Abstract
OBJECTIVES
To evaluate the cytotoxicity of dimeric naphthoquinones (BiQs) in prostate cancer cells.
To assess the interaction of dimeric naphthoquinones with common therapies including radiation and docetaxel.
MATERIALS AND METHODS
The cytotoxicity of 12 different dimeric naphthoquinones was assessed in androgen-independent (PC-3, DU-145) and androgen-responsive (LNCaP, 22RV1) prostate cancer cell lines and in prostate epithelial cells (PrECs).
BiQ2 and BiQ11 were selected for determination of dose response, effects on colony formation and initial exploration into mechanism of action.
Synergistic effects with radiation and docetaxel were explored using colony-forming and MTT assays.
RESULTS
At concentrations of 15μM, BiQ2, BiQ3, BiQ11, BiQ12, and BiQ15 demonstrated cytotoxicity in all prostate cancer cell lines.
Treatment with BiQs limited the ability of prostate cancer cells to form colonies in clonogenic assays.
Exposure of prostate cancer to BiQs increased cellular reactive oxygen species (ROS), decreased ATP production, and promoted apoptosis.
BiQ cytotoxicity was independent of NADP(H):quinone oxidoreductase 1 (NQO1) activity in PrECs, PC-3 and 22RV1, but not DU-145 cells.
Exposure of prostate cancer cells to radiation before treatment with BiQs increased their activity allowing for inhibitory effects well below the IC50s of these compounds in PrECs.
Co-administration of BiQs with docetaxel had minimal additive effects.
CONCLUSIONS
Dimeric naphthoquinones represent a new class of compounds with prostate cancer cytotoxicity and synergistic effects with radiation. The cytotoxic effect of these agents is probably contributed to by the accumulation of ROS and mitochondrial dysfunction.
Further studies are warranted to better characterize this class of potential chemotherapeutics.
Keywords: dimeric naphthoquinones, oxidative stress, cytotoxicity, prostate cancer, radiation synergy
INTRODUCTION
Over the last two decades there have been major advances in both the detection of prostate cancer and in the treatment of localized disease, resulting in a roughly 30% decrease in prostate cancer mortality since its peak in 1991 [1]. Despite this significant progress, prostate cancer remains the second leading cause of cancer-related death among men, highlighting the need for new therapies, particularly those directed against aggressive and advanced disease [2].
Multimeric naphthoquinones represent unique molecules which have a diverse array of biological activities, including antineoplastic, antiprotozoal and antiviral effects [3]. Previously, Stagliano et al. [4] reported the regiocontrolled synthesis of a series of novel symmetrical and asymmetrical dimeric naphthoquinones (BiQs) with initial efforts being focused on the development of HIV-1 integrase inhibitors that would mimic the activity of conocurvone, a naturally occurring multimeric naphthoquinone [5]. While the resultant compounds inhibited HIV-1 mediated cytopathogenicity, they also showed cytotoxicity in CEM-T4 lymphoblastic leukaemia cells at different μM concentrations, suggesting possible antineoplastic activity. In addition, other dimeric naphthoquinone-based compounds were previously shown to possess growth inhibition and toxicity in squamous, melanoma, lung and breast cancer cell lines [6,7]. To elucidate the mechanism of BiQ cytotoxicity, we recently conducted a chemical genetic screen of a series of these compounds in yeast and found that, in these organisms, BiQ cytotoxicity is probably mediated by the ability of these compounds to undergo reduction-oxidation cycling, produce free radicals and cause mitochondrial dysfunction [8].
In the present study, we perform an initial analysis of the cytotoxicity of BiQs in prostate cancer cell lines. We show that treatment with BiQs impairs survival and colony formation in both androgen-independent and androgen-responsive cells, with LD50s in the μM range. We furthermore show that exposure of prostate cancer cells to BiQs results in the accumulation of reactive oxygen species, inhibition of ATP generation, and promotion of cell death by apoptosis. Finally, we explore the effects of BiQs administered in combination with ionizing radiation or docetaxel and report a synergistic sensitizing effect of ionizing radiation on BiQ activity.
MATERIALS AND METHODS
Dimeric naphthoquinones were synthesized and characterized as previously described [4,9–11]. Docetaxel and dicoumarol (Dic) (3,3′-methylene-bis(4-hydroycoumarin) were purchased from Sigma-Aldrich (Poole, UK) and dissolved in dimethyl sulphoxide (DMSO) or water alkalized with 0.1 N sodium hydroxide respectively.
Prostate cancer cell lines, PC-3, DU-145, LNCaP, 22RV1 (American Type Culture Collection, Manassas, VA, USA) were grown in a humidified incubator at 37 °C and 5% CO2 in RPMI media 1640 (Life Technology, Rockville, MD, USA) containing 10% foetal bovine serum and antibiotics. Prostate epithelial cells (PrECs) were purchased from Lonza and maintained in PrEGM medium (Lonza, Slough, UK) according to the manufacturer’s specifications.
