Abstract
We have identified host IQGAP1 as an interacting partner for Ebola virus (EBOV) VP40, and its expression is required for EBOV VP40 virus-like particle (VLP) budding. IQGAP1 is involved in actin cytoskeletal remodeling during cell migration and formation of filopodia. The physical interaction and the functional requirement for IQGAP1 in EBOV VP40 VLP egress link virus budding to the cytoskeletal remodeling machinery. Consequently, this interaction represents a novel target for development of therapeutics to block budding and transmission of filoviruses.
TEXT
Ebola virus (EBOV) and Marburg virus (MARV) are enveloped, negative-sense RNA viruses belonging to the family Filoviridae which cause hemorrhagic syndromes with high mortality rates in humans (1, 2). There are currently no licensed vaccines or therapeutics to control filovirus infection and transmission. The filovirus VP40 matrix protein plays a central role in virion assembly and egress such that independent expression of VP40 leads to the production of virus-like particles (VLPs) that accurately mimic budding of live virus (3–7). Late (L) budding domains of VP40, which recruit host proteins (e.g., Tsg101) required for efficient virus-cell separation (or ”pinching-off”), consist of core consensus amino acid motifs such as PPxY, P(T/S)AP, YxxL, or FPIV (x = any amino acid). The conservation of L-domains within matrix proteins of many RNA viruses suggests that they are generally important and required for efficient RNA virus budding (8), although they are not absolutely required for viral replication (9).
Unlike the events that contribute to the late stages of filovirus budding, little is known about the regulation of early stages of filovirus budding, but this likely involves cellular mechanisms that control cytoskeletal remodeling and membrane deformation/curvature. For example, filopodia significantly increase the ability of filoviruses to spread from cell to cell, thereby contributing to pathogenesis (10). One multifunctional host protein that plays key roles in regulating cell motility, cytoskeletal architecture, actin polymerization, and formation of filopodia is IQGAP1 (11–16). Indeed, IQGAP1 is a widely expressed scaffolding protein with multiple protein-protein interaction domains, including a WW-domain that may interact with viral PPxY type L-domains (12). Intriguingly, IQGAP1 has been detected in purified HIV-1 virions (17) and has been shown to interact with the Gag protein of Moloney murine leukemia virus (MuLV) (18) and the core protein of classical swine fever virus (CSFV) (19) as well as host Tsg101 (20).
Here we investigated whether endogenous IQGAP1 interacts with EBOV VP40 and whether this interaction regulates efficient egress of EBOV VP40 VLPs. We found that EBOV VP40 interacts with endogenous IQGAP1 and that the L-domain region of VP40 mediates this interaction. Importantly, we found that egress of EBOV VP40 VLPs from IQGAP1-suppressed cells is reduced. Together, our findings identify a functional requirement for IQGAP1 interactions with EBOV VP40 during budding and suggest that IQGAP1-regulated proteins/pathways may be generally important for filovirus egress. As such, an EBOV VP40-IQGAP1 interaction represents a novel target for therapeutics to block filovirus budding and transmission.
IQGAP1 (Fig. 1) is a ubiquitously expressed scaffolding protein that regulates processes that include cell motility and division, actin polymerization, and formation of filopodia. We sought to determine whether IQGAP1 expression is important for VP40 VLP egress. To test this, human 293T cells were transfected with IQGAP1-specific or random small interfering RNAs (siRNAs) or were mock transfected for 12 h. Cells were then transfected with the indicated siRNAs and pCAGGS-VP40 for an additional 12 h. Cell extracts and supernatants containing released VLPs were harvested, and proteins were analyzed by SDS-PAGE and Western analysis using IQGAP1- or VP40-specific antisera (Fig. 2). Expression of endogenous IQGAP1 was reduced by approximately 90% in cells receiving IQGAP1-specific siRNAs (Fig. 2, lane 1) compared to the expression seen after transfection with random siRNAs (lane 2) or mock transfection (lane 3) in control cells. Importantly, VP40 budding in IQGAP1-suppressed cells was reduced by approximately 10-fold (Fig. 2, bottom panel, lane 1) relative to control results (Fig. 2, bottom panel, lane 1 versus lanes 2 and 3), indicating that IQGAP1 is crucial for efficient egress of EBOV VP40 VLPs from HEK293T cells.
