Abstract
Herpes simplex virus 1 (HSV-1) protein ICP27 enables viral mRNA export by accessing the cellular mRNA export receptor TAP/NXF, which guides mRNA through the nuclear pore complex. ICP27 binds viral mRNAs and interacts with TAP/NXF, providing a link to the cellular mRNA export pathway. ICP27 also interacts with the mRNA export adaptor protein Aly/REF, which binds cellular mRNAs and also interacts with TAP/NXF. Studies using small interfering RNA (siRNA) knockdown indicated that Aly/REF is not required for cellular mRNA export, and similar knockdown studies during HSV-1 infection led us to conclude that Aly/REF may be dispensable for viral RNA export. Recently, the structural basis of the interaction of ICP27 with Aly/REF was elucidated at atomic resolution, and it was shown that three ICP27 residues, W105, R107, and L108, interface with the RNA recognition motif (RRM) domain of Aly/REF. Here, to determine the role the interaction of ICP27 and Aly/REF plays during infection, these residues were mutated to alanine, and a recombinant virus, WRL-A, was constructed. Virus production was reduced about 10-fold during WRL-A infection, and export of ICP27 protein and most viral mRNAs was less efficient. We conclude that interaction of ICP27 with Aly/REF contributes to efficient viral mRNA export.
INTRODUCTION
The export of mRNA from the nucleus to the cytoplasm in mammalian cells is a complex and well-orchestrated process that has been linked to pre-mRNA splicing (1–6). A multiprotein complex termed the TREX complex associates with the 5′ ends of mRNAs during splicing (3), and TREX complex components then bridge mRNA to the mRNA export receptor TAP/NXF (3, 7). The TREX complex associates at the 5′ ends of mRNAs through an interaction of Aly/REF (also called REF), a component of the TREX complex, with the cap-binding protein CBP80 (8). Aly/REF is an mRNA export adaptor protein that binds RNA and interacts with TAP/NXF (9–11). Aly/REF transfers bound mRNA to TAP/NXF in a series of steps (12). Specifically, Aly/REF bound to RNA interacts with TAP/NXF and forms a ternary complex, which increases the weak RNA binding affinity of TAP/NXF (12). Aly/REF interacts with TAP/NXF through a short arginine-rich region, which is also its RNA binding domain (9). Aly/REF becomes methylated on arginines within its RNA binding domain, which reduces its RNA binding affinity, thus displacing bound RNA from Aly/REF to TAP/NXF (13). TAP/NXF then escorts the mRNA cargo through the nuclear pore complex by interacting through its C terminus with nucleoporins that line the nuclear pore (14–16).
Herpes simplex virus 1 (HSV-1) mRNAs are predominantly intronless, yet they are exported to the cytoplasm through the TAP/NXF pathway (17–20). The HSV-1 immediate early protein ICP27 has been shown to act as an mRNA export adaptor protein that provides viral mRNAs access to the TAP/NXF pathway (17–19, 21). ICP27 binds viral RNA (22–24), interacts with Aly/REF (17, 18, 21), and interacts with TAP/NXF (18, 19, 25). Further, ICP27 homologues in Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) have also been found to interact with Aly/REF (26, 27), leading to a model in which ICP27 and its homologues promote intronless viral mRNA export by recruiting the TREX complex to viral RNA through an interaction with Aly/REF (28). However, studies using small interfering RNA (siRNA) knockdown have suggested that Aly/REF is not required for cellular mRNA export (29, 30). Moreover, we showed that reducing Aly/REF levels through siRNA knockdown had little effect on HSV-1 mRNA export, leading us to suggest that interaction of ICP27 with Aly/REF may be dispensable for viral mRNA export (20). Other proteins in the TREX complex have been found to interact with ICP27 homologues, including TREX protein UAP56, which was shown to interact with human cytomegalovirus (HCMV) pUL69 (31), and UIF, which interacts with KSHV ORF57 (32), indicating that there may be mRNA adaptor redundancy in mRNA export.
Aly/REF was first discovered as an interacting partner of ICP27 using yeast two-hybrid assays (17, 21), and the interaction was confirmed to occur in virus-infected cells by coimmunoprecipitation and colocalization experiments (17, 18). The region of ICP27 involved in the interaction with Aly/REF was mapped by in vitro binding studies to include amino acids 104 to 138 (17, 21). However, the region of Aly/REF involved in binding ICP27 and the manner in which ICP27 bound Aly/REF were not elucidated until the solution structure of a short peptide of ICP27 bound to Aly/REF was resolved at the atomic level using nuclear magnetic resonance (NMR) spectroscopy (33). It was found that the Aly/REF recognition region of ICP27 is very short consisting of residues 104 to 112, which binds in the cleft formed by two α-helices on the surface of the Aly/REF RNA recognition motif (RRM) domain (9, 33). Three ICP27 residues, W105, R107, and L108, appeared to be the most important for the interaction of ICP27 with Aly/REF forming a recognition triad, in which W105 contacted F98 of Aly/REF, R107 formed salt bridges with the acidic residues of Aly/REF α-helix 1, and L108 was positioned in a hydrophobic pocket composed of Aly/REF residues V86, L94, Y135, V138, L140, and M145. The ICP27 binding site showed a highly specific recognition with a very short sequence, and further, mutation of W105, R107, and L108 to alanine resulted in reduced binding of ICP27 to Aly/REF (33). Here we sought to determine the functional importance of ICP27 interaction with Aly/REF for viral mRNA export during HSV-1 infection. While we previously probed the role of Aly/REF by siRNA knockdown (20), this approach does not completely eliminate Aly/REF, and we may have missed more subtle effects on viral mRNA export due to residual levels of Aly/REF and possible redundancy in mRNA adaptor function, as well as compensatory cellular regulation mechanisms. To address this, in this study, we mutated the three ICP27 residues that directly contact Aly/REF and monitored the ability of ICP27 to interact with Aly/REF during infection as well as the effect on the export of viral transcripts. Mutation of ICP27 residues W105, R107, and L108 to alanine precluded ICP27-Aly/REF interaction during infection, and there was a measurable effect on the export efficiency of many HSV-1 transcripts.
MATERIALS AND METHODS
Cells, viruses, and recombinant plasmids.
HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Thermo Scientific) supplemented with 10% newborn calf serum (Life Technologies). Vero cells were grown on DMEM supplemented with 8% fetal bovine serum and 4% donor calf serum (Life Technologies). HSV-1 strain KOS and ICP27 null mutant virus 27-LacZ were previously described (34). HSV-1 mutants d2-3 and ΔRGG (d4-5) were generously provided by Steve Rice (35). Plasmid pGFP-Aly/REF was described previously (17, 18). ICP27 alanine substitution mutations in plasmid pD6-21-A (D6, D10, D14, D17, D19, and D21 were all changed to A) were made by site-specific mutagenesis using the QuikChange site-directed mutagenesis kit (Agilent Technologies). The D6-21-A mutant was constructed as follows: D6, D10, and D14 were changed to A using forward primer 5′-ACTGACATTGCGATGCTAATTGCGCTCGGCCTGGCGCTCTCCGAC-3′ and reverse primer 5′-GTCGGAGAGCGCCAGGCCGAGCGCAATTAGCATCGCAATGTCAGT-3′; for D17 to A, forward primer 5′-CTCGGCCTGGCGCTCTCCGCCAGCGATCTGGACGAGGAC-3′ and reverse primer 5′-GTCCTCGTCCAGATCGCTGGCGGAGAGCGCCAGGCCGAG-3′ were used; for D19 to A, forward primer 5′-CTGGCGCTCTCCGCCAGCGCTCTGGACGAGGACCCCCCC-3′ and reverse primer 5′-GGGGGGGTCCTCGTCCAGAGCGCTGGCGGAGAGCGCCAG were used; and for D21 to A, forward primer 5′-CTCTCCGCCAGCGCTCTGGCCGAGGACCCCCCCGAGCCG-3′ and reverse primer 5′-CGGCTCGGGGGGGTCCTCGGCCAGAGCGCTGGCGGA GAG-3′ were used. The ICP27 substitution mutations W105, R107, and L108 to alanine (WRL-A) were made by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Agilent Technologies). The WRL-A mutant was constructed by using forward primer 5′-ACAGTGTGGCCTCGGCTGCAGGGGCCCGGCGA-3′ and reverse primer 5′-TCGCCGGGCCCCTGCAGCCGAGGCCACACTGTG-3′. All mutations were verified by sequencing. The WRL-A mutation was introduced into the HSV-1 genome by marker transfer. Mutant WRL-A was plaque purified 4 times, and the ICP27 sequence in viral recombinant WRL-A was verified by sequencing the ICP27 gene.
Transfection and virus infection.
Cells were infected with HSV-1 KOS or mutant virus as indicated in the figure legends at a multiplicity of infection (MOI) of 10. Transfection of plasmid DNA was performed by using Lipofectamine 2000 reagent (Life Technologies) according to the manufacturer's protocol. In infections with ICP27 mutant 27-LacZ, cells were infected 24 h after transfection.
Immunofluorescence staining.
Cells were grown on coverslips and transfected and/or infected as indicated in the figure legends. Four or 8 h after infection as indicated, cells were fixed with 3.7% formaldehyde, and immunofluorescence staining was performed with anti-ICP27 antibody (P1119; Virusys). Green fluorescent protein (GFP)-Aly/REF fusion protein was visualized directly. Cells were viewed by fluorescence microscopy at a magnification of ×100 with a Zeiss Axiovert S100 microscope.
Immunoprecipitation and Western blot analysis.
Cells were lysed 8 h after infection in low-salt lysis buffer (10 mM Tris [pH 7.4], 3 mM CaCl2, 2 mM MgCl2, 0.5% NP-40, and protease inhibitor cocktail [Roche]). The cell lysate was passed through a syringe with a 25-gauge needle 10 times. The nuclei were pelleted by centrifugation at 14,000 × g for 30 s. The supernatant was transferred to a new tube and represented the cytoplasmic fraction. The nuclear pellet was resuspended in high-salt extraction buffer consisting of phosphate-buffered saline (PBS) containing 250 mM NaCl, 0.5% NP-40, and protease inhibitor cocktail. Immunoprecipitations were performed with anti-ICP27 monoclonal antibody P1119 using Dynabeads protein G magnetic beads (Life Technologies) according to the manufacturer's protocol. Protein samples were fractionated on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The blots were probed with anti-ICP27 antibody or anti-Aly/REF antibody (11G5; Abcam) and analyzed by SuperSignal chemiluminescent substrate (Thermo Scientific).
In situ hybridization.
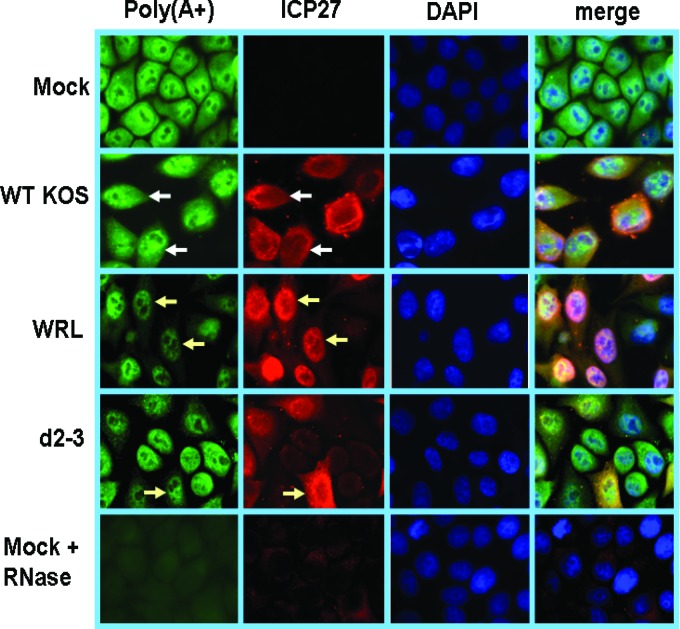
HeLa cells grown on coverslips in 24-well dishes were fixed in 3.7% formaldehyde 8 h after infection and then overlaid with 70% ethanol and stored at 4°C. For poly(A)+ RNA hybridizations, cells were rehydrated for 5 min at room temperature in 15% dimethylformamide in SSC (1× SSC is 150 mM NaCl and 15 mM sodium citrate [pH 7.0]) treated two times with diethyl pyrocarbonate (DEPC). Cells were overlaid with 75 μl hybridization solution [10% dextran sulfate, 0.5 μg/μl yeast tRNA, 0.2 μg/μl bovine serum albumin, 1.25 μM biotinylated oligo(dT)25, 1.6 U/μl RNAsin, 10 mM dithiothreitol, in SSC treated two times with DEPC] and incubated at 37°C for 2 h. Cells were washed twice for 30 min at 37°C in wash solution (15% formamide and 0.1% NP-40 in 2× SSC) and then immunostained with streptavidin-fluorescein isothiocyanate (FITC) and anti-ICP27 antibody. In the bottom row in Fig. 5, cells were treated with 6 μg/μl RNase A for 30 min at 37°C prior to hybridization as a negative control.
