Abstract
Type III interferons (IFNs), also called lambda interferons (IFN-λ), comprise three isoforms, IFN-λ1 (interleukin-29 [IL-29]), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B). Only limited information is available on their expression and biological functions in humans. Type I and type II IFNs protect human pancreatic islets against coxsackievirus infection, and this is important since such viruses have been proposed to play a role in the development of human type 1 diabetes. Here we investigated whether type III IFN is expressed during infection of human islet cells with coxsackievirus and if type III IFN regulates permissiveness to such infections. We show that human islets respond to a coxsackievirus serotype B3 (CVB3) infection by inducing the expression of type III IFNs. We also demonstrate that islet endocrine cells from nondiabetic individuals express the type III IFN receptor subunits IFN-λR1 and IL-10R2. Pancreatic alpha cells express both receptor subunits, while pancreatic beta cells express only IL-10R2. Type III IFN stimulation elicited a biological response in human islets as indicated by the upregulated expression of antiviral genes as well as pattern recognition receptors. We also show that type III IFN significantly reduces CVB3 replication. Our studies reveal that type III IFNs are expressed during CVB3 infection and that the expression of the type III IFN receptor by the human pancreatic islet allows this group of IFNs to regulate the islets' permissiveness to infection. Our novel observations suggest that type III IFNs may regulate viral replication and thereby contribute to reduced tissue damage and promote islet cell survival during coxsackievirus infection.
INTRODUCTION
Interferons (IFNs) are a class of proteins initially identified as molecules interfering with viral replication (1) but which are also now known to play an indispensable role in the immune response to infection. In humans, type I IFNs comprise several alpha interferon (IFN-α) subtypes and various single isoforms of IFN-β, -ε, -κ, -δ, -ω, -τ, and -ζ (2, 3), while the type II IFN family contains only a single known member, IFN-γ. The most recently identified group, type III IFNs or IFN-λs, comprises IFN-λ1, IFN-λ2, and IFN-λ3 (also designated interleukin-29 [IL-29], IL-28A, and IL28-B, respectively) (4, 5), but only limited information is available on their expression and biological functions.
IFN-λ signaling occurs via a specific cell surface receptor, consisting of two subunits: IFN-λ receptor 1 (IFN-λR1, also designated interleukin 28 receptor alpha [IL-28Rα]) and interleukin-10 receptor 2 (IL-10R2) (2, 4, 5). While most cells express the IL-10R2 subunit, the distribution of IFN-λR1 appears to be much more restricted. Accumulating data suggest that the IFN-λ receptor complex is expressed preferentially on cells of epithelial origin, as well as on certain immune cells (2, 6, 7), but this may be an oversimplification since IFN-λ receptor expression has not been investigated in many other human cell types.
Binding of IFN-λ to its receptor leads to activation of JAK1 and TYK2, which mediates the subsequent phosphorylation of STAT1, STAT2, and STAT3. Phosphorylated STATs dimerize and translocate to the nucleus, where they regulate the expression of selected genes (2, 6, 7). However, the biological actions of type III IFNs are still not fully understood. Type III IFNs have been described as being functionally similar to type I IFNs, since both display antiviral properties and can, for example, induce an antiviral state in responsive cells (2, 6, 7). In support of this, type III IFNs have been shown to mediate an antiviral response against several human pathogens, including hepatitis B virus, hepatitis C virus, rhinovirus, and human immunodeficiency virus (7–9). The restricted receptor expression, however, suggests that type III IFNs may exert this specialized antiviral defense role in only certain cell types (10, 11). Further studies on primary human cells and clinically relevant pathogens are thus needed to understand the full potential for type III IFNs in antiviral defense.
Coxsackieviruses (CVs) are single-stranded RNA viruses belonging to the Enterovirus genus in the family Picornaviridae. Most enterovirus infections are asymptomatic. Nevertheless, in some cases these viruses cause severe disease, including myocarditis, hepatitis, and pancreatitis (12). Infections with enteroviruses, particularly those with coxsackie B virus serotypes (CVBs), have also been associated with the development of type 1 diabetes (13, 14), a chronic metabolic disease that arises as a result of the loss of insulin-producing pancreatic beta cells. While rarely found in the pancreatic islets of healthy individuals, enterovirus protein and/or RNA has been identified in pancreatic endocrine cells of type 1 diabetes patients (15–17). This observation, coupled with in vitro studies demonstrating a gradual loss of function and viability in human pancreatic islet cells infected with enteroviruses (e.g., CVBs) (18), suggests that a direct infection of the pancreatic beta cell may be involved in the disease process. Studies in animal models have provided additional support for this hypothesis (19–22). However, irrespective of these observations, surprisingly little is known about the ways in which permissiveness to enterovirus infection is regulated in pancreatic islet cells (23). In previous studies, we have shown that both type I and II IFNs induce an antiviral state in human pancreatic islets and that they lower islet cell permissiveness to CVB (24). Moreover, studies in an animal model have suggested that the beta cell relies on IFN signaling in order to survive during a systemic CVB infection (19, 20). However, whether human pancreatic islets respond to type III IFN (IFN-λ) stimulation and whether such stimuli can modify their permissiveness to infection have not been investigated.
In the present study, we show that primary human pancreatic islets express type III IFN upon CVB3 infection. We also demonstrate that certain pancreatic islet endocrine cells express both subunits of the type III IFN receptor and that they respond to type III IFN treatment by upregulating genes involved in antiviral defense and the recognition of RNA viruses. Importantly, our study also shows that CVB3 replication is perturbed in type III IFN-treated pancreatic islets.
MATERIALS AND METHODS
Handling of human islets and in vitro stimulations.
Human islets were isolated from 19 human cadaver organ donors (5 female and 14 male) at The Nordic Network for Clinical Islet Transplantation, Uppsala University Hospital, as described previously (25). The average donor age was 58.8 years ± 11.3 years (range, 25 to 72 years), and the cold ischemic time was 9.9 ± 4.4 h (range, 2.1 to 17.2 h). The body mass index (BMI) was available for only nine of the donors and averaged 26.7 ± 2.9 kg/m2 (range, 22.9 to 31.1 kg/m2). The quality of the isolated islets was evaluated by measuring insulin release in response to glucose. The corresponding stimulation index averaged 6.0 ± 3.6 (range, 1.6 to 12.7) in a dynamic perfusion system (26). The islets had a purity of 73% ± 17% (range, 40 to 99%), determined by dithizone staining, and were further purified by hand picking before use in individual experiments. Islets were initially maintained in CMRL-1066 supplemented with 2 mM l-glutamine, 10% inactivated human serum, 10 mM HEPES, 0.25 μg/ml amphotericin B (Fungizone), 50 μg/ml gentamicin, 10 μg/ml ciprofloxacin, and 10 mM nicotinamide. Upon arrival at Karolinska Institutet, the islets were transferred to RPMI 1640 supplemented as described above but with inactivated fetal bovine serum instead of human serum and without nicotinamide. Human islets were transported to Karolinska Institutet 5 ± 2 days (range, 2 to 9 days) after isolation, and experiments were performed after a further 7 ± 3 days (range, 3 to 13 days) of culture. The experiments were approved by local ethics committees in Uppsala and Stockholm, Sweden, and performed in accordance with the principles of the Declaration of Helsinki 2000. Each experiment comprised control and stimulated islets from the same donor.
