Abstract
Dengue viruses are the most common arthropod-transmitted viral infection, with an estimated 390 million human infections annually and ∼3.6 billion people at risk. Currently, there are no approved vaccines or therapeutics available to control the global dengue virus disease burden. In this study, we demonstrate the binding, neutralizing activity, and therapeutic capacity of a novel bispecific dual-affinity retargeting molecule (DART) that limits infection of all four serotypes of dengue virus.
TEXT
Dengue virus (DENV) is a mosquito-transmitted, enveloped, positive-sense RNA virus and member of the Flavivirus genus of the Flaviviridae family. Infection by four closely related but serologically distinct viruses (DENV serotype 1 [DENV-1], DENV-2, DENV-3, and DENV-4) causes dengue fever (DF), an acute self-limiting yet severe febrile illness, or dengue hemorrhagic fever and shock syndrome (DHF/DSS), a potentially fatal vascular leakage syndrome. While primary infection is believed to confer long-term immunity against strains of the homologous DENV serotype, epidemiological studies suggest that secondary infection with a heterologous DENV serotype can enhance the risk of DHF/DSS due to preexisting and nonneutralizing, cross-reactive antibodies (1, 2) and/or T cells (3–5). A requirement for protection against all four serotypes has limited the development of vaccines and antibody-based therapies against DENV.
Most neutralizing antibodies against DENV recognize the structural E protein (reviewed in reference 6), which is divided into three domains. Several epitopes that elicit serotype-specific protective responses in mice and humans have been identified, with the most potently inhibitory monoclonal antibodies (MAbs) mapping to the lateral ridge of domain III (DIII) (7–9) and the hinge region between domain I (DI) and domain II (DII) (10, 11), respectively. Many neutralizing subcomplex- and complex-specific MAbs, which recognize several or all DENV serotypes, bind to an epitope on the A strand of DIII (7, 12–15). Finally, cross-reactive MAbs that bind to multiple flaviviruses map generally to the conserved fusion loop in DII (DII-FL) and neutralize most DENV serotypes, albeit with reduced potency relative to that of type-specific antibodies. Nonetheless, passive transfer of at least some DII-FL and DIII-A-strand MAbs had therapeutic activity in vivo in mouse models of DENV-2 infection (16–18), especially when the Fc region was modified to eliminate the capacity for antibody-dependent enhancement of infection (ADE) in myeloid cells expressing Fc-γ receptors (FcγR).
We hypothesized that a viable antibody treatment against DENV would need to neutralize infection of all four DENV serotypes, lack enhancing activity, and target two epitopes to prevent emergence of resistance or a single epitope in which escape mutants showed reduced fitness. We chose to target two spatially distinct epitopes on the surface of the DENV virion using a novel platform, antibody variable-region-based bispecific dual-affinity retargeting molecules (DARTs). For our DART, we selected E60, a cross-reactive MAb that binds the DII-FL (19), neutralizes DENV efficiently (17), and protects in vivo (17), and 4E11, a complex-specific MAb that binds the A-strand epitope on DIII, neutralizes all four DENV serotypes, and also demonstrates therapeutic efficacy in vivo (18, 20–23).
