Abstract
A library of neutral, hydrophobic reagents was synthesized for use as derivatizing agents in order to increase the ion abundance of N-linked glycans in electrospray ionization mass spectrometry (ESI MS). The glycans are derivatized via hydrazone formation and are shown to increase the ion abundance of a glycan standard more than 4-fold. Additionally, the data show that the systematic addition of hydrophobic surface area to the reagent increases the glycan ion abundance, a property that can be further exploited in the analysis of glycans. The results of this study will direct the future synthesis of hydrophobic reagents for glycan analysis using the correlation between hydrophobicity and theoretical non-polar surface area calculation to facilitate the development of an optimum tag for glycan derivatization. The compatibility and advantages of this method are demonstrated by cleaving and derivatizing N-linked glycans from human plasma proteins. The ESI-MS signal for the tagged glycans are shown to be significantly more abundant, and the detection of negatively charged sialylated glycans is enhanced.
Introduction
The inherent hydrophobic bias in electrospray ionization (ESI) has been exploited in numerous studies first for nucleic acids [1], then peptides and proteins [2–7], and most recently glycans [8–14] in order to increase the ion abundance of analytes in mass spectrometry (MS). While these techniques frequently introduce an additional wet chemistry step in sample preparation, the benefits have been shown to outweigh the time and cost; a >2000-fold improvement in peptide signal has been reported [7]. The addition of hydrophobic functionality to molecules via chemical derivatization will not only increase the ion abundance in ESI but can also have other beneficial aspects, such as the ability to incorporate stable isotopes for quantification [10, 15] and the enhanced information acquired from fragmentation spectra in the permethylation of glycans [16]. Analysis of glycans by ESI has been hampered by the hydrophilicity of the sugar functional groups causing them to have a higher free energy of solvation and be more difficult to desorb from the electrospray droplet in the generation of gas-phase ions. Thus, derivatization using hydrophobic reagents can be used to counter this obstacle. Imparting additional hydrophobic functions onto glycan molecules allows the glycans to be less solvated and have a higher surface activity in the precursor electrospray droplet. As this droplet is desolvated on the way to the MS, a series of Coulombic fission events occur where a number of smaller progeny droplets (estimated to be ~20 [17]) are ejected from the surface of the original droplet [17–19]. In comparison with the native glycans, the more hydrophobic, derivatized glycans are more likely to have a higher surface activity and are significantly enriched in the progeny droplets [20]. It is these progeny droplets that are further desolvated and eventually produce gas-phase ions [21], creating the hydrophobic bias in ESI.
Permethylation [22–24] and peracetylation [23, 25] represent two initial methods used to derivatize glycans for enhanced MS analysis. These methods have been shown to enhance fragmentation spectra [26, 27] and increase the glycan ion abundance. However, permethylation and peracetylation convert all hydroxyl, amino, and carboxylic acid groups to their methyl- and acetyl-ether functions, respectively and, thus, the m/z shift due to the derivatization is variable for different sized glycans. Additionally, 100% conversion has proven difficult and is variable for different N-linked glycans. For example, due to the numerous hydroxyl, amino, and carboxylic groups present in oligosaccharides (~5 per monosaccharide and 7–18 monosaccharides per N-linked glycan), a 0.1% change in the permethylation efficiency can result in a 3%–10% efficiency difference depending on the type and size of the glycan. In contrast, the reducing terminus is a convenient location for derivatization due to the availability of a free aldehyde group after the enzymatic cleavage of N-linked glycans from proteins using peptide: N-glycosidase F (PNGase F). Moreover, stable isotope labels with a small, fixed mass shift can be incorporated into the reagents while simultaneously increasing the hydrophobicity. Reductive amination is a prominent technique capable of reacting with the reducing terminus of N-linked glycans and has been used to enhance several analytical techniques including UV and fluorescence detection [28–31] and hydrophobic derivatization [9, 10, 32, 33]. Two studies have shown the ability to incorporate hydrophobic tagging and isotopic labeling using reductive amination derivatization [10, 15]. Though this method is effective, an additional significant clean-up step is necessary after derivatization due to salt contamination, which often increases sample preparation time, the opportunity for sample loss, and the analytical variability in the measurement.
Hydrazone formation is an attractive alternative derivatization strategy in which hydrazide reagents react at the reducing terminus of glycans much like reductive amination but does not involve reducing the glycan to a Schiff base using salts such as sodium borohydride [34]. Thus, when using hydrazone formation, the product can be directly injected onto the nano-LC column due to the lack of salts necessary in the reaction mixture. In addition, >95% reaction efficiency can be routinely achieved using hydrazone formation as a means of hydrophobic tagging of glycans [13]. Danzylhydrazine [35] was the first of many reagents to be coupled to glycans via hydrazone formation for enhanced fluorescence or UV detection [36–38]. More recently, hydrazone formation has been used for enhanced MS detection [8, 12, 13], though an abundance of these reagents is not readily available. Several different hydrazide reagents have been developed and used to increase glycan abundance [8, 12, 13]. Girard’s T reagent was first used to increase mass spectral detection in order to incorporate a permanent cationic charge onto the glycan [12]. However, recent studies have shown decreases in ion abundance when coupling Girard’s T reagent to glycans in nanoLC MS [8], and it has been shown that neutral reagents are significantly better than preformed cations when coupled to N-linked glycans [8, 13].
Herein, a small library of hydrazide reagents has been synthesized and is used to derivatize glycans in order to determine the type of hydrophobic structure that most effectively increases the ion abundance of glycans in ESI-MS. The reagents studied are exclusively neutral compounds due to the compatibility in positive and negative mode MS and due to recent studies showing decreased electrospray response when incorporating a permanent charge on the reagent (vide supra) [13]. Additionally, the non-polar surface areas (NPSA) for all the reagents have been calculated in order to give a theoretical estimate of the hydrophobicity of the reagents as it has been shown that the NPSA correlates directly with the hydrophobicity of the analytes in peptide analysis [5, 6]. Furthermore, the compatibility of the hydrazone reagents with complex mixtures and their inherent advantages are demonstrated by the derivatization of N-linked glycans found in human plasma.