Subjects included in immunohistochemistry and immunofluorescence studies.
Five human pancreases removed at autopsy from nondiabetic pediatric patients were selected randomly from a collection described previously (16). The specimens were studied with ethical approval and had been fixed in buffered formalin and paraffin embedded. The cohort consisted of patients with a mean age of 3.8 ± 1.6 years (range, 2 to 6 years).
Interferons, virus stock, and virus titration.
IFN-α2b (Intron A; Merck Sharp & Dohme [Sweden]) was added to the islets (500 islets/well) at a final concentration of 1,000 U/ml. IFN-λ1 and IFN-λ2 (PeproTech) were dissolved in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and added to the islets (500 islets/well) at a final concentration of 100 ng/ml. Due to the scarcity of human pancreatic islets, the concentration of IFN-λs used was selected based on the literature and our own work on dose-response studies using human cell lines showing that 100 ng/ml induces high expression levels of IFN-stimulated genes (ISGs) without causing cellular damage (e.g., see reference 27 and K. Lind and M. Flodström-Tullberg, unpublished data). Mock-treated cells were incubated with dilution buffer alone (PBS containing 0.1% BSA).
CVB3 Nancy was propagated in HeLa cells, and the titer was determined as described before (28). Viral titers in supernatant and in the last wash supernatant were determined by standard plaque assay using HeLa cells (each sample was run twice). Viral titers were determined as PFU/ml and presented as log10(PFU/ml). The lower detection limit for the plaque assay was 2.5 PFU/ml.
In vitro stimulation and infection of human islets.
Human islets were infected with CVB3 Nancy (4 × 104 PFU/islet) as described previously (19). In brief, islets were infected with virus for 90 min in 2 ml serum-free medium (Sterilin tissue culture-treated plates, 50 mm). Plates were gently rocked every 10 min during the infection. After infection, the islets were washed extensively in order to remove free virus particles and placed in Sterilin plates containing 3 ml complete medium. At indicated time points, supernatant alone (20 μl) or islets and supernatant were collected. Supernatants were stored at −80°C and later used for virus titration by a standard plaque assay. The virus titer in the supernatant from the last wash (following the 90-min incubation with virus) was below or just above the detection limit (data not shown). The harvested islets were lysed and homogenized using RLT buffer and QIAshredder (Qiagen); RNA was isolated and used for analysis of gene expression.
In some experiments, human islets were treated with IFN-α2b, IFN-λ1, or IFN-λ2 or mock treated for 6 h followed by lysis and homogenization using RLT buffer and QIAshredder (Qiagen). RNA was isolated and used for analysis of gene expression.
In other experiments, the human islets were treated with IFN-α2b, IFN-λ1, or IFN-λ2 or mock treated for 24 h. This was followed by either protein isolation for Western blot analysis or infection with CVB3 as described above. After infection, the islets were washed using complete medium; placed in 3 ml complete medium supplemented with IFNs, as described above; and incubated at 37°C for 48 h. At indicated time points, supernatants were collected and used for virus titration. The islets were lysed and homogenized using RLT buffer and QIAshredder (Qiagen) for RNA isolation or harvested in RIPA buffer and used for Western blot analysis.
RNA isolation, real-time RT-PCR, and PCR array for interferon response.
Total RNA was isolated using an RNeasy minikit (Qiagen) according to the manufacturer's instructions. The quantity and purity of the isolated RNA were assessed using a NanoDrop ND-1000 instrument (Saveen and Werner AB, Sweden). For individual genes, total RNA (0.27 μg) was treated with DNase and converted to cDNA using the Superscript III First Strand synthesis system for real-time reverse transcription-PCR (RT-PCR) (Invitrogen, Sweden). Quantification of IFN-λ1, IFN-λ2, IL-10R2, IFN-λR1, RIG-I, TLR3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was achieved using the Quantitech primer assay (Qiagen) and RT2 Real-time SYBR green/ROX PCR master mix (SuperArray, Sweden). The thermocycler parameters were 50°C for 20 s and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min.
The PCR array was performed according to the manufacturer's instructions. In brief, total RNA (1 μg) from three human islet donors was converted into cDNA using the RT2 First Strand kit (SABiosciences) followed by expression analysis using the RT2 Profiler PCR array for human IFN-α/β response (SABiosciences; catalog number PAHS-016A) and RT2 Real-time SYBR green/ROX PCR master mix (SuperArray, Sweden). The array contains a panel of 84 ISGs and five housekeeping genes. The thermocycler parameters were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min.
A threshold cycle (CT) value of ≥35 was considered below the detection level of the assay, and a gene with a CT value of ≥35 was considered to be not expressed. The mRNA expression for each gene was normalized to the expression level of GAPDH (individual genes) or five housekeeping genes (genes analyzed using PCR array) using the ΔCT method, and the data shown in the figures as “relative expression” represent 2−ΔCT values. Expression levels between control and experimental samples were compared using ΔΔCT, and the data shown in Fig. 3 as “fold induction” represent the 2−ΔΔCT values.
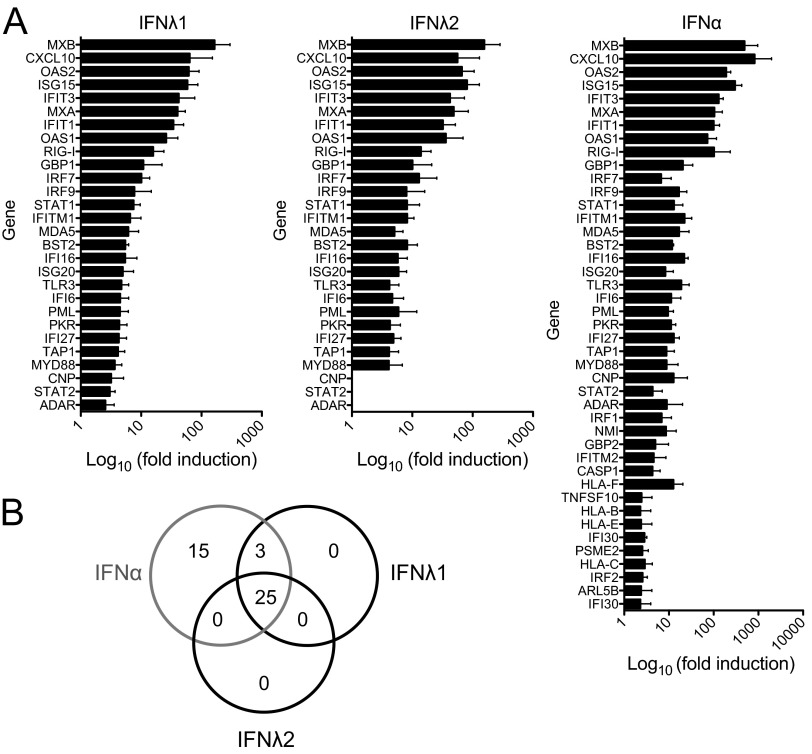
Fig 3.
