Abstract
Infants are protected from a severe respiratory syncytial virus (RSV) infection in the first months of life by maternal antibodies or by prophylactically administered neutralizing antibodies. Efforts are under way to produce RSV-specific antibodies with increased neutralizing capacity compared to the currently licensed palivizumab. While clearly beneficial during primary infections, preexisting antibodies might affect the onset of adaptive immune responses and the ability to resist subsequent RSV infections. Therefore, we addressed the question of how virus neutralizing antibodies influence the priming of subsequent adaptive immune responses. To test a possible role of the neonatal Fc receptor (FcRn) in this process, we compared the responses in C57BL/6 wild-type (WT) and FcRn−/− mice. We observed substantial virus-specific T-cell priming and B-cell responses in mice primed with RSV IgG immune complexes resulting in predominantly Th1-type CD4+ T-cell and IgG2c antibody responses upon live-virus challenge. RSV-specific CD8+ T cells were primed as well. Activation of these adaptive immune responses was independent of FcRn. Thus, neutralizing antibodies that localize to the airways and prevent infection-related routes of antigen processing can still facilitate antigen presentation of neutralized virus particles and initiate adaptive immune responses against RSV.
INTRODUCTION
Antibodies are an important correlate of protection for many viral infections. Neutralizing antibodies reduce viral load and virus-induced pathogenesis. Virus infection may be directly cytopathogenic or cause indirect tissue damage by host immune responses that follow viral exposure. In addition to lowering viral load, virus-specific antibodies might reduce or alter innate immune responses, affect antigen presentation and thereby the level of T-cell activation (1–3), and potentially enhance disease (4–7).
Children experiencing a primary respiratory syncytial virus (RSV) infection are protected against lower respiratory tract infections (LRTIs) by maternal antibodies. However, maternal antibodies decline rapidly within a few months after birth (8), and high levels of serum antibodies are required to provide efficient local protection in the airways. Adults with acquired immunity to RSV, including RSV neutralizing serum antibodies and memory T cells, still experience recurrent reinfections (9). Reduced titers of serum antibodies correlate with increased RSV-associated hospitalization in patients of all ages (9–11). Based on these observations current vaccine development is focused on a vaccine preparation that induces the production of highly neutralizing antibodies. Alternatively, palivizumab, a neutralizing antibody to the fusion protein of RSV, can be administered prophylactically to protect high-risk infants against LRTIs (12).
Because better neutralizing antibodies are being developed to protect against severe RSV disease (13), it is essential to understand the consequences of the presence of antibodies in vivo on the outcome of the immune response upon infection. Previously, we showed that the neutralizing capacity of RSV-specific antibodies influenced the level of virus-specific CD8+ and CD4+ T-cell responses in immune mice (14). Highly neutralizing serum antibodies decreased the CD8+ T-cell response, while both neutralizing and nonneutralizing antibodies increased CD4+ T-cell responses. The increased T-cell responses in the presence of antibodies was mediated by increased antigen presentation of RSV immune complexes (IC-RSV) to CD4+ T cells in an FcγR-dependent process (14).
Recently, yet another IgG Fc receptor, the neonatal Fc receptor (FcRn), was also shown to participate in phagocytosis processes of neutrophils, and it facilitates antigen presentation by dendritic cells (DCs) of soluble antigens opsonized by IgG (15, 16). FcRn was first described as a transporter of IgG across epithelial barriers, including transmission of IgG across the placenta from mother to infant. In addition, FcRn binds albumin and IgG Fc within endosomes at low pH and increases the serum half-life of these ligands by recycling them back to the cell surface, where the ligands are released from FcRn at higher pH (17, 18). FcRn is also responsible for antigen sampling from the airways and gut, by transporting IgG complexes across epithelium for uptake into macrophages and dendritic cells residing underneath the epithelial layer (19).
In the present work, we studied the effect of neutralizing antibodies to RSV on the induction of RSV-specific adaptive immune responses initiated after intranasal (i.n.) administration of antibody-bound RSV in mice. The role of FcRn in the local antigen presentation of RSV-palivizumab complexes was investigated by comparing the immune responses of C57BL/6 wild-type (WT) and FcRn−/− mice.
MATERIALS AND METHODS
Mice.
Pathogen-free 6- to 8-week-old C57BL/6cjo wild-type mice were purchased from Charles River (Maastricht, The Netherlands). FcR common-γ-chain−/− (γ−/−) mice and FcRn−/− mice on a C57BL/6 background (20) were bred and maintained at the central animal facility at Utrecht University. Study protocols were approved by the UMC Utrecht Animal Ethics Committee.
Virus and cell lines.
The RSV-A2 strain was grown in HEp-2 cells (ATCC CCL-23), purified by polyethylene glycol precipitation, and stored in liquid nitrogen in 10% sucrose in phosphate-buffered saline (PBS). The 50% tissue culture infective dose (TCID50) was determined after titration in HEp-2 cells. Detailed methods have been described previously (14).
D1, a mouse dendritic cell line derived from C57BL/6 mice (21) used in antigen presentation assays, was maintained in Iscove's modified Dulbecco's medium (IMDM) (Gibco, Invitrogen) containing 5% HyClone fetal calf serum (FCS) (Perbio, Logan, UT), 1% penicillin–streptomycin and 50 μM 2-mercaptoethanol (Bio-Rad, Hercules, CA) supplemented with 30% conditioned medium from granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing R1 cells (NIH 3T3 mouse fibroblasts transfected with the GM-CSF gene) (21). Bone marrow-derived dendritic cells (BM-DCs) were prepared as described before (14, 22). The percentage of CD11c+ cells (routinely >70%) was determined by staining with anti-CD11c (clone HL3; BD Pharmingen, San Diego, CA).
TNP labeling of RSV.
Trinitrophenol (TNP) labeling was performed as described by Hale et al. (23). In short, 200 μg/ml UV-inactivated RSV (UV-RSV) was incubated with 10 mM 2,4,6-trinitrobenzene sulfonic acid (TNBS) (Sigma-Aldrich, Steinheim, Germany) in 1 ml Hanks' buffer (Invitrogen) for 15 min at 37°C. Unbound TNBS was removed via dialysis against PBS overnight at 4°C while stirring. Precipitated RSV-TNP was removed by centrifugation at 1,500 rpm for 1 min. The concentration of stably linked TNP groups was measured by the optical density at 350 nm (OD350).
Generation of human anti-2,4,6-TNP antibodies.
