Abstract
Hepatitis D virus (HDV) superinfection of hepatitis B virus (HBV) carriers causes severe liver disease and a high rate of chronicity. Therefore, a vaccine protecting HBV carriers from HDV superinfection is needed. To protect from HDV infection an induction of virus-specific T cells is required, as antibodies to the two proteins of HDV, p24 and p27, do not neutralize the HBV-derived envelope of HDV. In mice, HDV-specific CD8+ and CD4+ T cell responses were induced by a DNA vaccine expressing HDV p27. In subsequent experiments, seven naive woodchucks were immunized with a DNA prime and adenoviral boost regimen prior to simultaneous woodchuck hepatitis virus (WHV) and HDV infection. Five of seven HDV-immunized woodchucks were protected against HDV infection, while acute self-limiting WHV infection occurred as expected. The two animals with the breakthrough had a shorter HDV viremia than the unvaccinated controls. The DNA prime and adenoviral vector boost vaccination protected woodchucks against HDV infection in the setting of simultaneous infection with WHV and HDV. In future experiments, the efficacy of this protocol to protect from HDV infection in the setting of HDV superinfection will need to be proven.
INTRODUCTION
Hepatitis delta virus (HDV) superinfection of hepatitis B virus (HBV) carriers causes the most severe hepatitis in humans. Nearly all patients develop chronic HDV infection that has a high probability of progressing to liver cirrhosis and hepatocellular carcinoma (1, 2). Worldwide, approximately 15 million patients are affected with HDV. About 8% of HBV surface antigen (HBsAg)-positive patients in several European countries have tested positive for antibodies against HDV (2). Therapeutic options for HBV/HDV carriers are limited. Only in about 25% of the patients does alpha interferon therapy result in sustained viral clearance (3).
HBV carriers are at risk of being superinfected with HDV. Therefore, a vaccine protecting HBV carriers from HDV superinfection would be eligible. A main obstacle for the design of a vaccine against HDV infection is the fact that antibodies to the two proteins of HDV, p24 and p27, do not neutralize the HDV particle. The HDV protein/RNA complex is covered by the envelope protein of HBV (HBsAg). Therefore, classical vaccines which induce neutralizing antibodies cannot be expected to prevent HDV infection.
Immunizations with nucleoproteins of, e.g., influenza A virus-, HBV-, or woodchuck hepatitis virus (WHV) induced virus-specific T cells and were able to suppress replication, e.g., by cytokine secretion. In a second step, these virus-specific T cells are able to eliminate infected cells by their cytolytic activity and thus prevent the spread of the virus (4–7). T cell vaccines may not provide sterile immunity, because they do not induce neutralizing antibodies. However, T cell vaccines may stop infection via the cellular immune response at a very early phase of infection. Most conventional vaccines for humans induce sufficient amounts of neutralizing antibodies which prevent infection. To protect against HDV infection, a T cell vaccine inducing a vigorous HDV-specific T cell response would be required to prevent the spread of the virus after infection by killing infected cells.
The importance of CD4+/CD8+ T cells for the elimination of HDV has been demonstrated in patients who cleared HDV RNA after superinfection. These patients showed an HDV-specific CD4+ or CD8+ T cell response (8, 9). So far, two HLA-A*0201 epitopes have been characterized. These HDV-specific T cell responses were absent in patients with persistence of HDV RNA. These findings imply that an HDV-specific T cell vaccine may be able to effectively suppress HDV replication and protect from infection.
Chronically WHV-infected woodchucks (Marmota monax) can be superinfected with HDV and therefore are a good model to investigate the protective capacity of different vaccines (reviewed in reference 10). Several previous attempts to protect woodchucks from HDV infection by immunization with either protein or DNA vaccines failed. In this study, we intended to improve the strength of the CD8+ T cell response by a DNA prime and adenoviral boost immunization protocol. Recombinant adenoviruses have been shown to induce vigorous T cell responses in many viral infections such as Ebola virus, simian immunodeficiency virus, and WHV (7, 11, 12). Recently, the adenoviral vector approach was shown to induce sustained T cell responses against hepatitis C virus in healthy human volunteers (13).
In this study, we show that a DNA prime and adenoviral boost regimen can prevent HDV infection in the setting of WHV/HDV coinfection in the woodchuck model.
MATERIALS AND METHODS
Animals.
Eight-week-old female C3H/HeN mice (genotype H-2k) were purchased from Charles River Laboratories (Sulzfeld, Germany). Woodchucks negative for markers of WHV and HDV infection (negative for WHV DNA and antibodies to WHV core antigen and HDV antigen) trapped in the state of New York were purchased from North Eastern Wildlife (Harrison, ID). Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (14) and were reviewed and approved by the local Animal Care and Use Committee (Animal Care Center, University of Duisburg-Essen, and district government, Düsseldorf, Germany).
Construction of plasmids encoding HDV antigen (HDAg).
For the generation of the DNA plasmids expressing the HDV open reading frame an HDV isolate originally obtained as a gift from John Gerin was used and passaged eight times in woodchucks. Construction, cloning, and purification of the plasmids pcDNA3p24 and pcDNA3p27 encoding the gene for HDAgp24 or p27 were described previously (15). Biologically active woodchuck-specific gamma interferon (IFN-γ) was cloned and characterized previously. The 560-bp woodchuck IFN-γ cDNA fragment was obtained from the plasmid pwIFNγ (16). The plasmids were dissolved in phosphate-buffered saline (PBS) at a concentration of 1 mg/ml.
Construction of recombinant adenoviral vectors expressing HDAg.
