEV041 | B2 | ||
| 7 | Unknown; similar to AMEV261 | AHEV015 | AMEV261 | CBEV030 | CREV011 | MySEV042 | |||
| 8 | DNA photolyase | AHEV028 | AMEV025 | CBEV053 | CREV035 | MySEV044 | MSEV235 | ||
| 9 | Unknown; similar to AMEV022 | AHEV027 | AMEV022 | CBEV055 | CREV036 | MySEV046 | |||
| 10 | Unknown; similar to AMEV028 | AHEV031 | AMEV028 | CBEV049 | CREV029 | MySEV048 | |||
| 11 | Unknown; similar to AMEV123 | AHEV114 | AMEV123 | CBEV173 | CREV141 | MySEV049 | |||
| 12 | Unknown; similar to AMEV032 | AHEV034 | AMEV032 | CBEV068 | CREV047 | MySEV056 | |||
| 13 | Entry-fusion complex component, myristylprotein | AHEV036 | AMEV035 | CBEV088 | CREV067 | MySEV060 | MSEV121 | VACV087 | |
| 14 | Unknown; similar to AMEV034 | AHEV035 | AMEV034 | CBEV086 | CREV065 | MySEV062 | |||
| 15 | Poly(A) polymerase catalytic subunit VP55 | AHEV038 | AMEV038 | CBEV085 | CREV064 | MySEV064 | MSEV143 | VACV057 | |
| 16 | NlpC/P60 superfamily protein (Cop-G6R) | AHEV040 | AMEV041 | CBEV082 | CREV061 | MySEV066 | MSEV039 | VACV084 | |
| 17 | Unknown; similar to AMEV040 | AHEV041 | AMEV040 | CBEV083 | CREV062 | MySEV067 | MSEV138 | ||
| 18 | Unknown; similar to AMEV042 | AHEV042 | AMEV042 | CBEV081 | CREV060 | MySEV068 | |||
| 19 | Unknown; similar to AMEV043 | AHEV043 | AMEV043 | CBEV080 | CREV059 | MySEV069 | MSEV188 | ||
| 20 | Unknown; similar to AMEV044 | AHEV044 | AMEV044 | CBEV079 | CREV058 | MySEV070 | MSEV140 | ||
| 21 | Unknown; similar to AMEV045 | AHEV045 | AMEV045 | CBEV078 | CREV057 | MySEV071 | MSEV077 | ||
| 22 | Late transcription factor VLTF-2 | AHEV046 | AMEV047 | CBEV077 | CREV056 | MySEV072 | MSEV187 | VACV119 | |
| 23 | Unknown; similar to AMEV048 | AHEV047 | AMEV048 | CBEV071 | CREV050 | MySEV073 | |||
| 24 | Unknown; similar to AMEV049 | AHEV048 | AMEV049 | CBEV070 | CREV049 | MySEV074 | |||
| 25 | DNA polymerase | AHEV049 | AMEV050 | CBEV069 | CREV048 | MySEV075 | MSEV036 | VACV065 | |
| 26 | RNA polymerase RPO35 | AHEV051 | AMEV051 | CBEV094 | CREV070 | MySEV076 | MSEV149 | VACV152 | B3 |
| 27 | DNA topoisomerase type I | AHEV052 | AMEV052 | CBEV095 | CREV071 | MySEV077 | MSEV130 | VACV104 | B3 |
| 28 | Unknown; similar to AMEV053 | AHEV053 | AMEV053 | CBEV096 | CREV072 | MySEV078 | MSEV120 | B3 | |
| 29 | RNA polymerase-associated protein RAP94 | AHEV054 | AMEV054 | CBEV097 | CREV073 | MySEV079 | MSEV118 | VACV102 | B3 |
| 30 | mRNA-decapping enzyme (Cop-D9R) | AHEV055 | AMEV058 | CBEV103 | CREV080 | MySEV081 | MSEV150 | VACV115 | B3 |
| 31 | DNA helicase, transcript release factor | AHEV056 | AMEV059 | CBEV104 | CREV081 | MySEV082 | MSEV148 | VACV138 | B3 |
| 32 | Poly(A) polymerase small subunit VP39 | AHEV057 | AMEV060 | CBEV105 | CREV082 | MySEV083 | MSEV041 | VACV095 | B3 |
| 33 | ssDNA/dsDNA binding protein VP8 (Cop-L4R) | AHEV061 | AMEV061 | CBEV108 | CREV083 | MySEV084 | MSEV158 | VACV091 | B3 |
| 34 | Unknown; similar to AMEV062 | AHEV062 | AMEV062 | CBEV109 | CREV084 | MySEV085 | MSEV160 | B3 | |
| 35 | Unknown; similar to AMEV072 | AHEV063 | AMEV072 | CBEV124 | CREV092 | MySEV088 | MSEV044 | ||
| 36 | Unknown; similar to AMEV071 | AHEV064 | AMEV071 | CBEV125 | CREV093 | MySEV089 | MSEV049 | ||
| 37 | Internal virion protein (Cop-L3L) | AHEV066 | AMEV069 | CBEV127 | CREV095 | MySEV092 | MSEV180 | VACV090 | |
| 38 | RNA polymerase RPO132 | AHEV068 | AMEV066 | CBEV129 | CREV097 | MySEV094 | MSEV155 | VACV144 | |
| 39 | Protein tyrosine phosphatase 2 | AHEV070 | AMEV078 | CBEV134 | CREV102 | MySEV098 | B4 | ||
| 40 | Putative thioredoxin | AHEV073 | AMEV079 | CBEV136 | CREV104 | MySEV102 | MSEV087 | B4 | |
| 41 | Unknown; similar to AMEV080 | AHEV074 | AMEV080 | CBEV137 | CREV105 | MySEV103 | MSEV085 | B4, E1 | |
| 42 | RNA helicase, DExH-NPH-II domain | AHEV079 | AMEV081 | CBEV138 | CREV106 | MySEV104 | MSEV086 | VACV077 | B4, E1 |
| 43 | Unknown; similar to AMEV082 | AHEV080 | AMEV082 | CBEV139 | CREV107 | MySEV105 | B4 | ||
| 44 | Entry and fusion IMV protein (Cop-L5R) | AHEV081 | AMEV083 | CBEV140 | CREV108 | MySEV106 | MSEV129 | VACV092 | B4 |
| 45 | Ser/Thr kinase (Cop-B1R) | AHEV082 | AMEV084 | CBEV141 | CREV109 | MySEV107 | MSEV154 | B4 | |
| 46 | Unknown; similar to AMEV085 | AHEV083 | AMEV085 | CBEV142 | CREV110 | MySEV108 | MSEV088 | B4, E2 | |
| 47 | Unknown; similar to AMEV086 | AHEV084 | AMEV086 | CBEV143 | CREV111 | MySEV109 | B4 | ||
| 48 | NTPase, DNA primase | AHEV085 | AMEV087 | CBEV144 | CREV112 | MySEV110 | MSEV089 | VACV110 | B4, E2 |
| 49 | Unknown; similar to AMEV088 | AHEV086 | AMEV088 | CBEV145 | CREV113 | MySEV111 | B4 | ||
| 50 | Unknown; similar to AMEV089 | AHEV087 | AMEV089 | CBEV146 | CREV114 | MySEV112 | B4 | ||
| 51 | Unknown; similar to AMEV090 | AHEV088 | AMEV090 | CBEV147 | CREV115 | MySEV113 | MSEV116 | B4 | |