Expression profile of ISGs after type III IFN treatment. (A) Human pancreatic islets from three donors were stimulated with IFN-λ1 (100 ng/ml), IFN-λ2 (100 ng/ml), or IFN-α (1,000 U/ml) or mock treated with buffer alone for 6 h; thereafter, mRNA expression of 86 ISGs was assessed by real-time RT-PCR. The mRNA expression levels were normalized to the expression level of GAPDH (individual genes; RIG-I and TLR3) or five housekeeping genes (genes analyzed using PCR array) and calculated relative to untreated control islets from the same donor. For each gene, a 2-fold-or-higher-increased expression in at least two out of the three donors was used as a criterion to classify the gene as being increasingly expressed following IFN stimulation. For each gene, the mean of the fold induction values for the three donors ± SD is shown as log10(fold induction). (B) Venn diagram of genes demonstrating increased expression after IFN treatment, showing the numbers of overlapping and individually expressed genes upon IFN-λ1, IFN-λ2, or IFN-α treatment.
All real-time RT-PCR analyses were performed using an ABI Prism 7500 sequence detecting system.
Antibodies and immunoblotting.
Cellular extracts for Western blot analysis were prepared using RIPA buffer (20 nM Tris, pH 7.5, 1 mM EDTA, 140 mM NaCl, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 1 mM activated Na3VO4) and mechanical disruption of the islets using a pellet pestle. Total protein concentrations were determined using the bicinchoninic acid (BCA) protein assay kit (Pierce, Sweden). Total protein extracts (5 μg) were diluted 1:1 in Laemmli buffer and boiled for 10 min followed by separation on an SDS-polyacrylamide gel (Bio-Rad) and transfer to nitrocellulose membrane (Bio-Rad). Primary antibodies were incubated overnight at 4°C: MDA5 (1:1,000; Alexis Biochemicals), RIG-I (1:1,000; Alexis Biochemicals), VP-1 (1:400, clone 5D8/1; Dako), or MxA (1:1,000; a kind gift from Otto Haller, University of Freiburg, Germany), an antibody which weakly cross-reacts with MxB. Binding of the primary antibody was detected using a horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit antibody (1:1,000; Bio-Rad, Sweden). To guarantee equal loading, membranes were probed with a primary antibody against actin (1:30,000; MP Biomedicals, Aurora, OH, USA). Binding of primary antibody was detected using an HRP-conjugated anti-mouse antibody (1:10,000; Bio-Rad, Sweden). Blots were developed using Supersignal West Dura extended-duration substrate (Pierce, Sweden) and the LAS-4000 imaging system (Fujifilm).
Immunohistochemistry.
Serial sections (4 μm) were cut from each case and mounted on glass slides coated in (3-aminopropyl)-triethoxysilane (Sigma, Dorset, United Kingdom). Sections were processed and labeled using a standard immunoperoxidase technique for paraffin sections. Antigens were unmasked by heat-induced epitope retrieval in 10 mM citrate buffer, pH 6.0. Primary antibodies (Abcam, Cambridge, United Kingdom) were applied for 1 h at room temperature (RT) (anti-IL-10R2; 1:1,000) or overnight at 4°C (anti-IFN-λR1; 1:1,500). The Dako Real Envision detection system (Dako, Cambridge, United Kingdom) was used for antigen detection.
Immunofluorescence.
To examine the islet cell subtypes expressing IL-10R2 and IFN-λR1, double immunofluorescence staining was performed. Rabbit antisera with specificity for IFN-λR1 or IL-10R2 were incubated as described for the immunoperoxidase method above and were detected with goat anti-rabbit Alexa Fluor 568-conjugated secondary antibody (1:400; Invitrogen, Paisley, United Kingdom). To detect and localize IFN-λR1, sections were incubated overnight with antireceptor serum and then washed and stained for islet hormones (guinea pig anti-insulin [Dako], mouse antiglucagon, or rat antisomatostatin [Abcam]) for 1 h. In the case of IL-10R2, antireceptor and antihormone sera were coincubated on the sections for 1 h. Immunopositivity was detected with an appropriate goat secondary antibody conjugated with Alexa Fluor 488 (1:400; Invitrogen). 4′,6-Diamidino-2-phenylindole (DAPI; 1:1,000; Invitrogen) was included in the final incubation step to stain cell nuclei. Sections were mounted in Vectashield hard-set mounting medium (Vector Laboratories, Peterborough, United Kingdom) under glass coverslips. The sections were analyzed using a Zeiss LSM510 Meta confocal microscope. Images were cropped and separated via Zeiss LSM Image software and ImageJ software, respectively.
Statistics.
Statistical analysis was performed using GraphPad Prism 5. The Wilcoxon matched pairs test (nonparametric) was used when comparisons were made between two groups representing paired observations. One-way analysis of variance (ANOVA) with Bonferroni correction was used when multiple comparisons were made. Statistical analysis on virus titers was performed prior to logarithmic transformation of data. Data are presented as means ± standard deviations (SDs).
RESULTS
CVB3 infection induces the expression of type III IFN in human pancreatic islets.
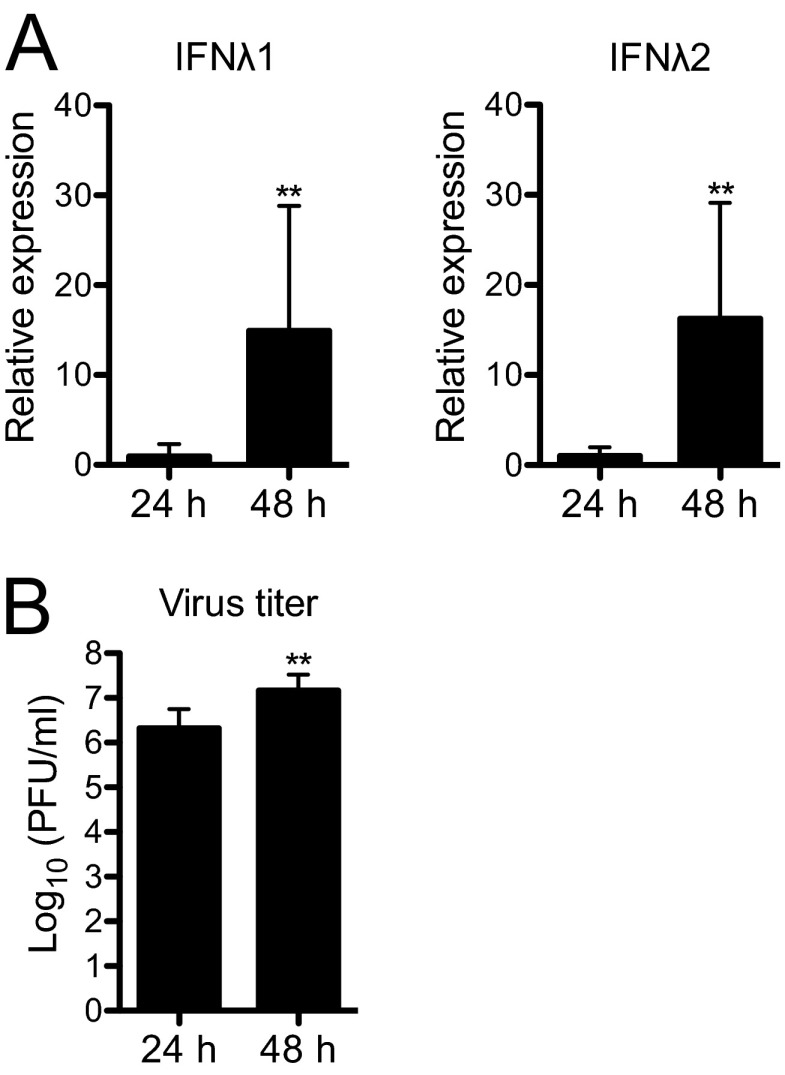
First, we investigated whether infection of human pancreatic islets with CVB3 induces the expression of type III IFN. To this end, islets (n = 450 to 500 per condition) from nine donors were either mock treated or infected with CVB3. Twenty-four and 48 h later, the IFN-λ1 and IFN-λ2 mRNA levels were measured using real-time RT-PCR. In uninfected islets, the expression levels of the two IFNs were below the detection limit of the assay (CT values for IFN-λ1 and IFN-λ2 were ≥35 for both time points in all control samples [data not shown], and CT values for GAPDH in control samples [means ± SDs, n = 9] were 17.89 ± 1.12 and 18.22 ± 1.40 for 24 h and 48 h, respectively). In contrast, IFN-λ1 and IFN-λ2 mRNAs were expressed by CVB3-infected islets from all donors as early as 24 h postinfection (p.i.), and their expression levels increased further by 48 h p.i. (Fig. 1A). The induction of IFN-λ1 and IFN-λ2 mRNAs correlated with virus replication as indicated by the increasing concentrations of infectious virus particles recovered from culture supernatants over time (Fig. 1B). Collectively, these observations suggest that CVB3 infection results in increased type III IFN expression by human islet cells.
