Abstract
Object
Glioblastoma is the most common and aggressive type of primary brain tumor in adults. These tumors recur regardless of intervention. This propensity to recur despite aggressive therapies has made many perceive that repeated resections have little utility. The goal of this study was to evaluate if patients who underwent repeat resections experienced improved survival as compared with patients with fewer numbers of resections, and whether the number of resections was an independent predictor of prolonged survival.
Methods
The records of adult patients who underwent surgery for an intracranial primary glioblastoma at an academic tertiary-care institution between 1997 and 2007 were retrospectively reviewed. Multivariate proportional-hazards regression analysis was used to identify an association between glioblastoma resection number and survival after controlling for factors known to be associated with survival, such as age, functional status, periventricular location, extent of resection, and adjuvant therapy. Survival as a function of time was plotted using the Kaplan-Meier method, and survival rates were compared using log-rank analysis.
Results
Five hundred seventy-eight patients with primary glioblastoma met the inclusion/exclusion criteria. At last follow-up, 354, 168, 41, and 15 patients underwent 1, 2, 3, or 4 resections, respectively. The median survival for patients who underwent 1, 2, 3, and 4 resections was 6.8, 15.5, 22.4, and 26.6 months (p < 0.05), respectively. In multivariate analysis, patients who underwent only 1 resection experienced shortened survival (relative risk [RR] 3.400, 95% CI 2.423–4.774; p < 0.0001) as compared with patients who underwent 2 (RR 0.688, 95% CI 0.525–0.898; p = 0.0006), 3 (RR 0.614, 95% CI 0.388–0.929; p = 0.02), or 4 (RR 0.600, 95% CI 0.238–0.853; p = 0.01) resections. These results were verified in a case-control evaluation, controlling for age, neurological function, periventricular tumor location, extent of resection, and adjuvant therapy. Patients who underwent 1, 2, or 3 resections had a median survival of 4.5, 16.2, and 24.4 months, respectively (p < 0.05). Additionally, the risk of infections or iatrogenic deficits did not increase with repeated resections in this patient population (p > 0.05).
Conclusions
Patients with glioblastoma will inevitably experience tumor recurrence. The present study shows that patients with recurrent glioblastoma can have improved survival with repeated resections. The findings of this study, however, may be limited by an intrinsic bias associated with patient selection. The authors attempted to minimize these biases by using strict inclusion criteria, multivariate analyses, and case-control evaluation.
Keywords: glioblastoma, prognosis, resection, survival, oncology
Glioblastoma is the most common malignant primary CNS tumors in adults.11,17 Despite advances in medical and surgical therapy, the median survival for patients harboring these tumors remains approximately 1 year.11,13 These tumors frequently invade and infiltrate surrounding normal parenchyma, making curative resection unlikely. In fact, Walter Dandy performed hemispherectomies for glioblastoma in the 1920s,10 and the tumors still recurred on the contralateral side. Despite extensive resection, these tumors will also continue to recur despite repeated resections.2,17,19,33 Some patients will undergo more than 3 resections and yet continue to experience tumor recurrence.2,17,19,33 The ability of glioblastoma to recur after extensive and repeated resection has made many question the utility of surgery for patients with these tumors.
There is growing evidence that extent of resection at the time of initial surgery is associated with prolonged survival,6,20,22,25,31 but studies on the association between repeated resections and survival are few and limited.1,2,17,19 This lack of clarity has led to the implementation of experimental therapies such as vaccines and salvage chemotherapy.28,32 A better understanding of the efficacy of repeated resection may help guide treatment strategies aimed at prolonging survival for patients with glioblastoma. The goal of this study was therefore to evaluate the role that repeated resections has on prolonging survival for patients with glioblastoma.
Methods
Patient Selection
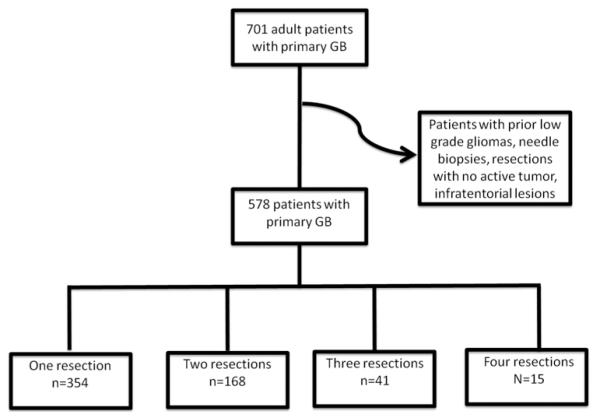
Johns Hopkins Institutional Review Board approval was obtained prior to conducting this study. A total of 701 patients underwent nonbiopsy surgery for an intracranial glioblastoma at a single academic tertiary-care institution between 1997 and 2007. The pathology was determined by a senior neuropathologist in all cases, and the grading criteria were based on the WHO classification system.21,24 Patients at least 18 years old with a tissue-proven diagnosis of a supratentorial glioblastoma (WHO Grade IV) were included in the study. Patients with infratentorial gliomas, multifocal and multicentric lesions, and prior lower-grade gliomas were excluded from the analysis. Patients who underwent biopsies and/or with incomplete medical records lacking clinical presentation, pre- and postoperative MRI, and/or adjuvant therapies were also excluded. Patients who underwent surgeries in which no active tumor was found were also excluded. These exclusions were made to create a more uniform patient population with similar tumor types, tumor location, and treatment strategies. In total, 578 patients met the inclusion and exclusion criteria (Fig. 1).
