Abstract
Background
Adiponectin, an insulin-sensitizing adipokine, is inversely associated with adiposity and prostate cancer risk and progression. However, the role of genetic variation in the adiponectin (ADIPOQ) and receptor genes (ADIPOR1/R2) in prostate cancer is largely unknown.
Methods
In a nested case-control study of 1286 cases and 1267 controls within the Physicians' Health Study, we evaluated 29 common single nucleotide polymorphisms (SNPs) in ADIPOQ (n=13), ADIPOR1(n=5) and ADIPOR2(n=11) in relation to the risk of prostate cancer. In subgroups, we also evaluated the association of genotype and circulating adiponectin levels (n=951) and prostate tumor expression of insulin receptor (IR) and insulin-like growth factor 1 (IGF-1R) receptor (n=181).
Results
Among the 12 tagging polymorphisms in ADIPOQ, four (rs266729, rs182052, rs822391, rs2082940) were significantly associated (p<0.05) with overall prostate cancer risk, with no significant difference by tumor grade or clinical stage. Two of the risk SNPs (rs266729, rs182052) plus four other SNPs (rs16861209, rs17366568, rs3774261, rs7639352) were also associated with plasma adiponectin levels and three of these (rs1686109, rs17366568, rs3774261) were also significantly associated with IR expression in prostate tumor tissue. One additional SNP was associated with IGF1-R tumor tissue expression (rs16861205). None of the 16 variants in ADIPOR1/R2 were related to cancer risk or circulating adiponectin levels.
Conclusions
Common variants in the adiponectin gene were associated with prostate cancer risk, plasma adiponectin levels, and IR or IGF-1R expression in the prostate tumor.
Impact
These genotype-phenotype associations support the biological relevance of adiponectin for prostate carcinogenesis, particularly in earlier stages of development.
Introduction
Emerging evidence suggests that high body mass index (BMI) and adiposity are linked to increased prostate cancer mortality (1, 2). Higher circulating levels of adiponectin, a protein secreted by adipose tissue and inversely correlated with BMI, may be associated with a reduced risk of prostate cancer (3, 4), lower Gleason score (3-6) and lower tumor stage (3-5). We had previously reported that low pre-diagnostic levels of circulating adiponectin were associated with increased risk of high-grade and lethal prostate cancer in the Physicians' Health Study (7), although another smaller prospective study did not yield similar results (8).
It remains unclear whether adiponectin acts directly on tumorigenesis or indirectly through its effects on insulin resistance (9), which is also highly regulated by the insulin-like growth factor 1 (IGF-1) signaling system. Adiponectin activates the AMP-activated protein kinase (AMPK), stimulates fatty acid oxidation, acts as a direct endogenous inhibitor of inflammation (10-12) and activates anti-inflammatory cytokines through the nuclear factor κB (NF-κB) pathway (13-15). It also reduces angiogenesis (10) and inhibits prostate cancer cell proliferation in vitro (16). Adiponectin receptor expression (ADIPOR1/R2) has been demonstrated in androgen-dependent and androgen-independent prostate cancer cell lines (16-18) and lower expression has been observed in prostate tissue of prostate cancer patients than that of healthy men or men with benign prostatic hyperplasia (5).
Several polymorphisms in the adiponectin and its receptor genes modulate levels and function and have been linked to obesity, insulin resistance (19-22) and prostate cancer risk (23). Three studies have evaluated single nucleotide polymorphisms (SNPs) in the adiponectin gene with respect to prostate cancer risk (23) of which one recently reported significant associations (rs266729, rs822395, rs822396, rs1510299 in ADIPOQ and rs12733285, rs7539452 in ADIPOR1; (23)). The other two studies - a case-control study of African Americans and a cohort of Finnish smokers - yielded no associations in candidate SNPs selected for potential functionality and prostate cancer (24). None of these studies evaluated concurrent associations between genotypes and circulating adiponectin levels. In the current case-control study nested within a long-term prospective cohort of U.S. male physicians, we comprehensively evaluated common haplotype tagging SNPs throughout genes encoding adiponectin (ADIPOQ) and its two receptors (ADIPOR1/R2). We examined these SNPs in relation to the risk of total prostate cancer, as well as high-grade and aggressive disease. In sub-groups with available data, we also assessed whether plasma adiponectin levels, and expression of tumor biomarkers involved in insulin sensitivity (insulin receptor (IR) and insulin-like growth factor 1 receptor (IGF-1R)), were correlated with these genotypes as potential mediators of risk.
Methods
Study population
We conducted a nested case-control study within the Physicians' Health Study (PHS), a randomized trial of aspirin and β-carotene for the primary prevention of cancer and cardiovascular disease among U.S. male physicians (25, 26). The study began in 1982 among 22,071 physicians aged 40-84 years free of baseline cardiovascular disease or cancer (except for non-melanoma skin cancer). Follow-up information and mortality data are 97% complete on all participants. The research protocol was approved by the Human Subjects Committee at Brigham and Women's Hospital, and all participants provided written informed consent.
