Role of Allogeneic Stem Cell Transplantation in Childhood and Adolescent Hodgkin’s Lymphoma (HL)
Introduction
Hodgkin’s lymphoma (HL) is the third most common malignancy in children less than 15 years of age but the most common cancer in adolescents 15- to 19 years of age. [1] According to the WHO classification, HL is subdivided into a classical variant (cHL), including four subtypes (nodular sclerosing, mixed cellularity, lymphocyte depleted and lymphocyte rich) and a nodular lymphocyte predominant variant (nLPHL) characterized by the presence of Reed Stanberg cells (RS) and lymphocyte and histocytic (L+H) cells, respectively. [1] In general, the prognosis is excellent for children and adolescents with newly diagnosed HL. Depending on stage, symptoms, risk groups and age, the estimated 5 year OS ranges from 80–95% in children and adolescents with newly diagnosed HL.[1] However, children and adolescents with relapsed/refractory HL following combined modality of chemotherapy and radiation therapy have a worse prognosis, often requiring reinduction therapy followed by some form of stem cell transplantation. [2]
Autologous stem cell transplantation in children and adolescents with recurrent/relapsed HL
Myeloablative therapy followed by autologous stem cell transplantation is considered the standard of care in children and adolescents with HL who have recurrent and/or relapsed disease. [2] Prognositc factors include chemoresponsiveness at relapse, disease status at relapse and performance status at relapse. [3] The most recent prospective trial performed by the Children’s Oncology Group analyzed children and adolescents with HL at relapse or progression and determined their EFS at the time of reinduction and through an autologous stem cell transplant following cyclophosphomide, BCNU and VP-16 conditioning. [4] In this study, Harris et al demonstrated a 45% 2 year EFS in children and adolescents with recurrent/relapsed HL following myeloablative therapy and autologous stem cell transplantation. These most recent results suggest that autologous stem cell transplantation is not curative in all children and adolescents with recurrent/relapsed HL and that new alternative strategies are needed.
Is there a graft vs. HL effect following allogeneic stem cell transplantation?
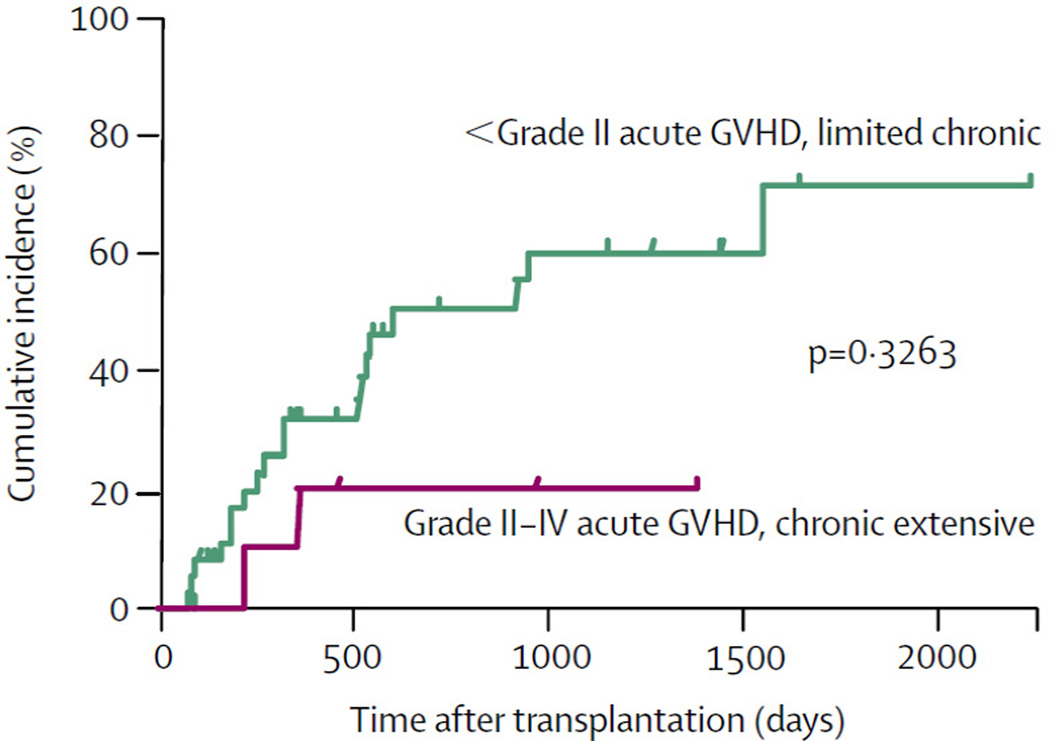
Jones et al first demonstrated a graft vs. lymphoma effect in patients with recurrent/refractory HL. [5] There was a 24% increase in the relapse rate following autologous vs. allogeneic stem cell transplantation in patients with recurrent/refractory HL. [5] Carella et al subsequently demonstrated the success of autologous stem cell transplantation followed by reduced intensity conditioning and allogeneic stem cell transplantation (alloSCT) in patients with recurrent/refractory HL. [6] More recently, Peggs et al demonstrated a significant graft vs. HL effect following reduced intensity conditioning and alloSCT in 49 adults with multiply relapsed HL (4 year OS and PFS was 55.7 and 39.0%, respectively). [7] Furthermore, the risk of relapse in these alloSCT recipients with either acute grade II-IV or extensive chronic GVHD was only 20.2% (CI95 5.9–69.6%) vs 50.7% (CI95 35.4–72.4%) in those without acute or limited chronic GVHD (Figure One). [7]
Figure 1. Cumulative incidences of relapse GVHD.
