Abstract
Dendritic cells (DC) are the professional antigen-presenting cells of the immune system. In their quiescent and mature form, the presentation of self-antigens by DC leads to tolerance; whereas, antigen presentation by mature DC, after stimulation by pathogen-associated molecular patterns, leads to the onset of antigen-specific immunity. DC have been found in many of the major organs in mammals (e.g. skin, heart, lungs, intestines and spleen), while the brain has long been considered devoid of DC in the absence of neuroinflammation. Consequently, microglia, the resident immune cell of the brain, have been charged with many functional attributes commonly ascribed to DC. Recent evidence has challenged the notion that DC are either absent or minimal players in brain immune surveillance. This review will discuss the recent literature examining DC involvement within both the young and aged steady-state brain. We will also examine DC contributions during various forms of neuroinflammation resulting from neurodegenerative autoimmune disease, injury, and CNS infections. This review also touches upon DC trafficking between the central nervous system and peripheral immune compartments during viral infections, the new molecular technologies that could be employed to enhance our current understanding of brain DC ontogeny, and some potential therapeutic uses of DC within the CNS.
Keywords: Dendritic cells, Steady-state, Neuroinflammation, Pathogens
Introduction
Dating back to the first half of the twentieth century, the central nervous system (CNS) has historically been considered a site devoid of immunological surveillance in the steady-state, and is often referred to as an immunologically privileged site [17, 112]. Several observations have contributed to this “privileged” status. The most notable is the presence of a blood–brain barrier (BBB), a complex layer of astrocyte sheathed endothelial cells stitched together by a network of tight junctions [1, 13]. The network of tight junctions primarily functions to control the flux of soluble factors between the blood and neuronal tissue, while the glia limitans (astrocytic endfeet) are also responsible for limiting the entry of immune cells into the CNS parenchyma in the absence of inflammation [6]. Additional evidence in support of the concept of immunological privilege stems from a 1981 study, which demonstrated that while other tissues were rich in cells expressing major histocompatibility complex class II (MHC II+), none were detected in the CNS parenchyma of young animals [61]. Finally, the steady-state brain is also associated with a lack of lymphocytes and limited access to the lymphatic system, and as such has largely been considered a poor site for immune priming [8, 67, 130].
Over time, the explanation for immunological privilege has evolved from a CNS lacking immune surveillance to one that describes the active regulation of the BBB and the afferent arm of adaptive immunity [49]. More specifically, immunologically privileged CNS regions have been redefined to refer to the brain parenchyma located beyond the glia limitans. This new concept also acknowledges the presence of discretely located and tightly controlled portals and passages into and around the CNS parenchyma, which allow for trafficking and functional interactions of leukocytes. The most studied of these anatomic structures are the meninges and ventricles. However, the circumventricular organs (i.e., the area postrema, intermediate and neural lobes of the pituitary, the organum vasculosum of the lamina terminalis, subcommissural organ, and subfornical organ) and choroid plexus are also gaining recognition as sites associated with CNS immunological surveillance during the steady-state, and primary points of entry for activated leukocytes during neuroinflammation [5, 23, 40, 44, 86, 105, 138, 146, 156]. The circumventricular organs also serve as the nexus of immune–endocrine interaction (specifically the hypothalamic–pituitary–adrenal axis) following inflammation and pathogenic insult [22, 40].
Another pathway that has attained prominence follows along olfactory nerve projections due to their interface between the CNS and the external environment. For example, olfactory nerves project from the nasal mucosa through the cribriform plate and terminate in the olfactory bulb (located within the CNS). Lymphatic vessels, found throughout the submucosa of the nasal turbinates, form a cuff around these nerves that permits the collection of cerebral spinal fluid (CSF), and estimates show that at least 40–50 % of the CSF exits through this route in some species [87, 120]. Furthermore, both pathogens and cells have been shown to use this vulnerability both to gain access into and exit from the brain parenchyma [33, 37, 38, 53, 78, 162].
Among leukocytes, dendritic cells (DC) have been well documented as important functional components of the immune system both in the steady-state and during the innate and adaptive immune responses. These key cellular components of immunity are found within the meninges and circumventricular organs of both healthy and immunologically challenged mice, rats, and humans [60, 106, 109, 125, 147]. Thus, while the focus of this review is on the presence of DC within the CNS, their involvement in CNS immunity is still a relatively new field of study. Consequently, we will first briefly describe the roles of microglia and perivascular macrophages (PVM)—cells more traditionally associated with CNS immune responses.