Fig 1.
Human pancreatic islets express type III IFN mRNA upon infection with CVB3. (A) Human pancreatic islets from nine donors were infected with CVB3 (4 × 104 PFU/islet), and the expression of IFN-λ1 and IFN-λ2 mRNA was measured at 24 and 48 h p.i. using real-time RT-PCR. The IFN-λ mRNA expression levels are presented relative to that of GAPDH. The expression of IFN-λ1 and IFN-λ2 mRNAs was below the detection limit in uninfected control islets (CT values of ≥35 [data not shown]). (B) Titers (PFU/ml) of infectious virus particles were measured in culture supernatants harvested from infected islets from the same donors at 24 h and 48 h p.i. using the plaque assay technique. Data are presented as log10(PFU/ml), mean ± SD. **, P < 0.01, 24 h versus 48 h for both panels A and B, Wilcoxon matched pairs test.
Human islet cells express the type III IFN receptor.
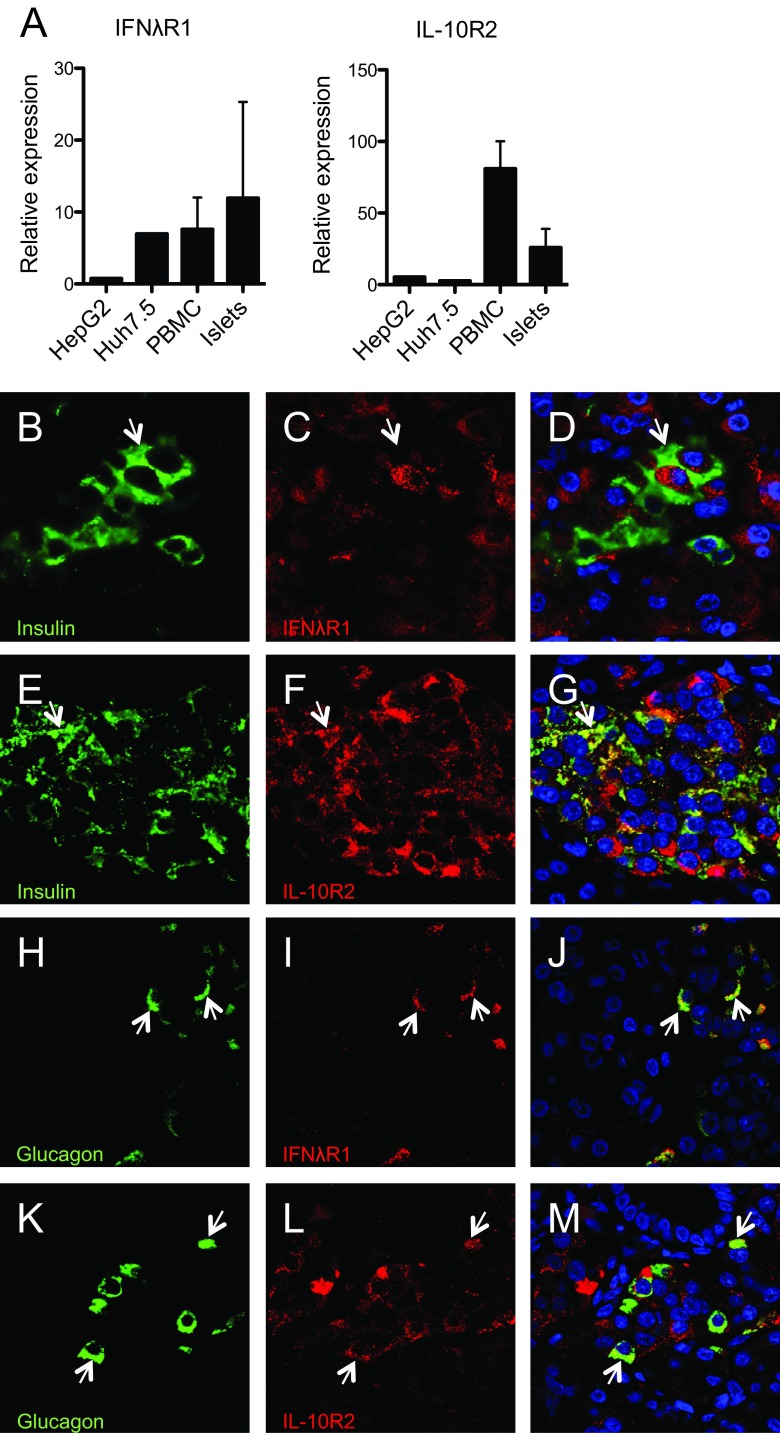
The expression of the two type III IFN receptor subunits is required for cells to respond to IFN-λs (2, 4, 5). In order to determine whether human pancreatic islets express the type III IFN receptor, we next evaluated the mRNA expression levels of the two receptor subunits in islets (n = 450 to 500 per condition) from seven donors using real-time RT-PCR. Two human hepatic cancer-derived cell lines and human peripheral blood mononuclear cells (PBMCs) were included as positive controls (11, 29). As expected, the hepatic cell lines and PBMCs were found to express both the IFN-λR1 and the IL-10R2 subunits (Fig. 2A). Human pancreatic islets also expressed mRNAs encoding IFN-λR1 and IL-10R2 (Fig. 2A).
Fig 2.
Human pancreatic islets express the type III IFN receptor. (A) mRNA expression levels of type III IFN receptor subunits IFN-λR1 and IL-10R2 were quantified in human pancreatic islet cells from seven donors using real-time RT-PCR. The expression levels were compared with those observed in two hepatic cell lines and four human PBMC donors. The receptor mRNA expression levels are presented relative to that of GAPDH. Data are presented as means ± SDs for islets and PBMCs and as means from three independent cell preparations for the cell lines. (B to M) Expression of each component of the type III IFN receptor (IFN-λR1 and IL-10R2) was assessed in parallel with islet hormone immunostaining in pancreas sections from five nondiabetic individuals, to determine their respective cellular localization. Representative images from a single pancreas section are shown, and arrows are used to illustrate relevant cells in each panel. (B to D) Sections were stained with anti-insulin (B), anti-IFN-λR1 (C), and both anti-insulin and anti-IFN-λR1 (merged image) (D). (E to G) Sections were stained with anti-insulin (E), anti-IL-10R2 (F), and both anti-insulin and anti-IL-10R2 (merged image) (G). (H to J) Sections were stained with antiglucagon (H), anti-IFN-λR1 (I), and both antiglucagon and anti-IFN-λR1 (merged image) (J). (K to M) Sections were stained with antiglucagon (K), anti-IL-10R2 (L), and both antiglucagon and anti-IL-10R2 (merged image) (M).