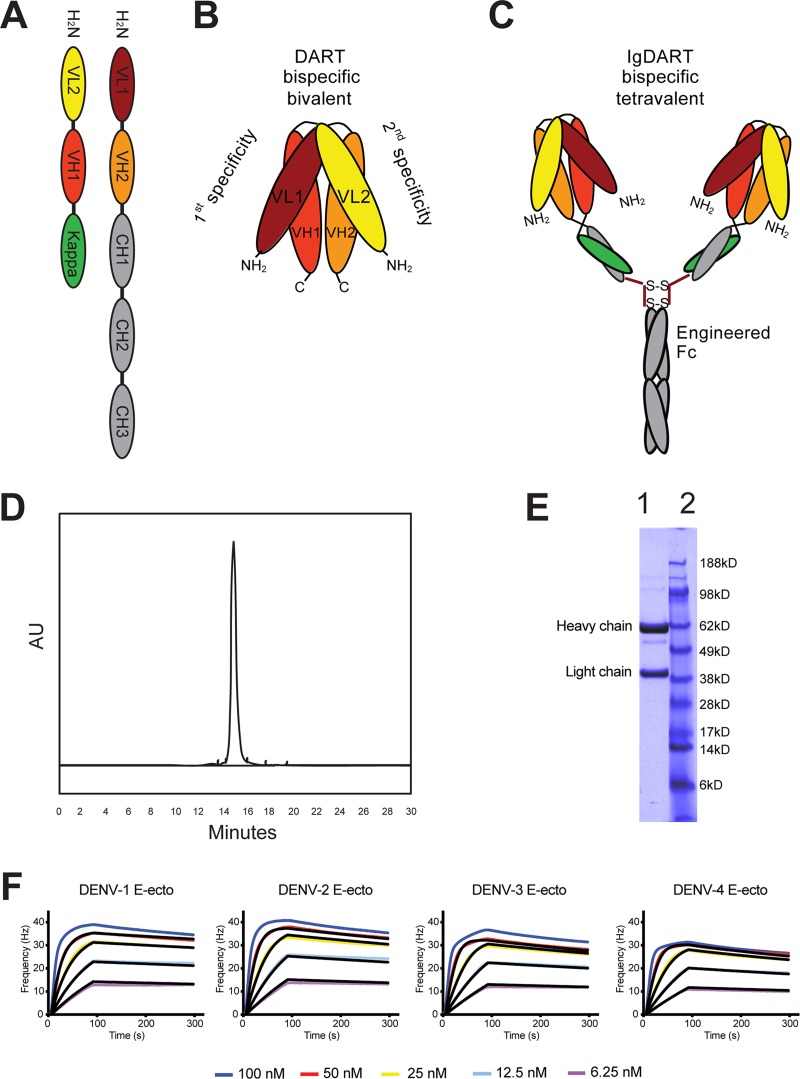
The amino acid sequence of the variable light (VL) and heavy (VH) regions of E60 was determined after isolation of RNA from the parent mouse hybridoma cells. The VL and VH sequences of the 4E11 antibody have been published (21). The complementarity-determining regions (CDRs) of E60 and 4E11 were cloned into the pCI-neo vector with a human IgG signal sequence and γ1 constant region, expressed in CHO-S cells, and purified from supernatants by serial protein A affinity and size exclusion (Superdex 200) chromatography steps to generate purified recombinant E60 and 4E11 MAbs (data not shown). We also produced and expressed E60 and 4E11 as a DART, which consists of two protein chains that dimerize to form two antibody-derived antigen-binding sites (Fv). The first DART chain was constructed by juxtaposing the mouse VL1 domain of E60 with the mouse VH2 domain of 4E11 and contains the human constant domains CH1, CH2, and CH3. A short Gly-Ser linker (GGGSGGGG) between the two domains prevents intramolecular association of the VL1-VH2 pair but does not affect assembly of the constant region. The second DART chain is the complement of the first, containing the VL2 of 4E11 and VH1 of E60 followed by the constant domain of the light chain, CLκ (Fig. 1A). Assembly of a DART requires heterodimerization, which is facilitated by the length of the linker and allows the development of a single protein that is bispecific and bivalent (Fig. 1B).
Fig 1.
Structure and function of Ig-DART. Antibody Fv regions are antigen-binding sites resulting from heterodimerization of the light and heavy chain variable domains (VL and VH). (A) Schematic representation of the linear sequences that assemble into an Ig-DART. (B) A representation of the bispecific, bivalent structure reconstituted by two Fv regions by encoding mismatched VL and VH domains on each of two complementary polypeptide chains. Upon heterodimerization of these chains, both Fvs are assembled and can bind their respective antigens. (C) The Ig-DART structure in the figure is bispecific and tetravalent. The Ig region is engineered with a point mutation (corresponding to N297Q in the intact MAb) to eliminate FcγR binding and prevent ADE in vivo. However, binding to neonatal Fc receptor is preserved, which confers an extended half-life in serum. (D) Size exclusion chromatography profile of the Ig-DART after affinity purification. (E) SDS-PAGE gel of the purified Ig-DART (lane 1) and protein ladder (lane 2, NuPAGE MES ladder) run under reduced, denaturing conditions. (F) Avidity measurements of Ig-DART where anti-human IgG was immobilized on a sensor chip to capture the Ig-DART and soluble dimeric DENV E ectodomain protein was made to flow over the chip at decreasing concentrations (100, 50, 25, 12.5, and 6.25 nM). A single representative sensogram is shown for each antibody. The raw data at each concentration are shown in color, and the fit for each curve is in black. A 1:1 simple/mass-transport limited model was used for fitting of kinetics parameters.