Cumulative-relapse incidence for patients with HL following RIC acute grade II–IV or extensive chronic GVHD (20·2% [95% CI 5·9–69·6%]) versus those with no GVHD or with only grade I acute or limited chronic GVHD (50·7% [35·4–72·4%]). Reprinted from The Lancet, 365, Peggs K.S., Hunter A., Chopra R., Parker A., Mahendra P., Milligan D., Craddock C., Pettengell R., Dogan A., Thomson K.J., Morris E.C., Hale G., Waldmann H., Goldstone A.H., Linch D.C., and Mackinnon S., Clinical evidence of a graft-versus-Hodgkin's-lymphoma effect after reduced-intensity allogeneic transplantation, 1934–1941, Copyright (2005), with permission from Elsevier.
Sureda et al recently compared reduced intensity (RIC) vs. myeloablative conditioning prior to alloSCT in patients with relapsed/refractory HL. [8] In this EBMT analysis, Sureda et al demonstrated a significant reduction in non-relapsed mortality (NRM) in the group receiving reduced intensity conditioning (hazard ratio [HR] 2.85 [CI95 1.62–5.02]), p<0.001.[8] Additionally, OS was significantly improved in the RIC vs. myeloablative group. [8] (HR 2.05 [CI95 1.27–3.29], p<0.04). Similarly, Peggs et al most recently demonstrated a durable salvage rate after donor lymphocyte infusions to improve mixed donor chimerism in patients with relapsed HL following in-vivo T-cell depletion with alemtuzumab followed by alloSCT. [9]
Allogeneic stem cell transplantation in children and adolescents with Hodgkin’s lymphoma
There are very few reports of large cohorts of children and adolescents with recurrent/relapsed HL receiving alloSCT. [1, Bradley, 2008 #77, 2] Claviez et al recently analyzed 91 children and adolescents with relapsed or refractory HL that underwent allogeneic stem cell transplantation that were registered in the EBMT registry. [10] Within this cohort, 51 of the children and adolescents received RIC and the remaining 40 patients received myeloablative conditioning. The 2 and 5 year OS rates were 54±6 and 45±6%, respectively. Most importantly, the subset of patients transplanted in the more recent period who had good performance status and chemosensitive disease had a 3 year PFS and OS of 60±27% and 83±15%, respectively. [10]
Cairo et al, have performed the only prospective series to date in children and adolescents with recurrent/refractory HL. [11] Ten patients, median age 18.4 years, with high-risk recurrent/refractory HL received a myeloablative conditioning with CBV as reported by Harris et al. [4] followed by a RIC with two days of busulfan and five days of fludarabine followed by an allogeneic stem cell transplant. [11] The probability of grade II-IV acute GVHD was 37.5% (CI95 0–63%) and probability of 2 year OS is 70% in this ultra high-risk group of children and adolescents. [11]
Future roles of allogeneic stem cell transplantation in children and adolescents with recurrent/relapsed HL
Prospective studies are needed to determine the safety and efficacy of allogeneic stem cell transplantation in children and adolescents with recurrent/relapsed HL. Besides the patients who relapse following an autologous stem cell transplantation, we need to determine which patients who have the poorest risk at relapse will benefit from an allogeneic vs. autologous stem cell transplant. It has yet to be determined whether there is a subgroup of children who may benefit from an allogeneic stem cell transplant at the time of first relapse or progression. We also need to determine the role of RIC vs. myeloablative conditioning prior to allogeneic stem cell transplantation in children and adolescents with relapsed/refractory HL.
