Abstract
Pancreatic ductal adenocarcinoma (PDA) is an aggressive malignancy with one of the worst outcomes among all cancers. PDA often recurs after initial treatment to result in patient death despite the use of chemotherapy or radiation therapy. PDA contains a subset of tumor-initiating cells capable of extensive self-renewal known as cancer stem cells (CSC), which may contribute to therapeutic resistance and metastasis. At present, conventional chemotherapy and radiotherapy are largely ineffective in depleting CSC pool, suggesting the need for novel therapies that specifically target the cancer-sustaining stem cells for tumor eradication and to improve the poor prognosis of PDA patients. In this study, we report that death receptor 5 (DR5) is enriched in pancreatic CSCs compared with the bulk of the tumor cells. Treating a collection of freshly generated patient-derived PDA xenografts with gemcitabine, the first-line chemotherapeutic agent for PDA, is initially effective in reducing tumor size, but largely ineffective in diminishing the CSC populations, and eventually culminated in tumor relapse. However, a combination of tigatuzumab, a fully humanized DR5 agonist monoclonal antibody, with gemcitabine proved to be more efficacious by providing a double hit to kill both CSCs and bulk tumor cells. The combination therapy produced remarkable reduction in pancreatic CSCs, tumor remissions, and significant improvements in time to tumor progression in a model that is considered more difficult to treat. These data provide the rationale to explore the DR5-directed therapies in combination with chemotherapy as a therapeutic option to improve the current standard of care for pancreatic cancer patients.
Introduction
Pancreatic ductal adenocarcinoma (PDA) is among the most lethal human cancers and its incidence is increasing in the United States (1). Resistance to chemotherapy is thought to be a major cause of treatment failure in PDA patients (2, 3). As our understanding of PDA evolves, evidence is growing to support a role for tumor-initiating cells, called cancer stem cells (CSC), in this devastating disease (4). Recent studies suggest that PDA is driven by a small population of CSCs that are responsible for tumor initiation and propagation (5, 6). At present, conventional chemotherapy and radiotherapy affect rapidly dividing PDA cells that constitute the tumor bulk, thus reducing tumor mass, but probably fail to target CSCs that drive tumorigenesis and metastasis, which might be responsible for treatment failure and tumor recurrence in many patients (7). Although the clinical relevance of CSCs beyond experimental models is still lacking, the high frequency of relapse after conventional cytotoxic chemotherapies in PDA suggests that CSCs survive standard treatments (8).
Decades of efforts have witnessed the failure of many chemotherapeutic regimens tested in PDA, and the current standard-of-care chemotherapeutic agent gemcitabine (GEM) extends patient survival by only a few weeks (9). In the last 20 years, a large number of patients have been treated in randomized, large phase III clinical trials, but results have been globally disappointing (10). A marked change in treatment paradigm is essential to move beyond the persistently dismal outcome for the majority of PDA patients (11). It is becoming evident that a cancer treatment that fails to eliminate CSCs may allow the regrowth of the tumor (12). Recent reports indicate that a subpopulation of PDA cells functionally resembling CSCs have strong resistance to GEM both in vitro and in vivo (13, 14). In addition, treatment with ionizing radiation and GEM resulted in the enrichment of CSC populations in human primary PDA xenografts (15, 16). For these reasons, targeting cancer-sustaining stem cells might be an attractive strategy for more effective cancer treatment.
In the quest to discover antitumor agents with greater specificity and potency, efforts have been directed toward developing monoclonal antibodies (mAb) that recognize antigens unique to or overexpressed by cancer cells. Tumor necrosis factor–related apoptosis-inducing ligand (Apo2L/TRAIL) and its agonistic antibodies, which are being evaluated clinically as anticancer therapies, selectively kill cancer cells through the death receptors DR4 and DR5 (17, 18). Importantly, purified recombinant human TRAIL suppresses tumor growth and shows little or no overt toxicity when systemically administered to animals (19). DR5 expression has been detected with high frequency in tumor cell lines and clinical tumor specimens (20). Cancer cell lines express DR5 more frequently than DR4 and studies showed that DR5 might contribute more than DR4 to TRAIL-induced apoptosis in cancer cells that express both death receptors (21). DR5 levels have been reported to be elevated in primary PDA tissues as compared with the normal pancreas (22). A novel murine anti-human DR5 mAb, TRA-8, has been reported to induce apoptosis in several tumor cell lines and inhibit the growth of tumors xenografted in mice (23, 24). Tigatuzumab, a humanized version of TRA-8, is currently in clinical trials as a therapy for solid tumors (25). Tigatuzumab has selective toxicity toward tumor cells expressing DR5 and showed robust antitumor efficacy in human malignancies without damage to other tissues or hepatocyte cytotoxicity (26).
In the present study, we investigated the in vivo efficacy of tigatuzumab monotherapy and in combination with GEM in a panel of pancreatic cancer xenografts, which were generated from PDA patients. We provide evidence for the first time that pancreatic CSCs are enriched with DR5. GEM, the first-line agent for PDA, is initially effective in reducing tumor size, but not capable of reducing tumorigenic CSCs, and eventually culminated in tumor recurrence. However, the combination of GEM and tigatuzumab markedly reduced pancreatic CSCs and subsequently prevented tumor recurrence. The present findings are supportive of the notion that targeting CSCs is an attractive avenue in cancer treatment and provides the rationale to explore DR5-directed therapies in combination with chemotherapy.
Materials and Methods
Animals and establishment of xenograft models
Female nu/nu athymic mice (Harlan) were used for the study. Animals were maintained under pathogen-free conditions and a 12-hour light/12-hour dark cycle. Animal experiments were conducted following approval and in accordance with the Animal Care and Use Committee guidelines of the Johns Hopkins University. Fresh pancreatic tumor pieces obtained from patients at the time of surgery, with informed written patient consent, were implanted s.c. into the flanks of 6-week-old mice. The patients had not undergone chemotherapy or radiation therapy before surgery. Grafted tumors were subsequently transplanted from mouse to mouse and maintained as a live PancXenoBank according to an Institutional Review Board–approved protocol (27).