Fig. 1.
Flow chart demonstrating selection of patients for this study. Seven hundred one adult patients underwent a craniotomy for resection of a primary glioblastoma (GB) at a tertiary-care institution during the reviewed period. Of these 701 patients, 578 met the inclusion/exclusion criteria. During the reviewed period, 354, 168, 41, and 15 patients underwent 1, 2, 3, or 4 resections, respectively, of their glioblastoma. Excluded from the study were patients with incomplete medical records and those who underwent biopsy procedures, had resections with no active tumors, or had infratentorial tumors.
Recorded Variables
The clinical, operative, and hospital course records of the patients who met the inclusion criteria were retrospectively reviewed. The information collected from neurosurgery and neurooncology clinical notes included patient demographics, comorbidities, presenting symptoms, neuroimaging, neurological function, and adjuvant therapy. The KPS score was used to classify the patients’ preoperative functional status.12 The KPS scores were assigned during a chart review by a reviewer blinded to patient outcomes at the clinical visit prior to surgery. A motor deficit was defined as decreased strength, while a language deficit was defined as any combination of receptive and/or expressive aphasia.
The MR images were obtained and reviewed. The characteristics that were recorded included lesion size (largest diameter based on Gd enhancement), specific lobe involvement, and adjacency to the lateral ventricles. The tumor was defined as periventricular if the tumor bordered the lateral or third ventricles on T1-weighted Gd-enhanced MRI, as previously defined,4,8 including basal ganglia and thalamic tumors.4,8 Extent of resection was classified from radiology reports obtained less than 48 hours after resection as either GTR or STR by an independent neuroradiologist blinded to patient outcomes. Subtotal resection and GTR were defined as having residual and no residual enhancement, respectively. This classification was performed by comparing preoperative and postoperative MR images. Resection number was defined as the number of times a patient with known glioblastoma underwent surgery for tumor resection. Patients who underwent biopsies were not classified as having undergone a resection. Surgeries in which final pathological analysis revealed treatment effect and no active tumor were excluded.
The date of death was recorded for any patient whose record was available from the social security index database (http://search.ancestry.com/search/db.aspx?dbid=3693). Time to death was defined as the time from initial glioblastoma diagnosis until death. Patients whose deaths were unconfirmed were classified as lost to follow-up at the time of the last clinic visit.
Perioperative Treatment
The general aim of surgery was to achieve GTR of the tumor when possible. Subtotal resection was achieved primarily when the tumor involved eloquent brain as confirmed by intraoperative mapping and/or monitoring (awake/speech language mapping, direct cortical motor stimulation, and motor evoked or somatosensory evoked potentials). Motor and somatosensory evoked potentials were routinely used in the majority of cases, whereas surgical navigation (CT and/or MRI wand) was used in all cases after 2001. The use of motor mapping or electrocorticography largely depended on the preference of the surgeon. Motor or speech mapping was primarily used when the tumor was near the motor or speech cortex, respectively.
Patients were typically offered repeat surgery for recurrent tumors and to establish diagnosis after case discussion in our Neuro-Oncology Tumor Board, which consists of neurosurgeons, neurooncologists, and radiation oncologists. Recurrent tumors were typically discovered on routine postoperative MRI that was performed at 3-month intervals following surgery, or when symptoms developed including increased headaches, weakness, or other deficits. The use of polifeprosan with carmustine (BCNU) implant therapy was determined by both the surgeon as well as the patient. These carmustine wafers were typically not implanted when tumors were multifocal, extended across the corpus callosum, or required large opening of the ventricle. Likewise, the particular use of adjuvant radiation and chemotherapy was determined by the surgeon, radiation oncologist, medical oncologist, and the patients themselves.
Statistical Analysis
Summary data were presented as mean ± SD for parametric data and as median (IQR) for nonparametric data. For intergroup comparison, the Student t-test was used for continuous data and the Fisher exact test for categorical data. The multivariate proportional hazards regression analysis was used to identify an association between resection number and survival. This analysis was conducted after controlling for preoperative (age, KPS score, and periventricular tumor location),4,8,22,23 intraoperative (extent of resection),6,20,22,25,31 and postoperative factors (carmustine wafer implantation,3,9,27,36 temozolomide chemotherapy,16,34 and radiation therapy14) known to be associated with survival. In addition, to evaluate the role of resection number and survival, a case-control study was performed. Each group was selected by an individual blinded to patient outcomes, and controlled for in terms of age (± 5 years), KPS score (± 0), periventricular tumor (yes/no), GTR (yes/no), and temozolomide/radiation (yes/no). Survival as a function of time was plotted using the Kaplan-Meier method, and a log-rank analysis was used to compare Kaplan-Meier plots (GraphPad Prism 5). Values with a probability < 0.05 in these analyses were considered statistically significant. The statistical program JMP (version 8, SAS Institute Inc.) was used unless otherwise specified.