Experimental
Materials
(Gal-GlcNAc)2Man3GlcNac2 (NA2) glycan, (NeuAc-Gal-GlcNAc)2Man3(Fuc)(GlcNAc)2 (A2F) glycan, trifluoroacetic acid (TFA), acetic acid, ammonium acetate, ethyl chloroacetate, and β-mercaptoethanol were all purchased from Sigma Aldrich (St. Louis, MO, USA). Phenylacetic acid, 3-phenylpropionic acid, 4-phenylbutanoic acid, 5-phenylpentanoic acid, ethynyl benzene, ethyl 2-(4-bromophenyl)acetate, hydrazine hydrate, 4-phenylpyridine, and ethyl phenylacetate were purchased from VWR (West Chester, PA, USA). Peptide: N-Glycosidase F (2.5 mU/µL), denaturing solution (1 M β-mercaptoethanol and 2% (w/w) SDS), and the detergent solution (15% Nonidet P40) were purchased from Prozyme (San Leandro, CA, USA). Pooled human plasma was purchased from Innovative Research (Novi, MI, USA). High performance liquid chromatography grade acetonitrile (ACN), water, and methanol (MeOH) were all purchased from Burdick and Jackson (Muskegon, MI, USA). IntegraFrit and PicoFrit columns were purchased from New Objective (Woburn, MA, USA) and the TSK-Gel Amide-80 stationary phase was from TOSOH Bioscience (San Jose, CA, USA). The graphitized solid phase extraction cartridges were purchased from Alltech (Deerfield, IL, USA).
Reagent Synthesis, Purification, and Characterization
Five hydrazide reagents were synthesized: 2-phenylacetohydrazide (GPN), 3-phenylpropanehydrazide (GPN2), 4-phenylbutanehydrazide (GPN3), 5-phenylpentanehydrazide (GPN4), and 4-phenethylbenzohydrazide (phenyl2-GPN). The mono-phenyl reagents were synthesized by Fischer esterification, converting a carboxylic acid to a methyl ester followed by reaction with hydrazine hydrate in absolute ethanol at 90 °C to form the hydrazide. The bi-phenyl reagent (phenyl2-GPN) was synthesized by reacting ethynyl benzene with ethyl 2-(4-bromophenyl)acetate in the presence of a catalyst and the triple bond was hydrogenated in absolute ethanol over Pd/C. As with the mono-phenyl reagents the ester was converted to a hydrazide function via reaction with hydrazine hydrate in absolute ethanol incubated at 90 °C. Synthesis schemes, purification methods, and characterization data have been extensively detailed in the supplemental material.
Reagent Analysis for Model Glycan
A 2 mg/mL solution of each individual reagent was made in 85:15 MeOH:acetic acid (vol/vol). The NA2 glycan was aliquotted into six microcentrifuge tubes (170 pmol in each tube), dried, and 100 µL (~3500 mol XS) of the synthesized reagents (GPN, GPN2, GPN3, GPN4, and phenyl2-GPN) were added to separate dried glycan samples so that five of the tubes have a different tag in each tube and the final glycan sample has no tag (only 100 µL of reaction solvent was added). The six samples were allowed to incubate at 75 °C for 3 h, and the samples were dried in vacuo. A reaction diagram with starting materials and the final derivatized glycan structure is presented in the supplemental material (Scheme S1). The samples were then reconstituted in HILIC initial conditions (80% ACN and 20% 50 mM ammonium acetate in water), and the six glycan samples were combined in an equimolar mixture so that ~1 pmol of each was injected on column in a 10 µL injection and analyzed using nanoLC LTQ-FTICR MS.
Cleavage, Derivatization, and Analysis of Plasma N-linked Glycans
Fifty microliters of lyophilized pooled human plasma was reconstituted in 250 µL of 50 mM tris-HCl buffer (pH 7.5) and denatured by adding 28 µL of 2% SDS/1 M β-mercaptoethanol and incubating at 95 °C for 5 min. The solution was allowed to cool to room temperature, and 5 µL of PNGase F (12.5 mU) was added. The reaction was allowed to proceed for 18 h at 37 °C. The reaction was then quenched with 500 µL of 0.1% TFA. The glycans were extracted using graphitized carbon solid phase extraction (SPE), which has been described in detail previously [39], and the glycan samples were dried in vacuo.
The derivatization procedure for plasma glycans was optimized to decrease the amount of peeling for labile capping sugar units such as sialic acid and fucose. A 1 mg/ml solution of phenyl2-GPN reagent in 75:25 MeOH:acetic acid solution was prepared. To the dried glycan sample, 100 µL of the reagent solution was added and allowed to react for 3 h at 56 °C in an incubator. The glycans were then dried in vacuo and reconstituted in HILIC initial conditions.
nano-Flow Liquid Chromatography (HILIC)
Separation of derivatized glycans was performed using online hydrophilic interaction (liquid) chromatography (HILIC) [39]. An Eksigent nanoLC-2D system equipped with an AS1 autosampler (Dublin, CA, USA) was used with a vented-column setup as described previously [40]. TSK-Gel Amide-80 stationary phase with a 5 µm particle size (TOSOH Bioscience) was used to pack the trap and analytical columns, and both were packed in house. The trap column (360 µm o.d., 100 µm i.d. IntegraFrit) was packed to ~3.2 cm, and the analytical column (360 µm o.d., 75 µm i.d. with a 15 µm PicoFrit tip) was packed to ~10 cm. Mobile phase A and B were 50 mM ammonium acetate (pH 4.5) and 100% ACN, respectively. Onto the trap, 10 µL of sample were injected at the initial gradient conditions (20% Solvent A), washed at high organic solvent (93% Solvent B), and eluted at 500 nL/min. The gradient was ramped from 20% to 60% Solvent A over 37 min with a total run time of 60 min. A shallower gradient was used with the plasma glycans that included an additional washing step in high organic solvent (93% Solvent B). The gradient for plasma glycans was ramped from 20% to 55% solvent A over 37 min, and the total run time was 70 min.
LTQ-FTICR Mass Spectrometry
A hybrid linear ion trap Fourier Transform ion cyclotron resonance mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) equipped with a 7 Tesla superconducting magnet was used to acquire precursor and product ion mass spectra. Electrospray ionization was performed by applying a 2 kV potential to a zero dead volume union preceding the trap column. The ions were introduced into a stainless steel capillary heated to 225 °C, and the capillary and tube lens voltages were set to 42 and 120 V, respectively. The AGC in the ICR cell was set to 1 × 106 ions with a maximum injection time of 1 s. In the LTQ, the AGC was set to 8 × 103 ions with a maximum injection time of 80 ms and a normalized collision energy of 22. The instrument was operated in data dependent mode where up to 3 MS/MS spectra were collected per precursor scan. An include list was generated consisting of the three most intense m/z values in the precursor scan (FTICR), and these ions were subsequently fragmented (LTQ). If the same m/z value is chosen twice in 30 s, the m/z was placed on an exclude list for 30 s. Additionally, a 5000 count threshold was used to ensure quality MS/MS spectra. The mass spectrometer was calibrated according to manufacturer protocol and Xcalibur software (ver. 2.0.5) was used for peak integration and data analysis.