Cell survival was assessed by MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium-bromide, Sigma-Aldrich) assays and trypan blue exclusion (Invitrogen, Carlsbad, CA, USA) [12]. For MTT assays, an equal number of cells were plated and allowed to attach overnight. Cells were then treated with vehicle (DMSO) or individual BiQs at the specified concentrations, with or without 40 μM Dic, in complete media for 4 h. Where indicated, cells were treated with the specified concentrations of either docetaxel or DMSO for 5 days before treatment with BiQs. After treatment, cells were incubated with 0.5 mg/mL MTT in media for 1 h, then the media was removed and DMSO was added as a solvent. Absorbance was assayed at 570 nm (signal) and 690 nm (background). Cell viability at 15 μM for all BiQs and at 10 μM for BiQ2 and BiQ11, with and without Dic, in three or more replicates after 4 h of treatment, was assessed by counting cells using a haemocytometer after staining with trypan blue (Invitrogen).
Clonogenic survival assays were performed using standard methods [13]. Equal numbers (10 000 per condition) of subconfluent prostate cancer cells were exposed to DMSO, BiQ2 or BiQ11 in the presence or absence of 40 μM Dic for 4 h. After incubation with drugs, cells were washed, trypsinized and plated at a limiting dilution so as to yield ~50–200 colonies per plate in DMSO-treated cells after 14 days. Scoring was performed after fixation in 50% methanol and staining with crystal violet. All experiments were performed at least in triplicate. Where indicated, cells were first exposed to ionizing radiation from a 137Cesium source delivering a measured dose of 0.51 Gy/min and then treated with BiQs as above after a 4-h incubation period.
ATP production was measured using the CellTiter-Glo Luminescent assay, which directly measures ATP content (Promega, Madison, WI, USA), and adjusted for viable cell number. Cells were plated at 10 000 cells per well of a 96-well plate and treated in replicates of six with either DMSO or 10 μM of BiQ2 or BiQ11 for 4 h. For each condition, cell viability was measured in triplicate experiments by trypan blue staining and additional triplicate samples were used for the CellTiter-Glo assay, as per the manufacturer’s specifications, and performed on a Spectramax M2 instrument (Molecular Devices, Wokingham, UK). The resultant luminescence was normalized to the average number of viable, trypan blue-excluding cells per treatment condition.
Flow cytometric analysis of production of reactive oxygen species (ROS) and apoptosis was performed using a FACScan instrument and CellQuest Pro software (Becton Dickinson, Oxford, UK). To measure ROS generation, prostate cancer cells were preloaded with 5μM of 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA; Molecular Probes, Invitrogen) for 30 min. Cells were then treated with DMSO or 10 μM of BiQ2 or BiQ11 for 2 h, collected and analysed using an excitation wavelength of 488 nm produced by an argon laser and measuring emission at 530 nm. Apoptosis was measured after 4-h treatment with 10μM of BiQ2 or DMSO using the FITC AnnexinV/ Dead Cell apoptosis Kit for Flow Cytometry (Molecular Probes, Invitrogen) with apoptotic cells being considered PI-negative and Annexin V-positive.
Data are presented as mean ± SD unless otherwise specified. Pairwise comparisons were performed using two-sided t-tests. Comparisons between groups were made using ANOVA analysis. Statistical calculations were performed using GraphPad Prism version 5.00 for Windows, (GraphPad Software, San Diego, CA, USA, http://www.graphpad.com). P < 0.05 was considered significant. Synergistic effects were determined by using the formula Ac/Ae + Bc/ Be = C where Ac and Bc are the concentration of drug or dose of radiation used in combination, Ae and Be are the concentration of drug or dose of radiation required to achieve the same effect if used alone, and C is the combination index. If C is <1 the treatments were considered to act synergistically, and if C ≥1 the treatments were considered to act in an additive or antagonistic manner [14].
