Abstract
Epstein-Barr virus (EBV) is associated with various malignancies, including epithelial cancers. In this study, we analyzed the effect of EBV infection on epithelial cells by using EBV-converted epithelial cells. In EBV-positive cells, the extracellular signal-regulated kinase (ERK) pathway is constitutively activated. Inhibition of ERK activity leads to reduced anoikis resistance; therefore, EBV-positive cells are more resistant to anoikis, a type of apoptosis induced by cell detachment, than are EBV-negative cells. Among the viral genes expressed in EBV-positive cells, the latent membrane protein 2A (LMP2A) is responsible for induction of ERK-mediated anoikis resistance, although the expression level of LMP2A is much lower in EBV-positive cells than in EBV-transformed B cells. Further analysis demonstrated that LMP2A downregulation of the proanoikis mediator Bim through proteasomal degradation is dependent on the immunoreceptor tyrosine-based activation motif (ITAM). These findings suggest that LMP2A-mediated ERK activation is involved in the generation of EBV-associated epithelial malignancies.
INTRODUCTION
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus that infects more than 90% of the world's populations. EBV infection is associated with a variety of malignancies, including endemic Burkitt's lymphoma, Hodgkin's lymphoma, gastric carcinoma (GC), and nasopharyngeal carcinoma (NPC) (1). These cancers are characterized by the proliferation of monoclonal EBV-infected cells, and viral gene expression in these cells is limited to a subset of latent genes. In EBV-positive epithelial cancers, both GC and NPC cells express EBV nuclear antigen 1 (EBNA1), a series of transcripts from the BamHI-A region of the EBV genome (BARTs), and EBV-encoded small RNAs (EBERs) (2). The latent membrane protein 2A (LMP2A) is expressed in approximately 40% of GC cases (3) and in most cases of NPC (4, 5). In approximately 35% of NPC cases, additional LMP1 expression can be detected (6).
LMP2A functions to maintain viral latency in B lymphocytes by blocking B cell receptor (BCR) signaling, which would otherwise reactivate the virus to enter the lytic cycle (7–9). Studies using LMP2A transgenic mice have revealed that LMP2A sends survival signals and allows B cells to bypass developmental checkpoints and escape the bone marrow to colonize peripheral lymphoid organs (10, 11). A more recent study demonstrated that LMP2A provides a surrogate pre-BCR signal through activation of the extracellular signal-regulated kinase (ERK) pathway (12). The immunoreceptor tyrosine-based activation motif (ITAM), located in the cytoplasmic amino (N) terminus of LMP2A, is essential for interaction with associated kinases; it is therefore considered to be critical for the above-mentioned functions of LMP2A (13, 14). The LMP2A N terminus also contains 2 proline-rich motifs known as PY motifs that enable LMP2A to interact with neural precursor cell-expressed developmentally downregulated gene 4 (Nedd4) family members of ubiquitin ligases in B cells to manipulate its turnover (15–17).
Despite the lack of BCR expression and BCR-associated kinases in epithelial cells, LMP2A has been reported to activate similar signaling in both epithelial cells and B lymphocytes. The phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway is activated by LMP2A, leading to the transformation of epithelial cells (18) and activation of β-catenin signaling in human foreskin keratinocyte (HFK) cells (19). The ITAM and PY motifs of LMP2A are essential for the activation of β-catenin signaling via PI3K (20). Another study demonstrated that LMP2A activates the ERK pathway and promotes cell mobility (21). However, it remains unclear whether the levels of LMP2A expression are sufficient for these activities in epithelial cancer tissues in vivo because previous studies used LMP2A-transfected cells, which express much higher levels of LMP2A than those in cancer tissues.
In this study, we used an in vitro system of EBV infection of epithelial cells that has been previously described (22). Because recombinant EBV (rEBV)-infected cell clones as well as EBV-positive GC or NPC cells expressed a limited number of EBV genes, this system is a useful model for analyzing the role of EBV in epithelial cancer development (23–25). We demonstrate that EBV infection leads to resistance to anoikis, a cell detachment-induced apoptosis, by activating the ERK signaling pathway. Further analysis revealed that LMP2A expression in EBV-infected cells is crucial for the induction of anoikis resistance.
MATERIALS AND METHODS
Cell culture and reagents.
Cells of the human fetal intestinal epithelial cell line Intestine 407 (kindly provided by Tsutomu Chiba, Kyoto University) (26) were cultured in Dulbecco's modified Eagle's medium (Sigma, St. Louis, MO) supplemented with 10% bovine serum (Invitrogen, Carlsbad, CA) and antibiotics. An Akata cell clone infected with rEBV (27) or LMP2A-knockout (KO) EBV (28), which carries the neomycin resistance (Neor) gene, was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Invitrogen), antibiotics, and G418 (700 μg/ml; Sigma). The MEK inhibitor U0126 was purchased from Cell Signaling Technologies, Beverly, MA, and the proteasome inhibitor MG132 was obtained from Sigma.
Establishment of EBV-infected Intestine 407 cells.
Intestine 407 cells were infected with rEBV and LMP2A-KO EBV by using a cell-to-cell infection procedure, as previously described (22). Briefly, cells were cocultured with rEBV-infected Akata cells that had previously been treated with rabbit polyclonal anti-human IgG antibody (Dako, Copenhagen, Denmark) to induce virus production. The culture was then incubated for 3 days at 37°C in 5% CO2, with half of the medium replaced with fresh medium on day 2. After 3 days of cocultivation, the culture was washed 4 times with phosphate-buffered saline to remove residual Akata cells, and 2 ml of fresh medium was added. On day 5, the cells were reseeded into 24-well plates at 104/ml per well in a culture medium containing G418 (500 μg/ml) for selection of EBV-infected clones.
Cell lysis and immunoblot analysis.
Cells were lysed with 1% Nonidet P-40 (NP-40) lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate (Na3VO4), 10 μg/ml pepstatin, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and a phosphatase inhibitor cocktail diluted at 1:100 (Sigma). After protein concentrations were determined by use of a Bradford protein assay kit (Bio-Rad, Hercules, CA), lysates were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and were then electrotransferred to a nitrocellulose membrane. The membrane was blocked with 5% bovine serum albumin (Sigma) or nonfat milk in Tris-buffered saline containing 0.05% Tween 20 and subsequently treated with the primary antibodies for Flag and β-actin (Sigma), green fluorescent protein (GFP; BD Biosciences, San Jose, CA), LMP2A (kindly provided by Richard Longnecker, Northwestern University) (29), Bim (Chemicon International Inc., Temecula, CA), and ERK and phospho-ERK (Cell Signaling Technology). The membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies, anti-rat IgG (for detection of LMP2A), anti-mouse IgG (for detection of Flag and β-actin), and anti-rabbit IgG (for detection of GFP, Bim, ERK, and phospho-ERK) (Amersham Biosciences, Buckinghamshire, United Kingdom). For detection of LMP2A, both primary and secondary antibodies were diluted in Can Get Signal solution (Toyobo, Osaka, Japan) before use. Protein bands were visualized using ECL-Plus Western blotting detection reagents (Amersham).