Fig 5.
Poly(A)+ RNA accumulates in the nucleus in cells infected with WRL-A. HeLa cells were either mock infected or infected with WT KOS, WRL-A, or d2-3 as indicated. Eight hours after infection, cells were fixed, and in situ hybridization was performed by using a biotinylated oligo(dT) probe to detect poly(A)+ RNA. ICP27 was detected by staining with anti-ICP27 antibody. In the bottom row, mock-infected cells were treated with RNase.
Microarray analysis.
HeLa cells were infected with HSV-1 KOS, WRL-A, d2-3, or ΔRGG. Eight hours after infection, cells were harvested, and nuclei were fractionated from the cytoplasm as described above. Total RNA was isolated with TRIzol (Life Technologies) from the nuclear and cytoplasmic fractions. RNA was extracted with chloroform and precipitated in ethanol. Poly(A)+ RNA was selected using a MicroPoly(A) Purist kit (Ambion) according to the manufacturer's instructions. The cDNA was reverse transcribed by SuperScript II reverse transcriptase (Life Technologies), and subsequent hybridization to HSV-1 transcript-specific chips was performed as previously described (19, 36).
RESULTS
The ICP27 substitution mutant WRL-A does not interact with Aly/REF.
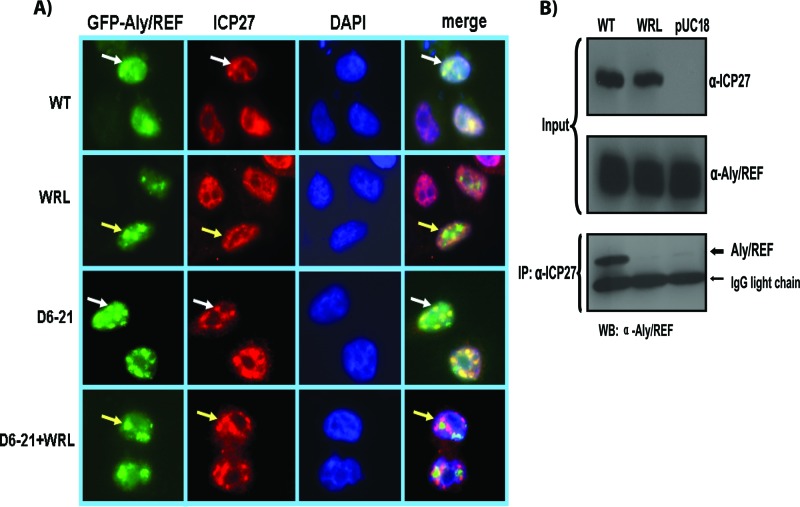
First, to determine how mutation of the ICP27 triad residues W105, R107, and L108, which were shown to contact the Aly/REF RRM domain in NMR studies (33), would affect ICP27 interaction with Aly/REF in vivo, site-specific mutagenesis was performed to mutate residues W105, R107, and L108 to alanine in full-length ICP27. For a control, substitution mutations were constructed in the N-terminal portion of ICP27, which interacts with TAP/NXF, but is not involved in the interaction with Aly/REF (37). Specifically, residues D6, D10, D14, D17, D19, and D21 were changed to alanine, and the resulting mutant was called D6-21-A. A double mutant was also constructed consisting of D6-21-A and WRL-A (D6-21-A+WRL-A). Cells were transfected with GFP-tagged Aly/REF and plasmids expressing wild-type (WT) ICP27 and mutant WRL-A, D6-21-A, or D6-21-A+WRL-A. Twenty-four hours after transfection, cells were infected with ICP27 null mutant 27-LacZ (34). This was done because the wild-type and mutant ICP27 constructs were under the control of the native ICP27 promoter, and infection with 27-LacZ would result in stimulation of ICP27 expression by the action of VP16. Cells were fixed and stained 4 h later. While there were clear regions of colocalization of GFP-Aly/REF and WT ICP27 (white arrows), little colocalization was observed for mutant WRL-A (Fig. 1A). Colocalization was seen with mutant D6-21-A and GFP-Aly/REF, confirming that the N terminus of ICP27 is not required for this interaction; however, in the double mutant D6-12-A+WRL-A, colocalization was not observed (Fig. 1A). The patterns of colocalization for WT ICP27 and little to no colocalization for WRL-A mutants were seen in greater than 80% of cells observed in these experiments. Similar results were seen 8 h after infection (data not shown).
Fig 1.
ICP27 mutant WRL-A does not interact with Aly/REF. (A) HeLa cells were cotransfected with pEGFP-Aly/REF and wild-type (WT) ICP27 or plasmids expressing ICP27 mutant WRL-A, D6-21-A, or D6-21-A+WRL-A as indicated. Transfected cells were infected with ICP27 null mutant virus 27Lac-Z 24 h after transfection. Four hours after infection, cells were fixed, and immunofluorescence staining was performed with anti-ICP27 antibody. Green fluorescent protein (GFP) fluorescence was visualized directly. The white arrows show the colocalization of Aly/REF and ICP27. The yellow arrows point to Aly/REF sites that do not colocalize with ICP27 harboring the WRL mutations. (B) HeLa cells were transfected with WT ICP27 or mutant WRL-A or with pUC18 as a negative control. Twenty-four hours after transfection, cells were infected with 27-LacZ. Eight hours after infection, cells were harvested, and immunoprecipitation (IP) was performed on cell lysates with anti-ICP27 antibody (α-ICP27). Western blots (WB) were probed with anti-Aly/REF and anti-ICP27 antibodies.
