Abstract
Hepatitis C Virus (HCV) infection and recurrence post-transplant (OLT) is associated with extracellular matrix (ECM) components remodeling, particularly collagen (Col), leading to fibrosis. Our aim was to determine whether development of antibodies (Abs) to self antigen Col in HCV infection correlates with fibrosis stage and peripheral cytokine response. Chronic HCV patients, those with recurrence after OLT undergoing biopsy and healthy control subjects were enrolled. HCV subjects (n=70) were stratified: 1) Non OLT No Fibrosis (Scheuer Stage 0–2) 2) Non OLT Fibrosis (Scheuer Stage 3–4) 3) Post OLT No Fibrosis (Scheuer 0–2) and 4) Post OLT Fibrosis (Scheuer 3–4). Serum was analyzed for Abs against Col-1, 2, 4, 5 and vimentin using ELISA. Serum levels of cytokines were measured using Multiplex Bead immunoassays. Levels of Abs to Col 1 were higher in fibrosis groups compared with no fibrosis groups and control both Non OLT (p<0.0001) and Post OLT (P=0.01). There were increased levels of Abs to Col 2, 4, 5 and vimentin, in fibrotic groups Non OLT (Col 2: p=0.0001, Col 4: p =0.09, Col 5: p<0.0001, vimentin: p=0.36) and Post OLT (Col 2: p=0.006, Col 4: p = 0.1, Col 5: p<0.0001, vimentin: p=0.24) compared with non fibrotic groups. Fibrotic groups non-OLT and post OLT demonstrated significantly higher Th2, Th17 cytokines and lower Th1 cytokines compared to non fibrotic groups. Our results demonstrate that in HCV infection, Abs to ECM Collagen 1, 2, 5 positively correlate with liver fibrosis which is associated with a predominant Th2 and Th17 cytokine profile.
Keywords: Autoantibodies, IL-17, Fibrosis, HCV Recurrence
Chronic hepatitis C (HCV) is the leading indication for orthotopic liver transplantation (OLT) in the United States, accounting for up to 40–45% of all transplants (1). HCV recurrence in the allograft is universal (2). The natural history of HCV in the native liver is characterized by a slow progression to cirrhosis, end-stage liver disease, and in some cases hepatocellular carcinoma (3). Many factors including but not limited to: age, ethnicity, concurrent alcohol use, duration of infection, inflammatory activity index (HAI) and steatosis (4, 5) account for the variability of progression. HCV following OLT has an accelerated course, with the development of cirrhosis in 20–30% of patients within 5 years (6, 7). In this setting, factors impacting progression of fibrosis include liver donor quality, recipient factors (older age, non-Caucasian race, higher Child-Pugh score prior to transplantation), and systemic immunosuppression (8–10).
Liver fibrosis due to different etiologies has been associated with an increased deposition of collagens (Col) and other connective tissue components (11, 12). In particular, HCV infection in the native liver and its recurrence post-transplant has been shown to significantly impact deposition and remodeling of extracellular matrix (ECM) components particularly Col, leading to enhanced fibrosis (13). Further, chronic rejection in the allograft is initiated by a host-anti-graft-immune response with both antigen-dependent and non-immune (antigen-independent) factors leading to fibroproliferative changes affecting graft function. Inflammation and tissue remodeling promoted by alloimmune mechanisms facilitate the induction of autoimmune responses against self-antigens. Studies in the arena of heart, lung, and kidney transplantation have identified putative mechanisms that contribute to the development of chronic rejection (14, 15). In these instances, an emerging paradigm is that inflammation and associated tissue remodeling attendant to the post-transplant state exposes cryptic self-antigens or their determinants that, along with a favorable cytokine milieu, allows for loss of peripheral tolerance and activation of cell-mediated immunity towards development of de novo immune responses to self antigens (16). Previous studies have shown a higher prevalence of auto-Abs to nuclear, smooth muscle and anti-mitochondrial antigens in patients with chronic HCV infection (17, 18). In addition, the presence of circulating Abs to ECM collagen has been well established in liver disease (19). However the clinical implications of their occurrence, in particular their relation to HCV induced fibrosis of the native and allograft liver and their functional significance in the induction of fibrosis needs better characterization.
In this investigation we postulated that chronic HCV infection resulting in liver parenchymal damage and progressive ECM remodeling may lead to the induction of an immune response to ECM proteins. This was assessed by determining Abs to ECM collagen (subtypes 1, 2, 4 and 5) and vimentin at various stages of HCV infection in the native liver and in the allograft post OLT. The study group was also examined for the presence of classic autoimmune markers such as anti-nuclear, anti-smooth muscle, anti-mitochondrial Abs, rheumatoid factor and cryoglobulins. In addition, we measured serum levels of pro-inflammatory cytokines (IL-17, IL-6, IL 1β, IFN-γ, IL-4, IL-5, and IL-10) that have previously been implicated in the pathogenesis of liver fibrosis in chronic HCV infection (20, 21). Our results demonstrate that Abs to self antigen ECM collagen positively correlate with stage of liver fibrosis as well as a Th2 and Th17 cytokine milieu both in non OLT and post-OLT HCV patients.
PATIENTS AND METHODS
HCV patient population
Patients followed in the Division of Gastroenterology and the Liver Transplant Program at Washington University School of Medicine and Barnes-Jewish Hospital, St Louis, Missouri, over a 4-year period (April, 2005 to July, 2009) were consecutively enrolled for study upon presentation for liver biopsy. Patients had documented HCV infection, confirmed by HCV RNA quantitative polymerase chain reaction assays with a lower detection limit of 50 IU/ml (Cobas Amplicor, Roche Diagnostics). Patients <18 years old, previously treated with anti-viral therapy within 24 weeks of enrollment, with other active liver disease (including hepatitis B, autoimmune hepatitis, biliary cirrhosis, sclerosing cholangitis, steatohepatitis or alcoholic liver disease) or with documented HIV infection were excluded.