In vivo tumor therapy studies
To establish the efficacy of tigatuzumab, GEM, and the combination of GEM and tigatuzumab, tumors from eight separate patient xenografts were implanted s.c. into the flanks of athymic mice. Cohorts of mice with tumor size of ~200 mm3 in each xenografts were randomized to four treatment groups (6 mice; 10 tumors per group): (a) vehicle (control); (b) 3 mg/kg tigatuzumab i.v. once weekly for 4 weeks; (c) 100 mg/kg GEM i.p. twice a week for 4 weeks; and (d) GEM plus tigatuzumab at the above-mentioned doses and frequencies for 4 weeks. Tigatuzumab (Daiichi Sankyo Co., Ltd.) and GEM (Eli Lilly and Company) doses were selected based on previously published reports (26, 28). Animals were sacrificed after the final dose of drug treatment (28th day) except for six to eight tumors in the GEM and the combination treatment groups, which were followed for up to 120 days to investigate whether the combination therapy results in a durable tumor inhibition. Tumor size was evaluated twice per week with calipers. Tumor volume was calculated using the following formula: tumor volume = (length × width2)/2. Relative tumor growth [treated versus control (T/C)] rate was calculated using the formula (mean tumor volume of drug-treated group/mean tumor volume of control group) × 100. Tumor doubling time was calculated as the time elapsed from the day of first treatment to double the initial tumor size on an individual tumor basis and analyzed using a log-rank test based on the Kaplan-Meier method.
Protein extraction and Western blot analysis
Protein extracts were prepared from tumors according to previously published methods (29). Briefly, tumors from two separate animals of the control and the treatment groups (on day 28) were minced on ice in prechilled lysis buffer. The minced tissue was homogenized using a Dounce homogenizer and centrifuged at 16,000 × g at 4°C for 10 minutes. Protein lysates (30 μg) were fractionated by SDS-PAGE, electrotransferred onto nitrocellulose membranes, and blotted with primary antibodies for TRAIL (Santa Cruz Biotechnology Inc.), DR5, Fas, Fas-associated death domain (FADD), tumor necrosis factor receptor 1–associated death domain (TRADD), procaspase-8 and cleaved caspase-8, procaspase-8 and cleaved caspase-3, Bid, Bax, cleaved poly(ADP-ribose) polymerase, X-linked inhibitor of apoptosis, p53, and β actin (Cell Signaling Technology). TRAIL and caspase-8 were mouse mAbs and the other antibodies used were of rabbit monoclonal or poyclonal origin. After washing thrice with TBS, the membranes were incubated for 1 hour at room temperature with horseradish peroxidase–conjugated secondary anti-bodies, rabbit or mouse IgG-horseradish peroxidase (Santa Cruz Biotechnology Inc.). After washing thrice with TBS, antibody binding was detected by enhanced chemiluminescence (GE Healthcare) as previously reported (29). Band intensities were quantitated by densitometric scanning.
Cancer stem cell quantification and DR5 expression by flow cytometry
Tumors grown subcutaneously in nude mice were harvested and single-cell suspensions were generated by mincing tumors using sterile razors, followed by incubation in dispase and collagenase type IV (both from Sigma) at 37°C for 2 hours with agitation. Debris was removed by passing the cell suspension through a 70-μm filter (BD Biosciences) and further purified by density centrifugation using Ficoll-Paque Plus (GE Healthcare). The purified cells were washed twice in cold DMEM. Cells were incubated with Aldefluor reagent (Stem Cell Technologies) in the presence or absence of diethylaminobenzalde-hyde following the manufacturer’s protocol. Cells were then stained for 15 minutes at 4°C with different combinations of the following antibodies: antimouse CD31-biotin (BD Biosciences), mouse lineage cocktail-biotin (Miltenyi Biotec), antimouse H-2Kd-biotin (BD Biosciences), antihuman CD44-allophycocyanin (BD Biosciences, clone G44-26), antihuman CD24-phycoerythrin (PE; BD Biosciences, clone ML5), antihuman CD24-FITC (BD Biosciences, clone ML5), antihuman DR5-PE (eBiosciences, clone DJR2-4), mouse specific IgG2b κ-allophycocyanin, mouse specific IgG2a κ-PE, and mouse specific IgG1 κ-PE. The cells were washed and then incubated with streptavidin-peridinin chlorophyll for 10 minutes at 4°C. Finally, the cells were washed once again and resuspended in Aldefluor buffer containing 2 μg/mL propidium iodide. The cells were analyzed using a FACSAria flow cytometer (BD Biosciences). The cells were gated based on forward scatter and side scatter properties followed by exclusion of mouse-derived and nonviable cells. Aldefluor+, CD24+CD44+, and DR5+ tumor cells were determined based on the fluoresce as previously reported (30). In a separate experiment, Panc219 tumor–bearing mice were treated with tigatuzumab, GEM, and GEM plus tigatuzumab in the mentioned doses and frequencies for 4 weeks. Tumors were harvested and stem cell populations were purified and analyzed using a FACSAria flow cytometer as described above.