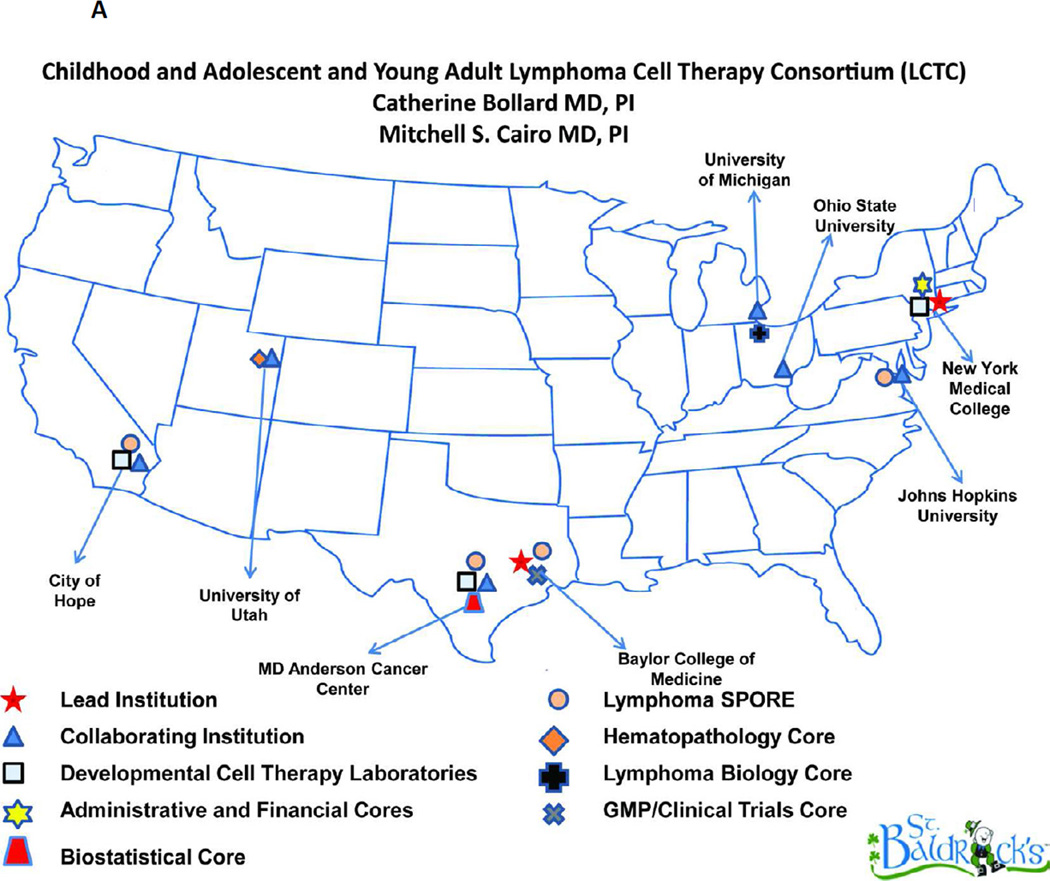
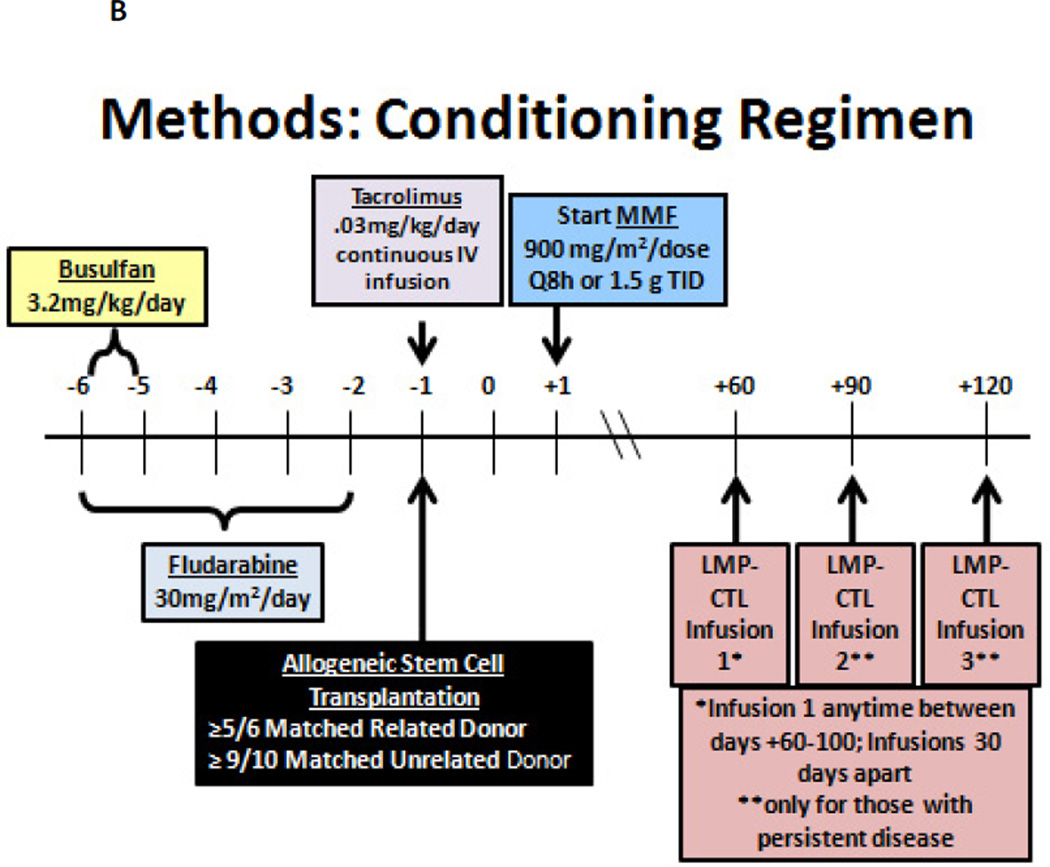
There is also a need to investigate the role of additional allogeneic cellular therapy after an allogeneic stem cell transplantation in patients with recurrent/relapsed HL. Since DLI has had some promising result but is associated with a high risk of acute GVHD, other targeted allogeneic cellular therapies are needed. In this regard, Drs Catherine Bollard, MD and Mitchell S. Cairo, MD have developed an eight center “Childhood, Adolescent and Young Adult Lymphoma Cell Therapy Consortium” (LCTC) (Figure 2A). Since EBV infection is present in up to 40% of patients with HL, targeting EBV proteins may be a potential cellular therapeutic strategy. We have therefore developed a new protocol of RIC followed by EBV-positive matched related or unrelated donor alloSCT in children, adolescents and young adults with EBV positive recurrent/refractory HL who have failed an autologous stem cell transplant. Following RIC and allogeneic stem cell transplantation, these patients will also receive donor derived LMP-CTLs from their EBV positive donor under IND# 15104 and clinicaltrials.gov # NCT01636388 (Figure 2B). The results of this prospective study will determine the safety and efficacy of RIC and allogeneic stem cell transplantation in this relapsed/recurrent HL patient group and the safety and efficacy of donor-derived LMP/CTL infusions. There is a need for other prospective RIC alloScT trials in children and adolescents with recurrent/relapsed poor-risk HL.