Fig 1.

Schematic diagram of IQGAP1. The amino acid (aa) numbers are shown for the following IQGAP1 domains: calponin homology (CHD), polyproline binding domain (WW), IQ domain containing four IQ motifs (IQ), Ras GTPase-activating protein-related domain (GRD), and RasGAP C-terminal domain (RGCT). Amino acids 7 to 13 from EBOV VP40 (PTAPPEY) are shown. Solid arrows indicate reported interactions between Tsg101 and the RGCT domain of IQGAP1 (20) as well as with the PTAP motif of EBOV VP40 (25). The dashed arrow linking the PPEY motif of EBOV VP40 and the WW domain of IQGAP1 indicates a putative interaction.
Fig 2.

siRNA knockdown of IQGAP1 reduces egress of EBOV VP40 VLPs. Western analysis was performed using monoclonal anti-IQGAP1 antiserum (Invitrogen) or polyclonal anti-VP40 antiserum (26) to detect endogenous IQGAP1 in human 293T cells and EBOV VP40 in cells and VLPs as indicated. Cells were transfected with the indicated siRNAs (Santa Cruz).
We then questioned whether the observed decrease in VP40 VLP egress from IQGAP1-suppressed cells correlated with a direct interaction between IQGAP1 and VP40. To test this, cells were transfected with vector alone or pCAGGS-VP40 and then lysed in nondenaturing buffer 30 h posttransfection. Cell proteins were immunoprecipitated with either mouse preimmune serum or an IQGAP1-specific monoclonal antibody, and VP40 levels in precipitates were quantified by Western analysis using VP40-specific antiserum (Fig. 3, top panel). EBOV VP40 was detected in IQGAP1 immunoprecipitates but not in precipitates produced with preimmune antiserum (Fig. 3, top panel; compare lanes 1 and 2). As expected, control cells receiving pCAGGS vector alone were negative for VP40 (Fig. 3, top panel, lanes 3 and 4). Although these findings are consistent with a direct IQGAP1-VP40 interaction, they do not rule out the possibility that these proteins are linked through other intermediates such as the actin cytoskeleton and/or Tsg101.
Fig 3.

EBOV VP40 interacts with endogenous IQGAP1. Cell proteins from mock-transfected cells (pCAGGS vector alone; lanes 3 and 4) or pCAGGS-VP40-transfected cells (lanes 1 and 2) were first immunoprecipitated (IP) with either mouse preimmune (control; lanes 2 and 4) serum (Invitrogen) or monoclonal anti-IQGAP1 (lanes 1 and 3) antiserum (Invitrogen) as indicated, and EBOV VP40 was detected in the precipitated samples by Western blot (WB) analysis using anti-VP40 antiserum (top panel). Controls for expression of endogenous IQGAP1, β-actin, and VP40 are shown. Ab, antibody.
Because IQGAP1 has been shown to interact with host Tsg101 (20), we sought to determine whether endogenous Tsg101 regulates IQGAP1 interactions with EBOV VP40. To test this, we performed an analysis similar to that described above in control and Tsg101-suppressed cells (Fig. 4). Suppression of endogenous Tsg101 resulted in a difference of <2-fold in the amount of VP40 pulled down by IQGAP1 (Fig. 4, top panel; compare lanes 1 and 3), and VP40 was not pulled down by preimmune control serum (Fig. 4, top panel, lanes 2 and 4). Taken together, these data strongly suggest that EBOV VP40 interacts directly with endogenous IQGAP1 and that this association is not mediated by Tsg101.