| 52 | Intermediate transcription factor VITF-3 45-kDa subunit (Cop-A23R) | AHEV089 | AMEV091 | CBEV148 | CREV116 | MySEV114 | MSEV052 | VACV143 | B4 |
| 53 | mRNA-capping enzyme small subunit | AHEV090 | AMEV093 | CBEV149 | CREV117 | MySEV115 | MSEV124 | VACV117 | B4 |
| 54 | Unknown; similar to AMEV096 | AHEV092 | AMEV096 | CBEV151 | CREV119 | MySEV117 | MSEV213 | B4 | |
| 55 | Unknown; similar to AMEV098 | AHEV093 | AMEV098 | CBEV152 | CREV120 | MySEV118 | MSEV136 | B4 | |
| 56 | Unknown; similar to AMEV102 | AHEV108 | AMEV102 | CBEV157 | CREV125 | MySEV119 | MSEV092 | ||
| 57 | Unknown; similar to AMEV104 | AHEV107 | AMEV104 | CBEV158 | CREV126 | MySEV120 | |||
| 58 | Early transcription factor large subunit VETF-L | AHEV106 | AMEV105 | CBEV159 | CREV127 | MySEV121 | MSEV063 | VACV126 | |
| 59 | Unknown; similar to AMEV107 | AHEV105 | AMEV107 | CBEV160 | CREV128 | MySEV126 | |||
| 60 | Unknown; similar to AMEV101 | AHEV109 | AMEV101 | CBEV156 | CREV124 | MySEV127 | MSEV079 | ||
| 61 | Protein phosphatase 1B | AHEV097 | AMEV119 | CBEV167 | CREV135 | MySEV129 | MSEV081 | ||
| 62 | Myristylated protein, essential for entry/fusion (Cop-A16L) | AHEV099 | AMEV118 | CBEV165 | CREV133 | MySEV131 | MSEV090 | VACV136 | |
| 63 | Unknown; similar to AMEV117 | AHEV100 | AMEV117 | CBEV164 | CREV132 | MySEV132 | |||
| 64 | Unknown; similar to AMEV116 | AHEV101 | AMEV116 | CBEV163 | CREV131 | MySEV133 | |||
| 65 | Unknown; similar to AMEV120 | AHEV096 | AMEV120 | CBEV168 | CREV136 | MySEV134 | MSEV082 | ||
| 66 | Unknown; similar to AMEV121 | AHEV111 | AMEV121 | CBEV169 | CREV137 | MySEV135 | MSEV064 | ||
| 67 | Unknown; similar to AMEV099 | AHEV095 | AMEV099 | CBEV154 | CREV122 | MySEV138 | MSEV071 | ||
| 68 | Conotoxin-like protein | AHEV128 | AMEV267 | CBEV182 | CREV150 | MySEV140 | |||
| 69 | Unknown; similar to AMEV126 | AHEV196 | AMEV126 | CBEV175 | CREV143 | MySEV141 | |||
| 70 | Lipase | AHEV192 | AMEV133 | CBEV184 | CREV152 | MySEV144 | MSEV048 | ||
| 71 | Unknown; similar to AMEV075 | AHEV012 | AMEV075 | CBEV193 | CREV161 | MySEV151 | |||
| 72 | Entry-fusion complex essential component (Cop-H2R) | AHEV194 | AMEV127 | CBEV181 | CREV149 | MySEV156 | MSEV060 | VACV100 | |
| 73 | Poly(A) polymerase small subunit VP39 | AHEV102 | AMEV115 | CBEV162 | CREV130 | MySEV168 | |||
| 74 | Sulfhydryl oxidase, FAD linked (Cop-E10R) | AHEV103 | AMEV114 | CBEV161 | CREV129 | MySEV170 | MSEV093 | VACV066 | |
| 75 | Trimeric virion coat protein; rifampin resistance | AHEV112 | AMEV122 | CBEV171 | CREV139 | MySEV176 | MSEV069 | VACV118 | |
| 76 | Unknown; similar to AMEV128 | AHEV154 | AMEV128 | CBEV180 | CREV148 | MySEV177 | |||
| 77 | mRNA-capping enzyme large subunit | AHEV190 | AMEV135 | CBEV186 | CREV154 | MySEV178 | MSEV066 | VACV106 | |
| 78 | Unknown; similar to AMEV137 | AHEV189 | AMEV137 | CBEV187 | CREV155 | MySEV179 | MSEV068 | ||
| 79 | Viral membrane formation (Cop-A11R) | AHEV188 | AMEV138 | CBEV188 | CREV156 | MySEV180 | MSEV151 | VACV130 | |
| 80 | P4a precursor | AHEV187 | AMEV139 | CBEV189 | CREV157 | MySEV181 | MSEV152 | VACV129 | |
| 81 | Unknown; similar to AMEV140 | AHEV186 | AMEV140 | CBEV190 | CREV158 | MySEV182 | MSEV170 | ||
| 82 | Unknown; similar to AMEV141 | AHEV185 | AMEV141 | CBEV191 | CREV159 | MySEV183 | MSEV050 | ||
| 83 | Unknown; similar to AMEV145 | AHEV183 | AMEV145 | CBEV195 | CREV163 | MySEV185 | MSEV167 | ||
| 84 | P4b precursor | AHEV182 | AMEV147 | CBEV194 | CREV162 | MySEV186 | MSEV164 | VACV122 | |
| 85 | ATPase/DNA-packaging protein | AHEV180 | AMEV150 | CBEV196 | CREV164 | MySEV190 | MSEV171 | VACV155 | |
| 86 | Unknown; similar to AMEV151 | AHEV179 | AMEV151 | CBEV197 | CREV165 | MySEV191 | |||
| 87 | Essential Ser/Thr kinase morph (Cop-F10L) | AHEV178 | AMEV153 | CBEV198 | CREV166 | MySEV192 | MSEV173 | VACV049 | |
| 88 | Unknown; similar to AMEV156 | AHEV177 | AMEV156 | CBEV199 | CREV167 | MySEV193 | |||
| 89 | Unknown; similar to AMEV157 | AHEV176 | AMEV157 | CBEV200 | CREV168 | MySEV194 | MSEV169 | ||
| 90 | Unknown; similar to AMEV159 | AHEV175 | AMEV159 | CBEV201 | CREV169 | MySEV195 | |||
| 91 | Unknown; similar to AMEV160 | AHEV174 | AMEV160 | CBEV202 | CREV170 | MySEV196 | |||
| 92 | Viral membrane-associated early morphogenesis protein (Cop-A9L) | AHEV173 | AMEV161 | CBEV203 | CREV171 | MySEV197 | MSEV108 | VACV128 | |
| 93 | Holliday junction resolvase | AHEV172 | AMEV162 | CBEV204 | CREV172 | MySEV198 | MSEV106 | VACV142 | |
| 94 | Unknown; similar to AMEV163 | AHEV171 | AMEV163 | CBEV205 | CREV173 | MySEV199 | MSEV112 | ||
| 95 | Unknown; similar to AMEV164 | AHEV170 | AMEV164 | CBEV206 | CREV174 | MySEV200 | MSEV107 | ||
| 96 | Unknown; similar to AMEV165 | AHEV169 | AMEV165 | CBEV207 | CREV175 | MySEV201 | |||
