Abstract
An important step in megakaryocyte maturation is the appropriate assembly of at least two distinct subsets of α-granules. The mechanism that sorts the α-granule components into distinct structures and mediates their release in response to specific stimuli is now emerging. P-selectin and von Willebrand factor are two proteins present in the α-granules that recognize P-selectin glycoprotein ligand on neutrophils and collagen in the subendothelial matrix. These proteins may play an important role in determining the differential release of the α-granule contents in response to external stimuli. If P-selectin and von Willebrand factor are localized in the same or different α-granules is not known. To clarify this question, we analyzed by immunoelectron microscopy the localization of von Willebrand factor and P-selectin during the maturation of wild-type and Gata1low megakaryocytes induced in vivo by treating animals with thrombopoietin. Gata1low is a hypomorphic mutation that blocks megakaryocyte maturation, reduces the levels of von Willebrand factor expression and displaces P-selectin on the demarcation membrane system. The maturation block induced by this mutation is partially rescued by treatment in vivo with thrombopoietin. In immature megakaryocytes, both wild-type and Gata1low, the two receptors were co-localized in the same cytoplasmic structures. By contrast, the two proteins were segregated to separate α-granule subsets as the megakaryocytes matured. These observations support the hypothesis that P-selectin and von Willebrand factor may ensure differential release of the α-granule content in response to external stimuli.
Keywords: Gata1low, megakaryocyte maturation, P-selectin, thrombopoietin, von Willebrand factor
Megakaryocyte (MK) maturation is a highly regulated process characterized by specific ultrastructural changes that are very similar between mice and humans. Based on these changes, MKs are considered to pass through five distinct stages of maturation (Zucker-Franklin 2003): promegakaryoblast (stage 0 MK), a small mono-nuclear cell expressing platelet-specific proteins; megakaryoblast (stage I MK), a cell 15–20 μm in diameter with a large oval or kidney-shaped nucleus and several nucleoli and whose cytoplasm contains abundant ribosomes and a well developed rough endoplasmic reticulum (RER); promegakaryocyte (stage II MK), a cell 20–80 μm in diameter with an irregularly shaped nucleus and more abundant cytoplasm containing a rudimentary demarcation membrane system (DMS); and mature MKs (stage III MKs) that contain a multilobed nucleus surrounded by abundant cytoplasm with many α-granules, a well-developed DMS and platelet territories. Stage IV MKs are mature and in the process of releasing platelets (Zucker-Franklin 2003). An integral part of the process of MK maturation is the assembly of the α-granules. These structures contain hemostatically active factors, such as fibrinogen, fibronectin, von Willebrand factor (VWF), P-selectin, pro-angiogenic [vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)] and anti-angiogenic growth factors (endostatin and thrombospondin-1) (Cramer 1989, Cramer et al. 1994, Daly et al. 2003, Kaplan et al. 1979, Salgado, et al. 2001, Schick, et al. 1996) that are released in response to external stimuli. Although the content of the α-granules is well characterized, how the different components are routed into these structures during maturation is only now emerging. Recently, it has been reported that in both MKs and platelets, pro- and anti-angiogenic factors are sorted into different subsets of α-granules possibly to be released in response to distinct stimuli. P-selectin and VWF, two of the components of the α-granules, recognize the P-selectin glycoprotein ligand present on neutrophils and collagen and may, therefore, mediate differential degranulation in response to inflammation or injury. Although it is known that VWF is associated specifically with α-granules containing anti-angiogenic growth factors, the α-granule distribution of P-selectin is not known. The observation that in endothelial cells, P-selectin localization to the Weibel-Palade bodies requires VWF expression suggests that P-selectin may also be associated with VWF in the α-granules of the megakaryocytes (Denis et al. 2001, Hop et al. 2000). Indirect support for this hypothesis is provided by the observation that MKs from mice carrying the hypomorphic Gata1low mutation, a mutation that lacks the regulatory sequences driving expression of the transcription factor Gata1 in MKs and that reduce VWF expression (VWF expression is controlled by Gata1), express normal levels of P-selectin, but the protein is abnormally accumulated on the DMS (Centurione et al. 2004, McDevitt et al. 1997a,b). Because of the immaturity of the MKs of Gata1low mice, however, it is not clear whether the abnormal localization of P-selectin to the DMS of these cells is due to their immaturity or to reduced expression of VWF that prevents its routing to α-granules.
To clarify the mechanism that determines the routing of P-selectin and VWF into the α-granules during MK maturation, we have investigated by immunoelectron microscopy the localization of the two proteins at different stages of MK maturation in wild type (WT) and Gata1low littermates. Because MKs are rare in bone marrow and spleen of mice under steady state conditions, MK maturation was induced in vivo by treating the mice with thrombopoietin (TPO), the most important extrinsic factor regulating MK development (Kaushansky et al. 1994). In fact, exogenous administration of TPO in mice induces a wave of MK maturation that increases their frequency at all stages of maturation in bone marrow and spleen and the numbers of platelets in the peripheral blood by days 7 and 15 after treatment (Broudy et al. 1995, Vannucchi et al. 2005a,b). In addition, TPO treatment partially rescues MK maturation in mice carrying the hypomorphic Gata1low mutation (Centurione et al. 2004), which allows investigation of the effect of the mutation on P-selectin localization in mature MKs.
Materials and methods
Mice
The Gata1low (neoΔHS) mutation was induced experimentally in mice by deleting the first enhancer (DNA hypersensitive site I) and the distal promoter of the gene (McDevitt et al. 1997a,b). A colony harboring the mutation in the CD1 background is bred at the animal facilities of the Istituto Superiore di Sanità. These mutants also are available from Jackson Laboratories (Barr Harbor, ME; JAX@Mice DATAbase-STOCK Gata1 <tm2Sho>). Littermates were genotyped at birth by PCR (Migliaccio et al. 2003, Vannucchi et al. 2001) and those found not to carry the mutation were used as normal controls. All the experiments were performed with sex- and age-matched mice under protocols approved by the institutional animal care committee.