CNS immune cells
Microglia
The CNS mononuclear phagocytic system is comprised mainly of a tissue resident population of macrophages known as microglia, characterized by high CD11b (integrin αM, a cell adhesion molecule that complexes with CD18 to form Mac-1/complement receptor 3) and intermediate expression of CD45 (member of the protein tyrosine phosphatase family found on all leukocytes). In the steady-state adult brain, microglia can range from 5 to 12 % of all CNS cells and are uniformly arranged independent of cell layers or blood vessel distribution throughout the parenchyma [93]. Similarly, microglia morphology varies considerably. White matter microglia have elongated somata and processes oriented preferentially along fiber tracts, microglia in the circumventricular organs exhibit compact morphology with few short processes, and in the gray matter microglia exhibit many elaborate radially oriented processes [93]. Although novel non-immunologic roles for microglia are being described (e.g. during steady-state homeostasis, synaptic plasticity, developmental and adult neurogenesis, learning and memory, and neurotoxicity prevention [143, 163]), little is known about the heterogeneity of microglia and the differences in functional capacities of individual microglial subpopulations among and within CNS regions [84]. Our knowledge gap is further amplified in pathological situations, where blood-derived monocytes/macrophages infiltrate the CNS. The extent of overlap and/or complementary functions between resident microglia and infiltrating mononuclear cells is currently being explored. What is well established is that microglia constitute the main arm of innate immune responses in the CNS [50]. Similar to peripheral macrophages, microglia exhibit various forms of activation [29] with distinct reactive microglial phenotypes corresponding to the background stimulus, i.e. infection, injury, neurodegeneration [29]. Classical activation is mediated by interferon gamma (IFN-γ), which promotes phagocytic and destructive functions. During infection, toll-like receptor (TLR) activation triggers a proinflammatory state, well characterized by expression of cytokines like tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-12, as well as chemokines, proteases, and redox proteins that aid in host defense [29]. Recently, the question of whether activated microglia participate in antigen presentation and the initiation and/or modulation of adaptive immune responses has become an active field of study.
Perivascular macrophages
PVM are myeloid cells found within the perivascular or Virchow-Robin space [129]. PVM juxtapose to cells of the tunica media, wrapping their processes around the vasculature and aid in the BBB formation [56, 68, 159]. PVM are continuously replaced by bone marrow (BM) progenitor cells during the steady-state [14, 15], but can accumulate during neuroinflammation [10, 91]. Aside from their anatomical location, PVM are similar in phenotype to other tissue macrophages, and their functions include phagocytosis of cellular and pathogenic debris, surveillance of the interstitial space via pinocytosis, and initiation of the CNS acute phase response through the production of prostaglandins [43, 85, 145, 148]. PVM, their origin, immunological function, and role in neurodegeneration have been reviewed extensively elsewhere [133, 168].
Dendritic cells
DC specialize in antigen capture, processing, and presentation to T lymphocytes to induce either tolerance or adaptive immunity [154]. DC represent distinct cell populations found in both lymphatic and non-lymphatic tissue, in which their ontogeny and functional roles are equally diverse. DC are currently believed to follow two developmental pathways, each originating from common hematopoietic stem cells. Monocyte/DC progenitor (MDP) sequentially gives rise to common DC progenitors, a precursor DC (pre-DC), and plasmacytoid DC (pDC) [16]. Pre-DC then egress into the circulation to populate most tissue, where they terminally differentiate into classical DC (cDC) [99]. cDC include CD8α+ DC in mice, termed “cross-presenting cells” due to functional specialization in presenting antigens from dead and dying cells and viral infections. Upon leaving the BM, pDC are widely distributed among all tissue, but are distinguished from cDC based on their morphological similarity to plasma cells, less effective antigen presentation ability, and rapid production of type I interferons after activation. An alternative DC developmental circuit occurring after the MDP stage involves monocytes. Under inflammatory conditions, monocytes undergo diapedesis and translocate into the afflicted tissue to differentiate into monocyte-derived DC or macrophages depending on the local cytokine milieu [98]. This heterogeneity in DC development is directly linked to DC function and results in a diverse array of transcriptional regulators and cell surface markers used in the identification and characterization of these cells [64].
The lack of detectible DC in the steady-state CNS was believed, by some, to be a consequence of immunological privilege. This concept was reinforced by studies that show when the BBB is compromised, such as during severe injury or inflammation, functional DC are identified within the parenchyma of the CNS [124]. Other hypotheses favor the existence of a “unique immune system” in the brain comprised of microglia, astrocytes, and PVM, which can respond to infection or brain trauma [68, 152, 170]. While microglia have been characterized for their morphological and cytokine responses following damage or infection in the CNS [136, 150], they have been shown to have poor antigen-presenting capacity, and thus do not fulfill the major requirement for an adaptive or maladaptive immune response [12, 26, 57]. Part of the problem in identifying immune competent antigen-presenting cells (APC) in the brain may lie in the misconception that microglia are a single population of cells in the steady-state. Several studies show that microglia are heterogeneous [24, 139], and subpopulations of these cells may serve as precursors for DC [47, 144].
DC in the steady-state CNS
In 1996, MHC II+ cells were observed in the meninges and choroid plexus of rodents [106]; however, at this time, DC were only poorly defined by phenotypic criteria and the precise nature of these cells was not well described. In humans, myeloid (CD11c+ HLA-DRhi, CD123lo) and plasmacytoid (CD11cneg HLA-DRmod, CD123hi) cells were observed in the CSF of patients with neurological disease [125, 132] (see Table 1 for descriptions of the various phenotypic markers used throughout this review). A low number of these cells are found within the parenchyma, similar to levels in circulation, suggesting that they might be involved in surveillance. However, the presence of DC in steady-state CNS tissue in humans is unclear, although several observations in the non-inflamed steady-state brain warrant further clarification.