The variable regions of the heavy (VH) and light (VL) chains were cloned from an anti-2,4,6-trinitrophenol (anti-TNP) hybridoma (24) and expressed as chimeric human IgG as previously described (25). RNA was amplified using the SMART Race cDNA amplification kit with CH- and CL-specific primers (Clontech, Inc., CA). Variable (V) genes were identified after sequencing of clones. Codon-optimized V genes, including 5′-HindIII and 3′-EcoRI restriction sites, Kozak sequence, and HAVT20 leader sequence (26), were then designed and ordered from Geneart (Life Technologies), along with codon-optimized human κ or γ1 constant regions for the variable light and heavy chains, respectively. The IHH IgG1 HC variant was created by inserting three mutations that negate binding to FcRn in the CH3 region (where IHH represents I253A, H435A, and H436A) (27). The HindIII-EcoRI fragment for the codon-optimized light chain was ligated into pEE14.4 (Lonza), and the HindIII-EcoRI fragment for the heavy chain was ligated into pEE6.4 (Lonza). A single-gene vector encoding either WT IgG1 or IHH-IgG1 was subsequently generated by ligation of the BamHI-NotI fragment from pEE6.4 [including a cytomegalovirus (CMV) promoter, IgG1 heavy chain, and poly(A)] into the light-chain-encoding pEE14.4 vector. The plasmids were then transfected in the FreeStyle 293 expression system (Life Technologies). Antibodies were purified on a protein A (WT IgG1) or protein G (IHH) HiTrap HP column (GE Life Sciences) and dialyzed against PBS overnight.
In vivo RSV infection, IC-RSV priming, and tissue sampling.
Mice were lightly anesthetized with isoflurane and i.n. inoculated with 2 × 106 PFU RSV in a volume of 50 μl. Lung cells were obtained from C57BL/6 mice 8 days after primary RSV infection at the peak of the T-cell response (28) when used as a readout in the in vitro antigen presentation assays.
In the in vivo experiments testing the effect of immune priming induced with RSV immune complexes (IC-RSV), mice were i.n. inoculated with IC-RSV or UV-inactivated RSV (UV-RSV) in a volume of 50 μl at day 0. A third group of mice was left untreated. IC-RSV was prepared by preincubation of 4.7 × 107 PFU with 50 μg/ml palivizumab [humanized IgG1 monoclonal antibody; Synagis, MedImmune, Gaithersburg, MD) (12) for 15 min in a volume of 200 μl. At day 35, all groups were i.n. challenged with RSV. Six days after challenge, T-cell responses in the lung were analyzed.
To obtain lung cell suspensions, mice were sacrificed by intraperitoneal (i.p.) injection of 300 μl pentobarbital. After bronchoalveolar lavage (BAL), lungs were perfused with 10 ml ice-cold PBS containing 100 U/ml heparin via the right ventricle. The lungs were removed, cut into pieces, and incubated with collagenase (2.4 mg/ml; Roche Applied Science, Basel, Switzerland) and DNase (1 mg/ml; Roche) for 20 min at 37°C. Single-cell suspensions were prepared by processing the tissue through 70-μm-pore cell strainers (BD Falcon, Franklin Lakes, NJ).
In vitro antigen presentation assay.
In vitro mouse antigen presentation assays were performed with D1 cells or, in experiments to study the role of FcγRs and FcRn, bone marrow-derived DCs (BM-DCs) obtained from FcγR−/−, FcRn−/−, or C57BL/6 WT mice.
To study antigen presentation of RSV immune complexes by BM-DCs or D1 cells, UV-inactivated RSV (UV-RSV) at multiplicities of infection (MOI) of 10, 3, 1, 0.1, and 0 was preincubated with either plasma derived from secondary RSV-infected mice or naive mice (preimmune serum) for 15 min at 37°C. UV-RSV was used to completely rule out infection-related effects. In parallel, RSV-TNP at MOI of 1, 0.3, 0.1, and 0 was preincubated with 0.1 μg/ml anti-TNP IgG1, anti-TNP-H435A mutant IgG1, or anti-TNP-IHH mutant IgG1 (IHH I253A, H310A, and H435A) (mutations that abolish FcRn binding capacity [29, 30]) or without antibodies as a control. RSV immune complexes were incubated with 5 × 104 BM-DCs or D1 cells for 24 h. Thereafter, antigen-presenting cells (APCs) were incubated with 5 × 105 total lung cells in the presence of 25 U/ml recombinant human interleukin-2 (hIL-2) (Roche) and 10 μg/ml brefeldin A (Sigma, St. Louis, MO) to facilitate intracellular accumulation of cytokines, for 5 h at 37°C in 5% CO2. Lung cells of mice 8 days after a primary RSV infection were used as a source of RSV-specific T cells. Antigen presentation of RSV was analyzed by measuring gamma interferon (IFN-γ) production in lung CD4+ T cells by intracellular fluorescence-activated cell sorter (FACS) staining.
Measurements of lung T-cell responses in IC-RSV priming experiments.
Single-cell suspensions of lung cells (106) from RSV-infected wild-type and FcRn−/− mice were stimulated for 5 h at 37°C in 5% CO2 with 2 × 105 RSV-infected D1 cells or uninfected D1 cells in 200 μl IMDM supplemented with 5% FCS, 1% penicillin–streptomycin, 50 μM 2-mercaptoethanol, 25 U/ml recombinant hIL-2, and 10 μg/ml brefeldin A. D1 cells were infected for 48 h with RSV at an MOI of 2 before addition to the lung cell suspension. Cytokine production by CD4+ and CD8+ T cells was analyzed by intracellular cytokine staining.
Intracellular cytokine staining.
Cytokine production by CD4+ and CD8+ T cells was measured by flow cytometry. Cells were stained for surface markers with anti-CD8 (clone 53-6.7; BD) and anti-CD4 (clone RM4-5; BD). Before intracellular staining, cells were fixed and permeabilized with CytoFix/CytoPerm (BD) solution and Perm/Wash buffer (BD). Intracellular cytokines were detected with anti-IFN-γ (clone XMG1.2; BD), anti-IL-5 (clone TRFK5; BD), anti-IL-4 (clone 11B11; BD), and anti-IL-13 (clone eBio13a; eBioscience). Stained samples were acquired on a FACSCanto flow cytometer (BD), and data were analyzed using FacsDiva software (BD).
RT-PCR. (i) RSV-specific real-time PCR.