To determine whether endogenous Aly/REF could be coimmunoprecipitated with ICP27, cells were transfected with plasmids expressing WT ICP27 or mutant WRL-A or with pUC18 as a negative control and were subsequently infected with 27-LacZ. Cell lysates prepared 8 h after infection were immunoprecipitated with a monoclonal antibody specific for ICP27 (Fig. 1B). The immunoblot was probed with antibody specific for Aly/REF, which was seen to coimmunoprecipitate with WT ICP27, but not with WRL-A or pUC18 (Fig. 1B). These results indicate that mutation of ICP27 residues W105, R107, and L108 in the context of full-length ICP27 eliminated the interaction with Aly/REF in vivo.
ICP27 viral mutant WRL-A exhibits a viral growth defect.
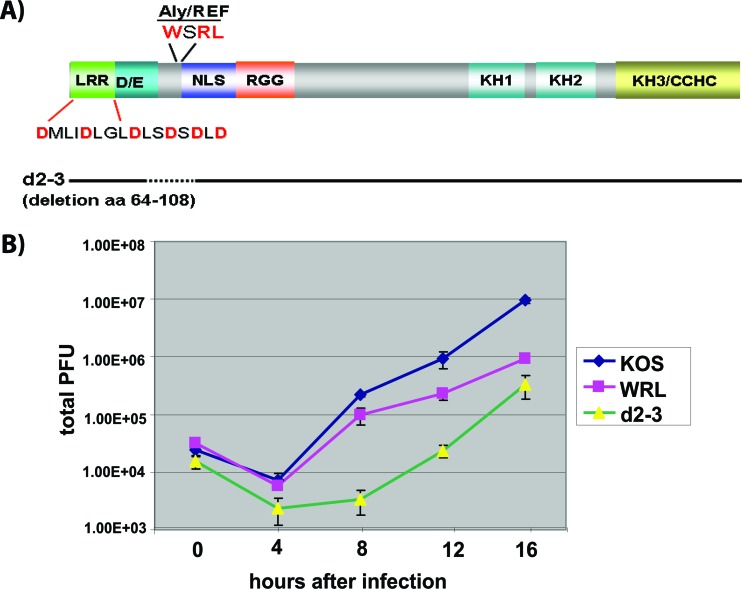
To determine the effect of the WRL-A mutation in the context of viral infection, we constructed a viral recombinant. A schematic showing the positions of the mutations is shown in Fig. 2A. One-step growth curves demonstrated that replication of viral mutant WRL-A was reduced compared with HSV-1 KOS, resulting in viral titers about 10-fold lower (Fig. 2B). Viral mutant d2-3 (35), which has a deletion of amino acids 64 to 108, encompassing the major part of the Aly/REF interaction site at positions 104 to 112, as well as flanking regions (Fig. 2A), was even more impaired than WRL-A (Fig. 2B). Because d2-3 has a relatively large deletion, it is possible that this ICP27 mutant may be conformationally altered, and thus, some other ICP27 interactions important for viral replication may also be affected.
Fig 2.
Virus production is reduced in ICP27 WRL-A mutant virus infection. (A) Schematic of the ICP27 coding sequence illustrating the leucine-rich region (LRR), acidic region (D/E), nuclear localization signal (NLS), RGG box RNA binding domain, predicted KH domains, and a zinc finger-like region (CCHC). The Aly/REF binding site is shown. The sites of the mutations in D6-21-A are also shown. HSV-1 mutant d2-3, which has a deletion of amino acids (aa) 64 to 108, is shown by the dotted line. (B) A one-step virus growth curve was performed by infecting Vero cells with wild-type (WT) HSV-1 KOS or ICP27 mutant WRL-A or d2-3 at an MOI of 1. Infected cells were harvested at 0, 4, 8, 12, and 16 h after infection. Virus titers were determined by plaque assays. The experiments were performed three times, and in each experiment, each time point was performed in triplicate. The data shown are from a representative experiment.
Aly/REF does not coimmunoprecipitate or colocalize with WRL-A during infection.
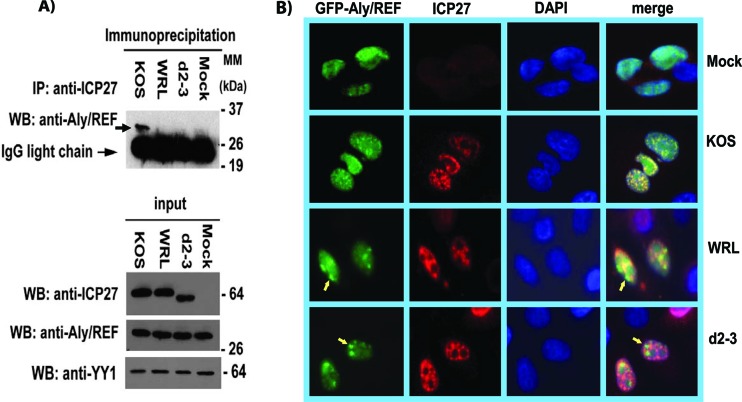
To analyze the interaction of Aly/REF with mutant WRL-A in the context of viral infection, cells were infected with HSV-1 KOS, mutant WRL-A, or d2-3 for 8 h. Cell lysates were immunoprecipitated with anti-ICP27 antibody, and the immunoblot was probed with anti-Aly/REF antibody to detect endogenous Aly/REF that was coprecipitated with ICP27 (Fig. 3A). While Aly/REF was clearly seen in the KOS sample, it was not detected in the WRL-A sample, showing that endogenous Aly/REF does not interact with ICP27 mutant WRL-A during infection, in agreement with the NMR results that indicated that residues W105, R107, and L108 were critical for ICP27-Aly/REF interaction (33). Aly/REF also was not found in the immunoprecipitation of the d2-3 sample as expected, since this mutant has a deletion that encompasses the main part of the Aly/REF recognition region.
Fig 3.