RESULTS
DIMERIC NAPHTHOQUINONES CAUSE CYTOTOXICITY AND IMPAIR THE CLONOGENIC SURVIVAL OF PROSTATE CANCER CELLS
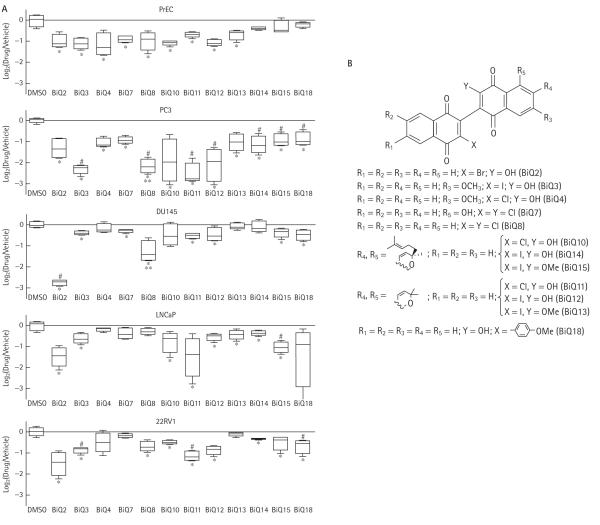
To determine whether treatment with BiQs affected the viability of prostate cancer cells, we first screened 12 structurally different representatives of this class in androgen-independent (PC-3, DU-145) and androgen-responsive (LNCaP, 22RV1) cell lines as well as in PrECs using the MTT assay (Fig. 1). At concentrations of 15 μM, BiQ2, BiQ3, BiQ11, BiQ12 and BiQ15 all significantly impaired conversion of MTT to formazan in all prostate cancer cell lines, suggesting cell death (Fig. 1). Trypan blue exclusion assays for cell viability confirmed cell death, showing 33–62% cell survival compared with the vehicle (DMSO)-treated cells after 4 h of exposure to these BiQs. Significant cytotoxicity was also seen in prostate cancer cell lines as early as 2 h after treatment, however, consistent results were best obtained after a treatment time of 4 h (data not shown). In addition to having inhibitory effects in prostate cancer cell lines, multiple BiQs also showed some degree of cytotoxicity in PrECs (Fig. 1). BiQ2 and BiQ11 were selected for further analysis based on their disparate chemical structures, effective killing and their preferential cytotoxicity when compared with PrECs in at least one prostate cancer cell line (Fig. 1B).
FIG. 1.
Log2 of the ratio of corrected absorbance of drug-treated cells to that of cells treated with vehicle alone are plotted for each condition in all cell types (A). Horizontal lines in boxes represent medians; vertical lines represent 1.5 times the interquartile range. * P < 0.05 for drug compared with DMSO; ** P < 0.05 for drug compared between androgen-independent and androgen-responsive cell lines; #P < 0.05 for drug compared with its effects in PrECs. (B) Chemical structures of BiQs.
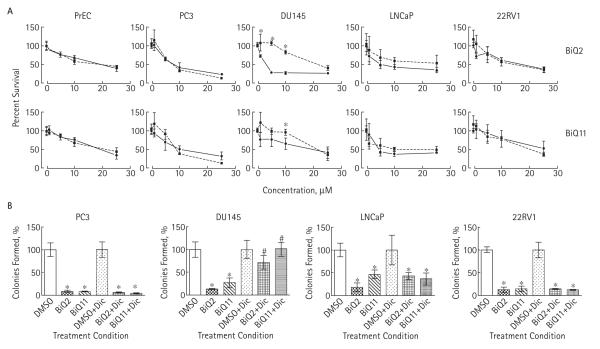
The effect of BiQ2 and BiQ11 on prostate cancer cells was dose-dependent with IC50s in the 2.5–25 μM range, depending on cell line and BiQ used (Fig. 2A). BiQ2 was particularly potent in androgen-independent cell lines with IC50s of 2.5 and 6μM in DU-145 and PC-3 compared with 20μM in PrECs. We next sought to determine whether BiQ treatment would impede clonogenic survival by using colony formation assays after drug exposure. Both BiQ2 and BiQ11 significantly reduced the ability of all prostate cancer cell lines to form colonies after treatment (Fig. 2B).
FIG. 2.
A, Results of MTT assays in PrECs and prostate cancer cell lines after exposure to vehicle (DMSO) or 1, 5, 10, or 25 μM of BiQ2 or BiQ11 for 4 h in the presence (dotted lines, squares) or absence (solid lines, circles) of co-treatment with 40 μM Dic. *P < 0.05 compared with mean of Dic-treated samples. B, Clonogenic survival assays following treatment with 10 μM of BiQ2 or BiQ11 in the presence or absence of 40 μM Dic. *P < 0.05 comparing drug treatment with DMSO or drug treatment with DMSO treatment in the presence of Dic; #P < 0.05 comparing drug treatment group with the corresponding drug-treated group in the presence of Dic.