A total of 2 × 105 A549 cells were incubated with RSV or UV-RSV (MOI of 2) in the presence or absence of palivizumab. RSV (4.7 × 107 PFU) was preincubated with 50 μg/ml palivizumab for 15 min before addition to the cells. After 24 h, total RNA was extracted from these cells using MagnaPure LC (Roche) equipment. cDNA was synthesized and viral loads were determined by real-time quantitative PCR (RT-qPCR) as previously described (31). In short, extracted RNA was reverse transcribed using a MultiScribe reverse transcriptase kit (Applied Biosystems, Foster City, CA) and random hexamers. Reverse transcriptase was inactivated followed by RT-qPCR performed on 20 μl cDNA using primers and probes specific for the N gene (31). Amplification and detection were performed with an ABI 7900HT fast RT-qPCR system for 2 min at 50°C, 10 min at 95°C, and 45 cycles of 15 s at 95°C and 1 min at 60°C. Sample threshold cycle (CT) values were compared with a standard curve of RSV-A2.
(ii) FcRn-specific RT-qPCR.
RNA was isolated from cultured BM-DCs of WT and FcRn−/− mice with the Qiagen RNA extraction kit, and cDNA was generated with random primers and Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen) according to the manufacturer's instructions. FcRn-specific mRNA was quantified by subsequent TaqMan RT-qPCR analysis with forward (5′-GTGGAAGGA-GCCGCCGTCTATG-3′) and reverse (5′-TGACCTCCAGCAATGACCATGCG-3′) primers and the 5′-ATCGTCATCGGTGTCTTGCTACTCACGG-3′ probe. FcRn expression was normalized for the expression of the housekeeping gene RPL27 (32).
ELISA. (i) RSV-specific IgG.
RSV-specific IgG was determined in serum samples obtained from C57BL/6 WT and FcRn−/− mice 28 days after i.n. inoculation with IC-RSV, UV-RSV, or control mice and from all groups at 6 days after i.n. challenge with live RSV, using an RSV enzyme-linked immunosorbent assay (ELISA) as described before (14). Denatured RSV lysate from RSV-infected HEp-2 cells in PBS was used as a coating. RSV-specific IgG was detected with secondary horseradish peroxidase (HRP)-labeled antibodies, anti-IgG1 (Invitrogen) or anti-IgG2c (Immunology Consultant Laboratory), and developed with the substrate 3,3′,5,5′-tetramethylbenzidine in NaAc (pH 5.5) and H2O2. The enzymatic activity was stopped by adding 9.8% H2SO4 and measured at OD450 (14). C57BL/6 mice express the Igh1-b allele encoding antibodies of the IgG2c isotype and lack the allele of the IgG2a isotype (33). Therefore, we used an IgG2c-specific secondary antibody.
(ii) IL-6 ELISA.
In the supernatant of A549 cells incubated for 24 h with RSV or UV-RSV (MOI of 2) in the presence or absence of palivizumab, IL-6 production was measured by ELISA (M9316; Sanquin Pelipair).
Statistical analysis.
Data were analyzed for statistical significance using a one-way or two-way analysis of variance (ANOVA), as indicated in the figure legends. Data are expressed as means ± standard errors of the means (SEM). A P value of <0.05 was taken as the level of significance.
RESULTS
H-2Ab-mediated antigen presentation of IC-RSV by DCs is facilitated by activating FcγRs and not FcRn.
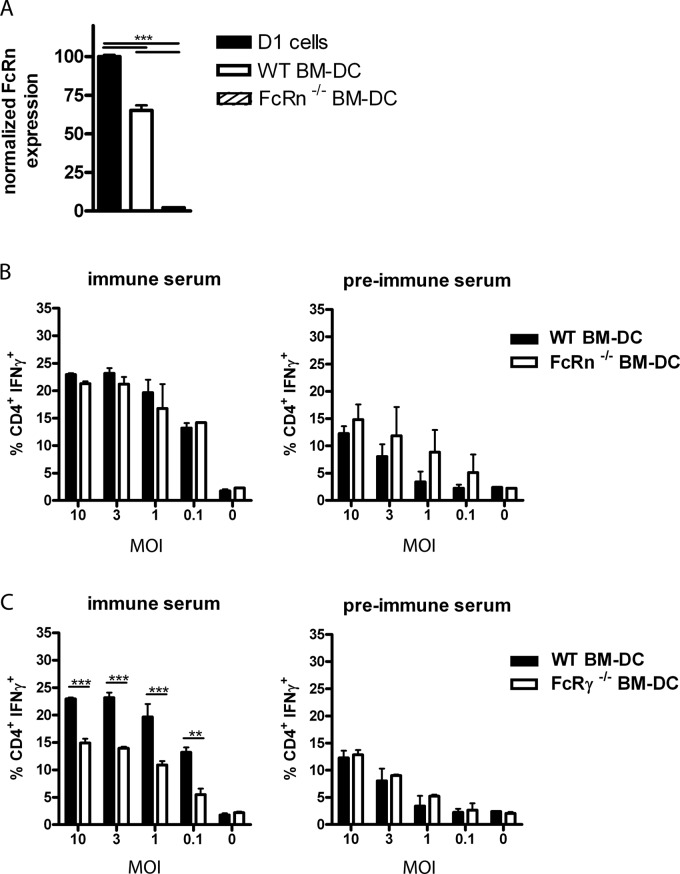
In previous work, we showed that activating FcγRs are involved in antigen presentation of RSV-derived antigens to CD4+ T cells during in vitro antigen presentation with dendritic cells and during in vivo RSV infection in mice (14). To determine whether FcRn also played a role during presentation of RSV-derived antigenic peptides, we first performed in vitro antigen presentation assays in the presence or absence of virus-specific antibodies, using BM-DCs as antigen-presenting cells obtained from WT, FcRn−/−, or FcR common-γ-chain−/− (γ−/−) mice. Expression of FcRn in WT BM-DCs and D1 cells and its absence in FcRn−/− BM-DCs were confirmed by RT-qPCR (Fig. 1A). As a source of polyclonal RSV-specific T cells, lung cells were harvested at day 8 after a primary i.n. RSV infection. T-cell activation was measured by intracellular staining for IFN-γ. A similar increased percentage of IFN-γ-producing CD4+ T cells was observed when WT BM-DCs or BM-DCs from FcRn−/− mice were pulsed with UV-inactivated RSV in the presence of serum derived from mice after secondary RSV infection (immune serum) compared to the response of preimmune serum (Fig. 1B). However, as described in our previous work, the IgG-mediated enhanced antigen presentation of IC-RSV to CD4+ T cells completely depended on functional expression of FcγRs on BM-DCs (14) (Fig. 1C).