Fig 4.

EBOV VP40 interacts with endogenous IQGAP1 in Tsg101-suppressed cells. Cells were cotransfected with EBOV VP40 and Tsg101-specific siRNAs (Santa Cruz) (lanes 1 and 2) or random siRNAs (Santa Cruz) (lanes 3 and 4). Transfected cell extracts were first immunoprecipitated with either mouse preimmune (control; lanes 2 and 4) serum (Invitrogen) or monoclonal anti-IQGAP1 (lanes 1 and 3) antiserum (Invitrogen) as indicated, and EBOV VP40 was detected in the precipitated samples by Western analysis using polyclonal anti-VP40 antiserum (top panel). Controls for expression of endogenous IQGAP1, β-actin, VP40, and Tsg101 are shown.
We next used BiMC (21–24) to visualize IQGAP1-VP40 complex formation in live mammalian cells and to confirm that IQGAP1 and VP40 directly interact. To do this, both an N-terminal enhanced yellow fluorescent protein (EYFP) fragment (residues 1 to 173, denoted NYFP) joined in-frame to human IQGAP1 and a previously generated C-terminal EYFP fragment (residues 174 to 239, denoted CYFP) joined to either the wild type (WT) (CYFP-eVP40-WT) or an L-domain deletion mutant of EBOV VP40 (CYFP-eVP40-ΔPT/PY) that produces at least 10-fold less VLPs than CYFP-eVP40-WT (23, 24) were coexpressed in 293T cells. Expression of all YFP fusion proteins was confirmed by Western analysis, and CYFP-eVP40-WT was confirmed to bud as a VLP (Y. Liu and R. N. Harty; data not shown) (23, 24). Cells were fixed 24 h posttransfection with acetone-methanol and stained with 4′,6′diamidino-2-phenylindole (DAPI), and YFP fluorescence, which is indicative of an interaction between the expressed protein pairs, was examined by confocal microscopy (Fig. 5). A strong fluorescent signal was observed in cells expressing NYFP-IQGAP1 plus CYFP-VP40-WT (Fig. 5, top left); however, little to no fluorescence was observed in cells expressing NYFP-IQGAP1 and CYFP-VP40-ΔPT/PY (Fig. 5, top right). To prove that CYFP-VP40-ΔPT/PY was indeed expressed in transfected cells, we cotransfected 293T cells with NYFP-VP40-WT and CYFP-VP40-ΔPT/PY (Fig. 5, bottom left). As expected, we observed a strong fluorescent signal in these cells, since deletion of the L-domain region of VP40 does not abolish a VP40-VP40 self-interaction (Fig. 5, bottom left). As an additional negative control, 293T cells were cotransfected with a plasmid expressing just the NYFP fragment alone plus CYFP-VP40-WT (Fig. 5, bottom right). These data confirm that EBOV VP40 and IQGAP1 interact directly and also demonstrate that the L-domain region of EBOV VP40 is critical for mediating this virus-host interaction.
Fig 5.