Results
Preoperative, Perioperative, and Postoperative Characteristics of all Patients
The preoperative characteristics of the patients in this study are summarized in Table 1. A total of 578 patients met the inclusion/exclusion criteria, of which 354 (61%), 168 (29%), 41 (7%), and 15 (3%) patients underwent 1, 2, 3, or 4 resections, respectively. The average age was 55 ± 14 years at the time of initial glioblastoma diagnosis, and 347 (60%) were male. The median preoperative KPS score was 80 (IQR 80–90), and the major presenting symptoms were headaches in 107 (19%), motor deficits in 162 (28%), language deficits in 102 (18%), and seizures in 73 (13%). The average size of the tumor was 4.5 ± 1.6 cm, and 259 (45%) involved the frontal lobe, 123 (21%) the parietal lobe, 164 (28%) the temporal lobe, and 36 (6%) the occipital lobe. The tumor was periventricular in 230 (40%).
TABLE 1.
Preoperative, perioperative, and postoperative characteristics of patients undergoing 1–4 resections of an intracranial glioblastoma*
| Resection No. |
||||
|---|---|---|---|---|
| Characteristics | 1 (n = 354) | 2 (n = 168) | 3 (n = 41) | 4 (n = 15) |
| preop characteristics | ||||
| mean age ± SD at diagnosis (yrs) | 57 ± 15 | 51 ± 12 | 46 ± 11 | 40 ± 11 |
| median KPS score (IQR) | 80 (80–90) | 80 (70–90) | 80 (70–90) | 80 (70–90) |
| motor deficit | 95 (27%) | 50 (30%) | 11 (27%) | 3 (20%) |
| language deficit | 65 (18%) | 24 (14%) | 8 (20%) | 4 (27%) |
| periventricular location | 120 (34%) | 80 (48%) | 16 (39%) | 10 (67%) |
| periop characteristics | ||||
| GTR | 102 (29%) | 35 (21%) | 12 (29%) | 1 (7%) |
| postop characteristics | ||||
| new motor deficit | 24 (7%) | 14 (8%) | 3 (7%) | 2 (13%) |
| new language deficit | 20 (6%) | 8 (5%) | 2 (5%) | 1 (7%) |
| adjuvant therapy | ||||
| carmustine wafer | 115 (32%) | 85 (51%) | 25 (61%) | 11 (73%) |
| temozolomide | 94 (27%) | 74 (44%) | 23 (56%) | 11 (73%) |
| radiation | 230 (65%) | 161 (96%) | 40 (98%) | 15 (100%) |
| surgical site infection survival |
2 (0.6%) | 4 (2%) | 1 (2%) | 1 (7%) |
| dead at last follow-up | 314 (89%) | 146 (87%) | 36 (88%) | 13 (87%) |
| median survival (mos) | 6.8 | 15.5 | 22.4 | 26.6 |
| 6-mo survival rate | 54% | 93% | 88% | 100% |
| 12-mo survival rate | 29% | 65% | 82% | 100% |
| 18-mo survival rate | 18% | 39% | 67% | 64% |
| 24-mo survival rate | 13% | 24% | 45% | 57% |
Preoperative and perioperative outcomes are relative to the last surgery unless otherwise specified. Survival is from the time of initial glioblastoma diagnosis until death. Values in boldface are statistically different from 1 or more of the other resection groups (p < 0.05).
The perioperative and postoperative outcomes are summarized in Table 1. All of these outcomes are listed relative to the last surgery. Gross-total resection was achieved in 150 patients (26%). Two hundred thirty-six patients (41%) had carmustine wafers placed at the time of any of their surgeries, and 202 (35%) underwent temozolomide chemotherapy. Of the 202 patients who received temozolomide, 127 (63%) underwent temozolomide/radiation therapy according to the protocol used by Stupp et al.34 Four hundred forty-six patients (77%) received radiotherapy, with a median dose of 6000 cGy (IQR 5940–6000 cGy).
At last follow-up, 509 (88%) patients had died. The median follow-up time for surviving patients was 10.5 months (IQR 6.5–20.7 months). The median survival of the entire cohort was 10.7 months from the time of glioblastoma diagnosis, where the 6-, 12-, 18-, and 24-month survival rates were 69%, 46%, 29%, and 20%, respectively.