Fig 1.
Activating FcγR, but not FcRn, expressed by BM-DCs is involved in antigen presentation of IC-RSV to RSV-specific CD4+ T cells. (A) WT BM-DCs and D1 cells but not BM-DCs from FcRn−/− mice express FcRn. FcRn expression levels were analyzed by RT-qPCR and normalized for RPL27 expression. The result of one representative culture out of three is shown. Error bars represent the SEM of a duplicate within one experiment. Significance was calculated using a one-way ANOVA. ***, P < 0.001. (B) BM-DCs obtained from WT, FcRn−/−, or (C) WT and γ−/− mice were incubated with UV-RSV (MOI of 10, 3, 1, 0.1, and 0) in the presence of 2% serum from naive mice or from RSV-immune mice for 24 h. Lung cells harvested 8 days after a primary RSV infection (a source of RSV-specific T cells) were added to the BM-DCs, and the responding CD4+ T cells were visualized by intracellular staining for IFN-γ. Significance was calculated using a two-way ANOVA. Error bars represent the SEM on data of one out of three individual experiments with similar results. **, P < 0.01; ***, P < 0.001.
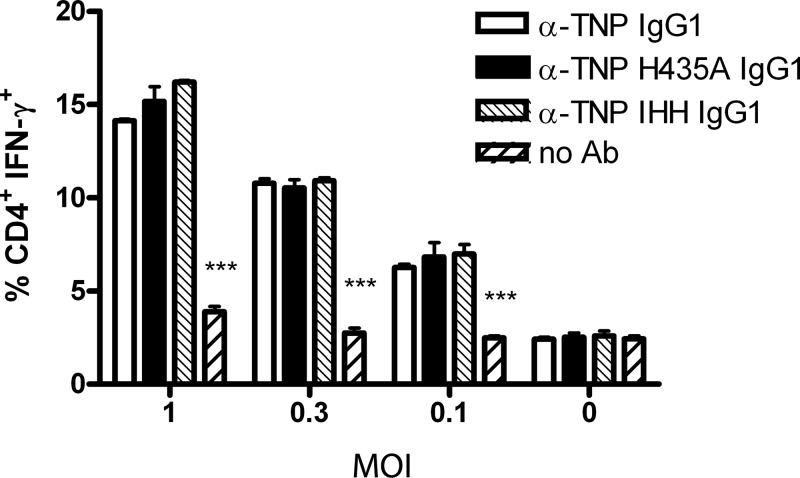
Individual DC cultures originating from bone marrow harvested from different mice might vary in the percentage of immature DCs. To exclude this experimental variation, we performed experiments with a dendritic cell line (D1) expressing functional FcRn (Fig. 1A) and used mutant antibodies unable to bind to FcRn in the in vitro antigen presentation assay. We used either WT anti-TNP or recombinant monoclonal antibodies with the same antigen specificity but mutated at a single amino acid residue (H435A) or three residues (IHH: I253A, H310A, and H345A) in the Fc domain. These positions are involved in FcRn binding but do not interfere with binding to FcγRs (15, 29). TNP-labeled RSV alone or opsonized with these recombinant WT or mutant (H435A and IHH) IgG1 monoclonal antibodies, was incubated with D1 cells, and antigen presentation was monitored using RSV-specific lung T cells. The anti-TNP WT and mutant antibodies all increased the percentage of IFN-γ-producing CD4+ T cells responding to D1 cells exposed to RSV-TNP to a similar extent compared to the samples in which nonopsonized virus was incubated with D1 cells (Fig. 2). These results confirmed that FcRn was not involved in the in vitro presentation by DCs of IC-RSV-derived antigenic peptides to virus-specific CD4+ T cells.
Fig 2.
Abrogated binding of IC-RSV to FcRn does not affect antigen presentation to RSV-specific CD4+ T cells. C57BL/6-derived D1 dendritic cells were incubated with haptenized RSV (RSV-TNP) at MOI of 1, 0.3, 0.1, and 0 in the presence of 0.1 μg/ml of either anti-TNP (α-TNP) IgG1, anti-TNP H435A mutant IgG1, or anti-TNP IHH or without antibodies (No Ab) for 24 h. The percentage of responding CD4+ T cells (in lung cells harvested at 8 days postinfection) was measured by intracellular staining for IFN-γ. One representative experiment out of three is shown. Significance was calculated using a two-way ANOVA. Error bars represent SEM of a duplicate within one experiment. ***, P < 0.001 comparing the no-antibody response to all three antibody-mediated responses.
Noninfectious IC-RSV efficiently primes virus-specific T-cell responses after i.n. inoculation.
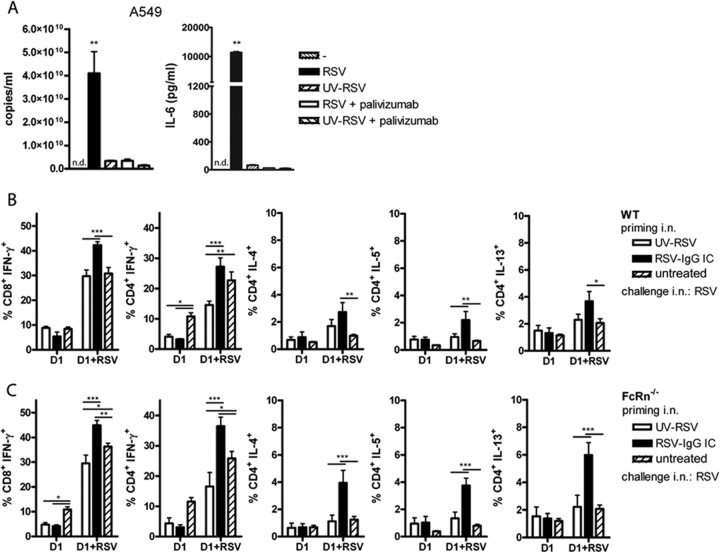
We next examined the impact of antibody neutralization of RSV in the airways on the initiation of the T- and B-cell responses by measuring recall responses during a virus challenge 5 weeks after immune priming. Abortive replication has been described as occurring in RSV-immune animals (34). This process of abortive replication is defined as viral penetration followed by replication of viral genetic material in the cytoplasm, without the production of infectious virus particles. Abortive replication in vivo can be monitored by comparing lung viral loads using the PFU assay and by the PCR detection technique and has been observed in animals treated prophylactically by virus neutralizing antibodies instilled systemically. Abortive replication still induces inflammatory immune responses via cytoplasmic recognition of genomic viral RNA and replication intermediates (35). Also, viral antigen expression might still occur. To focus only on the contribution of neutralized virus particles in priming of the immune response, we administered in vitro-preformed RSV immune complexes intranasally. Moreover, this approach also minimized the effect of increased antibody degradation in FcRn−/− mice that could lead to differences in virus neutralization in the lung, when antibody was administered parenterally. We used a monoclonal humanized IgG1 antibody, palivizumab (12), specific for the fusion protein of RSV in these experiments. Binding of palivizumab to RSV completely abolished virus infection of A549 epithelial cells in vitro (Fig. 3A). RSV-palivizumab complexes could still be detected by RT-qPCR at a level of one-third of the input virus in the washed A549 samples incubated for 24 h with the different virus preparations. However, the amount of viral material still detected represented noninfectious virus bound to A549 cells because expression of viral pathogen-associated molecular patterns in the cytoplasm, which leads to inflammatory cytokine production in RSV-infected A549 cells, was completely abolished. This was shown by the complete absence of IL-6 production by A549 cells exposed to RSV-palivizumab (Fig. 3A). We used these antibody-inactivated RSV immune complexes and UV-inactivated RSV to prime immune responses in vivo. Five weeks after intranasal inoculation of mice with RSV immune complexes (equivalent of 2 × 106 PFU and 50 μg/ml palivizumab in a total volume of 50 μl) or control UV-inactivated RSV (equivalent of 2 × 106 PFU in 50 μl), T- and B-cell responses were measured in the lungs at day 6 after an i.n. challenge with live RSV. A third group only received a primary RSV infection.