Demographic, clinical and laboratory data were extracted from the medical record, using values obtained within 1 month of liver biopsy. In addition, for OLT recipients, aminotransferase and bilirubin level at day 7 post transplant was collected. Liver biopsies were performed in non-transplant patients for evaluation of histologic severity prior to consideration of antiviral therapy. In post OLT patients, liver biopsies were obtained in the evaluation of abnormal liver tests or as part of protocol biopsies 1 year following OLT. OLT patients were maintained on stable doses of immunosuppression and had no episodes of acute cellular rejection within 6 months of study entry. Liver biopsies were scored for fibrosis using the Scheuer staging system [Stage 0: No Fibrosis-none; 1: Mild Fibrosis-enlarged, fibrotic portal tracts; 2: Moderate Fibrosis-periportal or portal-portal septa, but intact architecture; 3: Severe Fibrosis-fibrosis with architectural distortion but no obvious cirrhosis; and 4: Cirrhosis-probable or definite cirrhosis](22). Patients for which a fibrosis score was not available were excluded from the study. All patients non OLT and post OLT were classified into two groups based on fibrosis stage: No Fibrosis (Stage 0–2) and Fibrosis (Stage 3–4)
Blood samples were obtained at the time of liver biopsy. Serum was stored at −70°C. Serum samples from 25 normal healthy subjects with no known pre-existing liver or autoimmune disease was obtained at the time of blood donation at the HLA laboratory and stored. Informed consent was obtained from all subjects. No donor organs were obtained from executed prisoners or other institutionalized persons. This protocol was approved by the Human Research Protection Office (institutional review board) of Washington University School of Medicine, St Louis, Missouri.
Enzyme Linked Immunosorbent Assay (ELISA)
We used a standardized ELISA test for detecting human Abs to Col-1, 2, 4, 5 and vimentin as described previously (14, 15, 23, 24). In brief, Col and vimentin proteins were commercially obtained. Ninety-six well ELISA plates were coated with 100ng/100 μl of human Col 1 (Cell Sciences, Canton, MA), 50ng/100 μl of human Col 2, 2ng/100 μl of human Col 4 (Biodesign International, Saco, ME) 200ng/100 μl of human Col 5 (Sigma, St Louis, MO) and 200ng/100μl of vimentin (Sigma, St. Louis, MO). The plates were kept at 4°C overnight and blocked with 1% BSA for 2hrs at 37°C. A minimum of two dilutions of serum for each auto-Ab were used: 1/50 and 1/75 for Col 4, 1/100 and 1/200 for Col 1 and 2, 1/500 and 1/750 for Col 5 and 1/500 and 1/1000 for vimentin. Plates were incubated overnight at 4°C and washed. The secondary peroxidase conjugated goat anti-human IgA+IgM+IgG (Jackson immuno research, West grove, PA) were added the following day and incubated for 1 hr at room temperature. The plates were washed again and developed using 100μl/well of TMB substrate (Millipore, Temecula, CA). The reactions were stopped by 2N HCl and plates were read using ELISA plate reader (μ Quant, Bio-TEK instrument Inc, Winooski, VT) at 450 nm wave length. Concentration of Abs was determined based on a standard curve using the binding of known concentration of standard monoclonal mouse anti Col 4 and anti vimentin, rabbit anti Col 1 and 5 (Abcam, Cambridge, MA) and goat anti Col 2 (Santa Cruz Biotechnology Inc. CA) Abs. The results were normalized and expressed in μg/mL. Positive cut off for the level of Abs was set as +2 standard deviations of the mean obtained in normal healthy subjects. This was determined to be 14ng/mL for anti Col I, 2ng/mL for anti Col 2, 0.5ng/mL for Col 4, 160ng/mL for anti Col 5 and 35ng/mL for anti vimentin.
Luminex Assay
Serum cytokine and chemokine profile of the study cohort was analyzed by measuring concentration of cytokines (IL-1β, IL-1rα, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-13, IL-15, IL-17, TNF-α, IFN-α, IFN-γ, GM-CSF) and chemokines (MIP-1c, MIP-1β, IP-10, MIG, Eotaxin, RANTES, MCP-1)using the 25-plex Human Multiplex Bead immunoassay (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions as described (21). The Luminex xMAP™ was used to read 96 well plates (Fisher, Pittsburgh, PA) and determine the mean fluorescence intensity (MFI) of experimental and standard wells and concentrations were calculated by the standard curves.
Statistical Analysis
All analysis was performed using GraphPad Prism v5.0b (La Jolla, CA), SPSS v17 (Chicago, IL) and SYSTAT V11.0 (SYSTAT, Richmond, VA). Data was checked for normality using Schapiro-Wilks test and non-normal data was log transformed. Clinical demographics were compared using the Kruskal Wallis test with post-hoc Dunn’s multiple comparison. Mann-Whitney U test was used to determine differences in antibody and cytokine levels between groups. Correlation analyses were performed using the Spearman rank test. Two by two tables were analyzed with Fischer T tests. Relative risk with confidence intervals, specificity and sensitivity of presence of Abs and fibrosis was determined from contingency tables. Two sided level of significance was set at p<0.05. Results were expressed as mean (SEM).
RESULTS
Serum from HCV patients (n=70) [Non OLT no fibrosis (n=25); Non OLT fibrosis (n=25), Post OLT no fibrosis (n=10) and Post OLT fibrosis (n=10)] and 25 normal healthy controls were collected. No significant demographic difference was noted between non fibrotic or fibrotic groups non-OLT or post OLT with regards to: age, gender, race, HCV genotype, aminotransferase level, or viral load (Table 1 and Table 2). In the post OLT population with HCV recurrence time to biopsy from date of OLT was not significantly different among the non-fibrotic versus the fibrotic group (9.1 +/− 1.4 months vs 9.8 +/− .76, p=0.68). In addition, there was no difference in aminotransferase and bilirubin level 1 week post OLT, type of immunosuppression, incidence of biliary complication necessitating endoscopic or percutaneous cholangiogram, or episodes of acute rejection (Table 2).
Table 1.
Clinico-demographic profile of Chronic HCV patients with and without fibrosis of the native liver
| Demographic | No Fibrosis (Stage 0–2) (n=25) | Fibrosis (Stage 3–4) (n=25) | p value |
|---|---|---|---|
|
| |||
| Age (years)* | 49.5 (1.86) | 52.56(1.14) | 0.25 |
|
| |||
| Male: Female (n) | 60:40% (15:10) | 84:16% (21:4) | 0.14 |
|
| |||
| Race | |||
| Caucasian (n) | 72% (18) | 76% (19) | 0.81 |
| African American (n) | 28% (7) | 24% (6) | |
|
| |||
| BMI (kg/m2)* | 27.8(0.8) | 28.9(0.9) | 0.056 |
|
| |||
| Viral Genotype (n) | |||
| 1 | 80%(20) | 76%(19) | 1 |
| 3 | 4%(1) | ||
| Unknown | 20%(5) | 20%(5) | |
|
| |||
| Viral Load (in 106 IU/mL)* | 2.35 (0.529) | 2.51(0.585) | 0.95 |
|
| |||
| Bilirubin (mg/dL)* | 1.0(0.2) | 0.9(0.3) | 0.67 |
|
| |||
| AST (IU/mL)* | 81.92(16.78) | 97.54(11.36) | 0.45 |
|
| |||
| ALT (IU/mL)* | 110 (17.71) | 135.5(19.01) | 0.12 |
|
| |||
| ANA positive (n) | 8%(2) | 8%(2) | 0.432 |
|
| |||
| Anti Smooth Muscle positive(n) | 4%(1) | 4%(1) | 0.39 |
|
| |||
| Antimitochondrial Positive (n) | 0%(0) | 4%(1) | 0.213 |
|
| |||
| Cryoglobulin present (n) | 0%(0) | 4%(1) | 0.213 |
|
| |||
| Rheumatoid Factor positive (n) | 8%(2) | 4%(1) | 0.45 |
represented as mean(SEM)
Table 2.