ICP27 mutant WRL-A does not interact with Aly/REF during infection. (A) HeLa cells were infected with WT HSV-1 KOS, WRL-A, or d2-3. Eight hours after infection, cells were harvested, and immunoprecipitation was performed with anti-ICP27 antibody. Western blots were probed with anti-Aly/REF and anti-ICP27 antibodies. Antibody to YY1 was used as the loading control. The positions of molecular mass markers (MM) (in kilodaltons) are indicated to the right of the gels. (B) HeLa cells were transfected with pEGFP-Aly/REF, and 24 h later, the cells were infected with WT KOS, WRL-A, or d2-3. Four hours after infection, cells were fixed, and immunofluorescence staining was performed with anti-ICP27 antibody. GFP fluorescence was visualized directly. The arrows point to Aly/REF sites that do not colocalize with WRL-A and d2-3.
As we reported previously, cellular Aly/REF colocalizes with ICP27 at early times during KOS infection (18), and this was also the case in cells transfected with GFP-Aly/REF, which were subsequently infected with HSV-1 KOS (Fig. 3B). In contrast, GFP-Aly/REF and ICP27 from WRL-A mutant were not seen to colocalize during infection. In addition, GFP-Aly/REF and ICP27 mutant d2-3 were not colocalized in infected cells, in agreement with what we reported previously (18). These results confirm that ICP27 residues W105, R107, and L108 are the major contacts for ICP27 binding to Aly/REF during HSV-1 infection.
ICP27 export to the cytoplasm is less efficient during infection with WRL-A.
ICP27 is predominantly located in the nucleus early after infection, but it begins shuttling to the cytoplasm at about 5 h after infection and is largely cytoplasmic by 8 h after infection (18, 24). ICP27 interaction with TAP/NXF is required for its export to the cytoplasm (17–19). ICP27 interacts with TAP/NXF directly, as we demonstrated by bimolecular fluorescence complementation, through both its N and C termini, when ICP27 is in a head-to-tail configuration (25, 38). Nonetheless, in looking at the localization of mutant ICP27 during WRL-A infection, it was noted that ICP27 appeared to be nuclear in a significant proportion of infected cells even at 8 h after infection. We quantified the proportion of cells in which ICP27 in WRL-A-infected cells was nuclear, cytoplasmic, or nuclear/cytoplasmic compared to KOS at 8 h after infection (Fig. 4). In WRL-A-infected cells, of a total of 141 cells scored, it was observed that ICP27 was nuclear in 47% of the cells and nuclear/cytoplasmic in 21% of the cells, with only 32% of the cells displaying a largely cytoplasmic fluorescence. In contrast, 66% of KOS-infected cells showed strong cytoplasmic fluorescence, and predominantly nuclear fluorescence was seen in only 26% of KOS-infected cells (Fig. 4). Similar results were seen 12 h after infection (data not shown). This suggests that while ICP27 directly interacts with TAP/NXF, Aly/REF may also serve to bridge the interaction with TAP/NXF, which may increase the efficiency of ICP27 export.
Fig 4.
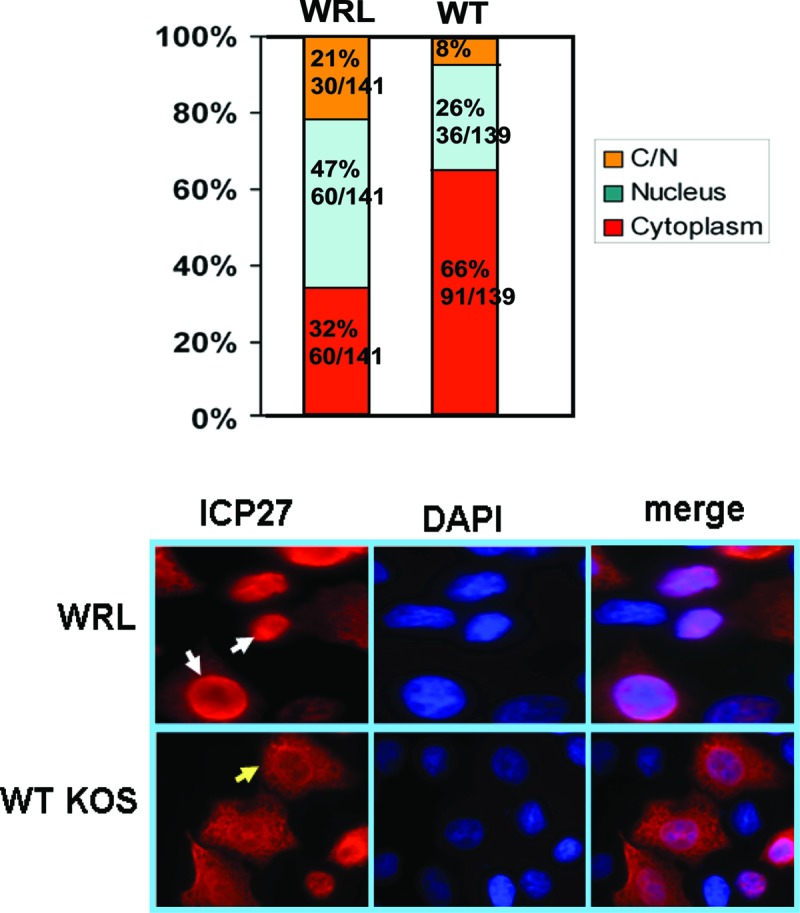
ICP27 is exported to the cytoplasm less efficiently in WRL-A-infected cells. HeLa cells were infected with WT KOS or ICP27 mutant WRL-A. Eight hours after infection, cells were fixed, and immunofluorescence staining was performed with anti-ICP27 antibody. (Top) Distribution of cells in which ICP27 was nuclear, cytoplasmic, or both cytoplasmic and nuclear (C/N). For WRL-A, 141 cells were scored, and for WT KOS, 139 cells were scored. (Bottom) Representative images of ICP27 cellular location. The two white arrows in WRL-infected cells show the nuclear localization, and the yellow arrow in KOS-infected cells shows the cytoplasmic localization.
Export of poly(A)+ RNA is curtailed during WRL-A infection.
