Abstract
Background and purpose
To examine the role of adjuvant chemoradiation (CRT) in patients with resected ampullary adenocarcinoma.
Materials and methods
The records of patients who underwent curative surgery for ampullary adenocarcinoma at a single institution between 1992 and 2007 were reviewed. Final analysis included 111 patients, 45% of which also received adjuvant CRT.
Results
Median overall survival (OS) was 36.2 months for all patients. Adverse prognostic factors for OS included T stage (T3/4 vs. T1/T2, p = 0.046), node status (positive vs. negative, p < 0.001), and histological grade (grade 3 vs. 1/2, p = 0.09). Patients receiving CRT were more likely to have advanced T-stage (p = 0.001), node positivity (p < 0.001), and poor histologic grade (p = 0.015). Patients who received CRT were also significantly younger (p = 0.001). On univariate analysis, adjuvant CRT failed to result in a significant difference in survival when compared to surgery alone (median OS: 33.4 vs. 36.2 months, p = 0.969). Patients with node-positive resections who underwent CRT had a non-significant improvement in survival (median OS: 21.6 vs. 13.0 months, p = 0.092). Thirty-three percent of patients developed distant metastasis. Common sites of distant metastasis included liver (23%) and peritoneum (7%).
Conclusions
Adjuvant chemoradiation following curative resection for ampullary adenocarcinoma did not lead to a statistically significant benefit in overall survival. A significant proportion of patients still developed distant metastatic disease suggesting a need for more effective systemic adjuvant therapy.
Keywords: Ampulla of Vater, Ampullary cancer, Radiotherapy, Adjuvant therapy, Chemoradiation
Carcinoma of the ampulla of Vater is a relatively uncommon malignancy, but it is the second most common cancer of the periampullary region with a proportional incidence of 6–20% [1–3]. Ampullary carcinoma is generally associated with better outcomes as well as resectability than primary pancreatic adenocarcinoma, which has a 5-year survival rate of approximately 20% for resected patients [4]. Pancreaticoduodenectomy (PD), the preferred curative surgical treatment for ampullary cancer, has yielded survival rates of 20–60%, averaging more than 35%, based on retrospective series over the past two decades [5–11].
Adjuvant CRT (or chemotherapy alone) has been shown to improve overall survival for patients with pancreatic adenocarcinoma [4,12,13]. Several institutions have extrapolated from the results of pancreatic cancer studies to incorporate adjuvant therapy in patients with ampullary cancer. However, the use of adjuvant therapy in this setting is controversial. The results of a 1999 European Organization for Research and Treatment of Cancer (EORTC) trial demonstrated no statistically significant benefit in survival with adjuvant CRT following resection for ampullary adenocarcinoma [13]. A more recent retrospective review from the Mayo Clinic found that the addition of adjuvant CRT resulted in a statistically significant improvement in overall survival only for patients with lymph node involvement [14]. Similarly, a 2007 study from MDACC demonstrated improved survival only in patients with advanced primary tumor stage (T3/T4) who received adjuvant CRT [19].
Theoretically, adjuvant chemoradiation (CRT) may improve survival after resection for ampullary cancers. However, there are limited data regarding the use of CRT as adjuvant treatment for ampullary cancer. In order to assess the impact of adjuvant therapy on ampullary carcinoma, we analyzed the results of our large single-institution retrospective series by comparing surgery alone to surgery followed by modern conformal 5-FU-based CRT.
Methods and materials
We reviewed electronic and paper records for all patients with ampulla of Vater carcinoma who underwent surgery for curative intent at Johns Hopkins Hospital from December 1992 to March 2007. A total of 290 patients with ampullary adenocarcinoma were identified from a prospectively collected database. Patients who died within 50 days of surgery (n = 6), had <60 days of follow-up (n = 6), or had metastatic disease at the time of surgery (n = 11) were excluded. Another 156 patients who were referred to institutions outside Johns Hopkins for adjuvant therapy were excluded because follow-up information was not available after referral, or data were not available on whether they received any adjuvant treatment. Our final cohort includes 111 patients who received potentially curative surgery at Johns Hopkins Hospital (50 with adjuvant CRT, 45% vs. 61 without adjuvant CRT, 55%).
Cancer of the ampulla of Vater was defined as adenocarcinoma directly involving or emanating from the ampulla, or papilla, or both, as evidenced by review of the final pathology report. Cancers arising from the duodenum, pancreatic head or common bile duct were excluded.