Immunohistochemical detection of CD24
Tumor tissues from the saline-treated and other treatment groups on day 28 were fixed in formalin and processed into paraffin blocks. Two tumors per treatment group were analyzed. Sections were deparaffinized in xylene and rehydrated in graded-alcohol washes. Antigen retrieval was done by incubating the slides in boiling sodium citrate buffer (10 mmol/L, pH 6.0) for 30 minutes, and endogenous peroxidases were quenched by incubating the slides in 3% hydrogen peroxide in methanol for 10 minutes at room temperature. The slides were then incubated with CD24 mouse mAb (clone SN3b, Thermo Scientific, Inc.). Envision plus dual-link polymer-horseradish peroxidase (DAKO) was used as a detection reagent and 3,3′-diaminobenzidine as a chromogen. After immunostaining, slides were counterstained with hematoxylin. The staining was scored as the number of CD24+ tumor cells (membranous and/or cytoplasmic staining for CD24) over total tumor cells (0–100%) multiplied by staining intensity. We used a 0 to 3 scale for staining intensity: 0, completely negative; 1, weak positivity; 2, moderate positivity; 3, strong positivity (16, 28). CD24 staining was scored in approximately 10 random fields with a minimum of 1,000 tumor cells by a pathologist in a blind way.
Quantitative real-time reverse transcription-PCR
RNA was purified from the Panc219 and Panc410 xenografts on day 28 using the RNeasy kit (Qiagen). Five micrograms of total RNA from two separate tumors in each group were used for cDNA synthesis and reverse transcription was done using Superscript II (Invitrogen) per standard protocol. Quantitative PCR was done on a MyIQ real-time PCR machine (Bio-Rad) and using TaqMan primer/probe sets (Applied Biosystems) for β-actin, ALDH1A1 (Hs00167445_m1), and CD44 (Hs00174139_m1). Comparative gene expression was done using the δδCT method.
Statistical analysis
All error bars represent the SEM. Significance levels for comparison between groups were analyzed using unpaired Student’s t test. All statistical testes were two sided and the differences were considered significant when P < 0.05.
Results
In vivo antitumor effects of tigatuzumab alone or in combination with GEM
When tested as a single agent, tigatuzumab showed variable efficacy in PDA xenografts. Tigatuzumab monotherapy showed comparable antitumor activity to that of GEM in 2 of 8 xenografts. T/C rates for Panc219 and Panc286 were 17.7% and 33.9% for tigatuzumab and 44.7% and 46.3% for GEM, respectively (Fig. 1A). Tigatuzumab monotherapy was not effective to achieve tumor regression even in the most sensitive xenografts (Panc219 and Panc286). Rather, the established tumors showed decreased growth compared with controls. GEM monotherapy showed greater efficacy resulting in tumor regression in 4 of 8 xenografts (Fig. 1A). The combined treatment of GEM with tigatuzumab suppressed the tumor growth in 7 of 8 xenografts and resulted in tumor regressions in 5 of 8 xenografts (Fig. 1A).
Figure 1.
The combination of GEM and tigatuzumab produces sustained tumor growth inhibition and induces the expression of cell-extrinsic apoptotic pathway proteins in pancreatic cancer xenografts. A, in vivo efficacy of tigatuzumab, GEM, and the combination of GEM and tigatuzumab on the tumor growth of established pancreatic adenocarcinoma xenografts. Tigatuzumab monotherapy was ineffective in controlling the tumor growth of xenografts. However, the combination of GEM and tigatuzumab was highly effective in preventing the tumor growth of xenografts. Eight individual patient-derived low-passage pancreatic cancer xenografts were implanted in athymic nude mice. Cohorts of mice with a tumor volume of 200 mm3 were randomized and treated with (a) saline (vehicle); (b) tigatuzumab (3 mg/kg i.v. once a week for 4 wk); (c) GEM (100 mg/kg i.p. twice a week for 4 wk); and (d) GEM + tigatuzumab at the above-mentioned doses and frequencies. Relative tumor growth rate on day 28 of treated animals was calculated versus the tumor volume of vehicle-treated mice (100%). Cases that showed maximum sensitivity to tigatuzumab were plotted on the left side of the graph. Bars, SEM; n = 10 tumors per group (4 mice with bilateral flank tumors and 2 mice with unilateral flank tumor). B, Panc219 Western blot showing that tigatuzumab or combination with GEM induces the expression of key proteins of the extrinsic apoptotic pathway. Tumors harvested on day 28 were used for immunoblotting. Equal amounts of tumor lysates (30 μg) from two separate animals in each group were analyzed by immunoblotting and probed with the indicated antibodies. Band intensities were measured by densitometry and normalized with respective β-actin loading controls. There was an average of 2.5- and 3.0-fold increases in DR5 expression in the tigatuzumab- and GEM plus tigatuzumab–treated tumors, respectively, as compared with the saline-treated tumors. The activation of DR5 in the tigatuzumab and the combination therapy groups was coupled with the upregulation of Fas, FADD, and TRADD. Tigatuzumab and GEM plus tigatuzumab treatments lead to the upregulation of Fas (1.8- and 2.4-fold), FADD (1.5- and 2.6-fold), and TRADD (2.2- and 2.1-fold) as compared with the control tumors. GEM treatment did not modulate the expression of the above-mentioned proteins. Decreases of 1.9- and 2.4-fold in the expression of full-length and cleaved caspase-8 (C-Casp-8) were observed in the GEM-treated tumors as compared with vehicle-treated tumors. A similar downregulation of TRAIL (1.8-fold) was noticed in GEM-treated tumors as compared with vehicle-treated tumors. Both agents alone and in combination were marginally effective in reducing X-linked inhibitor of apoptosis (XIAP) expression in tumors as compared with the control tumors. There was a 2.4-fold upregulation of cleaved poly(ADP-ribose) polymerase (C-PARP), a marker of apoptosis, in the GEM plus tigatuzumab–treated tumors as compared with the GEM-treated tumors.