To verify these data and to examine the cellular localization of each receptor subunit, pancreas sections from control (nondiabetic) subjects were studied by immunohistochemistry (Fig. 2B to M). The results revealed that IL-10R2 was expressed abundantly in many islet cells. Colocalization analysis revealed that IL-10R2 was present in both alpha (Fig. 2K to M) and beta (Fig. 2E to G) cells whereas it was absent from somatostatin-positive delta cells (data not shown). IFN-λR1 was highly expressed in alpha cells (Fig. 2H to J), while it was below the detection limit in most beta (Fig. 2B to D) and delta (data not shown) cells.
Type III IFN induces an antiviral state in human pancreatic islets.
Type I and II IFNs establish an antiviral state in human pancreatic islet cells by inducing the expression of numerous genes directly or indirectly involved in antiviral defense (24). To investigate whether type III IFNs also induce the expression of classical interferon-stimulated genes (ISGs) in primary human pancreatic islet cells, islets (n = 500 per condition) from three donors were stimulated with IFN-λ1 or IFN-λ2 for 6 h. Mock-treated islets served as controls. Using real-time RT-PCR and a PCR array, the expression of 84 genes known to be modulated by IFN-α and -β was examined. In separate real-time RT-PCR analyses, we also studied the mRNA expression levels of two pattern recognition receptors (PRRs), RIG-I and TLR3, both known to sense viral RNA (23). Pancreatic islets exposed to IFN-α served as a positive control (24). The gene expression levels in IFN-treated islets were compared to those in untreated control islets from the same donor, and a fold change of ≥2 for at least two of the three donors was set as the cutoff criterion for differentially expressed genes.
Of 86 genes studied, the expression of 28 and 25 genes was upregulated by IFN-λ1 or IFN-λ2, respectively (Fig. 3; see also Table S1 in the supplemental material). IFN-α treatment induced the expression of 43 of the 86 genes investigated (Fig. 3; see also Table S1).
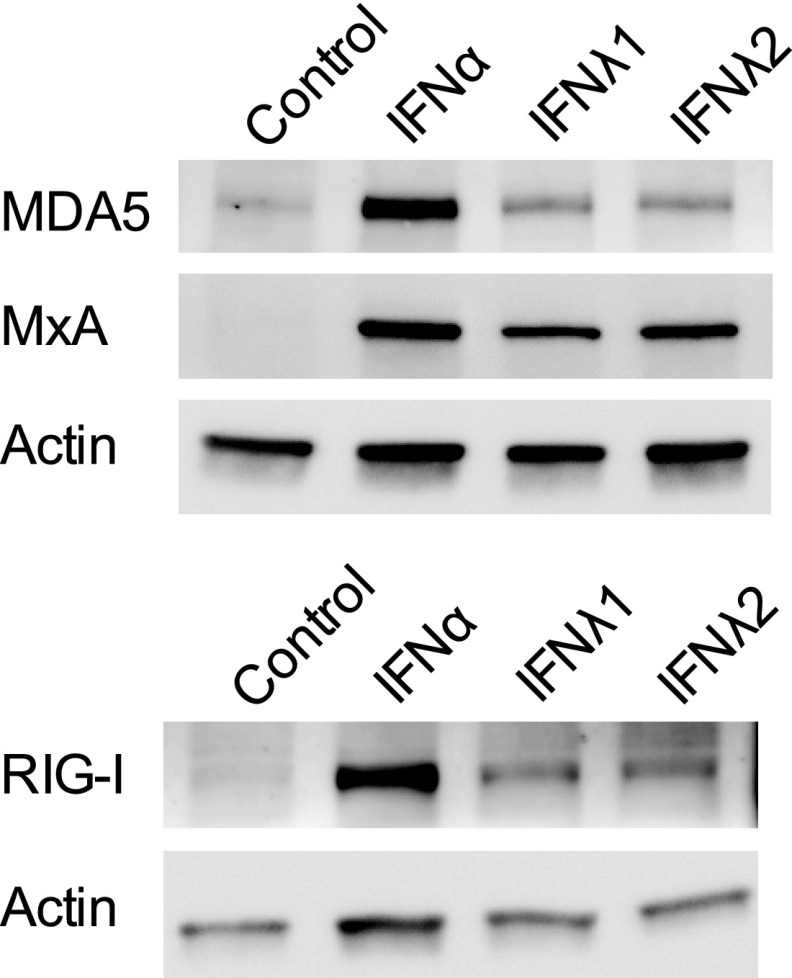
In order to determine whether the gene expression changes were translated into increased protein production, we exposed human pancreatic islets (n = 500 per condition) from two additional donors to IFN-λs for 24 h and compared the expression levels of selected proteins by Western blotting. As shown in Fig. 4, IFN-λ treatment upregulated the levels of MxA (encoded by the gene mx1), MDA5 (also called IFIH1), and RIG-I. Taken together, these results strongly indicate that IFN-λ1 and IFN-λ2 induce an antiviral state in human pancreatic islets.
Fig 4.
Type III IFN-treated human pancreatic islets upregulate the expression of proteins involved in antiviral defense and virus recognition. Human pancreatic islets from two donors were treated with IFN-λ1 (100 ng/ml), IFN-λ2 (100 ng/ml), or IFN-α (1,000 U/ml) for 24 h, and the protein expression levels of MDA5, MxA, and RIG-I were measured using Western blotting. Actin was used as a loading control. One representative experiment out of two is shown.
CVB3 replication is perturbed in human pancreatic islets treated with type III IFN.