Clinico-demographic profile among patients with and without allograft fibrosis following OLT with HCV recurrence
| Demographic | No Fibrosis (Stage 0–2) (n=10) | Fibrosis (Stage 3–4) (n=10) | p value |
|---|---|---|---|
|
| |||
| Age (years)* | 54.1 (1.46) | 51.4 (2.51) | 0.19 |
|
| |||
| Male: Female (n) | 60:40% (6:4) | 60:40% (6:4) | 1 |
|
| |||
| Race | |||
| Caucasian (n) | 80% (8) | 90% (9) | 0.68 |
| African American (n) | 10% (1) | 10% (1) | |
| Other (n) | 10% (1) | 0 | |
|
| |||
| BMI (kg/m2)* | 28.07(0.88) | 28.7(2.1) | 0.774 |
|
| |||
| Viral Genotype (n) | |||
| 1 | 60%(6) | 100%(10) | 1.25 |
| 3 | 10%(1) | ||
| Unknown | 30%(3) | ||
|
| |||
| Viral Load (in 106 IU/mL)* | 3.36 (1.25) | 2.58 (1.06) | 0.64 |
|
| |||
| Time to Biopsy after OLT (months)* | 9.13(1.4) | 9.8(0.76) | 0.68 |
|
| |||
| Bilirubin (mg/dL)* | 1.2(0.3) | 1.3(0.2) | 0.54 |
|
| |||
| AST (IU/mL)* | 71.5(14.5) | 76.7(18.6) | 0.83 |
|
| |||
| ALT (IU/mL)* | 132.7(32.7) | 99.7(21.6) | 0.4 |
|
| |||
| ANA positive (n) | 10%(1) | 10%(1) | 0.137 |
|
| |||
| Anti Smooth Muscle positive(n) | 10%(1) | 10%(1) | 0.137 |
|
| |||
| Antimitochondrial Positive (n) | 10%(1) | 10%(1) | 0.137 |
|
| |||
| Cryoglobulin present (n) | 0 | 10%(1) | 0.169 |
|
| |||
| Rheumatoid Factor positive (n) | 10%(1) | 0 | 0.169 |
|
| |||
| Donor Age (years)* | 42.5(3.01) | 38.3(5.2) | 0.501 |
|
| |||
| First week Bilirubin (mg/dL)* | 2.9(0.4) | 3(1.1) | 0.685 |
|
| |||
| First week AST (IU/mL)* | 86.5(9.98) | 66.32(17.66) | 0.355 |
|
| |||
| First week ALT (IU/mL)* | 326.1(38.5) | 226(87.1) | 0.29 |
|
| |||
| First week ALP (IU/mL)* | 171.6(17.1) | 98.5(16.6) | 0.07 |
|
| |||
| Immunosuppression (n) | |||
| Tacrolimus | 50%(5) | 30%(3) | 0.314 |
| Sirolimus | 10%(1) | 10%(1) | |
| Tacrolimus + MMF | 20%(2) | 40%(4) | |
| Cyclosporine | 10%(1) | 10%(1) | |
| Cyclosporine + MMF | 10%(1) | 10%(1) | |
|
| |||
| Rejection episodes | 1 | 1 | 0.137 |
|
| |||
| Post transplant biliary obstruction (n) | 1 | 1 | 0.137 |
values represented as mean(SEM)
Among non OLT patients the overall prevalence of antinuclear, anti smooth muscle, antimitochondrial, cryoglobulins, and rheumatoid factor was 8%, 4%, 2%, 2% and 7.5%, respectively. There was no difference in the prevalence of these between the fibrotic and non fibrotic groups (Table 1). In post OLT patients, the overall prevelance of antinuclear, anti-smooth muscle, anti-mitochondrial, cryoglobulin and rheumatoid factor was 10%, 10%, 10%, 5% and 5%, respectively. The prevalence of these between the fibrotic and non fibrotic groups did not differ (Table 2). The control group consisted of 25 healthy subjects with a mean age of 45 ± 12.4 years and a male:female ratio of 15:10. They had no known pre-existing history of liver or autoimmune disease and were HCV, HBV and HIV negative.