| 97 | RNA polymerase RPO19 | AHEV168 | AMEV166 | CBEV208 | CREV176 | MySEV202 | MSEV101 | VACV124 | |
| 98 | Ubiquitin | AHEV166 | AMEV167 | CBEV209 | CREV177 | MySEV206 | MSEV144 | ||
| 99 | Unknown; similar to AMEV168 | AHEV165 | AMEV168 | CBEV210 | CREV178 | MySEV207 | MSEV165 | ||
| 100 | Unknown; similar to AMEV169 | AHEV164 | AMEV169 | CBEV212 | CREV180 | MySEV209 | MSEV163 | ||
| 101 | Unknown; similar to AMEV173 | AHEV160 | AMEV173 | CBEV216 | CREV184 | MySEV210 | MSEV157 | ||
| 102 | Unknown; similar to AMEV171 | AHEV162 | AMEV171 | CBEV214 | CREV182 | MySEV211 | MSEV166 | ||
| 103 | Unknown; similar to AMEV172 | AHEV161 | AMEV172 | CBEV215 | CREV183 | MySEV212 | MSEV098 | ||
| 104 | Virion protein (Cop-E6R) | AHEV163 | AMEV170 | CBEV213 | CREV181 | MySEV213 | MSEV145 | VACV062 | |
| 105 | Morph, early transcription factor small subunit (VETF-s) | AHEV159 | AMEV174 | CBEV217 | CREV185 | MySEV214 | MSEV113 | VACV111 | |
| 106 | FEN1-like nuclease (Cop-G5R) | AHEV157 | AMEV179 | CBEV218 | CREV186 | MySEV215 | MSEV115 | VACV082 | |
| 107 | Virion core cysteine protease | AHEV156 | AMEV181 | CBEV223 | CREV191 | MySEV217 | MSEV189 | VACV076 | |
| 108 | Unknown; similar to AMEV183 | AHEV155 | AMEV183 | CBEV224 | CREV192 | MySEV218 | MSEV190 | ||
| 109 | Unknown; similar to AMEV185 | AHEV153 | AMEV185 | CBEV225 | CREV193 | MySEV219 | |||
| 110 | IMV MP/virus entry (Cop-A28L) | AHEV152 | AMEV186 | CBEV226 | CREV194 | MySEV220 | MSEV132 | VACV151 | |
| 111 | Spheroidin | AHEV151 | AMEV187 | CBEV227 | CREV195 | MySEV221 | MSEV073 | ||
| 112 | ATPase, NPH1 | AHEV150 | AMEV192 | CBEV228 | CREV196 | MySEV222 | MSEV053 | VACV116 | |
| 113 | Unknown; similar to AMEV198 | AHEV149 | AMEV198 | CBEV229 | CREV197 | MySEV223 | MSEV161 | ||
| 114 | NAD-dependent DNA ligase | AHEV148 | AMEV199 | CBEV230 | CREV198 | MySEV224 | MSEV162 | ||
| 115 | Unknown; similar to AMEV200 | AHEV147 | AMEV200 | CBEV231 | CREV199 | MySEV225 | MSEV159 | ||
| 116 | Unknown; similar to AMEV203 | AHEV145 | AMEV203 | CBEV233 | CREV201 | MySEV228 | MSEV168 | ||
| 117 | Unknown; similar to AMEV204 | AHEV144 | AMEV204 | CBEV234 | CREV202 | MySEV229 | MSEV095 | ||
| 118 | Late transcription factor VLTF-3 | AHEV142 | AMEV205 | CBEV235 | CREV203 | MySEV231 | MSEV065 | VACV120 | |
| 119 | Unknown; similar to AMEV206 | AHEV141 | AMEV206 | CBEV236 | CREV204 | MySEV232 | |||
| 120 | DNA polymerase-beta/AP polymerase | AHEV140 | AMEV210 | CBEV237 | CREV205 | MySEV233 | MSEV117 | ||
| 121 | Unknown; similar to AMEV211 | AHEV139 | AMEV211 | CBEV239 | CREV207 | MySEV234 | MSEV137 | ||
| 122 | Unknown; similar to AMEV219 | AHEV138 | AMEV219 | CBEV245 | CREV212 | MySEV235 | MSEV072 | ||
| 123 | Unknown; similar to AMEV218 | AHEV136 | AMEV218 | CBEV244 | CREV213 | MySEV239 | |||
| 124 | IMV membrane protein (Cop-L1R) | AHEV135 | AMEV217 | CBEV246 | CREV211 | MySEV240 | MSEV183 | VACV088 | |
| 125 | Unknown; similar to AMEV216 | AHEV134 | AMEV216 | CBEV247 | CREV210 | MySEV241 | MSEV099 | ||
| 126 | Unknown; similar to AMEV214 | AHEV133 | AMEV214 | CBEV248 | CREV209 | MySEV242 | MSEV184 | ||
| 127 | RNA polymerase RPO147 | AHEV199 | AMEV221 | CBEV256 | CREV222 | MySEV244 | MSEV042 | VACV098 | B5 |
| 128 | Unknown; similar to AMEV224 | AHEV201 | AMEV224 | CBEV260 | CREV226 | MySEV245 | B5 | ||
| 129 | Unknown; similar to AMEV225 | AHEV203 | AMEV225 | CBEV261 | CREV228 | MySEV248 | B5 | ||
| 130 | Unknown; similar to AMEV226 | AHEV204 | AMEV226 | CBEV262 | CREV229 | MySEV251 | MSEV031 | B5 | |
| 131 | Ca2+ BP | AHEV205 | AMEV228 | CBEV263 | CREV230 | MySEV252 | MSEV097 | B5 | |
| 132 | RNA polymerase RPO18 | AHEV207 | AMEV230 | CBEV265 | CREV232 | MySEV253 | MSEV245 | VACV112 | |
| 133 | Unknown; similar to AMEV229 | AHEV206 | AMEV229 | CBEV264 | CREV231 | MySEV254 | |||
| 134 | Uracil-DNA glycosylase, DNA polymerase processivity factor | AHEV210 | AMEV231 | CBEV267 | CREV234 | MySEV257 | MSEV208 | VACV109 | B6 |
| 135 | Putative late 16-kDa membrane protein (Cop-J5L) | AHEV211 | AMEV232 | CBEV268 | CREV235 | MySEV258 | MSEV142 | VACV097 | B6 |
| 136 | Unknown; similar to AMEV233 | AHEV212 | AMEV233 | CBEV269 | CREV236 | MySEV259 | MSEV033 | B6 | |
| 137 | Protein phosphatase 2C | AHEV213 | AMEV234 | CBEV272 | CREV238 | MySEV260 | MSEV135 | B6 | |
| 138 | Unknown; similar to AMEV235 | AHEV214 | AMEV235 | CBEV273 | CREV239 | MySEV261 | MSEV123 | B6 | |
| 139 | Unknown; similar to AMEV240 | AHEV218 | AMEV240 | CBEV276 | CREV243 | MySEV264 | |||
| 140 | Unknown; similar to AMEV238 | AHEV217 | AMEV238 | CBEV275 | CREV242 | MySEV265 | MSEV055 | ||
| 141 | Unknown; similar to AMEV241 | AHEV219 | AMEV241 | CBEV277 | CREV244 | MySEV266 | |||
| 142 | Unknown; similar to AMEV245 | AHEV224 | AMEV245 | CBEV280 | CREV247 | MySEV267 | |||