Thrombopoietin treatment
Recombinant murine TPO was dissolved in sterile saline as described (Vannucchi et al. 2005a) and injected i.p. for five consecutive days (100 μg TPO/Kg of body weight/day). Mice then were sacrificed sequentially at days 7, 10, 14 and 21 after the first injection of TPO. Because in WT mice the number of MKs in the spleen reaches its maximum on day 7 after the first injection (data not shown; Vannucchi et al. 2005a), all observations reported were obtained on sections from mice at day 7 after TPO treatment. Control mice were injected with saline alone. Data obtained from control animals are not shown, because they were identical to those observed before treatment.
Transmission electron microscopy
Spleen samples for transmission electron microscopy (TEM) were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.6, for 2 h at 4° C and postfixed in osmium tetroxide for 60 min at 4° C. The samples then were dehydrated in alcohol at progressively higher concentrations and embedded in Spurr resin (Polysciences, Warrington, PA). Consecutive semithin and ultrathin sections were cut using a Reichert ultramicrotome. Ultrathin sections were collected on 200 mesh copper grids, counterstained with uranyl acetate and lead citrate, and observed with an EM 109 Zeiss (Oberkochen, Germany).
Immunoelectron microscopy
Spleen samples were fixed for 3 h at 4° C in a mixture of 2% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.6. They were dehydrated in alcohol at progressively higher concentrations and embedded in Bioacryl resin (British Biocell, Cardiff, UK) followed by UV polymerization according to standard procedures (Falcieri et al. 2000). Ultrathin sections were cut and mounted on 300 mesh nickel grids. To block nonspecific binding sites, the grids were treated with a blocking buffer made of phosphate buffer saline supplemented with 0.1% Tween-20, 0.1% bovine serum albumin and 4% normal rabbit serum. All primary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). For single localization experiments, grids were incubated overnight in the presence of goat anti-P-selectin or anti-VWF (catalogue nos. sc-8068 and sc-6941, respectively). The two antibodies recognize antigens expressed by human, mouse and rat cells. The grids were incubated for 1 h with rabbit anti-goat IgG conjugated with 15 nm colloidal gold particles (British Biocell, Cardiff, UK). For double localization experiments, grids first were incubated with rabbit-anti VWF (catalogue no. sc-14014) and subsequently with goat anti-rabbit IgM conjugated with 10 nm colloidal gold particles (British Biocell, Cardiff, UK). After washings, the grids were incubated again with goat anti-P-selectin (catalogue no. sc-6941) followed by incubation with rabbit-anti-goat IgG conjugated with 20 nm colloidal gold particles. Sections then were counterstained in uranyl acetate to display cell morphology and observed with EM 109 Zeiss. Negative controls were cells treated as above, but not exposed to the primary antibody.
Statistical analysis
Results are presented as the mean (± SD) of three separate experiments and statistical analysis was performed by unpaired t-test using Prism 3.1 software (Microcal Software Inc., Northampton, MA).
Results
MKs at all stages were detected in the spleen of both WT and Gata1low mice at day 7 after TPO treatment
In mice, MK precursors are found in both the bone marrow and spleen, and no morphological difference has been reported between the MKs located in the two organs (Schmitt et al. 2001). Because bone marrow is difficult to process for ultrastructural studies with TEM, the morphological changes induced by TPO treatment of WT and Gata1low mice were investigated primarily in the spleen.
As expected (Broudy et al. 1995, Vannucchi et al. 2005a, Zucker-Franklin and Kaushansky 1996), MKs were scarce (0.33 MKs/field) in the spleens of untreated WT mice and, as described earlier (Centurione et al. 2004, Zucker-Franklin 2003), predominantly mature (Figs. 1 and 3). By contrast, spleen sections of WT mice after treatment with TPO were hypercellular with numerous small MKs organized in clusters (6.3 MKs/field; (Figs. 1 and 3). Ultrastructural examination of the spleen of TPO-treated WT mice revealed the presence of both light and heavy electron dense MKs (Figs. 1 and 3). The light electron dense MKs were prevalent at stage I/II of maturation and contained a multilobed nucleus with extensive heterochromatic areas surrounding the nucleoli (Figs. 2 and 3). In addition, these MKs displayed cytoplasmic alterations including dystrophic and dilated DMS, and empty α-granules with prominent vacuolization (Fig. 2). The heavy electron dense MKs resemble para-apoptotic cells described first in tissues from Gata1low mice (Centurione et al. 2004) (Figs. 2 and 3).
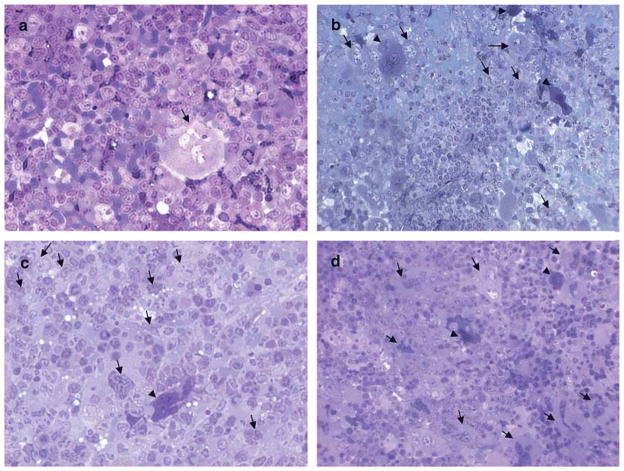
Fig. 1.
MK morphology in the spleens of untreated and TPO-treated WT and Gata1low littermates. Representative semithin sections from the spleen of untreated a,c) and TPO-treated b,d) WT a,b) and Gata1low c,d) mice. Representative megakaryocytes are indicated by arrows. Only one MK is present in the semithin section from WT spleen a). By contrast, the semithin section of TPO-treated WT spleen b) shows nine MKs, six light (whole arrows) and three heavy electron dense (arrowheads). The sections from the spleen of untreated and TPO-treated Gata1low mice are similar c,d). Original magnification: × 40.