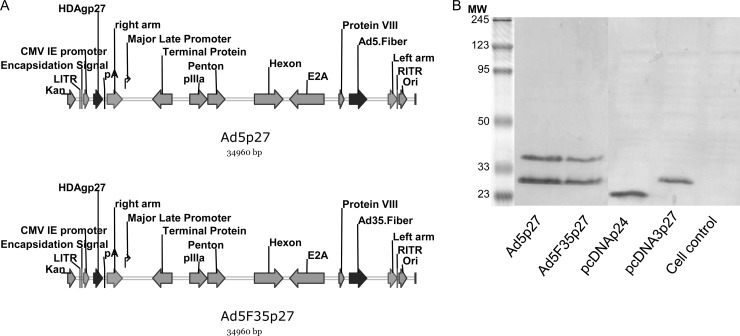
The adenoviral vectors Ad5p27 and Ad5F35p27 expressing HDAgp27 were constructed using the AdEasy system (Qbiogene, Carlsbad, CA) (Fig. 1A). For the construction of a pShuttle plasmid expressing HDAgp27 the pcDNA3p27 plasmid was employed. The insert was amplified by PCR using specific primers introducing BglII (5′-CGCTAGAGATCTATGAGCCGGTCCGAGTCGAGG-3′) and HindIII (5′-ATCTTATCTAGAAGCTTTCACTGGGGTCGACAACTCTGGGGAG-3′) restriction sites. Recombinant Ad5- and Ad35-based vectors were obtained by homologous recombination of pShuttlep27 with pAdEasy-1 and pAdEasy-1/F35 using the AdEasy system, respectively, transfected into 293 cells, and purified with Vivapure AdenoPACK 100 kit (Vivascience, Hannover, Germany). The adenovirus particle concentrations were determined by spectrophotometry as described previously and expressed as number of viral particles/ml (17).
Fig 1.
Construction and expression of Ad5p27 and AdF35p27. (A) HDVp27 was inserted under the control of the CMV immediate early promoter. The two vectors express different fibers (top, Ad5.Fiber; bottom, Ad35.Fiber). (B) Expression of HDAgp24 and HDAgp27 by adenoviral vectors and plasmids was verified by Western blotting using a human polyclonal serum of anti-HDV.
Transient expression of HDAg and detection of HDAg by Western blotting.
The human embryonic kidney (HEK) cell line 293 (Microbix Biosystems, Toronto, Ontario, Canada) was propagated as presented previously followed by infection with 5 × 107 PFU of Ad5p27 or Ad5F35p27 (multiplicity of infection [MOI], 10) (7). BHK-21 cells (baby hamster kidney cells; ATCC CCL-10) were handled as described previously (7), and 104 cells were transfected with 1 μg of pcDNA3p24 or pcDNA3p27 using the Lipofectamine reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturers' instructions. For Western blot analysis, cultured cells were handled as usual (7). Expression of HDAgp27 was verified using a polyclonal human anti-HDV serum (Fig. 1B).
Immunization of mice by intramuscular injection of pcDNA3 plasmid.
Mice were pretreated by intramuscular (i.m.) injection of 50 μl of cardiotoxin (10 μM in PBS; Latoxan, Valence, France) into the musculi tibiales anteriores, followed by three plasmid immunizations (100 μg of pcDNA3p27, 50 μg each) at 2-week intervals, according to the protocol described previously (6). Mice in the control group received sterile PBS i.m. (same volume). Mice were sacrificed 2 weeks after the last immunization.
Immunization of woodchucks with plasmid DNA and recombinant adenoviral vectors.
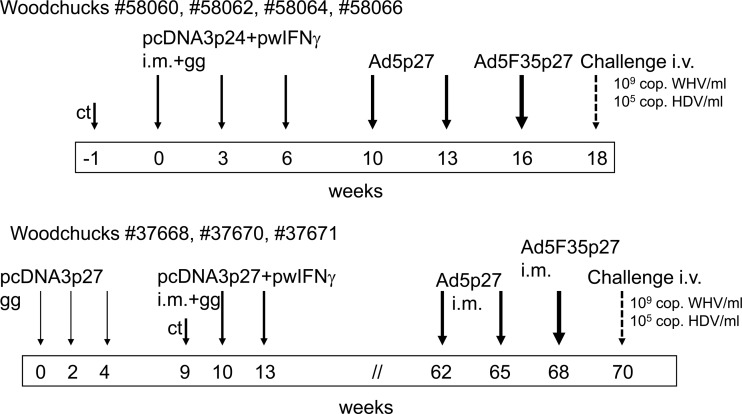
Four naive woodchucks (no. 58060, 58062, 58064, and 58066) were immunized with pcDNA3p24 and pwIFNγ i.m. (500 μg/500 μl each in a total volume of 1 ml) in the musculi tibiales anteriores and with pcDNA3p24 intradermally via gene gun (Helios gene gun; Bio-Rad, Hercules, CA) in weeks 0, 3, and 6 (Fig. 2). Woodchucks were pretreated with 250 μl of cardiotoxin (10 μM in PBS; Latoxan) 1 week before the first i.m. immunization. For the gene gun immunization, the plasmid was used to coat gold microcarriers as recommended by the manufacturer and as described previously (15). Booster immunizations (i.m.) with Ad5p27 followed in weeks 10 and 13 and with Ad5F35p27 in week 16. A second set of experiments was conducted to confirm and possibly improve the results. Three naive woodchucks (no. 37668, 37670, and 37671) were immunized at weeks 0, 2, and 4 with pcDNA3p27 intradermally with the gene gun. In weeks 10 and 13, gene gun immunization was repeated and combined with i.m. injections of pcDNA3p27 and pwIFNγ after cardiotoxin pretreatment. After their hibernation period, the woodchucks were given boosters of Ad5p27 in weeks 62 and 65 and Ad5F35p27 in week 68.
Fig 2.
Immunization schedule. Altogether seven naive woodchucks were immunized with a DNA prime and adenoviral boost regimen, three of them with a prolonged protocol (bottom). Two weeks after the last immunization, the woodchucks were challenged with WHV and HDV intravenously. ct, cardiotoxin; cop., copies; i.m., intramuscularly; gg, gene gun; i.v., intravenously.
