Abstract
Adolescents display high levels of interactions with peers relative to other age groups, with these interactions further enhanced by ethanol under some circumstances. Understanding of the neural mechanisms underlying these high levels of social interactions is important given that alcohol use is initiated during adolescence and adolescents tend to report drinking for social reasons. Given that ethanol’s effects are associated in part with functional antagonism of the NMDA receptor system, the current experiment explored the role of NMDA antagonists for facilitating adolescent social behavior. Adolescent male Sprague-Dawley rats were challenged acutely with either the non-competitive NMDA antagonist, MK-801 (0.01, 0.03 mg/kg), the NR2A antagonist, PEAQX (1.25, 3.75 mg/kg) or the NR2B antagonist, ifenprodil (0.75, 2.25 mg/kg) 30 min prior to a 10-min social interaction test. All compounds generally increased overall social activity (i.e., sum of social investigation, contact behavior, and play), with ifenprodil also significantly enhancing play and social contact behaviors. Although the frequencies of peer-directed social behaviors were typically greater following administration with these NMDA antagonists, social preference, indexed via the number of crossovers to the side with the partner relative to crossovers away, was significantly reduced in MK-801 and PEAQX-treated rats. None of these changes were associated with concomitant alterations in overall locomotor activity under these test circumstances. These data support the suggestion that the increases in social interactions observed in adolescents following acute ethanol may be driven in part by NMDA receptor antagonism—particularly of the NR2B subunit—given that ifenprodil stimulated social behavior in a manner similar to that produced by low doses of ethanol.
Keywords: Adolescence, social interactions, rat, MK-801, PEAQX, ifenprodil
The developmental period of adolescence is characterized as a time during which interactions with peers are particularly high in humans and other mammalian species such as rodents [1–4]. In addition to the high levels of social interactions observed during this time, alcohol consumption is also higher during adolescence relative to adults in both humans [5] and laboratory animals [6, 7]. Human adolescents not only spend more time interacting with peers, but report consuming alcohol mostly for social reasons (i.e., for social facilitation and to make social situations more enjoyable) (see [8], for review). Adolescent rats are likewise more sensitive to the social facilitation induced by low doses of ethanol [4, 9–11], although they are conversely less sensitive than adults to many other effects of ethanol that emerge at higher doses, including ethanol-induced social inhibition [4]. For example, in Sprague-Dawley rats, low doses of ethanol (0.25–0.5 g/kg) have been found to increase social interactions under familiar test circumstances in adolescents, an effect not normally observed in adults, whereas adults are more sensitive to the suppression in social behavior that emerges at higher ethanol doses, as well as to the anxiolytic effects of ethanol and other anxiolytic drugs [4, 9, 10]. For an example of the latter, the reductions in overall social interactions of adolescent and adult rats that were observed when tested in an unfamiliar, anxiogenic environment were reversed by ethanol in adults, but not adolescent rats [4]. Likewise, total time spent in overall social interactions in an unfamiliar environment was increased in adults after treatment with the benzodiazepine agonist, diazepam, whereas behavior was unaffected in adolescents [12].
Although several neural systems (e.g., opioid, cannabinoid) have been shown to modulate social facilitation in young rodents [3, 13], the current experiment explored the potential for N-methyl-D-aspartate (NMDA) receptor antagonists to facilitate social interactions, given that ethanol’s effects are partly associated with NMDA receptor antagonism [14] and given the relationship between this neural system and many ethanol-related behaviors [15, 16]. Indeed, NMDA antagonists have consistently been shown to have social inhibitory effects in adult rodents [17, 18] (similar to those observed at moderate or higher doses of ethanol [4]), whereas a biphasic effect with a low dose stimulation of social behavior was reported in one of the few studies that investigated effects of a NMDA antagonist on social behavior in young rodents [19]. In that study, the non-competitive NMDA antagonist, MK-801 induced an increase in play behavior in juveniles at a low dose (0.025 mg/kg), but a suppression in social behavior at higher (0.1 and 0.2 mg/kg) doses [19]. These results resemble biphasic effects of ethanol on social behavior that are observed in adolescent rats [4, 9]. Given that ethanol’s effects on NMDA function are subunit specific, with the NR2A and NR2B subunits having the strongest evidence for ethanol’s actions [20], the current experiment investigated the potential socially facilitating effects not only of MK-801, but also antagonists selective for the NR2A and NR2B subunits (PEAQX and ifenprodil, respectively) in adolescent male rats.