At baseline, all study participants completed questionnaires with information on age, height, weight, cigarette smoking and the presence of diabetes, and 14,916 men (68%) provided a baseline blood sample in 1982. Annual questionnaires updated medical information, including diagnoses of prostate cancer, which were confirmed through medical records and pathology reports reviewed by an End Point Committee of physicians. Deaths were identified through the postal system and next of kin, and cause of death was determined through medical record review and death certificates with nearly 100% mortality follow-up. All participants who provided a baseline blood specimen and who were later diagnosed with a confirmed prostate adenocarcinoma from 1982-2004 were eligible cases for this study. For each case, one control was selected at random from those who provided blood, had not had a prostatectomy and had not reported a diagnosis of prostate cancer at the time of the matched case's diagnosis date Controls were individually matched to cases by age (within 1 year, if feasible, or 5 years for older men) and cigarette smoking status (never, current, former).
Clinical and demographic characteristics
Information on Gleason score, tumor-node-metastasis (TNM) stage, and PSA at diagnosis was obtained from medical records for all cases. Development of bony metastases was ascertained through annual mailed questionnaires to consenting participants and confirmed by treating physicians. If data on clinical characteristics were not available from medical records, self-reported stage and Gleason score were used from follow-up questionnaires. All clinical covariates were collected while blinded to genotype and plasma level status.
Adiponectin genotyping
We used the web-based tagger application (27) to select linkage disequilibrium (LD) tag SNPs capturing genetic variation in the international HapMap database (HapMap Phase II, build 35 data) in the adiponectin (ADIPOQ, Chr3q27) and both receptor genes (ADIPOR1, Chr1p36.13-q41 and ADIPOR2, Chr12p13.31) as well as 5 kb up- and down-stream for each gene. An aggressive tagging approach, a maximum combination of three SNPs, was used to tag SNPs with a minor allele frequency (MAF) greater than 0.05 with a minimum r2 of 0.80 in CEU (27). Evaluation of our tag SNPs demonstrated sufficient genetic coverage using pair-wise only tagging allowing for evaluation of single SNP effects. Genotyping was performed using MassARRAY high-throughput DNA analysis with matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Sequenom, San Diego, CA, USA) and genotypes were called using the automated analysis SpectroTYPER-RT software. We selected 29 SNPs across the three genes: 13 in ADIPOQ, 5 in ADIPOR1 and 12 in ADIPOR2. Blind duplicates were run to assess genotyping quality yielding a concordance rate greater than 98% for duplicate samples. All SNPs had >90% genotype passing rates.
Adiponectin plasma levels
Plasma adiponectin concentrations were measured by competitive radioimmunoassay (Linco Research, St. Charles, MO) in the laboratory of Dr. Nader Rifai (Children's Hospital, Boston MA) for a subset of 565 men diagnosed with prostate cancer and 440 controls who also had genotype data. Specimens from cases and matched control(s) were analyzed together and placed in random order so the case status remained unknown to laboratory personnel. The median intra-individual coefficient of variation for blinded duplicate quality control samples was 11%. Previously, we showed that two adiponectin measurements over a 1-year period had a high BMI-adjusted intra-class correlation (r = 0.84, 95% CI 0.65-0.94) (28), suggesting that a single blood measurement of adiponectin is reasonably accurate and stable, over time and over different transport conditions.
Tissue microarrays
Archival formalin-fixed paraffin embedded tissue specimens were available for a subset of surgically treated cases (90.1% radical prostatectomy, n=1171; 8.9% TURP, n=115). Tissue microarrays (TMAs) were created using a manual tissue arrayer (Beecher Instruments, Silver Spring MD). For each patient, a study pathologist circled the dominant nodule or nodule with the highest Gleason score on a Hematoxylin and Eosin-stained slide, and three or more replicate 0.6 mm tissue cores were transferred from the corresponding area on the tissue block into the recipient TMA blocks. The pathologist had no a priori knowledge of the clinical or pathological status of the cases. There were three tissue microarrays with 1 to 14 cores per TMA block. We had immunohistochemical data for a subset of the cases for whom we had genotyping information for insulin receptor (IR) and IGF-1 receptor on the TMA's (n=169 for IR, n=190 for IGF1-R &)
Insulin receptor (IR) and Insulin-like growth factor 1 receptor (IGF-1R)
Immunohistochemical staining was conducted on 5 um sections of each TMA using the Anti-Insulin Receptor, B subunit, Rabbit immunoaffinity purified IgG (Upstate Cell Signaling Solutions, Lake Placid) and the IGF-1R beta R rabbit polyclonal antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) with enzyme labeled biotin streptavidin system and solvent resistant DAB Map kit (Ventana autostainer model Discover XT ™, Vantana Medical System, Tuscan, Arizona). Nonspecific reactivity was assessed by omission of the primary antibody. The specificity of staining for IR was confirmed by using placenta as a positive control. The slide was scanned with the BLISS system (Bacus Lab, North Lombard, IL) and scored manually by two pathologists who were blinded to clinical outcomes. Immunohistochemical analysis of IR showed homogenous cytoplasmic staining in the cancer cells while that of IGF-1R yielded mostly membranous and occasional cytoplasmic staining. For both receptors, intensity was scored from 0 to +3 – 0 (no staining by any tumor cells, 1 (faint or focal staining), 2 (moderate intensity in a convincing number of cells), and 3 (intense staining by a sufficient number of cells). Immunohistochemical staining was completed for 169 men for IR and for 190 men for IGF-1R.
Statistical Analyses
We restricted our analyses to Caucasians to reduce spurious associations by population stratification (29). Using the SAS program package, version 9.1 (SAS Institute, Cary, NC), we evaluated Hardy-Weinberg equilibrium (HWE) and removed one adiponectin SNP (rs7649121) from the analysis because it was severely out of HWE (p<0.0001).