Fig. 3.
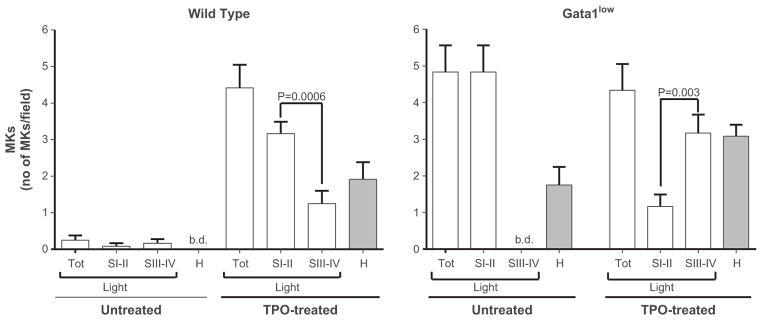
Effect of TPO treatment on the frequencies of MKs at different stages of maturation in the spleen of untreated and TPO-treated WT and Gata1low mice. Megakaryocyte frequencies were analyzed in semithin sections from wild type and Gata1low mice using light microscopy before and on day 7 after TPO-treatment. Results are presented as the mean6SD of determinations in 12 high power fields for each experimental group. In WT mice, TPO treatment resulted in a statistically significant increase (p < 0.001) in total number of MKs and the number of immature MKs (stage I/II) was significantly greater (p = 0.0006) than that of mature MKs (stage III/IV). TPO treatment also resulted in the appearance of heavy electron dense MKs, normally not present in WT spleen. No difference was observed in the total number of MKs present in the spleen of untreated and TPO-treated Gata1low mice. Moreover, in Gata1low mice, no stage III/IV MKs were found before TPO treatment (b.d. = below detection), while after treatment these cells were found in significantly greater frequency than in the earlier stages (I/II) (p = 0.003).
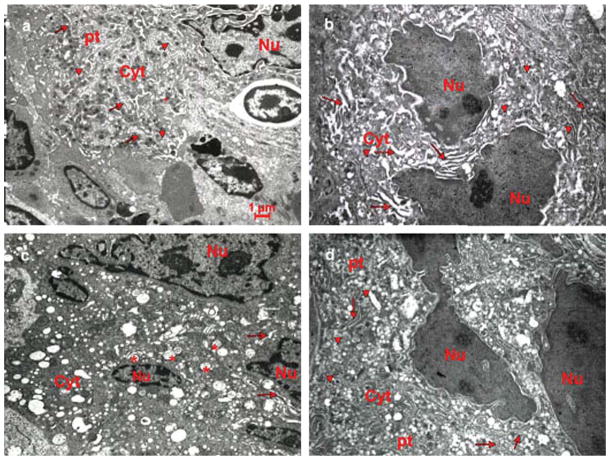
Fig. 2.
Ultrastructure of MKs from the spleen of untreated and TPO-treated WT and Gata1low littermates. Representative ultrathin sections of spleen from untreated a,c), and TPO-treated b,d) WT a,b) and Gata1low c,d) mice. A MK from an untreated WT spleen a) shows a multilobed nucleus, surrounded by abundant cytoplasm (cyt) rich in α-granules (arrowheads), DMS (whole arrows), platelet territories (pt) and containing cytoskeletal proteins and microtubules peripherally. The ultrathin section from TPO-treated WT spleen b) shows a heavy electron dense MK with a multilobed nucleus with prominent heterochromatic area. The DMS appears dilated, surrounding poorly developed platelet territories and α-granules are scarce. The MK in the ultrathin section from a Gata1low spleen c) is similar to the MK in the TPO-treated WT section. The α-granules are replaced by vacuoles (*) distributed throughout the cytoplasm. The ultrathin section from a TPO-treated Gata1low spleen d) shows reversion toward the ultrastructure of the untreated WT MK. The TPO-treated Gata1low section contains recognizable platelet territories, α-granules and a normalized DMS. Original magnification: × 4,400.
Spleen sections from untreated Gata1low mice also were hypercellular with an increased number of MKs compared to sections from untreated WT mice (5.1 vs. 0.33 MKs/field, respectively) and did not contain detectable mature MKs (Centurione et al. 2004). These spleens contained large numbers of heavy electron dense MKs that represented cells that had undergone cell death (para-apoptosis) triggered by pathological emperipolesis of neutrophils that engulf the cytoplasm of MKs in large numbers. Up to 30–50% of the Gata1low MKs are engaged in the pathological emperipolesis process as revealed by the large numbers of MKs with the distinctive high electron density para-apoptotic morphology (a large DMS area organized in long hollows with completely empty vacuoles) detected by electron microscopy (Figs. 2 and 3). After TPO treatment, mature MKs were detected in the spleen of Gata1low mice in numbers significantly greater than that of immature MKs (3.2 vs. 1.2 MKs/field, p = 0.003; Figs. 1 and 3). The mature morphological features of TPO-treated Gata1low MKs included the presence of a developed DMS, several α-granules and platelet territories in the cytoplasm (Figs. 2 and 3).
Localization of P-selectin and VWF during MK maturation in the spleen of WT and Gata1low littermates
The expression and cell localization of P-selectin and VWF during MK maturation were investigated by immunoelectron microscopy using goat polyclonal anti-P-selectin or anti-VWF antibodies, respectively (Figs. 4–6). In mature MKs from untreated WT spleen, P-selectin gold particles were found scattered mainly throughout the cytoplasm and only 10% were associated with the membrane of the α-granules (Fig. 4). By contrast, VWF gold particles were distributed evenly between the cytoplasm and the α-granules. In fact, 45% of the particles were asymmetrically stored in clusters at one pole of the α-granule (Fig. 5). Because of the low number of mature MKs from TPO-treated mice, the cellular localization of P-selectin and VWF in these cells could not be determined by our single staining experiments (Fig. 6). Therefore, the relative distribution of P-selectin and VWF was assessed by comparing the immature MKs from TPO-treated mice with mature MKs from untreated animals. Immature MKs in the spleen from TPO-treated WT mice expressed numbers of P-selectin gold particles similar to those expressed by mature MKs of untreated animals. By contrast, significantly fewer VWF-gold particles were seen in these cells than in mature MKs from untreated WT mice (6.7 ± 2 vs. 27 ± 0.7 particles/MK, respectively, p < 0.001; Fig. 6).