Differences Between Patients Undergoing Different Numbers of Resection
The differences between patients undergoing 1, 2, 3, or 4 resections are summarized in Table 1. Preoperatively, patients undergoing only 1 resection were older than patients undergoing 2 (p = 0.0001), 3 (p = 0.0001), or 4 resections (p = 0.0001) at the time of glioblastoma diagnosis. Likewise, patients undergoing 2 resections were older than patients undergoing 3 (p = 0.01) or 4 resections (p = 0.0001). Patients who underwent 3 resections were not significantly older than patients undergoing 4 resections (p = 0.06). Patients who underwent 1 resection had tumors that were less commonly located adjacent to the ventricles as compared with patients who underwent 2 (p = 0.003) or 4 (p = 0.01) resections, but not 3 resections (p = 0.60). There were no differences between the cohorts in regards to KPS score, motor deficits, and language deficits.
Perioperatively, there were no statistical differences between patients who underwent 1, 2, 3, or 4 resections in regards to extent of resection. Postoperatively, patients who underwent 1 resection had carmustine wafers placed less frequently than patients who underwent 2 (p = 0.0001), 3 (p = 0.0005), or 4 (p = 0.002) resections. Likewise, patients who underwent 1 resection underwent temozolomide therapy less frequently as compared with patients who underwent 2 (p = 0.0001), 3 (p = 0.0002), or 4 (p = 0.0003) resections. Patients who underwent 2 resections also underwent temozolomide therapy less frequently than patients who underwent 4 resections (p = 0.03). Additionally, patients who underwent 1 resection underwent radiation therapy less frequently as compared with patients who underwent 2 (p = 0.0001), 3 (p = 0.0001), or 4 (p = 0.003) resections. There were no other differences in perioperative and postoperative characteristics between the patient resection groups. Notably, this included no significant differences in perioperative deficits or wound infections. Patients who underwent 1 resection did not have a decreased risk of surgical site infection as compared with patients who underwent 2 (p = 0.10), 3 (p = 0.28), or 4 (p = 0.12) resections. Similarly, patients who had 1 resection did not have a decreased risk of new postoperative deficits compared with patients who had 2 (p = 0.89), 3 (p = 0.99), or 4 (p = 0.42) resections.
At last follow-up, there were no significant differences in the percentage of patients who died between the groups. The median survival times of all patients who underwent 1, 2, 3, or 4 resections were 6.8, 15.5, 22.4 and 26.6 months, respectively. However, patients who underwent 1 resection had a shorter survival as compared with patients who underwent 2 (p < 0.0001), 3 (p < 0.0001), or 4 (p = 0.0006) resections. Patients who underwent 2 resections had shorter survival as compared with patients who underwent 3 (p = 0.04) or 4 (p = 0.02) resections. Likewise, patients who underwent 3 resections had poorer survival than patients who underwent 4 resections, but this difference did not reach statistical significance (p = 0.30). The Kaplan-Meier survival curves are illustrated in Fig. 2.
Fig. 2.
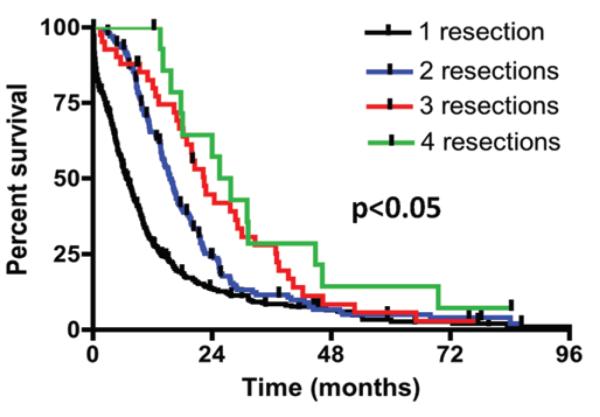
Kaplan-Meier curves for the different number of resections from the time of initial glioblastoma diagnosis. The median survival times for patients undergoing 1, 2, 3, or 4 resections were 6.8, 15.5, 22.4, and 26.6 months, respectively. Patients who underwent 1 resection had significantly shorter survival times than patients who had 2 resections (p < 0.0001), 3 resections (p < 0.0001), or 4 resections (p = 0.0006). Likewise, patients who underwent 2 resections had significantly shorter survival times than patients who had 3 (p = 0.04) or 4 (p = 0.02) resections. Patients who underwent 3 resections had survival times that trended lower than patients who underwent 4 resections, but this difference did not reach statistical significance (p = 0.30).
Association Between Resection Number and Survival
The association between resection number and survival was assessed after controlling for factors known to be associated with survival (age, KPS score, periventricular tumor location, GTR, carmustine wafer implantation, and temozolomide/radiation) in a multivariate proportional hazards regression analysis (Table 2). All resection numbers were significantly associated with survival for all patients with glioblastoma. Patients who underwent only 1 resection experienced significantly decreased survival as compared with patients who underwent a greater number of resections (RR 3.400, 95% CI 2.423–4.774; p < 0.0001). Patients with 2 (RR 0.688, 95% CI 0.525–0.898; p = 0.0006), 3 (RR 0.614, 95% CI 0.388–0.929; p = 0.02), or 4 resections (RR 0.600, 95% CI 0.238–0.853; p = 0.01) had significantly improved chances of prolonged survival as compared with patients with fewer resections.