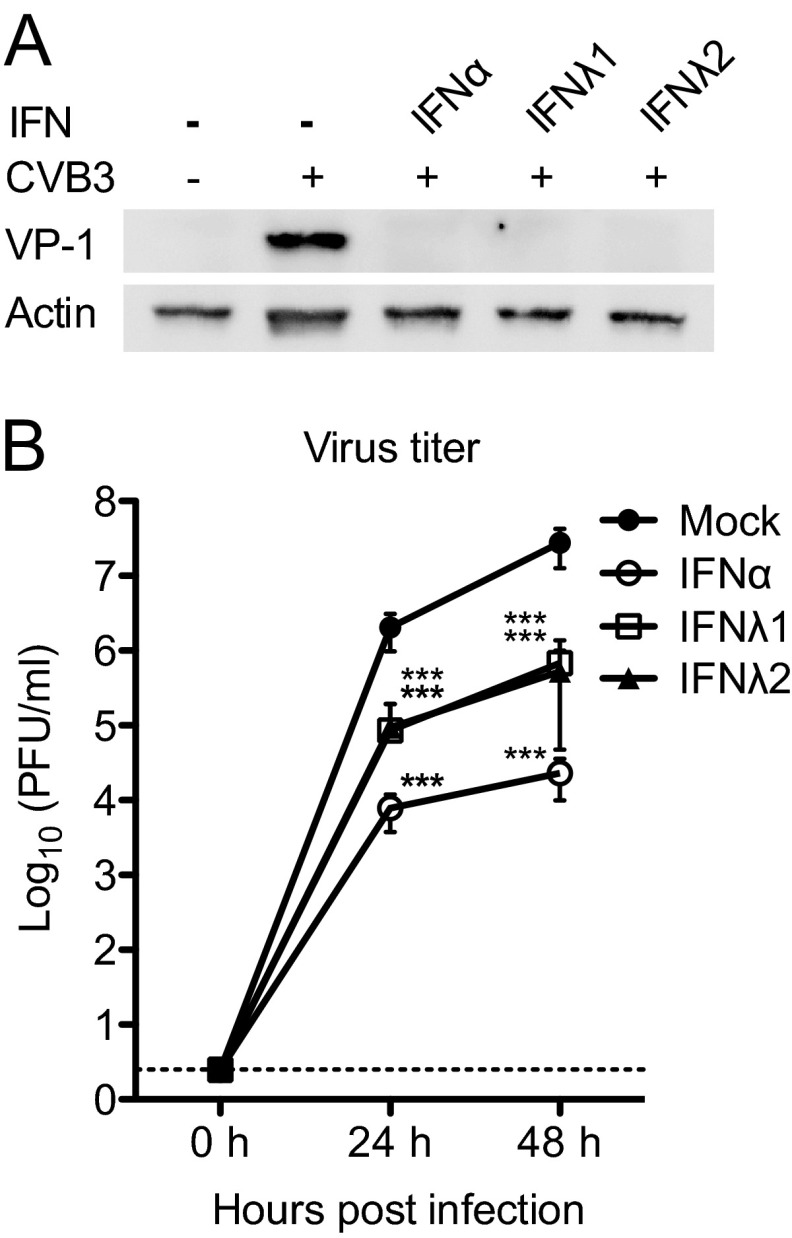
Type III IFN exerts antiviral activity against several viruses (7–9). It is not known, however, if it also inhibits CVB infection. We therefore set out to investigate whether treatment with IFN-λs would suppress CVB3 replication in human pancreatic islet cells. To this end, we made use of a previously established infection protocol where the islet cells are exposed to IFNs for 24 h before exposure to CVB (24). In order to determine whether type III IFN exerts antiviral activity against CVB3, human islets (n = 500 per condition) from 4 or 5 donors were pretreated with IFN-λ1, IFN-λ2, or IFN-α for 24 h prior to challenge with virus. The expression of viral protein 1 (VP-1) in infected cells at 48 h p.i. and the release of viral particles into the supernatant at 24 and 48 h p.i. were measured by Western blotting and a standard plaque assay, respectively.
A clear cytopathic effect could not be observed in any of the cultured islets (data not shown). VP-1 was, however, strongly expressed in mock-treated CVB3-infected pancreatic islet cells (Fig. 5A). In contrast, the expression of this viral protein was below the detection limit in islets treated with IFN-α or IFN-λ (Fig. 5A).
Fig 5.
Type III IFN inhibits CVB3 protein expression and replication. (A) Human islets from five donors were treated with IFN-λ1 (100 ng/ml, n = 5), IFN-λ2 (100 ng/ml, n = 5), or IFN-α (1,000 U/ml, n = 4) for 24 h prior to infection with CVB3 (4 × 104 PFU/islet). Total protein was isolated at 48 h p.i. Five micrograms of protein from each sample was added to SDS-polyacrylamide gels, and the expression of VP-1 was measured using Western blotting. Actin was used as a loading control. One representative experiment out of five is shown. (B) Supernatant was collected at 0, 24, and 48 h p.i., from experiments described for panel A, and viral titers (PFU/ml) were determined using plaque assay. Data are presented as log10(PFU/ml), means ± SDs of five independent experiments; ***, P < 0.001, versus untreated infected islets, one-way ANOVA with Bonferroni correction.
To determine whether type III IFN also prevented the release of new viral particles, we measured the accumulation of infectious virus in media harvested at 24 h and 48 h p.i. As shown previously (24), IFN-α exerted a powerful antiviral activity in the pancreatic islets (Fig. 5B, 259- and 1,203-fold difference in viral titers at 24 and 48 h, respectively). Importantly, our analysis also revealed a significantly lower accumulation of virus in media from islets treated with IFN-λ1 (24- and 40-fold difference in viral titers at 24 and 48 h, respectively) and IFN-λ2 (22- and 53-fold difference in viral titers at 24 and 48 h, respectively) compared to untreated islets (Fig. 5B). These data demonstrate that type III IFNs suppress CVB3 replication in human pancreatic islet cells.
DISCUSSION
The present study demonstrates that primary human pancreatic islets express both components required to constitute the functional type III IFN receptor, that they respond to type III IFN stimulation, and that such stimulation affords protection from CVB3 replication. To our knowledge, this is the first time that a detailed study has been conducted on receptor expression and responsiveness to IFN-λ in primary pancreatic endocrine cells. Moreover, our study reveals, for the first time, that this family of IFNs interferes with CVB3 replication in primary human cells.
Type III IFN is known to become expressed during infection of mammalian cells with viruses both in vitro and in vivo (7–9). Here, we show that human pancreatic islets express both IFN-λ1 and IFN-λ2 in response to infection with CVB3. However, this response may not be limited to the B3 serotype, as a very recent study has demonstrated a similar response in human islets infected with the B5 serotype (30). It still remains to be investigated whether type III IFN is produced by islet cells during a systemic CVB infection, but since this family of IFNs is expressed during in vivo infections with other picornaviruses (10, 31), it is likely that type III IFN is also expressed during CVB infection.
In order to understand whether human pancreatic islet cells can respond to type III IFN, we first studied whether isolated islets express the two receptor units IFN-λR1 and IL-10R2. We found that both were present at the mRNA level. We also took advantage of access to human pancreases retrieved from control subjects to study the production and distribution of each receptor component at the protein level. This revealed that IL-10R2 is present in the majority of islet cells and that it colocalized with either glucagon or insulin, suggesting its expression in both alpha and beta cells (Fig. 2E to G and K to M). IL-10R2 was not detected in somatostatin-positive delta cells (data not shown). Interestingly, a different situation obtained for IFN-λR1, since this protein was labeled to a high intensity in alpha cells (Fig. 2H to J) but was undetectable in beta cells (Fig. 2B to D) and delta cells (data not shown). Collectively, these observations indicate that only certain islet cell types are likely to respond to type III IFN stimulation in nondiabetic patients. Further studies are required to clarify if beta cells are universally unresponsive to type III IFNs or whether they may become responsive during, for example, exposure to a proinflammatory environment in vivo (such as may be encountered during the development of type 1 diabetes) or following the stress of islet isolation.
Our study also demonstrates that human pancreatic islets respond to type III IFN by upregulating the expression of classical ISGs, at both the mRNA and the protein levels. The expression of genes involved in IFN-induced intracellular signaling (e.g., STAT1, STAT2, IRF7, and IRF9) and the recognition of viral RNA (e.g., IFIH1/MDA, RIG-I, and TLR3) as well as genes involved in antiviral defense (e.g., ISG15, PKR, OAS, and MxA) and recruitment of immune cells (e.g., CXCL10) was all upregulated. Consistent with a previous report (24), we also confirmed, in positive-control studies, that human islets respond to IFN-α by upregulating a number of ISGs and PRRs.