Non-Polar Surface Area Calculations
The NPSA were calculated as reported previously [5]. Due to the numerous hydrophilic functions, such as hydroxyl groups, that comprise glycans, the NPSA of the sugar units are deemed negligible. Thus, only the NPSA of the hydrophobic tagging reagents are taken into account. The NPSA of the reagents were calculated by using standard Van der Waal’s radii and bond lengths [41]. Other previous studies have used computer modeling to integrate the NPSA of reagents [13] and alternate assumptions [6, 8] in the determination of NPSA; thus, a detailed description of the assumptions, formulas, and calculations is included in the supplemental material (Figure S1) in order to present a single method for NPSA calculations of hydrophobic tagging reagents.
Results and Discussion
Characterization of Hydrazide Reagents
Five neutral reagents were synthesized in order to increase the relative abundance of N-linked glycans in ESI MS, analyze the types of hydrophobic groups that most effectively increase the electrospray response of glycans in MS, and direct the future synthesis of hydrazide reagents en route to determining an optimal tag for enhanced glycan analysis and quantification. Neutral reagents were chosen due to former studies showing the decreased ion abundance of glycans derivatized with permanently charged (+) reagents [13]. Furthermore, neutral reagents are compatible in both the positive and negative ion modes in ESI MS. Since the negative ion mode has shown to be advantageous to glycan analysis (more informative fragmentation patterns and the enhanced analysis of glycans with acidic sugar residues such as sialic acid) [42–48], the neutral reagents allow one to explore more experimental space without any additional sample preparation when switching between positive and negative modes. Two types of reagents have been synthesized: mono-phenyl and bi-phenyl (Figure 1a). A set of mono-phenyl reagents was synthesized in order to vary the alkyl chain length from 1 to 4 methylene units between the reactive site and the phenyl group. This will allow one to evaluate the affect of lengthening the hydrocarbon chain length with respect to the relative glycan response in ESI-MS. In addition, another set of molecules has been synthesized that contains a bi-phenyl reagent. These two types of hydrophobic groups differ in their types of bonds (σ versus π), geometry (tetrahedral versus planar), chemical structure, and localization of the electrons. The different characteristics of the reagents will allow for different solvent-analyte interactions in the electrospray droplet, and the characteristics most beneficial will be incorporated into future reagents in order to further enhance the ionization efficiency of glycans.
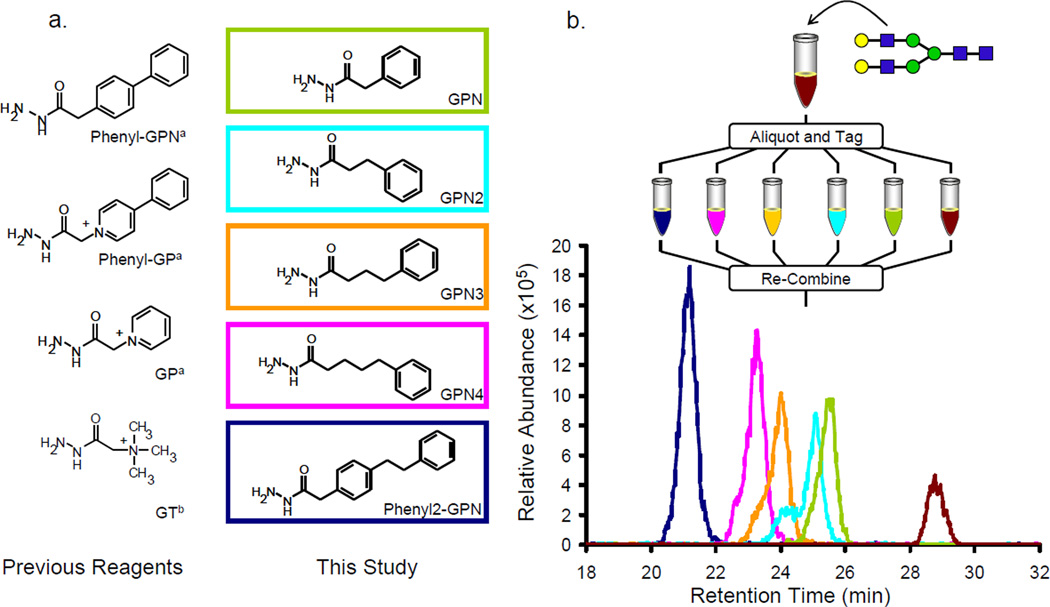
Figure 1.
(a) The reagents analyzed in this and in previous experiments (aRef. [13]; bRef. [8]). (b) The EIC’s of each of the current (boxed) reagents coupled to the NA2 glycan. The HILIC elution order is from the most hydrophobic reagents to the least (phenyl2-GPN, GPN4, GPN3, GPN2, GPN, native glycan). The relative abundances follow the same trend, as expected.
The synthesis and purification of the reagents were efficient and were confirmed by the accurate mass spectra of the derivatized NA2 glycan in the FT-ICR MS. Each reagent was coupled to the glycan and run individually in order to determine reaction efficiency and purity (data not shown). All reagents reacted nearly stoichiometrically with >95% efficiency, and there were no spectra in which there were more than two N-linked glycan peaks (tagged and free glycan), implying that there were no impurities or partial synthetic products present capable of reaction with the glycans. In addition, this is also evidence that the tags do not degrade in the reaction conditions or in the ionization source before analysis in the MS. Several characterization methods have been employed in order to confirm the synthesis of the reagents including 1H NMR, 13C NMR, IR, and high resolution MS, and are included in the supplemental material.