We used unconditional logistic regression to calculate the multivariate odds ratio (OR) and 95% confidence interval (CI) of prostate cancer associated with each tag SNP genotype, adjusted for the original matching factors of age at randomization (years), cigarette smoking status at baseline (never, former, current) and time between blood draw and diagnosis (years). We used analysis of variance to evaluate the association with each tag SNP genotype and mean plasma adiponectin levels, adjusted for age at baseline and body mass index; p-for-trend values were calculated assuming linear changes in risk with additive models.
For total prostate cancer risk and plasma adiponectin levels, we used additive models and the SNPs were divided into three categories, with the most common genotype selected as the reference group, the heterozygous genotype as a second group and the rare homozygous variant as the third group; p-for-trend values were presented, assuming linear changes in risk. For high-grade and aggressive prostate cancer risk and tumor biomarker expression, we used dominant genetic models due to much smaller numbers, and the SNPs were modeled dichotomously with the same reference group and with heterozygous and rare alleles combined into one category. For calculating the risk of high-grade disease, we restricted cases to those with a Gleason of 4+3, 8, 9 or 10, and for aggressive disease, we considered men who had extra-prostatic disease (T3 or T4 or N1 or M1) or men who developed bony metastases or fatal disease during follow-up (end of follow-up for prostate cancer mortality = 03/30/09). The impact of ADIPOQ variation on insulin sensitivity is dependent on adiposity (30). Thus, we examined whether associations between genotype and risk differed according to baseline body mass index (BMI, kg/m2) and plasma adiponectin levels (p-for-interaction<0.05).
We used analysis of variance for the comparison of continuous variables by genotype, including baseline age (years) and log-transformed adiponectin levels (ug/mL) among cases and controls. To evaluate adiponectin differences, we adjusted for age and BMI. To reduce bias from pre-clinical disease influencing adiponectin levels at baseline, we excluded cases whose time between blood draw and diagnosis was less than five years. To evaluate differences of mean intensity expression levels (scores were averaged across multiple cores per subject) of IGF-1R and IR by genotype, we analyzed cases with available tumor expression levels (IR, n=169; IGF1-R, n=190) and adjusted for age and TMA to account for batch variation by TMA.
We conducted permutation testing to better interpret nominally statistical associations by randomly assigning case-control status 5,000 times for the 29 tagging SNPs. Unadjusted P-values were examined in relation to the permuted distribution. We used the SAS program package, version 9.1 (SAS Institute, Cary, NC) to carry out statistical analyses with a significance level of .05. This study was approved by the Institutional Review Board at Partners Healthcare.
Results
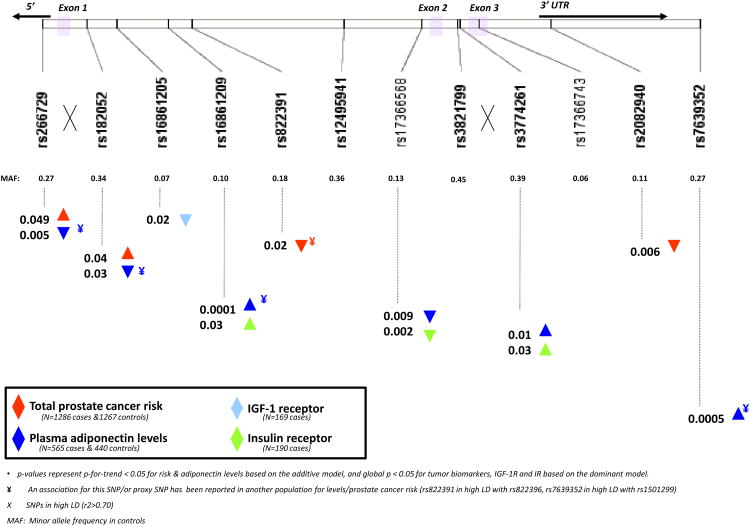
Baseline characteristics are provided in Table 1; men were on average, 57 years at the beginning of follow-up. Table 2 shows the frequency distribution of the 12 ADIPOQ SNPs and their associations with prostate cancer risk and plasma levels. Figure 1 summarizes the major findings of the associations of the 12 SNPs with risk of prostate cancer (p-for-trend < 0.05), plasma adiponectin levels (p-for-trend < 0.05) and tumor tissue expressions of IGF-1 receptor or insulin receptor (global p < 0.05) with the upwards triangle representing a positive association and downwards triangle an inverse association with the rare genotype. Each of these associations is presented in detail in the following sections.
Table 1.
Distribution of characteristics at baseline among cases and controls and clinical characteristics at diagnosis (cases) in the Physician's Health Study (PHS), 1982-2004.