Figure 2.
A Lymphoma Cell Therapy Consortium. Geographical map of core sites and collaborating centers.
B Treatment Schema: Reduced Intensity Conditioning (RIC) and Allogeneic Stem Cell Transplantation (AlloSCT) Followed by Latent Membrane Protein (LMP) Specific-Cytotoxic T cell Lymphocytes (CTLs) in Patients with Epstein Barr Virus (EBV) Positive Refractory/Recurrent Hodgkin Lymphoma (HL)
Recurrent/Refractory ALCL in Children and Adolescents: Autologous, Allogeneic or No Stem Cell Transplantation?
Introduction
ALK-positive anaplastic large cell lymphoma (ALCL) accounts for 10 – 15% of pediatric and adolescent Non-Hodgkin Lymphomas. ALCL are characterized by a high incidence of B-symptoms and extranodal involvement. The relapse rate is 25–35% with current first line strategies often based on short-pulse chemotherapy courses.
Available data on patients with relapse of an ALCL are until now limited to retrospective analyses. Children and adolescents with ALCL who relapse have a 30 – 60% chance to reach a second continuous remission with relapse therapies as different as maintenance treatment with Vinblastine, autoSCT or alloSCT. [12] Considering the general efficacy of these highly variable relapse therapies, identification of risk factors for further relapse and death is critical to allow for treatment stratification upon relapse.
Risk factors
The time from initial diagnosis to relapse or progression has been described as major risk factor in children and adolescents with relapsed ALCL [12] [13] . In the French series of 41 children and adolescents, 24 patients relapsing within 12 months after diagnosis had a significantly lower disease-free survival compared to 17 patients with later relapses (DFS 28% and 68%, respectively, p=0.01) [12]. A prognostic impact of the time of relapse was not replicated in a series of 26 relapse patients reported from Japan[14]. In a BFM-group report on 74 ALCL-relapse patients With comparable first-line therapy and recommended consolidation with autoSCT, survival again correlated with time of relapse‥ Only four of 16 patients (25±11%) with progression during initial therapy survived compared to 38 of 58 patients with later relapses (66±6%; p=0.002) [13]. Among patients with progression during first-line therapy survivors have only been reported after alloSCT with very few exceptions [13, 15–19].
Approximately 15% of children and adolescents present with clinically advanced dissemination in relapse documented by bone marrow or CNS-involvement [12] [13] which correlated with reduced survival in one analysis[13].
Expression of the T-cell antigen CD3 was associated with a high risk of failure among patients consolidated by autoSCT in the retrospective BFM-series [13]. Only 1 of 11 children with relapse of a CD3-positive ALCL remained in remission after autoSCT, whereas 9 of 10 CD3-positive patients who received an alloSCT for primary or further relapse survived. The prognostic impact of CD3-positivity, however, needs to be interpreted cautiously due to low patient numbers and CD3-expression data taken retrospectively from pathology reports.
Based on these data, patients in the prospective ALCL-Relapse trial of the European Inter-Group for Childhood Non-Hodgkin Lymphoma (EICNHL) are stratified primarily according to the time of relapse and then to the immunophenotype.
Therapeutic approaches
The efficacy of the various consolidation therapies – chemotherapy, long-term low-dose consolidation, autoSCT and alloSCT – for relapsed ALK-positive ALCL cannot be compared between and within reported studies due to selection bias in patients receiving SCT and the retrospective nature of the studies [12].