All patients received preoperative staging by one or more of the following modalities: abdominal and pelvic-computed tomography (CT), endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasonography (EUS), and percutaneous transhepatic cholangiography or percutaneous biliary drainage (PTC/PBD). The majority of patients received both CT and ERCP. Complete laboratory tests for patients included a full blood count, serum electrolytes, creatinine, urea, liver transaminases, alkaline phosphatase, total bilirubin, and CEA and CA-19.
Patient characteristics are outlined in Table 1. Of the 111 patients in this study, 44 (40%) were women and 67 (60%) were men. The median age was 66 years (range, 29–90 years). Patient demographics and pathologic characteristics are summarized in Table 1 according to whether the patients received adjuvant or no adjuvant treatment. “High risk” pathologic characteristics were defined as: T3/T4 primary tumors, positive regional lymph nodes, poor histologic differentiation, and positive resection margins.
Table 1.
Baseline characteristics between treatment groups.
| Demographic | Observation only N = 61 |
Adjuvant chemoradiation therapy N = 50 |
P-value |
|---|---|---|---|
| Age at Surgery (yr) | |||
| Mean, (SD) | 69.8 (12.3) | 62.4 (10.5) | 0.001 |
| Median (range) | 72.4 (35.0–90.3) | 64.2 (29.3–81.5) | |
| Gender | |||
| Male, No. (%) | 36 (59) | 31 (62) | 0.749 |
| Treatment | |||
| Surgery type | |||
| Classic PD | 12 (20) | 12 (24) | 0.582 |
| Pylorus preserving PD | 49 (80) | 38 (76) | |
| Tumor characteristics | |||
| T stage | |||
| 1 | 16 (26) | 2 (4) | |
| 2 | 27 (44) | 17 (34) | 0.001 |
| 3 | 16 (26) | 27 (54) | |
| 4 | 2 (3) | 4 (8) | |
| Tumor diameter | |||
| <3 cm | 44 (72) | 34 (68) | |
| >=3 cm | 16 (26) | 13 (26) | 0.773* |
| Missing | 1 (2) | 3 (6) | |
| Nodal status | |||
| N0 | 42 (69) | 14 (28) | <0.001 |
| N1 | 19 (31) | 36 (72) | |
| Histologic grading | |||
| 1 | 6 (10) | 0 (0) | |
| 2 | 33 (54) | 22 (44) | 0.015* |
| 3 | 19 (31) | 26 (52) | |
| Missing | 3 (5) | 2 (4) | |
| Surgical margins | |||
| Positive | 0 (0) | 2 (4) | 0.115 |
| Negative | 61 (100) | 48 (96) | |
| Lymphovascular invasion | |||
| Yes | 14 (23) | 26 (52) | |
| No | 32 (52) | 17 (34) | 0.004* |
| Missing | 15 (25) | 7 (14) | |
| Perineural invasion | |||
| Yes | 13 (21) | 23 (46) | 0.026* |
| No | 28 (46) | 18 (36) | |
| Missing | 20 (33) | 9 (18) |
Chi-square compares only non-missing values.
All patients underwent PD as a potentially curative procedure. Eighty-seven patients (78%) underwent a pylorus-preserving PD and 24 (22%) underwent a classic PD. Pathologic specimens were reviewed and staged according to American Joint Committee on Cancer (AJCC) guidelines. Pathologic data regarding T stage, tumor size, histologic grade, lymph node involvement, lymphovascular invasion, perineural invasion, and surgical margins were recorded.
The 50 patients who received adjuvant therapy included concurrent chemotherapy with radiotherapy. Radiotherapy was delivered using a four- or five-field coplanar beam arrangement for 40 patients (80%), intensity-modulated radiation therapy (IMRT) for 7 patients (14%) and 3-field for three patients (6%). Median total radiation dose was 50.4 Gy (range, 38.7–54.0 Gy). Most patients received 45 Gy to the tumor bed, anastamoses, and tumor draining lymph nodes followed by a boost to the tumor bed plus a margin. The median boost dose was 5.4 Gy (range, 3.0–9.0 Gy). All patients except one received 15-MV photons. The median duration of radiotherapy was 41 days (range, 30–64 days), which began a mean of 74 days after surgery (range, 36–145 days). There was one planned interruption (14 days) in radiotherapy at 20 Gy for the 17 patients who were treated as per the Gastrointestinal Study Group (GITSG) protocol. No patients received neoadjuvant or intraoperative radiation therapy. Concurrent chemotherapy for most patients included continuous infusion 5-fluorouracil (5-FU) (37, 76%) or capecitabine (10, 20%). Three patients (4%) received concurrent gemcitabine.