While a DART recapitulates the antigen-binding pattern of the parent MAbs, its serum half-life is shorter (S. Johnson, unpublished data). To circumvent this limitation, the DART constructs were engineered with the human IgG constant regions (CH1, CH2, and CH3); thus, two DART molecules join into a single complex to form a chimeric bispecific and tetravalent Ig-DART (Fig. 1C). The Ig-DART was isolated after serial protein A affinity and size exclusion (Superdex 200) chromatography steps (Fig. 1D), which resulted in a purified preparation (Fig. 1E), with a minor degradation product. The chimeric Ig-DART contains the variable chains from mice and the constant chain domains 1, 2, and 3 from human IgG1. This complex forms a structure similar to a MAb and can bind neonatal Fc receptors, which confers an extended half-life in vivo. The constant heavy domains also can interact with FcγR on immune effector cells and the complement component C1q in solution. However, our Ig-DARTs and MAbs were engineered with a point mutation, N297Q, which abrogates binding to the FcγR and eliminates the potential of ADE and enhanced DENV disease (17, 24, 25). To determine if the Ig-DART had binding properties similar to those of the parent recombinant MAbs, kinetic measurements were obtained using an Attana quartz crystal microbalance. The association (ka) and dissociation (kd) rates of E60, 4E11, and the Ig-DART binding to recombinant DENV E proteins were determined (Table 1 and Fig. 1F). As DENV E proteins form dimers in solution (26), kinetic binding analysis to bivalent MAbs or tetravalent Ig-DARTs reflects avidity (Kapp) rather than monovalent affinity measurements.
Table 1.
Binding avidity of recombinant MAb and Ig-DARTs to DENV E proteinsa
| Virus | Variable | 4E11 | E60 | Ig-DART |
|---|---|---|---|---|
| DENV-1 | ka (M−1 s−1) | 2.6E+06 ± 5.4E+02 | 4.0E+06 ± 8.1E+01 | 2.3E+06 ± 1.0E+03 |
| kd (s−1) | 1.6E−03 ± 2.1E−06 | 4.0E−03 ± 3.0E−06 | 9.1E−04 ± 9.9E−07 | |
| Kapp (M) | 1.3E−09 ± 1.2E−12 | 2.0E−09 ± 2.0E−12 | 7.8E−10 ± 1.0E−12 | |
| DENV-2 | ka (M−1 s−1) | 4.1E+06 ± 9.1E+02 | 4.4E+06 ± 6.3E+02 | 3.2E+06 ± 1.0E+03 |
| kd (s−1) | 4.3E−03 ± 2.0E−06 | 3.9E−03 ± 4.1E−07 | 1.4E−03 ± 6.0E−07 | |
| Kapp (M) | 2.1E−09 ± 1.0E−12 | 1.8E−09 ± 3.2E−13 | 8.8E−10 ± 5.0E−13 | |
| DENV-3 | ka (M−1 s−1) | 2.0E+05 ± 5.4E+03 | 3.3E+06 ± 2.0E+03 | 3.2E+06 ± 4.0E+04 |
| kd (s−1) | 4.2E−03 ± 5.0E−06 | 3.4E−03 ± 5.0E−06 | 1.6E−03 ± 9.2E−05 | |
| Kapp (M) | 4.3E−08 ± 9.2E−10 | 2.1E−09 ± 3.0E−12 | 1.1E−09 ± 5.1E−11 | |
| DENV-4 | ka (M−1 s−1) | 5.9E+04 ± 3.0E+03 | 2.7E+06 ± 8.9E+02 | 2.8E+06 ± 5.7E+02 |
| kd (s−1) | 1.8E−03 ± 4.2E−05 | 3.8E−03 ± 1.0E−05 | 2.0E−03 ± 2.2E−05 | |
| Kapp (M) | 7.4E−08 ± 2.2E−09 | 2.8E−09 ± 1.0E−11 | 1.5E−09 ± 2.1E−11 |
Avidity measurements were performed on an Attana biosensor. Anti-human IgG was immobilized on a sensor chip, and equal molar amounts of 4E11 N297Q, E60 N297Q, or Ig-DART (4E11 plus E60) were bound to the chip. Soluble DENV-1 (strain 2543-63), DENV-2 (16681), DENV-3 (Phil 9609), and DENV-4 (Thailand 1984) E proteins were produced in CHO-S cells, purified by affinity chromatography, and made to flow over the chip at decreasing concentrations (100, 50, 25, 12.5, and 6.25 nM). The table values represent the means of two independent experiments with standard deviations shown. A 1:1 simple/mass-transport limited model was used for fitting and calculation of kinetics parameters.