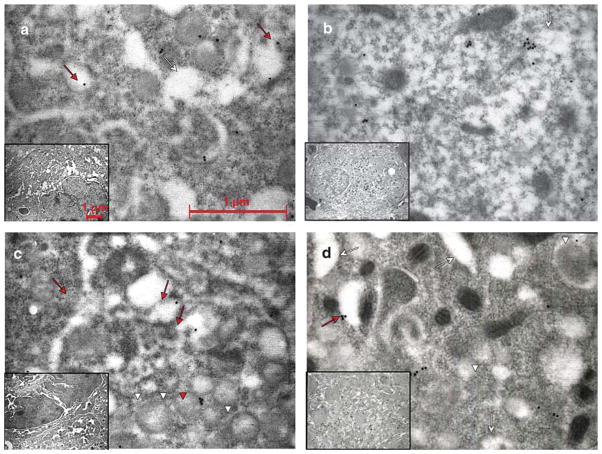
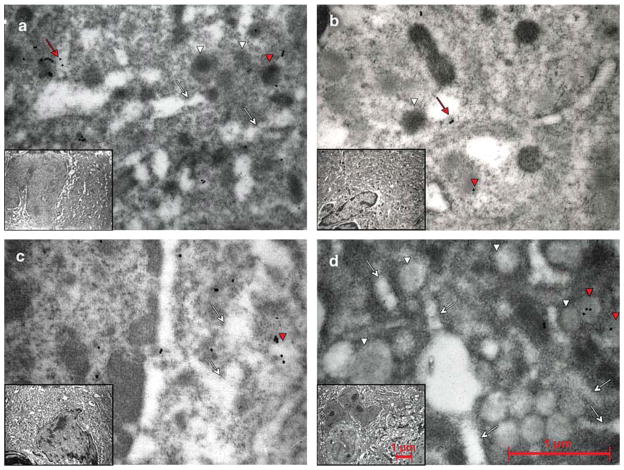
Fig. 4.
P-selectin distribution in MKs of WT and Gata1low mice before and after TPO treatment. Representative semithin sections of spleen from untreated a,c), and TPO-treated b,d) WT a,b) and Gata1low c,d) mice immunostained for P-selectin and analyzed by immunoelectron microscopy. Representative MKs and the corresponding cytoplasm are shown in the inset and in the panel, respectively. P-selectin staining appears as dots. Red arrows and arrowheads point to structures containing the protein, while the white ones point to the structure as such. As seen in a), P-selectin was found in the α-granule membrane as well as in the cytoplasm in the vicinity of the DMS (whole arrows) in untreated WT mice. After treatment with TPO b), most MKs were immature with only rudimentary DMS and very few granules. a) In these MKs, the major part of P-selectin staining was localized to the cytoplasm. In the immature MKs from the untreated Gata 1 low mice c), P-selectin staining was found both in the cytoplasm and in the scarce α-granules (arrow heads). In the mature forms a), P-selectin was localized primarily in the cytoplasm, often delineating the DMS, but also, atypically, in membranes of vacuolar structures. Original magnifications: × 30,000 for panels; × 4,400 for insets.
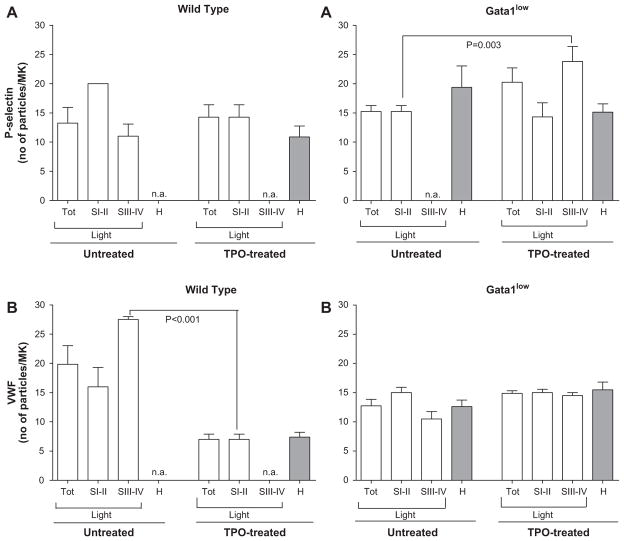
Fig. 6.
Quantification of the effects of TPO on P-selectin A) and VWF B) expression at different stages of maturation in the spleen of WT and Gata1low mice. Representative semithin sections from the spleens of WT and Gata1low mice were immunostained for P-selectin (Fig. 4) and VWF (Fig. 5) and numbers of gold particles within each MK determined, before and on day 7 of TPO-treatment. MKs were classified as immature (stage I/II), immature (stage III/IV) or heavy electron dense (H) and numbers of P-selectin and VWF gold particles per MK in each group determined. Results are presented as the mean6SD of determinations in eight MKs for each experimental group except for stage I/II MKs in untreated WT mice for which only two MKs were available. As seen in the upper left panel, TPO-treatment did not induce a statistically significant difference in P-selectin expression in MKs from the spleen of WT mice. The upper right panel shows that significantly more P-selectin gold particles were observed in the mature MKs in the spleen of TPO-treated Gata1low mice than in the immature MKs of untreated Gata1low mice (p = 0.003). As seen in the lower left panel, immature MKs in the spleen from TPO-treated WT mice expressed significantly fewer VWF-gold particles than mature MKs from untreated WT mice (p < 0.001). As seen in the lower right panel, TPO did not significantly alter the expression of VWF in MKs from the spleen of Gata1low mice.
Fig. 5.