| Cases (n=1286) | Controls (n=1267) | ||
|---|---|---|---|
|
|
|||
| Characteristics at baseline | N (%) or mean (SD) | N (%) or mean (SD) | p-value |
| Age at randomization (yrs) | 57.9 (8.4) | 57.5 (8.4) | n/a |
| Cigarette smoking status at baseline | |||
| Never | 627 (48.8) | 636 (50.2) | n/a |
| Former | 553 (43.0) | 532 (42.0) | |
| Current | 106 (8.2) | 99 (7.8) | |
| Body mass index (kg/m2, baseline) | |||
| <25 kg/m2 | 746 (58.0) | 761 (60.1) | 0.56 a |
| 25-29 kg/m2 | 499 (38.8) | 466 (36.8) | |
| 30+ kg/m2 | 41 (3.2) | 40 (3.2) | |
| Adiponectin levels (mg/ml) | 7.12 (4.2) | 7.18 (4.4) | 0.83 b |
| Diabetes at baseline | 21 (1.6) | 17 (1.3) | 0.54 a |
| Time b/w blood draw and diagnosis (yrs) | 12.2 (5.1) | 12.1 (5.0) | 0.61 a |
| Gleason score at diagnosis | |||
| 4-6 | 750 (58.3) | -- | |
| 3+4/7 c | 234 (18.2) | ||
| 4+3 | 103 (8.0) | -- | |
| 8-10 | 173 (13.5) | -- | |
| missing | 26 (2.0) | -- | |
| PSA at diagnosis (ng/mL, median (IQR)) | 7.3 (5.1-12.7) | -- | |
| missing d | 350 (27) | -- | |
| Clinical stagee at diagnosis | |||
| T1/T2, NX/NO | 1079 (83.9) | -- | |
| T3, NX/NO | 63 (4.9) | -- | |
| T4 or N1 or M1 | 79 (6.1) | -- | |
| Missing | 65 (5.1) | -- | |
global chi-square test (except for “n/a” where age and cigarette smoking status are matching factors)
p>0.05 in F-test for analysis of variance comparing cases and controls
includes n=45 (3.5%) men with Gleason=7 but no information on major/minor score
61% of men with missing PSA at diagnosis (n=213) were diagnosed in the pre-PSA period (<1992)
Pathological stage available for n=465 cases (27%): n=333 (25.9%) T2, n=116 (9.0%) T3/T4 and n=18(1.4%) N1/M1
Table 2.
Multivariate odds ratios of prostate cancer and adiponectin levels by sequence variants in the adiponectin (ADIPOQ) gene in the Physicians' Health Study, 1982-2009.
| Total prostate cancer | log(Adiponectin) - μg/mL b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Cases (n=1286) N (%) | Controls (n=1267) N (%) | OR a | 95% CI | p-for-trend | N | (n = 951) Mean (SD) | p-for-trend | |||
| 1 | rs266729 | CC | 636 (53) | 675 (57) | 1.00 | reference | -- | 483 | 8.78 (0.61) | ||
| CG | 477 (40) | 443 (37) | 1.14 | 0.97 | 1.36 | 340 | 8.67 (0.62) | ||||
| GG | 79 (7) | 65 (5) | 1.30 | 0.92 | 1.84 | 57 | 8.58 (0.53) | 0.001 | |||
| 0.049 | |||||||||||
| 2 | rs182052 | GG | 545 (45) | 564 (47) | 1.00 | reference | -- | 416 | 8.79 (0.63) | ||
| AG | 527 (43) | 524 (44) | 1.03 | 0.87 | 1.22 | 406 | 8.67 (0.62) | ||||
| AA | 147 (12) | 108 (9) | 1.42 | 1.08 | 1.87 | 90 | 8.61 (0.51) | 0.0009 | |||
| 0.04 | |||||||||||
| 3 | rs168681205 | GG | 1077 (87) | 1079 (88) | 1.00 | reference | 812 | 8.73 (0.63) | |||
| AG | 161 (13) | 138 (11) | 1.17 | 0.92 | 1.49 | 111 | 8.62 (0.58) | ||||
| AA | 5 (0.4) | 7 (0.6) | 0.74 | 0.23 | 2.33 | 3 | 8.93 (0.33) | 0.14 | |||
| 0.34 | |||||||||||
| 4 | rs168681209 | CC | 1014 (82) | 986 (80) | 1.00 | reference | 744 | 8.66 (0.63) | |||
| AC | 206 (17) | 230 (19) | 0.87 | 0.71 | 1.07 | 163 | 8.93 (0.52) | ||||
| AA | 13 (1) | 11 (1) | 1.13 | 0.51 | 2.55 | 10 | 9.17 (0.61) | <.0001 | |||
| 0.30 | |||||||||||
| 5 | rs822391 | TT | 855 (69) | 779 (64) | 1.00 | reference | -- | 622 | 8.71 (0.62) | ||
| CT | 346 (28) | 389 (32) | 0.81 | 0.68 | 0.96 | 270 | 8.74 (0.61) | ||||
| CC | 47 (4) | 52 (4) | 0.83 | 0.55 | 1.24 | 36 | 8.66 (0.77) | 0.82 | |||
| 0.02 | |||||||||||
| 6 | rs12495941 | GG | 508 (42) | 496 (42) | 1.00 | reference | 358 | 8.72 (0.61) | |||
| GT | 539 (45) | 531 (46) | 0.99 | 0.84 | 1.18 | 397 | 8.72 (0.65) | ||||
| TT | 162 (13) | 148 (13) | 1.07 | 0.83 | 1.39 | 127 | 8.74 (0.49) | 0.87 | |||
| 0.70 | |||||||||||