The binding characteristics of E60 for purified E proteins corresponding to all four DENV serotypes were similar, with a range in avidity (Kapp) of 2.8 × 10−9 M for DENV-4 to 1.8 × 10−9 M for DENV-2 (Table 1). The recombinant 4E11 had similar avidities for DENV-1 and DENV-2 (1.3 × 10−9 M and 2.1 × 10−9 M, respectively) but reduced avidity for DENV-3 (4.2 × 10−8 M) and DENV-4 (7.4 × 10−8 M), as reported previously (21). The Ig-DART had greater avidity for isolated E protein than for either E60 or 4E11 for all serotypes with Kapp values of 7.8 × 10−10 M (DENV-1), 8.8 × 10−10 M (DENV-2), 1.1 × 10−9 M (DENV-3), and 1.5 × 10−9 M (DENV-4) (Fig. 1D and Table 1).
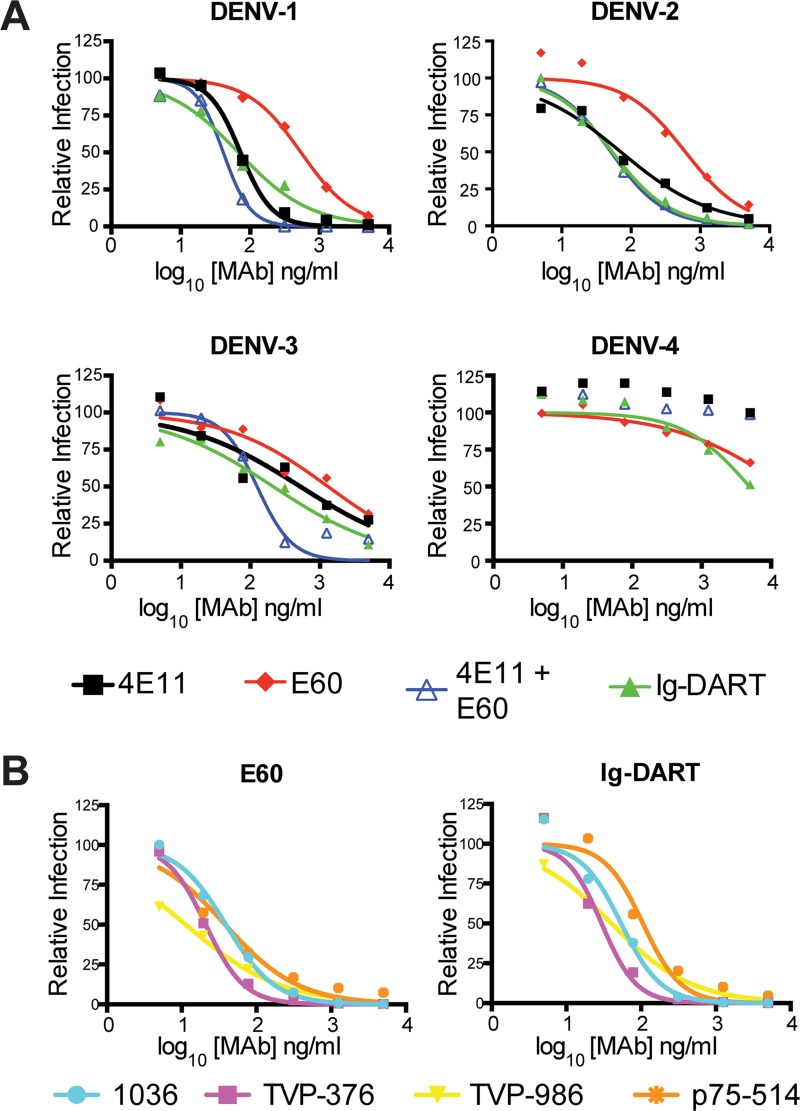
We next investigated the neutralization potential of the Ig-DART and compared it to the individual or combinations of parental recombinant 4E11 and E60 MAbs using a focus reduction neutralization test (FRNT) on Vero cells. Representative viruses from all four DENV serotypes included DENV-1 (strain Western Pacific-74), DENV-2 (strain New Guinea C [NGC]), DENV-3 (strain UNC3043), and DENV-4 (strain 1036). The 4E11 MAb efficiently neutralized DENV-1 (50% effective concentration [EC50] of 72 ng/ml) and DENV-2 (EC50 of 71 ng/ml) but neutralized DENV-3 less well (EC50 of 523 ng/ml) and DENV-4 not at all (EC50 of >5,000 ng/ml) at 37°C (Fig. 2A and Table 2). The E60 MAb neutralized all four DENV serotypes to various degrees at 37°C: DENV-1, EC50 of 538 ng/ml; DENV-2, EC50 of 607 ng/ml; DENV-3, EC50 of 1,308 ng/ml; and DENV-4, EC50 of 2,773 ng/ml. The neutralization potential of the combination of 4E11 and E60 MAbs or the Ig-DART was similar to the best of the two individual MAbs for DENV-1 and DENV-2 and somewhat improved for DENV-3 and DENV-4 (Table 2).