The effects of the hydrophobic hydrazide reagents were analyzed by creating an equimolar mixture of the NA2 glycan derivatized with each synthesized reagent, and the derivatized glycans show significant increases in ion abundance in comparison to the native glycan (Figure 1b). The extracted ion chromatograms (EIC) of each reagent are displayed to show the retention time and relative abundance of each tagged glycan. The reagents synthesized in this experiment (Figure 1a) were chosen to observe the effectiveness of incorporating linear alkane hydrophobic groups and additional phenyl groups onto the reagents. One phenyl group on the tagging reagent is necessary for the incorporation of stable isotopes (13C6) for future quantification studies, but as previously shown, a second phenyl group significantly increases the ion abundance of tagged glycans [13]. It was hypothesized that alkyl groups are more hydrophobic than phenyl groups due to the delocalization of the π bonds in the phenyl ring, and a larger increase in electrospray response would be expected for the reagents with linear alkanes. As the number of methylene groups is increased, the ion abundance increased, although it was not until the addition of three additional methylene groups (GPN4) that the electrospray response of the derivatized NA2 glycan increased equivalently to that of the reagent with two phenyl groups and no methylene groups (phenyl-GPN). In addition, the combination of two additional alkyl groups and two phenyl groups produced a reagent that outperformed any reagent synthesized to date. This evidence leads one to infer that the best method to increase the electrospray response of glycans is to add several phenyl rings to the reagents with the possibility of incorporating short alkyl chains between the rings.
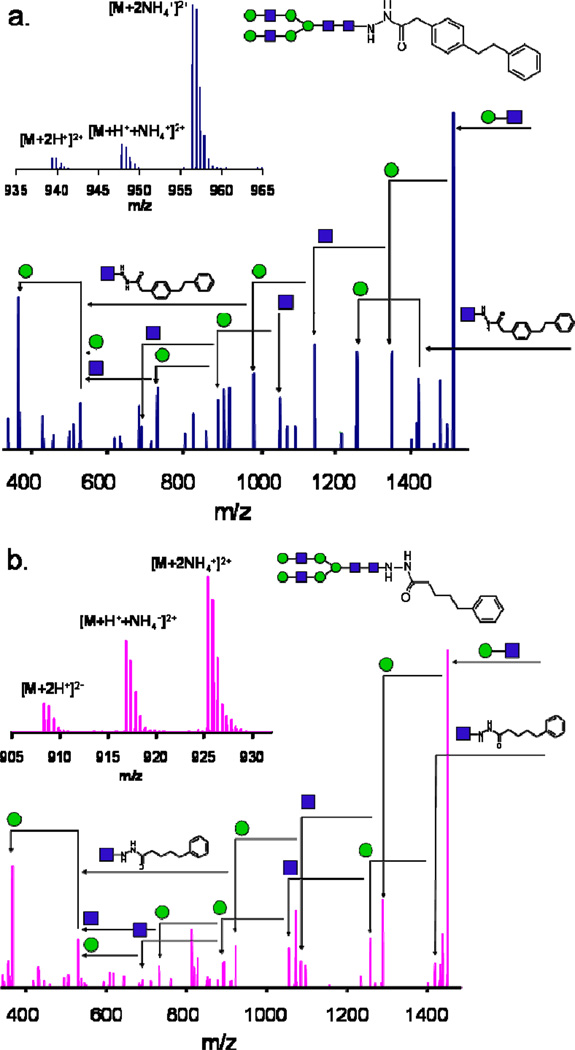
In order to further characterize the derivatized glycans and to confirm the stability of the reagents throughout the sample preparation and MS analysis, the MS/MS spectra of the two most successful reagents, phenyl2-GPN and GPN4, are shown in Figure 2a and b, respectively. These spectra show similar fragmentation in both types of reagents, mono- and bi-phenyl. In no spectrum has fragmentation of the reagent been observed, and the tag is never cleaved from the terminal GlcNAc residue, which allows for the determination of y and b glycosidic bond cleavages per Domon and Costello [26]. These fragmentation spectra show that the composition of unknown glycans can be determined using either the high mass measurement accuracy of the precursor scan, by the MS/MS spectra, or a combination of both. The signal-to-noise (S/N) ratios of the fragmentation spectra show good quality with only ~1 pmol introduced onto column, and nearly all the fragmentation products are present. This was not the case for the native glycans, where several fragmentation peaks were not able to be distinguished from the noise.
Figure 2.
The MS/MS data of the (a) phenyl2-GPN and (b) GPN4 reagents. The two fragmentation spectra show similar patterns, and in no case was a loss or fragmentation of the tagging reagent observed. In addition, the composition of the glycan was able to be determined using the MS/MS spectra. The insets are the high mass measurement accuracy FT-ICR precursor ions, showing the monoisotopic peak ([M + 2H+]2+) and the ammonium adduction peaks ([M + H+ + NH4+]2+ and [M + 2NH4+]2+). In both (a) and (b), squares and circles denote N-acetyllactosamine (HexNAc) and hexose (Hex) monosaccharide residues, respectively.
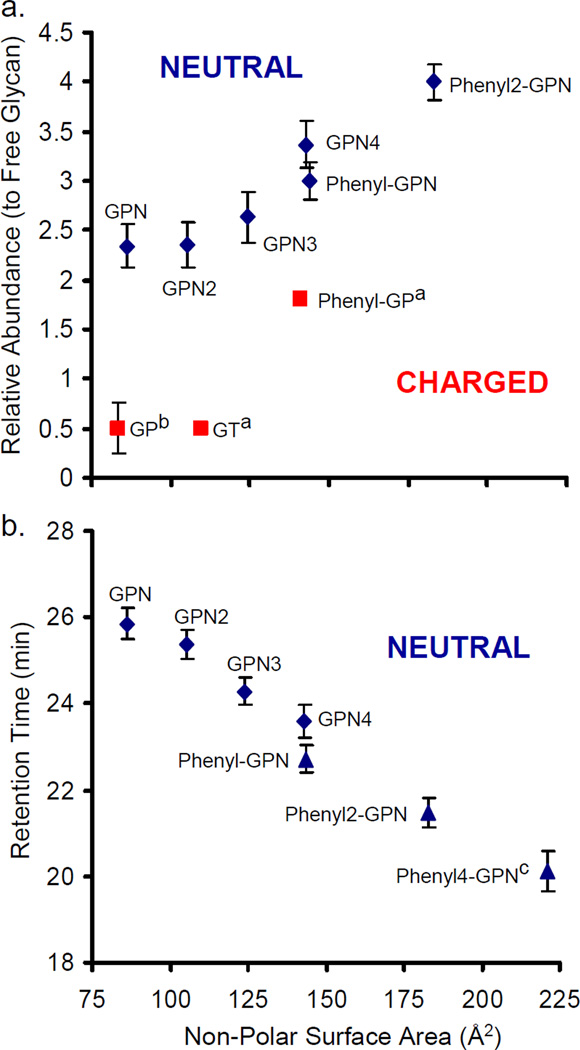
Non-polar surface area (NPSA) calculations are used to estimate the hydrophobicity of the hydrazide reagents. By estimating the hydrophobicity of the reagents, the most successful reagent will be able to be predicted. Figure 3a demonstrates the correlation between the NPSA calculations and the experimental ion abundances for the NA2 glycan derivatized with several different reagents. In general, as the NPSA, or hydrophobicity, of the reagent is increased, the signal of the derivatized glycan is increased as well. Based on this experimental data, future synthetic routes can be developed based on the NPSA and predict the types of hydrophobic reagents that will most effectively enhance the glycan ion abundance. This directed synthetic approach will allow only the reagents with the best chance of further increasing glycan ion abundance to be tested, and as the library continues to grow, the correlation between NPSA and relative glycan abundance will become more evident and lead toward the best possible hydrophobic reagent. However, increasing the hydrophobicity too much will be possible, leading to analytes that are not retained on a HILIC column or reagents that are not soluble in the reaction conditions.