| 7 | rs17366568 | GG | 920 (75) | 924 (77) | 1.00 | reference | 689 | 8.76 (0.60) | |||
| AG | 282 (23) | 256 (21) | 1.11 | 0.91 | 1.34 | 187 | 8.61 (0.63) | ||||
| AA | 17 (1) | 13 (1) | 1.31 | 0.63 | 2.71 | 12 | 8.44 (0.61) | 0.0003 | |||
| 0.23 | |||||||||||
| 8 | rs3821799 | CC | 383 (31) | 350 (29) | 1.00 | reference | -- | 282 | 8.66 (0.66) | ||
| CT | 601 (49) | 606 (50) | 0.90 | 0.75 | 1.08 | 441 | 8.73 (0.60) | ||||
| TT | 249 (20) | 259 (21) | 0.87 | 0.69 | 1.09 | 190 | 8.76 (0.62) | 0.09 | |||
| 0.20 | |||||||||||
| 9 | rs3774261 | GG | 462 (37) | 441 (37) | 1.00 | reference | -- | 343 | 8.63 (0.67) | ||
| AG | 596 (48) | 574 (48) | 0.98 | 0.83 | 1.17 | 431 | 8.74 (0.59) | ||||
| AA | 183 (15) | 193 (16) | 0.90 | 0.70 | 1.14 | 147 | 8.86 (0.59) | 0.0001 | |||
| 0.43 | |||||||||||
| 10 | rs17366743 | TT | 1154 (94) | 1136 (94) | 1.00 | reference | 850 | 8.72 (0.61) | |||
| CT/CC | 78 (6) | 73 (6.1) | 1.07 | 0.77 | 1.49 | 55 | 8.83 (0.63) | ||||
| 0.19 | |||||||||||
| 0.76 | |||||||||||
| 11 | rs2082940 | CC | 969 (80) | 907 (76) | 1.00 | reference | -- | 706 | 8.72 (0.64) | ||
| CT | 226 (19) | 260 (22) | 0.81 | 0.66 | 0.99 | 189 | 8.67 (0.58) | ||||
| TT | 14 (1) | 26 (2) | 0.51 | 0.27 | 0.98 | 17 | 9.05 (0.50) | 0.7 | |||
| 0.006 | |||||||||||
| 12 | rs7639352 | CC | 652 (54) | 650 (55) | 1.00 | reference | -- | 514 | 8.65 (0.65) | ||
| CT | 453 (38) | 428 (37) | 1.05 | 0.88 | 1.24 | 313 | 8.78 (0.54) | ||||
| TT | 98 (8) | 94 (8) | 1.04 | 0.76 | 1.40 | 70 | 8.90 (0.61) | <.0001 | |||
| 0.66 | |||||||||||
among Caucasians only, adjusted for age at randomization (yrs, continuous), cigarette smoking status (never, current, past) and time between blood draw & event date (yrs, continuous))
adjusted for age at randomization and body mass index among Caucasians, includes all controls and cases with > 5 years between blood draw & diagnosis
Figure 1.
Single nucleotide polymorphisms in ADIPOQ and associations* with prostate cancer risk, plasma adiponectin levels & tumor expression of IGF-1R and IR.
Genetic variation and prostate cancer risk
Among the 1286 cases and 1267 controls, four of the 12 tagging SNPs in the adiponectin gene were significantly associated with overall prostate cancer risk using the additive model (rs266729, rs182052, rs822391 and rs2082940) adjusting for age, cigarette smoking status and time since blood draw (Table 2, Figure 1). SNP rs266729 is located in the promoter region and is in high linkage disequilibrium (r2=0.73) with the intronic SNP rs182052. Increased risks were associated with the rare genotype for both SNPs (rs266729, p-for-trend = 0.049 and rs182052, p-for-trend = 0.04). For rs822391, the rare C allele was associated with a decreased cancer risk (p-for-trend = 0.04). Rs2082940, located in the 3′ UTR region, showed an allele dosage association with significantly decreasing per-allele ORs (OR (CT) =0.81, 95% CI: 0.66, 0.99; OR(TT)=0.51, 95% CI: 0.27, 0.98; p-for-trend=0.006). Adjustment for multiple comparisons by imputing a new null distribution for the test statistic (based on 50,000 imputations for the 12 SNPs in ADIPOQ) yielded an adjusted borderline significant p-value = 0.05 for this SNP. None of these polymorphisms were significantly associated with the risk of high-grade (Gleason 4+3, 8-10), regional or metastatic prostate cancer (T3-T4 or N1 or M1, Table 3). We found no significant risk associations for any of the 16 SNPs in the ADIPOR1/R2 regions (Supplemental Tables 1 and 2).
Table 3.
Multivariate odds ratiosa of high-grade and aggressive prostate cancer by ADIPOQ sequence variants using the dominant model in the Physicians Health Study, 1982-2009.