Tigatuzumab triggers apoptosis through the cell-extrinsic death pathway
Western blot analysis of tumor lysate from Panc219 xenograft showed that tigatuzumab induces DR5 expression. DR5 levels were elevated in the tigatuzumab and the combination treatment groups compared with control and GEM-treated animals (Fig. 1B). There was an average of 2.5- and 3.0-fold increase in DR5 expression in the tigatuzumab- and GEM plus tigatuzumab–treated tumors, respectively, as compared with the saline-treated tumors. The activation of DR5 in the tigatuzumab and the combination therapy groups was coupled with the upregulation of Fas, FADD, and TRADD (Fig. 1B). Tigatuzumab and GEM plus tigatuzumab treatments led to the upregulation of Fas (1.8- and 2.4-fold), FADD (1.5- and 2.6-fold), and TRADD (2.2- and 2.1-fold), respectively, as compared with the control tumors. GEM treatment did not modulate the expression of the above-mentioned proteins (Fig. 1B). There were 1.9- and 2.4-fold decreases in the expression of full-length and cleaved caspase-8, respectively, in the GEM-treated tumors as compared with the vehicle-treated tumors. A similar decrease in TRAIL levels (1.8-fold) was noticed in GEM-treated tumors as compared with control tumors. Both agents alone and in combination were marginally effective in reducing the X-linked inhibitor of apoptosis expression in tumors as compared with the control tumors (Fig. 1B). There was a 2.4-fold upregulation of cleaved poly(ADP-ribose) polymerase, a marker of apoptosis, in the GEM plus tigatuzumab–treated tumors as compared with the GEM-treated tumors (Fig. 1B). Caspase-3, BID, BAX, and p53 expression were unchanged in various treatment groups as compared with the control tumors (Fig. 1B).
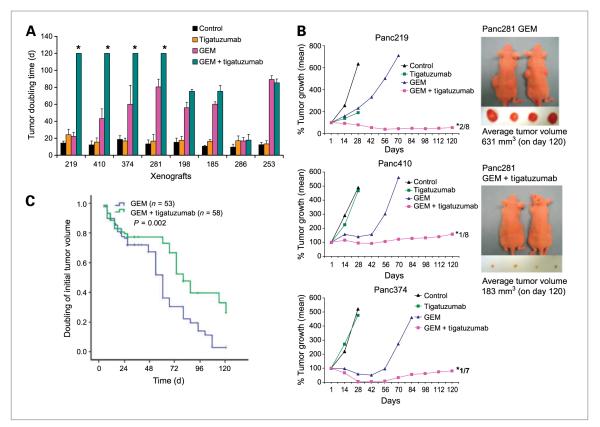
Combination of GEM with tigatuzumab leads to cooperative tumor growth inhibition and prolongs the tumor doubling time
GEM treatment resulted in rapid tumor shrinkage in PDA xenografts (Fig. 1A). However, tumor growth resumed within 2 to 4 weeks after the cessation of treatment, indicating that GEM treatment does not result in long-term cures (Fig. 2A and B) as observed in the clinic. There was no significant difference in the mean initial tumor doubling time of vehicle- and tigatuzumab-treated animals (13 ± 3 and 17 ± 3 days, respectively). The mean initial tumor doubling time of tumors in GEM treatment was 54 ± 26 days. However, the mean initial tumor doubling time in the combination therapy group was 92 ± 36 days. Aggregate analysis of the initial tumor doubling time of all tumors in the eight xenografts showed a significant increase in tumor doubling time in the combination treatment group as compared with the GEM group (Fig. 2C). In addition, tumors in the Panc219, Panc410, Panc374, and Panc281 xenografts of the combination group did not recur up to 120 days of follow-up (Fig. 2B). There was a complete remission of some tumors in Panc219 (2 of 8 on day 35), Panc410 (1 of 8 on day 45), and Panc374 (1 of 7 on day 31). Histologic examination of tumor injection sites of mice (on day 120), in which complete tumor remission occurred, failed to detect tumor cells (data not shown), indicating that combination therapy resulted in complete pathologic responses in these animals. The average tumor volume of Panc281 on sacrifice on day 120 was 631 and 183 mm3 for the GEM alone and the GEM plus tigatuzumab groups, respectively (Fig. 2B). This suggests that the combination therapy is very efficacious in preventing tumor recurrence and in achieving tumor remission.
Figure 2.
Combination therapy produces durable tumor growth inhibition and prolongs the tumor doubling time of pancreatic cancer xenografts. A, initial tumor doubling time of eight xenografts treated with tigatuzumab, GEM, and the combination of GEM and tigatuzumab. Tumors in the combination treatment group of Panc219, Panc410, Panc374, and Panc281 did not double its size as on day 120 compared with the initial tumor size. *, tumors in that group did not double its size on necropsy. There were 10 tumors each in various treatment groups until day 28 and thereafter 6 to 8 tumors in the GEM and the GEM plus tigatuzumab groups in each xenografts. Bars, SEM. B, the combination of GEM and tigatuzumab produces durable tumor growth inhibition in pancreatic cancer xenografts. Tumor growth curves representative of Panc219, Panc410, and Panc374. Number near to the asterisk denotes the number of tumors vanished during treatment. There were 10 tumors each in various treatment groups until day 28 and thereafter 6 to 8 tumors in the GEM and the GEM plus tigatuzumab groups in each xenografts. Representative images of live, anesthetized mice treated with GEM and GEM plus tigatuzumab of Panc281; excised tumors on day 120 are shown on the right. C, log-rank comparison of aggregate initial tumor doubling time of GEM-treated (n = 53) and GEM plus tigatuzumab–treated (n = 58) tumors of eight xenografts. Animals treated with the combination showed significant increase in time to tumor doubling compared with the GEM-treated mice (P = 0.002).