All statistical endpoints including overall survival (OS), local control (LC), and distant control (DC) were calculated from the date of surgery. Local control was defined as the absence of recurrence within the tumor bed or local and regional lymph nodes. Distant control was defined as the absence of metastatic disease outside the sites defined for local failures. Local and distant control was assessed on the basis of the last available CT scan or biopsy. Survival time was censored at the date of last follow-up if death was not observed.
Statistical analyses were performed using STATA, version 9 (Stata, College Station, TX). Summary statistics for continuous and dichotomous variables are provided. The distribution of prognostic variables between treatment groups was compared by using Fisher’s exact test. The Kaplan-Meier product-limit method was used to estimate overall and disease free survival [15]. Log–rank testing was used to calculate univariate differences in outcomes, and a proportional hazards model was used to estimate relative risk (RR) [16]. All tests were two-sided, and a value of p < 0.05 was considered to be statistically significant.
Results
Median follow-up time for surviving patients was 19.3 months (range, 2.0–160.1). Fifty-one patients (46%) were still alive at the time of the analysis. Thirty-four patients (57%) died from recurrence of their disease, and 26 (43%) died from other or unknown causes.
Univariate analysis of prognostic factors for all patients showed that T3/T4 disease (p = 0.046), node positivity (p < 0.001), and poor histologic grade (p = 0.093) had inferior survival (Table 2). Median OS was 19.4 months for patients with node-positive disease and 62.2 months for patients with node-negative disease (p < 0.001). Age, gender, tumor size, and surgery type (PPPD vs. PD) were not associated with decreased OS. Resected margin status was not analyzed due to small numbers of involved margins (n = 2).
Table 2.
Associations of overall survival with patient tumor and treatment characteristics.
| No. (%) | 2-year survival (%) |
5-year survival (%) |
Median survival (95% CI), mos | Univariate RR (95% CI) |
P-value | |
|---|---|---|---|---|---|---|
| Age 75 yrs+ | ||||||
| No | 84 (76) | 61 | 38 | 36.2 (21.6–55.4) | 1.00 | 0.669 |
| Yes | 27 (24) | 68 | 30 | 39.9 (14.0–60.0) | 1.14 (0.62–2.12) | |
| Gender | ||||||
| Female | 44 (40) | 63 | 34 | 36.9 (18.5–60.0) | 1.00 | |
| Male | 67 (60) | 63 | 38 | 32.2 (21.6–61.6) | 0.92 (0.55–1.54) | 0.749 |
| Adjuvant treatment | ||||||
| None | 61 (55) | 66 | 38 | 36.2 (21.0–61.6) | 1.00 | |
| Adjuvant CRT | 50 (45) | 60 | 35 | 33.4 (18.9–46.0) | 1.01 (0.61–1.68) | 0.969 |
| Surgery type | ||||||
| Pylorus preserving PD | 87 (78) | 66 | 37 | 36.9 (24.5–60.0) | 1.00 | |
| Classic PD | 24 (22) | 51 | 34 | 22.7 (18.9–61.6) | 1.46 (0.80–2.67) | 0.223 |
| T stage | ||||||
| 1/2 | 62 (56) | 72 | 43 | 53.6 (25.6–62.2) | 1.00 | |
| 3/4 | 49 (44) | 51 | 28 | 21.7 (15.0–41.7) | 1.69 (1.01–2.82) | 0.046 |
| Tumor diameter (>3 cm) | ||||||
| No | 78 (72) | 68 | 40 | 36.5 (25.0–62.2) | 1.00 | |
| Yes | 30 (28) | 53 | 33 | 22.7 (19.4–53.6) | 1.22 (0.70–2.12) | 0.480 |
| Node status | ||||||
| Node-negative | 56 (53) | 85 | 60 | 62.2 (41.7–undefined) | 1.00 | |
| Node-positive | 50 (47) | 44 | 17 | 19.4 (14.0–32.1) | 3.27 (1.88–5.69) | <0.001 |
| Histologic grading | ||||||
| G1/2 | 61 (58) | 75 | 43 | 40.3 (30.0–62.2) | 1.00 | |
| G3 | 45 (42) | 51 | 30 | 22.7 (14.9–46.0) | 1.56 (0.93–2.64) | 0.093 |
Comparing population characteristics between the group which received chemoradiation and no CRT, it can be seen that patients who received CRT were more likely to have high-risk prognostic factors (Table 1). Specifically, patients receiving CRT were more likely to have higher T-stage (62% vs. 29% stage 3/4, p = 0.001), node positivity (72% vs. 31%, p < 0.001) and poor histologic grade (96% vs. 85% grade 2/3, p = 0.015). Patients who received CRT were also significantly younger than those who did not, with a mean age of 62.4 (median 64.2) vs. 69.8 (median 72.4), p = 0.001.