To determine what effect the loss of the interaction between ICP27 and Aly/REF may have on mRNA export, in situ hybridization with an oligo(dT) probe to detect poly(A)+ RNA was performed on cells infected with KOS, WRL-A, and d2-3 at 8 h after infection, when mostly viral transcripts are produced. Strong cytoplasmic fluorescence was seen for poly(A)+ RNA in KOS-infected cells, and ICP27 was also seen to be largely cytoplasmic (Fig. 5). In WRL-A-infected cells, poly(A)+ RNA appeared to be mostly located in the nucleus with very weak cytoplasmic poly(A)+ staining, and the same was true for ICP27 staining (Fig. 5), and d2-3 behaved similarly to WRL-A. Therefore, the interaction of ICP27 with Aly/REF appears to be important for efficient export of poly(A)+ RNA during infection.
Viral transcripts are exported less efficiently during WRL-A infection.
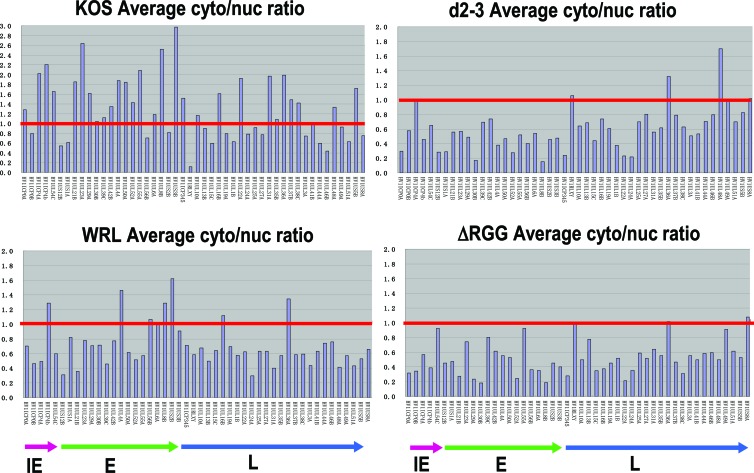
To analyze the export of HSV-1 transcripts during WRL-A infection compared to KOS infection, nuclear and cytoplasmic fractions of WRL-A- and KOS-infected cells were prepared 8 h after infection, and poly(A)+ RNA was selected and hybridized to microarrays with single-stranded DNA probes specific for 48 viral transcripts corresponding to immediate early, early, and late mRNAs, as described previously (19). Each probe was represented in triplicate on the microarrays, and the average cytoplasmic-to-nuclear distribution ratio was calculated for each experiment. The experiments were performed three times. The results of a representative experiment are shown in Fig. 6. The red line indicates a cytoplasmic/nuclear ratio of 1.0, and transcripts above the line were more abundant in the cytoplasm than in the nucleus, indicating efficient export. Transcripts with a ratio of 1.0 were exported less efficiently, and those below the line accumulated in the nucleus. Note that the scale for the cytoplasmic/nuclear ratio extends to 3.0 for WT KOS, but the scale extends to 2.0 for mutants WRL-A, d2-3, and ΔRGG. In WRL-A-infected cells, many viral transcripts were seen to be exported less efficiently than in KOS-infected cells (Fig. 6). Whereas 25 of the 48 transcripts represented had a cytoplasmic/nuclear ratio greater than 1.0 in KOS-infected cells, only 6 transcripts had a ratio greater than 1.0 in WRL-A-infected cells with an additional 2 transcripts with a ratio around 1.0, indicating that the interaction of ICP27 with Aly/REF does contribute to the efficient export of HSV-1 mRNA. The defect in viral RNA export was not as severe as that seen with d2-3, in which most viral transcripts accumulated in the nucleus (Fig. 6). This is likely due to the more drastic deletion of 44 amino acids in d2-3, which not only eliminates the ICP27 interaction site with Aly/REF but which may hinder its interaction with other partners as well by altering the conformational properties of the N-terminal portion of the protein. Viral RNA also accumulated in the nucleus in cells infected with ΔRGG, which deletes the RGG box RNA binding domain of ICP27, and which we have shown previously severely impairs viral mRNA export (19, 22, 23). These results indicate that the interaction of ICP27 with Aly/REF contributes to efficient export of HSV-1 mRNA.
Fig 6.
Viral mRNA export is less efficient for many HSV-1 transcripts in WRL-A infection. HeLa cells were infected with WT KOS or with WRL-A, d2-3, or ΔRGG. Eight hours after infection, cells were harvested, and nuclear and cytoplasmic viral poly(A)+ RNA was isolated and reverse transcribed. HSV-1 transcripts from each fraction were hybridized in triplicate to HSV-1-specific microarray chips. After quantification using Array Vision software, the average cytoplasmic/nuclear (cyto/nuc) ratio was calculated and plotted, with the y axis representing the ratio for each transcript and the x axis representing individual HSV-1 transcripts. The experiments were performed in triplicate, and the results of a representative experiment are shown. The red line shows a 1:1 ratio of cytoplasmic/nuclear poly(A)+ RNA. Note that the scale for the y axis for KOS is from 0 to 3.0, whereas the scale for the WRL-A, d2-3, and ΔRGG mutants is from 0 to 2.0. HSV-1 transcripts are shown as immediate early (IE), early (E), and late (L).
DISCUSSION
ICP27 is a multifunctional protein that plays a number of important roles during HSV-1 infection. One of its roles is to facilitate viral mRNA export, a task that ICP27 accomplishes by binding viral RNA (22–24, 39) and interacting with cellular mRNA export factors, including the TREX component Aly/REF (17, 21); SR proteins SRp20 and 9G8 (40), which function in the export of cellular intronless RNA (41, 42), and the mammalian mRNA export receptor TAP/NXF (18, 25). To determine the importance of each of its interactions to viral infection, studies with ICP27 mutants have been performed previously. Two problems have arisen with this approach. First, many of ICP27's interactions occur in the N-terminal 155 amino acids of the protein, so that ICP27 interaction regions with several partners overlap (37, 43), and thus, mutations may impair more than one interaction, making it difficult to ascribe the effect to a particular partner. Second, most ICP27 mutations that have been studied in the N-terminal 155 residues are deletions, which typically have removed more than 10 amino acids (35). Deletion of certain protein regions may cause structure perturbation, which may lead indirectly to loss of function of the whole domain or loss of more than one specific interaction. This appears to be the case with mutant d2-3, which has a deletion of 44 amino acids, and which had a more severe defect in mRNA export than the WRL-A mutant.