Development of Abs against Col-1, 2, 4, 5 and vimentin in patients with HCV infection of
Native Liver
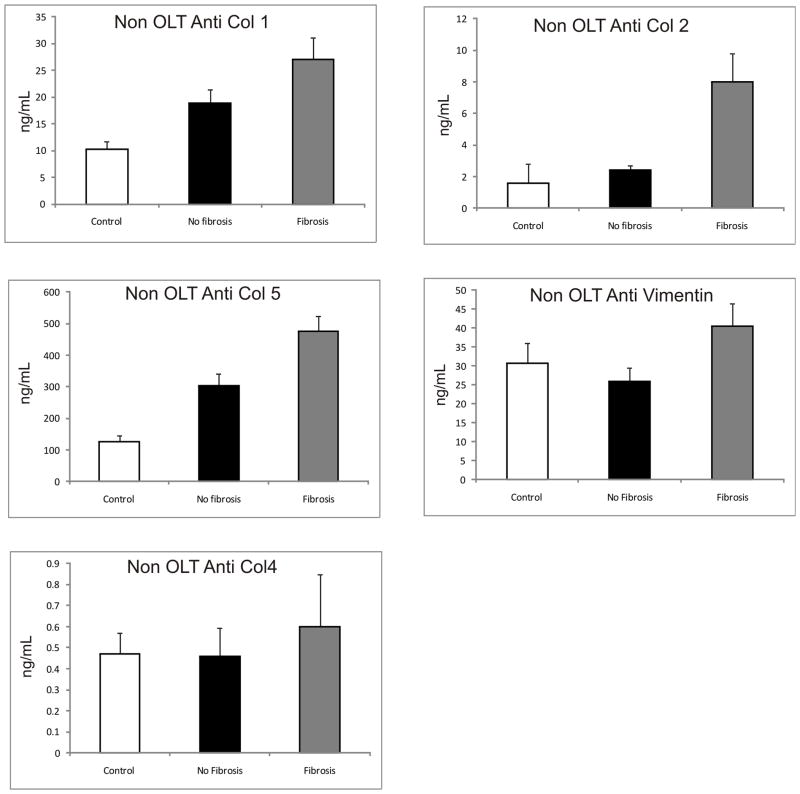
In order to determine whether Abs against ECM are developed during chronic HCV infection in the native liver, we analyzed serum samples from 25 HCV+ non-OLT subjects with no fibrosis (Scheuer 0–2), 25 HCV+ non-OLT with fibrosis (Scheuer 3–4), and 25 healthy controls (Figure 1). Subjects in the fibrosis group had significantly higher Abs to Col 1, 2, 5 compared to the non-fibrotic and control groups (expressed as mean(SEM) ng/mL – Anti Col1: 27(4.1) vs 18.8(1.6) vs 10.2(0.5), p<0.001; anti Col2: 8(1.8) vs 2.4(0.3) vs 1.6(0.2), p<0.01; anti Col5: 474.9(48.4) vs 302.8(37.4) vs 124.5(20), p<0.05. Abs to Col4 and vimentin were elevated in the fibrosis group but this did not reach statistical significance (in mean (SEM) ng/mL: anti Col4 – 0.6(0.25) vs 0.46(0.135) vs 0.47(0.1), p=0.122, anti vimentin – 40.5(6.03) vs 25.8(3.6) vs 30.7(5.35), p=0.36). In addition, anti Col 1, 2 and 5 Abs positively correlated with increasing fibrosis stage (expressed as Spearman rho(significance); Anti Col 1 – 0.58(0.05); anti Col 2 – 0.29(0.013); anti Col 5 – 0.712(0.037). Abs to Col 4 and vimentin did not show significant correlation to fibrosis (Spearman rho(significance); anti Col4 – 0.035(0.732), anti vimentin – 0.062(0.53).
Figure 1. Increased Ab levels to ECM Col-1, 2 and 5 in Non OLT Patients with Advanced Fibrosis.
25 HCV+ non OLT subjects with no fibrosis (Scheuer 0–2), 25 HCV+ non OLT with fibrosis (Scheuer 3–4), and 25 healthy controls were compared. Col 1 Ab levels in the Fibrosis group (27(4.1)) were significantly higher than in the no fibrosis group (18.8(1.6)) and healthy controls (12.2(0.5)) (p<0.0001). A similar trend was seen for Col 2: fibrosis group (8(1.8)), no fibrosis group (2.4(0.3)) and controls (1.6(0.2)) (p=0.0001) and Col 5: fibrosis group (474.9(48.4)), no fibrosis (302.8(37.4)) and controls (124.5(20)) (p<0.0001). There was no difference in the antibodies to Col 4 and vimentin among the three groups (Values expressed as mean (SEM) in ng/mL)
Development of Abs against Col-1, 2, 4 and 5 in patients with HCV recurrence in the allograft
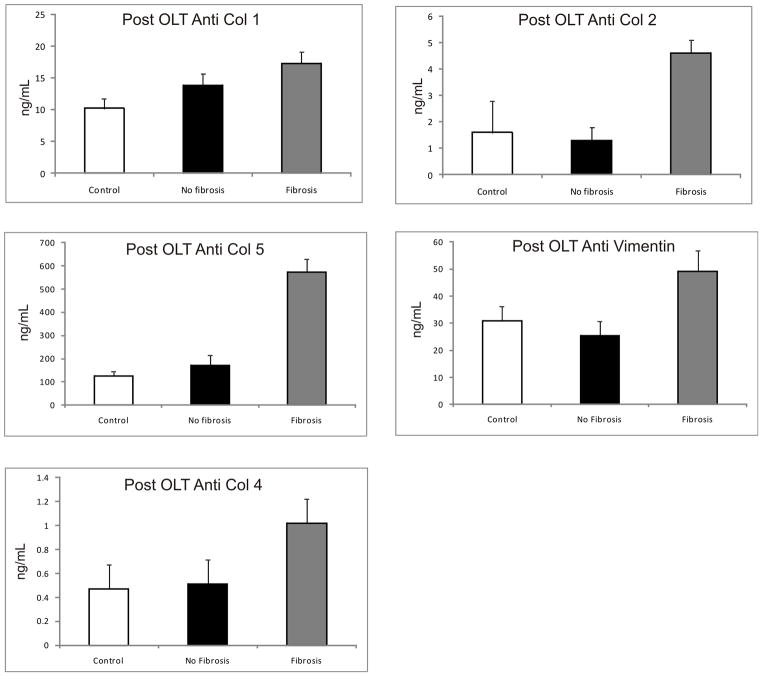
In order to determine whether Abs against ECM are developed during HCV recurrence in the allograft post OLT, we analyzed serum samples from 10 HCV+ Post-OLT subjects with no fibrosis (Scheuer 0–2), 10 HCV+ Post-OLT with fibrosis (Scheuer 3–4), and compared with 25 healthy controls (Figure 2). Post-OLT subjects with fibrosis had significantly higher abs to Col1, 2 and 5 in comparison to those without fibrosis and controls (in mean(SEM) ng/mL: anti Col1 – 17.3(1.8) vs 13.8(1.8) vs 10.2(0.5), p<0.05; anti Col2 – 4.6(1.5) vs 1.3(0.5) vs 1.6(0.2), p=0.05; anti Col5 – 573.5(56.3) vs 169.9(44.5) vs 124.5(20), p<0.01). Abs to Col 4 and vimentin were elevated in the post OLT group with fibrosis but again did not reach statistical significance (in mean(SEM) ng/mL - anti Col4: 1.02(0.3) vs 0.51(0.24) vs 0.47(0.1), p = 0.19, anti-vimentin: 49.17(7.71) vs 25.38(5.3) vs 30.76(5.35), p =0.24). The presence of abs to Col1, Col2 and Col5 positively correlated with the extent of allograft fibrosis post OLT (expressed as Spearman rho(significance): Anti Col1 0.353(0.05), Anti Col2 0.317(0.032); anti Col5 0.059(<0.01)). Abs to Col 4 and vimentin however did not correlate with fibrosis (Spearman rho(significance); anti Col4: 0.243(0.09), anti vimentin – 0.259(0.082)).
Figure 2. Increased Ab levels to ECM Col-1, 2 and 5 in Post OLT Patients with Advanced Fibrosis.