Acute effects of low doses of the three NMDA antagonists (MK-801: 0.01, 0.03 mg/kg; PEAQX: 1.25, 3.75 mg/kg; ifenprodil: 0.75, 2.25 mg/kg) on social behavior was investigated in adolescent male Sprague-Dawley rats (initial n=10/dose; after outliers n=8–9/dose for MK-801; n=9–10/dose for ifenprodil; n=10/dose for PEAQX) using a modified social interaction test [4] and a repeated test design. The non-competitive NMDA antagonist, (+) MK 801(Dizocilpine) (Tocris) and the NR2A antagonist, PEAQX tetrasodium hydrate (Sigma Aldrich), were dissolved in 0.9% saline, whereas sterile water was used as a vehicle for the NR2B antagonist, ifenprodil hemitartrate (Tocris). All drugs were injected at a volume of 2 ml/kg, with MK-801 and ifenprodil injected intraperitoneally and PEAQX administered subcutaneously at room temperature. The social testing apparatus consisted of two compartments with an aperture in the partition that allowed animals to move between compartments [4]. To ensure that testing occurred in a familiar environment, experimental and partner animals were habituated individually to the social testing apparatus for 30 min on postnatal day (P) 33. On P34, (baseline), each experimental animal was weighed, injected with the route and vehicle associated with its drug assignment, and immediately placed into the social testing apparatus alone for 30 min. At 30 min post-injection, a non-manipulated unfamiliar partner of approximately the same weight (within 10 g) was placed into the apparatus for the 10-min videotaped session. Upon completion of the 10-min social interaction test, animals were housed back together with their littermate until testing the next day. On the following day (P35: test), the same procedure was used as on baseline, except that experimental animals were challenged with the appropriate drug dose and exposed to the same partner as on baseline. This two-day test procedure has been previously shown to reveal similar levels of social behavior across days in vehicle-treated adolescents (baseline: 115.44±13.24; test: 115.67±9.82) and adults (baseline: 87.60±4.08; test: 81.30±6.01) under these familiar test circumstances, while resulting in significant decreases in social behaviors following high doses of ethanol or NMDA antagonists on test day relative to baseline [21]. Thus, while experimental animals were exposed to the same partner on test day, their social behavior remained unchanged across the two days and therefore any differences in behavior (i.e., increases on test day) would be attributed to the compound administered and not general to changes in social behavior.
Social behaviors were later scored by an experimenter blind to the dose/drug condition of each animal and included play: pinning, chasing, pouncing; social contact: crawling over/under a social partner; and social investigation: sniffing any part of the partner’s body. In addition, a social preference/avoidance coefficient [Coefficient (%) = (crossovers to the same side of the apparatus as the partner − crossovers away from the side where the partner was located)/(crossovers to the partner + crossovers away from the partner) × 100] was calculated and used as an index of social motivation, with positive scores reflecting a relative preference and negative scores indicating relative avoidance of the social partner [4, 10]. To confirm that any changes in social behavior following administration of these antagonists was not due to changes in motor activity, an index of locomotor activity under these social test circumstances was calculated by summing the number of crossovers between compartments made by the experimental animal during the session [4, 10].