| 143 | S-S bond formation pathway protein substrate (Cop-F9L) | AHEV221 | AMEV243 | CBEV279 | CREV246 | MySEV268 | MSEV094 | VACV048 | |
| 144 | Unknown; similar to AMEV242 | AHEV220 | AMEV242 | CBEV278 | CREV245 | MySEV269 | |||
| 145 | Unknown; similar to AMEV247 | AHEV222 | AMEV247 | CBEV283 | CREV250 | MySEV272 | MSEV139 | ||
| 146 | IMV heparin binding surface protein | AHEV225 | AMEV248 | CBEV285 | CREV252 | MySEV274 | MSEV206 | VACV101 | B7 |
| 147 | IMV membrane protein entry/fusion complex component (Cop-A21L) | AHEV226 | AMEV249 | CBEV286 | CREV253 | MySEV275 | MSEV209 | VACV140 | B7 |
| 148 | Unknown; similar to AMEV013 | AHEV019 | AMEV013 | CBEV061 | CREV042 | MySEV279 | |||
ssDNA, single-stranded DNA; FAD, flavin adenine dinucleotide; morph, morphogenesis; MP, membrane protein; AP, apurinic/apyrimidinic.
To determine if there were strict physical constraints on the order of the core genes, we analyzed the relative positions of the clusters in all poxvirus genomes. We were not able to identify any colocalized core genes at the level of the Poxviridae family. In contrast, within the BetaEPVs, we found seven clusters of strict gene order conservation containing 2 to 17 adjacent genes (clusters B1 [n = 2], B2 [n = 2], B3 [n = 9], B4 [n = 17], B5 [n = 5], B6 [n = 5], and B7 [n = 2]) (Table 2). Cluster B1 includes genes involved in metal ion cell detoxification. Cluster B2 contains genes of unknown function. Cluster B3 includes genes involved in transcription/mRNA modification. Cluster B4 includes genes involved in DNA replication, transcription/mRNA modification, and virus-host interactions. Cluster B5 includes the RNA polymerase RPO147 and the Ca2+ binding protein. Cluster B6 includes the uracil-DNA glycosylase, DNA polymerase processivity factor and the putative late 16-kDa membrane protein (Cop-J5L). Finally, cluster B7 includes two surface/membrane proteins of the intracellular mature virion (IMV). None of these clusters are conserved in MSEV. At the EPV level, only two clusters of two adjacent genes could be found. The first cluster (E1) includes the RNA helicase DExH-NPH-II domain and an unknown gene, AMEV080, and the second cluster (E2) includes the nucleoside triphosphatase (NTPase), DNA primase, and an unknown gene, AMEV085.
Whole-genome poxvirus phylogeny.
Phylogenetic analysis was conducted on the 49 poxvirus core genes (31) for which homologs were identified in 12 poxvirus species representative of each poxvirus genus and in AHEV, CBEV, CREV, and MySEV (Table 3). A concatenated multiple alignment of the 49 poxvirus core genes was used to reconstruct the poxvirus phylogeny by maximum likelihood inference. In accordance with previous studies (47–49), we obtained a highly supported phylogeny (Fig. 3) showing two major monophyletic clades corresponding to the chordopoxvirus and EPV subfamilies. AMEV, AHEV, CBEV, CREV, and MySEV grouped in a well-supported monophyletic lineage corroborating their affiliation within a single genus. Within the BetaEPVs, AHEV, CBEV, and CREV are closer to AMEV than MySEV. Moreover, CBEV and CREV, infecting hosts belonging to the same genus, are very closely related, even though C. biennis is a forestry pest while C. rosaceana is a pest of apple orchards.
Table 3.
Poxvirus genomes used in the phylogenomic analysis
| Subfamily | Genus | Genome | Abbreviation | Genome accession no. |
|---|---|---|---|---|
| Chordopoxvirinae | Avipoxvirus | Fowlpox virus strain Iowa | FWPV | NC_002188 |
| Capripoxvirus | Sheeppox virus strain 17077-99 | SPPV | NC_004002 | |
| Cervidpoxvirus | Deerpox virus strain W-848-83 | DPV | NC_006966 | |
| Leporipoxvirus | Myxoma virus strain Lausanne | MYXV | NC_001132 | |
| Molluscipoxvirus | Molluscum contagiosum virus strain subtype 1 | MOCV | NC_001731 | |
| Orthopoxvirus | Vaccinia virus strain Western Reserve | VACV | NC_006998 | |
| Parapoxvirus | Orf virus strain OV-SA00 | ORFV | NC_005336 | |
| Suipoxvirus | Swinepox virus strain Nebraska 17077–99 | SWPV | NC_003389 | |
| Yatapoxvirus | Yaba monkey tumor virus strain Amano | YMTV | NC_005179 | |
| Crocodylipoxvirus | Nile crocodile poxvirus strain Zimbabwe | CRV | NC_008030 | |
| Entomopoxvirinae | Betaentomopoxvirus | Adoxophyes honmai entomopoxvirus“L” strain Japan | AHEV | HF679131 |
| Amsacta moorei entomopoxvirus “L” strain Moyer | AMEV | NC_002520 | ||
| Choristoneura biennis entomopoxvirus “L” strain Canada | CBEV | HF679132 | ||
| Choristoneura rosaceana entomopoxvirus “L” strain Canada | CREV | HF679133 | ||
| Mythimna separata entomopoxvirus “L” strain China | MySEV | HF679134 | ||
| Unclassified | Melanoplus sanguinipes entomopoxvirus strain Tucson | MSEV | NC_001993 |
Fig 3.