For all 111 patients, median OS was 36.2 months, 95% CI (22.7–53.6). The estimated unadjusted median overall survival time was 33.4 months for the adjuvant group and 36.2 months for the surgery alone group (p = 0.969). Actuarial 2-year and 5-year survival for the CRT vs. No CRT groups were 60% vs. 66%, p = 0.969, and 35% vs. 38%, p = 0.223, respectively (Fig. 1).
Fig. 1.
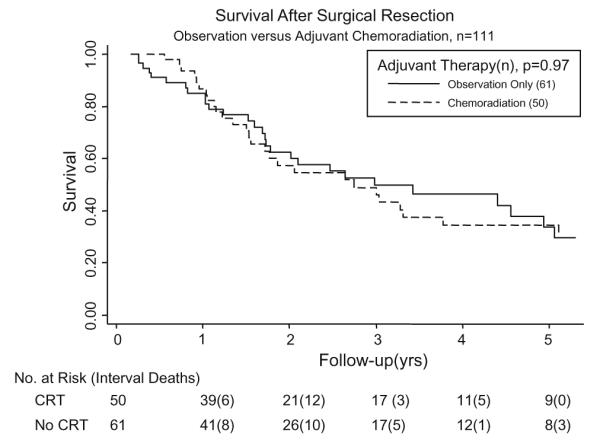
Overall survival of entire group of 111 patients, stratified based on adjuvant chemoradiation treatment.
The effect of adjuvant chemoradiation therapy was evaluated after stratifying patients into categories based on known poor prognostic factors (Table 3). There was no effect on survival from adjuvant therapy despite a statistical trend that was seen in the node-positive (21.6 vs. 13.0 mos, p = 0.092) patients (Fig. 2). Adjuvant CRT did not influence survival in patients with a negative node status (p = 0.21), tumor size >3 cm (p = 0.495), T-stage 3/4 (p = 0.863), or poor (grade 3) histology (p = 0.282).
Table 3.
Survival between treatment groups by patient, tumor, and treatment characteristics.
| No. of patients (%) |
Overall survival, median, mo |
5-year survival (%) |
|||||
|---|---|---|---|---|---|---|---|
| Observation | Adjuvant CRT | Observation | Adjuvant CRT | P-value | Observation | Adjuvant CRT | |
| All patients | 61 (55) | 50 (45) | 36.2 | 33.4 | 0.969 | 38 | 35 |
| Age, yrs | |||||||
| <75 | 39 (46) | 45 (54) | 36.2 | 36.5 | 0.688 | 35 | 41 |
| P75 | 22 (81) | 5 (19) | 60.0 | 25.0 | 0.181 | 47 | 0 |
| Gender | |||||||
| Female | 25 (57) | 19 (43) | 41.7 | 36.9 | 0.581 | 36 | 32 |
| Male | 36 (54) | 31 (46) | 32.2 | 33.4 | 0.776 | 41 | 36 |
| Node | |||||||
| Negative | 42 (75) | 14 (25) | 55.4 | 103.2 | 0.199 | 52 | 83 |
| Positive | 19 (35) | 36 (65) | 13.0 | 21.6 | 0.092 | 13 | 20 |
| Primary tumor | |||||||
| Tumor<=3 cm | 44 (56) | 34 (44) | 55.4 | 36.5 | 0.779 | 43 | 37 |
| Tumor > 3 cm | 16 (53) | 14 (47) | 21.7 | 39.9 | 0.495 | 31 | 34 |
| T-stage 1/2 | 43 (69) | 19 (31) | 53.6 | 40.3 | 0.336 | 40 | 48 |
| T-stage 3/4 | 18 (37) | 31 (63) | 18.5 | 25.0 | 0.863 | 35 | 23 |
| Histology | |||||||
| Grade 1–2 | 39 (64) | 22 (36) | 32.2 | 62.2 | 0.110 | 37 | 54 |
| Grade 3 | 19 (42) | 26 (58) | 41.7 | 20.8 | 0.282 | 42 | 22 |
Fig. 2.