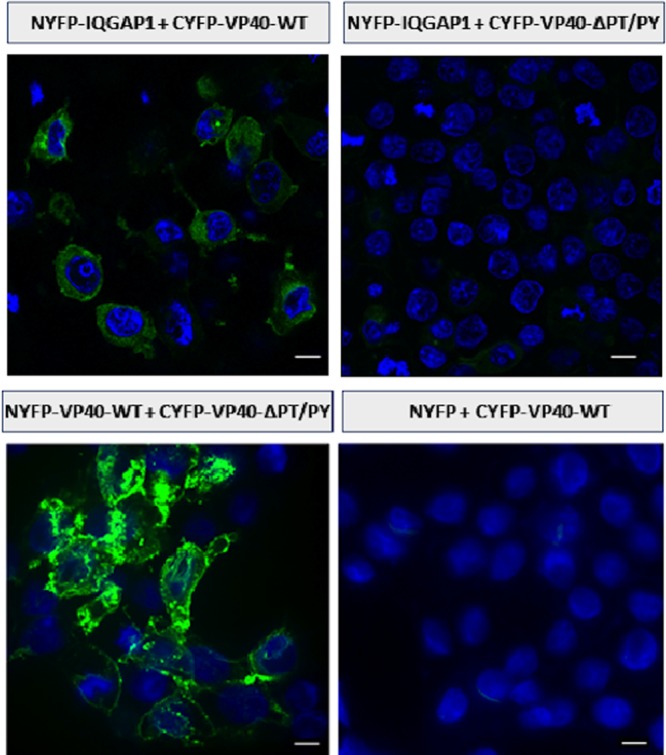
Use of BiMC to detect and visualize IQGAP1-VP40 interactions. Human 293T cells were cotransfected with the indicated plasmid pairs: NYFP-IQGAP1 plus CYFP-VP40-WT (top left), NYFP-IQGAP1 plus CYFP-VP40-ΔPT/PY (top right), NYFP-VP40-WT plus CYFP-VP40-ΔPT/PY (bottom left), and NYFP plus CYFP-VP40-WT (bottom right). Cells were fixed at 24 h posttransfection, stained with DAPI, and examined by confocal microscopy for YFP fluorescence. Bar = 2.0 μm.
Although IQGAP1 has been shown to interact with MuLV Gag during infection (18), our findings here are the first to demonstrate that IQGAP1 associates with VP40 and furthermore are the first to link IQGAP1 expression to filovirus egress. IQGAP1 is a central and crucial scaffolding protein that assembles, coordinates, and regulates formation of a multiprotein complex that includes actin, calmodulin, and Cdc42 to promote cell migration and actin microspikes and formation of filopodia (12). Our working model is that IQGAP1 is required by VP40 to initiate recruitment of other proteins (e.g., Cdc42) that promote early (e.g., bud protrusion) virus budding. We speculate that filoviral L-domains may recruit host proteins in a temporal and/or sequential manner to promote both early (e.g., bud protrusion) and late (e.g., pinching-off) stages of virion egress. This requirement of IQGAP1 for virus budding suggests that cellular proteins/pathways linked to IQGAP1 and involved in formation of filopodia/cytoskeletal rearrangements/cell motility are important for efficient EBOV egress. We further speculate that the IQGAP1 WW-domain region is involved in mediating interactions with EBOV VP40, although a full understanding of these domains and the mechanism by which IQGAP1 regulates the budding process of EBOV particles requires visualization of the spatial and temporal dynamics and trafficking of IQGAP1-VP40 complexes in live mammalian cells.
ACKNOWLEDGMENTS
We thank K. Kaibuchi (Nagoya University, Japan) for providing us with an IQGAP1-expression plasmid.
This work was supported in part by NIH grants U54-AI057168 and AI090284 to R.N.H. and AI060921-08 to B.F.