TABLE 2.
Multivariate analysis of the association between survival and number of resections*
| No. of Resections | RR (95% CI) | p Value |
|---|---|---|
| 1 | 3.400 (2.423–4.774) | <0.0001 |
| 2 | 0.688 (0.525–0.898) | 0.0006 |
| 3 | 0.614 (0.388–0.929) | 0.02 |
| 4 | 0.600 (0.238–0.853) | 0.01 |
The association between resection number and survival was assessed after controlling for age, KPS score, periventricular tumor location, extent of resection, carmustine wafer implantation, and temozolomide/radiation therapy.
Case-Control Evaluation of Survival
Even after evaluating the role of repeated resection for survival in a multivariate analysis, a case-control evaluation was also performed to better evaluate survival curves for patients with 1, 2, or 3 resections given the differences in patient preoperative and postoperative characteristics. The number of patients who underwent 4 resections was too small to perform case-control analyses. Each cohort (1, 2, and 3 resections) was controlled for in regard to age (± 5 years), KPS score (± 0), periventricular tumor (yes/no), GTR (yes/no), and temozolomide/ radiation therapy (yes/no). There were no significant differences in preoperative and perioperative characteristics between patients who had 1, 2, or 3 resections (Table 3), which notably included surgical site infection (p > 0.05) and iatrogenic deficits (p > 0.05). Patients who underwent 1 resection did not have significantly fewer surgical site infections than matched patients who had 2 (p = 0.45) or 3 (p = 0.99) resections. Likewise, patients who underwent 1 resection did not have significantly fewer iatrogenic deficits than patients who had 2 (p = 0.99) or 3 (p = 0.99) resections. The median survival for patients in this case-control evaluation who underwent 1, 2, or 3 resections was 4.5, 16.2, and 24.4 months (p < 0.05; Fig. 3). Patients who underwent 1 resection had a significantly shorter survival as compared with patients with 2 (p = 0.002) or 3 (p = 0.0001) resections. Patients with 2 resections had significantly shorter survival as compared with patients with 3 resections (p = 0.05).
TABLE 3.
Case-control evaluation*
| Resection No. |
|||
|---|---|---|---|
| Characteristics | 1 (n = 37) | 2 (n = 37) | 3 (n = 37) |
| preop characteristics | |||
| mean age ± SD at diag- nosis (yrs) |
48 ± 11 | 48 ± 11 | 47 ± 12 |
| median KPS score (IQR) | 80 (70–90) | 80 (70–90) | 80 (70–90) |
| motor deficit | 10 (27%) | 10 (27%) | 9 (24%) |
| language deficit | 6 (16%) | 5 (14%) | 6 (16%) |
| periventricular location | 14 (38%) | 14 (38%) | 14 (38%) |
| periop characteristics | |||
| GTR | 7 (19%) | 7 (19%) | 7 (19%) |
| postop characteristics | |||
| new motor deficit | 2 (5%) | 1 (3%) | 2 (5%) |
| new language deficit | 0 (0%) | 1 (3%) | 1 (3%) |
| adjuvant therapy | |||
| carmustine wafer | 11 (30%) | 12 (32%) | 10 (27%) |
| temozolomide | 21 (57%) | 21 (57%) | 21 (57%) |
| radiation | 37 (100%) | 37 (100%) | 37 (100%) |
| surgical site infection survival |
0 (0%) | 2 (5%) | 1 (3%) |
| dead at last follow-up | 31 (84%) | 32 (86%) | 32 (86%) |
| median survival (mos) | 4.5 | 16.2 | 24.4 |
| 6-mo survival rate | 40% | 94% | 86% |
| 12-mo survival rate | 26% | 69% | 81% |
| 18-mo survival rate | 16% | 43% | 66% |
| 24-mo survival rate | 12% | 34% | 50% |
Groups were matched for age, preoperative KPS score, periventricular tumor location, extent of resection, and temozolomide/radiation therapy. Preoperative and perioperative outcomes are relative to the last surgery. Survival is from the time of initial glioblastoma diagnosis until death. With the exception of overall survival (p < 0.05), there were no significant differences between the groups.
Fig. 3.
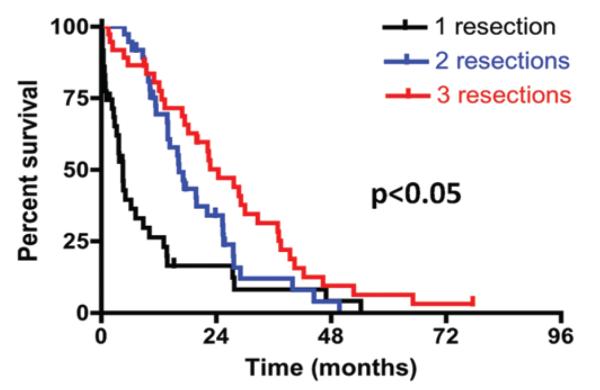
Kaplan-Meier curves for patients who underwent 1, 2, or 3 resections from the time of initial glioblastoma diagnosis. Groups were matched for age, preoperative KPS score, periventricular tumor location, extent of resection, and temozolomide/radiation therapy. The median survival was 4.5, 16.2, and 24.4 months for patients who underwent 1, 2, or 3 resections, respectively. Patients who underwent 1 resection experienced significantly shorter survival than patients with 2 (p = 0.002) or 3 (p = 0.0001) resections. Patients who underwent 2 resections had significantly shorter survival times than patients with 3 resections (p = 0.05).