Immunoprecipitation.
Cell lysates (1 mg) were precleared with protein G-Sepharose beads (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom), and samples were incubated overnight with 1 μg anti-Flag M2 antibody or anti-GFP antibody (diluted 1:200). The immune complexes were then precipitated by protein G-Sepharose at 4°C for 3 h and washed 3 times with 1% NP-40 lysis buffer. Finally, the immunoprecipitates were resolved by SDS-PAGE, and Western blot analysis was carried out as described above.
RNA extraction and reverse transcription-PCR (RT-PCR) analysis.
Total RNA was isolated from cells using the TRIzol reagent (Invitrogen) according to the manufacturer's protocol. For cDNA synthesis, 100 pmol of a random hexamer (Invitrogen) was added to 1 μg RNA, followed by heating at 94°C for 10 min. RNA was then reverse transcribed using 200 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen) in a reaction buffer containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mm MgCl2, 10 mM dithiothreitol, 0.5 mM each deoxynucleoside triphosphate, and 10 U of RNasin (Promega, Madison, WI) at 37°C for 60 min. cDNA aliquots were then subjected to PCR analyses by using primer pairs and probes specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), LMP2A, EBER, and BamHI-A rightward frame 0 (BARF0), as previously described (2). Primers used for Bim detection were as follows: forward, 5′-ATGGCAAAGCAACCTTCTGA-3′, and reverse, 5′-TCAATGCATTCTCCACACC-3′. cDNA amplification was performed as follows: denaturing at 94°C for 2 min, primer annealing at 58°C for 1 min, and an extension at 72°C for 1 min for 30 cycles.
Anoikis assays.
Anoikis assays were performed using established procedures (30). For determining cell viability or caspase 3/7 assays, cells were detached and maintained in suspension in low-cell-binding plates (Nunc). After 48 h, cells were harvested and the percentage of dead cells was assessed by trypan blue staining and by the Cell Titer-Glo luminescent cell viability assay system (Promega) according to the manufacturer's protocol. Caspase 3/7 activity in cell lysates was assayed by Caspase-Glo assays (Promega).
Plasmids, transfection, and cell cloning.
The LMP2A gene derived from Akata cell EBV was subcloned into the pTAT vector and pCMV-Tag2A expression vector (Stratagene, La Jolla, CA) carrying the Flag tag or the pEGFP-N1 expression vector (BD biosciences) carrying the enhanced GFP (EGFP) gene. LMP2A deletion mutants were generated by PCR and subcloned into expression vectors. LMP2A point mutations were generated by a QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. All plasmids were transfected into Intestine 407 cells by using the Lipofectamine plus reagent (Invitrogen). For isolation of stable transfectants, transfected cells were selected with complete culture medium containing 500 μg/ml G418.
RESULTS
EBV infection confers anoikis resistance in epithelial cells.
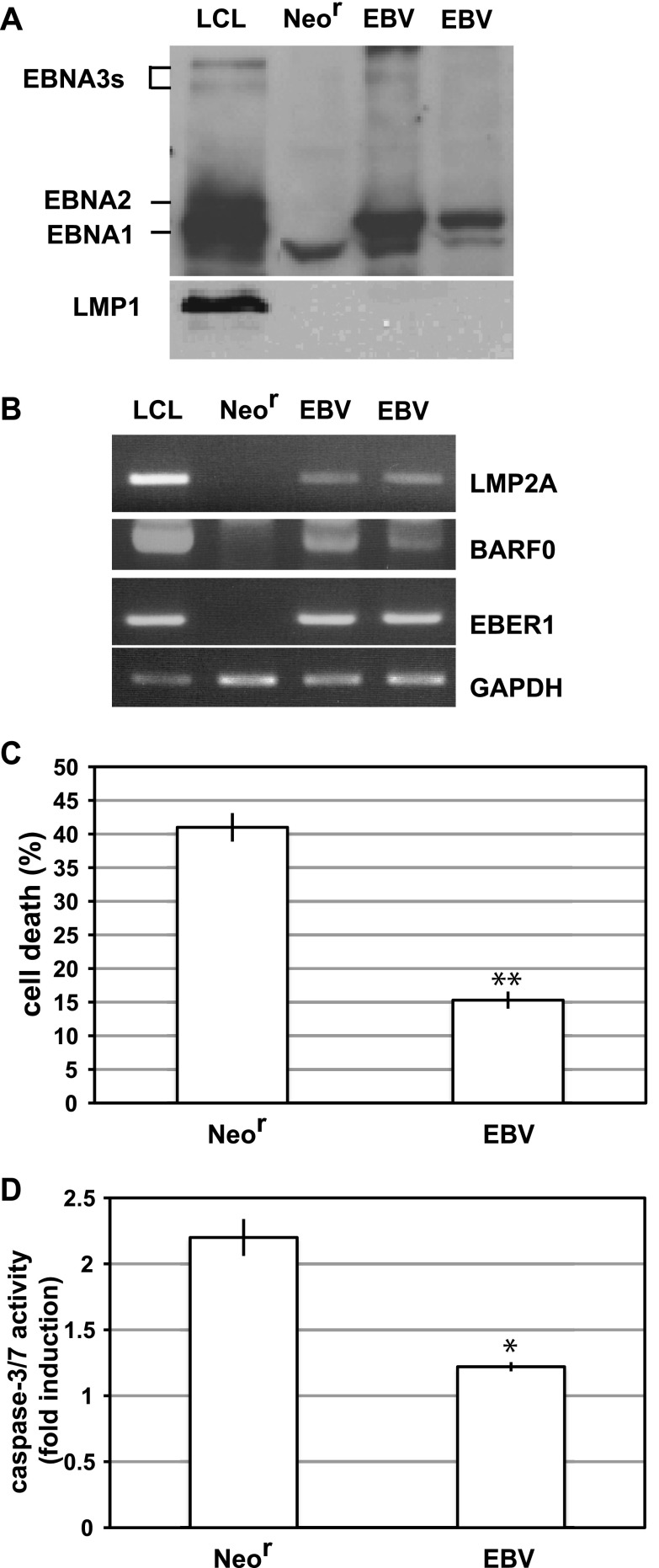
We established an EBV-converted human epithelial cell line, Intestine 407, by using the cell-to-cell infection method to analyze the effect of EBV infection on epithelial cells. rEBV isolates carried the Neor gene, and EBV-positive cell clones were selected by growth in culture medium containing G418 (22). After G418 selection, 10 representative cell clones were analyzed to determine whether cells were infected with EBV. All of the cell clones were 100% positive for EBNA, as determined by the immunofluorescence assay (data not shown). Western blot analysis demonstrated that EBV-positive Intestine 407 cells expressed EBNA1, but not EBNA2, EBNA3s, or LMP1 (Fig. 1A). RT-PCR analysis revealed that EBV-positive clones expressed LMP2A, although the mRNA level was much lower than that in a B lymphoblastoid cell line immortalized with rEBV (LCL) as a positive control (Fig. 1B). Consistent with the result of protein analysis, LMP1 mRNA expression was not observed (data not shown). Because EBER and BARF0 expression was also observed in EBV-positive clones, the viral gene expression patterns were identical to those of EBV-associated GC, i.e., type I latency (Fig. 1B). Subsequently, we cultured both EBV-positive and EBV-negative Intestine 407 cells by using low-binding culture plates, wherein cells cannot attach to the plate and are maintained in suspension. After 48 h of suspension culture, cell death was induced in EBV-negative Neor-transfected (Neor) cells (Fig. 1C); this was caused by the induction of apoptosis (anoikis) as a result of the increased activities of caspase-3/7 in these cells (Fig. 1D). In contrast, EBV-positive cells showed much less induction of cell death and a little increase in caspase3/7 activities (Fig. 1D). These results suggest that EBV confers resistance to anoikis in Intestine 407 cells.