Footnotes
Published ahead of print 1 May 2013
REFERENCES
- 1. Feldmann H, Klenk HD. 1996. Filoviruses, p 877–888 In Baron S. (editor), Medical microbiology, 4th ed University of Texas Medical Branch at Galveston, Galveston, TX: [PubMed] [Google Scholar]
- 2. Feldmann H, Slenczka W, Klenk HD. 1996. Emerging and reemerging of filoviruses. Arch. Virol. Suppl. 11:77–100 [DOI] [PubMed] [Google Scholar]
- 3. Hartlieb B, Weissenhorn W. 2006. Filovirus assembly and budding. Virology 344:64–70 [DOI] [PubMed] [Google Scholar]
- 4. Jasenosky LD, Kawaoka Y. 2004. Filovirus budding. Virus Res. 106:181–188 [DOI] [PubMed] [Google Scholar]
- 5. Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. 2000. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. U. S. A. 97:13871–13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Harty RN. 2010. Viral and host proteins that modulate filovirus budding. Future Virol. 5:481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson RF, Bell P, Harty RN. 2006. Effect of Ebola virus proteins GP, NP and VP35 on VP40 VLP morphology. Virol. J. 3:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen BJ, Lamb RA. 2008. Mechanisms for enveloped virus budding: can some viruses do without an ESCRT? Virology 372:221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neumann G, Ebihara H, Takada A, Noda T, Kobasa D, Jasenosky LD, Watanabe S, Kim JH, Feldmann H, Kawaoka Y. 2005. Ebola virus VP40 late domains are not essential for viral replication in cell culture. J. Virol. 79:10300–10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolesnikova L, Bohil AB, Cheney RE, Becker S. 2007. Budding of Marburgvirus is associated with filopodia. Cell. Microbiol. 9:939–951 [DOI] [PubMed] [Google Scholar]
- 11. Jausoro I, Mestres I, Remedi M, Sanchez M, Caceres A. 2012. IQGAP1: a microtubule-microfilament scaffolding protein with multiple roles in nerve cell development and synaptic plasticity. Histol. Histopathol. 27:1385–1394 [DOI] [PubMed] [Google Scholar]
- 12. White CD, Erdemir HH, Sacks DB. 2012. IQGAP1 and its binding proteins control diverse biological functions. Cell. Signal. 24:826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim H, White CD, Sacks DB. 2011. IQGAP1 in microbial pathogenesis: targeting the actin cytoskeleton. FEBS Lett. 585:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osman M. 2010. An emerging role for IQGAP1 in regulating protein traffic. ScientificWorldJournal 10:944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandt DT, Grosse R. 2007. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 8:1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. 2005. IQGAP1: a key regulator of adhesion and migration. J. Cell Sci. 118(Pt 10):2085–2092 [DOI] [PubMed] [Google Scholar]
- 17. Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Jr, Sowder RC, II, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE. 2006. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J. Virol. 80:9039–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung J, Yueh A, Appah FS, Jr, Yuan B, de los Santos K, Goff SP. 2006. Interaction of Moloney murine leukemia virus matrix protein with IQGAP. EMBO J. 25:2155–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gladue DP, Holinka LG, Fernandez-Sainz IJ, Prarat MV, O'Donnell V, Vepkhvadze NG, Lu Z, Risatti GR, Borca MV. 2011. Interaction between Core protein of classical swine fever virus with cellular IQGAP1 protein appears essential for virulence in swine. Virology 412:68–74 [DOI] [PubMed] [Google Scholar]
- 20. Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. 2007. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 26:4215–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu CD, Grinberg AV, Kerppola TK. 2006. Visualization of protein interactions in living cells using bimolecular fluorescence complementation (BiFC) analysis. Curr. Protoc. Cell Biol. 29:21.3.1–21.3.21 [DOI] [PubMed] [Google Scholar]
- 22. Kerppola TK. 2006. Visualization of molecular interactions by fluorescence complementation. Nat. Rev. Mol. Cell Biol. 7:449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Lee MS, Olson MA, Harty RN. 2011. Bimolecular complementation to visualize filovirus VP40-host complexes in live mammalian cells: toward the identification of budding inhibitors. Adv. Virol. 2011:341816. 10.1155/2011/341816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Stone S, Harty RN. 2011. Characterization of filovirus protein-protein interactions in mammalian cells using bimolecular complementation. J. Infect. Dis. 204(Suppl 3):S817–S824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Licata JM, Simpson-Holley M, Wright NT, Han Z, Paragas J, Harty RN. 2003. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J. Virol. 77:1812–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCarthy SE, Johnson RF, Zhang YA, Sunyer JO, Harty RN. 2007. Role for amino acids 212KLR214 of Ebola virus VP40 in assembly and budding. J. Virol. 81:11452–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]