Discussion
In this study of 578 patients with primary glioblastoma, 354, 168, 41, and 15 patients underwent 1, 2, 3, or 4 resections, respectively. At last follow-up, the median survival for patients who underwent 1, 2, 3, or 4 resections was 6.8, 15.5, 22.4, and 26.6 months, respectively. This difference in survival between groups was statistically significant; patients who underwent a greater number of resections had significantly longer survival times. In multivariate analysis, even after controlling for factors known to be associated with survival, patients who underwent more resections survived longer. Additionally, in the case-control study, after selecting for factors known to be associated with survival, patients with more resections survived longer as compared with patients with fewer resections. Importantly, there were no significant differences in the development of postoperative deficits and/or surgical site infections among the resection cohorts.
Glioblastomas are unique tumors composed of a heterogeneous population of cells with differential reproductive, invasive, and migratory potential.30 Glioblastoma cells have the propensity to migrate and invade normal brain parenchyma well beyond radiographic boundaries.30 This individual cell invasion makes curative resection difficult. These tumors will still recur even after resection beyond radiographic boundaries.10,25 Despite this recurrence, there is an increasing body of data supporting extensive resection for patients with glioblastoma.6,20,22,25,31 These studies show that GTR of these tumors results in prolonged survival as compared with STR and even near-total resection.6,20,22,25,31 This better survival is believed to be due in part to reduced tumor burden, which prolongs recurrence and possibly makes adjuvant therapy more effective, including radiation and chemotherapy.6,20,22,25,31 However, an immediate risk of extensive resection is the development of iatrogenic neurological deficits. The development of postoperative deficits is associated with poor survival for patients with glioblastoma.5,7,26 This risk of iatrogenic deficits, combined with inevitable recurrence, presumably minimizes the utility of repeated resection.
Previous studies evaluating the role of repeat resections in prolonging survival are few and limited (Table 4).1,2,17,19,28,33,37 Barker and colleagues2 found that the median survival for 46 patients who underwent a 1-time reoperation for glioblastoma was 36 weeks as compared with 23 weeks for patients undergoing only 1 surgery (p = 0.03). Likewise, Helseth et al.19 found that survival was longer for 65 patients who underwent repeat surgery than for 451 patients who only underwent 1 surgery or biopsy (18.4 vs 8.6 months, respectively). Hau and colleagues17 used a matched-pair analysis to evaluate whether salvage therapy prolonged survival. Groups were selected for age, KPS score, extent of resection, and use of chemotherapy, and salvage therapy consisted of some combination of surgery, radiation, and second-line chemotherapeutic drugs.17 Forty-six patients who underwent reintervention or salvage therapy had a median survival of 65.5 weeks, whereas 46 matched patients undergoing only 1 intervention had a median survival of 28.5 weeks (p = 0.05).17 Additionally, Stark et al.33 found that reoperation was significantly associated with improved survival among 72 of 267 patients with glioblastoma who underwent repeat surgery in multivariate analysis. Patients with multiple reoperations (> 2) were not routinely included in these studies.2,17,19,33
TABLE 4.
Summary of studies of repeated resections and survival for patients with glioblastoma
| Authors & Year | No. of Patients | Multivariate Analysis | Included >2 Resections | Studied >2 Resections Separately |
|---|---|---|---|---|
| studies supporting repeat resections | ||||
| present study | 578 | yes | yes | yes |
| Barker et al., 1998 | 301 | yes | no | no |
| Hau et al., 2003 | 168 | no | no | no |
| Helseth et al., 2010 | 516 | yes | no | no |
| Stark et al., 2005 | 267 | yes | no | no |
| studies opposing repeat resections | ||||
| Azizi et al., 2001 | 112 | no | yes | no |
| Wong et al., 1999 | 225 | yes | yes | no |
Despite these studies, other studies have found no association between repeat surgery and survival.1,37 Azizi et al.1 found no statistical difference in survival among 162 total patients with either glioblastomas or anaplastic astrocytomas who underwent biopsy, 1 resection, or multiple resections when comparing survival curves. Wong and colleagues37 studied 375 patients with high-grade gliomas (225 glioblastomas, 150 anaplastic astrocytomas), and found that patients who had 2 or more surgeries or chemotherapeutic regimens had poorer overall survival. Nieder and colleagues28 performed a review of the literature and found no evidence to suggest that reoperation would prolong survival. Other studies only evaluate the role of chemotherapeutics at the time of reoperation, making it difficult to discern if repeated resection has an effect on survival.3,15,35
Clinical Recommendations
This study demonstrates the efficacy of repeated resections for patients with glioblastoma. After a discussion with the patients, their families, and treatment teams, the majority of patients who can tolerate surgery should be offered repeated resections. The goals of these repeated resections should be to safely operate on these patients with recurrent tumors, obtain tissue for diagnosis, and resect as much tumor safely without endangering neurological function. These repeated debulking procedures may not only decrease tumor burden, but also increase the efficacy of adjuvant therapies including radiation, temozolomide, and other types of chemotherapy. Repeated resections can be achieved with no significant increase in postoperative deficits or wound infections.