Fig 3.
Intranasal inoculation of IC-RSV efficiently primes RSV-specific CD4+ and CD8+ T-cell responses in both WT and FcRn-deficient mice. (A) Palivizumab neutralizes RSV. RSV (4.7 × 107 PFU) was preincubated for 15 min with 50 μg/ml palivizumab. UV-RSV was used as a negative control for replication. RSV, RSV plus palivizumab, UV-RSV, or UV-RSV plus palivizumab was added to epithelial A549 cells (equivalent of an MOI of 2), and the cells were incubated for 24 h. The results of the RT-qPCR performed on the RSV N gene are shown for one representative experiment of two. Error bars represent the SEM of a duplicate within one experiment. IL-6 production was measured in the 24-h supernatant of A549 cells treated with similar live- and inactivated-RSV preparations. One representative experiment out of 6 is shown. Significance was calculated using a one-way ANOVA. **, P < 0.01. n.d., nondetectable. (B) WT mice and (C) FcRn−/− mice were primed with UV-RSV or IC-RSV at day 0. A third group was untreated. At day 35, all groups were challenged with RSV, and 6 days later, lungs were analyzed for T-cell responses in the presence of uninfected (D1) or RSV-infected D1 (D1+RSV) cells. The percentage of cytokine-producing CD8+ and CD4+ T cells is shown. Error bars represent the SEM of 5 individual mice per group. Results are shown for five mice per group of one of two representative experiments. Significance was calculated using a two-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Despite the failure of FcRn to enhance antigen presentation of IgG-complexed RSV in vitro, the possibility remained that FcRn might mediate translocation of ICs across the respiratory epithelial layer and increase antigen presentation in the lungs of mice (36). Therefore, we determined whether FcRn was involved in the in vivo priming of RSV-specific immune responses upon i.n. exposure to IC-RSV by comparing the priming of immune responses in C57BL/6 WT and FcRn−/− mice. Palivizumab contains the complementarity-determining regions of a mouse neutralizing anti-RSV-F antibody grafted into the framework of a human IgG1 constant region (37). Human IgG1 (and thus palivizumab) binds mouse FcRn via its constant region with high affinity at pH 6.0 (34). In addition, mouse FcRn has been shown to extend the half-life of human IgG1 to a similar extent to mouse IgG (17, 38, 39). Importantly, mouse FcRn has also been demonstrated to enhance antigen presentation of immune complexes through human IgG1 (15). The activation of T cells in the lungs after viral challenge was measured by in vitro restimulation with untreated or RSV-infected D1 cells and subsequent intracellular cytokine staining. T cells from mice inoculated with IC-RSV showed an increased percentage of RSV-specific CD8+ IFN-γ+ T cells compared to T cells from UV-RSV-inoculated mice and nonimmunized mice when restimulated with RSV-infected D1 cells. An increase in IFN-γ- and IL-5-producing CD4+ T cells was also observed for IC-RSV-immunized mice in comparison to UV-inoculated mice. For IL-4+ and IL-13+ CD4+ T cells, the increases were only significant in comparison to untreated mice but not in comparison to UV-inoculated mice (Fig. 3B). The slight increase in the percentage of Th2 cytokine-producing cells in IC-RSV-inoculated mice was not accompanied by an increase in the percentage of eosinophils in BAL specimens (data not shown). In both WT and FcRn−/− mice, UV-RSV inoculation did not prime Th1 or Th2 responses against RSV. A slight decrease in IFN-γ production was noticed in UV-RSV-immunized mice after challenge compared to primary RSV-infected mice. Similar increased Th1 and Th2 responses after immune complex priming were observed in FcRn−/− mice (Fig. 3C) and WT mice (Fig. 3B), suggesting again that FcRn-mediated processes were not involved in the IgG-mediated enhancement of the immune responses to RSV.
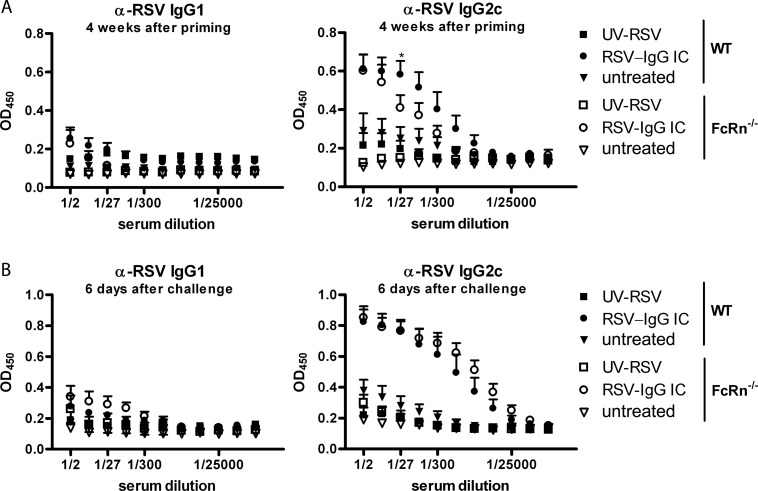
In addition to increasing virus-specific T-cell responses, binding of palivizumab to RSV strongly potentiated RSV-specific IgG2c antibody responses (Fig. 4). In FcRn−/− mice, the antibody responses were slightly lower than those in wild-type mice at day 28 after priming, probably due to the shorter antibody half-life in the knockout mice (Fig. 4A) (18). However, at day 41 (i.e., 6 days after challenge), the virus-specific antibody response was robust and the responses were of equal magnitude in WT and FcRn−/− mice inoculated with immune complexes (Fig. 4B). Due to the shorter half-life of IgG2c in FcRn−/− mice, this may indicate that the relative net production of anti-RSV IgG in these mice may be increased.