VWF distribution in MKs of WT and Gata1low mice before and after TPO treatment. Representative semithin sections of spleen from untreated a,c) and TPO-treated b,d) WT a,b) and Gata1low c,d) mice were immunostained for VWF and analyzed by immunoelectron microscopy. Representative MKs and the corresponding cytoplasm are shown in the insets and panels, respectively. VWF staining appears in a punctuate pattern. Red arrows and arrowheads point to structures containing the protein, while the white ones point to the structure itself. In mature MKs from untreated WT mice a), VWF staining was found almost equally distributed between α-granules (arrowheads) and the cytoplasm. After TPO treatment b), only immature WT MKs could be analyzed, but the proportion of VWF in α-granules and cytoplasm remained nearly the same. Staining often was found near the DMS (whole arrows). In untreated Gata1low mice c), only immature MKs could be studied and only a very minor portion of the VWF was bound to the α-granules. Treatment with TPO d) induced the appearance of mature MKs and in these, most of the VWF was dispersed in the cytoplasm, but also occasionally found in α-granules. Original magnifications: × 30,000 for panels; × 4,400 for insets.
The immature MKs of the spleen from untreated Gata1low mice showed similar numbers of P-selectin gold particles and lower numbers of VWF-gold particles than mature MKs from untreated WT mice (Fig. 6). The portion of P-selectin associated with the α-granules was greater in Gata1low than in WT MKs (29 vs. 10%, respectively) (Fig. 4), while that of VWF gold particles localized to the α-granules was 5% vs. 45% in untreated Gata1low mice and WT mice, respectively (Fig. 5). Therefore, the Gata1low mutation not only reduced VWF expression, but also its localization to the α-granules. On the other hand, although the mutation did not affect P-selectin expression, it increased its localization to the α-granules.
TPO treatment did not significantly affect the expression and distribution of P-selectin and VWF in immature MKs of Gata1low mice (Figs. 4–6). In the mature MKs observed in the spleen of TPO-treated Gata1low mice, however, more P-selectin gold particles were observed than in immature MKs of untreated Gata1low mice (24 ± 7 vs. 15 ± 3 particles/MK, respectively, p = 0.003; Fig. 6). Interestingly, TPO treatment normalized (3% vs. 10%) the portion of P-selectin associated with the α-granules of Gata1low MKs, but it did not change the localization of VWF in the α-granules, which remained low (< gold particles/MK; Fig. 5). Thus the localization of P-selectin, but not that of VWF, was restored in the mature MKs observed in Gata1low mice after TPO treatment.
Co-localization of P-selectin and VWF was limited to the early stages of MK maturation
The distinctive effects of TPO treatment on P-selectin and VWF localization in MKs from WT and Gata1low mice suggested that, unlike the co-routing to the Weibel-Palade bodies observed in endothelial cells (Denis et al. 2001, Hop et al. 2000) the two proteins are routed independently in MKs. To test this hypothesis, we performed double immunolabeling with both anti-P-selectin and anti-VWF polyclonal antibodies of MKs from untreated and TPO-treated WT and Gata1low mice (Figs. 7–10).
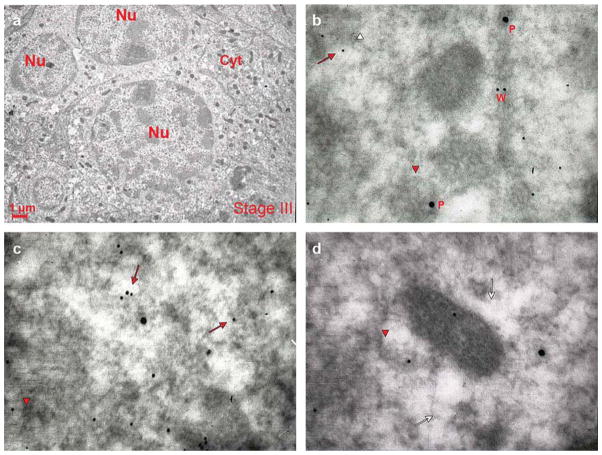
Fig. 7.
Co-localization of VWF and P-selectin in a mature MK from an untreated WT mouse. Representative sections from a WT mouse spleen were double stained for P-selectin (P, large gold particles) and VWF (W, small gold particles). A representative stage III MK is shown in a) and co-localization of P-selectin and VWF in three areas of its cytoplasm are shown in b–d). Red arrows and arrowheads point to structures containing the proteins, while the white ones point to the structure itself. As seen in (a), the mature MK is characterized by a multilobed nucleus and abundant cytoplasm with many α-granules in the perinuclear zone. As seen in b–d), VWF and P-selectin appear separately in the mature WT MK. Both proteins were found either dispersed in the cytoplasm or adjacent to the DMS (whole arrows). P-selectin was found also in the α-granule membrane (arrowheads), while VWF was localized to one pole of the granule. Original magnifications: a) × 4,400; b,c,d) × 30,000.
Fig. 10.
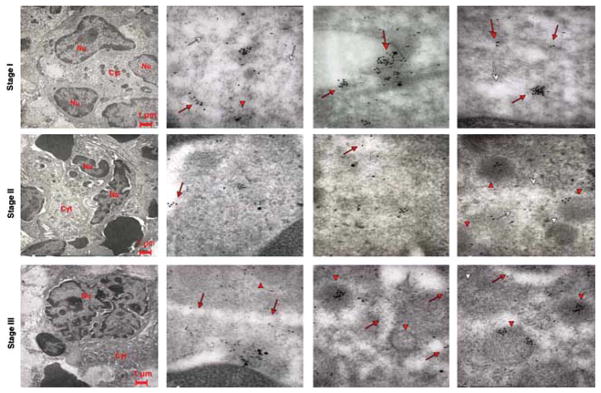
Co-localization of VWF and P-selectin in Gata1low MKs at different stages of maturation. Representative sections of spleen from Gata1low mice treated with TPO were double stained for P-selectin (large gold particles) and VWF (small gold particles) and analyzed by immunoelectron microscopy. Red arrows and arrowheads point to structures containing the protein, while the white ones point to the structure itself. In the upper left panel, the nucleus is multilobed, but contains multiple vacuoles while the cytoplasm is immature with abundant RER. Stage II and III Gata1low MKs showed similar ultrastructural features as the cells at the corresponding stage of maturation observed in TPO-treated WT mice (Fig. 8). In the three upper panels, P-selectin and VWF co-localize in the cytoplasm of stage I Gata1low MKs; as in the immature MKs from WT mice (Fig. 8), both proteins can be found in the cytoplasm close to the DMS (whole arrows) and in the primitive α-granules (arrowheads). With progressing maturation, the proteins become increasingly separated, imitating the pattern observed in mature WT MKs. Original magnifications: × 4,400 for the three panels farthest left and × 30,000 for the others.