Fig 2.
Ig-DART and MAb-mediated neutralization of DENV. (A) FRNT curves for one representative strain of each DENV serotype (DENV-1, Western Pacific-74; DENV-2, NGC; DENV-3, UNC3043; and DENV-4, 1036). Virus and antibody were incubated at 37°C for 1 h prior to infection with 100 focus-forming units (FFU) of each virus. The data are the means of three independent experiments completed in triplicate. EC50 values are shown in Table 2. (B) FRNT curves of four different DENV-4 strains after preincubation at 40°C for 1 h. The virus strains represent DENV-4 genotype 2 and a sylvatic strain. The data are the means of three independent experiments completed in triplicate. EC50 values are shown in Table 2.
Table 2.
Neutralizing activity of MAbs and Ig-DART against DENV serotypesa
| Serotype and strain | EC50, ng/ml (CI) |
|||
|---|---|---|---|---|
| 4E11 | E60 | 4E11 + E60 | Ig-DART | |
| DENV-1, Western Pacific-74 | 72 (60–87) | 538 (356–812) | 41 (31–54) | 68 (38–119) |
| DENV-2, NGC | 71 (43–118) | 607 (391–941) | 51 (41–64) | 54 (38–75) |
| DENV-3, UNC3043 | 523 (213–1,284) | 1,308 (599–2,857) | 125 (89–175) | 213 (94–481) |
| DENV-4, 1036 | >5,000 | 2,773 (1,683–4,570) | 2,452 (1,813–3,316) | 1,790 (1,257–2,550) |
Neutralizing activity was determined by focus reduction neutralization test (FRNT) on Vero cells after increasing concentrations of purified MAbs or Ig-DARTs (maximum of 5,000 ng/ml) were incubated at 37°C with 100 focus-forming units (FFU) of the indicated DENV strains. The data were derived from three independent experiments performed in triplicate. The EC50 was calculated by nonlinear regression analysis and is expressed as ng/ml of antibody. Confidence intervals (CIs) for each value are listed in parentheses.
Neutralization of flaviviruses by some classes of MAbs is modulated by time and temperature of preincubation, due to the effects on epitope accessibility on the virion (27). We evaluated the ability of E60 and Ig-DART to neutralize several DENV-4 strains at 37°C for 1 and 3 h as well as at 40°C for 1 h. Multiple virus strains were tested because variation in neutralization potency even in sequence-conserved epitopes has been observed across genotypes within a serotype due to differential accessibility on the virion surface (28, 29). DENV-4 strains, particularly, show differences in neutralization by some MAbs when the preincubation conditions are varied, although others, including 4E11, showed no appreciable effect (S. Sukulpolvi-Petty, J. Brien, and M. Diamond, unpublished results). At 37°C, E60 neutralized DENV-4 strain p75-514 (sylvatic genotype, EC50 of 261 ng/ml) most efficiently, followed by strains TVP-986 (genotype 2, EC50 of 908 ng/ml), TVP-376 (genotype 2, EC50 of 1,186 ng/ml), and 1036 (genotype 2, EC50 of 2,773 ng/ml) (Fig. 2B and Table 3). Increasing the time of preincubation enhanced the neutralizing activity of E60 or the Ig-DART for DENV-4 strains 1036 (4- to 5-fold, P < 0.0001) and TVP-986 (15- to 27-fold, P < 0.0001). However, the most significant improvement in neutralization for E60 and the Ig-DART occurred under 40°C preincubation conditions with increased inhibition of strains TVP-986 (9- to 10-fold, P < 0.0001), TVP-376 (24- to 40-fold, P < 0.0001), and 1036 (33- to 70-fold, P < 0.0001).
Table 3.