TREATMENT WITH BIQS RESULTS IN INCREASED CELLULAR PRODUCTION OF ROS, DECREASED CELLULAR ATP FORMATION AND INCREASED APOPTOTIC CELL DEATH
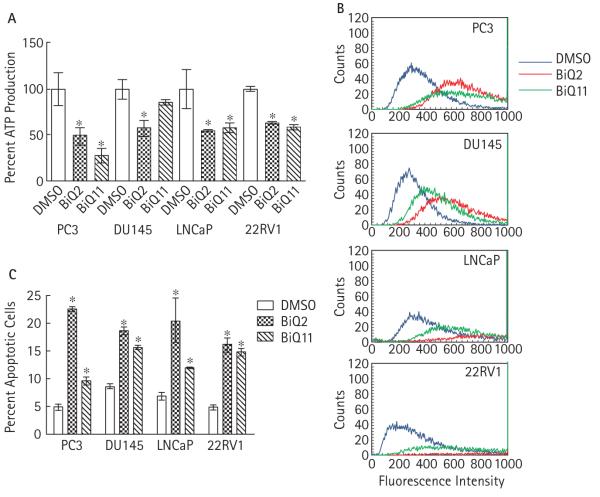
Our previous studies of genetic modulators to BiQ cytotoxicity in yeast suggested that one mechanism by which these drugs affect cell survival may be through causing mitochondrial dysfunction and free radical generation [8]. We hypothesized that this mechanism of BiQ action would be paralleled in prostate cancer. Indeed, after BiQ treatment, ATP production was decreased among remaining viable cells, suggesting mitochondrial dysfunction (Fig. 3A). To test whether ROS formation increased in prostate cancer cells treated with BiQ, we pre-loaded cell lines with DCFDA, which when intracellularly oxidized becomes fluorescent, and then exposed them to BiQs [8]. BiQ2 or BiQ11 treatment increased production of ROS as shown by an 18–80% absolute increase in the percentage of fluorescent cells (P < 0.001) (Fig. 3B).
FIG. 3.
A, ATP production after adjustment for viable cell number after treatment of prostate cancer cell lines with vehicle or 10 μM of BiQ2 or BiQ11 for 4 h. *P < 0.05 compared with DMSO-treated samples. B, Flow cytometric analysis to detect the accumulation of fluorescent, oxidized DCFDA (right shifted) after treatment with DMSO or 10μM BiQ2 or BiQ11 for 2 h. C, Percent of cells undergoing apoptosis (Annexin V-positive, PI-negative) comparing treatment with DMSO with that with 10μM BiQ2 or BiQ11 for 4 h. *P < 0.05 compared with DMSO treated samples.
Given the rapid effect of BiQs on cell survival we assessed whether BiQs could cause apoptosis by using a flow-cytometric-based assay for Annexin V-positive and PI-negative cells. After 4 h of treatment with BiQ2 or BiQ11, the fraction of apoptotic cells was significantly increased compared with treatment with vehicle alone (Fig. 3C).
DEPENDENCE OF BIQ CYTOTOXICITY ON NQO1
Formation of ROS by BiQs and cyclic voltammetry experiments suggested that these compounds can participate in futile redox cycling, in which they undergo reduction to hydronaphthoquinones and subsequent oxidation, thus generating ROS [11]. In addition, via a genetic screen for BiQ resistance we found that the toxicity of BiQs in yeast was dependent upon the yeast external NADH dehydrogenase, NDE1, which probably bioactivates these compounds [8]. Given these findings we tested whether BiQ toxicity would similarly be dependent upon NAD(P)H : quinone reductase (NQO1), a mammalian functional homologue of NDE1 which has been shown to activate quinine-containing compounds in prostate cancer cells [15–18]. Co-treatment of prostate cancer cells with the NQO1 inhibitor Dic abrogated the effects of both BiQ2 and BiQ11 in the DU-145 cell line but did not affect their inhibition of cell survival or colony formation in PC-3, 22RV1 or LNCaP cells (Fig. 2). Furthermore, Dic had no effect on BiQ cytotoxicity in PrECs (Fig. 2A). In contrast, consistent with previously published data, exposure of prostate cancer cells to beta-lapachone (which contains an orthonaphthoquinone) resulted in NQO1-dependent decreases in prostate cancer survival and colony formation in PC-3, DU-145 and 22RV1 cell lines but not in LNCaP, which possesses low expression and activity of NQO1 [18] (data not shown).