Given the scarcity of human islet material, it was not possible to perform dose-response curves for the different IFNs in the present work. Because of this, our studies do not allow a direct comparison between the effects of IFN-α and IFN-λ (or between the various IFN-λ isoforms) on individual genes. Some conclusions can, however, be drawn from our PCR array and RT-PCR analyses. First, we noted that the nine most highly upregulated genes were common to IFN-α and IFN-λs, although the orders of magnitude of induction were different. Second, there were no striking differences in the gene signatures induced by IFN-λ1 and IFN-λ2 (Fig. 3). Collectively, these observations confirm previous studies showing that IFN-α and IFN-λs have overlapping effects (7–9). Moreover, they suggest that the different subtypes of IFN-λs may exert similar, though not identical, effects on human pancreatic islet cells. It has been hypothesized that IFN-λ and IFN-α exert similar functions due to the fact that they use the same signaling molecules (7–9). Our data on a selected number of genes affirm this notion, although future studies with a broadened approach are needed to determine if other genes may be regulated differentially by IFN-α and IFN-λ, as well as by the different IFN-λs. In addition, since the kinetics of induction may differ for individual genes between type I and III IFNs (6, 32), future time course studies will also be required to assess this in islets.
Collectively, our data suggested that human pancreatic islets enter an antiviral state following exposure to type III IFN. To investigate if this transition resulted in protection from infection, we studied the enterovirus CVB3, a common human pathogen that is mainly associated with mild infections but which can, occasionally, cause more severe disease such as myocarditis, hepatitis, and pancreatitis (12). Our studies on CVB3 are of particular relevance as enterovirus proteins and RNA have been found frequently in the pancreatic islets of type 1 diabetes patients (15–17). Here, we found that both IFN-λ1 and IFN-λ2 induce antiviral activity in human pancreatic islets. Treatment with IFN-λ1 and IFN-λ2 prevented the synthesis of viral protein (VP-1) and the release of infectious particles into the culture medium. The accumulation of newly synthesized virus particles in the culture supernatant was 22- to 53-fold lower in type III IFN-treated islets than in untreated islets. Previous studies have shown a protective effect of type III IFN in other picornavirus infections, including those with foot-and-mouth disease virus (33, 34) and rhinovirus (9). To our knowledge, this is, however, the first time that type III IFN has been shown to promote antiviral activity against CVB.
Although the precise mechanism by which type III IFN inhibits CVB3 replication remains to be determined, we show that multiple genes known to be involved in antiviral defense against CVBs are upregulated following type III IFN treatment. For example, the expression of PKR and OAS was increased under these conditions, and each of these enzymes is important for preventing viral replication. PKR activation leads to the attenuation of protein translation, while OAS generates 2′,5′-oligoadenylates to activate RNase L as a means to enhance the degradation of viral RNA (35). Both proteins have been shown to be protective against CVBs during systemic infections and during infections of isolated murine pancreatic islets (36). Type III IFN also induced the expression of MxA (encoded by the mx1 gene), a protein involved in inhibition of the replication of several viruses, including CVB (37). Moreover, we noted that type III IFN induced the expression of the PRRs TLR3 and MDA5, both of which have been proposed to be of importance in the host response to CVBs (23). Although unlikely to contribute to the direct antiviral effects, increased sensing of viral RNA by these PRRs may heighten the cellular responses to infection (e.g., production of IFNs) and thereby contribute further to the induction of antiviral activity.
A high proportion of patients with type 1 diabetes have pancreatic islets that stain positively with antibodies directed against the enteroviral protein VP-1 and/or are positive for enterovirus RNA by in situ hybridization (15–17). This suggests that such islets harbor a viral infection, but it remains to be established whether this is important for the disease process. Previous studies have suggested that type I IFNs play an important role to limit viral infection and subsequent cell damage in pancreatic islets (24). The present study now broadens this view by revealing that type III IFNs are also regulators of the permissiveness of human islet cells to CVB infection. If a failure to regulate this process is a feature of the beta cells in human type 1 diabetes, then this could be important for the etiology of the disease. However, this remains to be confirmed, and it is also important to determine more directly whether beta cells are (or can become) responsive to type III IFN. Nevertheless, an indication that patients with type 1 diabetes may have an altered response to enterovirus infection comes from observations that nonsynonymous single nucleotide polymorphisms (nsSNPs) in the ifih1 gene that encodes the PRR MDA5 modulate the risk for diabetes development (38, 39). Studies in mice deficient in MDA5 have demonstrated that this PRR is crucial for an intact response to CVB infection (40, 41). Moreover, some of the nsSNPs associated with an altered risk for type 1 diabetes development are also associated with changes in type I IFN production in response to a viral mimetic [poly(I·C)] (42). Whether this results in different handling of the virus or in altered production of IFNs (including type III IFN) during the development of type 1 diabetes will need to be addressed in future studies.
In summary, our study shows that primary human pancreatic islets express both components of the type III IFN receptor and that they respond to type III IFN treatment by upregulating classical IFN-inducible genes, including PRRs important for the detection of RNA viruses. We also show for the first time that type III IFN treatment limits CVB3 replication in primary human cells, and we propose that type III IFN may regulate human islet permissiveness to CVB infection. Thus, type III IFN could contribute to reduced tissue damage and promote beta cell survival during an enteroviral infection. This information may be useful for the development of improved therapeutic strategies aiming at reducing the severity of enterovirus infections in humans, as is currently being used in trials in the case of foot-and-mouth disease in cattle (34).
Supplementary Material
ACKNOWLEDGMENTS
We thank K. Loré, Karolinska Institutet, Stockholm, and O. Haller, University of Freiburg, Germany, for help with the MxA antibody. We are grateful to A. Foulis, University of Glasgow, for access to human pancreas sections.
This work was supported by the Karolinska Institutet (K.L. and M.F.-T.); the Diabetes Research and Wellness Foundation, United Kingdom (S.J.R.); EU FP7 (grant agreement 260441/PEVNET) (M.F.-T., N.G.M., and O.K.); the Strategic Research Programme in Diabetes at Karolinska Institutet (M.F.-T.); the Swedish National Strategic Research Initiative EXODIAB (O.K.); the Swedish Child Diabetes Foundation (M.F.-T.); the Swedish Diabetes Association Research Foundation (M.F.-T.); the Swedish Research Council (M.F.-T. and O.K.); and the JDRF. M.F.-T is in part supported by a VINNMER fellowship from VINNOVA, Sweden. O.K.'s position is in part supported by the National Institutes of Health (2U01AI065192-06).
Footnotes
Published ahead of print 1 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03431-12.