Neutralizing activity of E60 and Ig-DART against DENV-4 at different times and temperaturesa
| DENV-4 strain (genotype) | EC50, ng/ml (CI) |
|||||
|---|---|---|---|---|---|---|
| 37°C, 1 h |
37°C, 3 h |
40°C, 1 h |
||||
| E60 | Ig-DART | E60 | Ig-DART | E60 | Ig-DART | |
| 1036 (2) | 2,773 (1,683–4,570) | 1,790 (1,257–2,550) | 624 (430–904) | 349 (262–464) | 39 (29–52) | 54 (43–68) |
| TVP-376 (2) | 1,186 (858–1,641) | 721 (473–1,098) | 89 (77–103) | 74 (65–85) | 21 (15–30) | 30 (18–47) |
| TVP-986 (2) | 908 (567–1,454) | 1,045 (643–1,699) | 58 (42–81) | 39 (31–48) | 11 (8–17) | 106 (46–243) |
| p75-514 (sylvatic) | 261 (156–438) | 147 (99–220) | 47 (32–69) | 33 (21–51) | 38 (18–80) | 40 (28–58) |
E60 and Ig-DART neutralization assays were performed after preincubation at the indicated temperatures and times. EC50 values were determined by FRNT on Vero cells. Increasing concentrations of MAbs or Ig-DART (maximum of 5,000 ng/ml) were mixed with 100 focus-forming units (FFU) of the indicated DENV-4 strains for either 1 or 3 h at 37°C or 1 h at 40°C. The data were derived from three independent experiments performed in triplicate. The EC50 was calculated by nonlinear regression analysis and is expressed as ng/ml of antibody. Confidence intervals (CIs) for each value are listed in parentheses.
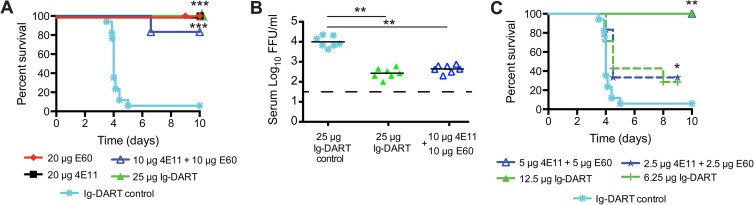
To begin to determine the physiological significance of these findings, we compared the therapeutic potential of the Ig-DART to that of its parent recombinant MAbs in a DENV-2 model of polyclonal antibody-enhanced disease (17); for these studies, all therapeutic MAbs and Ig-DARTs contained amino acid substitutions (e.g., N297Q) that abolished FcγR binding. A single dose of the individual MAbs 4E11 (20 μg, P = 0.0004) and E60 (20 μg, P = 0.0001) or a combination of the individual MAbs (4E11 plus E60, 10 μg each, P = 0.0008) administered 48 h after infection protected against lethal disease compared to a control Ig-DART specific for smallpox (Fig. 3A) or isotype control MAbs (reference 17 and data not shown). Equimolar amounts of the Ig-DART (25 μg, P = 0.0001) also protected against lethal DENV infection. Postexposure treatment with a single dose of Ig-DART (25 μg, P < 0.007) or a molar equivalent combination of the individual MAbs (4E11 plus E60, 10 μg each, P = 0.007) also reduced viral titer in the serum 36 h later, as determined by focus-forming assay (Fig. 3B). To further compare the therapeutic capacity of a traditional 4E11-plus-E60 MAb cocktail to that of the Ig-DART, we tested limiting doses. MAb combination treatment (4E11 plus E60) at 5 μg each (P = 0.006) and 2.5 μg each (P = 0.02) was equivalent in protective capacity to Ig-DART treatment at 12 μg (P = 0.006) and 6 μg (P = 0.03), when administered 48 h after infection (Fig. 3C).
Fig 3.