WHV/HDV challenge experiment.
To establish simultaneous WHV/HDV infection, two naive woodchucks (no. 37669 and 46950) were infected intravenously with 109 copies of HDV and 105 or 109 copies of WHV, respectively. The HDV inoculum had been preliminarily passaged eight times in woodchucks and has been used for HDV superinfection before (15). The WHV inoculum was described previously (7).
The 7 immunized woodchucks were challenged with 105 copies of HDV and 109 copies of WHV 2 weeks after the last immunization. Four unvaccinated woodchucks (no. 46955, 48160, 58058, and 58065) were challenged with the same dose and served as controls.
Preparation, in vitro stimulation, and staining of murine splenocytes.
Single-cell suspensions of murine splenocytes were prepared according to the procedure described previously (18). Splenic lymphocytes were stimulated for 7 days with a panel of 27 16-mer peptides (8-mer overlapping) spanning the whole HDAg at a final concentration of 2 μg/ml per peptide (EMC Microcollections, Tübingen, Germany). Unstimulated cells and cells stimulated with a cytomegalovirus (CMV)-derived peptide (YILEETSVM) served as negative controls. Cell surface and intracellular IFN-γ staining were performed as described in detail before (7). Analyses were performed using FlowJo software (Tree Star, Ashland, OR).
CD107a degranulation assay of woodchuck PBMCs.
Woodchuck peripheral blood mononuclear cells (PBMCs) were cultivated, stimulated, and stained as described previously (7, 18). For stimulation, the previously identified WHV core antigen-derived epitope c96-110, the WHV surface antigen derived epitope s220-234, and 27 HDV-related peptides (described above) were used (18). Unstimulated cells and cells stimulated with the CMV-derived peptide served as negative controls.
Measurement of liver cell damage.
The aspartate transaminase (AST) level was quantified according to the standard diagnostic procedure at the Central Laboratory of University Hospital Essen. Values above 50 IU/ml were considered elevated.
Serology and detection of WHV DNA and HDV RNA.
Antibodies to HDV (anti-HDV) were measured by enzyme linked immunoassay (ETI-AB-DELTAK-2; DiaSorin, Dietzenbach, Germany) according to the manufacturer's instructions. PCRs to detect WHV DNA and HDV RNA in woodchuck sera were performed as described earlier (7, 15, 18). Sera of woodchucks which tested positive for HDV RNA in the nested PCR were quantified on the LightCycler 2.0 instrument (Roche, Basel, Switzerland) using a recently described protocol (19). Markers of infection and immune response were monitored weekly after challenge.
In silico prediction of major histocompatibility complex class I (MHC-I)-restricted epitopes.
The MHC class I-restricted epitopes of HDAg for the mouse haplotype H-2k were predicted by two independent algorithms: SYFPEITHI (20) (http://www.syfpeithi.de) and the Bioinformatics and Molecular Analysis Section (BIMAS) MHC peptide binding prediction program (21) (http://www-bimas.cit.nih.gov/molbio/hla_bind/). A score of ≥21 for the SYFPEITHI program and a score of ≥200.000 for the BIMAS algorithm were considered good prediction scores.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism version 5 (GraphPad Software, Inc., San Diego, CA). Statistical differences were analyzed by one-way analysis of variance test using the Newman-Keuls multiple comparison post-test. P values of <0.05 were considered significant.
RESULTS
Establishment of simultaneous WHV/HDV infection in woodchucks.
As simultaneous infection with WHV and HDV in woodchucks had not been established before, we infected two animals simultaneously with 109 copies of HDV and 105 (no. 37669) or 109 (no. 46950) copies of WHV. We used a serum with high HDV concentration to ensure the propagation of HDV in these first simultaneous infections performed in woodchucks. Both animals were infected with WHV and HDV. However, in woodchuck no. 37669, HDV RNA became positive in serum only in week 3 (Table 1). In woodchuck no. 46950, HDV RNA could be measured by PCR from week 1 onwards after infection. This animal had to be killed in week 5 due to bacterial sepsis. The high dose of the WHV inoculum was accompanied by an earlier onset of WHV replication than the lower dose and therefore promoted better HDV replication. We demonstrated that simultaneous infection is similar to that in humans (1). The WHV copy number in the inoculum determines the onset of WHV and HDV viremia.
Table 1.
Kinetics of HDV RNA and WHV DNA after WHV/HDV coinfectiona
| Woodchuck (inoculum) and RNA or DNA | Result at week: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 37669 (109 copies of HDV and 105 copies of WHV) | |||||||||||
| HDV RNA | − | − | − | + | − | − | − | − | − | − | − |
| WHV DNA (PCR) | − | − | − | + | ++ | + | + | + | − | − | − |
| WHV DNA (spot blot) | − | − | − | − | − | − | − | − | − | − | − |
| 46950 (109 copies of HDV and WHV) | |||||||||||
| HDV RNA | − | + | + | + | + | + | NT | NT | NT | NT | NT |
| WHV DNA (PCR) | − | + | + | ++ | + | + | NT | NT | NT | NT | NT |
| WHV DNA (spot blot) | − | − | − | + | + | − | NT | NT | NT | NT | NT |
HDV RNA and WHV DNA were tested by PCR; WHV DNA was also tested by spot blot hybridization. −, not detectable; +, positive; ++, strong positive; NT, not tested.
Characterization of HDV-specific T cell responses in mice.