Fig 4.
Intranasal administration of IC-RSV efficiently primes RSV-specific IgG2c responses in both WT and FcRn-deficient mice. WT mice and FcRn−/− mice were inoculated with UV-RSV or IC-RSV (palivizumab) at day 0. A third group was left untreated. At day 35, all groups were challenged with RSV. Blood was collected to analyze serum antibodies 7 days prior to RSV challenge (4 weeks after priming in panel A and 6 days after challenge in panel B). (A) RSV-specific IgG2c levels were significantly increased in both WT and FcRn−/− mice (P < 0.001) after inoculation with IC-RSV. However, the responses were lower in FcRn−/− mice compared to those in WT mice (*, P < 0.05 at 1/27 serum dilution). (B) At day 6 after challenge in both WT and FcRn−/− mice, virus-specific IgG2c levels differed significantly in IC-RSV-treated mice from nonprimed and UV-RSV-inoculated mice (P < 0.001), but no significant differences were found between the WT and FcRn−/− mice who received IC-RSV priming. These experiments were performed twice with similar results. Error bars represent the SEM of 5 individual mice per group. Significance was calculated using a two-way ANOVA.
In conclusion, these experiments showed strong immune priming with opsonized RSV that was negligible when nonopsonized, noninfectious RSV was used. The enhanced immune priming was independent of FcRn.
DISCUSSION
In the present work, we showed that intranasal administration of noninfectious IC-RSV primed both virus-specific T- and B-cell responses in mice, while administration of nonopsonized UV-inactivated RSV did not. The increased RSV-specific immune response observed after RSV challenge in IC-RSV-primed mice was independent of FcRn. Therefore, FcRn-mediated transcytosis of these immune complexes across epithelium was not the route by which priming by IC-RSV was facilitated, and also a role in antigen processing of FcRn during in vivo priming can be ruled out. These observations were further substantiated in in vitro experiments using IC-RSV-pulsed DCs as antigen-presenting cells to stimulate RSV-specific T cells. Comparing WT BM-DCs with FcRn−/− BM-DCs (Fig. 1) and WT IgG with mutant IgG1 unable to bind FcRn (Fig. 2), we did not find a contribution of FcRn in antigen processing.
Previously, we found that neutralizing antibodies present in immune mice affected RSV-specific T-cell responses by increasing CD4+ and decreasing CD8+ T-cell responses in vivo after an i.n. live-RSV challenge (14). These findings suggested that virus-specific CD8+ T cells are most efficiently primed upon live-virus exposure, presumably via the classical cytoplasmic route of antigen presentation and in the context of an innate immune response triggered by the virus. Depending on the titer and affinity of serum neutralizing antibodies, the efficacies of virus neutralization in the airways may differ. In previous work, we studied the secondary T- and B-cell responses in mice preexposed to RSV (14). In those studies, both B-cell and T-cell responses were primed. The situation is different in infants before primary RSV exposure when only antiviral antibodies are present without primed T cells (i.e., maternal IgG obtained via the placenta and/or prophylactically administered antibody in children with a high risk of developing severe RSV disease). It has been shown in both laboratory animals and humans, that systemic antibodies do not completely prevent early viral replication steps and innate immune responses (34, 35, 40). This may be explained by inadequate serum titers and/or insufficient access of antibodies to the airway lumen. Systemic antibodies need to access the airways from the serum through a process in which basolateral-to-apical transport across airway epithelium facilitated by FcRn might play a role (19). Furthermore, the half-life of IgG antibodies in serum is affected by FcRn. Therefore, several mechanisms might contribute to altered immune priming when RSV neutralizing antibody is administered parenterally in FcRn−/− compared to WT mice. To circumvent the factors potentially affecting the efficacy of RSV neutralization and to exclude residual RSV (abortive) infection in the lung by systemically administered palivizumab the way in which earlier studies have been performed (41), we opted for the intranasal application of in vitro neutralized virus.
In the present study, we show that in comparison to an i.n. challenge with nonopsonized UV-inactivated virus particles, both CD4+ and CD8+ virus-specific T-cell responses were enhanced if the virus was opsonized with palivizumab. Thus, while live-virus infection might be the most effective way of priming CD8+ T-cell responses in this mouse model due to viral replication, we demonstrate that preexisting neutralizing antibodies do not block initiation of virus-specific CD8+ T responses completely. It can be envisioned that the inflammatory environment during T-cell priming is different when live RSV or immune complexes are present in the airways. This difference in innate immune response might affect the quality of the memory response and the functionality of effector cells recalled during subsequent infections. Low numbers of CD4+ T cells producing Th2 cytokines were detected in RSV-palivizumab-primed mice after a challenge with live virus. The lack of infection of RSV-palivizumab complexes might alter early innate immune responses that affect the nature of the local adaptive immune response during challenge. However, these numbers of Th2 CD4+ T cells are 1 to 4% higher than we usually find after secondary RSV infections in C57BL/6 mouse lungs and much lower than after intramuscular vaccination with inactivated-RSV vaccines (30 to 40%) (14, 42).
Similar T-cell responses were observed after i.n. RSV challenge with or without prior intranasal priming with UV-RSV. Thus, intranasal exposure with noninfectious virus appears to be an ineffective route of T-cell priming and even may slightly decrease Th1 priming (Fig. 3). We used UV-inactivated virus in these i.n. challenge experiments, because we wanted to evaluate the role of antibodies in the in vivo antigen presentation of noninfectious virus (i.e., mimicking the situation when the virus is bound to serum-derived neutralizing antibodies). It could be argued that UV inactivation of RSV might damage the virus proteins or the viral RNA, decreasing the efficiency of antigen presentation or interfering with virus-induced innate immune responses. However, in in vitro antigen presentation experiments, we found that antigen presentation to virus-specific T cells was equally efficient when antibody-bound live RSV or antibody-bound UV-RSV was incubated with antigen-presenting cells (14). Furthermore, the pattern and intensity of cytokines produced by antigen-presenting cells (monocytes, myeloid DCs [mDCs], and plasmacytoid DCs [pDCs]) were similar when these cells were exposed to live or UV-inactivated opsonized virus particles (data not shown).