The French group has reported long-term survival of ALCL-relapse patients with 12 months CVA-chemotherapy (CCNU, vinblastine, Cytosine-arabinoside) and long-term Vinblastine monotherapy [12]. They recently updated their experience on single drug Vinblastine for 36 ALCL-relapse patients[20]. More than 80% of patients reached a CR. Of the 25 children who received Vinblastine alone for a median of 14 months, 9 stayed in long-term remission. Further seven patients were again treated with weekly Vinblastine in re-relapse for at least two years of whom six survived long-term. The patients were not a selection of good-risk relapse patients as 15 patients had re-relapsed after autoSCT and several patients presented with early first relapse. Based on the French data, Vinblastine monotherapy for 24 months was chosen as therapy for patients with a late first relapse in the ALCL-Relapse trial of the EICNHL.
In the French series autoSCT was not associated with a higher disease-free survival compared to CVA chemotherapy (DFS 45% versus 52%, respectively, p=0.55) [12]. Interpretation is complicated since 14 of 15 patients who were consolidated by autoSCT experienced their relapse after current intensive first-line therapy compared to only 3 of 21 who did not. Among 26 Japanese relapse patients the rate of second relapses also was not different between those who received chemotherapy (relapses 3/10) or autoSCT (relapses 3/8) [14]. The efficacy of autoSCT varied widely between retrospective analyses with EFS-rates between <30% and almost 60% in reported series [12–13, 15, 21]. This variation may be explained by different approaches to ALCL-relapse and variable conditioning regimens. In most groups, the decision for the type of consolidation was individually based, and those who got an autoSCT were rather high-risk patients. In the BFM-group, autoSCT was recommended consolidation for all relapsed patients from 1990. Therefore, 39 of 74 relapse patients who reached autoSCT constituted a selection of good risk relapse patients [13]. The efficacy of autoSCT was, therefore, tested for patients with a medium risk of relapse in the ALCL- relapse trial.
Data on the use of alloSCT for relapsed ALCL are limited to the Japanese and BFM series as well as an IBMTR-analysis (together 38 patients) and case reports [14, 16, 21–22]. Taken together, there is a very low further relapse rate after alloSCT for relapsed ALCL (0–20%) raising the possibility that a graft-versus lymphoma effect may exist for this disease. The failure rate does not seem to be associated with donor type[16]. The conditioning regimens for alloSCT predominantly used 12 Gy total body irradiation. Thererfore, it is not possible to draw any definitive conclusions regarding the type of preparative regimen that is most beneficial for this disease. Nevertheless, the high efficacy of alloSCT was the basis to choose alloSCT for consolidation of high-risk relapse patients in the ALCL-Relapse study.
ALCL-Relapse trial and future prospective
The first prospective ALCL-Relapse trial of the EICNHL started in 2004 (NCT00317408). Stratification is based on the time of relapse followed by the expression of CD3. The primary objectives are: 1. To improve pEFS for patients with progression during first-line therapy or relapse of a CD3-positive ALCL by alloSCT. 2. To test the efficacy of BEAM-conditioning and autoSCT for patients with CD3-negative ALCL after first-line therapy. 3. To test the efficacy of weekly Vinblastine monotherapy for two years for patients with late relapse of a CD3-negative ALCL. Results of an interim analysis after inclusion of 90 patients are presented.
Brentuximab vedotin and Crizotinib are new targeted treatment options emerging for CD30-positive and ALK-positive malignancies [23–24]. Initial data regarding the safety profile and response rates are promising. However, given the high response rate and efficacy of Vinblastine monotherapy for relapsed ALCL almost without risk of late effects, clinical trials are necessary to define the role of these new agents in the therapeutic management for ALCL.
Novel Radioimmunotherapy Conditioning Prior to Hematopoietic Cell Transplantation in Patients with Recurrent/Refractory Lymphoma
Overview
A fraction of relapsed pediatric patients may have a significant improvement in survival following hematopoietic stem cell transplantation (HSCT), however, high-dose therapy and HSCT may still result in disease recurrence. One approach to reduce relapse has focused on attempts to intensify the preparative regimen prior to HSCT. This approach, in general, has been limited by associated toxicities due to the non-specific nature of most conditioning agents. To overcome this limitation, radiolabeled antibodies (Ab) have been investigated to deliver targeted therapy to decrease the risk of relapse after HSCT. Radionuclide conjugated Abs appear particularly attractive for lymphoma therapy for pediatric and adolescent patients because: 1) their surface antigens are well delineated, 2) multiple high quality Abs are available, 3) the best results of clinical trials with unmodified Abs and with radiolabeled Abs have been observed with hematologic malignancies, and 4) lymphomas are exquisitely sensitive to radiation therapy. Thus, varieties of novel HSCT strategies using RIT prior to HSCT in pediatric patients with recurrent/refractory lymphoma warrant continued investigation.