The amino-terminal 155 amino acids of ICP27 appear to be largely unstructured or intrinsically disordered (33, 44), making it difficult to predict the effect of extensive deletions on the ability of ICP27 to interact with specific partners. Here we made use of solution-state NMR studies that determined the structure of the Aly/REF-ICP27 interaction interface at atomic resolution and provided the precise binding interfaces between ICP27 and Aly/REF (33). Three residues that contact the Aly/REF RRM, namely, W105, R107, and L108, and that were found to contribute most to protein binding in vitro were changed here to alanine. A viral recombinant bearing these point mutations showed a viral growth defect, and both mutant ICP27 protein and many viral transcripts were less efficiently exported to the cytoplasm during infection with the WRL-A mutant. By performing targeted point mutation of the critical interface residues, it was possible to measure the consequences of precluding the interaction of ICP27 with Aly/REF without perturbing other interactions. As a result, it was demonstrated that the interaction of ICP27 and Aly/REF does play a role in the export of viral mRNA. In addition, Aly/REF interaction with ICP27 may also contribute to viral RNA accumulation in the cytoplasm by stabilizing some viral transcripts as has been demonstrated for Aly/REF interaction with ORF57 during KSHV infection (45–47). These results further illustrate that there is redundancy in RNA export factors because while export of many viral transcripts was less efficient compared to WT KOS infection, export was not abolished. This suggests that ICP27 interaction with other RNA export factors, such as SR proteins and perhaps other TREX components may also contribute to viral RNA export. In addition, because ICP27 binds viral RNA and interacts with TAP/NXF, it may itself function as the export adaptor for some viral transcripts. In sum, these studies show that combining structural information that defines interface “hot spot” residues can inform mutational analysis such that the importance of an individual ICP27 interaction can be parsed to determine its contribution to HSV-1 infection.
ACKNOWLEDGMENTS
We thank Richard B. Tunnicliffe for valuable discussions.
This work was supported by National Institute of Allergy and Infectious Diseases (NIAID) grant AI21215 to R.M.S.-G. X.T. was funded by a scholarship from the China Scholarship Council for part of these studies and was the recipient of a George E. Hewitt Foundation Postdoctoral Fellowship.
Footnotes
Published ahead of print 1 May 2013
REFERENCES
- 1. Luo MJ, Reed R. 1999. Splicing is required for rapid and efficient mRNA export in metazoans. Proc. Natl. Acad. Sci. U. S. A. 96:14937–14942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luo MJ, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, Reed R. 2001. Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature 413:644–647 [DOI] [PubMed] [Google Scholar]
- 3. Masuda S, Das R, Cheng H, Hurt E, Dorman N, Reed R. 2005. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev. 19:1512–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reed R. 2003. Coupling transcription, splicing and mRNA export. Curr. Opin. Cell Biol. 15:326–331 [DOI] [PubMed] [Google Scholar]
- 5. Reed R, Hurt E. 2002. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell 108:523–531 [DOI] [PubMed] [Google Scholar]
- 6. Zhou Z, Luo MJ, Straesser K, Katahira J, Hurt E, Reed R. 2000. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 407:401–405 [DOI] [PubMed] [Google Scholar]
- 7. Reed R, Cheng H. 2005. TREX, SR proteins and export of mRNA. Curr. Opin. Cell Biol. 17:269–273 [DOI] [PubMed] [Google Scholar]
- 8. Nojima T, Hirose T, Kimura H, Hagiwara M. 2007. The interaction between cap-binding complex and RNA export factor (REF) is required for intronless mRNA export. J. Biol. Chem. 282:15645–15651 [DOI] [PubMed] [Google Scholar]
- 9. Golovanov AP, Hautbergue GM, Tintaru AM, Lian L-Y, Wilson SA. 2006. The solution structure of REF2-1 reveals interdomain interactions and regions involved in binding mRNA export factors and RNA. RNA 12:1933–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodrigues JP, Rode M, Gatfield D, Blencowe BJ, Carmo-Fonseca M, Izaurralde E. 2001. REF proteins mediate the export of spliced and unspliced mRNAs from the nucleus. Proc. Natl. Acad. Sci. U. S. A. 98:1030–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E. 2000. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6:638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hautbergue GM, Hung M-L, Golovanov AP, Lian L-Y, Wilson SA. 2008. Mutually exclusive interactions drive handover of mRNA from export adaptors to TAP. Proc. Natl. Acad. Sci. U. S. A. 105:5154–5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hung M-L, Hautbergue GM, Snijders APL, Dickman MJ, Wilson SA. 2010. Arginine methylation of REF/Aly promotes efficient handover of mRNA to TAP/NXF1. Nucleic Acids Res. 38:3351–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bachi A, Braun IC, Rodrigues JP, Pante N, Ribbeck K, von Kobbe C, Kutay U, Wilm M, Gorlich D, Carmo-Fonseca M, Izaurralde E. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6:136–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fribourg S, Braun IC, Izaurralde E, Conti E. 2001. Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol. Cell 8:645–656 [DOI] [PubMed] [Google Scholar]
- 16. Grant RP, Hurt E, Neuhaus D, Stewart M. 2002. Structure of the C-terminal FG-nucleoporin binding domain of TAP/NXF1. Nat. Struct. Biol. 9:247–251 [DOI] [PubMed] [Google Scholar]