Cancer stem cell population varies from xenografts to xenografts
CSCs are currently identified by the expression of cell surface and functional markers. Several studies have reported that CSCs from primary human PDA can be identified based on the expression of specific cell surface antigens including CD44 and CD24 (5, 13). Due to the molecular heterogeneity among CSCs, a single marker is unlikely to identify all CSCs even within tumors of the same clinical grade from the same organ (31). Here, we used the expression of ALDH+ and CD24+CD44+, which are reliable markers of CSCs (5, 32). ALDH+, CD24+CD44+, as well as ALDH+CD24+CD44+ tumor cells (1,000 cells) have been shown to initiate tumors on injection into a nonobese diabetic/severe combined immunodeficient mouse, whereas tumor did not develop in a mouse injected with 10,000 bulk tumor cells (30). These three markers were widely used to identify putative CSCs in most common cancers and in the NCI60 panel of tumor cell lines (33). When examined individually, putative stem cell markers were heterogeneously expressed across the xenografts. There was a marked variation in CD24+CD44+ tumor cells (0.1–10.8%) among the xenografts (Table 1). Similarly, wide variations in ALDH+ cells (1.4–9.3%) were also noticed among the xenografts (Table 1). We have recently reported that the ALDH+CD24+CD44+ population was more tumorigenic than both the ALDH+ and the CD24+CD44+ tumor cells, but the differences were not statistically significant (30). In addition to analyzing the CD24+CD44+ and ALDH+ tumor cell populations, we also analyzed the ALDH+CD24+CD44+ population in pancreatic cancer xenografts. Similar to the findings of our recent work (30), we found that the percentages of ALDH+CD24+CD44+ tumor cells were too low to be accurately measured (<0.015%) and these populations were largely nonoverlapping with CD24+CD44+ and ALDH+ tumor cells (data not shown). The four xenografts (Panc219, Panc410, Panc374, and Panc281), which did not recur up to 120+ days of follow-up in the combination therapy, have relatively higher percentages of CSC population as compared with the other xenografts (Table 1).
Table 1.
Stem cell quantification in xenografts by FACS
| Xenografts | % ALDH+ | % CD44+CD24+ |
|---|---|---|
| Panc219 | 5.30 | 6.20 |
| Panc281 | 9.05 | 3.13 |
| Panc185 | 1.40 | 0.10 |
| Panc198 | 4.70 | 10.10 |
| Panc374 | 4.40 | 9.30 |
| Panc410 | 9.30 | 0.34 |
| Panc253 | 2.30 | 0.10 |
| Panc286 | ND | ND |
NOTE: Eight patient-derived pancreatic tumors grown separately (s.c.) in nude mice were harvested. Single-cell suspensions were generated by mincing tumors using sterile razors, followed by incubation in dispase (Sigma) and collagenase IV (Sigma) at 37°C for 2 h. The cell suspension was filtered using a 70-μm filter (BD Biosciences) and further purified by density centrifugation using Ficoll-Paque Plus (GE Healthcare). Cells were labeled using the Aldefluor reagent (Stem Cell Technologies) and then stained with antibodies against mouse CD31 (BD Biosciences), lineage cocktail (Miltenyi Biotec), and H-2Kd (BD Biosciences). Non-mouse cells were separated and stained with antibodies against human CD44 and CD24 (BD Biosciences). Cells were analyzed using a FACSAria flow cytometer (BD Biosciences). Due to the abundance of necrosis in Panc286, we did not have enough cells to sort this case for stem cell assessment.
Abbreviation: ND, not determined.
DR5 levels are enriched in PDA stem cells
We analyzed the DR5 expression in pancreatic CSCs and non–stem cell bulk tumor cells by flow cytometry. Pancreatic CSCs are indeed enriched with DR5 (94% of ALDH+ tumor cells and 89% of CD24+CD44+ tumor cells) as compared with non–stem cell bulk tumor cells (30%) in Panc219 (Fig. 3). A similar enrichment of DR5 in ALDH+ and CD24+CD44+ tumor cells was noticed in the other xenografts used in this study (data not shown).
Figure 3.
DR5 is enriched in PDA stem cells as compared with the bulk tumor cells. Tumors from mice bearing Panc219 were harvested and single-cell suspensions were generated by mincing tumors using sterile razors, followed by incubation in dispase and collagenase type IV at 37°C for 2 h with agitation. DR5+ tumor cells in the bulk tumor populations and in the ALDH+ and CD44+CD24+ tumor cell populations were measured by FACS as described in Materials and Methods. The frames represent the gates that depict respective antigen-positive tumor cells. DR5 is expressed in only 30% of bulk tumor cells. However, CSCs are relatively enriched with DR5 (94% of ALDH+ cells and 89% of CD24+CD44+ cells) as compared with the bulk tumor cells.
Combination therapy reduces CSC populations
Flow cytometry of tumor cells sorted from Panc219 showed that GEM treatment was not capable of diminishing the CSC pool as compared with the control mice (Fig. 4). However, the combination of GEM and tigatuzumab resulted in a 14.3-fold decrease in CD24+CD44+ tumor cells compared with GEM treatment (Fig. 4). Similarly, there was a 2.31-fold decrease in ALDH+ tumor cells in the combination therapy as compared with GEM treatment (Fig. 4). Tigatuzumab monotherapy reduced the cancer stem populations to nearly half of that of GEM and control mice (Fig. 4).
Figure 4.
The combination of GEM and tigatuzumab markedly enhances the elimination of PDA stem cells. Panc219 tumor–bearing mice were treated with tigatuzumab, GEM, and GEM plus tigatuzumab as mentioned in Materials and Methods. The tumors were harvested on day 28 and a single-cell suspension was generated; cells positive for CSC markers were measured using flow cytometry. The frames represent the gates that depict respective antigen-positive tumor cells. Remarkable decreases in CD24+CD44+ and ALDH+ tumor cells (14.3- and 2.31-fold, respectively) were noticed in animals that received combination therapy as compared with animals treated with GEM.
CD24 staining index indicates that GEM treatment did not reduce CD24+ tumor cells as compared with control tumors (Fig. 5A). However, tigatuzumab and the combination of GEM and tigatuzumab reduced the CD24 staining index in tumor cells as compared with the control- and GEM-treated groups (Fig. 5A and B). Combination treatment resulted in the complete elimination of CD24+ tumor cells in Panc410 and Panc374 (Fig. 5B).
Figure 5.