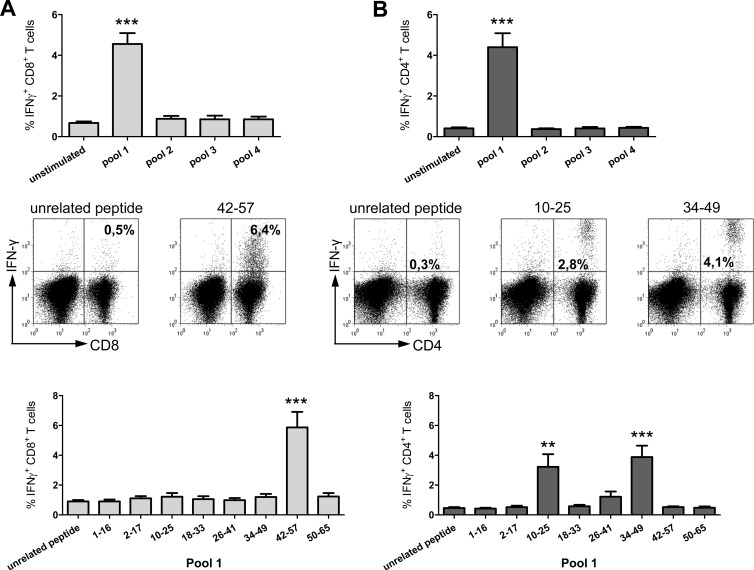
Due to the outbred status of woodchucks, it is difficult to assess the cellular immune response after immunization in this model. Mice are a convenient model to test the capability of DNA plasmids to induce cellular immune responses. Therefore, we characterized the T cell immune response to HDAgp27 after immunization first in mice. The computational prediction of potential epitopes within HDAg for different mouse H-2 haplotypes assigned a high score for one MHC class I-restricted epitope (amino acids [aa] 46 to 54, referred to here as peptide aa46-54) for the haplotype H-2k. Thus, we performed DNA immunization trials in C3H/HeN mice, which have the haplotype H-2k. We used the classical approach and stimulated spleen cells with 27 16-mer HDAg-derived overlapping peptides combined in 4 pools with up to 8 peptides each. Stimulation with pool 1 induced significant IFN-γ releases of CD8+ and CD4+ T cells (Fig. 3A and B, top). Restimulation of the spleen cells with the single peptides of pool 1 induced IFN-γ production of CD8+ T cells in response to peptide aa42-57 containing the predicted epitope aa46-54. The IFN-γ release of CD8+ T cells was significantly higher after stimulation with peptide aa42-57 (mean frequency, 5.9%) than after stimulation with the unrelated peptide (0.9%) (P < 0.0005) (Fig. 3A, bottom). A representative dot plot for one mouse is shown in Fig. 3A (middle). Positive IFN-γ responses of CD4+ T cells were also detected in pool 1 for the peptides aa10-25 and aa34-49; mean frequencies were 3.2% and 3.9%, respectively, versus 0.5% detected for the unrelated peptide control (P < 0.005 and < 0.0005, respectively [Fig. 3B, bottom]). Again, a representative dot plot of one mouse is shown in Fig. 3B (middle).
Fig 3.
Immune response in mice. Representative dot plots and summaric HDV-specific IFN-γ+ CD8+ (A) and IFN-γ+ CD4+ (B) responses are presented. The upper panels show the summaric results after stimulation of spleen cells with 4 pools containing up to 8 peptides each. The lower panels present the percentages of CD8+ or CD4+ and IFN-γ+ cells after restimulation with the single peptides of pool 1 (**, P < 0.005; ***, P < 0.0005). A representative dot plot for one mouse is presented in the middle panels.
Characterization of plasmids and recombinant adenoviral vectors.
After we had proven the immunogenicity of our HDAgp27 plasmid in mice, we inserted it into Ad5 and Ad5F35 vectors. For the immunization of woodchucks, a combination of plasmid and adenoviral vector immunization was selected in order to induce a strong cellular immune response. Recently, we showed in mice that this combination induces an immune response to WHV core-specific epitopes superior to that after plasmid immunization alone (7). For this purpose, HDAgp27 was inserted into the adenoviral vectors Ad5 and Ad5F35 as described above (Fig. 1A). The particle-to-PFU ratio of all vector preparations was ∼30:1. Titration of both vectors in HEK-293 cells revealed titers of 1 × 1010 to 1 × 1011 PFU/ml. After transient infection of HEK-293 cells, the expression of HDAg was verified by Western blotting using a human anti-HDV positive serum. Both, Ad5p27 and Ad5F35p27 expressed HDAg with the expected size of 27 kDa (Fig. 1B). The expression of HDAgp24 and HDAgp27 after transfection of BHK cells is also shown.
pcDNA3p27 DNA prime and Ad5p27/Ad5F35p27 booster immunizations protect woodchucks from HDV infection after challenge with WHV/HDV infection.
Seven naive woodchucks (no. 37668, 37670, 37671, 58060, 58062, 58064, and 58066) were immunized with HDV DNA via gene gun and intramuscularly followed by Ad vector injections as described (Fig. 2). The experiments were conducted in two series: one group was immunized over a longer time, including the hibernation period. Recently, we were able to show that woodchucks which were immunized against WHVpreS1 exhibited a stronger immune response if the booster immunizations were carried out after several months, which included the hibernation period of about 10 to 12 weeks, and not after 2 to 4 weeks (A. Schumann, unpublished data). Three out of four woodchucks immunized with the shorter immunization protocol and 2 out of 3 immunized with the longer immunization protocol were protected against HDV infection. In this setting, the prolonged immunization protocol showed no obvious advantage. Immunized woodchucks and four naive controls (no. 46955, 48160, 58058, and 58065) were intravenously challenged with 105 copies of HDV and 109 copies of WHV. The amount of HDV was reduced to a common concentration used before (10, 15) compared to that used in the first simultaneous infections. An unnaturally high dosage of HDV entails the risk of inducing tolerance and overrunning the immune system.
WHV replication.