Radioimmunotherapy and Autologous HSCT
Non-myeloablative approaches prior to autoSCT that combine a radiolabeled anti-CD20 Ab with a conditioning regimen was recently examined in a randomized study examined 90Y-ibritumomab tiuxetan combined with BEAM chemotherapy versus BEAM alone prior to autoSCT for adult non-Hodgkin lymphoma (NHL) patients. [25] After 2 years of follow-up, the progression-free survival (PFS) was approximately 25% better for the patients who received both 90Y-ibritumomab tiuxetan and BEAM chemotherapy instead of BEAM alone (49% versus 33% for high-risk patients). While this approach was proven to be feasible, the question remains whether adding the radiolabeled Ab can provide significant benefit over the use of standard high-dose chemotherapy alone in pediatric patients. The problem of relapse in adult and pediatric NHL patients has led investigators to explore the use of escalated doses of a radiolabeled Ab combined with autoSCT. Early single-agent studies with escalated-doses of radiolabeled Ab delivered very promising tumor-to-whole-body ratios of at least 10 to 1. [3, 20, 26] A non-randomized analysis suggested that myeloablative doses of targeted radiation as part of the HSCT conditioning regimen may lead to significant improvement in OS compared with total body irradiation for those with advanced NHL, including pediatric patients.
Safety and feasibility trials for the treatment of primarily pediatric and adolescent patients with advanced Hodgkin lymphoma (HL) have also been performed using anti-ferritin (a HL-associated antigen) immunoglobulin radiolabeled at myeloablative doses with 90Y, delivered either as stand-alone treatment or in combination with high-dose chemotherapy, followed by autologous marrow support. Despite results suggesting that a HSCT approach using 90Y-anti-ferritin alone provides objective responses in approximately half of patients with chemotherapy refractory disease, the majority of survivors studied developed HL recurrence. [27]
Radioimmunotherapy and AlloSCT Approaches
AlloSCT still may not be particularly effective in pediatric patients with high-risk lymphoma features, such as seen in those with rapidly growing disease, bulky lymphoma, or multiple-relapsed disease with either chemotherapy-resistant or chemotherapy-refractory NHL. Standard doses of 90Y-ibritumomab tiuxetan have been added prior to a reduced-intensity alloSCT conditioning regimen using fludarabine and 200 cGy of TBI. [28] This strategy was well-tolerated, as the 100-day non-relapse mortality rate was <5% and this primarily adult advanced NHL patient population had an encouraging overall survival rate of 31% at a median follow-up of 30 months after HSCT. Current approaches using up to 4 times the standard dose of 90Y-ibritumomab tiuxetan for adult and pediatric patients with relapsed NHL who need additional measure to control aggressive disease are on-going.
New Directions for Targeted Radiation Delivery and HSCT
Alternative Antigenic Targets
Although the most widely utilized target to date remains CD20, pre-clinical data indicate that CD20 blocking may impair the ability to target CD20 with RIT. [29] One alternative is to circumvent the potential of CD20 blocking (and the exclusion of CD20-negative NHL histologies) by targeting alternate targets such as the pan-hematopoietic antigen CD45. Preclinical and clinical data using radiolabeled anti-CD45 Ab support this strategy for continuing human studies. [29]
Alternative Radionuclides
RIT has primarily utilized beta-emitting radionuclides (particularly 131I and 90Y) for the treatment of lymphomas. Relapses in NHL studies may be due, however, to the low energy transfer characteristics of beta-particles resulting in suboptimal killing of tumor cells and the long path length of the beta-particle may lead to dose-limiting toxicities as it circulates in the blood stream. An attractive alternative might employ an alpha-emitter since their radiobiological properties may be more suited for HSCT of lymphoma (i.e., conditioning and treatment of minimal residual disease) than a beta-emitter. The alpha-emitting radionuclides exhibit very high cytotoxicity due to the release of large amounts of energy in a linear fashion within a few cell diameters (~50–90 µm). Clinical investigations using alpha-emitters with favorable radiobiological characteristics are continuing, however, other issues such as cost, availability, labeling chemistry, and in vivo stability have impacted their use in RIT.