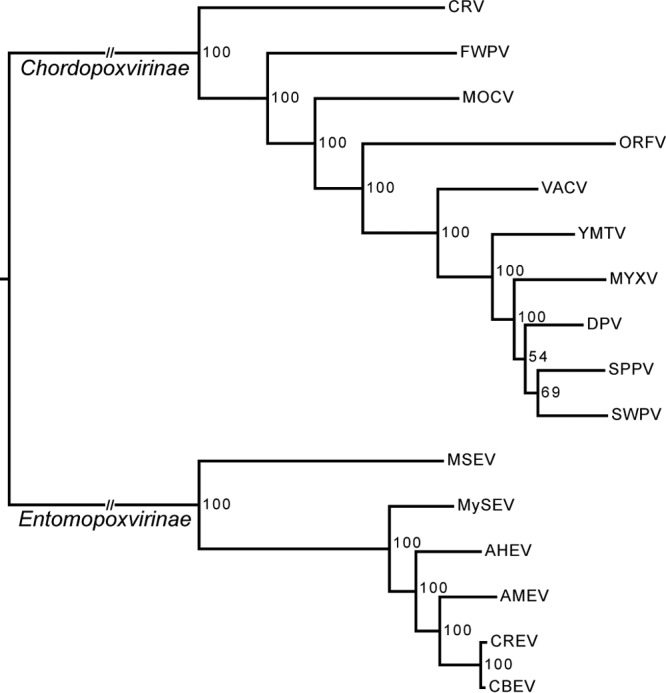
Whole-genome poxvirus phylogeny. The tree was obtained from maximum likelihood inference analysis of a concatenated amino acid multiple alignment of the 49 poxvirus core genes. Support for nodes indicates maximum likelihood nonparametric bootstraps (100 replicates). Full virus names are listed in Table 3.
Spheroidin phylogeny.
The spheroidin amino acid sequence phylogeny based on a larger sampling of EPV taxa (Fig. 4) showed strong phylogenetic similarity in terms of tree topology as well as branch length with the whole-genome EPV phylogeny (Fig. 3). This suggests that the spheroidin gene bears a good phylogenetic signal reflecting EPV species phylogeny. The phylogeny of all the spheroidin proteins available in public databases included sequences from coleopteran EPVs of the genus Alphaentomopoxvirus. Strikingly, the tree showed a clear division of the EPVs according to the orders of their insect hosts.
Fig 4.
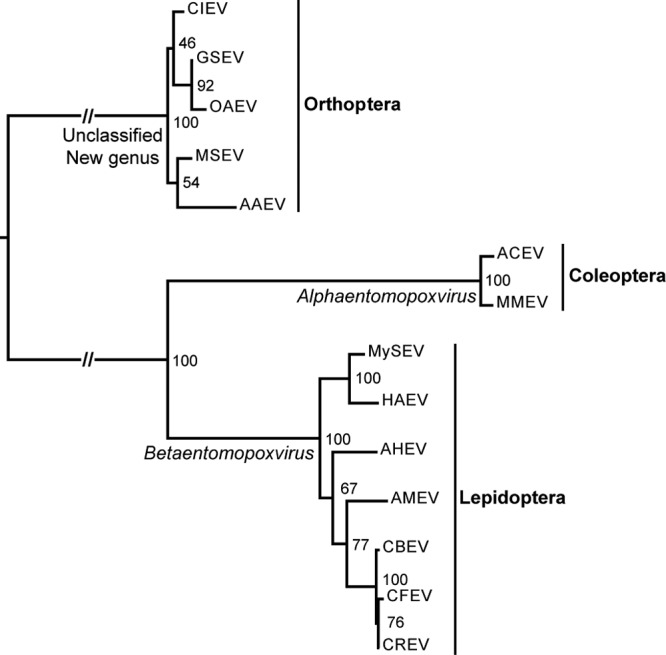
Spheroidin gene phylogeny. The tree was obtained from maximum likelihood inference analysis of the spheroidin gene amino acid alignment. Support for nodes indicates maximum likelihood nonparametric bootstraps (100 replicates). Abbreviations: CIEV, Calliptamus italicus entomopoxvirus; GSEV, Gomphocerus sibiricus entomopoxvirus; OAEV, Oedaleus asiaticus entomopoxvirus; AAEV, Anacridium aegyptium entomopoxvirus; ACEV, Anomala cuprea entomopoxvirus; MMEV, Melolontha melolontha entomopoxvirus; HAEV, Heliothis armigera entomopoxvirus “L”; CFEV, Choristoneura fumiferana entomopoxvirus “L.”
DISCUSSION
Here we report the complete genome sequences of four entomopoxviruses. This is long overdue, since the previous two EPV genomes were published more than 10 years ago (25, 26). The AHEV, CBEV, CREV, and MySEV genomes have general characteristics similar to those of the two EPV genomes sequenced previously. They are extremely AT rich, a reason why obtaining and assembling their sequences had been problematic (11).
EPV comparative genomics.
Like other poxvirus genomes, EPV genomes possess a central region encoding essential core proteins and terminal regions containing less conserved, nonessential, and orphan proteins, possibly involved in virus-host responses. Colinearity analyses showed that the five lepidopteran BetaEPV genomes are similar and that the orthopteran EPV MSEV is evolutionarily divergent (Fig. 1).
The sizes of BetaEPV genomes are extremely variable; CBEV, CREV, and MySEV are at least 50 kbp larger than the average size of other known EPVs. Larger genome sizes are due mainly to large protein families of unknown functions with many members repeated in tandem and predominantly clustered in terminal regions but also dispersed all along the genomes. The N1R/p28 genes are the most abundant gene family (>150 members found in all 5 BetaEPV genomes). These genes have also been identified in other NCLDV, such as iridoviruses and mimiviruses (50, 51), and some contain baculovirus repeated ORF (bro) domains. Considering the number of repeated members present in genomes, they could have important adaptive roles as virulence factors (52). Moreover, we identified several orphan genes found in other insect viruses, notably in baculoviruses, that could be involved in adaptation.
As observed within the Chordopoxvirinae subfamily (46, 53), global genome synteny is highly conserved among lepidopteran EPVs but less conserved at the level of the Entomopoxvirinae subfamily. There is, however, no gene synteny between chordopoxviruses and EPVs, pointing to significant gene rearrangements after the division and radiation of the two subfamilies. In contrast, with 49 conserved genes shared by all poxvirus genomes (31) and 104 shared by all EPV genomes, conservation of gene content remains remarkably substantial (Fig. 2; Table 2). This suggests that poxviruses need a relatively large number of core genes to perform complex functions. Yet gene order conservation does not appear to be crucial. The minimum poxvirus gene set of 49 is doubled for the EPV subfamily and encompasses additional genes related to EPV ecology, such as the spheroidin and DNA photolyase genes, both protecting virions from environmental degradation (11, 54). The number of BetaEPV core genes is 148, accounting for half to two-thirds of the overall number of genes predicted in each genome. Many of these genes, notably those encoding replication, transcription/mRNA modification, and envelope proteins, are arranged in a strict order within this genus, which may indicate that strong conservative selection pressure has kept the genes in this particular order. A similar trend has been observed in chordopoxviruses (48). The poxvirus linear genome structure could support sequential gene expression to ensure essential morphogenesis pathways, which may still be perceptible at the genus level but may be lost at higher taxonomic levels.