Fig 1.
EBV confers resistance to anoikis in Intestine 407 cells. (A, B) EBV latent gene expression in EBV-positive Intestine 407 cells. (A) Western blot analysis of EBV-positive (EBV) or EBV-negative (Neor) cells. The membrane was probed with EBV-positive human serum for EBNAs and with S12 monoclonal antibody for LMP1. (B) RT-PCR analysis. LCL, a B-lymphoblastoid cell line immortalized with rEBV, was used as a positive control for detection of EBNAs, LMP2A, BARF0, and EBER. (C) Detection of anoikis. EBV-positive (EBV) or EBV-negative (Neor) cells were cultured in suspension for 48 h, and the percentage of dead cells was assessed by Cell Titer-Glo assay. The representative results of 3 separate experiments using 2 independent clones are represented as mean values ± SDs. Statistically significant differences were evaluated by Student's t test (**, P < 0.01). (D) The caspase 3/7 activities of detached cells were measured, and results are represented as the fold induction of the activities of normally cultured cells. The representative results of 3 separate experiments are represented as mean values ± SDs. Statistically significant differences were evaluated by Student's t test (*, P < 0.05).
EBV prevents anoikis through activation of the MEK-ERK pathway.
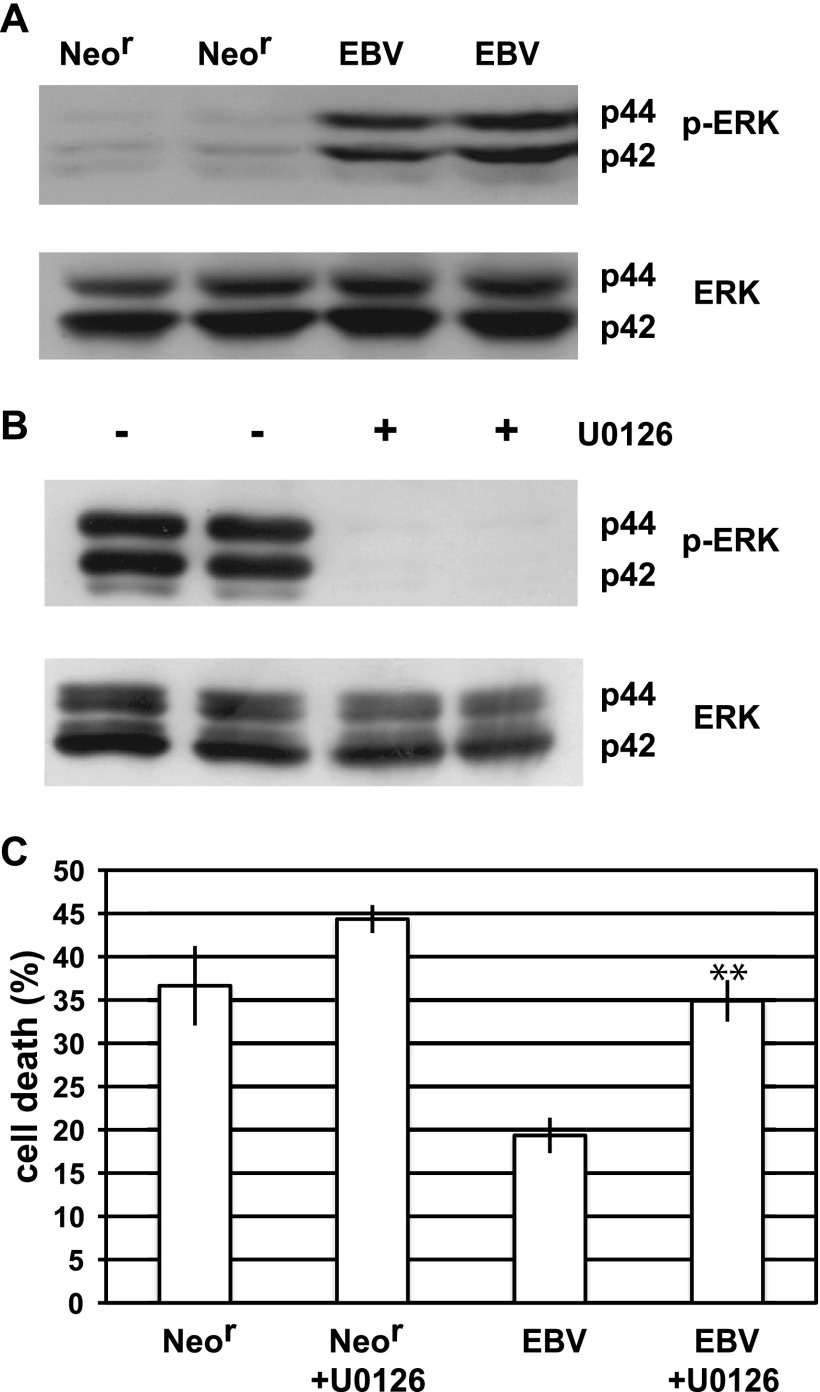
Previous studies have reported that activation of the MEK-ERK pathway plays a crucial role in the induction of resistance to anoikis (31, 32). We examined the strong activation of this pathway in EBV-positive cells and compared it to the activation in EBV-negative (Neor) cells (Fig. 2A). Treatment with the MEK inhibitor U0126 resulted in reduced ERK activity in EBV-positive cells, indicating that EBV induces activation of the MEK-ERK signaling pathway (Fig. 2B). To investigate the effect of U0126 treatment on the induction of anoikis, we cultured EBV-positive and Neor cells in suspension by using the U0126-containing medium. Although U0126 treatment led to a little induction of anoikis in Neor cells, U0126 resulted in considerable induction of anoikis in EBV-positive cells (Fig. 2C), indicating that EBV induces anoikis resistance through activation of the MEK-ERK pathway.