Nanoparticle Delivery Platforms for RIT
Increased attention has been placed on nanomaterials since the nanostructured surfaces offer a large surface area and greater reactivity, making nanovectors potentially ideal for targeted RIT. The characteristics necessary for a successful carrier nanoparticle are that it: 1) should be targeted for delivery of its payload, 2) have a sufficient contact time with the target, and 3) not be removed by the reticuloendothelial system so that toxic levels of radioparticles are not accumulated in the liver and spleen. [30] A number of recent studies to determine the efficacy of nanoparticle "carriers" for radiopharmaceutical delivery have shown that there are many factors that determine circulation time and clearance; these factors include nanoparticle size, surface coating and surface charge, presence of high immunological protein concentration on the particle surface, as well as particle size. [30–31]
Pretargeted RIT
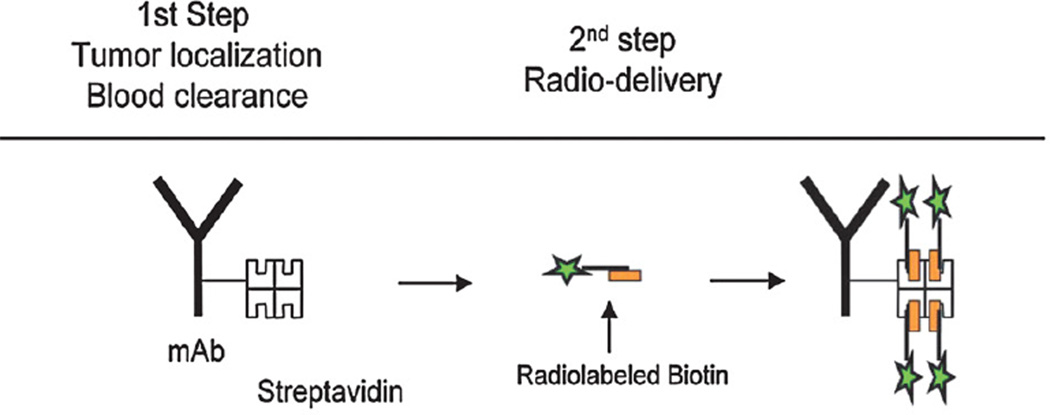
Many groups have explored a pretargeted RIT (PRIT) approach as a mechanism to further boost targeted radiation doses while minimizing non-specific radiation exposure to normal organs. In one PRIT strategy, the targeting Ab can be conjugated to streptavidin (SA) in an attempt to uncouple the delivery of the Ab from the delivery of the radiation. [32] The Ab-SA conjugate, delivered as an unlabeled agent, can be allowed to localize to hematological targets over 24 to 48 hours. Unbound Ab-SA may be then cleared from the circulation by way of Ashwell receptors in the liver using a biotinylated clearing agent prior to a therapeutic dose of radiobiotin. In this model, the biotin moiety can be conjugated to a radiometal via a DOTA chelate. Radiobiotin can subsequently localize rapidly to the Ab-SA conjugate pretargeted to malignant cells. Unbound radiobiotin can be rapidly eliminated through the kidneys into the urine, further reducing prolonged exposure of free radionuclide in normal organ tissues (Figure Three). Variations of this approach have also been explored to further optimize PRIT, including the development of bi-specific Ab fragments where one arm of the single-chain is engineered for its specific target, which may improve upon the current PRIT approaches.
Figure 3. Schema of PRIT approach.
Ab-SA conjugate followed by clearing agent before infusion of therapeutic radiolabeled biotin.
Acknowledgements
The authors would like to thank Lauren Harrison, RN, MSN for her expert assistance in the preparation of this manuscript.
Supported by a grant from the Pediatric Cancer Research Foundation, St. Baldrick’s Foundation and Marisa Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hochberg J, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144(1):24–40. doi: 10.1111/j.1365-2141.2008.07393.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradley MB, Cairo MS. Stem cell transplantation for pediatric lymphoma: past, present and future. Bone Marrow Transplant. 2008;41(2):149–158. doi: 10.1038/sj.bmt.1705948. [DOI] [PubMed] [Google Scholar]
- 3.Johnston LJ, Horning SJ. Autologous hematopoietic cell transplantation in Hodgkin's disease. Biol Blood Marrow Transplant. 2000;6(3A):289–300. doi: 10.1016/s1083-8791(00)70054-1. [DOI] [PubMed] [Google Scholar]
- 4.Harris RE, et al. Autologous peripheral blood stem cell transplantation in children with refractory or relapsed lymphoma: results of Children's Oncology Group study A5962. Biol Blood Marrow Transplant. 2011;17(2):249–258. doi: 10.1016/j.bbmt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RJ, et al. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood. 1991;77(3):649–653. [PubMed] [Google Scholar]
- 6.Carella AM, et al. Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin's disease and non-Hodgkin's lymphoma. J Clin Oncol. 2000;18(23):3918–3924. doi: 10.1200/JCO.2000.18.23.3918. [DOI] [PubMed] [Google Scholar]
- 7.Peggs KS, et al. Clinical evidence of a graft-versus-Hodgkin's-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365(9475):1934–1941. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]
- 8.Sureda A, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(3):455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 9.Peggs KS, et al. Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin's lymphoma. J Clin Oncol. 2011;29(8):971–978. doi: 10.1200/JCO.2010.32.1711. [DOI] [PubMed] [Google Scholar]
- 10.Claviez A, et al. Allogeneic hematopoietic stem cell transplantation in children and adolescents with recurrent and refractory Hodgkin lymphoma: an analysis of the European Group for Blood and Marrow Transplantation. Blood. 2009;114(10):2060–2067. doi: 10.1182/blood-2008-11-189399. [DOI] [PubMed] [Google Scholar]
- 11.Cairo MS, et al. Myeloablative (MAC) autologous stem cell transplantation (AutoSCT) followed by reduced Intensity (RIC) allogeneic stem cell transplantation (AlloSCT) in children, adolescents and young adults (CAYA) with poor risk Hodgkin’s lymphoma (HL): Induction of long term GVHL effect. Biology of Blood and Marrow Transplantation. 2010;16(2):S181. [Google Scholar]