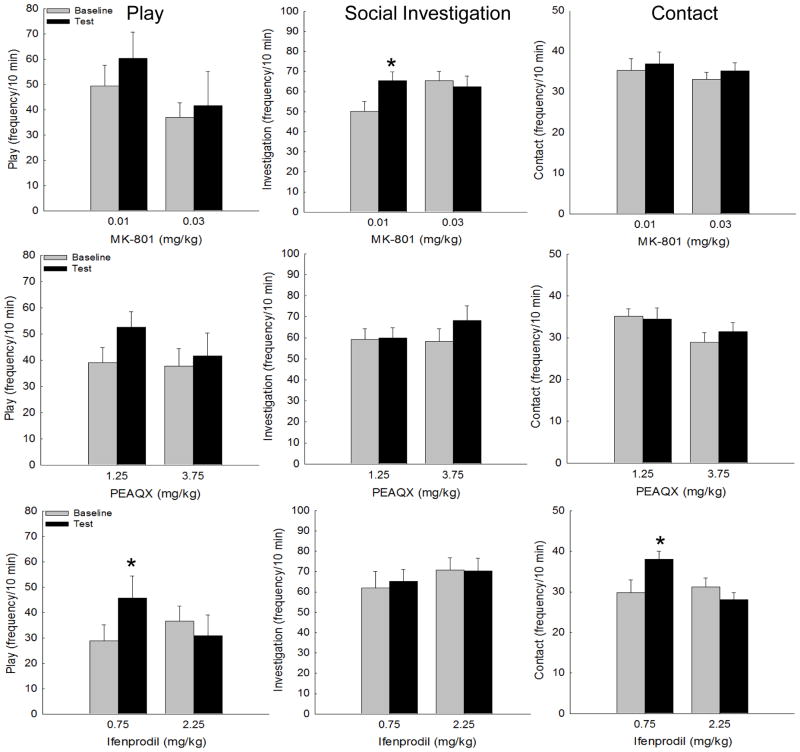
The repeated measures ANOVA for overall social activity (sum of social investigation, contact behavior, and play frequencies) for MK-801-challenged rats revealed a main effect of day [F(1,15) = 4.39, p≤0.05], with overall social activity significantly higher on the test day (MK-801 challenge) than on the vehicle-exposed baseline day, although this effect appears to be driven primarily by the lower dose. A similar finding was observed for adolescents injected with the NR2A antagonist, PEAQX: main effect of day [F(1,18) = 6.62, p<0.05]. In ifenprodil-challenged adolescents, a day X dose interaction emerged [F(1,17) = 5.16, p<0.05], with only the 0.75 mg/kg dose increasing overall social activity on the ifenprodil challenge test day (Figure 1). MK-801 at either dose did not impact play or contact behavior; however, a day X dose interaction emerged for social investigation [F(1,15) = 7.72, p<0.05], with 0.01 mg/kg MK-801 significantly increasing this behavior on test day (Figure 2, top middle panel). While there was a trend for PEAQX to increase play behavior at the 1.25 mg/kg dose, no significant main effects or interactions emerged for any of the social behaviors examined at any dose tested. The ifenprodil doses examined were ineffective at altering social investigation, although both play and contact behaviors were increased following 0.75 mg/kg of this NR2B antagonist [day X dose interaction: F(1,17) = 5.99, p<0.05] and F(1,17) = 5.80, p<0.05, for play and contact behavior, respectively] (Figure 2, bottom left and right panels). Both doses of MK-801 and PEAQX reduced social preference on test day [main effect of day: F(1,17) = 7.65, p<0.05 and F(1,16) = 4.35, p<0.05, respectively], whereas ifenprodil was without effect (Table 1). Ifenprodil was the only drug that altered locomotion under social circumstances, with both doses significantly reducing total crossovers on test day relative to the vehicle baseline day [main effect of day F(1,17) = 10.13, p<0.01] (Table 1).
Figure 1.
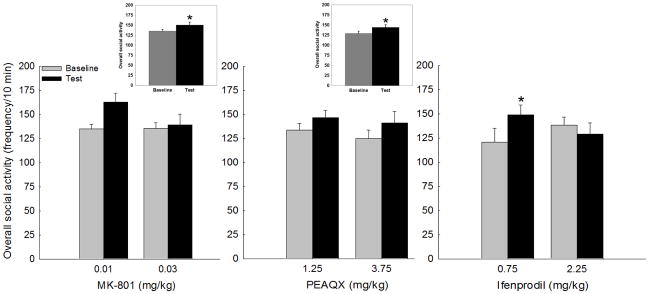
Overall social activity (frequency of play, social investigation and contact) in adolescent male rats following an acute challenge with MK-801, PEAQX, or ifenprodil. Regardless of dose, MK-801 and PEAQX resulted in a significant increase in overall social activity (see inserts), whereas only the 0.75 mg/kg dose of ifenprodil increased social interactions. * indicates a significant difference between baseline and test day. p<0.05
Figure 2.