Fig 2.
EBV activates the MEK-ERK pathway. (A) Detection of phosphorylated ERK (p-ERK) and total ERK (ERK) in EBV-positive (EBV) or EBV-negative (Neor) cells by Western blot analysis. (B) Effect of U0126 treatment on ERK activation in EBV-positive cells. EBV-positive cells treated (+) or untreated (−) with U0126 were lysed, and phosphorylation of ERK was analyzed. (C) Effect of U0126 on anoikis resistance. EBV-positive and EBV-negative cells were treated or untreated with U0126 and cultured in suspension, and cell death was analyzed. The representative results of 3 separate experiments using 2 independent clones are represented as the mean percent ± SD. Statistically significant differences were evaluated by Student's t test (**, P < 0.01).
LMP2A induces anoikis resistance through ERK activation.
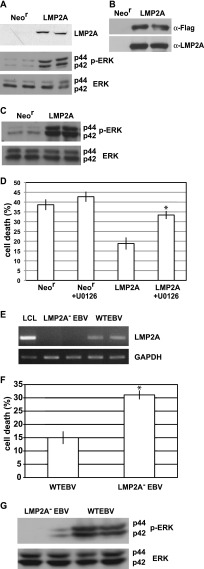
Among the viral genes that are expressed in EBV-positive Intestine 407 cells, LMP2A is known to activate ERK in epithelial cells (21). To clarify whether LMP2A is responsible for activation of the ERK, we introduced LMP2A-expressing plasmids to Intestine 407 cells. Both transient and stable LMP2A expression resulted in ERK activation in Intestine 407 cells (Fig. 3A to C). An anoikis assay revealed that LMP2A-expressing cells were more resistant to anoikis than were Neor cells, as the resistance was clearly reduced by U0126 treatment (Fig. 3D). These findings indicate that LMP2A contributes to anoikis resistance through ERK activation.
Fig 3.
LMP2A induces anoikis resistance through ERK activation. (A) Effect of transient LMP2A expression on ERK activation in Intestine 407 cells. Phosphorylated ERK (p-ERK) and total ERK (ERK) in LMP2A-transfected cells or control vector-transfected (Neor) cells were analyzed by Western blot analysis. (B, C) Effect of stable LMP2A expression on ERK activation. (B) Flag-tagged LMP2A (Flag-LMP2A) was stably expressed in Intestine 407 cells. Flag-LMP2A was immunoprecipitated by anti-Flag antibody, followed by Western blotting using anti-Flag antibody (top) or anti-LMP2A antibody (bottom). (C) Phosphorylated ERK and ERK in Flag-LMP2A-transfected cells or control vector-transfected (Neor) cells were analyzed by Western blotting. (D) Detection of anoikis. Flag-LMP2A-expressing cells, U0126-treated Flag-LMP2A-expressing cells, and Neor cells were cultured in suspension for 48 h, and cell death was analyzed. The representative results of 3 separate experiments using 2 independent clones are represented as the mean percent ± SD. Statistically significant differences were evaluated by Student's t test (*, P < 0.05). (E) LMP2A is responsible for induction of anoikis resistance in EBV-positive Intestine 407 cells. LMP2A mRNA expression was analyzed in WT EBV-positive cells and LMP2A-KO (LMP2A−) EBV-positive cells by RT-PCR. LCL was used as a positive control for LMP2A mRNA expression. (F) LMP2A− EBV- and WT EBV-positive cells were cultured in suspension for 48 h, and cell death was analyzed. The representative results of 3 separate experiments using 2 independent clones are represented as the mean percent ± SD. Statistically significant differences were evaluated by Student's t test (*, P < 0.05). (G) Phosphorylated ERK and total ERK protein in LMP2A− EBV- and WT EBV-positive cells were analyzed by Western blotting.
LMP2A-KO EBV does not induce anoikis resistance.
Although our results demonstrate that LMP2A induces anoikis resistance, the level of LMP2A expression in EBV-positive Intestine 407 cells was much lower than that in LMP2A-transfected cells. To exclude the possibility that anoikis resistance was caused by LMP2A overexpression, we established LMP2A-KO EBV-converted Intestine 407 cells. Cells were infected with recombinant LMP2A-KO EBV (28) carrying the Neor gene by using the cell-to-cell infection method, and EBV-positive cell clones were isolated by G418 selection. Because all representative cell clones showed the same patterns of EBV gene expression as wild-type (WT) EBV-positive cells (data not shown), with the exception of LMP2A expression (Fig. 3E), we believe that LMP2A-KO EBV-positive Intestine 407 cells should be further studied. As shown in Fig. 3F, LMP2A-KO EBV-positive cells were more susceptible to anoikis than WT EBV-positive cells. In addition, ERK activity in LMP2A-KO EBV-positive Intestine 407 cells was significantly lower than that in WT EBV-positive cells (Fig. 3G). Thus, we concluded that the LMP2A expressed in EBV-positive Intestine 407 cells is responsible for ERK-mediated induction of anoikis resistance.
LMP2A downregulates Bim expression through ERK activation.