10 HCV+ Post OLT subjects with no fibrosis (Scheuer 0–2), 10 HCV+ Post OLT with fibrosis (Scheuer 3–4), and compared with 25 healthy controls Col 1 Ab levels in the Fibrosis group (17.3(1.8)) were significantly higher than in the no fibrosis group (13.8(1.8)) and healthy controls (10.2(5)) (p=0.01). A similar trend was seen Col 2 Ab: fibrosis group (4.6(1.5)), no fibrosis group (1.3(0.5)) and controls (1.6(.2)) (p=0.006). Col 5 Ab: fibrosis group (573.5(56.3)) no fibrosis (169.9(44.5)) and controls (124.5(20)) (p<0.0001). There were no difference in the antibodies to Col4 and vimentin among the three groups. (Values expressed as mean(SEM) in ng/mL)
In order to evaluate the relative risk, specificity and sensitivity of anti-Col Abs in predicting fibrosis, we plotted two by two tables classifying presence or absence of Abs based on cut offs obtained from normal healthy individuals. Among the non OLT, we found that there was an increased risk of development of fibrosis with the development of anti-Col 1 (relative risk - 7.94, 95% CI – 3.56 – 15.54, P value < 0.001, Specificity 92%, Sensitivity 88%), anti Col 2 (Relative Risk – 4.69, 95% CI – 2.44 – 7.93, P value <0.001, Specificity 88%, Sensitivity 80%), and anti Col 5 Abs (Relative Risk – 8.09, 95% CI – 4.02 – 12.23, P value <0.001, Specificity 96%, Sensitivity 88%) (Table 3A). In the post OLT group as well, we found a increased risk of fibrosis with the development of anti Col 1(Relative Risk – 4.5, 95% CI – 1.69 – 8.15, P value – 0.002, Specificity 80%, Sensitivity 90%), anti Col 2 (Relative Risk – 4.0, 95% CI – 1.4 – 11.39, P value – 0.007, Specificity 80%, Sensitivity 80%) and anti Col 5 (Relative risk – 6.0, 95% CI – 1.48 – 34.0, P value – 0.006, Specificity 70%, Sensitivity 90%) Abs (Table 3B).
Table 3.
Two by two contingency tables to determine the relative risk, sensitivity and specificity of Abs to Col and presence of Fibrosis in non OLT (3A) and Post- OLT(3B) HCV patients. Non-OLT and Post- OLT HCV patients with Abs to Col 1, 2 and 5 have an increased risk of fibrosis. (percentages are based on column totals).
| A) i. | |||
|---|---|---|---|
| Fibrosis (Stage 3–4) | No Fibrosis (Stage 0–2) | Total | |
| Anti Col 1+ | 22 (88%) | 2 (8%) | 24 (48%) |
| Anti Col 1 − | 3 (12%) | 23 (92%) | 26 (52%) |
| Total | 25 (100%) | 25 (100%) | 50 (100%) |
| A) ii | |||
|---|---|---|---|
| Fibrosis (Stage 3–4) | No Fibrosis (Stage 0–2) | Total | |
| Anti Col 2+ | 20 (80%) | 3 (12%) | 23 (46%) |
| Anti Col 2 − | 5 (20%) | 22 (88%) | 27 (54%) |
| Total | 25 (100%) | 25 (100%) | 50 (100%) |
| A) iii | |||
|---|---|---|---|
| Fibrosis (Stage 3–4) | No Fibrosis (Stage 0–2) | Total | |
| Anti Col 5+ | 20 (80%) | 3 (12%) | 23 (46%) |
| Anti Col 5 − | 5 (20%) | 22 (88%) | 27 (54%) |
| Total | 25 (100%) | 25 (100%) | 50 (100%) |
| B) i | |||
|---|---|---|---|
| Fibrosis (Stage 3–4) | No Fibrosis (Stage 0–2) | Total | |
| Anti Col 1+ | 9 (90%) | 2 (20%) | 11 (55%) |
| Anti Col 1 − | 1 (10%) | 8 (80%) | 9 (45%) |
| Total | 10 (100%) | 10 (100%) | 20 (100%) |
| B) ii | |||
|---|---|---|---|
| Fibrosis (Stage 3–4) | No Fibrosis (Stage 0–2) | Total | |
| Anti Col 2+ | 8 (80%) | 2 (20%) | 10 (50%) |
| Anti Col 1 − | 2 (20%) | 8 (80%) | 10 (50%) |
| Total | 10 (100%) | 10 (100%) | 20 (100%) |
| B) iii | |||
|---|---|---|---|
| Fibrosis (Stage 3–4) | No Fibrosis (Stage 0–2) | Total | |
| Anti Col 5+ | 9 (90%) | 3 (30%) | 12 (60%) |
| Anti Col 5 − | 1 (10%) | 7 (70%) | 8 (40%) |
| Total | 10 (100%) | 10 (100%) | 20 (100%) |
Relative risk – 7.94, 95% CI – 3.56 – 15.54, P value < 0.001, Specificity 92%, Sensitivity 88%
Relative Risk – 4.69, 95% CI – 2.44 – 7.93, P value <0.001, Specificity 88%, Sensitivity 80%
Relative Risk – 8.09, 95% CI – 4.02 – 12.23, P value <0.001, Specificity 96%, Sensitivity 88%
Relative Risk – 4.5, 95% CI – 1.69 – 8.15, P value – 0.002, Specificity 80%, Sensitivity 90%
Relative Risk – 4.0, 95% CI – 1.4 – 11.39, P value – 0.007, Specificity 80%, Sensitivity 80%
Relative risk – 6.0, 95% CI – 1.48 – 34.01, P value – 0.006, Specificity 70%, Sensitivity 90%
Analysis of circulating cytokine profile in non OLT and post OLT HCV patients
In order to determine serum cytokine levels, we performed solid-phase sandwich Multiplex Bead immunoassays to measure 25 different cytokine and chemokines in the study cohort. The Post OLT group and 10 randomly selected Non-OLT no fibrosis and Non-OLT Fibrosis were analyzed [Table 4A and 4B]. There was a significantly higher level of Th1 cytokine (IFN-γ) in patients without fibrosis compared to those with fibrosis both non-OLT (expressed as mean(SEM) in pg/mL: 74(4.89) vs 42.4(7.01), p=0.01) and post OLT (90.3(6.2) vs 50.2(8.1) pg/mL, p=0.04). In contrast, the fibrotic groups demonstrated increased Th2(IL-10, IL-4) and Th-17(IL-17) cytokines non-OLT (in mean(SEM) pg/mL; IL-10 - 48.2(10.11) vs 28.4(4.29) p=0.033; IL-4 - 112.4(9.87) vs 88.2(6.4), p=0.05; IL-17 - 87.2(3.02) vs 68.4(8.23), p=0.041) and post OLT (in mean(SEM) pg/mL; IL-10 – 62.4(7.1) vs 38.2(4.2), p=0.05; IL-4 – 99.9(7.9) vs 76.3(10.4), p=0.05; IL-17 – 76.3(3.2) vs 36.1(4.2), p = 0.03). The fibrotic groups also demonstrated increased levels of pro-Th17 cytokines –IL-6, IL-1β and IL-5 (Table 4A, 4B). IL-1RA, IL-2, IL-2R, IL-7, IL-8, IL-13, IL-12, IL-15, TNF-a, IFN-a, GM-CSF) and chemokines (MIP-1c, MIP-1 b, IP-10, MIG, Eotaxin, RANTES, MCP-1) were not significantly different between controls and non OLT and post OLT patients with and without fibrosis.