Play, social investigation, and contact behaviors (frequency) in adolescent rats. Social investigation was significantly increased by 0.01 mg/kg MK-801 (top, middle panel). While no significant main effects or interactions emerged for PEAQX, adolescents challenged with 0.75 mg/kg ifenprodil demonstrated increases in play and contact behaviors (bottom, left and right panels). * indicates a significant difference between baseline and test day.
Table 1.
Total crossovers (mean±SEM) and social preference in adolescent male rats challenged acutely with either MK-801, PEAQX, or ifenprodil
| Crossovers | ||||||
|---|---|---|---|---|---|---|
| MK-801 (0.01 mg/kg) | MK-801 (0.03 mg/kg) | PEAQX (1.25 mg/kg) | PEAQX (3.75 mg/kg) | Ifenprodil (0.75 mg/kg) | Ifenprodil (2.25 mg/kg) | |
| Baseline | 46.00±4.77 | 37.36±4.74 | 49.70±3.72 | 39.50±2.94 | 41.40±4.93 | 38.11±4.06 |
| Test | 43.50±3.57 | 35.50±1.97 | 47.50±3.61 | 38.00±3.82 | 38.00±3.77* | 29.78±4.80* |
| Social Preference Coefficient | ||||||
|---|---|---|---|---|---|---|
| MK-801 (0.01 mg/kg) | MK-801 (0.03 mg/kg) | PEAQX (1.25 mg/kg) | PEAQX (3.75 mg/kg) | Ifenprodil (0.75 mg/kg) | Ifenprodil (2.25 mg/kg) | |
| Baseline | 36.87±6.53 | 47.59±5.44 | 35.96±3.88 | 30.52±5.56 | 39.86±7.62 | 40.52±6.08 |
| Test | 34.24±5.25* | 30.31±3.01* | 30.29±5.59* | 22.41±6.18* | 32.10±8.21 | 35.15±7.15 |
NOTE: Asterisk indicates a main effect of day, reflecting a significant difference between baseline and test day collapsed across dose (p<0.05).
These results support a role for the NMDA receptor in modulating social behaviors in males during the adolescent period. The data are reminiscent of the previously reported finding that low doses of MK-801 increase social behavior in juvenile rats [19], and extend those results by demonstrating that antagonists targeting the NR2A and NR2B subunits also produce social facilitation in adolescents. Furthermore, although all three antagonists were generally found to significantly increase overall social activity, when specific social behaviors were examined, only the NR2B antagonist ifenprodil increased play and contact behaviors. Play and contact are social behaviors that are particularly prevalent early in life, gradually declining thereafter, whereas social investigation typically increases into adulthood [22]. Of these behaviors, play is generally the most sensitive to facilitation by ethanol in adolescent animals [4]. The different responses observed in the specific social behaviors may be due to ontogenetic differences in expression of the NR2 subunits, with the NR2A subunit being barely detectable at birth, then increasing with age, whereas the NR2B subunit is strongly expressed at birth, and at levels higher than those seen in adulthood in some brain regions [23]. Unfortunately, little is known of the functional ontogeny of NR2A subunit-containing NMDA receptors, in part because of the relative lack of NR2A-specific antagonists, making it difficult to draw strong conclusions on age differences in sensitivity to pharmacological blockade of this subunit at this time. For instance, although PEAQX was originally described as having a strong selectivity for the NR2A subunit, it has recently been revealed that its ability to discriminate between the NR2B-containing subunits is actually small (~5 fold) [24]. More work has investigated the role of the NR2B subunit in developmental research and its relationship with ethanol-related behaviors, and the present findings complement and extend those findings. For example, our laboratory has found that adolescents require a higher dose of the NR2B antagonist ifenprodil than adults to block ethanol-induced acute tolerance when indexed via motor impairment [16]. Similar to the adolescent insensitivity observed in that study, we have recently observed that adolescents were also less sensitive than adults to the social inhibition emerging at higher doses of ifenprodil (Morales and Spear, in prep), differences that may be due to the ongoing alterations in NMDA receptor systems occurring throughout adolescence [23]. These data, in conjunction with the present results, are consistent with the suggestion that developmental differences in sensitivity to ethanol’s effects may be associated in part with ontogenetic differences in sensitivity to NR2B subunit manipulations—including not only the adolescent insensitivity to aversive effects seen at higher doses (i.e., social inhibition, motor impairment), but also to the social facilitation that emerges at low doses.
