Abstract
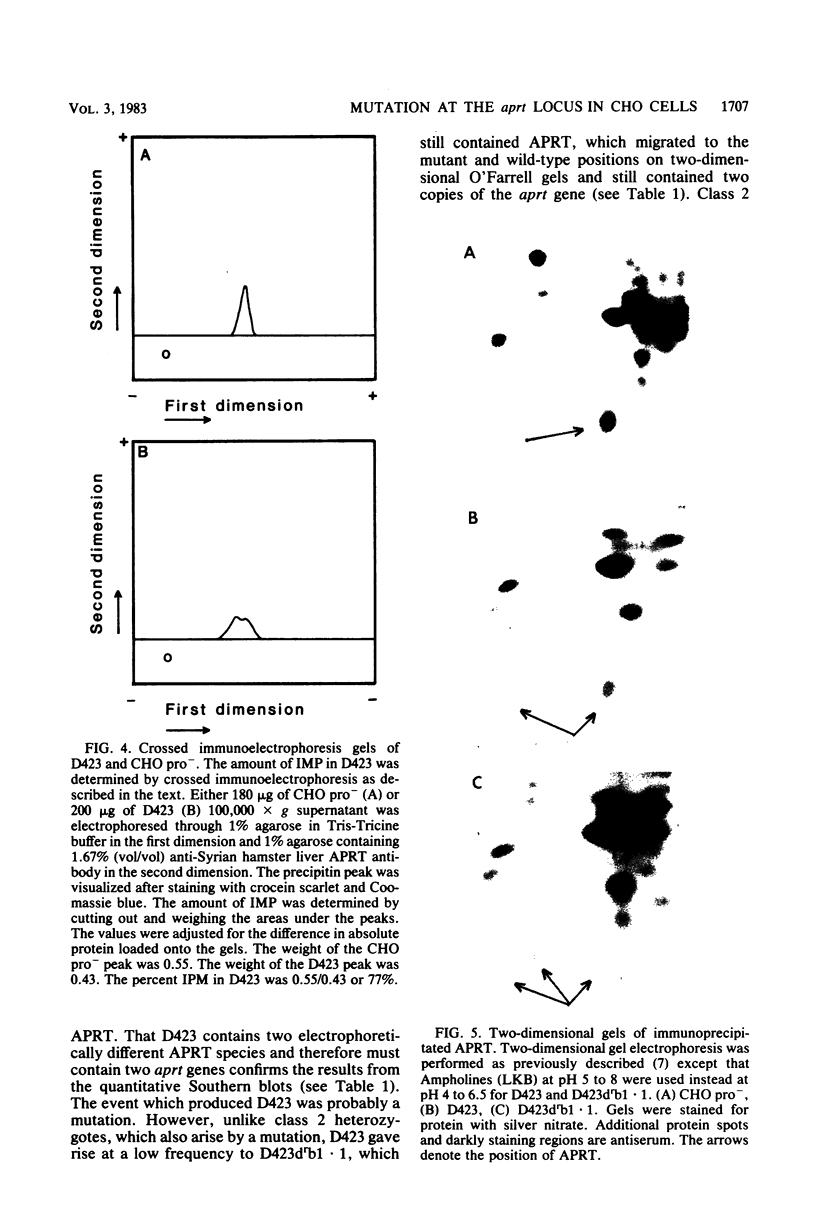
A two-step model to explain the high frequency of mutation at the diploid adenine phosphoribosyltransferase (aprt) locus in CHO cells has been proposed previously (Simon et al., Mol. Cell. Biol. 2:1126-1133, 1982). This model indicates that two distinct classes of aprt heterozygotes can be isolated. Class 1 heterozygotes, the most abundant class, were defined as those which arose spontaneously and were capable of undergoing mutation to the APRT- phenotype only at a low frequency (putative point mutation). Class 2 heterozygotes arose from a mutation and gave rise at a high frequency to APRT- cells. This high-frequency event has been identified as a deletion of the wild-type allele (A. E. Simon and M. W. Taylor, Proc. Natl. Acad. Sci. U.S.A. 80:810-814, 1983). In this paper we report further analysis of class 1 heterozygotes with respect to genetic structure, gene products, and karyotype. Our study indicated that class 1 heterozygotes contain two different types of mutants. About half have only one copy of the aprt gene and an unaltered karyotype, indicating that a deletion (similar to the high-frequency second-step event observed for class 2 heterozygotes) rather than a loss of the chromosome was responsible for the generation of the aprt+/- genotype. The remainder of the previously designated class 1 heterozygotes still contained two copies of the aprt gene (within the limits of the quantitation technique used) and arose presumably by a point mutation. One of this group, D423, was characterized with respect to aprt gene products and found to produce an electrophoretic variant in addition to the wild-type protein. APRT- mutants derived from D423 retained the same number of aprt gene copies as D423 and still synthesized a protein that comigrated with wild type, unlike APRT- mutants derived from class 2 heterozygotes. D423 and the other heterozygotes with two aprt genes therefore did not fit into either class 1 or 2 and are now designated class 3. The model we present suggests that only one of the two aprt alleles present in wild-type cells can undergo the deletion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley W. E., Letovanec D. High-frequency nonrandom mutational event at the adenine phosphoribosyltransferase (aprt) locus of sib-selected CHO variants heterozygous for aprt. Somatic Cell Genet. 1982 Jan;8(1):51–66. doi: 10.1007/BF01538650. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Green M. M. Genetic instability in Drosophila melanogaster: deletion induction by insertion sequences. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5367–5369. doi: 10.1073/pnas.79.17.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Meuth M., Arrand J. E. Alterations of gene structure in ethyl methane sulfonate-induced mutants of mammalian cells. Mol Cell Biol. 1982 Nov;2(11):1459–1462. doi: 10.1128/mcb.2.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W., Bradley W. E., Thompson L. H. Model involving gene inactivation in the generation of autosomal recessive mutants in mammalian cells in culture. Mol Cell Biol. 1982 Sep;2(9):1126–1133. doi: 10.1128/mcb.2.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Duff C. Karyotyping. Methods Enzymol. 1979;58:322–344. doi: 10.1016/s0076-6879(79)58148-8. [DOI] [PubMed] [Google Scholar]