Combination therapy reduces PDA stem cells as shown by immunohistochemical staining and quantitative reverse transcription-PCR. A, CD24 staining index of Panc219, Panc410, and Panc374. Immunohistochemical staining of CD24-positive tumor cells was done as described in Materials and Methods. Index means percent of positively stained tumor cells × staining intensity (0, 1, 2, and 3). GEM treatment was not capable of reducing CD24+ tumor cells as compared with vehicle-treated tumors. Tig., tigatuzumab. B, representative micrographs of CD24 immunohistochemical staining of Panc219, Panc410, and Panc374 xenografts showing low immunoreactivity for CD24 in the tigatuzumab and the GEM and tigatuzumab combination groups as compared with vehicle-treated and GEM-treated tumors. Combination therapy leads to the complete elimination of CD24-stained tumor cells in Panc410 and Panc374 xenograft tumors. C, the combination of GEM and tigatuzumab downregulates the mRNA expression of CSC markers as compared with GEM-treated tumors. RNA isolated from Panc219 and Panc410 treatment groups was used for quantitative reverse transcription-PCR, and comparative mRNA expression of ALDH, CD44, and CD44 were calculated using the δδCT method. In Panc219, there was 6- and 3.3-fold upregulation of ALDH and CD44 mRNA in GEM-treated tumors, respectively, as compared with the control tumors. However, the combination therapy led to 4.2-, 4.0-, and 3.6-fold decreases in the expression of ALDH, CD24, and CD44 mRNA, respectively, as compared with GEM treatment (C). Similarly, mRNA expression of ALDH and CD44 were increased 8.4- and 2.3-fold, respectively, in the GEM-treated tumors of Panc410 as compared with the control tumors. Combination therapy resulted in 5.3-, 2.3-, and 3.3-fold decreases in the expression of ALDH, CD24, and CD44 mRNA, respectively, in Panc410 as compared with GEM-treated tumors (C).
The quantitative real-time reverse transcription-PCR results of Panc219 and Panc410 clearly showed that the combination of GEM and tigatuzumab downregulated the mRNA expression of CSC markers as compared with GEM-treated tumors. GEM treatment resulted in the upregulation of ALDH and CD44 mRNA expression as compared with control tumors (Fig. 5C). However, the combination of GEM and tigatuzumab reduced the elevated ALDH and CD44 levels as compared with GEM treatment (Fig. 5C). There were 4.2-, 4.0-, and 3.6-fold decreases in the expression of ALDH, CD24, and CD44 mRNA, respectively, in combination therapy as compared with GEM treatment (Fig. 5C). Similarly, combination therapy resulted in 5.3-, 2.3-, and 3.3-fold decreases in the expression of ALDH, CD24, and CD44 mRNA, respectively, in Panc410 as compared with GEM-treated tumors (Fig. 5C). Tigatuzumab monotherapy did not alter the ALDH, CD24, and CD44 mRNA expression as compared with vehicle-treated tumors (data not shown).
Discussion
Despite the rapid advances in many fronts, PDA remains one of the most difficult human malignancies to treat, indicating the need for novel therapeutic strategies to improve the poor prognosis of patients with this disease. The existence of CSCs probably influences the intractable nature of PDA, explaining why conventional cancer therapy fails in the vast majority of patients. CSCs have been shown to be highly resistant to chemotherapy and radiotherapy. Thus, from a clinical standpoint, targeting cancer-sustaining pancreatic CSCs will be of paramount significance because there are few effective therapies for PDA and most of the patients die within the first year of diagnosis.
In this report, we provide evidence that pancreatic CSCs are relatively enriched with DR5 compared with the non–stem cell bulk tumor populations. A combination therapy using DR5 agonist mAb, tigatuzumab, and GEM produced robust antitumor activity in freshly generated PDA xenografts. More importantly, combination therapy resulted in the reduction of pancreatic CSCs, tumor remission, prevention of tumor recurrence, and significant prolongation of the time to tumor progression. The combination therapy tested here has a wide potential therapeutic role, which may possibly kill cancer-sustaining stem cell pool to prevent tumor recurrence and as an adjuvant therapy in clearing CSC populations following primary tumor resection and radiation therapy.
DR5 agonists represent a new class of therapeutics that selectively target apoptosis. Significant effort has been devoted to the discovery and development of mAbs against DR5, based on the impressive tumor growth inhibition and superior survival advantage obtained in a broad range of human tumor xenografts treated with DR5 agonist mAbs in combination with chemotherapy or radiation therapy (34–37). Activation of the DR5 pathway triggers programmed cell death through the extrinsic apoptotic pathway, independent of p53, which involves the direct recruitment and activation of caspase-8 through FADD (38). The extrinsic apoptosis pathway is an intriguing target for cancer therapy because it can circumvent a common apoptosis resistance mechanism associated with mutations in the p53 tumor suppressor gene, which account for 50% of human cancers (39, 40). On the other hand, many conventional chemotherapeutic agents generally require the function of p53 to initiate apoptosis by engaging the intrinsic apoptotic pathway (41). Combinations of the two approaches may facilitate tumor cell death that resists death induction through either one of the pathways as well as reduces the probability of tumor cells to develop resistance to either therapy.
Mounting data over the recent years have indicated the robust preclinical activity of TRAIL receptor–targeted human agonistic mAbs across a broad range of tumor types. Based on this, multiple clinical trials of either single agents or in combination with chemotherapeutics are currently at various stages (42). Early-phase clinical trials using agonistic anti-TRAIL receptor antibodies indicate that these agents can be delivered safely, are generally well tolerated, and seem to mediate some clinical benefit in terms of disease stabilization or objective responses (43). Death receptor agonists were safely combined with standard doses of cancer chemotherapeutics in small cohorts of patients. These combinations include single-agent cytotoxics, cytotoxic combinations, targeted agents, and cytotoxic-targeted agent combinations (25). Our results clearly show that GEM treatment initially results in rapid tumor shrinkage in human primary PDA xenografts. GEM treatment did not deplete the CSC population and the tumors progressed after an interim in all xenografts. However, tumors in 50% (4 of 8) of the xenografts treated with GEM and tigatuzumab not only did not relapse but also did not double its size until sacrificed on day 120. Specifically, this notable cooperativity between tigatuzumab and GEM in tumor growth inhibition as well as CSC reduction suggests that this therapeutically attractive combination therapy could be efficacious in the clinical setting.