REFERENCES
- 1. Isaacs A, Lindenmann J. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:258–267 [PubMed] [Google Scholar]
- 2. de Weerd NA, Nguyen T. 2012. The interferons and their receptors—distribution and regulation. Immunol. Cell Biol. 90:483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stetson DB, Medzhitov R. 2006. Type I interferons in host defense. Immunity 25:373–381 [DOI] [PubMed] [Google Scholar]
- 4. Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69–77 [DOI] [PubMed] [Google Scholar]
- 5. Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63–68 [DOI] [PubMed] [Google Scholar]
- 6. Kotenko SV. 2011. IFN-lambdas. Curr. Opin. Immunol. 23:583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li M, Liu X, Zhou Y, Su SB. 2009. Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses. J. Leukoc. Biol. 86:23–32 [DOI] [PubMed] [Google Scholar]
- 8. Hou W, Wang X, Ye L, Zhou L, Yang ZQ, Riedel E, Ho WZ. 2009. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J. Virol. 83:3834–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vareille M, Kieninger E, Alves MP, Kopf BS, Moller A, Geiser T, Johnston SL, Edwards MR, Regamey N. 2012. Impaired type I and type III interferon induction and rhinovirus control in human cystic fibrosis airway epithelial cells. Thorax 67:517–525 [DOI] [PubMed] [Google Scholar]
- 10. Sommereyns C, Paul S, Staeheli P, Michiels T. 2008. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 4:e1000017. 10.1371/journal.ppat.1000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K. 2009. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 10:702–714 [DOI] [PubMed] [Google Scholar]
- 12. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed). 2007. Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 13. Knip M, Simell O. 2012. Environmental triggers of type 1 diabetes. Cold Spring Harbor Perspect. Med. 2:a007690. 10.1101/cshperspect.a007690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeung WC, Rawlinson WD, Craig ME. 2011. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 342:d35. 10.1136/bmj.d35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P. 2007. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. U. S. A. 104:5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. 2009. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52:1143–1151 [DOI] [PubMed] [Google Scholar]
- 17. Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M. 2004. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47:225–239 [DOI] [PubMed] [Google Scholar]
- 18. Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T. 2002. Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 45:693–702 [DOI] [PubMed] [Google Scholar]
- 19. Flodstrom M, Maday A, Balakrishna D, Cleary MM, Yoshimura A, Sarvetnick N. 2002. Target cell defense prevents the development of diabetes after viral infection. Nat. Immunol. 3:373–382 [DOI] [PubMed] [Google Scholar]
- 20. Flodstrom M, Tsai D, Fine C, Maday A, Sarvetnick N. 2003. Diabetogenic potential of human pathogens uncovered in experimentally permissive beta-cells. Diabetes 52:2025–2034 [DOI] [PubMed] [Google Scholar]
- 21. Horwitz MS, Fine C, Ilic A, Sarvetnick N. 2001. Requirements for viral-mediated autoimmune diabetes: beta-cell damage and immune infiltration. J. Autoimmun. 16:211–217 [DOI] [PubMed] [Google Scholar]
- 22. Kanno T, Kim K, Kono K, Drescher KM, Chapman NM, Tracy S. 2006. Group B coxsackievirus diabetogenic phenotype correlates with replication efficiency. J. Virol. 80:5637–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lind K, Huhn MH, Flodstrom-Tullberg M. 2012. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the innate immune response to enteroviruses and its possible role in regulating type 1 diabetes. Clin. Exp. Immunol. 168:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hultcrantz M, Huhn MH, Wolf M, Olsson A, Jacobson S, Williams BR, Korsgren O, Flodstrom-Tullberg M. 2007. Interferons induce an antiviral state in human pancreatic islet cells. Virology 367:92–101 [DOI] [PubMed] [Google Scholar]
- 25. Goto M, Eich TM, Felldin M, Foss A, Kallen R, Salmela K, Tibell A, Tufveson G, Fujimori K, Engkvist M, Korsgren O. 2004. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation 78:1367–1375 [DOI] [PubMed] [Google Scholar]
- 26. Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. 2003. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem. Biophys. Res. Commun. 308:474–479 [DOI] [PubMed] [Google Scholar]
- 27. Tian RR, Guo HX, Wei JF, Yang CK, He SH, Wang JH. 2012. IFN-lambda inhibits HIV-1 integration and post-transcriptional events in vitro, but there is only limited in vivo repression of viral production. Antiviral Res. 95:57–65 [DOI] [PubMed] [Google Scholar]
- 28. Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. 1998. Diabetes induced by coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 4:781–785 [DOI] [PubMed] [Google Scholar]
- 29. Diegelmann J, Beigel F, Zitzmann K, Kaul A, Goke B, Auernhammer CJ, Bartenschlager R, Diepolder HM, Brand S. 2010. Comparative analysis of the lambda-interferons IL-28A and IL-29 regarding their transcriptome and their antiviral properties against hepatitis C virus. PLoS One 5:e15200. 10.1371/journal.pone.0015200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ylipaasto P, Smura T, Gopalacharyulu P, Paananen A, Seppanen-Laakso T, Kaijalainen S, Ahlfors H, Korsgren O, Lakey JR, Lahesmaa R, Piemonti L, Oresic M, Galama J, Roivainen M. 2012. Enterovirus-induced gene expression profile is critical for human pancreatic islet destruction. Diabetologia 55:3273–3283 [DOI] [PubMed] [Google Scholar]
- 31. Rosenthal LA, Szakaly RJ, Amineva SP, Xing Y, Hill MR, Palmenberg AC, Gern JE, Sorkness RL. 2012. Lower respiratory tract infection induced by a genetically modified picornavirus in its natural murine host. PLoS One 7:e32061. 10.1371/journal.pone.0032061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, Rotman Y, Liang TJ. 2012. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology 142:978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diaz-San Segundo F, Weiss M, Perez-Martin E, Koster MJ, Zhu J, Grubman MJ, de los Santos T. 2011. Antiviral activity of bovine type III interferon against foot-and-mouth disease virus. Virology 413:283–292 [DOI] [PubMed] [Google Scholar]
- 34. Perez-Martin E, Weiss M, Diaz-San Segundo F, Pacheco JM, Arzt J, Grubman MJ, de los Santos T. 2012. Bovine type III interferon significantly delays and reduces the severity of foot-and-mouth disease in cattle. J. Virol. 86:4477–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goodbourn S, Didcock L, Randall RE. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341–2364 [DOI] [PubMed] [Google Scholar]
- 36. Flodstrom-Tullberg M, Hultcrantz M, Stotland A, Maday A, Tsai D, Fine C, Williams B, Silverman R, Sarvetnick N. 2005. RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J. Immunol. 174:1171–1177 [DOI] [PubMed] [Google Scholar]
- 37. Haller O, Kochs G. 2011. Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity. J. Interferon Cytokine Res. 31:79–87 [DOI] [PubMed] [Google Scholar]
- 38. Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. 2009. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 324:387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA. 2006. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat. Genet. 38:617–619 [DOI] [PubMed] [Google Scholar]
- 40. Huhn MH, McCartney SA, Lind K, Svedin E, Colonna M, Flodstrom-Tullberg M. 2010. Melanoma differentiation-associated protein-5 (MDA-5) limits early viral replication but is not essential for the induction of type 1 interferons after coxsackievirus infection. Virology 401:42–48 [DOI] [PubMed] [Google Scholar]
- 41. Wang JP, Cerny A, Asher DR, Kurt-Jones EA, Bronson RT, Finberg RW. 2010. MDA5 and MAVS mediate type I interferon responses to coxsackie B virus. J. Virol. 84:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shigemoto T, Kageyama M, Hirai R, Zheng J, Yoneyama M, Fujita T. 2009. Identification of loss of function mutations in human genes encoding RIG-I and MDA5: implications for resistance to type I diabetes. J. Biol. Chem. 284:13348–13354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.