Figure 3.
(a) The abundance of the derivatized glycan (relative to the free glycan) has been plotted versus the NPSA of the reagents. Diamonds denote neutral reagents, and squares represent charged reagents (aRef. [8]; bRef. [13]). (b) The retention time of the derivatized glycan is plotted versus the NPSA of the reagents. Diamonds represent mono-phenyl reagents, and triangles represent bi-phenyl reagents. The error bars in both (a) and (b) indicate 95% confidence intervals (3 ≤ n ≤ 5). cThe phenyl4-GPN reagent was synthesized (identical to phenyl2-GPN, except that it contains a 4-C chain between the phenyl rings), but the recrystallization purification was only minimally successful, and thus, it was analyzed for retention time purposes only and not for quantitative analysis.
Separation by HILIC allows for another metric for the estimation of the hydrophobicity of the species present. In HILIC, the more hydrophobic species elute earlier, allowing for an estimation of the hydrophobicity of the analytes based on the retention time. Though HILIC is considered to be a “mixed-mode” separation method where both partitioning and adsorption mechanisms are involved in separation [49–52], the reagents used in this experiment are chemically similar and, thus, the retention mechanisms are assumed to be similar. Figure 3b is a plot of retention time (RT) versus NPSA and shows an additional correlation between the experimental (RT) and calculated (NPSA) hydrophobicity. The two types of reagents, mono- and bi-phenyl, both show linear trends in which the retention time increases as the reagents become less hydrophobic. However, the slopes and intercepts of these lines differ, which implies slightly different separation mechanisms between the two types of reagents coupled to glycans. Though the reagents GPN4 and phenyl-GPN have nearly identical NPSA, the retention time between the two derivatized glycans is significantly different. Thus, the different types of bonds, the localization of electrons, and the geometry of the reagents are the differences between the two analytes and interact in different ways with the stationary phase. Though HILIC separation mechanism, retention times, and stationary phase interactions are not fully understood, this study shows that with all other properties held constant, the glycans derivatized with reagents primarily composed of alkyl chains interact more with the stationary phase than glycans derivatized with the bi-phenyl reagents.
Analysis of the Human Plasma N-Linked Glycome Using the Phenyl2-GPN Reagent
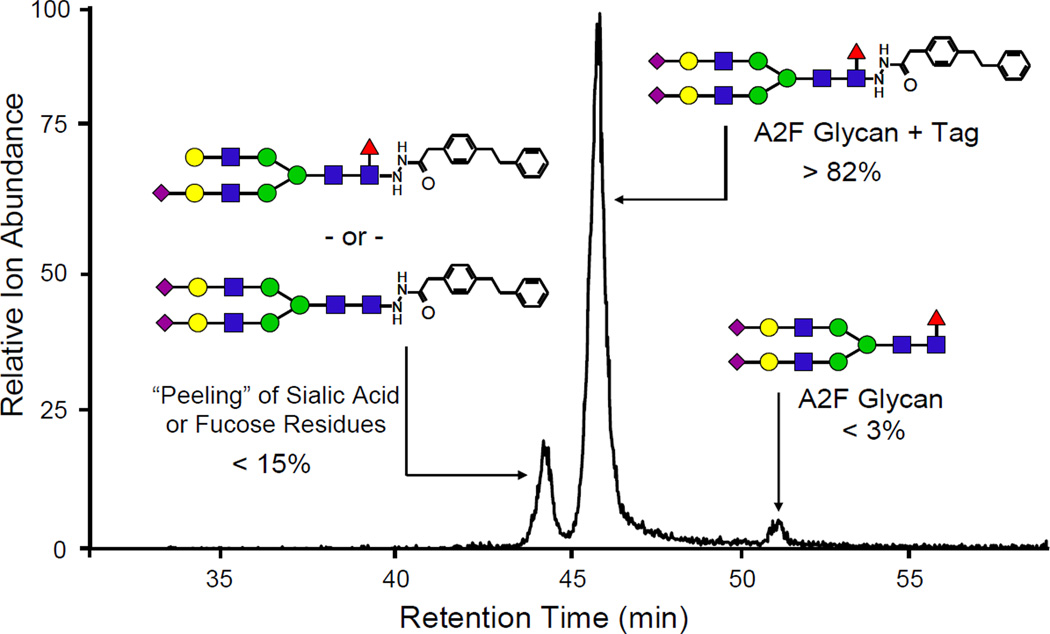
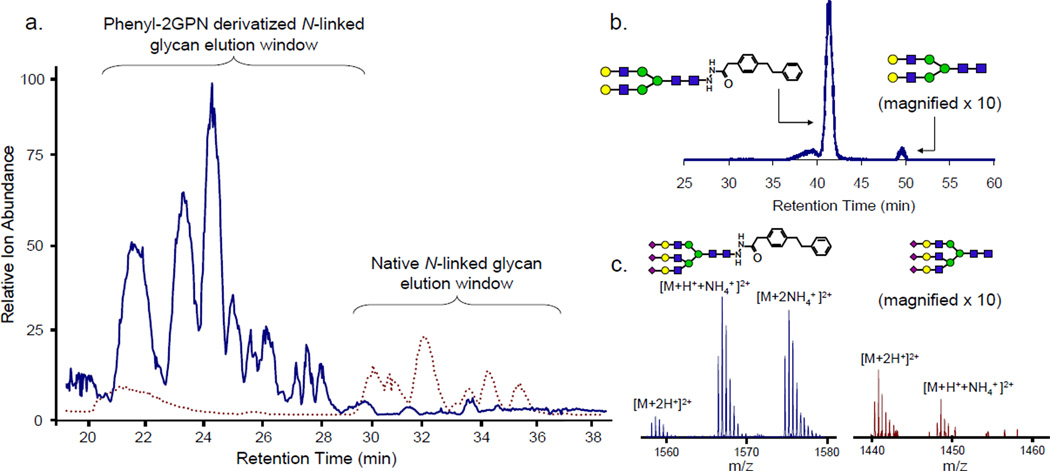
In order to demonstrate the compatibility and effectiveness of the novel glycan hydrophobic reagents in complex mixtures, the N-linked glycans from pooled human plasma were cleaved, derivatized with phenyl2-GPN, and analyzed. Initially, intact sialylated glycans were not able to be detected. Upon further investigation, it was determined that the high reaction temperature combined with acidic conditions (75 °C in 15% acetic acid for 3 h) was causing peeling, or loss of labile sugar residues (most notably NeuAc). Thus, numerous reaction conditions were tested using a model glycan, A2F, which contains two terminal sialic acid residues and one core fucose. It was found that a lower temperature and higher acidity (56 °C in 25:75 acetic acid:MeOH for 3 h) provided near complete conversion to the derivatized glycan (>95%) and minimized the peeling (Figure 4). These conditions were used in all the subsequent studies containing complex glycans with sialic acid or fucose residues.