FcRn-mediated transport across the airway epithelium has been reported by others to be an effective way to introduce Ig-antigen fusion proteins into the systemic circulation (36) and a route of immune priming (43). Herpes simplex virus 2 (HSV-2)-specific T- and B-cell responses were primed upon i.n. administration of an IgG Fc-gD fusion protein in an FcRn-dependent way, resulting in protection against a lethal dose of an intravaginal challenge with HSV-2 (43). These observations might be explained by FcRn-mediated transcytosis of gD-Fc across respiratory epithelium. The findings with respect to the role of FcRn in immune priming with gD-Fc fusion proteins and reports showing a role of FcRn in antigen processing in DC of ovalbumin immune complex (OVA-IC) contrast with our observations (15, 44, 45).
The observed difference between our findings and those published with OVA and Fc-gD might be explained by the soluble nature of the antigens; an Fc fusion protein or ovalbumin versus RSV viral particles in our study, or perhaps due to inherent yet unknown properties of RSV itself. However, a different explanation might be that there was a difference in the antigen-presenting cell populations involved in the processes described in the different studies. A recent publication showed that FcRn exclusively facilitated processing of multimeric Ig-complexed OVA in macrophages and not in DCs (46). Apparently, the phagosomes in DCs did not reach the low pH required for IgG binding to FcRn, while in macrophages the pH rapidly dropped to <6.5, allowing FcRn binding. Monovalent internalization of IgG-OVA was, however, FcRn dependent in both DCs and macrophages. Thus, FcRn-independent IC-RSV antigen presentation might be simply due to different mechanisms of uptake and presentation (phagocytosis versus endocytosis), or as described by Liu et al. (46) due to the neutral pH in the phagosomes of some types of DCs. In our studies, it might be possible that lung DCs efficiently captured IC-RSV via FcγR but did not (efficiently) process them and only transported captured viral material to the draining lymph nodes. Here antigen presentation could occur via cross-priming, possibly mediated by lymph node resident DCs (47). Dendritic cells located underneath the epithelial barrier in lung tissue contribute to the initiation of adaptive immune responses specific for RSV. DCs internalize antigen via a specific phagocytosis or through FcγRs when the antigen is opsonized by IgGs. Here we confirm previous work, in a secondary RSV infection model in mice, that antigen presentation and subsequent RSV-specific T-cell activation were mediated via FcγRs in the presence of acquired antibodies (14). Therefore, it might be hypothesized that IC-RSV is sampled through FcγRs that are present on CD103+ DCs in the airways. Moreover, antigen presentation in lymph nodes might depend on DCs that can more readily migrate to the lymph nodes compared to macrophages.
In conclusion, our study suggests that prophylactic RSV antibodies that suppress viral infection and the classical major histocompatibility complex (MHC) class I route of antigen presentation in the lung can boost the induction of adaptive CD4+ T-cell responses through FcγR, while a significant virus-specific CD8+ T-cell response is elicited, presumably via cross-priming.
ACKNOWLEDGMENTS
D. Kruijsen was financially supported by grant WK016 from the Wilhelmina Research Fund, H. Einarsdottir by grant 0721 from the Landsteiner Foundation for Blood Transfusion Research, and M. Schijf by the Dutch Top Institute Pharma Project (D1-101-0). We thank Derry Roopenian for the kind contribution of the FcRn−/− mice.
The authors of this article declare they have no financial or commercial conflicts of interest.
Footnotes
Published ahead of print 1 May 2013
REFERENCES
- 1. Hamano Y, Arase H, Saisho H, Saito T. 2000. Immune complex and Fc receptor-mediated augmentation of antigen presentation for in vivo Th cell responses. J. Immunol. 164:6113–6119 [DOI] [PubMed] [Google Scholar]
- 2. Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. 1999. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, van Schip JJ, Sedlik C, Melief CJ, Verbeek JS, Ossendorp F. 2002. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J. Immunol. 168:2240–2246 [DOI] [PubMed] [Google Scholar]
- 4. Ponnuraj EM, Springer J, Hayward AR, Wilson H, Simoes EA. 2003. Antibody-dependent enhancement, a possible mechanism in augmented pulmonary disease of respiratory syncytial virus in the Bonnet monkey model. J. Infect. Dis. 187:1257–1263 [DOI] [PubMed] [Google Scholar]
- 5. Polack FP, Teng MN, Collins PL, Prince GA, Exner M, Regele H, Lirman DD, Rabold R, Hoffman SJ, Karp CL, Kleeberger SR, Wills-Karp M, Karron RA. 2002. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 196:859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gimenez HB, Chisholm S, Dornan J, Cash P. 1996. Neutralizing and enhancing activities of human respiratory syncytial virus-specific antibodies. Clin. Diagn. Lab. Immunol. 3:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ubol S, Halstead SB. 2010. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin. Vaccine Immunol. 17:1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brandenburg AH, Groen J, van Steensel-Moll HA, Claas EC, Rothbarth PH, Neijens HJ, Osterhaus AD. 1997. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J. Med. Virol. 52:97–104 [DOI] [PubMed] [Google Scholar]
- 9. Hall CB, Walsh EE, Long CE, Schnabel KC. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163:693–698 [DOI] [PubMed] [Google Scholar]
- 10. Glezen WP, Taber LH, Frank AL, Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140:543–546 [DOI] [PubMed] [Google Scholar]
- 11. Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. 2003. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 21:3479–3482 [DOI] [PubMed] [Google Scholar]
- 12. IMpact Study Group-RSV 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531–537 [PubMed] [Google Scholar]
- 13. Carbonell-Estrany X, Simões EA, Dagan R, Hall CB, Harris B, Hultquist M, Connor EM, Losonsky GA, Motavizumab Study Group 2010. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics 125:e35–e51 [DOI] [PubMed] [Google Scholar]
- 14. Kruijsen D, Bakkers MJ, van Uden NO, Viveen MC, van der Sluis TC, Kimpen JL, Leusen JH, Coenjaerts FE, van Bleek GM. 2010. Serum antibodies critically affect virus-specific CD4+/CD8+ T cell balance during respiratory syncytial virus infections. J. Immunol. 185:6489–6498 [DOI] [PubMed] [Google Scholar]