EPV phylogeny and taxonomy.
Phylogenetic analyses of the 49 poxvirus core genes (Fig. 3) show that the four new genomes are more closely related to AMEV than to any other poxvirus. This confirms that AHEV, CBEV, CREV, and MySEV, isolated from lepidopteran hosts, belong to the genus Betaentomopoxvirus. The spheroidin phylogeny, including more EPV isolates, indicates that EPVs infecting insects from the same taxonomic order (Lepidoptera, Orthoptera, or Coleoptera) group together. There is thus a clear partition of the EPVs according to the orders of their insect hosts. The EPV genera were historically based on host range and virion morphology. The Betaentomopoxvirus genus was established as comprising viruses infecting Orthoptera and Lepidoptera. However, based on genomic divergence, the species Melanoplus sanguinipes entomopoxvirus “O,” infecting Orthoptera, was removed from the genus (4). Our phylogenetic analyses showed that orthopteran EPVs are excluded from the Betaentomopoxvirus genus. This suggests that host order could be a good criterion for defining EPV genera and that a new genus should be established for orthopteran EPVs (Fig. 4). This implies an ancient coevolution of EPVs with their insect hosts similar to that observed with baculoviruses and other large DNA viruses of insects (55, 56). The genome tree also shows that the subfamily Chordopoxvirinae is phylogenetically structured according to the taxonomic class of the host (mammals, birds, and reptiles). The coevolution between poxviruses and their hosts that culminated in their present distribution and host range suggests a remote virus origin, presumably going back to the common ancestors of vertebrates and insects: the first bilaterian Metazoa (57, 58).
Although the Entomopoxvirinae are structured according to the orders of the insect hosts, this virus clustering according to host taxonomy is not observable within the genus Betaentomopoxvirus. The phylogenies show an entanglement of EPVs infecting different lepidopteran host families (Arctiidae, Noctuidae, and Tortricidae) (59). EPVs, and large DNA viruses in general, tend to exhibit a fairly narrow host range (60), but the close phylogenetic relationships of EPVs infecting distant hosts suggest that large host shifts can occur. Current pathology data on EPVs show their relative host specificities (e.g., AHEV) (24). But generalists, such as the Heliothis armigera entomopoxvirus “L” (HAEV) (David Dall, personal communication), could promote host shifts, explaining the tangled phylogenetic relationships within the BetaEPVs.
Comparison of CREV and CBEV.
Within the Betaentomopoxvirus genus, AHEV, AMEV, MySEV, and HAEV are phylogenetically well differentiated, as should be expected for viruses belonging to different species (Fig. 3 and 4). In contrast, CBEV and CREV are quite closely related phylogenetically, calling for a closer examination to determine if they are the same or distinct species.
CBEV and CREV were both isolated in Canada from phytophagous pests belonging to the same genus. CBEV was isolated from C. biennis, the 2-year-cycle budworm, a forest pest feeding mostly on spruce trees, and CREV from C. rosaceana, the oblique-banded leafroller, a pest of orchard trees, such as apples, prunes, and cherries, and some hardwood. If the two viruses infect closely related hosts and share the same geographical range, they appear to be linked to different ecological habitats. The 49 core poxvirus gene nucleotide sequences are 97.2% identical in CREV and CBEV. This is well within the 96% identity proposed to differentiate among orthopoxvirus species but below the 98% accepted within-strain variation (4), suggesting that CREV and CBEV could be different strains of the same viral species.
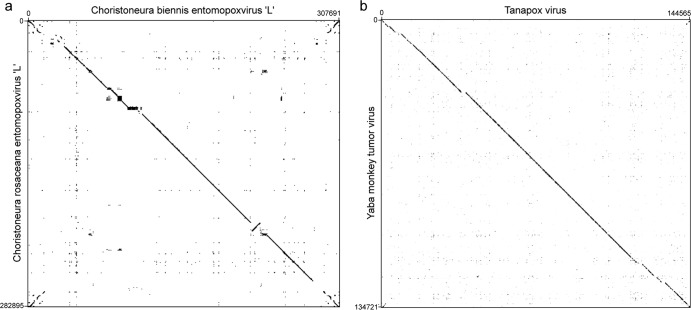
The genomes of CREV and CBEV are, however, quite different in size and gene content (Table 1). CBEV is ∼25 kb larger than CREV; the difference is mostly explained by the large CBEV ITRs containing several N1R/p28 gene copies and genes coding for hypothetical proteins. The remaining difference corresponds to genes coding for hypothetical proteins spread all along both genomes. Overall, 35 genes are different in the two genomes, corresponding to around 10% of both genomes. Furthermore, using dot plots (created with the Gepard program [61]), we compared genome synteny between the two genomes infecting Choristoneura species (CBEV and CREV) (Fig. 5a) and between genomes of two different chordopoxvirus species belonging to the same genus (Tanapox virus and Yaba monkey tumor virus, both species of the Yatapoxvirus genus) (Fig. 5b). We observed more rearrangements, deletions, and insertions between the CBEV and CREV genomes than between the Yatapoxvirus species (Fig. 5). These differences in genomic content and organization suggest that CBEV and CREV should be classified into different species, even if this classification was not corroborated by phylogenetic relationships and core gene nucleotide distances.
Fig 5.
Genome synteny visualization by dot plots. The dot plots were obtained from whole-genome DNA homology alignments, using the Gepard program, between CBEV and CREV (a) and between the Tanapox virus and Yaba monkey tumor virus species (b).
This discrepancy implies that we cannot apply the orthopoxvirus species genetic distance to define entomopoxvirus species. Although phylogenetic relationships and core gene nucleotide distances show the closeness of CBEV and CREV, they infect different hosts of the same genus and are specialized to clearly different ecological niches, implying that the two viruses are very likely to belong to two separate species.
Conclusions.
The genome sequences of AHEV, CBEV, CREV, and MySEV have provided new insights into EPV genomic organization and evolution. Our results allow certain generalizations on the structure of poxvirus genomes. Like those of chordopoxviruses, EPV genomes are structured in two parts, which appear to have evolved quite differently: the central core region and the more divergent terminal regions. Genetic diversity within the central core is relatively low in the BetaEPVs, resulting in high genome colinearity, both in terms of gene content and in terms of synteny conservation. However, the central core is much less diverse at the Entomopoxvirinae subfamily and Poxviridae family levels. The terminal regions, containing large gene families, as well as orphan genes, could play an important role in the adaptation of viruses to their hosts. In particular, the N1R/p28 gene family could play an adaptive role similar to that of the K3L antihost factor in orthopoxviruses, which was recently described as forming adaptive genomic accordions (62, 63).