Strength and Limitations of the Study
We believe this study provides several useful insights. First, the role of repeated surgeries in prolonging survival is poorly understood. This study shows that patients with more resections have improved survival as compared with patients with fewer resections. These patients also do not have an increased risk of postoperative deficits and/or surgical site infections. Second, studies that do evaluate the role of repeated surgeries are limited by small patient numbers and lack of multivariate analyses.1,2,17,19,28,33 The present study is the largest study to date to evaluate the association between repeated resection and survival. It also uses multivariate analyses and controls for factors known to be associated with survival to better understand the utility of repeated resections. Third, studies on repeated surgeries often only focus on second surgeries or else group repeated surgeries into the same cohort, which may obscure findings.1,2,17,19,28,33 This study evaluates patients who underwent up to 4 resections, and each resection number represents its own cohort. This study was designed in this way to better understand if increasing number of resections was associated with improved survival. Lastly, this study may provide useful information that may help guide treatment strategies aimed at prolonging survival for patients harboring glioblastoma.
This study, however, has some limitations. One limitation is that these findings only apply to patients undergoing craniotomy for surgery in which active tumor is found. Patients who underwent craniotomies where no active tumor was found were excluded. These findings are also not necessarily applicable to patients undergoing biopsies of their lesion or those undergoing conservative management of their tumors. This study also does not evaluate patients with previously diagnosed lower-grade gliomas; it only evaluates those patients who were diagnosed initially with glioblastoma to minimize the confounding effect for improved survival for patients with lower-grade lesions. An additional limitation is that this study does not analyze the prognostic implication of molecular markers and genotypes, which may be a better indicator of patient outcomes. In recent studies, patients with glioblastoma and O6-methylguanine–DNA methyltransferase promoter methylation had prolonged survival after temozolomide and radiation therapy as compared with patients without this molecular marker.18 More recently, Parsons et al.29 performed a genomic analysis on patients with glioblastoma and found that patients with isocitrate dehydrogenase 1 mutations had prolonged survival times. These molecular markers and others may also be associated with survival, but were not analyzed in this study. Furthermore, the patients in this study underwent disparate treatment regimens. The majority of patients in this study did not undergo GTR and/or receive triple combinatorial adjuvant therapy (carmustine wafer, temozolomide, and radiation). Patients who underwent more resections were treated with more aggressive adjuvant therapies. As a result, the relevance of repeated resections may be altered in the context of those patients receiving the most aggressive of treatment regimens. Finally, this study is inherently limited by its retrospective design, and, as a result, it is not appropriate to infer direct causal relationships. We acknowledge that there may be an inherent bias associated with patient selection, in which patients who were offered repeated surgeries may have a propensity for better outcomes. However, we tried to create a uniform patient population by utilizing strict inclusion and exclusion criteria, thus providing more relevant information for patients undergoing repeat resections for glioblastoma. We included only patients who underwent nonbiopsy resection of active intracranial tumors. In addition, we excluded patients with incomplete medical records, prior history of lower-grade gliomas, and pediatric patients. Furthermore, we performed multivariate analyses to control for potential confounding variables, which included disparate treatment regimens and factors associated with survival. We also performed a case-control analysis to try to minimize these biases. Given these statistical controls and a relatively precise outcome measure, we believe our findings offer useful insights into the role that repeat surgery has for patients with glioblastoma. However, prospective studies are needed to provide better data to guide clinical decision making.
Conclusions
Patients with glioblastoma have tumors that will inevitably recur regardless of the extent of resection. This propensity to recur has made many surgeons hesitant to offer repeated resections. The present study shows that repeated resections, at least up to 4 times, can significantly prolong survival for patients with glioblastoma. Patients who underwent an increasing number of resections had increased survival benefit regardless of age, functional status, and other factors. These resections can occur with no increase in morbidity or death. These findings, however, may be limited by an inherent bias in patient selection, which may favor patients with more benign tumor biology. We attempted to minimize these limitations by using strict inclusion criteria, multivariate analyses, and case-control evaluations. Therefore, despite this potential source of bias, these findings may help guide treatment strategies aimed at prolonging survival for patients with this devastating disease.