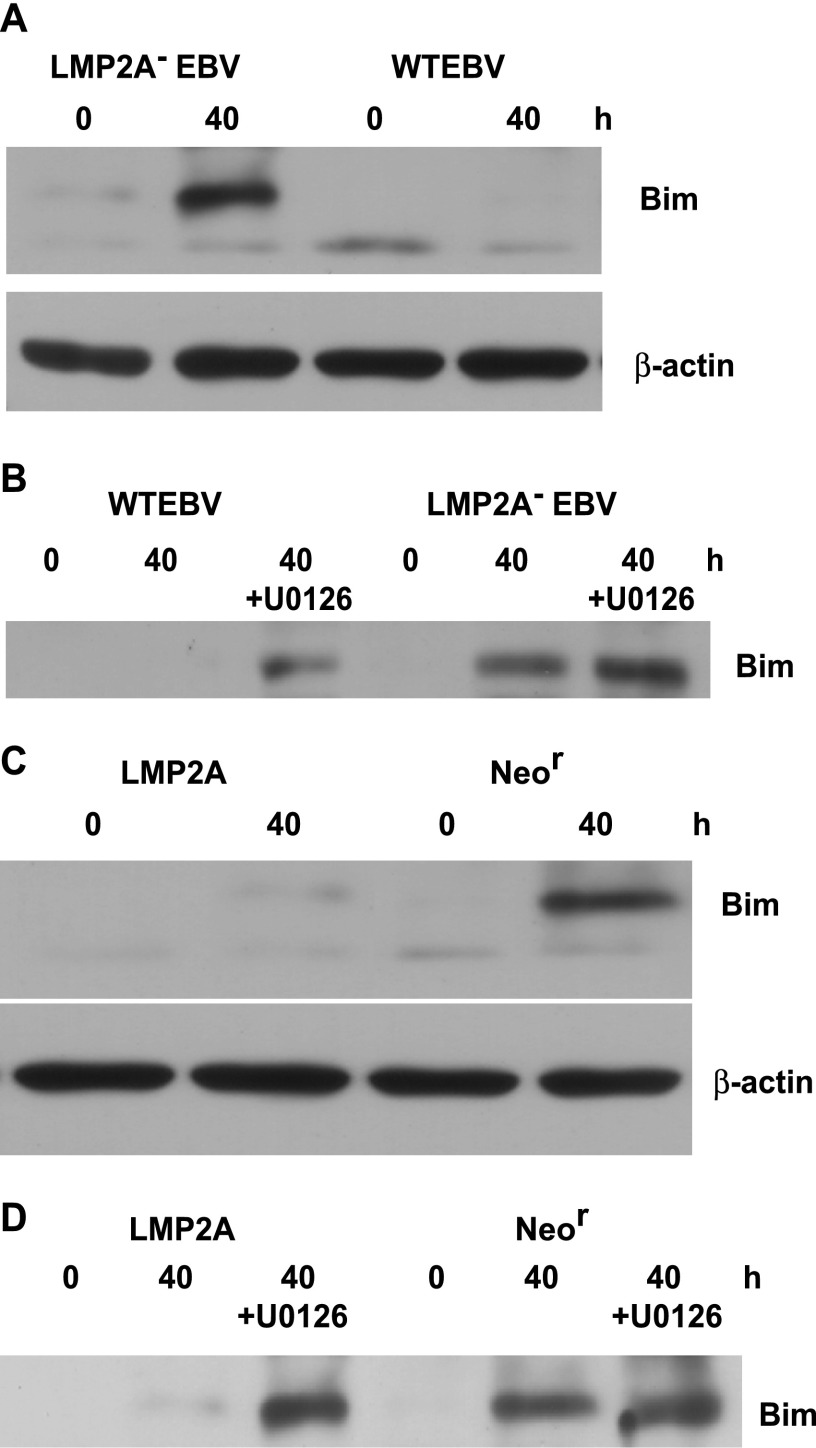
It was reported that detachment of epithelial cells induces anoikis through upregulation of the Bcl-2 homology 3 (BH-3) domain-only protein Bim, which is known to be a proapoptotic gene (33). In addition, a previous study demonstrated that Bim is downregulated by ERK activation (34). This led us to hypothesize that LMP2A-mediated ERK activation regulates Bim to prevent anoikis. To clarify whether LMP2A downregulates Bim, we analyzed Bim expression during the induction of anoikis. Western blot analysis demonstrated that Bim expression was clearly induced by cell detachment in LMP2A-KO EBV-positive cells, whereas Bim induction was significantly inhibited in WT EBV-positive cells (Fig. 4A). We also treated both cell lines with U0126 to assess whether downregulation of Bim depends on ERK activity. Bim was induced in WT EBV-positive cells by U0126 treatment, whereas U0126 had little effect on Bim induction in LMP2A-KO EBV-positive cells, indicating that LMP2A downregulates Bim expression through ERK activation (Fig. 4B). To confirm this conclusion, we repeated this experiment using LMP2A-tansfected cells and Neor cells. As shown in Fig. 4C, although Bim expression was clearly induced by cell detachment in Neor cells, it was strongly inhibited in LMP2A-transfected cells. Finally, U0126 treatment resulted in a significant induction of Bim in LMP2A-transfected cells (Fig. 4D), thus confirming our conclusion.
Fig 4.
LMP2A downregulates Bim expression during cell detachment. (A) LMP2A− EBV- and WT EBV-positive cells were cultured in suspension for 40 h, and Bim protein expression was analyzed by Western blotting. β-Actin protein was analyzed as an internal control. (B) Effect of U0126 on Bim induction in LMP2A− EBV- and WT EBV-positive cells. U0126-treated or untreated cells were cultured in suspension for 40 h, and then Bim expression was analyzed. (C) LMP2A-transfected and nontransfected (Neor) cells were cultured in suspension for 40 h, and Bim protein expression was analyzed. β-Actin expression was analyzed as an internal control. (D) Effect of U0126 on Bim induction in LMP2A-transfected and Neor cells. U0126-treated or untreated cells were cultured in suspension for 40 h and were then analyzed for Bim expression.