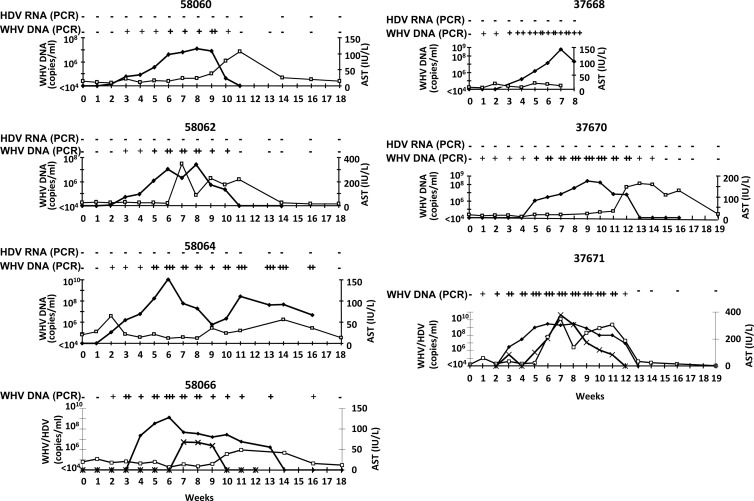
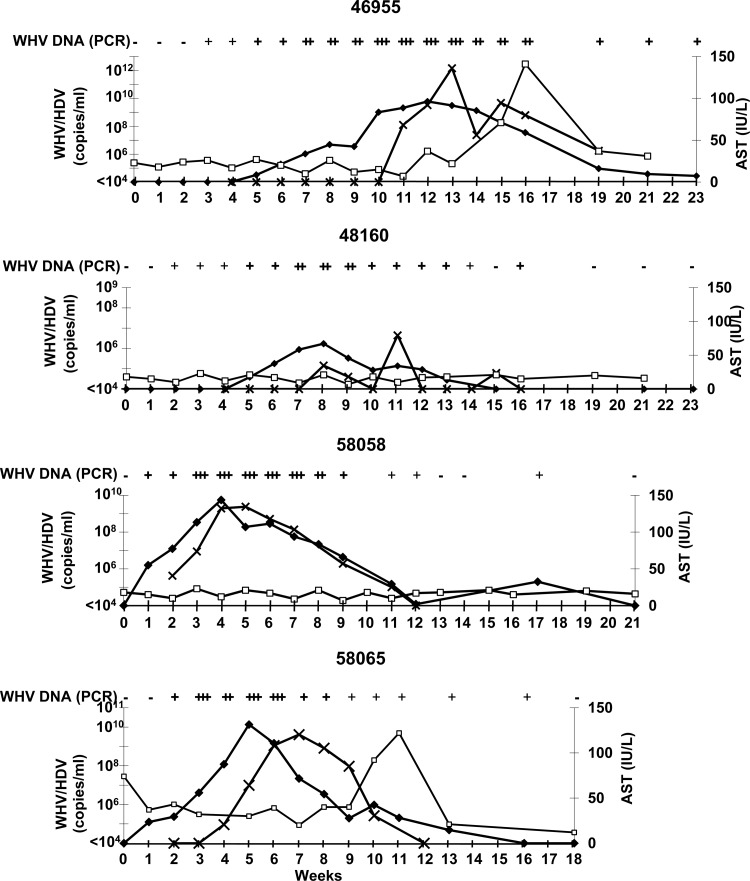
WHV DNA became detectable by qualitative PCR 1 to 3 weeks after inoculation (Fig. 4 and 5). WHV DNA persisted for 8 weeks (no. 58060 and no. 58062), 12 weeks (no. 37671), 14 weeks (no. 37670), or 15 weeks (no. 58064 and no. 58066) in the vaccinated animals. In the controls, WHV DNA was measurable for 15 weeks (no. 48160 and no. 58065), 17 weeks (no. 58058), and more than 21 weeks (no. 46955). WHV replication was quantitatively comparable in vaccinated woodchucks and controls. In all woodchucks, WHV replication was sufficient to assist HDV replication.
Fig 4.
Course of simultaneous WHV/HDV infection in seven woodchucks after vaccination. Kinetics of HDV RNA (×) (qualitative nested RT-PCR for negative animals, quantitative light cycler (LC)-PCR values for woodchucks with HDV breakthrough), WHV DNA (♦) (qualitative and quantitative PCRs in copies/ml), and AST (□) (IU/liter) starting with challenge in week 0, are shown (−, not detectable; +, ++, and +++, different strengths of positive signals).
Fig 5.
Course of simultaneous WHV/HDV infection in four naive controls. Kinetics of HDV RNA (x) (LC-PCR), WHV DNA (♦) (qualitative and quantitative PCRs in copies/ml), and AST (□) (IU/liter) starting with challenge in week 0 are shown (−, not detectable; +, ++, and +++, different strengths of positive signals).
HDV replication.
In 5 out of 7 immunized woodchucks, HDV RNA could not be detected during the follow up (no. 37668, 37670, 58060, 58062, and 58064) (Fig. 4). Woodchuck no. 37668 could not be observed beyond week 8 after challenge due to bacterial sepsis. Two woodchucks showed a breakthrough of HDV infection. HDV infection presented with a variable courses in all animals. In vaccinated woodchucks with breakthrough of HDV (no. 37671 and no. 58066), HDV RNA was measurable for 9 and 3 weeks, respectively. HDV replication in the controls lasted for 7, 8, 9, and 10 weeks. The highest levels of HDV RNA detected were 4 × 1010 (no. 37671) and 5 × 106 (no. 58066) in the vaccinated woodchucks and 1 × 1012 (no. 46955), 4 × 106 (no. 48160), 2 × 109 (no. 58058), and 4 × 109 (no. 58065) in the unvaccinated woodchucks. In conclusion, HDV replication in the two immunized woodchucks with viral breakthrough presented with a course similar to that in controls; however, the viremia in one animal (no. 58066) persisted for a shorter period than in controls.
Course of hepatitis.
AST as a marker for liver injury was elevated in 4/7 vaccinated woodchucks (no. 37670, 37671, 58060, and 58062) (Fig. 4) and in 2/4 controls (no. 46955 and 58065) (Fig. 5). Peak values of AST followed peak values of WHV DNA. The AST course was similar in vaccinated and nonvaccinated woodchucks.