Phylogenies showed the long history of coevolution between poxviruses and their hosts. The Entomopoxvirinae are grouped based on the orders of their insect hosts, suggesting that taxonomic revision is necessary. Basic pathological and genomic knowledge of EPVs, however, remains sparse, particularly for alpha- and gammaentomopoxviruses. This diverse, understudied group of viruses could find new applications as microbial biocontrol agents for sustainable agriculture. Finally, a better understanding of the early origin and evolution of the Poxviridae could shed new light on the evolutionary history of all large DNA viruses.
ACKNOWLEDGMENTS
European Research Council grant 205206 GENOVIR funded J. Thézé, J. Gallais, and E. A. Herniou. The sequencing of the AHEV and MySEV genomes was supported by JSPS KAKENHI grant 21380038, and that of CBEV and CREV genomes by a grant from Genome Canada through the Ontario Genomics Institute.
We thank David Dall for discussions on entomopoxvirus host range.
Footnotes
Published ahead of print 15 May 2013
REFERENCES
- 1. Iyer LM, Aravind L, Koonin EV. 2001. Common origin of four diverse families of large eukaryotic DNA viruses. J. Virol. 75:11720–11734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wittek R, Menna A, Müller HK, Schümperli D, Boseley PG, Wyler R. 1978. Inverted terminal repeats in rabbit poxvirus and vaccinia virus DNA. J. Virol. 28:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schramm B, Locker JK. 2005. Cytoplasmic organization of poxvirus DNA replication. Traffic 6:839–846 [DOI] [PubMed] [Google Scholar]
- 4. Skinner MA, Buller RM, Damon IK, Lefkowitz EJ, McFadden G, McInnes CJ, Mercer AA, Moyer RW, Upton C. 2011. Poxviridae, p 291–309 King AM, Lefkowitz E, Adams MJ, Carstens EB. (ed), Virus taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses Elsevier, Amsterdam, Netherlands [Google Scholar]
- 5. Mercer AA, Schmidt A, Weber OF. 2007. Poxviruses. Birkhäuser, Basel, Switzerland [Google Scholar]
- 6. McFadden G. 2005. Poxvirus tropism. Nat. Rev. Microbiol. 3:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawrence PO. 28 May 2002. Purification and partial characterization of an entomopoxvirus (DLEPV) from a parasitic wasp of tephritid fruit flies. J. Insect Sci. 2:10 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC355910/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawrence PO. 2005. Morphogenesis and cytopathic effects of the Diachasmimorpha longicaudata entomopoxvirus in host haemocytes. J. Insect Physiol. 51:221–233 [DOI] [PubMed] [Google Scholar]
- 9. Clark TB. 1982. Entomopoxvirus-like particles in three species of bumblebees. J. Invertebr. Pathol. 39:119–122 [Google Scholar]
- 10. Radek R, Fabel P. 2000. A new entomopoxvirus from a cockroach: light and electron microscopy. J. Invertebr. Pathol. 75:19–27 [DOI] [PubMed] [Google Scholar]
- 11. Perera S, Li Z, Pvalik L, Arif B. 2010. Entomopoxviruses, p 83–115 Asgari S, Johnson KN. (ed), Insect virology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 12. Rohrmann GF. 1986. Polyhedrin structure. J. Gen. Virol. 67:1499–1513 [DOI] [PubMed] [Google Scholar]
- 13. Arif BM. 1995. Recent advances in the molecular biology of entomopoxviruses. J. Gen. Virol. 76:1–13 [DOI] [PubMed] [Google Scholar]
- 14. Bilimoria SL, Arif BM. 1979. Subunit protein and alkaline protease of entomopoxvirus spheroids. Virology 96:596–603 [DOI] [PubMed] [Google Scholar]
- 15. Roberts DW, Granados RR. 1968. A poxlike virus from Amsacta moorei (Lepidoptera: Arctiidae). J. Invertebr. Pathol. 12:141–143 [Google Scholar]
- 16. Volkman LE. 2007. Baculovirus infectivity and the actin cytoskeleton. Curr. Drug Targets 8:1075–1083 [DOI] [PubMed] [Google Scholar]
- 17. Ishii T, Takatsuka J, Nakai M, Kunimi Y. 2002. Growth characteristics and competitive abilities of a nucleopolyhedrovirus and an entomopoxvirus in larvae of the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae). Biol. Control 23:96–105 [Google Scholar]
- 18. Goodwin RH. 1991. Replacement of vertebrate serum with lipids and other factors in the culture of invertebrate cells, tissues, parasites, and pathogens. In Vitro Cell. Dev. Biol. 27A:470–478 [DOI] [PubMed] [Google Scholar]
- 19. Nakai M, Sakai T, Kunimi Y. 1997. Effect of entomopoxvirus infection of the smaller tea tortrix, Adoxophyes sp. on the development of the endoparasitoid, Ascogaster reticulatus. Entomol. Exp. Appl. 84:27–32 [Google Scholar]
- 20. Nakai M, Kunimi Y. 1998. Effects of the timing of entomopoxvirus administration to the smaller tea tortrix, Adoxophyes sp. (Lepidoptera: Tortricidae) on the survival of the endoparasitoid, Ascogaster reticulatus (Hymenoptera: Braconidae). Biol. Control 13:63–69 [Google Scholar]
- 21. Bird FT, Sanders CJ, Burke JM. 1971. A newly discovered virus disease of the spruce budworm, Choristoneura biennis (Lepidoptera: Tortricidae). J. Invertebr. Pathol. 18:159–161 [DOI] [PubMed] [Google Scholar]
- 22. Hukuhara T, Xu JH, Yano K. 1990. Replication of an entomopoxvirus in two lepidopteran cell lines. J. Invertebr. Pathol. 56:222–232 [DOI] [PubMed] [Google Scholar]
- 23. Cory J. 1997. Use of baculoviruses as biological insecticides. Mol. Biotechnol. 7:303–313 [DOI] [PubMed] [Google Scholar]
- 24. Takatsuka J, Okuno S, Ishii T, Nakai M, Kunimi Y. 2010. Fitness-related traits of entomopoxviruses isolated from Adoxophyes honmai (Lepidoptera: Tortricidae) at three localities in Japan. J. Invertebr. Pathol. 105:121–131 [DOI] [PubMed] [Google Scholar]
- 25. Afonso CL, Tulman ER, Lu Z, Oma E, Kutish GF, Rock DL. 1999. The genome of Melanoplus sanguinipes entomopoxvirus. J. Virol. 73:533–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bawden AL, Glassberg KJ, Diggans J, Shaw R, Farmerie W, Moyer RW. 2000. Complete genomic sequence of the Amsacta moorei entomopoxvirus: analysis and comparison with other poxviruses. Virology 274:120–139 [DOI] [PubMed] [Google Scholar]
- 27. Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanger F, Coulson AR. 1978. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 87:107–110 [DOI] [PubMed] [Google Scholar]
- 29. Salzberg SL, Delcher AL, Kasif S, White O. 1998. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 26:544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ehlers A, Osborne J, Slack S, Roper RL, Upton C. 2002. Poxvirus Orthologous Clusters (POCs). Bioinformatics 18:1544–1545 [DOI] [PubMed] [Google Scholar]