Table 4A.
Serum Cytokine concentrations in Chronic HCV patients with and without fibrosis of the native liver
| Cytokine | No Fibrosis (Stage 0–2) (n=10) | Fibrosis (Stage 3–4) (n=10) | p value |
|---|---|---|---|
| IL-17 (pg/ml) | 68.4(8.23) | 87.2(3.02) | 0.041 |
| IL-6 (pg/ml) | 44.2(2.05) | 66.4(1.33) | 0.049 |
| IL-1β (pg/ml) | 76.8(14.11) | 126(16.14) | 0.009 |
| IFN-γ (pg/ml) | 74 (4.89) | 42.4(7.01) | 0.01 |
| IL-5 (pg/ml) | 45.4(6.22) | 93.2(10.47) | 0.032 |
| IL-10 (pg/ml) | 28.4(4.29) | 48.2(10.11) | 0.033 |
| IL-4 (pg/ml) | 88.2(6.4) | 112.4(9.87) | 0.05 |
Values represented as mean (SEM)
Table 4B.
Serum Cytokine concentrations in patients with and without allograft fibrosis following OLT with HCV recurrence
| Cytokine | No Fibrosis (Stage 0–2) (n=10) | Fibrosis (Stage 3–4) (n=10) | p value |
|---|---|---|---|
| IL-17 (pg/ml) | 36.1(4.2) | 76.3(3.2) | 0.03 |
| IL-6 (pg/ml) | 43.2(3.1) | 75.4(4.1) | 0.02 |
| IL-1β (pg/ml) | 85.9(1.9) | 110.1(4.1) | 0.03 |
| IFN-γ (pg/ml) | 90.3(6.2) | 50.2(8.1) | 0.01 |
| IL-5 (pg/ml) | 75(3.2) | 83.1(2.2) | 0.04 |
| IL-10 (pg/ml) | 38.2(4.2) | 62.4(7.1) | 0.05 |
| IL-4 (pg/ml) | 76.3(10.4) | 99.9(7.9) | 0.05 |
Values represented as mean (SEM)
The serum level of IFN-γ negatively correlated both with the grade of fibrosis and with ab to Col 5 both non-OLT (Spearman rho(significance); Fibrosis: −0.382(0.032), anti Col 5: −0.63(0.02)) and post OLT (Spearman rho(significance); Fibrosis: −0.43(0.022), anti Col 5: −0.39(0.04)). Serum levels of IL-10 and IL-17 positively correlated with grade of fibrosis and ab to Col 5 both non-OLT (Spearman rho(significance); IL-10-Fibrosis: 0.55(0.023), IL-10 Anti Col 5: 0.48(0.029); IL-17- Fibrosis: 0.51(0.01), IL-17 Anti Col 5: 0.366(0.047)) and post OLT (Spearman rho(significance); IL-10 Fibrosis: 0.49 (0.04), IL-10 Anti Col 5: 0.36 (0.05); IL-17- Fibrosis 0.67(0.03), IL-17 Anti Col 5: 0.42 (0.05)).
DISCUSSION
Chronic HCV infection has been associated with the development of auto-Abs including cryoglobulins (25), rheumatoid factor (26), anti-cardiolipin (27), anti-nuclear (28, 29), anti-smooth muscle (29) and anti-liver-kidney microsome 1 (28). Recent studies have provided evidence for the role of circulating Abs to self antigens in the development of hepatic inflammation and subsequent fibrosis during chronic HCV infection (30, 31). With disease progression, HCV induced inflammation can lead to necroinflammatory damage and the development of fibrosis. Such an inflammatory milieu can lead to the exposure and release of sequestered antigens or their antigenic determinants from the ECM. Previous studies from our laboratory have shown that Abs to self antigens developed following human lung transplantation can induce secretion of fibrogenic growth factors, cytokines, chemokines and matrix metalloproteinases following binding with their cognate antigens expressed on epithelial and endothelial surfaces (32, 33). T cell mediated immune responses to self antigens such as to cardiac myosin (34) heat shock protein 60 (35) and Col-5 (23) have been shown to lead to fibrosis following heart, kidney and lung transplantation respectively. Based on these findings we hypothesized that the HCV infection leads to the development of Abs to ECM antigens both in non OLT and post OLT patients and may play a role in the development of liver fibrosis.
In the present study, we demonstrate that HCV infection is associated with the development of Abs to Col-1, 2 and 5 in both non- and post-OLT patients. Although we did detect Abs to other ECM proteins such as Col 4 and vimentin, we did not find a significant difference between histologic groups. Interestingly, we also found that both non-OLT and post-OLT subjects had a low prevalence of other classical autoimmune markers such as antinuclear, antimitochondrial, antismooth muscle, cryoglobulins, rheumatoid factor, and these did not differ between the groups. This suggests that the development of Abs to Col is not part of a global autoimmune response and but rather a more specific to response related to liver fibrosis. While post-OLT patients demonstrated increased levels of Abs to Col-1, 2 and 5 when compared to normal controls, these were generally at overall lower levels when compared with non- OLT patient. This is possibly due to the replacement of fibrotic liver with a healthy allograft, and also possibly due to immunosuppression during the post-OLT period. In cirrhotic livers, higher levels of Col-1 are found in fibrotic septa while Col-5 is seen in the walls of proliferating bile ductules, vessels and along periportal sinusoids (36, 37). Col- 5 is thought to play a role in anchoring parenchymal Col- 4 and fibrous septa Col-1. Therefore, Ab mediated responses against ECM self antigens, particularly Col, may play a role in the development of fibrosis in chronic HCV patients.