The observed increases in social interactions seen following these drugs as well as the decreases in social motivation seen after MK-801 and PEAQX are not attributable to general changes in locomotor activity under these test circumstances. MK-801 and PEAQX had no effect on total number of crossovers, whereas ifenprodil increased social behaviors while also significantly reducing crossovers in the social test regardless of dose (although this effect appears to be driven primarily by the 2.25 mg/kg dose). This suggests that the social facilitation induced by these NMDA antagonists is separable from general alterations in locomotor activity.
Interestingly, social preference was significantly reduced after administration of both MK-801 and PEAQX, but not ifenprodil. While our laboratory has consistently reported that the social preference/avoidance coefficient is sensitive to anxiogenic manipulations (e.g., restraint stress or testing in an unfamiliar environment) [4, 10, 25], the amount of social avoidance induced by the manipulations in those studies was considerably more pronounced than the modest declines in social preference observed in the current experiments, suggesting that these slight reductions in social motivation were unlikely to be associated with notable drug-induced anxiogenesis. Further support for the idea that the lower levels of social motivation did not reflect anxiogenic effects, per se, is that the reductions in social motivation observed previously in our laboratory following anxiety-provoking manipulations were also accompanied by significant decreases in social investigation, another behavioral measure sensitive to anxiogenic manipulations and anxiolytic compounds (e.g., ethanol) [4, 10, 25]. In contrast, no declines in social investigation were seen following MK-801 or PEAQX, with adolescents even showing a significant increase in social investigation at the 0.01 mg/kg dose of MK-801—although the elevated levels of social investigation on test day after the low dose of MK-801 was likely driven by baseline differences. Nevertheless, although uncommon, other reports of drugs eliciting both an anxiolytic and anxiogenic effect at the same dose have been reported [26]. For example, the same dose (5 mg/kg) of MDMA (‘ecstasy’) has been shown both to increase time spent in social interactions (an anxiolytic effect) as well as to reduce time spent in the open arms of an elevated plus maze test (an anxiogenic effect) [26], supporting the possibility that under some circumstances drugs may be able to induce both anxiolytic and anxiogenic effects.
Given that we generally do not observe sex differences in social activity [4, 9], only males were used in the present experiment, so that females could be utilized in other studies. Still, it should be noted that sex differences in responsivity to NMDA antagonists have been observed using other behavioral tests. For instance, female mice were shown to be less sensitive to NMDA antagonism of analgesic responses than males [27]. In terms of locomotor activity, adolescent (P30) females challenged with 0.1 mg/kg MK-801 showed significantly higher levels of locomotion and for a longer duration relative to adolescent males [28]. The literature suggests that sex differences do exist in response to NMDA antagonists—at least under the cited conditions—and should be considered in future studies examining the role of this receptor system on behaviors.
The overall findings of this experiment are consistent with the suggestion that the increases in social interactions observed after low doses of ethanol in adolescents may be associated, at least in part, with ethanol-induced inhibition of the NMDA receptor system. The NR2B subunit appears to be of particular importance given that similar socially facilitating effects were observed with ifenprodil that are observed after low doses of ethanol [4]. Such developmental differences in sensitivity to the socially facilitating effects of ethanol along with insensitivity to the aversive effects may help promote the enhanced ethanol intake that often characterizes this developmental period. Understanding the social motives underlying drinking and their associated neural mechanisms are likely to prove key for determining why adolescent alcohol consumption patterns differ from other age populations.