| High-grade/Poorly differentiated PCa (Gleason 4+3 & 8-10, n=276) | Aggressive PCa (T3, N1, M1 or boney metastases during follow-up, n=252) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | N b (cases) | OR a | 95% CI | p-value | N b (cases) | OR a | 95% CI | p-value | ||||
| 1 | rs266729 | CC | 138 | 1.00 | reference | -- | 117 | 1.00 | reference | -- | ||
| CG/GG | 113 | 1.09 | 0.83 | 1.44 | 0.53 | 104 | 1.17 | 0.87 | 1.57 | 0.31 | ||
| 2 | rs182052 | GG | 125 | 1.00 | reference | -- | 109 | 1.00 | reference | -- | ||
| AG/AA | 136 | 0.94 | 0.72 | 1.23 | 0.66 | 128 | 1.00 | 0.75 | 1.34 | 0.98 | ||
| 3 | rs168681205 | GG | 234 | 1.00 | reference | -- | 209 | 1.00 | reference | -- | ||
| AG/AA | 35 | 1.08 | 0.72 | 1.61 | 0.71 | 38 | 1.26 | 0.84 | 1.88 | 0.26 | ||
| 4 | rs168681209 | CC | 220 | 1.00 | reference | -- | 205 | 1.00 | reference | -- | ||
| AC/AA | 46 | 0.85 | 0.6 | 1.21 | 0.36 | 38 | 0.81 | 0.55 | 1.19 | 0.27 | ||
| 5 | rs822391 | TT | 180 | 1.00 | reference | -- | 166 | 1.00 | reference | -- | ||
| CT/CC | 86 | 0.84 | 0.64 | 1.12 | 0.24 | 76 | 0.79 | 0.59 | 1.07 | 0.13 | ||
| 6 | rs12495941 | GG | 112 | 1.00 | reference | -- | 98 | 1.00 | reference | -- | ||
| GT/TT | 139 | 0.93 | 0.7 | 1.22 | 0.58 | 126 | 0.94 | 0.70 | 1.26 | 0.66 | ||
| 7 | rs17366568 | GG | 202 | 1.00 | reference | -- | 176 | 1.00 | reference | -- | ||
| AG/AA | 54 | 0.9 | 0.65 | 1.26 | 0.55 | 51 | 1.00 | 0.70 | 1.42 | 0.99 | ||
| 8 | rs3821799 | CC | 86 | 1.00 | reference | -- | 79 | 1.00 | reference | -- | ||
| CT/TT | 180 | 0.8 | 0.6 | 1.07 | 0.13 | 164 | 0.83 | 0.61 | 1.12 | 0.22 | ||
| 9 | rs3774261 | GG | 103 | 1.00 | reference | -- | 97 | 1.00 | reference | -- | ||
| AG/AA | 166 | 0.89 | 0.67 | 1.17 | 0.39 | 147 | 0.88 | 0.66 | 1.18 | 0.39 | ||
| 10 | rs17366743 | TT | 245 | 1.00 | reference | -- | 215 | 1.00 | reference | -- | ||
| CT/CC | 12 | 0.76 | 0.4 | 1.43 | 0.39 | 16 | 1.23 | 0.69 | 2.19 | 0.49 | ||
| 11 | rs2082940 | CC | 212 | 1.00 | reference | -- | 198 | 1.00 | reference | -- | ||
| CT/TT | 52 | 0.78 | 0.56 | 1.08 | 0.13 | 46 | 0.76 | 0.53 | 1.08 | 0.12 | ||
| 12 | rs7639352 | CC | 143 | 1.00 | reference | -- | 135 | 1.00 | reference | -- | ||
| CT/TT | 118 | 0.98 | 0.74 | 1.29 | 0.87 | 100 | 0.96 | 0.72 | 1.28 | 0.77 | ||
among Caucasians only, adjusted for age at randomization (yrs, continuous), cigarette smoking status (never, current, past) and time between blood draw & event date (yrs, continuous))
may not add up to total number of cases (n=276 & n=252) if genotype is missing for some participants.
Genetic variation and plasma adiponectin levels
We next evaluated variation in adiponectin levels by genotype in all controls and cases whose prostate cancer was diagnosed at least 5 years after time of blood draw (n=951, Table 2, supplementary Table 1). Six of the 12 SNPs in ADIPOQ were significantly associated (p-for-trend < 0.05) with circulating adiponectin levels (rs266729, rs182052, rs168681209, rs17366568, rs3774261, rs7639352) even after adjusting for baseline age and BMI. Two of these SNPs (rs266729, rs182052), which were highly linked and significantly inversely associated with plasma adiponectin levels, were also positively associated with the risk of overall prostate cancer (p-for-trend≤0.001); these genotype-plasma and genotype-risk associations were in the expected inverse directions to each other, suggesting a biological causal link. No variants in ADIPOR1/R2 were associated with plasma adiponectin levels (supplementary Table 1).
Genetic variation and receptor expressions in the prostate tumors
In a subset of cases whose prostate tumor tissue was available for immunohistochemical analysis, we evaluated tumor expression of insulin receptor (IR, n = 169) and IGF-1 receptor (IGF-1R, n = 190) to assess whether alleles associated with risk or circulating levels also yielded differential expression of receptors in tumors. Supplementary Table 3 summarizes tumor expression levels of IGF-1R and IR for each genotype using the dominant model. We observed three polymorphisms in ADIPOQ (rs168681209, rs3774261, and rs17366568) that were significantly associated with mean intensity levels of IR expression (p-for-trend≤0.02). One variant in ADIPOQ (rs16861205, p=0.02) was associated with IGF-1R expression (Supplementary Table 3). There were no significant associations between ADIPOR1/R2 polymorphisms and IR or IGF-1R protein expression.
Effect modification
We assessed whether genotype associations with risk varied according to baseline BMI and plasma adiponectin levels. We found that the inverse trend between rs2082940 in ADIPOQ and prostate cancer risk (Table 2) was stronger in men with normal/low (<10 ug/mL) baseline plasma adiponectin levels (OR's (OR (CT) = 0.76, 95% CI: 0.53, 1.07 and OR (TT) = 0.31, 95% CI: 0.08, 1.21, p-for-trend=0.03; data not shown). We also observed a significant interaction for two SNPs in ADIPOR1 (rs10920531, rs7539542) whereby an increased risk for the rare allele was observed (p-for-interaction=0.02 for rs10920531 and p-for-interaction=0.04 for rs7539542, Supplementary Table 4). We found no significant differences in risk by BMI.