In mature MKs from untreated WT mice, P-selectin and VWF gold particles were not co-localized (Fig. 7). By contrast, in MKs from TPO-treated WT mice, a dynamic picture of P-selectin and VWF localization was observed with maturation (Fig. 8). In the most immature (stage I) MKs, P-selectin and VWF gold particles frequently were associated. In stage II MKs, the two proteins were both associated and separated in the cytoplasm. In stage III MKs, like mature MKs from untreated WT mice, the two proteins were found in separate structures. These results suggest that these two proteins are associated in the same compartments at early stages of maturation, but that their localization diverges to separate α-granules as these structures mature.
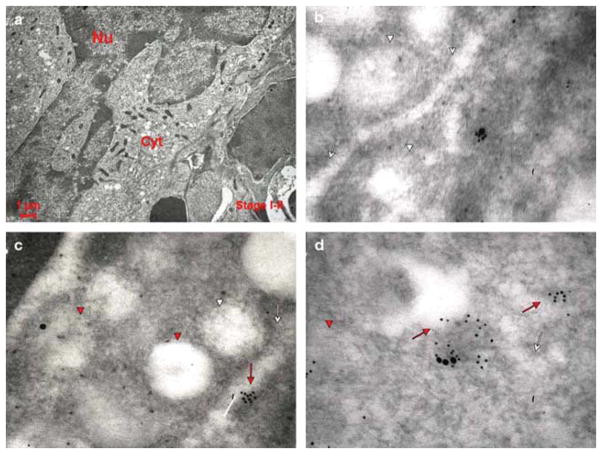
Fig. 8.
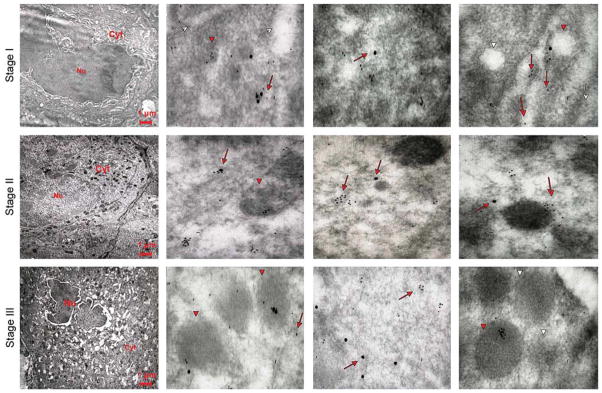
Co-localization of VWF and P-selectin in MKs of different stages of maturation observed in WT mice after TPO treatment. Representative sections from WT mice spleens were double stained for P-selectin (large gold particles) and VWF (small gold particles) and analyzed by immunoelectron microscopy. Three representative MKs at different stages of maturation are shown in the left panels, while three representative areas of their cytoplasms are shown in the other panels of each row. Red arrows and arrowheads point to structures containing the proteins, while the white ones point to the structure itself. P-selectin staining appears as isolated dots, and VWF staining shows a punctuate pattern. In a stage I MK, P-selectin and VWF are mostly co-localized, because both proteins are found both in the cytoplasm, often along the DMS (whole arrows), and in the rudimentary α-granule membrane (arrowheads). With maturation, the localizations of the two proteins progressively diverge, and become totally separated in the mature stage III MK (lower panels). Original magnifications: × 4,400 for the three panels on the far left and × 30,000 for the others.
In the MKs of untreated Gata1low mice, there was co-localization of P-selectin and VWF both in the α-granules and cytoplasm/DMS (Fig. 9). This co-localization was similar to that observed in immature MKs of TPO-treated WT mice and is, therefore, consistent with the immature nature of Gata1low MKs. On the other hand, P-selectin and VWF were not co-localized in the mature MKs from Gata1low mice observed after TPO treatment (Fig. 10). Therefore, although TPO treatment does not rescue the quantitative deficiency of VWF expression (Figs. 5 and 6), it induces formation of α-granules that, as observed in WT MKs, contained either P-selectin or VWF.
Fig. 9.
Co-localization of VWF and P-selectin in a MK from the spleen of an untreated Gata1low mouse. Representative sections from Gata1low mice spleen were double stained for P-selectin (large gold particles) and VWF (small gold particles). Because the Gata1 mutation blocks maturation (Centurione et al. 2004), only stages I/II MKs were available for analysis. Red arrows and arrowheads point to structures containing the proteins, while the white ones point to the structure itself. In a), cells are characterized by a large nucleus with several nucleoli, with a cytoplasm that contains ribosomes and well developed RER. In b,c,d), P-selectin and VWF co-localize in the cytoplasm in the immature MKs of Gata1low mice. Both proteins were found in proximity to the DMS (whole arrows) and in the vacuoles representing degenerated granules (arrowheads). Original magnifications: a) × 4,400; b,c,d) × 30,000.