Notwithstanding our ability to sequence cancer genome and to create personalized targeted therapies, it is apparent that combination therapies, which target the CSC subpopulation as well as the bulk of the tumor cells, will be required to effectively manage cancer treatment (44, 45). Emerging studies show that CSCs are indeed more resistant to therapy than other cancer cells and injection of a small number of CSCs is able to reproduce an entire tumor (46). It is thought that CSCs are relatively drug and radiation resistant by virtue of quiescence, expression of drug resistance mechanisms, and possibly their location in tissue niches with restricted drug access. Therefore, development of novel therapeutic strategy to combat CSCs is an attractive strategy for more effective cancer treatment. Although the true relevance of the CSC is yet to be revealed, there are tantalizing reports that the CSC can be selectively targeted without ablating normal stem cells (47). As the concept of CSC is becoming scientifically accepted, there is increasing interest in evaluating potential therapeutic targets in these cells. Initial studies have focused on the evaluation of developmental pathways such as the Wnt and the Sonic Hedgehog pathways (48). Indeed, a recent report from our group showed that the Sonic HH inhibitor cyclopamine induced tumor regression in combination with GEM in one patient PDA xenograft (16). Another study showed that a combination of cyclopamine, rapamycin, and GEM was capable of eliminating pancreatic CSCs (49). The present results indicate that there may be other targets in CSCs, and target-focused as well as systematic approaches are needed to investigate this aspect. As novel CSC directed therapies emerge, studies such as the one presented here can be of great value when trying to prioritize drugs with anti-CSC activity for preclinical testing and validation. This may allow us to better select targeted agents directed to CSCs, which may ultimately improve the chances that the chosen therapy successfully completes the clinical development.
The quest for potent targeted therapeutic approaches to combat CSCs is an imperative clinical issue. A recent report from our group suggests that pancreatic CSCs play a key role in the development of metastatic disease that negatively affects the overall survival of patients with PDA (30). An important question is how we move forward and translate these findings to the clinic. By the time tumors are diagnosed, the disease is usually advanced and does not respond to treatment. Adding CSC-directed therapeutics to conventional agents in this advanced stage may unlikely be effective. A more appropriate scenario can be patients with resectable disease, who, despite treatment with GEM, uniformly develop disease progression. Here, the most important clinical goal is the elimination of micrometastatic disease, in which CSCs probably have a critical role. Combination of CSC-directed treatment with chemotherapy can be tested with the primary objective of preventing disease progression, relapse, and metastasis. Importantly, agents that eliminate the CSCs within a tumor may bring little or no immediate reduction in tumor size. However, tumor growth is not sustainable without CSCs to replenish the bulk population and the tumor will eventually degenerate as bulk tumor cells are depleted.
Our results agree with a previous report (16) that ALDH and CD44 mRNA levels were elevated in the GEM-treated tumors as compared with the saline-treated tumors (Fig. 5B). However, increased average mRNA levels did not correspond to an increase in the number of ALDH+ and CD24+CD44+ tumor cells as revealed by fluorescence-activated cell sorting (FACS) data (Fig. 4). At present, we do not know the reason behind this discrepancy. Additional studies are needed to investigate whether posttranscriptional modification or trafficking of antigens between cell membrane/cytoplasm and nucleus plays a role on this aspect. There is now abundant evidence that stem cell properties are highly relevant to the biology of several human cancers. An obvious question is whether CSCs in other tumor types have DR5 expression. Studies are needed to investigate whether DR5 is also a CSC target in other tumor types besides pancreas cancer.
Taken together, our results provide strong evidence that pancreatic CSCs are enriched with DR5. Combination therapy using GEM plus tigatuzumab results in long-term cures in an otherwise incurable direct PDA model. Furthermore, the novel combination proved to be more efficacious than either single agent alone by providing a double hit to kill both CSCs and non–stem cell bulk tumor populations. Considering that combination therapy produced remarkable reduction in PDA stem cells, tumor remission, and significant improvement in time to tumor progression, this innovative approach may represent a valuable treatment option to improve the current standard of care for PDA patients. Further investigation of this promising approach in PDA is warranted.
Acknowledgments
Grant Support Daiichi Sankyo Co., Ltd., Tokyo, Japan (M. Hidalgo).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest K. Fujiwara is an employee of Daiichi Sankyo Co., Ltd., Tokyo, Japan. The other authors declared no potential conflicts of interest.