Determination of WHV- and HDV-specific cellular immune responses.
Due to the outbred status of woodchucks, it was difficult to characterize the T cell response in this animal model. Recently, we established a flow-cytometric CD107a degranulation assay to monitor the WHV-specific T cell response in PBMC (18). With this assay, however, an HDV-specific cellular immune response could not be detected during vaccination or after WHV/HDV challenge (data not shown).
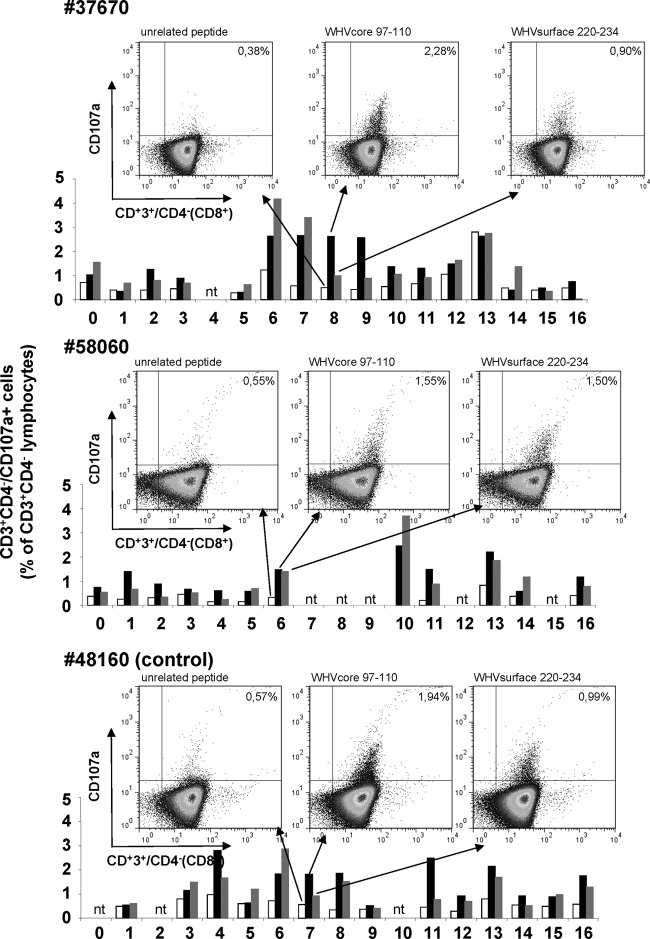
T cell responses to WHV core epitope c96-110 and WHV surface epitope s220-234 were detectable after simultaneous infection but not after immunization by the CD107a degranulation assay in all woodchucks. The population of CD3+ CD4− lymphocytes was considered CD8+ T cells. In Fig. 6, some dot plots are shown as examples for two vaccinated and one control woodchuck. The degranulation response against WHV core epitope c96-110 is shown for woodchuck no. 37670 in week 8 (2.28% CD107a+ CD8+ T cells), for woodchuck no. 58060 in week 6 (1.55%), and for control woodchuck no. 48160 in week 7 (1.94%). The responses were significantly higher than background values in unstimulated controls (0.38%, 0.55%, and 0.57%, respectively). The WHV surface antigen-specific T cell response after stimulation with the epitope s220-234 could be detected at similar or lower levels (0.90%, 1.50%, and 0.99%, respectively). The time course of the amount of CD107a+ CD8+ T cells is shown as examples for these three woodchucks in the graphs under the dot plots. In woodchuck no. 37670, e.g., the increase of the WHV core antigen-specific cells in week 6 is followed by a steady decrease of WHV DNA from week 10 onwards and loss of WHV DNA in week 15 (Fig. 4). As shown previously, the CD107a degranulation assay is a useful tool to monitor the WHV-specific immune response (18). The cellular immune response against WHV was the same in vaccinated and unvaccinated animals.
Fig 6.
WHV-specific cellular immune response. The T cell response was evaluated by the flow cytometric CD107a degranulation assay after stimulation of woodchuck PBMCs with WHV core (aa97-110) and surface (aa220-234) antigen-derived peptides. Representative dot plots and the course of the T-cell responses of two vaccinated and one control woodchuck are shown. The population of the CD3+ CD4− lymphocytes was considered the CD8+ T cells (nt, not tested).
DISCUSSION
The humoral and cellular immune responses to HDV proteins p24 and p27 are directed exclusively to an intracellular protein. Anti-HDV antibodies are not neutralizing due to the viral envelope, which is composed of the 3 forms of HBsAg (10). The induction of a T cell response against nucleoproteins can be protective and has been demonstrated previously in several viral infections (4–7). In HDV-infected patients, T cell responses were detected, which were correlated with self-limiting HDV superinfection. In contrast, T cell responses were not measurable in patients with persistence of HDV and a chronic course of infection (8, 9). Therefore, the induction of a protective HDV-specific T cell response seems feasible by vaccination with HDAg.
It was shown previously that DNA vaccination mounts HDV-specific cellular immune responses in mice (22, 23). In one study, the T cell response was vigorous enough to protect against tumor challenge in a syngeneic tumor model (23). We characterized the HDV-specific CD8+ and CD4+ T cell epitopes in mice using the classical approach with the stimulation of spleen cells with overlapping peptides spanning the whole HDAg. An H-2k-restricted HDV-specific CD8+ T cell epitope is located in the region from aa 42 to 57. The predicted response was directed to aa 46 to 54. Specific CD4+ T cell epitopes were located on the peptides aa10-25 and aa34-49. However, the CD8+ T cell response after immunization of mice was not as strong as that induced with a plasmid expressing the WHV core antigen (7).