Figure 4.
The EIC of the native and tagged A2F glycan after derivatization with phenyl2-GPN at 56 °C for 3 h in 25:75 acetic acid:MeOH. This shows >95% conversion to the derivatized glycan, however there is still a small amount of peeling, or loss of sialic acid or fucose.
Figure 5 shows the analysis of N-linked glycans cleaved from plasma, both native and derivatized. The base peak chromatograms have been overlaid in Figure 5a and the derivatized glycans show on average a 4-fold increase in total ion abundance with certain negatively charged glycans showing up to a 10-fold increase. Figure 5b shows the derivatization efficiency of the glycans in a complex mixture. The >97% reaction efficiency in plasma displays the robustness of the derivatization even in the most complex of samples, plasma. Additionally, Figure 5c demonstrates the advantage of derivatizing negatively charged sialylated glycans, which are often difficult to ionize in the positive ion mode. When analyzing native glycans, the tri-sialylated A3 glycan is only minutely detected. It is shown that the signal of the native A3 glycan is only slightly above the noise, whereas >10-fold S/N is detected for the derivatized A3 glycan. This provides more statistically accurate isotopic ratios, which yield higher quality data and will lead to the accurate quantification of N-linked glycans over a wider dynamic range.
Figure 5.
The compatibility of the phenyl2-GPN hydrophobic hydrazide reagent with glycans cleaved from complex mixtures: (a) the base peak chromatogram of the derivatized N-linked glycans (solid line) overlaid with the base peak chromatogram of the native N-linked glycans (dotted line); an average of 4-fold increase in glycan ion abundance is observed for all glycan species. (b) the EIC and reaction efficiency of the NA2 glycan cleaved from plasma proteins; (c) the mass spectra of the tri-sialylated A3 glycan both native and derivatized.
Conclusion
The hydrazide reagents are effective in increasing the glycan ion abundance in ESI FT-ICR MS, and as the reagents become more hydrophobic, the ion abundance is increased further. The addition of a second phenyl ring has been found to have the greatest effect on the glycan response, and future studies will continue to incorporate this multi-phenyl structure into the tagging reagents. To date, no reagents have suffered from degradation or decreased ion abundance due to solubility issues. This is evidence that there are still possibilities to make reagents that are even more hydrophobic, will remain compatible with the reaction solvents and nanoLC conditions, and can further increase the ion abundance of glycans.
The increased total ion abundance of the derivatized glycans cleaved from plasma proteins imparts several advantages for glycan analysis, including the facilitated detection of lower abundant glycan species. By increasing the ion abundance of these glycans by an average of 4-fold and some negatively charged glycans up to 10-fold, the number of N-linked glycans detected in plasma was increased by ~10%. This glycan derivatization procedure, which adds <4 h total of sample preparation time, is an effective tool in enhancing the profiling of N-linked glycans in complex samples. The success of this approach will ultimately lead to stable isotope labeled tags that improve ESI response and facilitate comparative glycomics.
Supplementary Material
Acknowledgments
The authors are grateful for the funding provided by the NSF Research Experience for Undergraduates (L.M.L.) program at North Carolina State University, the NIH/NCSU Molecular Biotechnology Training Program (S.H.W.), the W. M. Keck Foundation, and North Carolina State University.