Highlights.
Low doses of MK-801, PEAQX, and ifenprodil increased overall social interactions
Only the NR2B antagonist, ifenprodil, increased play and social contact
These effects on social interactions were not due to alterations in locomotion
Increases in ethanol-induced social behavior may be driven partly by NMDA blockade
Acknowledgments
The work presented in this manuscript was funded by grant P50-AA017823 to LPS
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. Journal of Youth and Adolescence. 1997;6:281–4. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- 2.Berndt TJ. The Features and Effects of Friendship in Early Adolescence. Child Development. 1982;53:1447–60. [Google Scholar]
- 3.Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcoholism, clinical and experimental research. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism, clinical and experimental research. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 5.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- 6.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism, clinical and experimental research. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 7.Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism, clinical and experimental research. 2007;31:1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clinical psychology review. 2005;25:841–61. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Varlinskaya EI, Spear LP. Sensitization to social anxiolytic effects of ethanol in adolescent and adult Sprague-Dawley rats after repeated ethanol exposure. Alcohol. 2010;44:99–110. doi: 10.1016/j.alcohol.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacology, biochemistry, and behavior. 2012;100:440–50. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trezza V, Baarendse PJ, Vanderschuren LJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:2560–73. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Primus RJ, Kellogg CK. Developmental influence of gonadal function on the anxiolytic effect of diazepam on environment-related social interaction in the male rat. Behavioural pharmacology. 1990;1:437–46. [PubMed] [Google Scholar]
- 13.Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. European journal of pharmacology. 1995;276:257–66. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- 14.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–4. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 15.Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcoholism, clinical and experimental research. 2002;26:449–56. [PubMed] [Google Scholar]
- 16.Ramirez RL, Varlinskaya EI, Spear LP. Effect of the selective NMDA NR2B antagonist, ifenprodil, on acute tolerance to ethanol-induced motor impairment in adolescent and adult rats. Alcoholism, clinical and experimental research. 2011;35:1149–59. doi: 10.1111/j.1530-0277.2011.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvestre JS, Nadal R, Pallares M, Ferre N. Acute effects of ketamine in the holeboard, the elevated-plus maze, and the social interaction test in Wistar rats. Depress Anxiety. 1997;5:29–33. [PubMed] [Google Scholar]
- 18.Snigdha S, Neill JC. Improvement of phencyclidine-induced social behaviour deficits in rats: involvement of 5-HT1A receptors. Behavioural brain research. 2008;191:26–31. doi: 10.1016/j.bbr.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiology & behavior. 1995;57:843–7. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- 20.Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg(2+)-insensitive mutants. Neuropharmacology. 1995;34:347–55. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- 21.Morales M, Varlinskaya EI, Spear LP. Age-related differences in sensitivity to social suppression induced by the NMDA receptor antagonist, MK-801. Society for Neuroscience; New Orleans, LA: 2012. Online. [Google Scholar]
- 22.Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behavioural brain research. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:8885–95. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frizelle PA, Chen PE, Wyllie DJ. Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5 -yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-D-aspartate receptors: Implications for studies of synaptic transmission. Molecular pharmacology. 2006;70:1022–32. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- 25.Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacology, biochemistry, and behavior. 2010;96:228–35. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley KC, McGregor IS. (+/−)-3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) increases social interaction in rats. European journal of pharmacology. 2000;408:41–9. doi: 10.1016/s0014-2999(00)00749-4. [DOI] [PubMed] [Google Scholar]
- 27.Kavaliers M, Choleris E. Sex differences in N-methyl-D-aspartate involvement in kappa opioid and non-opioid predator-induced analgesia in mice. Brain research. 1997;768:30–6. doi: 10.1016/s0006-8993(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 28.Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. European journal of pharmacology. 1999;369:145–57. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]