Whether such a T cell response is strong enough to protect against HDV infection was tested in the woodchuck model. Since the 1980s, several vaccine strategies have been investigated in woodchucks (reviewed in reference 10). Only after immunization with vaccines that are known to induce a CD8+ T cell response, namely, vaccinia virus and DNA-based vaccines, was a modulation of the course of superinfection described (15, 24). However, none of the animals had been protected against HDV infection so far.
To augment the CD8+ T cell response, we decided to immunize the woodchucks with a DNA prime and adenoviral boost schedule. Recently, a DNA prime and adenoviral boost protocol has been shown to induce a stronger CD8+ T cell response to WHV core in mice superior to that after DNA immunization alone (7). Additionally, this protocol induced a strong T cell response in chronically WHV-infected woodchucks that was able to suppress WHV replication (25). Immunization with DNA plasmid prime administered via gene gun and Ad5/Ad5F35-vector boost expressing HDAg conferred protection against HDV in WHV/HDV coinfection in 5 out of 7 immunized animals. This vaccination protocol did not alter the course of WHV infection. We used a recently established CD107a degranulation assay for the detection of WHV-specific CD8+ T cell responses in woodchucks (18). This assay showed WHV core- and surface-specific T cell responses in our WHV/HDV coinfected woodchucks. However, an HDV-specific T cell response was detectable neither after vaccination nor after challenge. As described above, the results in mice already showed a less vigorous HDV-specific T cell response than the WHV-specific response after WHV DNA immunization. In addition, studies in mice demonstrated that the CD107a degranulation assay is less sensitive than the intracellular IFN-γ staining (7). Taking these findings together, HDV-specific CD8+ T cells most probably mediated the protection against HDV infection; however, our assay was not sensitive enough to detect this response. We assume that the CD8+ T cell response mounted in the two immunized but not protected woodchucks may have been weaker than that in the five protected animals and, therefore, was able only to modulate HDV replication.
HDV-specific antibodies were detected neither in controls nor in protected woodchucks in this simultaneous infection with WHV and HDV. HDV superinfection of WHV carriers, however, results in the production of anti-HDV in all woodchucks (10). Only a minor percentage of patients with HBV/HDV coinfection produce HDV-specific antibodies in the acute phase of hepatitis. In the majority of patients, the antibodies are detected 1 month or even later after coinfection (26). We can only speculate that the B-cell response might be too low to detect antibodies or even more delayed in woodchucks than in humans.
In this study, we achieved protection against HDV infection after simultaneous infection with WHV and HDV. The two different courses of HDV infection result in different outcomes. HBV/HDV coinfection resembles HBV infection alone, with a high rate of elimination of both viruses. An HBV-specific cellular immune response results in a downregulation of HBV replication by cytokines and an elimination of infected cells by cytotoxic cells (27). The role of an HDV-specific immune response for the elimination of HDV-infected hepatocytes has not been clarified so far. In HDV superinfection of HBV carriers and WHV carrier woodchucks, less than 10% of the patients or animals are able to eliminate HDV (1, 10). In HDV superinfection of WHV carrier woodchucks, HDV RNA became detectable as early as 1 week after superinfection (15). A quick expression of WHV surface antigen in a large number of hepatocytes may facilitate the release of HDV particles within this short period. Probably, the expansion of the hypothesized T cell response was overrun by HDV replication. For the protection of chronically HBV- or WHV-infected patients or woodchucks from HDV superinfection virus-specific T cells would have to circulate in high numbers at the time point of HDV superinfection to inhibit rapid infection of numerous hepatocytes. This could explain why only a delay of the appearance of HDV RNA and not long-term protection was observed after immunization with DNA or vaccinia virus (15, 24). Another explanation for the different outcomes of vaccination prior to superinfection or to simultaneous infection may be a dysfunction of HDV-specific T cells in the state of chronic HBV infection by the presence of regulatory T cells (Treg cells). It was shown that in chronically HBV-infected patients, circulating Treg cells suppressed the function of HBV-specific and unspecific CD8+ T cells ex vivo (28). We have chosen the setting of simultaneous WHV and HDV infection to investigate whether HDV-specific T cells may in principle prevent HDV infection.
Presumably, a DNA prime and adenoviral boost regimen can induce a strong T cell response that may be sufficient to protect not only from HDV in the setting of simultaneous infection, but also from HDV superinfection. In future experiments, the efficiency of this immunization protocol to induce an immune response that is able to confer protection in the setting of HDV superinfection will need to be verified.
ACKNOWLEDGMENTS
This work was supported by the Dr. Mildred Scheel Stiftung für Krebsforschung (grant 107740). Anja Mayer was paid by this grant. Anna Kosinska was supported by the DFG (GK1045 and TRR60), Alexandra Schumann by the DFG (KFO117), and Olena Brovko by the German reference center for hepatitis C virus.
We thank L. Vollbracht (Institute of Clinical Chemistry, University Clinic Essen, Germany) for the performance of the AST assays and Thekla Kemper and Inga Möller for technical assistance.