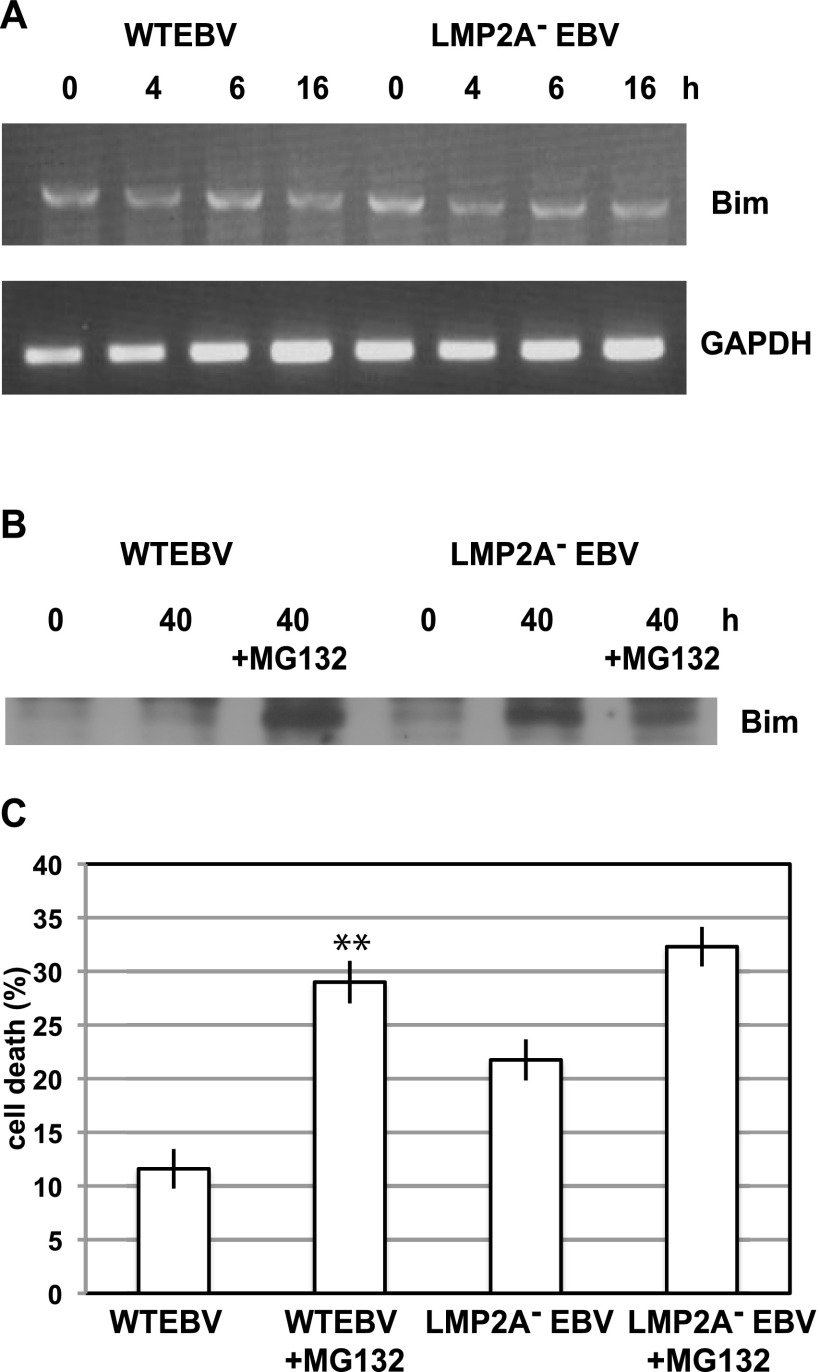
Bim is downregulated by LMP2A through proteasomal degradation.
To assess the mechanism by which LMP2A blocks Bim induction, we analyzed Bim mRNA expression during cell detachment. RT-PCR analysis demonstrated comparable basal Bim mRNA expression in both WT EBV-positive and LMP2A-KO EBV-positive cells. In addition, no significant Bim mRNA upregulation was observed in either cell line during cell detachment (Fig. 5A). RT-PCR using RNA from LMP2A-transfected and Neor cells cultured in suspension showed similar results (data not shown). Thus, we concluded that Bim is downregulated by LMP2A at the posttranscriptional level. To determine whether Bim is downregulated by LMP2A in the proteasome, we used the proteasome inhibitor MG132. Western blot analysis demonstrated that MG132 treatment resulted in WT EBV-positive cells showing Bim expression comparable to that of LMP2A-KO EBV-positive cells (Fig. 5B). Moreover, the effect of MG132 treatment on anoikis induction in WT EBV-positive cells was significant compared with that in LMP2A-KO EBV-positive cells (Fig. 5C). These findings suggest that LMP2A induces anoikis resistance through the proteasomal degradation of Bim.
Fig 5.
LMP2A downregulates Bim through proteasomal degradation. (A) Bim mRNA expression during cell detachment. LMP2A− EBV- and WT EBV-positive cells were cultured in suspension, and total RNA was extracted at the indicated times. Extracted RNA was analyzed for Bim expression by RT-PCR. GAPDH mRNA was analyzed as an internal control. (B) Effect of MG132 on Bim expression. Cells were treated or untreated with MG132, cultured in suspension for 40 h, and then lysed. Bim protein expression was analyzed by Western blotting. (C) Effect of MG132 on anoikis resistance. Cells were treated or untreated with MG132 and cultured in suspension for 48 h, and then cell death was analyzed. The representative results of 3 separate experiments using 2 independent clones are represented as the mean percent ± SD. Statistically significant differences were evaluated by Student's t test (**, P < 0.01).
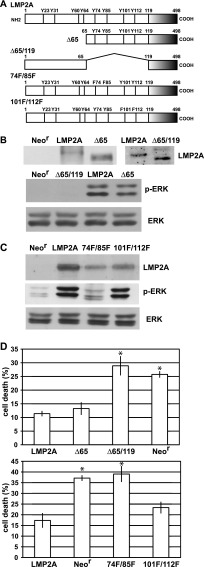
ITAM of LMP2A is required for ERK activation and prevention of anoikis.
It has been reported that LMP2A activates the intracellular signaling pathway in epithelial cells through the interaction between LMP2A N-terminal tyrosine residues and cellular proteins. To assess which tyrosine contributes to LMP2A-mediated ERK activation, we generated plasmids that express GFP-fused LMP2A mutants. The various LMP2A mutants are illustrated in Fig. 6A. Tyrosines 74 and 85 constitute the LMP2A ITAM, and tyrosine 101 is considered to interact with Csk. Tyrosine 112, within the YEEA motif, resembles the Src family kinase SH2 domain interaction motif. Tyrosines 74, 85, 101, and 112 were deleted in the Δ65/119-LMP2A mutant, whereas tyrosines 21, 31, and 64 were deleted in the Δ65-LMP2A mutant. These plasmids were transfected into Intestine 407 cells, and stable clones were isolated by G418 selection. As shown in Fig. 6B, ERK activity was significantly reduced in cells that expressed Δ65/119-LMP2A, whereas Δ65-LMP2A-expressing cells showed ERK activity comparable to that of WT LMP2A-expressing cells, suggesting that the LMP2A N-terminal regions containing tyrosines 74, 85, 101, and 112 are required for ERK activation. For further analysis, mutants with point mutations of double tyrosines, 74F/85F or 101F/112F (Fig. 6A), were generated. 74F/85F-LMP2A expression resulted in remarkably reduced ERK activity, whereas 101F/112F-LMP2A expression was not affected, indicating that ITAM is required for ERK activation (Fig. 6C). Finally, we assessed whether ITAM is required for LMP2A-mediated prevention of anoikis. The Δ65-LMP2A- and 101F/112F-LMP2A-expressing cells showed anoikis resistance similar to that of WT LMP2A-expressing cells, whereas anoikis was clearly induced in Δ65/119-LMP2A- and 74F/85F-LMP2A-expressing cells (Fig. 6D). Therefore, we concluded that ITAM is required for anoikis resistance.