- 12.Brugieres L, et al. Relapses of childhood anaplastic large-cell lymphoma: treatment results in a series of 41 children--a report from the French Society of Pediatric Oncology. Ann Oncol. 2000;11(1):53–58. doi: 10.1023/a:1008352726155. [DOI] [PubMed] [Google Scholar]
- 13.Woessmann W, et al. Relapsed or refractory anaplastic large-cell lymphoma in children and adolescents after Berlin-Frankfurt-Muenster (BFM)-type first-line therapy: a BFM-group study. J Clin Oncol. 2011;29(22):3065–3071. doi: 10.1200/JCO.2011.34.8417. [DOI] [PubMed] [Google Scholar]
- 14.Mori T, et al. Recurrent childhood anaplastic large cell lymphoma: a retrospective analysis of registered cases in Japan. Br J Haematol. 2006;132(5):594–597. doi: 10.1111/j.1365-2141.2005.05910.x. [DOI] [PubMed] [Google Scholar]
- 15.Williams DM, et al. Anaplastic large cell lymphoma in childhood: analysis of 72 patients treated on The United Kingdom Children's Cancer Study Group chemotherapy regimens. Br J Haematol. 2002;117(4):812–820. doi: 10.1046/j.1365-2141.2002.03482.x. [DOI] [PubMed] [Google Scholar]
- 16.Woessmann W, et al. Allogeneic haematopoietic stem cell transplantation in relapsed or refractory anaplastic large cell lymphoma of children and adolescents--a Berlin-Frankfurt-Munster group report. Br J Haematol. 2006;133(2):176–182. doi: 10.1111/j.1365-2141.2006.06004.x. [DOI] [PubMed] [Google Scholar]
- 17.Mori T, et al. Anaplastic large cell lymphoma in Japanese children: retrospective analysis of 34 patients diagnosed at the National Research Institute for Child Health and Development. Br J Haematol. 2003;121(1):94–96. doi: 10.1046/j.1365-2141.2003.04249.x. [DOI] [PubMed] [Google Scholar]
- 18.Reiter A, et al. Successful treatment strategy for Ki-1 anaplastic large-cell lymphoma of childhood: a prospective analysis of 62 patients enrolled in three consecutive Berlin-Frankfurt-Munster group studies. J Clin Oncol. 1994;12(5):899–908. doi: 10.1200/JCO.1994.12.5.899. [DOI] [PubMed] [Google Scholar]
- 19.Seidemann K, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 2001;97(12):3699–3706. doi: 10.1182/blood.v97.12.3699. [DOI] [PubMed] [Google Scholar]
- 20.Brugieres L, et al. Single-drug vinblastine as salvage treatment for refractory or relapsed anaplastic large-cell lymphoma: a report from the French Society of Pediatric Oncology. J Clin Oncol. 2009;27(30):5056–5061. doi: 10.1200/JCO.2008.20.1764. [DOI] [PubMed] [Google Scholar]
- 21.Gross TG, et al. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol Blood Marrow Transplant. 2010;16(2):223–230. doi: 10.1016/j.bbmt.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesaro S, et al. Unrelated bone marrow transplantation for high-risk anaplastic large cell lymphoma in pediatric patients: a single center case series. Eur J Haematol. 2005;75(1):22–26. doi: 10.1111/j.1600-0609.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- 23.Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med. 2011;364(8):775–776. doi: 10.1056/NEJMc1013224. [DOI] [PubMed] [Google Scholar]
- 24.Pro B, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 25.Shimoni A, et al. A multi-centere prospective randomized study comparing ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherpay (Z-BEAM) vs. BEAM alone as the conditioning regimen prior to autologous stem cell transplantation in paitents with aggressive lymphoma; possible advantage for Z-BEAM in low-risk paitents. Blood. 2010;116(suppl 21) Abstract 686. [Google Scholar]
- 26.Liu SY, et al. Follow-up of relapsed B-cell lymphoma patients treated with iodine-131-labeled anti-CD20 antibody and autologous stem-cell rescue. J Clin Oncol. 1998;16(10):3270–3278. doi: 10.1200/JCO.1998.16.10.3270. [DOI] [PubMed] [Google Scholar]
- 27.Bierman PJ, et al. Yttrium 90-labeled antiferritin followed by high-dose chemotherapy and autologous bone marrow transplantation for poor-prognosis Hodgkin's disease. J Clin Oncol. 1993;11(4):698–703. doi: 10.1200/JCO.1993.11.4.698. [DOI] [PubMed] [Google Scholar]
- 28.Gopal AK, et al. (9)(0)Y-Ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood. 2011;118(4):1132–1139. doi: 10.1182/blood-2010-12-324392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gopal AK, et al. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008;112(3):830–835. doi: 10.1182/blood-2008-01-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 31.Zhang H, et al. Quantitative proteomics analysis of adsorbed plasma proteins classifies nanoparticles with different surface properties and size. Proteomics. 2011;11(23):4569–4577. doi: 10.1002/pmic.201100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter RB, Press OW, Pagel JM. Pretargeted radioimmunotherapy for hematologic and other malignancies. Cancer Biother Radiopharm. 2010;25(2):125–142. doi: 10.1089/cbr.2010.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]