Abbreviations used in this paper
- GTR
gross-total resection
- IQR
interquartile range
- KPS
Karnofsky Performance Scale
- RR
relative risk
- STR
subtotal resection
Footnotes
Disclosure The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Chaichana. Acquisition of data: Chaichana, Zadnik. Analysis and interpretation of data: Chaichana, Zadnik, Weingart, Blakeley, Lim, Brem, Quiñones-Hinojosa. Drafting the article: Chaichana, Zadnik, Olivi, Gallia, Lim, Quiñones-Hinojosa. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Chaichana. Statistical analysis: Chaichana, Zadnik. Administrative/technical/material support: Quiñones-Hinojosa. Study supervision: Chaichana, Quiñones-Hinojosa.
References
- 1.Azizi A, Black P, Miyamoto C, Croul SE. Treatment of malignant astrocytomas with repetitive resections: a longitudinal study. Isr Med Assoc J. 2001;3:254–257. [PubMed] [Google Scholar]
- 2.Barker FG, II, Chang SM, Gutin PH, Malec MK, McDermott MW, Prados MD, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42:709–723. doi: 10.1097/00006123-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 4.Chaichana K, Parker S, Olivi A, Quiñones-Hinojosa A. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. Clinical article. J Neurosurg. 2010;112:997–1004. doi: 10.3171/2009.9.JNS09805. [DOI] [PubMed] [Google Scholar]
- 5.Chaichana KL, Chaichana KK, Olivi A, Weingart JD, Bennett R, Brem H, et al. Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Clinical article. J Neurosurg. 2011;114:587–594. doi: 10.3171/2010.8.JNS1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaichana KL, Garzon-Muvdi T, Parker S, Weingart JD, Olivi A, Bennett R, et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. 2011;18:239–245. doi: 10.1245/s10434-010-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaichana KL, Halthore AN, Parker SL, Olivi A, Weingart JD, Brem H, et al. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg. 2011;114:604–612. doi: 10.3171/2010.4.JNS091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 9.Chaichana KL, Zaidi H, Pendleton C, McGirt MJ, Grossman R, Weingart JD, et al. The efficacy of carmustine wafers for older patients with glioblastoma multiforme: prolonging survival. Neurol Res. 2011;33:759–764. doi: 10.1179/1743132811Y.0000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA. 1928;90:823–825. [Google Scholar]
- 11.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 12.Dutta D, Vanere P, Gupta T, Munshi A, Jalali R. Factors influencing activities of daily living using FIM-FAM scoring system before starting adjuvant treatment in patients with brain tumors: results from a prospective study. J Neurooncol. 2009;94:103–110. doi: 10.1007/s11060-009-9810-y. [DOI] [PubMed] [Google Scholar]
- 13.Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 2005;3:721–728. doi: 10.6004/jnccn.2005.0042. [DOI] [PubMed] [Google Scholar]
- 14.Genc M, Zorlu AF, Atahan IL. Accelerated hyperfractionated radiotherapy in supratentorial malignant astrocytomas. Radiother Oncol. 2000;56:233–238. doi: 10.1016/s0167-8140(00)00198-5. [DOI] [PubMed] [Google Scholar]
- 15.Harsh GR, IV, Levin VA, Gutin PH, Seager M, Silver P, Wilson CB. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615–621. doi: 10.1227/00006123-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD007415. CD007415. [DOI] [PubMed] [Google Scholar]
- 17.Hau P, Baumgart U, Pfeifer K, Bock A, Jauch T, Dietrich J, et al. Salvage therapy in patients with glioblastoma: is there any benefit? Cancer. 2003;98:2678–2686. doi: 10.1002/cncr.11845. [DOI] [PubMed] [Google Scholar]
- 18.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 19.Helseth R, Helseth E, Johannesen TB, Langberg CW, Lote K, Rønning P, et al. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand. 2010;122:159–167. doi: 10.1111/j.1600-0404.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 20.Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52:371–379. doi: 10.1016/s0090-3019(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 21.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–229. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 23.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. Clinical article. J Neurosurg. 2009;110:156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 26.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–470. doi: 10.1227/01.NEU.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 27.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. Clinical article. J Neurosurg. 2009;110:583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26:397–409. doi: 10.1053/ctrv.2000.0191. [DOI] [PubMed] [Google Scholar]
- 29.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiñones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. Clinical article. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 32.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6:1087–1104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- 33.Stark AM, Nabavi A, Mehdorn HM, Blömer U. Glioblastoma multiforme—report of 267 cases treated at a single institution. Surg Neurol. 2005;63:162–169. doi: 10.1016/j.surneu.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 35.Subach BR, Witham TF, Kondziolka D, Lunsford LD, Bozik M, Schiff D. Morbidity and survival after 1,3-bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case-matched cohort series. Neurosurgery. 1999;45:17–23. doi: 10.1097/00006123-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term followup of a multicenter controlled trial. Acta Neurochir (Wien) 2006;148:269–275. doi: 10.1007/s00701-005-0707-z. [DOI] [PubMed] [Google Scholar]
- 37.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:257–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]