Discussion
In this large prospective analysis, we evaluated the associations of common tagging variants in the genes encoding adiponectin (ADIPOQ), adiponectin receptors 1 and 2 (ADIPOR1/R2) with pre-diagnostic plasma levels of adiponectin, future risk of prostate cancer and receptor (IR and IGF-1R) expressions in prostate tumors. We found that among the 12 genotyped tagging ADIPOQ SNP's, four (rs266729, rs182052, rs822391, rs2082940) were significantly associated with the risk of developing prostate cancer. Of these 4 SNPs, two highly linked SNPs (rs266729 in the promoter and rs182052 in intron 1, r2=0.73, Supplementary Figure 1) were associated with both lower adiponectin levels and higher prostate cancer risk. In addition, three other SNPs (rs168681209, rs17366568, rs3774261) were significantly associated with insulin receptor expression in the prostate and plasma adiponectin levels. There were no associations with the risk of high-grade or aggressive disease, which may suggest these loci are more relevant for the development rather than progression of prostate carcinomas. We observed no variants in ADIPOR1/R2 in relation to prostate cancer risk.
To our knowledge, three published studies have evaluated genetic variation in the adiponectin gene with respect to prostate cancer risk, selecting SNPs targeted for potential functionality (24). Kaklamani et al. (23) recruited Caucasians (n=465 cases and 441 controls in New York City) to evaluate 10 SNPs selected for functionality in ADIPOQ and ADIPOR1, and reported four significant SNPs in the adiponectin gene (rs266729, rs822395, rs822396 and rs1501299), of which one was in the same reported direction as our findings (rs266729) and another two were in high LD (r2= 0.77 and 0.92) with our significant findings (rs822396 and rs1501299, respectively; Figure 1). In the other two studies, African Americans were evaluated on 10 SNPs (31) and Finnish smokers were evaluated on 4 SNPs - all targeted for potential functionality (25); neither study yielded associations with prostate cancer risk. The smaller study size and African ancestry of participants in Beebe-Dimmer et al. (24) may contribute to the differences in our findings. For the Finnish study (25), there were two overlapping SNPs (rs182052, rs17366743), one of which yielded a significant association in our study (rs182052, OR=1.41; 95% CI: 1.07, 1.86, p=0.02). Compared with all of the previous studies, we used a more comprehensive tagging approach for SNP selections and none of the previous findings linked SNPs to circulating adiponectin levels and receptor expressions in prostate tumors. For the promoter SNP, rs266729, the rare G allele was associated with an increased risk in our population, which is consistent with other studies that have found this allele to be correlated with lower adiponectin levels (19, 20, 32) and higher prostate cancer risk (23). Rs 266729 may map to a polymorphic regulatory element in this region with a nucleotide sequence similar to that of an enhancer element, which may explain its influence on serum levels in several study populations (20).
One strength of this study is the comprehensive evaluation of common polymorphisms in three genes in the adiponectin pathway in relation to prostate cancer risk, circulating adiponectin levels and tissue biomarkers expressed in the prostate tumor; these markers are related to the regulation of insulin resistance, which is a critical function of both the adiponectin and IGF-1 signaling systems, and which may play a role in prostate carcinogenesis. We found three variants (rs16861209, rs17366568, rs3774261) that were associated with both circulating adiponectin levels and IR expression in prostate tumor tissue. Variants in the adiponectin gene have been linked to plasma levels in various studies (19-21, 32, 33). Our findings are consistent with prior evidence for rs16861209 and rs3774261, for which we also found significant differences in insulin receptor expression (Figure 1). We found a novel SNP, rs17366568 in intron 2, related to plasma levels, which was also related to insulin receptor expression in prostate tumor tissue. In vitro data also show that adiponectin can increase the migration activity of prostate cancer cells through up-regulation of several pathways, including AdipoR1, p38, NF-kappaB and AMPK pathways (34). The absence of associations with high-grade or advanced stage disease suggests that potential pathway(s) for risk may be different than those for fatal disease, providing further evidence for heterogeneous etiologies of this disease (Giovannucci et al. 2007). Our data add to increasing evidence that suggests prostate cancer may be an insulin-responsive disease, although the specific mechanisms are unclear.
One limitation is that our findings are restricted to Caucasians due to the small number of men of other races/ethnicities in our study population (n = 128) - the frequency ADIPOQ SNP rs266729 is considerably different across populations (MAF is 47% in Japanese and 35% in persons of European ancestry, International HapMap). Although we cannot rule out chance findings due to limited sample size and multiple comparisons, several lines of evidence suggest the validity of our findings for ADIPOQ. First, we have comprehensive coverage (12 tagging SNPs) of ADIPOQ as compared with other published data and the CGEMS. Second, we observed no significant risk associations with ADIPOR1/R2, where we examined a greater number of SNPs (n=16), and chance findings are more likely. Third, we identified multiple genotype-phenotype associations (e.g., prostate cancer risk, circulating plasma levels and prostate tumor expression) with suggested biologically plausible directions. Also, in a multiple-SNP model with three variants covering the regions of significance (rs266729, rs822391, rs2082940), all SNP's remained significant at the p<0.05 level (data not shown). Fourth, one of the risk SNPs identified in our study (rs822391) was in high LD with one of the highest ranking SNPs from CGEMS stage one (rs822396). Finally, our observed interaction between rs2082940 and plasma adiponectin levels is consistent with previous evidence suggesting (30) that ADIPOQ variants may have a stronger impact on risk among individuals with a higher adiposity (or lower circulating adiponectin levels).