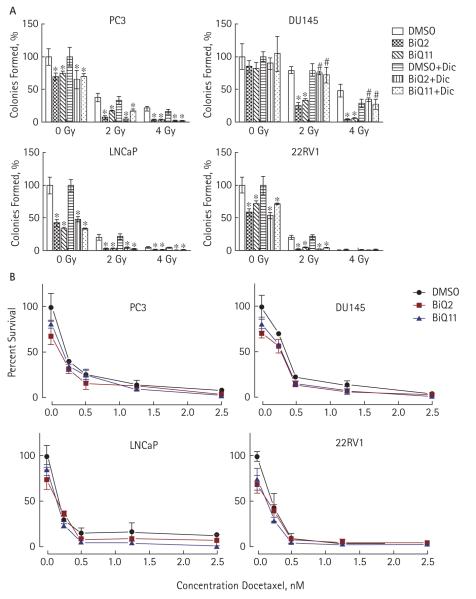
SYNERGISTIC EFFECTS OF RADIATION AND BIQ TREATMENT IN PROSTATE CANCER CELL LINES
Although the BiQs caused prostate cancer cell death and impeded colony formation, their toxicity in PrECs possibly narrows their therapeutic index. One mechanism by which this therapeutic index might be increased, however, would be through synergy with other drugs or treatments. To this end, we next explored whether treatment with radiation or docetaxel could sensitize prostate cancer cells to BiQs. Prostate cancer cell lines were exposed to ionizing radiation at 2 or 4 Gy and then subsequently treated with BiQs at doses at or below their LD50 (Fig. 4A). Radiation treatment at either dose acted synergistically with BiQ treatment to impair the clonogenic survival of prostate cancer cells (Fig. 4A). As with their use as single agents, BiQ2 and BiQ11 toxicity, and their augmentation by radiation, was dependent on NQO1 activity only in DU-145 cells. In contrast to their synergistic effects with ionizing radiation, co-treatment of prostate cancer cells with docetaxel and BiQs had only minimal additive effects on cell survival (Fig. 4B).
FIG. 4.
A, Clonogenic survival assays performed after treatment with BiQ2 (at 5 μM in all cell lines but DU-145 which was treated at 2.5 μM) or BiQ11 (at 5 μM for all cell lines) in the presence or absence of 40 μM Dic after exposure to 0, 2 or 4 Gy of ionizing radiation. *P < 0.05 comparing drug treatment with DMSO or drug treatment with DMSO treatment in the presence of Dic at each level of radiation. **P < 0.05 comparing the drug treatment group with the corresponding drug-treated group in the presence of Dic. B, MTT assays after exposure to vehicle (DMSO) or 0.25, 0.5, 1.25, or 2.5 nM of docetaxel in the absence (solid lines, circles) or presence of BiQ2 (at 5 μM for all cell lines but DU-145 which was treated at 2.5 μM, (red lines, squares) or BiQ11 (all cell lines treated at 5 μM (blue lines, triangles).
DISCUSSION
Treatment of four prostate cancer cells lines with 12 different BiQs revealed that the class, in general, possesses cytotoxic effects, with BiQ2, BiQ3, BiQ11, BiQ12, and BiQ15 showing significant activity in both androgen-independent and androgen-dependent cell lines. Many of the BiQs, however, also possessed inhibitory activity in cells derived from normal prostate epithelium. Why particular BiQs exhibit enhanced cytotoxicity in certain prostate cancer cell lines compared with cells from normal prostate, and why BiQ8 exhibited increased cytotoxicity in androgen-independent cells, is not obvious from comparing the chemical structures of these molecules. However, some correlations of BiQ structure and activity can be made. For example, pyranylated iodo biquinones (BiQ12, BiQ13, BiQ14 and BiQ15) are slightly less active than pyranylated chloro biquinones (BiQ10, BiQ11). In addition, the introduction of an aryl ring in lieu of a halogen in BiQ18 did not change the activity significantly. BiQ2 contains a bromo group which could enhance its cytotoxicity. BiQ8 is a completely symmetrical dichloro BiQ, which may be more permissive for nucleophilic reaction on the quinone core and this could have contributed to its increased activity in PC-3 and DU-145 cells. Even taking these features into account, the cytotoxicity of individual BiQs most probably represents a multi-factorial interplay between innate chemical properties of the BiQ and the metabolic pathways that are active in the different cell lines.
The mechanism of BiQ cytotoxicity in mammalian cells has not been described but may be related in part to the presence of quinone moieties. Quinones are present in multiple anti-neoplastic drugs, including doxorubicin and mitomycin C, and quinone cytotoxicity has been ascribed to a myriad of cellular effects including DNA and protein modification, topoisomerase inhibition, caspase activation and generation of oxidative stress [3,19–21]. We recently carried out a high throughput chemical genetic screen of BiQ cytotoxicity using a yeast isogenic deletion mutant array and found enhanced sensitivity of yeast mitochondrial mutants to these drugs. We also showed the ability of these drugs to depolarize the mitochondrial membrane and produce intracellular oxidative stress [8]. These effects appear to be recapitulated in prostate cancer cell lines, as exposure to BiQs resulted in decreased levels of cellular ATP and increased levels of ROS.