Footnotes
Supplementary Material: Supplementary material associated with this article may be found in the online version at doi:10.1016/j.jasms.xxxx.xx.xxx
References
- 1.Null AP, Nepomuceno AI, Muddiman DC. Implications of hydrophobicity and free energy of solvation for characterization of nucleic acids by electrospray ionization mass spectrometry. Anal. Chem. 2003;75(6):1331–1339. doi: 10.1021/ac026217o. [DOI] [PubMed] [Google Scholar]
- 2.Frahm JL, Bori ID, Comins DL, et al. Achieving augmented limits of detection for peptides with hydrophobic alkyl tags. Anal. Chem. 2007;79(11):3989–3995. doi: 10.1021/ac070558q. [DOI] [PubMed] [Google Scholar]
- 3.Mirzaei H, Regnier F. Enhancing electrospray ionization efficiency of peptides by derivatization. Anal. Chem. 2006;78(12):4175–4183. doi: 10.1021/ac0602266. [DOI] [PubMed] [Google Scholar]
- 4.Yang WC, Mirzaei H, Liu XP, et al. Enhancement of amino acid detection and quantification by electrospray ionization mass spectrometry. Anal. Chem. 2006;78(13):4702–4708. doi: 10.1021/ac0600510. [DOI] [PubMed] [Google Scholar]
- 5.Shuford CM, Comins DL, Whitten JL, et al. Improving limits of detection for B-type natriuretic peptide using PC-IDMS: An application of the ALiPHAT strategy. Analyst. 2010;135(1):36–41. doi: 10.1039/b919484c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DK, Comins DL, Whitten JL, et al. Evaluation of the ALiPHAT method for PC-IDMS and correlation of limits-of-detection with nonpolar surface area. J. Am. Soc. Mass. Spectrom. 2009;20(11):2006–2012. doi: 10.1016/j.jasms.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DK, Meadows CW, Bori ID, et al. Synthesis, characterization, and application of iodoacetamide derivatives utilized for the ALiPHAT strategy. J. Am. Chem. Soc. 2008;130(7):2122–2123. doi: 10.1021/ja076849y. [DOI] [PubMed] [Google Scholar]
- 8.Bereman MS, Comins DL, Muddiman DC. Increasing the hydrophobicity and electrospray response of glycans through derivatization with novel cationic hydrazides. Chem. Commun. 2010;46(2):237–239. doi: 10.1039/b915589a. [DOI] [PubMed] [Google Scholar]
- 9.Bowman MJ, Zaia J. Tags for the stable isotopic labeling of carbohydrates and quantitative analysis by mass spectrometry. Anal. Chem. 2007;79(15):5777–5784. doi: 10.1021/ac070581b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowman MJ, Zaia J. Comparative glycomics using a tetraplex stable-isotope coded tag. Anal. Chem. 2010;82(7):3023–3031. doi: 10.1021/ac100108w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouw JW, Burgers PC, Trikoupis MA, et al. Derivatization of small oligosaccharides prior to analysis by matrix-assisted laser desorption/ionization using glycidyltrimethylammonium chloride and Girard's reagent T. RCMS. 2002;16(10):905–912. doi: 10.1002/rcm.654. [DOI] [PubMed] [Google Scholar]
- 12.Naven TJP, Harvey DJ. Cationic derivatization of oligosaccharides with Girard's T reagent for improved performance in matrix-assisted laser desorption/ionization and electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 1996;10(7):829–834. [Google Scholar]
- 13.Walker SH, Papas BN, Comins DL, et al. The interplay of permanent charge and hydrophobicity in the electrospray ionization of glycans. Anal. Chem. 2010;82(15):6636–6642. doi: 10.1021/ac101227a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhaak LR, Zauner G, Huhn C, et al. Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 2010 doi: 10.1007/s00216-010-3532-z. published online 12 March 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia BY, Feasley CL, Sachdev GP, et al. Glycan reductive isotope labeling for quantitative glycomics. Anal. Biochem. 2009;387(2):162–170. doi: 10.1016/j.ab.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhold VN, Reinhold BB, Costello CE. Carbohydrate Molecular-Weight Profiling, Sequence, Linkage, and Branching Data—ES-MS and CID. Anal. Chem. 1995;67(11):1772–1784. doi: 10.1021/ac00107a005. [DOI] [PubMed] [Google Scholar]
- 17.Kebarle P, Tang L. From Ions in Solution to Ions in the Gas-Phase—the Mechanism of Electrospray Mass-Spectrometry. Anal. Chem. 1993;65(22):A972–A986. [Google Scholar]
- 18.Kebarle P, Verkerk UH. Electrospray: From Ions in Solution to Ions in the Gas Phase. What We Know Now. Mass Spectrom. Rev. 2009;28(6):898–917. doi: 10.1002/mas.20247. [DOI] [PubMed] [Google Scholar]
- 19.Gomez A, Tang KQ. Charge and Fission of Droplets in Electrostatic Sprays. Phys. Fluids. 1994;6(1):404–414. [Google Scholar]
- 20.Cech NB, Enke CG. Effect of affinity for droplet surfaces on the fraction of analyte molecules charged during electrospray droplet fission. Anal. Chem. 2001;73(19):4632–4639. doi: 10.1021/ac001267j. [DOI] [PubMed] [Google Scholar]
- 21.Fenn JB. Ion Formation from Charged Droplets—Roles of Geometry, Energy, and Time. J. Am. Soc. Mass Spectrom. 1993;4(7):524–535. doi: 10.1016/1044-0305(93)85014-O. [DOI] [PubMed] [Google Scholar]
- 22.Ciucanu I, Kerek F. A Simple and Rapid Method for the Permethylation of Carbohydrates. Carbohydr. Res. 1984;131(2):209–217. [Google Scholar]
- 23.Dell A. Preparation and Desorption Mass-Spectrometry of Permethyl and Peracetyl Derivatives of Oligosaccharides. Methods Enzymol. 1990;193:647–660. doi: 10.1016/0076-6879(90)93443-o. [DOI] [PubMed] [Google Scholar]
- 24.Guillard M, Gloerich J, Wessels HJCT, et al. Automated measurement of permethylated serum N-glycans by MALDI-linear ion trap mass spectrometry. Carbohydr. Res. 2009;344(12):1550–1557. doi: 10.1016/j.carres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Bourne EJ, Stacey M, Tatlow JC, et al. Studies on Trifluoroacetic Acid .1. Trifluoroacetic Anhydride as a Promoter of Ester Formation between Hydroxy-Compounds and Carboxylic Acids. J. Chem. Soc. 1949 Nov;:2976–2979. [Google Scholar]
- 26.Domon B, Costello CE. A Systematic Nomenclature for Carbohydrate Fragmentations in FAB-MS-MS Spectra of Glycoconjugates. Glycoconj. J. 1988;5(4):397–409. [Google Scholar]