Fig 6.
ITAM is required for LMP2A-mediated induction of anoikis resistance. (A) The LMP2A deletion and point mutants used in this study are illustrated. For the Δ65 mutant, 64 N-terminal amino acids were deleted, and for the Δ65/119 mutant, amino acids 66 to 118 were deleted. The 74F/85F and 101F/112F mutants had double mutations of tyrosine (Y) to phenylalanine (F). The Δ65/119 and 74F/85F mutants had the ITAM mutation. (B) (Top) Mutant LMP2A expression. Cells stably expressing GFP-tagged mutated LMP2A (Δ65 or Δ65/119 mutant cells) were lysed and subjected to immunoprecipitation, followed by Western blotting. Mutated LMP2As were detected by anti-GFP antibody. (Bottom) Results of ERK activation analysis. Neor cells and LMP2A (WT or mutant)-expressing cells were lysed and analyzed by Western blotting. (C) Effect of LMP2A double tyrosine mutation on ERK activation. Cells stably expressing double tyrosine-mutated LMP2A (74F/85F or 101F/112F mutant cells) were lysed and subjected to Western analysis for LMP2A expression (top) and ERK activation (middle and bottom). (D) Analysis of anoikis resistance. Cells with the Δ65 and Δ65/119 mutations (top) and cells with the 74F/85F and 101F/112F mutations (bottom) were cultured in suspension for 48 h, and cell death was analyzed. The representative results of 3 separate experiments using 2 independent clones are represented as the mean percent ± SD. Statistically significant differences were evaluated by Student's t test (*, P < 0.05).
DISCUSSION
Anoikis is a common event in untransformed adherent cells such as epithelial (35, 36), endothelial (37) and fibroblastoid (38) cell lines. The dependence on matrix interaction for cell survival is important in preventing inappropriate population expansion and metastatic spread of normal epithelial cells; therefore, resistance to anoikis is indicative of a phenotype of malignant cells. Here, we demonstrated that EBV induces anoikis resistance in Intestine 407 cells through ERK activation by LMP2A. A previous study demonstrated that expression of LMP2A and LMP2B resulted in promotion of epithelial cell spreading and motility (39). Although the level of LMP2A expression in EBV-positive Intestine 407 cells was much lower than that of LCLs or LMP2A-transfected cells, the studies using LMP2A-KO EBV-positive cells have indicated that the LMP2A level in EBV-positive cells is sufficient for the prevention of anoikis. In this regard, our study strongly suggests that LMP2A physiologically expressed in EBV-associated GC and NPC tissues contributes to a malignant phenotype in vivo, in contrast to findings from previous studies using LMP2A-overexpressing epithelial cells.
Several signal transduction pathways have been shown to modulate the anoikis sensitivity of various cells. Survival of fibroblastoid and epithelial cells in suspension is in part mediated by the MEK-ERK pathway (38, 40). The ERK-mediated anoikis resistance caused by LMP2A in epithelial cells is consistent with these reports. A previous study demonstrated that signaling from β1-integrin and epidermal growth factor receptor (EGFR) coordinately regulates Bim expression for the prevention of anoikis in epithelial cells via ERK activation (34). A lack of these signals causes cell detachment, which results in inhibition of the ERK-dependent pathway, leading to Bim upregulation. However, it is possible to maintain ERK activity in cells with LMP2A expression to prevent Bim induction by cell detachment even after detachment from the extracellular matrix. It has been shown in transgenic mice that LMP2A can provide a potent survival signal to B cells, which is analogous to the action of functional BCR (10). Our results suggest that LMP2A provides a functional signal to detached epithelial cells on behalf of the integrin and EGFR.
The mechanisms of Bim downregulation have been previously reported. BimEL is phosphorylated by various kinases, including the MAP kinases Jun N-terminal protein kinase (JNK) and ERK (41–46). Phosphorylation by ERK leads to proteasome-dependent degradation of BimEL (41, 42, 47). Our data are consistent with these observations and indicate that the control of Bim expression through the proteasome pathway is due to LMP2A-mediated ERK activation. Interestingly, it was reported that EBV infection of B lymphocytes leads to BimEL downregulation and prevents apoptosis (48). EBV may downregulate Bim in both epithelial cells and B lymphocytes.
Previous studies have established that ITAM plays a crucial role in LMP2A function. ITAM is required for association with Syk, which provides B cells with survival signals, including PI3K and ERK activation (14, 49). A recent study demonstrated that Shb, which is recruited to ITAM, is involved in the regulation of PI3K activity by LMP2A (50). The present study demonstrated that ITAM is also required for ERK-dependent anoikis resistance mediated by LMP2A in epithelial cells. Further studies are required to elucidate the mechanisms of ITAM-mediated ERK activation.
ACKNOWLEDGMENTS
We thank Y. Ando and I. Miura for technical assistance.
This work was supported by grants in aid from the Ministry of Education, Science, Sports, Culture and Technology, Japan (to D.I.). D.I. was also supported by the Takeda Science Foundation and the Akiyama Foundation.