Our studies imply that BiQs undergo futile redox cycles to produce cytotoxic levels of ROS. Similar mechanisms have been described for beta-lapachone which requires the NAD(P)H : quinone reductase NQO1 for its bioactivation [15,18]. Interestingly, we only observed dependency of BiQ cytotoxic effects on NQ01 in the DU-145 cell line and not in PC-3 cells, although both cell lines have high levels of NQO1 expression and activity [18]. In addition, the synergistic effects of radiation on BiQ cytotoxicity were also only dependent on NQO1 in DU-145 cells, despite increases in the expression and activity of this enzyme with ionizing radiation (suggesting that NQO1 does not serve as an alternative pathway for BiQ activation) [22]. This implies that, in contrast to beta-lapachone, BiQs are activated by other cellular reductases in PC-3 and 22RV1 cell lines.
We found that ionizing radiation had a strong synergistic effect on BiQ cytotoxicity, such that doses with limited cytotoxicity in both normal and neoplastic prostate epithelial cells lines could substantially impair prostate cancer clonogenic survival. The mechanism by which this synergy occurs will be the subject of a subsequent study, but is probably mediated both by an increase in DNA damage and an inhibition of its repair. As with treatment with BiQs, ionizing radiation can cause mitochondrial dysfunction and the generation of ROS which can increase DNA damage [23–25]. Furthermore, treatment with BiQs may inhibit the repair of potentially lethal DNA damage caused by radiation, as such mechanisms have been shown to occur with other naphthoquinone-containing compounds such as beta-lapachone [22,26]. In addition to these mechanisms, exposure to radiation might act to activate BiQs by potentiating their redox cycling, both by facilitating the formation of semiquinones and through the activation of cellular reductases [22,27,28]. Finally, radiation might facilitate the autodimerization of BiQs to potentially more toxic tetrameric molecules [29]. Interestingly, the synergistic effects of radiation and BiQs were present in all prostate cancer cell lines tested, including those with (22RV1, LNCaP) and without (PC-3,DU-145) functional p53 activity [30,31], suggesting that BiQs may promote p53-independent apoptosis in prostate cancer. Indeed such mechanisms have been reported with beta-lapachone [32].
While their cytotoxic effects in prostate cancer cell lines, and particularly in androgen-independent cell lines, is encouraging, this study also highlights potential limitations to the development of these compounds as anti-neoplastic agents. First, while multiple members of the class showed prostate cancer toxicity, many BiQs also had effects in non-neoplastic prostate cells. Although the effects on PrECs might not be truly reflective of normal tissue response in vivo, this and the striking effects of BiQs given in combination with radiation suggest that the utility of these compounds might be primarily realized when used as one of several treatment methods. Another concern is that the LD50s of these compounds are in the μM range, which might be difficult to obtain in vivo. While this issue remains to be addressed, recent advances in the use of nanomaterials such as micelles and gold nanoparticles have allowed for potentially clinically viable formulations of naphthoquinone-containing compounds with efficacy shown in in vivo animal models of solid tumours [33,34]. Furthermore, given the apparent rapid onset of action of BiQs, their synergy with radiation therapy (which is by its nature localizing), and the accessibility of the prostate, one could also envision increasing the local drug concentration by direct injection of these compounds into the prostate, for instance in the setting of brachytherapy [15].
In summary, we present an initial evaluation of the effects of dimeric naphthoquinones in prostate cancer cells and find that multiple members of this class can decrease prostate cancer cell survival and clonogenicity in vitro. Cell death was found to occur rapidly and accordingly, BiQs were shown to be able to promote apoptosis. Exposure to ionizing radiation resulted in synergistic activity of BiQs, potentially increasing their therapeutic index. These drugs are able to increase the amount of intracellular reactive oxygen species generation and decrease cellular ATP production, possibly contributing to their cytotoxic activity and their synergistic effects with ionizing radiation. This is the first characterization of dimeric naphthoquinones in prostate cancer cells, and further studies are warranted to continue to explore the mechanism of action of these drugs, their synergy with radiation, and to characterize this class of potential chemo-therapeutics in in vivo models.
What’s known on the subject? and What does the study add?
Sexual function is often impaired after radical prostatectomy (RP) resulting in reduced sexual activity and sexual bother. The main focus in the literature concerning sexual adverse effects has been on erectile dysfunction and impairment of sexual function rather than the actual sexual bother it causes, although the sexual bother is most important to the individual patient’s quality of life. The relation between these measures, and in particular pre-operative prediction of post-operative sexual bother, has only been studied limitedly and with varying results. Some studies have found good mental health, low levels of pre-operative sexual bother, and higher education to be associated with absence of post-operative sexual bother, but another study could not identify any pre-operative predictors of a post-operative sexual bother. Severe sexual bother after RP were reported by 64% to 95% of the patients three years after operation, and the prevalence was associated with the level of pre-treatment sexual bother and pre-operative nerve-preservation. On the other hand, others have reported that only 43% of men have sexual bother 2 years after RP. However, none of these studies stratified patients according to their pre-operative sexual activity and most of them were American. It has been shown that American findings concerning sexual bother may not always be valid for non-American patients due to differing sex role expectations thus warranting the need for more non-American studies.