In conclusion, we observed multiple polymorphic loci in the adiponectin gene associated with prostate cancer risk. Six loci were associated with circulating adiponectin levels, of which two overlapped with risk in the expected opposite direction (rs266729, rs182052) and an additional three associated with IR tumor tissue expression (rs16861209, rs17366568, rs3774261), suggesting potential biological consequences. We did not find any evidence to suggest that variation in the receptor genes, ADIPOR1/R2, plays a major role in prostate cancer risk. In our data, several susceptibility loci in ADIPOQ were associated with multiple phenotypes and may represent genomic regions with functional variants involved in prostate carcinogenesis that require replication in other studies and a careful evaluation of underlying mechanisms.
Supplementary Material
Acknowledgments
Funding sources: This work was funded by the Prostate Cancer Foundation Award, “ Obesity, Adiponectin Angiogenesis and Prostate Cancer Survival” the Department of Defense (W81XWH-01-1-0322), the National Institutes of Health (CA 42182 and CA 097193, R01CA131945, RO1CA141298 Bethesda, MD), Department of Defense (PC073618), and the NCIC - Canadian Breast Cancer Research Initiative (019894). Dr. Dhillon was supported by a Cancer Epidemiology Training Grant NCI T32 CA009001.
References
- 1.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 2.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. (2008/10/07) 2008:1039–47. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 4.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65:1168–72. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 5.Michalakis K, Williams CJ, Mitsiades N, et al. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:308–13. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- 6.Sher DJ, Oh WK, Jacobus S, Regan MM, Lee GS, Mantzoros C. Relationship between serum adiponectin and prostate cancer grade. Prostate. 2008;68:1592–8. doi: 10.1002/pros.20823. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Stampfer MJ, Mucci L, et al. A 25 Year Prospective Study of Plasma Adiponectin and Leptin Concentrations and Prostate Cancer Risk and Survival. Clin Chem. 2010;56:34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baillargeon J, Platz EA, Rose DP, et al. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15:1331–5. doi: 10.1158/1055-9965.EPI-06-0082. [DOI] [PubMed] [Google Scholar]
- 9.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–66. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 10.Brakenhielm E, Veitonmaki N, Cao R, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:2476–81. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi N, Argueta JG, Masuhiro Y, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 13.Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 14.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 15.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 16.Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun. 2006;340:1158–66. doi: 10.1016/j.bbrc.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 17.Mistry T, Digby JE, Chen J, Desai KM, Randeva HS. The regulation of adiponectin receptors in human prostate cancer cell lines. Biochem Biophys Res Commun. 2006;348:832–8. doi: 10.1016/j.bbrc.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki T, Bub JD, Uzuki M, Iwamoto Y. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem Biophys Res Commun. 2005;333:79–87. doi: 10.1016/j.bbrc.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 19.Qi L, Doria A, Manson JE, et al. Adiponectin genetic variability, plasma adiponectin, and cardiovascular risk in patients with type 2 diabetes. Diabetes. 2006;55:1512–6. doi: 10.2337/db05-1520. [DOI] [PubMed] [Google Scholar]
- 20.Vasseur F, Helbecque N, Dina C, et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607–14. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- 21.Filippi E, Sentinelli F, Romeo S, et al. The adiponectin gene SNP+276G>T associates with early-onset coronary artery disease and with lower levels of adiponectin in younger coronary artery disease patients (age <or=50 years) J Mol Med. 2005;83:711–9. doi: 10.1007/s00109-005-0667-z. [DOI] [PubMed] [Google Scholar]
- 22.Hara K, Boutin P, Mori Y, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–40. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- 23.Kaklamani V, Yi N, Zhang K, et al. Polymorphisms of ADIPOQ and ADIPOR1 and prostate cancer risk. Metabolism. doi: 10.1016/j.metabol.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore SC, Leitzmann MF, Albanes D, et al. Adipokine genes and prostate cancer risk. Int J Cancer. 2009;124:869–76. doi: 10.1002/ijc.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 26.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians' Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–34. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 27.de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–23. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 28.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003;49:650–2. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]
- 29.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- 30.Vasseur F, Meyre D, Froguel P. Adiponectin, type 2 diabetes and the metabolic syndrome: lessons from human genetic studies. Expert Rev Mol Med. 2006;8:1–12. doi: 10.1017/S1462399406000147. [DOI] [PubMed] [Google Scholar]
- 31.Beebe-Dimmer JL, Zuhlke KA, Ray AM, Lange EM, Cooney KA. Genetic variation in adiponectin (ADIPOQ) and the type 1 receptor (ADIPOR1), obesity and prostate cancer in African Americans. Prostate Cancer Prostatic Dis. 13:362–8. doi: 10.1038/pcan.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heid IM, Wagner SA, Gohlke H, et al. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes. 2006;55:375–84. doi: 10.2337/diabetes.55.02.06.db05-0747. [DOI] [PubMed] [Google Scholar]
- 33.Crimmins NA, Martin LJ. Polymorphisms in adiponectin receptor genes ADIPOR1 and ADIPOR2 and insulin resistance. Obes Rev. 2007;8:419–23. doi: 10.1111/j.1467-789X.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 34.Tang CH, Lu ME. Adiponectin increases motility of human prostate cancer cells via adipoR, p38, AMPK, and NF-kappaB pathways. Prostate. 2009;69:1781–9. doi: 10.1002/pros.21029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.