Therapeutic efficacy of MAb and Ig-DART against DENV-2 in an ADE disease model. The ADE model of DENV-2 infection with polyclonal mouse serum containing heterologous DENV-1-enhancing antibodies was performed as described previously (17). (A) AG129 mice were passively administered 12.5 μl of anti-DENV-1 polyclonal sera via the intraperitoneal route, and 24 h later, mice were infected with 2 × 105 PFU of DENV-2 strain D2S10 via an intravenous route. Forty-eight hours after infection, mice were treated with equimolar amounts of individual MAbs (20 μg, n = 6 to 10), combinations of MAbs (10 μg plus 10 μg, n = 6), or a single Ig-DART (25 μg, n = 8), and mortality was monitored. All MAbs and Ig-DARTs contained the N297Q mutation, which prevents ADE in vivo. The control is an Ig-DART specific for two poxvirus envelope proteins (Johnson, unpublished). Kaplan-Meier survival curves for all experiments are shown. The results reflect at least two independent experiments for each group, and the Ig-DART control was performed in every experiment. (B) Viral titers in the serum were evaluated 36 h after treatment by a focus-forming assay (31) after passive transfer of single Ig-DART control (25 μg, n = 7), Ig-DART (25 μg, n = 7), or combinations of MAbs (10 μg plus 10 μg, n = 7) using an experimental protocol identical to that for the experiment shown in panel A. The results are pooled from three independent experiments. (C) Dose-limiting studies with a combination of 4E11 plus E60 (5 μg plus 5 μg, n = 3, or 2.5 μg plus 2.5 μg, n = 6) or Ig-DART (12.5 μg, n = 3, or 6.25 μg, n = 7) were performed at 48 h after infection. Kaplan-Meier survival curves for all experiments are shown. The results reflect one experiment for the intermediate dose of 4E11 plus E60 (5 μg each) and Ig-DART (12.5 μg) and two independent experiments for each group at the lowest dose. Protection by individual antibodies (4E11 or E60) has been established previously (18). The Ig-DART control was performed in every experiment. Asterisks indicate differences that are statistically significant by the log rank (A and C) or Mann-Whitney (B) test (*, P < 0.05; **, P < 0.001; ***, P < 0.0001). All mouse studies were approved and performed according to the guidelines of the Washington University School of Medicine and the University of California, Berkeley, Animal Safety Committees.
In summary, we show as a proof of principle that a bispecific dual-affinity retargeting molecule (Ig-DART) targeting two spatially distinct cross-reactive and complex-specific epitopes on DII and DIII of the DENV E protein can retain its neutralizing activity in vitro and therapeutic activity in vivo. The Ig-DART, which was engineered to lack FcγR binding capacity, performed as well as did the combination of two parent MAbs of identical specificities. Although we observed improved avidity of the tetravalent Ig-DART for the isolated recombinant DENV E protein, this did not translate into greater inhibitory activity in vitro and in vivo, possibly because the quasi-icosahedral structure of the DENV virion (30) precludes binding geometries necessary for simultaneous recognition of adjacent epitopes. Nonetheless, the Ig-DART has advantages over traditional MAb combination therapy; as a single manufactured and purified product, it will have reduced cost of goods and regulatory requirements. In comparison, an antibody cocktail requires independent manufacturing and approval processes for each component antibody as well as the combinations. Future studies are planned to define the optimal pair of cross-reactive antibodies to engineer into the Ig-DART against DENV, ideally with additive or synergistic interactions; to expand testing to other DENV serotypes in vivo as models become available; and to test the platform with other viruses in which the rapid emergence of resistance with antibody monotherapy would be anticipated.
ACKNOWLEDGMENTS
This work was supported in part by the Burroughs Wellcome Fund and NIH grants R01-AI077955 (M.S.D.), U01-AI061373 (M.S.D.), R01-AI089588 (S.J.), and U54AI065359 (E.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 8 May 2013
REFERENCES
- 1. Kliks SC, Nimmanitya S, Nisalak A, Burke DS. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 38:411–419 [DOI] [PubMed] [Google Scholar]
- 2. Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, Dung NT, Lien le B, Quy NT, Hieu NT, Hieu LT, Hien TT, Hung NT, Farrar J, Simmons CP. 2008. Dengue in Vietnamese infants—results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J. Infect. Dis. 198:516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A, Khemnu N, Tangthawornchaikul N, Chotiyarnwong P, Sae-Jang K, Koch M, Jones Y, McMichael A, Xu X, Malasit P, Screaton G. 2006. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J. Immunol. 176:3821–3829 [DOI] [PubMed] [Google Scholar]
- 4. Zivna I, Green S, Vaughn DW, Kalayanarooj S, Stephens HA, Chandanayingyong D, Nisalak A, Ennis FA, Rothman AL. 2002. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J. Immunol. 168:5959–5965 [DOI] [PubMed] [Google Scholar]
- 5. Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 9:921–927 [DOI] [PubMed] [Google Scholar]