Overall survival in node-positive patients, stratified based on adjuvant chemoradiation treatment.
Treatment-related toxicities as a result of radiation in the CRT group were nausea (28%), diarrhea (12%), pain (10%), fatigue (8%), weight loss (8%), mucositis (6%), hematologic (6%), and skin (4%). There were no known treatment-related deaths.
The site of first recurrence was local in 2 patients (2%), distant in 28 patients (25%) and both local and distant in 9 patients (8%). The liver (23%) and peritoneum (7%) were the common sites of metastatic disease. Three patients had involvement of both the liver and peritoneum (3%). Patterns of failure were similar in both the CRT and the observation-only groups.
Discussion
The parameters used to classify ampullary tumors into high-risk categories varies between studies, but is largely based on primary tumor stage, pancreatic invasion, node status, margin status, and tumor size. A 27-year retrospective study by Talamini et al. [7] on the surgical resection of ampullary tumors at JHH found that lymph node status and tumor differentiation influenced survival. The study did not find an associated survival benefit with adjuvant therapy but the number of patients who received chemoradiation was small (n = 13).
On univariate analysis, the high-risk categories including primary tumor stage (T3/T4 vs. T1/T2), node-positive status, and histological grade (2/3 vs. 1) were all associated with poor overall survival. In our study patients receiving CRT had a significantly greater proportion of patients with these poor prognostic factors compared to the no CRT group. For example, patients receiving CRT were more likely to have node-positive disease (p < 0.001). Overall survival was not statistically different for those who received adjuvant therapy vs. surgery alone (p = 0.969). However, the fact that the two patient groups (adjuvant vs. surgery only) had comparable OS outcomes while differing in the number of adverse prognostic factors suggested the possibility that adjuvant therapy may improve survival of patients with poor prognostic factors. Nevertheless, after controlling for the various high-risk factors, a significant survival benefit could not be demonstrated. The two potential interpretations are that adjuvant therapy does not increase overall survival and the sample size was underpowered.
Earlier studies have previously advocated the benefit of adjuvant therapy in patients with high-risk features. A study by Willet et al. [17] found that high-risk patients who underwent adjuvant therapy had better 5-year actuarial local control compared with high-risk patients who did not receive adjuvant therapy (83% vs. 50%) although this finding was not statistically significant. Similarly, Lee et al. [18] demonstrated that among the high-risk patient population who had node-positive disease or pancreatic invasion (T3 disease), there was a statistically significant OS difference attributable to adjuvant RT. However, both of these studies were limited by small patient numbers (<20 in each arm in Willet et al. and <10 in each arm in Lee et al.).
In the more recent study by Bhatia et al. [14], high-risk patients exhibiting node-positive status who underwent adjuvant CRT had a significantly improved 5-year OS than node-positive patients who underwent resection alone (p = 0.01). In our study, on univariate analysis, there was only a trend toward improved survival from adjuvant CRT among node-positive patients compared with surgery alone. Similarly, higher primary tumor stage (T3/T4) has been identified as independent adverse-risk factor. Krishnan et al. [19] demonstrated improved survival with adjuvant CRT in patients with advanced primary tumor stage. However, in our study median overall survival was not significantly altered by adjuvant CRT status when stratified by T-stage (p > 0.35).
Unfortunately a large number of our patients developed distant metastases (n = 37, 33%). The majority of such metastases were to the liver (23%) and peritoneum (7%), which are consistent with those found by Bhatia et al. In that study, 33% of the patients had distant failure, in which 27% were found to have metastatic disease to the liver, and 8% with peritoneal disease. The large number of patients with distant progression of disease suggests that more effective systemic treatment is needed for those patients with high-risk disease. These studies do not, however, address the controversy as to whether adjuvant CRT is superior to chemotherapy alone.
This study is retrospective in nature and therefore the results may be altered by patient selection and confounding factors. Patients receiving adjuvant therapy were younger than those patients not receiving CRT suggesting younger and healthier patients were more likely to be offered adjuvant CRT. It appears that patients with high-risk features were more likely to receive adjuvant therapy while those with favorable features were not offered CRT. This is especially the case with node positivity and poor histologic grade which have been shown to be an adverse prognostic factor in both ampullary and pancreatic adenocarcinoma [14,19]. A second limitation of this study is the relatively limited power due to a small cohort of patients. In this study a trend toward improved survival was seen for node-positive patients who underwent CRT. However, with a larger patient population it may be possible to detect a statistically significant difference.