This study has shown that two thirds of patients experienced sexual bother one year after RP. We have identified patients with increased risk of experiencing overall sexual bother post-operatively: those who report pre-operative sexual bother, those who are sexually active before RP, and those who display neurotic personality-traits. Another important finding was that the proportion of patients who experienced bother relevant to having impaired post-operative SF was significantly higher among pre-operatively sexually active patients than those who had been inactive. This study adds knowledge that patients’ pre-operative sexual activity, sexual bother and personality should be taken into account to be able to give individualized information about the risk of getting sexual bother after RP.
ACKNOWLEDGEMENTS
Funding for this study was provided in part by a Johns Hopkins Hospital Department of Pathology Start-up Fund (to M.V.R.).
Abbreviations
- PrECs
prostate epithelial cells
- Dic
dicoumarol
- DMSO
dimethyl sulphoxide
- ROS
reactive oxygen species
- DCFDA
5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate
Footnotes
CONFLICT OF INTEREST Ashkan Emadi shares a patent on drugs used in this study.
REFERENCES
- 1.ACS . Cancer Facts and Figures. Atlanta American Cancer Society; Atlanta, GA: 2009. [Google Scholar]
- 2.Horner MJRL, Krapcho M, Neyman N, et al. [Accessed 2009];SEER Cancer Statistics Review, 1975-2006. Available at: http://seer.cancer.gov/csr/1975_2006.
- 3.O’Brien PJ. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- 4.Stagliano KW, Emadi A, Lu Z, et al. Regiocontrolled synthesis and HIV inhibitory activity of unsymmetrical binaphthoquinone and trimeric naphthoquinone derivatives of conocurvone. Bioorg Med Chem. 2006;14:5651–65. doi: 10.1016/j.bmc.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Dai JR, Decosterd LA, Gustafson KR, Cardellina JH, 2nd, Gray GN, Boyd MR. Novel naphthoquinones from Conospermum incurvum. J Nat Prod. 1994;57:1511–6. doi: 10.1021/np50113a006. [DOI] [PubMed] [Google Scholar]
- 6.Kayser O, Kiderlen AF, Laatsch H, Croft SL. In vitro leishmanicidal activity of monomeric and dimeric naphthoquinones. Acta Trop. 2000;76:131–8. doi: 10.1016/s0001-706x(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 7.Lim ES, Rhee YH, Park MK, et al. DMNQ S-64 induces apoptosis via caspase activation and cyclooxygenase-2 inhibition in human nonsmall lung cancer cells. Ann N Y Acad Sci. 2007;1095:7–18. doi: 10.1196/annals.1397.002. [DOI] [PubMed] [Google Scholar]
- 8.Emadi A, Ross AE, Cowan KM, Fortenberry YM, Vuica-Ross M. A chemical genetic screen for modulators of asymmetrical 2,2′-dimeric naphthoquinones cytotoxicity in yeast. PLoS One. 2010;5:e10846. doi: 10.1371/journal.pone.0010846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stagliano KW, Lu Z, Emadi A, Harwood JS, Harwood CA. Effect of methoxyl group position on the regioselectivity of ammonia substitution reactions involving 3,3′-dichloro-2,2′-binaphthoquinones. J Org Chem. 2004;69:5128–31. doi: 10.1021/jo049713g. [DOI] [PubMed] [Google Scholar]
- 10.Emadi A, Harwood JS, Kohanim S, Stagliano KW. Regiocontrolled synthesis of the trimeric quinone framework of conocurvone. Org Lett. 2002;4:521–4. doi: 10.1021/ol010272m. [DOI] [PubMed] [Google Scholar]
- 11.Emadi A. Regiocontrolled Synthesis and HIV Inhibitory Properties of Binapthoquinone and Trimeric Naphthoqinone Derivates of Conocurvone. Illinois institute of Technology; Chicago, IL: 2004. [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Ezekwudo D, Shashidharamurthy R, Devineni D, Bozeman E, Palaniappan R, Selvaraj P. Inhibition of expression of anti-apoptotic protein Bcl-2 and induction of cell death in radioresistant human prostate adenocarcinoma cell line (PC-3) by methyl jasmonate. Cancer Lett. 2008;270:277–85. doi: 10.1016/j.canlet.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Berenbaum MC. Criteria for analyzing interactions between biologically active agents. Adv Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Chin SF, Blanco E, et al. Intratumoral delivery of beta-lapachone via polymer implants for prostate cancer therapy. Clin Cancer Res. 2009;15:131–9. doi: 10.1158/1078-0432.CCR-08-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez Villamil S, Stoppani AO, Dubin M. Redox cycling of beta-lapachone and structural analogues in microsomal and cytosol liver preparations. Methods Enzymol. 2004;378:67–87. doi: 10.1016/S0076-6879(04)78004-0. [DOI] [PubMed] [Google Scholar]