- 15. Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. 2008. Dependence of antibody-mediated presentation of antigen on FcRn. Proc. Natl. Acad. Sci. U. S. A. 105:9337–9342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, de Haas M, van de Winkel JG. 2006. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood 108:3573–3579 [DOI] [PubMed] [Google Scholar]
- 17. Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, Zhao Y, Kleijer M, Sandlie I, de Haas M, Jonsdottir I, van der Schoot E, Vidarsson G. 2011. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun. 2:599. 10.1038/ncomms1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. 2003. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 197:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. 2004. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20:769–783 [DOI] [PubMed] [Google Scholar]
- 20. Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76:519–529 [DOI] [PubMed] [Google Scholar]
- 21. Foti M, Granucci F, Aggujaro D, Liboi E, Luini W, Minardi S, Mantovani A, Sozzani S, Ricciardi-Castagnoli P. 1999. Upon dendritic cell (DC) activation chemokines and chemokine receptor expression are rapidly regulated for recruitment and maintenance of DC at the inflammatory site. Int. Immunol. 11:979–986 [DOI] [PubMed] [Google Scholar]
- 22. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hale AH. 1980. Molecular requirements for trinitrophenyl recognition by antihapten cytotoxic T lymphocytes. Cell. Immunol. 49:408–414 [DOI] [PubMed] [Google Scholar]
- 24. Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, van Lent PL, Hofhuis FM, van Ojik HH, Sedlik C, da Silveira SA, Gerber J, de Jong YF, Roozendaal R, Aarden LA, van den Berg WB, Saito T, Mosser D, Amigorena S, Izui S, van Ommen GJ, van Vugt M, van de Winkel JG, Verbeek SJ. 2002. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity 16:391–402 [DOI] [PubMed] [Google Scholar]
- 25. Vidarsson G, van de Winkel JG, van Dijk MA. 2001. Multiplex screening for functionally rearranged immunoglobulin variable regions reveals expression of hybridoma-specific aberrant V-genes. J. Immunol. Methods 249:245–252 [DOI] [PubMed] [Google Scholar]
- 26. Kimura N, Toyonaga B, Yoshikai Y, Du RP, Mak TW. 1987. Sequences and repertoire of the human T cell receptor alpha and beta chain variable region genes in thymocytes. Eur. J. Immunol. 17:375–383 [DOI] [PubMed] [Google Scholar]
- 27. Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, Palombella VJ, Stattel JM, Lu Y, Tan CA, Song JJ, Garcia AM, Simister NE, Spiekermann GM, Lencer WI, Blumberg RS. 2004. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc. Natl. Acad. Sci. U. S. A. 101:9763–9768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lukens MV, Claassen EA, de Graaff PM, van Dijk ME, Hoogerhout P, Toebes M, Schumacher TN, van der Most RG, Kimpen JL, van Bleek GM. 2006. Characterization of the CD8+ T cell responses directed against respiratory syncytial virus during primary and secondary infection in C57BL/6 mice. Virology 352:157–168 [DOI] [PubMed] [Google Scholar]
- 29. Kim JK, Firan M, Radu CG, Kim CH, Ghetie V, Ward ES. 1999. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur. J. Immunol. 29:2819–2825 [DOI] [PubMed] [Google Scholar]
- 30. Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES. 1997. Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J. Immunol. 158:2211–2217 [PubMed] [Google Scholar]
- 31. Houben ML, Coenjaerts FE, Rossen JW, Belderbos ME, Hofland RW, Kimpen JL, Bont L. 2010. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J. Med. Virol. 82:1266–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te Meerman GJ, ter Elst A. 2007. Evidence based selection of housekeeping genes. PLoS One 2:e898. 10.1371/journal.pone.0000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin RM, Brady JL, Lew AM. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212:187–192 [DOI] [PubMed] [Google Scholar]
- 34. Boukhvalova MS, Prince GA, Blanco JC. 2007. Respiratory syncytial virus infects and abortively replicates in the lungs in spite of pre-existing immunity. J. Virol. 81:9443–9450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pletneva LM, Haller O, Porter DD, Prince GA, Blanco JC. 2008. Induction of type I interferons and interferon-inducible Mx genes during respiratory syncytial virus infection and reinfection in cotton rats. J. Gen. Virol. 89:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. 2002. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 196:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. 1997. Development of a humanized monoclonal antibody (MED-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 176:1215–1224 [DOI] [PubMed] [Google Scholar]
- 38. Ober RJ, Radu CG, Ghetie V, Ward ES. 2001. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int. Immunol. 13:1551–1559 [DOI] [PubMed] [Google Scholar]
- 39. Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. 2002. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J. Immunol. 169:5171–5180 [DOI] [PubMed] [Google Scholar]
- 40. Falsey AR, Formica MA, Walsh EE. 2002. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J. Clin. Microbiol. 40:817–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siber GR, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, Thompson CM, Walsh EE, Piedra PA, Hemming VG, Prince GA. 1994. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J. Infect. Dis. 169:1368–1373 [DOI] [PubMed] [Google Scholar]
- 42. Kruijsen D, Schijf MA, Lukens MV, van Uden NO, Kimpen JL, Coenjaerts FE, van Bleek GM. 2011. Local innate and adaptive immune responses regulate inflammatory cell influx into the lungs after vaccination with formalin inactivated RSV. Vaccine 29:2730–2741 [DOI] [PubMed] [Google Scholar]
- 43. Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. 2011. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat. Biotechnol. 29:158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, Chen Z, de Haar C, Lencer WI, Fiebiger E, Blumber RS. 2011. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 108:9927–9932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mi W, Wanjie S, Lo ST, Gan Z, Pickl-Herk B, Ober RJ, Ward ES. 2008. Targeting the neonatal Fc receptor for antigen delivery using engineered Fc fragments. J. Immunol. 181:7550–7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu X, Lu L, Yang Z, Palaniyandi S, Zeng R, Gao LY, Mosser DM, Roopenian DC, Zhu X. 2011. The neonatal FcR-mediated presentation of immune-complexed antigen is associated with endosomal and phagosomal pH and antigen stability in macrophages and dendritic cells. J. Immunol. 186:4674–4686 [DOI] [PubMed] [Google Scholar]
- 47. Lukens MV, Kruijsen D, Coenjaerts FE, Kimpen JL, van Bleek GM. 2009. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J. Virol. 83:7235–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]