Studies from our lab have shown that both non OLT and post OLT patients who develop fibrosis display a predominant Th2 (20, 21) and Th-17 response (accepted manuscript – Am J Transplant 2011) with an increase in IL-10, IL-4, IL-5, IL-1β and IL-6. In contrast patients who demonstrate an HCV specific Th1 (IFN-γ) response are resistant to fibrosis (20, 21). In addition, IL-17 has been shown to be a critical inflammatory mediator facilitating the development of germinal center B cells involved in the production of Abs to self antigens (38). Autoreactive B cells infiltrating the liver (39) and Th17 cells (40) have been shown to be a significant source of the pro-fibrotic cytokine IL-6, which is involved in differentiation of hepatic stellate cells to myofibroblasts, induction of fibroblast proliferation, and increased synthesis of ECM collagen and tissue inhibitors of metalloproteinases. Studies have also shown that patients with active chronic HCV have increased serum IL-4, IL-5, IL-6, IL-10 and IL-1β (41–44).
In this study the occurrence of Abs to ECM collagen positively correlated both with the stage of fibrosis on biopsy and an increased Th2 (IL-4, IL-5, IL-10), Th17 (IL-17) and pro-Th-17(IL-1β, IL-6) cytokine profile in the serum (Table 4A, 4B). In addition, abs to collagen negatively correlated with IFN-γ levels. These results support our hypothesis that IL-6, IL-4, IL-5 and IL-10 may augment humoral responses to Col. IL-6 and IL-1β polarize a Th-17 response which may perpetuate the production of abs to self antigens. In post OLT patients, binding of abs to self antigens in the allograft can results in activation of Kupffer cells to release pro-fibrogenic growth factos such as TGF-β which has also shown to be responsible for ECM production and fibrosis (45). A limitation of our study is that given its cross sectional nature, the development of Abs cannot be temporally correlated with progression of fibrosis. In addition, although our study suggests a definite correlation between the detection of serum abs to Col and liver fibrosis, the exact role of these in the pathogenesis of fibrosis still remains unclear. Further studies to evaluate a mechanistic correlation between Th17 immunity, development of abs to ECM Col and fibrosis progress in HCV infection of the native liver and allograft are warranted.
In conclusion, chronic HCV infection leads to development of an immune response to self-antigens which may play a role in liver fibrosis in non-OLT and post OLT HCV patients. Elevated levels of proinflammatory mediators IL-17, IL-6, IL-4, IL-5, IL-10 and IL-1β may facilitate a breakdown of peripheral tolerance to various self antigens and specifically induce the development of pathogenic autoreactive Th17 cells which promote the development of Abs to ECM collagen. This strong correlation of abs to Col and liver fibrosis will provide the basis to conduct prospective longitudinal studies to validate if serial measurements of anti-Col ab titers can serve as an early, non invasive marker for fibrosis progression in HCV patients.
Acknowledgments
This publication was made possible by a grant from NIH/NIDDK DK065982 and the Barnes-Jewish Foundation (TM), and NRSA 5-T32-DK07301 (BB, AS) from the National Institutes of Health and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors have no financial interests to disclose. No writing assistance was obtained.
The authors would like to thank Ms. Billie Glasscock for help in the preparation of this manuscript.
Abbreviations
- HCV
Hepatitis C Virus
- HBV
Hepatitis B Virus
- OLT
Orthotopic Liver Transplantation
- ECM
Extracellular Matrix
- Col
Collagen Abs-Antibodies
- ANA
Antinuclear antibody
- HIV
Human Immunodeficiency Virus
- ELISA
Enzyme Linked Immunosorbent Assay
- Th
T helper
- BSA
bovine serum albumin
- TMB
Tetramethylbenzidine
- CI
Confidence interval
- BMI
Body mass index
- SEM
Standard Error of mean
Literature Cited
- 1.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90(10):1562–9. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992;103(1):317–22. doi: 10.1016/0016-5085(92)91129-r. [DOI] [PubMed] [Google Scholar]
- 3.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 4.Hissar SS, Kumar M, Tyagi P, Goyal A, Suneetha PV, Agarwal S, et al. Natural history of hepatic fibrosis progression in chronic hepatitis C virus infection in India. J Gastroenterol Hepatol. 2009;24(4):581–7. doi: 10.1111/j.1440-1746.2008.05649.x. [DOI] [PubMed] [Google Scholar]
- 5.Cross TJ, Quaglia A, Hughes S, Joshi D, Harrison PM. The impact of hepatic steatosis on the natural history of chronic hepatitis C infection. J Viral Hepat. 2009;16(7):492–9. doi: 10.1111/j.1365-2893.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 6.Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14 (Suppl 2):S36–44. doi: 10.1002/lt.21646. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Luna H, Vargas HE. Natural history of hepatitis C and outcomes following liver transplantation. Minerva Gastroenterol Dietol. 2004;50(1):51–9. [PubMed] [Google Scholar]
- 8.Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann BC, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110(1):167–77. doi: 10.1053/gast.1996.v110.pm8536853. [DOI] [PubMed] [Google Scholar]
- 9.Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology. 1995;21(1):30–4. [PubMed] [Google Scholar]
- 10.Charlton M, Wiesner R. Natural history and management of hepatitis C infection after liver transplantation. Semin Liver Dis. 2004;24 (Suppl 2):79–88. doi: 10.1055/s-2004-832932. [DOI] [PubMed] [Google Scholar]
- 11.Rauterberg J, Voss B, Pott G, Gerlach U. Connective tissue components of the normal and fibrotic liver. I. Structure, local distribution and metabolism of connective tissue components in the normal liver and changes in chronic liver diseases. Klin Wochenschr. 1981;59(14):767–79. doi: 10.1007/BF01724682. [DOI] [PubMed] [Google Scholar]
- 12.Seyer JM, Hutcheson ET, Kang AH. Collagen polymorphism in normal and cirrhotic human liver. J Clin Invest. 1977;59(2):241–8. doi: 10.1172/JCI108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giannelli G, Antonaci S. Immunological and molecular aspects of liver fibrosis in chronic hepatitis C virus infection. Histol Histopathol. 2005;20(3):939–44. doi: 10.14670/HH-20.939. [DOI] [PubMed] [Google Scholar]