Note: Presented in part as a poster (abstract #1069) at the 100th Annual Meeting of AACR, April 18-22, 2009, Denver, Colorado.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ischenko I, Seeliger H, Jauch KW, Bruns CJ. Metastatic activity and chemotherapy resistance in human pancreatic cancer-influence of cancer stem cells. Surgery. 2009;146:430–4. doi: 10.1016/j.surg.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Bednar F, Simeone DM. Pancreatic cancer stem cells and relevance to cancer treatments. J Cell Biochem. 2009;107:40–5. doi: 10.1002/jcb.22093. [DOI] [PubMed] [Google Scholar]
- 4.Hermann PC, Mueller MT, Heeschen C. Pancreatic cancer stem cells-insights and perspectives. Expert Opin Biol Ther. 2009;9:1271–8. doi: 10.1517/14712590903246362. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 6.Simeone DM. Pancreatic cancer stem cells: implications for the treatment of pancreatic cancer. Clin Cancer Res. 2008;14:5646–8. doi: 10.1158/1078-0432.CCR-08-0584. [DOI] [PubMed] [Google Scholar]
- 7.Sergeant G, Vankelecom H, Gremeaux L, Topal B. Role of cancer stem cells in pancreatic ductal adenocarcinoma. Nat Rev Clin Oncol. 2009;6:580–6. doi: 10.1038/nrclinonc.2009.127. [DOI] [PubMed] [Google Scholar]
- 8.Jordan CT. Cancer stem cells: controversial or just misunderstood? Cell Stem Cell. 2009;4:203–5. doi: 10.1016/j.stem.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 10.Tabernero J, Macarulla T. Changing the paradigm in conducting randomized clinical studies in advanced pancreatic cancer: an opportunity for better clinical development. J Clin Oncol. 2009;27:5487–91. doi: 10.1200/JCO.2009.23.3098. [DOI] [PubMed] [Google Scholar]
- 11.Berlin J, Benson AB., III Chemotherapy: gemcitabine remains the standard of care for pancreatic cancer. Nat Rev Clin Oncol. 2010;7:135–7. doi: 10.1038/nrclinonc.2010.16. [DOI] [PubMed] [Google Scholar]
- 12.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 13.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–31. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 15.Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806–12. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- 16.Jimeno A, Feldmann G, Suarez-Gauthier A, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–4. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan G, O’Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–3. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 19.Naka T, Sugamura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients’ colon tumors grown in SCID mice. Cancer Res. 2002;62:5800–6. [PubMed] [Google Scholar]
- 20.Daniels RA, Turley H, Kimberley FC, et al. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res. 2005;15:430–8. doi: 10.1038/sj.cr.7290311. [DOI] [PubMed] [Google Scholar]
- 21.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–30. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 22.Ozawa F, Friess H, Kleeff J, et al. Effects and expression of TRAIL and its apoptosis-promoting receptors in human pancreatic cancer. Cancer Lett. 2001;163:71–81. doi: 10.1016/s0304-3835(00)00660-1. [DOI] [PubMed] [Google Scholar]
- 23.Ichikawa K, Liu W, Zhao L, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–60. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 24.Kaliberov S, Stackhouse MA, Kaliberova L, Zhou T, Buchsbaum DJ. Enhanced apoptosis following treatment with TRA-8 anti-human DR5 monoclonal antibody and overexpression of exogenous Bax in human glioma cells. Gene Ther. 2004;11:658–67. doi: 10.1038/sj.gt.3302215. [DOI] [PubMed] [Google Scholar]
- 25.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16:1701–8. doi: 10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- 26.Yada A, Yazawa M, Ishida S, et al. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–7. doi: 10.1093/annonc/mdn015. [DOI] [PubMed] [Google Scholar]
- 27.Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–61. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 28.Jimeno A, Rubio-Viqueira B, Rajeshkumar NV, Chan A, Solomon A, Hidalgo M. A fine-needle aspirate-based vulnerability assay identifies polo-like kinase 1 as a mediator of gemcitabine resistance in pancreatic cancer. Mol Cancer Ther. 2010;9:311–8. doi: 10.1158/1535-7163.MCT-09-0693. [DOI] [PubMed] [Google Scholar]
- 29.Rajeshkumar NV, Tan AC, De Oliveira E, et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. 2009;15:4138–46. doi: 10.1158/1078-0432.CCR-08-3021. [DOI] [PubMed] [Google Scholar]
- 30.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–51. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris MA, Yang H, Low BE, et al. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68:10051–9. doi: 10.1158/0008-5472.CAN-08-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–9. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuelten CH, Mertins SD, Busch JI, et al. Complex display of putative tumor stem cell markers in the NCI60 tumor cell line panel. Stem Cells. 2010;28:649–60. doi: 10.1002/stem.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchsbaum DJ, Zhou T, Grizzle WE, et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–41. [PubMed] [Google Scholar]
- 35.Jin H, Yang R, Ross J, et al. Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res. 2008;14:7733–40. doi: 10.1158/1078-0432.CCR-08-0670. [DOI] [PubMed] [Google Scholar]
- 36.Kendrick JE, Straughn JM, Jr., Oliver PG, et al. Anti-tumor activity of the TRA-8 anti-DR5 antibody in combination with cisplatin in an ex vivo human cervical cancer model. Gynecol Oncol. 2008;108:591–7. doi: 10.1016/j.ygyno.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 37.Ren B, Song K, Parangi S, et al. A double hit to kill tumor and endothelial cells by TRAIL and antiangiogenic 3TSR. Cancer Res. 2009;69:3856–65. doi: 10.1158/0008-5472.CAN-08-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–20. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 39.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–85. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 40.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–88. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 41.Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 42.Carlo-Stella C, Lavazza C, Locatelli A, Vigano L, Gianni AM, Gianni L. Targeting TRAIL agonistic receptors for cancer therapy. Clin Cancer Res. 2007;13:2313–7. doi: 10.1158/1078-0432.CCR-06-2774. [DOI] [PubMed] [Google Scholar]
- 43.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 44.Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–94. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 45.Jones RJ, Matsui WH, Smith BD. Cancer stem cells: are we missing the target? J Natl Cancer Inst. 2004;96:583–5. doi: 10.1093/jnci/djh095. [DOI] [PubMed] [Google Scholar]
- 46.Zucchi I, Sanzone S, Astigiano S, et al. The properties of a mammary gland cancer stem cell. Proc Natl Acad Sci U S A. 2007;104:10476–81. doi: 10.1073/pnas.0703071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 48.Hidalgo M, Maitra A. The hedgehog pathway and pancreatic cancer. N Engl J Med. 2009;361:2094–6. doi: 10.1056/NEJMcibr0905857. [DOI] [PubMed] [Google Scholar]
- 49.Mueller MT, Hermann PC, Witthauer J, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–13. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]