- 17.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)H : Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem. 2000;275:5416–24. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 18.Planchon SM, Pink JJ, Tagliarino C, Bornmann WG, Varnes ME, Boothman DA. beta-Lapachone-induced apoptosis in human prostate cancer cells: involvement of NQO1/xip3. Exp Cell Res. 2001;267:95–106. doi: 10.1006/excr.2001.5234. [DOI] [PubMed] [Google Scholar]
- 19.Kanaan YM, Das JR, Bakare O, et al. Biological evaluation of 2,3-dichloro-5,8-dimethoxy-1,4-naphthoquinone as an anti-breast cancer agent. Anticancer Res. 2009;29:191–9. [PubMed] [Google Scholar]
- 20.Rockwell S, Sartorelli AC, Tomasz M, Kennedy KA. Cellular pharmacology of quinone bioreductive alkylating agents. Cancer Metastasis Rev. 1993;12:165–76. doi: 10.1007/BF00689808. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Thomas B, Sachdeva R, et al. Mechanism of arylating quinone toxicity involving Michael adduct formation and induction of endoplasmic reticulum stress. Proc Natl Acad Sci U S A. 2006;103:3604–9. doi: 10.1073/pnas.0510962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki M, Amano M, Choi J, et al. Synergistic effects of radiation and beta-lapachone in DU-145 human prostate cancer cells in vitro. Radiat Res. 2006;165:525–31. doi: 10.1667/RR3554.1. [DOI] [PubMed] [Google Scholar]
- 23.Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704:152–9. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayal D, Martin SM, Owens KM, et al. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res. 2009;172:737–45. doi: 10.1667/RR1617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol. 2004;14:259–66. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Boothman DA, Trask DK, Pardee AB. Inhibition of potentially lethal DNA damage repair in human tumor cells by beta-lapachone, an activator of topoisomerase I. Cancer Res. 1989;49:605–12. [PubMed] [Google Scholar]
- 27.Boothman DA, Meyers M, Fukunaga N, Lee SW. Isolation of x-ray-inducible transcripts from radioresistant human melanoma cells. Proc Natl Acad Sci U S A. 1993;90:7200–4. doi: 10.1073/pnas.90.15.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simic M, Hayon E. A model of radiation sensitization by quinones. Int J Radiat Biol Relat Stud Phys Chem Med. 1972;22:507–11. doi: 10.1080/09553007214551391. [DOI] [PubMed] [Google Scholar]
- 29.Werbin H, Strom ET. Photochemistry of electron-transport quinones. I. Model studies with 2-methyl-1,4-naphthoquinone (vitamin K3) J Am Chem Soc. 1968;90:7296–301. doi: 10.1021/ja01028a022. [DOI] [PubMed] [Google Scholar]
- 30.Wu CT, Chen WC, Liao SK, Hsu CL, Lee KD, Chen MF. The radiation response of hormone-resistant prostate cancer induced by long-term hormone therapy. Endocr Relat Cancer. 2007;14:633–43. doi: 10.1677/ERC-07-0073. [DOI] [PubMed] [Google Scholar]
- 31.Cronauer MV, Schulz WA, Burchardt T, Ackermann R, Burchardt M. Inhibition of p53 function diminishes androgen receptor-mediated signaling in prostate cancer cell lines. Oncogene. 2004;23:3541–9. doi: 10.1038/sj.onc.1207346. [DOI] [PubMed] [Google Scholar]
- 32.Tagliarino C, Pink JJ, Reinicke KE, Simmers SM, Wuerzberger-Davis SM, Boothman DA. Mu-calpain activation in beta-lapachone-mediated apoptosis. Cancer Biol Ther. 2003;2:141–52. doi: 10.4161/cbt.2.2.237. [DOI] [PubMed] [Google Scholar]
- 33.Jeong SY, Park SJ, Yoon SM, et al. Systemic delivery and preclinical evaluation of Au nanoparticle containing beta-lapachone for radiosensitization. J Control Release. 2009;139:239–45. doi: 10.1016/j.jconrel.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Blanco E, Bey EA, Khemtong C, et al. Beta-lapachone micellar nanotherapeutics for non-small cell lung cancer therapy. Cancer Res. 2010;70:3896–904. doi: 10.1158/0008-5472.CAN-09-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]