Given the current controversy regarding adjuvant therapy in periampullary malignancies, it is important to identify which patients are more or less likely to benefit from the addition of adjuvant radiation therapy. The patient survival rates reported in our study also compare favorably to the survival data from other retrospective adjuvant treatment series despite having a large proportion of patients with known adverse prognostic features [13,14,18,19].
Conclusion
Patients with resected ampullary adenocarcinoma do not appear to benefit from adjuvant CRT in our study. Additional studies are needed to confirm the role of adjuvant therapy in the setting of high-risk disease. Furthermore, a significant proportion of patients still develop metastatic disease to the liver suggesting a need for more effective adjuvant therapy.
Footnotes
Presented as a poster at the 50th annual meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO), Boston, MA 2008.
References
- [1].Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s. Pathology, complication and outcomes. Ann Surg. 1997;226:248–60. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bouvet M, Gamagami RA, Gilpin EA, et al. Factors influencing survival after resection for periampullary neoplasms. Am J Surg. 2000;180:13–7. doi: 10.1016/s0002-9610(00)00405-0. [DOI] [PubMed] [Google Scholar]
- [3].Stephens J, Kuhn J, O’Brien J, et al. Surgical morbidity, mortality, and long term survival in patients with pancreatic cancer following pancreaticoduodenectomy. Am J Surg. 1997;174:600–4. doi: 10.1016/s0002-9610(97)00204-3. [DOI] [PubMed] [Google Scholar]
- [4].Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- [5].Matory YL, Gaynor J, Brennan M. Carcinoma of the ampulla of Vator. Surg Gynecol Obstet. 1993;177:366–70. [PubMed] [Google Scholar]
- [6].Monson JRT, Donohue JH, McEntee GP, et al. Radical resection for carcinoma of the ampulla of Vater. Arch Surg. 1991;126:353–7. doi: 10.1001/archsurg.1991.01410270099016. [DOI] [PubMed] [Google Scholar]
- [7].Talamini MA, Moesinger RC, Pitt HA, et al. Adenocarcinoma of the ampulla of Vater: a 28-year experience. Ann Surg. 1997;225:590–600. doi: 10.1097/00000658-199705000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].De Castro SMM, Kuhlmann KFD, van Heek NT, et al. Recurrent disease after microscopically radical (R0) resection of periampullary adenocarcinoma in patients without adjuvant therapy. J Gastrointest Surg. 2004;8:775–84. doi: 10.1016/j.gassur.2004.08.006. [DOI] [PubMed] [Google Scholar]
- [9].Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228:87–94. doi: 10.1097/00000658-199807000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brown KM, Tompkins AJ, Yong S, Aranha GV, Shoup M. Pancreaticoduodenectomy is curative in the majority of patients with node-negative ampullary cancer. Arch Surg. 2005;140:529–33. doi: 10.1001/archsurg.140.6.529. [DOI] [PubMed] [Google Scholar]
- [11].El-Ghazzawy AG, Wade TP, Virgo KS, Johnson FE. Recent experience with the cancer of the ampulla of Vater in a national hospital group. Am Surg. 1995;61:607–11. [PubMed] [Google Scholar]
- [12].Gastrointestinal Tumor Study Group Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–10. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [13].Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-Fluorouracil after curative resection of cancer of the pancreas and periampullary region. Phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776–84. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. Adjuvant therapy for ampullary carcinomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2006;66:514–9. doi: 10.1016/j.ijrobp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- [15].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- [16].Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:1187–220. [Google Scholar]
- [17].Willett CG, Warshaw AL, Convery K, Compton CC. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg Gynecol Obstet. 1993;176:33–8. [PubMed] [Google Scholar]
- [18].Lee JH, Whittington R, Williams NN, et al. Outcome of pancreaticoduodenectomy and impact of adjuvant therapy for ampullary carcinomas. Int J Radiat Oncol Biol Phys. 2000;47:945–53. doi: 10.1016/s0360-3016(00)00537-x. [DOI] [PubMed] [Google Scholar]
- [19].Krishnan S, Rana V, Evans DB, et al. Role of adjuvant chemoradiation therapy in adenocarcinomas of the ampulla of vater. Int J Radiat Oncol Biol Phys. 2008;70:735–43. doi: 10.1016/j.ijrobp.2007.07.2327. [DOI] [PubMed] [Google Scholar]