Footnotes
Published ahead of print 22 May 2013
REFERENCES
- 1. Young LS, Rickinson AB. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 4:757–768 [DOI] [PubMed] [Google Scholar]
- 2. Rickinson AB, Kieff E. 2001. Epstein-Barr virus, p 2575–2627 Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 3. Takada K. 2000. Epstein-Barr virus and gastric carcinoma. Mol. Pathol. 53:255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Busson P, McCoy R, Sadler R, Gilligan K, Tursz T, Raab-Traub N. 1992. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J. Virol. 66:3257–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks L, Yao QY, Rickinson AB, Young LS. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young LS, Dawson CW, Clark D, Rupani H, Busson P, Tursz T, Johnson A, Rickinson AB. 1988. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J. Gen. Virol. 69:1051–1065 [DOI] [PubMed] [Google Scholar]
- 7. Miller CL, Burkhardt AL, Lee JH, Stealey B, Longnecker R, Bolen JB, Kieff E. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155–166 [DOI] [PubMed] [Google Scholar]
- 8. Miller CL, Lee JH, Kieff E, Longnecker R. 1994. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc. Natl. Acad. Sci. U. S. A. 91:772–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller CL, Longnecker R, Kieff E. 1993. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J. Virol. 67:3087–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405–411 [DOI] [PubMed] [Google Scholar]
- 11. Caldwell RG, Brown RC, Longnecker R. 2000. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J. Virol. 74:1101–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson LJ, Longnecker R. 2008. EBV LMP2A provides a surrogate pre-B cell receptor signal through constitutive activation of the ERK/MAPK pathway. J. Gen. Virol. 89:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fruehling S, Longnecker R. 1997. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology 235:241–251 [DOI] [PubMed] [Google Scholar]
- 14. Swart R, Ruf IK, Sample J, Longnecker R. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838–10845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikeda M, Ikeda A, Longan LC, Longnecker R. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178–191 [DOI] [PubMed] [Google Scholar]
- 16. Ikeda M, Ikeda A, Longnecker R. 2001. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J. Virol. 75:5711–5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winberg G, Matskova L, Chen F, Plant P, Rotin D, Gish G, Ingham R, Ernberg I, Pawson T. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526–8535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scholle F, Bendt KM, Raab-Traub N. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681–10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrison JA, Klingelhutz AJ, Raab-Traub N. 2003. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J. Virol. 77:12276–12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrison JA, Raab-Traub N. 2005. Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of β-catenin signaling. J. Virol. 79:2375–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen SY, Lu J, Shih YC, Tsai CH. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J. Virol. 76:9556–9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imai S, Nishikawa J, Takada K. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishikawa J, Imai S, Oda T, Kojima T, Okita K, Takada K. 1999. Epstein-Barr virus promotes epithelial cell growth in the absence of EBNA2 and LMP1 expression. J. Virol. 73:1286–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iwakiri D, Eizuru Y, Tokunaga M, Takada K. 2003. Autocrine growth of Epstein-Barr virus-positive gastric carcinoma cells mediated by an Epstein-Barr virus-encoded small RNA. Cancer Res. 63:7062–7067 [PubMed] [Google Scholar]
- 25. Iwakiri D, Sheen TS, Chen JY, Huang DP, Takada K. 2005. Epstein-Barr virus-encoded small RNA induces insulin-like growth factor 1 and supports growth of nasopharyngeal carcinoma-derived cell lines. Oncogene 24:1767–1773 [DOI] [PubMed] [Google Scholar]
- 26. Hazama A, Okada Y. 1988. Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J. Physiol. 402:687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimizu N, Yoshiyama H, Takada K. 1996. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J. Virol. 70:7260–7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konishi K, Maruo S, Katoh H, Takada K. 2001. Role of Epstein-Barr virus-encoded latent membrane protein 2A on virus-induced immortalization and virus activation. J. Gen. Virol. 82:1451–1456 [DOI] [PubMed] [Google Scholar]
- 29. Fruehling S, Lee SK, Herrold R, Frech B, Laux G, Kremmer E, Grässer FA, Longnecker R. 1996. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 70:6216–6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frisch SM, Francis H. 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994 124:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aoudjit F, Vuori K. 2001. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 152:633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collins NL, Reginato MJ, Paulus JK, Sgroi DC, Labaer J, Brugge JS. 2005. G1/S cell cycle arrest provides anoikis resistance through Erk-mediated Bim suppression. Mol. Cell. Biol. 25:5282–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puthalakath H, Strasser A. 2002. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 9:505–512 [DOI] [PubMed] [Google Scholar]
- 34. Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. 2003. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 5:733–740 [DOI] [PubMed] [Google Scholar]
- 35. Rytömaa M, Lehmann K, Downward J. 2000. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: inhibition by PKB/Akt but not Raf signaling. Oncogene 19:4461–4468 [DOI] [PubMed] [Google Scholar]
- 36. Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J. 2001. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 15:981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meredith JE, Jr, Fazeli B, Schwartz MA. 1993. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 9:953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Gall M, Chambard JC, Breittmayer JP, Grall D, Pouysségur J, Van Obberghen-Schilling E. 2000. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol. Biol. Cell 11:1103–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Allen MD, Young LS, Dawson CW. 2005. The Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote epithelial cell spreading and motility. J. Virol. 79:1789–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilmore AP, Valentijn AJ, Wang P, Ranger AM, Bundred N, O'Hare MJ, Wakeling A, Korsmeyer SJ, Streuli CH. 2002. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J. Biol. Chem. 277:27643–27650 [DOI] [PubMed] [Google Scholar]
- 41. Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. 2003. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 22:6785–6793 [DOI] [PubMed] [Google Scholar]
- 42. Mouhamad S, Besnault L, Auffredou MT, Leprince C, Bourgeade MF, Leca G, Vazquez A. 2004. B cell receptor-mediated apoptosis of human lymphocytes is associated with a new regulatory pathway of Bim isoform expression. J. Immunol. 172:2084–2091 [DOI] [PubMed] [Google Scholar]
- 43. Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM. 2001. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron 29:615–628 [DOI] [PubMed] [Google Scholar]
- 44. Biswas SC, Greene LA. 2002. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J. Biol. Chem. 277:49511–49516 [DOI] [PubMed] [Google Scholar]
- 45. Lei K, Davis RJ. 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. U. S. A. 100:2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weston CR, Balmanno K, Chalmers C, Hadfield K, Molton SA, Ley R, Wanger EF, Cook SJ. 2003. Activation of ERK1/2 by Raf-1: ER represses Bim expression independently of the JNK or PI3K pathways. Oncogene 22:1281–1293 [DOI] [PubMed] [Google Scholar]
- 47. Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. 2003. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278:18811–18816 [DOI] [PubMed] [Google Scholar]
- 48. Clybouw C, McHichi B, Mouhamad S, Auffredou MT, Bourgeade MF, Shrma S, Leca G, Vazquez A. 2005. EBV infection of human B lymphocytes leads to down-regulation of Bim expression: relationship to resistance to apoptosis. J. Immunol. 175:2968–2973 [DOI] [PubMed] [Google Scholar]
- 49. Merchant M, Caldwall RG, Longnecker R. 2000. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J. Virol. 74:9115–9124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matskova LV, Helmstetter C, Ingham RJ, Gish G, Lindholm CK, Ernberg I, Pawson T, Winberg G. 2007. The Shb signaling scaffold binds to and regulates constitutive signals from the Epstein-Barr virus LMP2A membrane protein. Oncogene 26:4908–4917 [DOI] [PubMed] [Google Scholar]