- 17. Chen IB, Sciabica KS, Sandri-Goldin RM. 2002. ICP27 interacts with the export factor Aly/REF to direct herpes simplex virus 1 intronless RNAs to the TAP export pathway. J. Virol. 76:12877–12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen IB, Li L, Silva L, Sandri-Goldin RM. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 79:3949–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson LA, Sandri-Goldin RM. 2009. Efficient nuclear export of herpes simplex virus 1 transcripts requires both RNA binding by ICP27 and ICP27 interaction with TAP/NXF1. J. Virol. 83:1184–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson LA, Li L, Sandri-Goldin RM. 2009. The cellular RNA export receptor TAP/NXF1 is required for ICP27-mediated export of herpes simplex virus 1 RNA, whereas the TREX-complex adaptor protein Aly/REF appears to be dispensable. J. Virol. 83:6335–6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koffa MD, Clements JB, Izaurralde E, Wadd S, Wilson SA, Mattaj IW, Kuersten S. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769–5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corbin-Lickfett K, Chen IB, Cocco MJ, Sandri-Goldin RM. 2009. The HSV-1 ICP27 RGG box specifically binds flexible, GC-rich sequences but not G-quartet structures. Nucleic Acids Res. 37:7290–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corbin-Lickfett K, Souki SK, Cocco MJ, Sandri-Goldin RM. 2010. Three arginine residues within the RGG box are crucial for ICP27 binding to herpes simplex virus 1 GC-rich sequences and for efficient viral RNA export. J. Virol. 84:6367–6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandri-Goldin RM. 1998. ICP27 mediates herpes simplex virus RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hernandez FP, Sandri-Goldin RM. 2010. Head-to-tail intramolecular interaction of herpes simplex virus 1 regulatory protein ICP27 is important for its interaction with cellular mRNA export receptor TAP/NXF1. mBio 1(5):e00268–10. 10.1128/mBio.00268-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hiriart E, Farjot G, Gruffat H, Nguyen MVC, Sergeant A, Manet E. 2003. A novel nuclear export signal and a REF interaction domain both promote mRNA export by the Epstein-Barr virus EB2 protein. J. Biol. Chem. 278:335–342 [DOI] [PubMed] [Google Scholar]
- 27. Malik P, Blackbourn DJ, Clements JB. 2004. The evolutionarily conserved Kaposi's sarcoma-associated ORF57 protein interacts with REF protein and acts as an RNA export factor. J. Biol. Chem. 279:33001–33011 [DOI] [PubMed] [Google Scholar]
- 28. Boyne JR, Colgan KJ, Whitehouse A. 2008. Recruitment of the complete hTREX complex is required for Kaposi's sarcoma-associated herpesvirus intronless mRNA nuclear export and virus replication. PLoS Pathog. 4:e1000194. 10.1371/journal.ppat.1000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gatfield D, Izaurralde E. 2002. REF/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J. Cell Biol. 159:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Longman D, Johnstone IL, Caceres JF. 2003. The Ref/Aly proteins are dispensable for mRNA export and development in Caenorhabditis elegans. RNA 9:881–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lischka P, Toth Z, Thomas M, Mueller R, Stamminger T. 2006. The UL69 transactivator protein of human cytomegalovirus interacts with DEAD/H-box RNA helicase UAP56 to promote cytoplasmic accumulation of unspliced RNA. Mol. Cell. Biol. 26:1631–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson BR, Boyne JR, Norerenberg M, Taylor A, Hautbergue GM, Walsh MJ, Wheat R, Blackbourn DJ, Wilson SA, Whitehouse A. 2011. An interaction between KSHV ORF57 and UIF provides mRNA adaptor redundancy in herpesvirus intronless mRNA export. PLoS Pathog. 7:e1002138. 10.1371/journal.ppat.1002138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tunnicliffe RB, Hautbergue GM, Kalra P, Jackson BR, Whitehouse A, Wilson SA, Golovanov AP. 2011. Structural basis for the recognition of cellular mRNA export factor REF by herpes viral proteins HSV-1 ICP27 and HVS ORF57. PLoS Pathog. 7:e1001244. 10.1371/journal.ppat.1001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith IL, Hardwicke MA, Sandri-Goldin RM. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74–86 [DOI] [PubMed] [Google Scholar]
- 35. Lengyel J, Guy C, Leong V, Borge S, Rice SA. 2002. Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG box RNA-binding domain. J. Virol. 76:11866–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stingley SW, Ramirez JJ, Aguilar SA, Simmen K, Sandri-Goldin RM, Ghazal P, Wagner EK. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916–9927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sandri-Goldin RM. 2011. The many roles of the highly interactive HSV protein ICP27, a key regulator of infection. Future Microbiol. 6:1261–1277 [DOI] [PubMed] [Google Scholar]
- 38. Hernandez FP, Sandri-Goldin RM. 2010. Herpes simplex virus 1 regulatory protein ICP27 undergoes a head-to-tail intramolecular interaction. J. Virol. 84:4124–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sokolowski M, Scott JE, Heaney RP, Patel AH, Clements JB. 2003. Identification of herpes simplex virus RNAs that interact specifically with regulatory protein ICP27 in vivo. J. Biol. Chem. 278:33540–33549 [DOI] [PubMed] [Google Scholar]
- 40. Escudero-Paunetto L, Li L, Hernandez FP, Sandri-Goldin RM. 2010. SR proteins SRp20 and 9G8 contribute to efficient export of herpes simplex virus 1 mRNAs. Virology 401:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang Y, Gattoni R, Stevenin J, Steitz JA. 2003. SR splicing factors serve as adaptor proteins for TAP-dependent mRNA export. Mol. Cell 11:837–843 [DOI] [PubMed] [Google Scholar]
- 42. Huang Y, Steitz JA. 2001. Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol. Cell 7:899–905 [DOI] [PubMed] [Google Scholar]
- 43. Sandri-Goldin RM. 2008. The many roles of the regulatory protein ICP27 during herpes simplex virus infection. Front. Biosci. 13:5241–5256 [DOI] [PubMed] [Google Scholar]
- 44. Corbin-Lickfett K, Rojas S, Li L, Cocco MJ, Sandri-Goldin RM. 2010. ICP27 phosphorylation site mutants display altered functional interactions with cellular export factors Aly/REF and TAP/NXF1 but are able to bind herpes simplex virus 1 RNA. J. Virol. 84:2212–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nekorchuk M, Han Z, Hsieh TT, Swaminathan S. 2007. Kaposi's sarcoma-associated herpesvirus ORF57 protein enhances mRNA accumulation independent of effects on nuclear RNA export. J. Virol. 81:9990–9998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li D-L, Verma D, Swaminathan S. 2012. Binding of cellular export factor Ref/Aly by Kaposi's sarcoma-associated herpesvirus (KSHV) by ORF57 protein is not required for efficient KSHV lytic replication. J. Virol. 86:9866–9874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stubbs SH, Hunter OV, Hoover A, Conrad NK. 2012. Viral factors reveal a role for REF/Aly in nuclear RNA stability. Mol. Cell. Biol. 32:1260–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]