- 6. Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. 2008. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sukupolvi-Petty S, Austin SK, Purtha WE, Oliphant T, Nybakken G, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS. 2007. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J. Virol. 81:12816–12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shrestha B, Brien JD, Sukupolvi-Petty S, Austin SK, Edeling MA, Kim T, O'Brien KM, Nelson CA, Johnson S, Fremont DH, Diamond MS. 2010. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog. 6:e1000823. 10.1371/journal.ppat.1000823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gromowski GD, Barrett AD. 2007. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 366:349–360 [DOI] [PubMed] [Google Scholar]
- 10. Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci. Transl. Med. 4:139ra183. 10.1126/scitranslmed.3003888 [DOI] [PubMed] [Google Scholar]
- 11. de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brien JD, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. 2011. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl. Trop. Dis. 5:e1188. 10.1371/journal.pntd.0001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, Sedlak D, Fremont DH, Chipman PR, Roehrig JT, Diamond MS, Kuhn RJ, Rossmann MG. 2008. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat. Struct. Mol. Biol. 15:312–317 [DOI] [PubMed] [Google Scholar]
- 13. Gromowski GD, Barrett ND, Barrett AD. 2008. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J. Virol. 82:8828–8837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rajamanonmani R, Nkenfou C, Clancy P, Yau YH, Shochat SG, Sukupolvi-Petty S, Schul W, Diamond MS, Vasudevan SG, Lescar J. 2009. On a mouse monoclonal antibody that neutralizes all four dengue virus serotypes. J. Gen. Virol. 90:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thullier P, Demangel C, Bedouelle H, Megret F, Jouan A, Deubel V, Mazie JC, Lafaye P. 2001. Mapping of a dengue virus neutralizing epitope critical for the infectivity of all serotypes: insight into the neutralization mechanism. J. Gen. Virol. 82:1885–1892 [DOI] [PubMed] [Google Scholar]
- 16. Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E. 2010. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 6:e1000790. 10.1371/journal.ppat.1000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams KL, Sukupolvi-Petty S, Beltramello M, Johnson S, Sallusto F, Lanzavecchia A, Diamond MS, Harris E. 2013. Therapeutic efficacy of antibodies lacking FcgammaR against lethal dengue virus infection is due to neutralizing potency and blocking of enhancing antibodies. PLoS Pathog. 9:e1003157. 10.1371/journal.ppat.1003157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliphant T, Nybakken G, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi B, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. 2006. Determinants of West Nile virus envelope protein domains I and II antibody recognition and neutralization. J. Virol. 80:12149–12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lisova O, Hardy F, Petit V, Bedouelle H. 2007. Mapping to completeness and transplantation of a group-specific, discontinuous, neutralizing epitope in the envelope protein of dengue virus. J. Gen. Virol. 88:2387–2397 [DOI] [PubMed] [Google Scholar]
- 21. Cockburn JJ, Navarro Sanchez ME, Fretes N, Urvoas A, Staropoli I, Kikuti CM, Coffey LL, Arenzana Seisdedos F, Bedouelle H, Rey FA. 2012. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure 20:303–314 [DOI] [PubMed] [Google Scholar]
- 22. Thullier P, Lafaye P, Megret F, Deubel V, Jouan A, Mazie JC. 1999. A recombinant Fab neutralizes dengue virus in vitro. J. Biotechnol. 69:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Megret F, Hugnot JP, Falconar A, Gentry MK, Morens DM, Murray JM, Schlesinger JJ, Wright PJ, Young P, Van Regenmortel MHV, Deubel V. 1992. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue virus envelope glycoprotein. Virology 187:480–491 [DOI] [PubMed] [Google Scholar]
- 24. Zellweger RM, Prestwood TR, Shresta S. 2010. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 7:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. 2007. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. U. S. A. 104:9422–9427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Modis Y, Ogata S, Clements D, Harrison SC. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 100:6986–6991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dowd KA, Jost CA, Durbin AP, Whitehead SS, Pierson TC. 2011. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog. 7:e1002111. 10.1371/journal.ppat.1002111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Austin SK, Dowd KA, Shrestha B, Nelson CA, Edeling MA, Johnson S, Pierson TC, Diamond MS, Fremont DH. 2012. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog. 8:e1002930. 10.1371/journal.ppat.1002930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wahala WM, Donaldson EF, de Alwis R, Accavitti-Loper MA, Baric RS, de Silva AM. 2010. Natural strain variation and antibody neutralization of Dengue serotype 3 viruses. PLoS Pathog. 6:e1000821. 10.1371/journal.ppat.1000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brien JD, Austin SK, Sukupolvi-Petty S, O'Brien KM, Johnson S, Fremont DH, Diamond MS. 2010. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J. Virol. 84:10630–10643 [DOI] [PMC free article] [PubMed] [Google Scholar]