- 14.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181(8):5738–47. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170(4):1415–27. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352(6):558–69. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 17.Zauli D, Cassani F, Bianchi FB. Auto-antibodies in hepatitis C. Biomed Pharmacother. 1999;53(5–6):234–41. doi: 10.1016/S0753-3322(99)80094-8. [DOI] [PubMed] [Google Scholar]
- 18.Luo JC, Hwang SJ, Li CP, Lu RH, Chan CY, Wu JC, et al. Clinical significance of serum auto-antibodies in Chinese patients with chronic hepatitis C: negative role of serum viral titre and genotype. J Gastroenterol Hepatol. 1998;13(5):475–9. doi: 10.1111/j.1440-1746.1998.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 19.Suou T, Hirayama C. Antibodies to denatured bovine collagens in sera of patients with liver disease. Clin Exp Immunol. 1980;39(1):119–24. [PMC free article] [PubMed] [Google Scholar]
- 20.Sreenarasimhaiah J, Jaramillo A, Crippin J, Lisker-Melman M, Chapman WC, Mohanakumar T. Lack of optimal T-cell reactivity against the hepatitis C virus is associated with the development of fibrosis/cirrhosis during chronic hepatitis. Hum Immunol. 2003;64(2):224–30. doi: 10.1016/s0198-8859(02)00781-4. [DOI] [PubMed] [Google Scholar]
- 21.Bharat A, Barros F, Narayanan K, Borg B, Lisker-Melman M, Shenoy S, et al. Characterization of virus-specific T-cell immunity in liver allograft recipients with HCV-induced cirrhosis. Am J Transplant. 2008;8(6):1214–20. doi: 10.1111/j.1600-6143.2008.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19(12):1409–17. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 90(4):1094–101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, et al. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. 29(11):1277–85. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonelli A, Ferri C, Ferrari SM, Colaci M, Fallahi P. Immunopathogenesis of HCV-related endocrine manifestations in chronic hepatitis and mixed cryoglobulinemia. Autoimmun Rev. 2008;8(1):18–23. doi: 10.1016/j.autrev.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Buskila D. Hepatitis C-associated rheumatic disorders. Rheum Dis Clin North Am. 2009;35(1):111–23. doi: 10.1016/j.rdc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Zachou K, Liaskos C, Christodoulou DK, Kardasi M, Papadamou G, Gatselis N, et al. Anti-cardiolipin antibodies in patients with chronic viral hepatitis are independent of beta2-glycoprotein I cofactor or features of antiphospholipid syndrome. Eur J Clin Invest. 2003;33(2):161–8. doi: 10.1046/j.1365-2362.2003.01110.x. [DOI] [PubMed] [Google Scholar]
- 28.Bai L, Feng ZR, Lu HY, Li WG, Yu M, Xu XY. Prevalence of antinuclear and anti-liver-kidney-microsome type-1 antibodies in patients with chronic hepatitis C in China. Chin Med J (Engl) 2009;122(1):5–9. [PubMed] [Google Scholar]
- 29.Williams MJ, Lawson A, Neal KR, Ryder SD, Irving WL. Autoantibodies in chronic hepatitis C virus infection and their association with disease profile. J Viral Hepat. 2009;16(5):325–31. doi: 10.1111/j.1365-2893.2008.01035.x. [DOI] [PubMed] [Google Scholar]
- 30.Chretien P, Chousterman M, Abd Alsamad I, Ozenne V, Rosa I, Barrault C, et al. Non-organ-specific autoantibodies in chronic hepatitis C patients: association with histological activity and fibrosis. J Autoimmun. 2009;32(3–4):201–5. doi: 10.1016/j.jaut.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh MY, Dai CY, Lee LP, Huang JF, Tsai WC, Hou NJ, et al. Antinuclear antibody is associated with a more advanced fibrosis and lower RNA levels of hepatitis C virus in patients with chronic hepatitis C. J Clin Pathol. 2008;61(3):333–7. doi: 10.1136/jcp.2006.046276. [DOI] [PubMed] [Google Scholar]
- 32.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309–18. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–94. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162(11):6836–42. [PubMed] [Google Scholar]
- 35.Caldas C, Luna E, Spadafora-Ferreira M, Porto G, Iwai LK, Oshiro SE, et al. Cellular autoreactivity against heat shock protein 60 in renal transplant patients: peripheral and graft-infiltrating responses. Clin Exp Immunol. 2006;146(1):66–75. doi: 10.1111/j.1365-2249.2006.03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takai KK, Hattori S, Irie S. Type V collagen distribution in liver is reconstructed in coculture system of hepatocytes and stellate cells; the possible functions of type V collagen in liver under normal and pathological conditions. Cell Struct Funct. 2001;26(5):289–302. doi: 10.1247/csf.26.289. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M, Sumiyoshi H, Nakagami K, Tahara E. Distribution of collagen types I, III, and V in fibrotic and neoplastic human liver. Acta Pathol Jpn. 1984;34(1):77–86. doi: 10.1111/j.1440-1827.1984.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 38.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9(2):166–75. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 39.Bhogal RK, Bona CA. B cells: no longer bystanders in liver fibrosis. J Clin Invest. 2005;115(11):2962–5. doi: 10.1172/JCI26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang SY, Kim JY, Kim KW, Park MK, Moon Y, Kim WU, et al. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 2004;6(2):R120–8. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bortolami M, Kotsafti A, Cardin R, Farinati F. Fas/FasL system, IL-1beta expression and apoptosis in chronic HBV and HCV liver disease. J Viral Hepat. 2008;15(7):515–22. doi: 10.1111/j.1365-2893.2008.00974.x. [DOI] [PubMed] [Google Scholar]
- 42.Oyanagi Y, Takahashi T, Matsui S, Takahashi S, Boku S, Takahashi K, et al. Enhanced expression of interleukin-6 in chronic hepatitis C. Liver. 1999;19(6):464–72. doi: 10.1111/j.1478-3231.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 43.Reiser M, Marousis CG, Nelson DR, Lauer G, Gonzalez-Peralta RP, Davis GL, et al. Serum interleukin 4 and interleukin 10 levels in patients with chronic hepatitis C virus infection. J Hepatol. 1997;26(3):471–8. doi: 10.1016/s0168-8278(97)80409-6. [DOI] [PubMed] [Google Scholar]
- 44.Wan L, Kung YJ, Lin YJ, Liao CC, Sheu JJ, Tsai Y, et al. Th1 and Th2 cytokines are elevated in HCV-infected SVR(−) patients treated with interferon-alpha. Biochem Biophys Res Commun. 2009;379(4):855–60. doi: 10.1016/j.bbrc.2008.12.114. [DOI] [PubMed] [Google Scholar]
- 45.Gangadharan B, Antrobus R, Dwek RA, Zitzmann N. Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clin Chem. 2007;53(10):1792–9. doi: 10.1373/clinchem.2007.089144. [DOI] [PubMed] [Google Scholar]