- 27.Zhao C, Xie B, Chan SY, et al. Collisionally activated dissociation and electron capture dissociation provide complementary structural information for branched permethylated oligosaccharides. J. Am. Soc. Mass Spectrom. 2008;19(1):138–150. doi: 10.1016/j.jasms.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Hase S, Ikenaka T, Matsushima Y. A Highly Sensitive Method for Analyses of Sugar Moieties of Glycoproteins by Fluorescence Labeling. J. Biochem. 1981;90(2):407–414. doi: 10.1093/oxfordjournals.jbchem.a133487. [DOI] [PubMed] [Google Scholar]
- 29.Pabst M, Kolarich D, Poltl G, et al. Comparison of fluorescent labels for oligosaccharides and introduction of a new post-labeling purification method. Anal. Biochem. 2009;384(2):263–273. doi: 10.1016/j.ab.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 30.Schwaiger H, Oefner PJ, Huber C, et al. Capillary Zone Electrophoresis and Micellar Electrokinetic Chromatography of 4-Aminobenzonitrile Carbohydrate-Derivatives. Electrophoresis. 1994;15(7):941–952. doi: 10.1002/elps.11501501138. [DOI] [PubMed] [Google Scholar]
- 31.Yoshino K, Takao T, Murata H, et al. Use of the Derivatizing Agent 4-Aminobenzoic Acid 2-(Diethylamino)Ethyl Ester for High-Sensitivity Detection of Oligosaccharides by Electrospray-Ionization Mass-Spectrometry. Anal. Chem. 1995;67(21):4028–4031. doi: 10.1021/ac00117a034. [DOI] [PubMed] [Google Scholar]
- 32.Harvey DJ. Electrospray mass spectrometry and fragmentation of N-linked carbohydrates derivatized at the reducing terminus. J. Am. Soc. Mass Spectrom. 2000;11(10):900–915. doi: 10.1016/S1044-0305(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 33.Poulter L, Burlingame AL. Desorption Mass-Spectrometry of Oligosaccharides Coupled with Hydrophobic Chromophores. Methods Enzymol. 1990;193:661–688. doi: 10.1016/0076-6879(90)93444-p. [DOI] [PubMed] [Google Scholar]
- 34.Lamari FN, Kuhn R, Karamanos NK. Derivatization of carbohydrates for chromatographic, electrophoretic, and mass spectrometric structure analysis. J. Chromatogr. B. 2003;793(1):15–36. doi: 10.1016/s1570-0232(03)00362-3. [DOI] [PubMed] [Google Scholar]
- 35.Avigad G. Dansyl Hydrazine as a Fluorimetric Reagent for Thin-Layer Chromatographic Analysis of Reducing Sugars. J. Chromatogr. 1977;139(2):343–347. doi: 10.1016/s0021-9673(00)89330-9. [DOI] [PubMed] [Google Scholar]
- 36.Karamanos NK, Tsegenidis T, Antonopoulos CA. Analysis of Neutral Sugars as Dinitrophenyl-Hydrazones by High-Performance Liquid-Chromatography. J. Chromatogr. 1987;405:221–228. [Google Scholar]
- 37.Miksik I, Gabriel J, Deyl Z. Microemulsion electrokinetic chromatography of diphenylhydrazones of dicarbonyl sugars. J. Chromatogr. A. 1997;772(1/2):297–303. [Google Scholar]
- 38.Mopper K, Johnson L. Reversed-Phase Liquid-Chromatographic Analysis of DNS-Sugars—Optimization of Derivatization and Chromatographic Procedures and Applications to Natural Samples. J. Chromatogr. 1983;256(1):27–38. [Google Scholar]
- 39.Bereman MS, Young DD, Deiters A, et al. Development of a Robust and High Throughput Method for Profiling N-Linked Glycans Derived from Plasma Glycoproteins by NanoLC-FTICR Mass Spectrometry. J. Proteome Res. 2009;8(7):3764–3770. doi: 10.1021/pr9002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews GL, Shuford CM, Burnett JC, et al. Coupling of a vented column with splitless nanoRPLC-ESI-MS for the improved separation and detection of brain natriuretic peptide-32 and its proteolytic peptides. J. Chromatogr. B. 2009;877(10):948–954. doi: 10.1016/j.jchromb.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oxtoby DW, Gillis HP, Nachtrieb NH. Principles of Modern Chemistry. 4th ed. Fort Worth: Saunders College Pub.; 1999. p. xxiii-876. [Google Scholar]
- 42.Wheeler SF, Harvey DJ. Negative ion mass spectrometry of sialylated carbohydrates: Discrimination of N-acetylneuraminic acid linkages by MALDI-TOF and ESI-TOF mass spectrometry. Anal. Chem. 2000;72(20):5027–5039. doi: 10.1021/ac000436x. [DOI] [PubMed] [Google Scholar]
- 43.Chai WG, Piskarev V, Lawson AM. Branching pattern and sequence analysis of underivatized oligosaccharides by combined MS/MS of singly and doubly charged molecular ions in negative-ion electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2002;13(6):670–679. doi: 10.1016/S1044-0305(02)00363-X. [DOI] [PubMed] [Google Scholar]
- 44.Chai WG, Piskarev V, Lawson AM. Negative ion electrospray mass spectrometry of neutral underivatized oligosaccharides. Anal. Chem. 2001;73(3):651–657. doi: 10.1021/ac0010126. [DOI] [PubMed] [Google Scholar]
- 45.Pfenninger A, Karas M, Finke B, et al. Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MSn. Part 2. Application to isomeric mixtures. J. Am. Soc. Mass Spectrom. 2002;13(11):1341–1348. doi: 10.1016/S1044-0305(02)00646-3. [DOI] [PubMed] [Google Scholar]
- 46.Harvey DJ. Fragmentation of negative ions from carbohydrates: Part 3. Fragmentation of hybrid and complex N-linked glycans. J. Am. Soc. Mass Spectrom. 2005;16(5):647–659. doi: 10.1016/j.jasms.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Harvey DJ. Fragmentation of negative ions from carbohydrates: Part 2. Fragmentation of high-mannose N-linked glycans. J. Am. Soc. Mass Spectrom. 2005;16(5):631–646. doi: 10.1016/j.jasms.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Harvey DJ. Fragmentation of negative ions from carbohydrates: Part 1. Use of nitrate and other anionic adducts for the production of negative ion electrospray spectra from N-linked carbohydrates. J. Am. Soc. Mass Spectrom. 2005;16(5):622–630. doi: 10.1016/j.jasms.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Alpert AJ. Hydrophilic-Interaction Chromatography for the Separation of Peptides, Nucleic Acids, and Other Polar Compounds. J. Chromatogr. 1990;499:177–196. doi: 10.1016/s0021-9673(00)96972-3. [DOI] [PubMed] [Google Scholar]
- 50.Hemstrom P, Irgum K. Hydrophilic interaction chromatography. J. Sep. Sci. 2006;29(12):1784–1821. doi: 10.1002/jssc.200600199. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen HP, Schug KA. The advantages of ESI-MS detection in conjunction with HILIC mode separations: Fundamentals and applications. J. Sep. Sci. 2008;31(9):1465–1480. doi: 10.1002/jssc.200700630. [DOI] [PubMed] [Google Scholar]
- 52.Dejaegher B, Heyden YV. HILIC methods in pharmaceutical analysis. J. Sep. Sci. 2010;33(6–7):698–715. doi: 10.1002/jssc.200900742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.