Footnotes
Published ahead of print 1 May 2013
REFERENCES
- 1. Buti M, Homs M, Rodriguez-Frias F, Funalleras G, Jardi R, Sauleda S, Tabernero D, Schaper M, Esteban R. 2011. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J. Viral Hepat. 18:434–442 [DOI] [PubMed] [Google Scholar]
- 2. Rizzetto M. 2009. Hepatitis D: thirty years after. J. Hepatol. 50:1043–1050 [DOI] [PubMed] [Google Scholar]
- 3. Wedemeyer H, Yurdaydin C, Dalekos GN, Erhardt A, Cakaloglu Y, Degertekin H, Gurel S, Zeuzem S, Zachou K, Bozkaya H, Koch A, Bock T, Dienes HP, Manns MP. 2011. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N. Engl. J. Med. 364:322–331 [DOI] [PubMed] [Google Scholar]
- 4. Fu TM, Guan L, Friedman A, Schofield TL, Ulmer JB, Liu MA, Donnelly JJ. 1999. Dose dependence of CTL precursor frequency by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J. Immunol. 162:4163–4170 [PubMed] [Google Scholar]
- 5. Kuhrober A, Wild J, Pudollek HP, Chisari FV, Reimann J. 1997. DNA vaccination with plasmids encoding the intracellular (HBcAg) or secreted (HBeAg) form of the core protein of hepatitis B virus primes T cell responses to two overlapping Kb- and Kd-restricted epitopes. Int. Immunol. 9:1203–1212 [DOI] [PubMed] [Google Scholar]
- 6. Lu M, Hilken G, Kruppenbacher J, Kemper T, Schirmbeck R, Reimann J, Roggendorf M. 1999. Immunization of woodchucks with plasmids expressing woodchuck hepatitis virus (WHV) core antigen and surface antigen suppresses WHV infection. J. Virol. 73:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kosinska AD, Johrden L, Zhang E, Fiedler M, Mayer A, Wildner O, Lu M, Roggendorf M. 2012. DNA prime-adenovirus boost immunization induces a vigorous and multifunctional T-cell response against hepadnaviral proteins in the mouse and woodchuck model. J. Virol. 86:9297–9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nisini R, Paroli M, Accapezzato D, Bonino F, Rosina F, Santantonio T, Sallusto F, Amoroso A, Houghton M, Barnaba V. 1997. Human CD4+ T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profiles. J. Virol. 71:2241–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Y-H, Tao M-H, Hu C, Syu W-J, Wu J-C. 2004. Identification of novel HLA-A*0201-restricted CD8+ T-cell epitopes on hepatitis delta virus. J. Gen. Virol. 85:3089–3098 [DOI] [PubMed] [Google Scholar]
- 10. Fiedler M, Roggendorf M. 2001. Vaccination against hepatitis delta virus infection: studies in the woodchuck (Marmota monax) model. Intervirology 44:154–161 [DOI] [PubMed] [Google Scholar]
- 11. Richardson JS, Yao MK, Tran KN, Croyle MA, Strong JE, Feldmann H, Kobinger GP. 2009. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS One 4:e5308. 10.1371/journal.pone.0005308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZG, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331–335 [DOI] [PubMed] [Google Scholar]
- 13. Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O'Hara G, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. 2012. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 4:115ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC [Google Scholar]
- 15. Fiedler M, Lu M, Siegel F, Whipple J, Roggendorf M. 2001. Immunization of woodchucks (Marmota monax) with hepatitis delta virus DNA vaccine. Vaccine 19:4618–4626 [DOI] [PubMed] [Google Scholar]
- 16. Lohrengel B, Lu M, Roggendorf M. 1998. Molecular cloning of the woodchuck cytokines: TNF-α, IFN-γ, and IL-6. Immunogenetics 47:332–335 [DOI] [PubMed] [Google Scholar]
- 17. Bayer W, Schimmer S, Hoffmann D, Dittmer U, Wildner O. 2008. Evaluation of the Friend Virus model for the development of improved adenovirus-vectored anti-retroviral vaccination strategies. Vaccine 26:716–726 [DOI] [PubMed] [Google Scholar]
- 18. Frank I, Budde C, Fiedler M, Dahmen U, Viazov S, Lu M, Dittmer U, Roggendorf M. 2007. Acute resolving woodchuck hepatitis virus (WHV) infection is associated with a strong cytotoxic T-lymphocyte response to a single WHV core peptide. J. Virol. 81:7156–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hofmann J, Frenzel K, Minh BQ, von Haeseler A, Edelmann A, Ross SR, Berg T, Kruger DH, Meisel H. 2010. Quantitative detection and typing of hepatitis D virus in human serum by real-time polymerase chain reaction and melting curve analysis. Diagn. Microbiol. Infect. Dis. 67:172–179 [DOI] [PubMed] [Google Scholar]
- 20. Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213–219 [DOI] [PubMed] [Google Scholar]
- 21. Parker KC, Bednarek MA, Coligan JE. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163–175 [PubMed] [Google Scholar]
- 22. Huang Y, Wu J, Hsu S, Syu W. 2003. Varied immunity generated in mice by DNA vaccines with large and small hepatitis delta antigens. J. Virol. 77:12980–12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mauch C, Grimm C, Meckel S, Wands J, Blum H, Roggendorf M, Geissler M. 2001. Induction of cytotoxic T lymphocyte responses against hepatitis delta virus antigens which protect against tumor formation in mice. Vaccine 20:170–180 [DOI] [PubMed] [Google Scholar]
- 24. Karayiannis P, Saldanha J, Jackson AM, Luther S, Goldin R, Monjardino J, Thomas HC. 1993. Partial control of hepatitis delta virus superinfection by immunisation of woodchucks (Marmota Monax) with hepatitis delta antigen expressed by a recombinant vaccinia or baculovirus. J. Med. Virol. 41:210–214 [DOI] [PubMed] [Google Scholar]
- 25. Kosinska A, Zhang E, Johrden L, Liu JB, Seiz P, Zhang X, Ma Z, Kemper T, Fiedler M, Glebe D, Wildner O, Dittmer U, Lu M, Roggendorf M. Combination of DNA prime-adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salassa B, Daziano E, Bonino F, Lavarini C, Smedile A, Chiaberge E, Rosina F, Brunetto MR, Pessione E, Spezia C, et al. 1991. Serological diagnosis of hepatitis B and delta virus (HBV/HDV) coinfection. J. Hepatol. 12:10–13 [DOI] [PubMed] [Google Scholar]
- 27. Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. 2003. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franzese O, Kennedy PT, Gehring AJ, Gotto J, Williams R, Maini MK, Bertoletti A. 2005. Modulation of the CD8+-T-cell response by CD4+ CD25+ regulatory T cells in patients with hepatitis B virus infection. J. Virol. 79:3322–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]