Discussion
We demonstrated that P-selectin and VWF are co-localized within the same intracellular compartments (both cytoplasm and rudimentary α-granules) in immature MKs in both WT mice after TPO treatment and untreated Gata1low mice. The two proteins, however, are not co-localized in α-granules of mature MKs in the spleen of either untreated WT mice or from WT and Gata1low mice after TPO treatment. These results suggest that P-selectin and VWF are associated in the MK cytoplasm at early stages of maturation, but that they are routed into separate α-granule subtypes as these cells mature. The different routing of P-selectin and VWF is consistent with previous immunoelectron microscopy observations that indicated that the two proteins are localized in different regions within the α-granules: VWF was found mainly in peripherally located internal vesicles, while P-selectin was found in both internal vesicles and the limiting membrane of the α-granules (Schmitt et al. 2000). These investigators did not perform double immunoelectron microscopy, however, and they could not assess whether, in addition, these proteins are localized into distinct α-granule subtypes. The data presented here imply that the mechanisms that route P-selectin and VWF to the α-granules of the MK are at least partially different from those involved in the localization of the two proteins to the Weibel-Palade bodies of endothelial cells. It is possible that they resemble those involved in the formation of the few endothelial Weibel-Palade bodies that contain either VWF or P-selectin, but not both (Cleator et al. 2006).
It has been shown earlier that VWF is associated specifically with the α-granules containing endostatin, i.e., with anti-angiogenic α-granules (Italiano et al. 2008). It is tempting, therefore, to speculate that the localization of P-selectin is restricted instead to α-granules that contain pro-angiogenic factors. This hypothesis, which will be investigated in future studies, allows us to build a model for stimulus-specific platelet activation based on the distinctive receptor functions of P-selectin and VWF. It is well established that platelets are involved in the regulation of the entire response to injury (Nurden et al. 2008). In fact, in addition to their role in initiating clot formation, these cells initiate late responses such as inflammation and wound healing. We propose that the differential association of VWF and P-selectin with anti-angiogenic or pro-angiogenic factors provides a mechanism that regulates the sequential release of different α-granule subtypes during tissue repair. In the initial phases of the response, vessel injury exposes collagen that binds soluble VWF present in plasma. VWF then forms a bridge between collagen fibers and platelets by binding to the platelet receptor glycoprotein Ib (GPIb) (Varga-Szabo et al. 2008). After arrival at the site of injury, platelets are activated by both collagen binding to glycoprotein VI on the platelets and small amounts of thrombin formed when exposed tissue factor activates FVII, FX and prothrombin, sequentially (Furie and Furie 2008). Because, at the initial phase of activation, platelets move negatively charged phospholipids, predominantly phosphatidylserine, from the inner plasma membrane leaflet to the outer leaflet, exposing the contents of the α-granules, we propose that collagen binding to VWF present in the anti-angiogenic α-granules triggers the release of these factors and prevents inappropriate endothelial proliferation. Once hemostasis has occurred, the process of wound healing can be initiated. In this phase, neutrophils recruited to the wound site bind to P-selectin localized to pro-angiogenic α-granules and promote the release of these factors into the microenvironment to neutralize the anti-angiogenic factors released earlier, promoting neo-Angiogenesis Indirect support for the possibility that VWF- and P-selectin-α-granules are released independently is provided by the observation that cultured endothelial cells release VWF, but not P-selectin, after activating the cells with forskolin (a labdane diterpene usually used as cell activator because it raises intracellular levels of cyclic AMP) (Cleator et al. 2006).
The abnormalities in VWF expression and P-selectin localization observed in MKs from Gata1low mice described here explain some of the pleiotropic defects expressed by these mutants. Because it is conceivable that the ultrastructural abnormalities of MKs and Gata1low platelets are similar, the selective absence of VWF in the anti-angiogenic α-granules of platelets would explain the prolonged bleeding time beyond that expected for the degree of thrombocytopenia described in these mice (Vyas et al. 1999). On the other hand, the increased angiogenesis observed in Gata1low mice (Zetterberg et al. 2007) would be the result of the combination of increased numbers of P-selectin-pro-angiogenic α-granules and decreased numbers of VWF-anti-angiogenic ones. Therefore, upon stimulation, Gata1low platelets would preferentially release pro-angiogenic factors, thus explaining why high levels of VEGF and transforming factor- are present in the micro-environment of these mutants. On the other hand, thrombopoietin, by restoring MK maturation and P-selectin localization, would reduce neutrophil emperipolesis, partially blocking the release of pro-angiogenic α-granule content and rescuing, at least in part, as published previously, the phenotype induced by the hypomorphic Gata1low mutation (Centurione et al. 2004).
Patients with primary myelofibrosis (Vannucchi et al. 2002) and X-linked gray platelet syndrome (Tubman et al. 2007 ) are characterized, through at least partially different mechanisms, by reduced expression of GATA1 in their MKs. The importance of normal GATA1 expression for normal α-granule assembly is further underscored by the fact that platelets from patients with these syndromes show several abnormalities including abnormal assembly of α-granule proteins (Centurione et al. 2004, Tubman et al. 2007). The data presented here associate reduced expression of a transcription factor with abnormal localization of P-selectin and highlight the clinical relevance of the fact that low expression of GATA1 may affect, in addition to mRNA and protein expression levels, appropriate protein routing within the cells.
Acknowledgments
The authors gratefully acknowledge Professor Jan Palmblad for advice and discussion when preparing the manuscript as well as Dr Antonio Di Virgilio for managing the animal colony. Murine TPO was provided by Kirin (transfer of material agreement of March 29, 2002). This study was supported by a project from Alleanza sul Cancro from the Ministero per la salute, Italy and from the grant no. P01-CA108671, National Cancer Institute, USA. Eva Zetterberg is supported by a grant from the Swedish Research Council. Maria Elena Fabucci is the recipient of Marie Curie Erythron Fellowship.