- 31. Upton C, Slack S, Hunter AL, Ehlers A, Roper RL. 2003. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J. Virol. 77:7590–7600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang ZQ, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res. 19:1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eddy SR. 1998. Profile hidden Markov models. Bioinformatics 14:755–763 [DOI] [PubMed] [Google Scholar]
- 35. Pavesi G, Mauri G, Iannelli F, Gissi C, Pesole G. 2004. GeneSyn: a tool for detecting conserved gene order across genomes. Bioinformatics 20:1472–1474 [DOI] [PubMed] [Google Scholar]
- 36. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7:539. 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keane TM, Creevey CJ, Pentony MM, Naughton TJ, Mclnerney JO. 2006. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol. Biol. 6:29. 10.1186/1471-2148-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690 [DOI] [PubMed] [Google Scholar]
- 39. Sriskantha A, Osborne RJ, Dall DJ. 1997. Mapping of the Heliothis armigera entomopoxvirus (HaEPV) genome, and analysis of genes encoding the HaEPV spheroidin and nucleoside triphosphate phosphohydrolase I proteins. J. Gen. Virol. 78:3115–3123 [DOI] [PubMed] [Google Scholar]
- 40. Li X, Barrett JW, Yuen L, Arif BM. 1997. Cloning, sequencing and transcriptional analysis of the Choristoneura fumiferana entomopoxvirus spheroidin gene. Virus Res. 47:143–154 [DOI] [PubMed] [Google Scholar]
- 41. Sanz P, Veyrunes JC, Cousserans F, Bergoin M. 1994. Cloning and sequencing of the spherulin gene, the occlusion body major polypeptide of the Melolontha melolontha entomopoxvirus (MmEPV). Virology 202:449–457 [DOI] [PubMed] [Google Scholar]
- 42. Mitsuhashi W, Saito H, Sato M, Nakashima N, Noda H. 1998. Complete nucleotide sequence of spheroidin gene of Anomala cuprea entomopoxvirus. Virus Res. 55:61–69 [DOI] [PubMed] [Google Scholar]
- 43. Hernandez-Crespo P, Veyrunes JC, Cousserans F, Bergoin M. 2000. The spheroidin of an entomopoxvirus isolated from the grasshopper Anacridium aegyptium (AaEPV) shares low homology with spheroidins from lepidopteran or coleopteran EPVs. Virus Res. 67:203–213 [DOI] [PubMed] [Google Scholar]
- 44. Zhao C, Wang L, Li Y, Yun G. 2003. Cloning and analysis of Oedaleus asiaticus entomopoxvirus spheroidin gene. Virol. Sin. 18:593–596 [Google Scholar]
- 45. Li YD, Wang LY, Gaol XW, Zhao CY, Tian ZF. 2004. Complete nucleotide sequence of spheroidin genes of Calliptamus italicus entomopoxvirus (CiEPV) and Gomphocerus sibiricus entomopoxvirus (GsEPV). Insect Sci. 11:173–182 [Google Scholar]
- 46. McLysaght A, Baldi PF, Gaut BS. 2003. Extensive gene gain associated with adaptive evolution of poxviruses. Proc. Natl. Acad. Sci. U. S. A. 100:15655–15660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xing K, Deng R, Wang J, Feng J, Huang M, Wang X. 2006. Genome-based phylogeny of poxvirus. Intervirology 49:207–214 [DOI] [PubMed] [Google Scholar]
- 48. Bratke KA, McLysaght A. 2008. Identification of multiple independent horizontal gene transfers into poxviruses using a comparative genomics approach. BMC Evol. Biol. 8:67. 10.1186/1471-2148-8-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu GA, Jun SR, Sims GE, Kim SH. 2009. Whole-proteome phylogeny of large dsDNA virus families by an alignment-free method. Proc. Natl. Acad. Sci. U. S. A. 106:12826–12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wong CK, Young VL, Kleffmann T, Ward VK. 2011. Genomic and proteomic analysis of invertebrate iridovirus type 9. J. Virol. 85:7900–7911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Legendre M, Santini S, Rico A, Abergel C, Claverie J-M. 2011. Breaking the 1000-gene barrier for Mimivirus using ultra-deep genome and transcriptome sequencing. Virol. J. 8:99. 10.1186/1743-422X-8-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nicholls R, Gray T. 2004. Cellular source of the poxviral N1R/p28 gene family. Virus Genes 29:359–364 [DOI] [PubMed] [Google Scholar]
- 53. Lefkowitz EJ, Wang C, Upton C. 2006. Poxviruses: past, present and future. Virus Res. 117:105–118 [DOI] [PubMed] [Google Scholar]
- 54. Nalcacioglu R, Dizman YA, Vlak JM, Demirbag Z, van Oers MM. 2010. Amsacta moorei entomopoxvirus encodes a functional DNA photolyase (AMV025). J. Invertebr. Pathol. 105:363–365 [DOI] [PubMed] [Google Scholar]
- 55. Herniou EA, Olszewski JA, O'Reilly DR, Cory JS. 2004. Ancient coevolution of baculoviruses and their insect hosts. J. Virol. 78:3244–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thézé J, Bézier A, Periquet G, Drezen JM, Herniou EA. 2011. Paleozoic origin of insect large dsDNA viruses. Proc. Natl. Acad. Sci. U. S. A. 108:15931–15935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruiz-Trillo I, Riutort M, Littlewood DTJ, Herniou EA, Baguna J. 1999. Acoel flatworms: earliest extant bilaterian metazoans, not members of Platyhelminthes. Science 283:1919–1923 [DOI] [PubMed] [Google Scholar]
- 58. Peterson KJ, Lyons JB, Nowak KS, Takacs CM, Wargo MJ, McPeek MA. 2004. Estimating metazoan divergence times with a molecular clock. Proc. Natl. Acad. Sci. U. S. A. 101:6536–6541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mutanen M, Wahlberg N, Kaila L. 2010. Comprehensive gene and taxon coverage elucidates radiation patterns in moths and butterflies. Proc. R. Soc. B 277:2839–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Villarreal LP, Defilippis VR, Gottlieb KA. 2000. Acute and persistent viral life strategies and their relationship to emerging diseases. Virology 272:1–6 [DOI] [PubMed] [Google Scholar]
- 61. Krumsiek J, Arnold R, Rattei T. 2007. Gepard: a rapid and sensitive tool for creating dotplots on genome scale. Bioinformatics 23:1026–1028 [DOI] [PubMed] [Google Scholar]
- 62. Elde NC, Child SJ, Eickbush MT, Kitzman JO, Rogers KS, Shendure J, Geballe AP, Malik HS. 2012. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 150:831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Anderson RP, Roth JR. 1977. Tandem genetic duplications in phage and bacteria. Annu. Rev. Microbiol. 31:473–505 [DOI] [PubMed] [Google Scholar]