References
- Broudy VC, Lin NL, Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85:1719–1726. [PubMed] [Google Scholar]
- Centurione L, Di Baldassarre A, Zingariello M, Bosco D, Gatta V, Rana RA, Langella V, Di Virgilio A, Vannucchi AM, Migliaccio AR. Increased and pathologic emperipolesis of neutrophils within megakaryocytes associated with marrow fibrosis in GATA-1(low) mice. Blood. 2004;104:3573–3580. doi: 10.1182/blood-2004-01-0193. [DOI] [PubMed] [Google Scholar]
- Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2006;107:2736–2744. doi: 10.1182/blood-2004-07-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer EM, Berger G, Berndt MC. Platelet α-granule and plasma membrane share two new components: CD9 and PECAM-1. Blood. 1994;84:1722–1730. [PubMed] [Google Scholar]
- Cramer EM, Debili N, Martin JF, Gladwin AM, Breton-Gorius J, Harrison P, Savidge GF, Vainchenker W. Uncoordinated expression of fibrinogen compared with thrombospondin and von Willebrand factor in maturing human megakaryocytes. Blood. 1989;73:1123–1129. [PubMed] [Google Scholar]
- Daly ME, Makris A, Reed M, Lewis CE. Hemostatic regulators of tumor angiogenesis: a source of antiangiogenic agents for cancer treatment? J Natl Cancer Inst. 2003;95:1660–1673. doi: 10.1093/jnci/djg101. [DOI] [PubMed] [Google Scholar]
- Denis CV, Andre P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:4072–4077. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcieri E, Bassini A, Pierpaoli S, Luchetti F, Zamai L, Vitale M, Guidotti L, Zauli G. Ultrastructural characterization of maturation, platelet release, and senescence of human cultured megakaryocytes. Anat Rec. 2000;258:90–99. doi: 10.1002/(SICI)1097-0185(20000101)258:1<90::AID-AR10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- Hop C, Guilliatt A, Daly M, de Leeuw HP, Brinkman HJ, Peake IR, van Mourik JA, Pannekoek H. Assembly of multimeric von Willebrand factor directs sorting of P-selectin. Arterioscler Thromb Vasc Biol. 2000;20:1763–1768. doi: 10.1161/01.atv.20.7.1763. [DOI] [PubMed] [Google Scholar]
- Italiano JE, Richardson JL, Jr, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Chao FC, Stiles CD, Antoniades HN, Scher CD. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979;53:1043–1052. [PubMed] [Google Scholar]
- Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, Forstrom JW, Buddle MM, Oort PJ, Hagen FS, Roth GJ, Papayannopoulou T, Foster DC. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Fujiwara Y, Shivdasani RA, Orkin SH. An upstream, DNase I hypersensitive region of the hematopoietic-expressed transcription factor GATA-1 gene confers developmental specificity in transgenic mice. Proc Natl Acad Sci USA. 1997a;94:7976–7981. doi: 10.1073/pnas.94.15.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Shivdasani RA, Fujiwara Y, Yang H, Orkin SH. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc Natl Acad Sci USA. 1997b;94:6781–6785. doi: 10.1073/pnas.94.13.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AR, Rana RA, Sanchez M, Lorenzini R, Centurione L, Bianchi L, Vannucchi AM, Migliaccio G, Orkin SH. GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J Exp Med. 2003;197:281–296. doi: 10.1084/jem.20021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- Salgado R, Benoy I, Bogers J, Weytjens R, Vermeulen P, Dirix L, Van Marck E. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis. 2001;4:37–43. doi: 10.1023/a:1016611230747. [DOI] [PubMed] [Google Scholar]
- Schick PK, Wojenski CM, Bennett VD, Ivanova T. The synthesis and localization of alternatively spliced fibronectin EIIIB in resting and thrombin-treated megakaryocytes. Blood. 1996;87:1817–1823. [PubMed] [Google Scholar]
- Schmitt A, Guichard J, Masse JM, Debili N, Cramer EM. Of mice and men: comparison of the ultrastructure of megakaryocytes and platelets. Exp Hematol. 2001;29:1295–1302. doi: 10.1016/s0301-472x(01)00733-0. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Jouault H, Guichard J, Wendling F, Drouin A, Cramer EM. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood. 2000;96:1342–1347. [PubMed] [Google Scholar]
- Tubman VN, Levine JE, Campagna DR, Monahan-Earley R, Dvorak AM, Neufeld EJ, Fleming MD. X-linked gray platelet syndrome due to a GATA1 Arg216Gln mutation. Blood. 2007;109:3297–3299. doi: 10.1182/blood-2006-02-004101. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Bianchi L, Cellai C, Paoletti F, Carrai V, Calzolari A, Centurione L, Lorenzini R, Carta C, Alfani E, Sanchez M, Migliaccio G, Migliaccio AR. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression (GATA-1(low) mice) Blood. 2001;97:3040–3050. doi: 10.1182/blood.v97.10.3040. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Bianchi L, Cellai C, Paoletti F, Rana RA, Lorenzini R, Migliaccio G, Migliaccio AR. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice) Blood. 2002;100:1123–1132. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Bianchi L, Paoletti F, Pancrazzi A, Torre E, Nishikawa M, Zingariello M, Di Baldassarre A, Rana RA, Lorenzini R, Alfani E, Migliaccio G, Migliaccio AR. A pathobiologic pathway linking thrombopoietin, GATA-1, and TGF-beta1 in the development of myelofibrosis. Blood. 2005a;105:3493–3501. doi: 10.1182/blood-2004-04-1320. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Pancrazzi A, Guglielmelli P, Di Lollo S, Bogani C, Baroni G, Bianchi L, Migliaccio AR, Bosi A, Paoletti F. Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis. Am J Pathol. 2005b;167:849–858. doi: 10.1016/S0002-9440(10)62056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28:403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93:2867–2875. [PubMed] [Google Scholar]
- Zetterberg E, Vannucchi AM, Migliaccio AR, Vainchenker W, Tulliez M, Dickie R, Hasselbalch H, Rogers R, Palmblad J. Pericyte coverage of abnormal blood vessels in myelofibrotic bone marrows. Haematologica. 2007;92:597–604. doi: 10.3324/haematol.11013. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D. Megakaryocytes and Platelets. In: Zucker Franklin D, Grossi CE, editors. Atlas of Blood Cells, Function and Pathology. Edi Emes; Bologna, Italy: 2003. pp. 753–857. [Google Scholar]
- Zucker-Franklin D, Kaushansky K. Effect of thrombopoietin on the development of megakaryocytes and platelets: an ultrastructural analysis. Blood. 1996;88:1632–1638. [PubMed] [Google Scholar]