Table 1.
List of cell surface markers, and their reported functions, used to phenotype cell populations
| Name | Definition | Expression | Function |
|---|---|---|---|
| 33D1 | Dendritic cell-specific antigen | Dendritic cells | Unknown |
| CD103 | Integrin alpha-E | T lymphocytes, dendritic cells | Lymphocyte homing |
| CD115 (c-fms) | Macrophage colony stimulating factor receptor | Monocytes, macrophages | Proliferation and differentiation of cells in the monocytic lineage |
| CD11b/Mac-1 | Integrin α-M | Myeloid cells | Cell adhesion molecule |
| CD11c | Integrin α-X | Dendritic cells, a subset of intestinal intraepithelial lymphocytes and some activated T cells | Leukocyte adhesive interaction |
| CD123 | IL3Ra | Hematopoietic progenitors, plasmacytoid dendritic cells, macrophages | IL-3 signaling |
| CD24a | Ly-52 | B lymphocytes, monocytes, macrophages | Cell adhesion molecule |
| CD36a | Fatty acid translocase | Monocytes, macrophages, B lymphocytes | Scavenger protein |
| CD4 | Cell surface receptor | Dendritic cells, T lymphocytes | T-cell development and optimal functioning of mature T cells |
| CD40 | Type I transmembrane glycoprotein | B lymphocytes, folicular dendritic cells, peripheral T cells | B-cell development and maturation |
| CD45 | Protein tyrosine phosphatase | Leukocytes | Leukocyte differentiation/activation |
| CD45R | B220 | B lymphocytes, natural killer cells, plasmacytoid dendritic cells | Tyrosine phosphatase |
| CD47 | Neurophilin | Hematopoietic cells | Thrombospondin receptor |
| CD49b | Integrin alpha subunit | Natural killer cells, natural killer T cells | Cell adhesion |
| CD80 (B7.1) | A member of the Ig superfamily | Macrophages, dendritic cells, activated B cells | Activation of T and B cells by interaction of CD28 and CD152 |
| CD86 (B7.2) | A member of the Ig superfamily | B lymphocytes, macrophages, dendritic cells | T–B crosstalk, T-cell costimulation, autoantibody production and Th2-mediated Ig production |
| CD8a | Cell surface receptor | Dendritic cells, T lymphocytes | T-cell development and activation of mature T cells |
| DCX | Doublecortin | Migrating neurons, axons of differentiating neurons | Microtubule binding protein required for normal neural migration and cortical layering during development |
| DEC-205 | Integral membrane glycoprotein | CD8+ dendritic cells, bone marrow derived dendritic cells | Antigen uptake, trafficking and presentation |
| Flt3 | CD135 | Hematopoietic progenitors, dendritic cells | Myeloid and and lymphoid development; binds Flt3L |
| F4/80 | Transmembrane protein | Mature macrophages | Maturation of macrophages |
| GFAP | Glial fibrillary acidic protein | Astrocytes and ependymal | Maintain mechanical strength and cell strength |
| Gr-1 (Ly-6G) | Glycophosphatidyl-inositol (GPI)-linked protein | Myeloid lineage, bone marrow granulocytes, peripheral neutrophils | Unknown |
| Iba-1 | Ionized calcium-binding adaptor molecule 1 | Macrophages, microglia, monocytes | Calcium binding peptide |
| Ly-6C | Glycophosphatidyl-inositol (GPI)-linked protein | T lymphocytes, natural killer cells, monocytes, macrophages | T-cell homing |
| MHC I | Major histocompatibility complex I | Ubiquitous | Presentation of fragments of proteins from within the cell to T cells |
| MHC II | Major histocompatibility complex I | B lymphocytes, monocytes, macrophages, dendritic cells, activated T lymphocytes | Presentation of extracellular pathogens to T cells |
| NeuN | Neuron-specific nuclear protein | Neurons | DNA-binding nuclear protein |
| NG2 | Chondroitin sulfate proteoglycan 4 | Oligodendrocytes | Unknown |
| Nk1.1 | Killer cell lectin-like receptor subfamily B, member 1 | Natural killer cells, natural killer T cells, natural killer dendritic cells | Regulation of NK cell function |
| PDCA-1 | A specific marker of pDC | Plasmacytoid dendritic cells | Trafficking, blocking of retroviral budding |
Anatomic studies that addressed the distribution of DC, or their precursors, in the mouse brain during the steady-state were conducted with the aid of a transgenic (Tg) reporter mouse in which the promoter for CD11c (integrin αx) was used to drive the expression of enhanced yellow fluorescence protein (EYFP) [95]. CD11c is similar to CD11b in that it also complexes with CD18 and is highly expressed on DC. The presence of this molecule has been used as a distinguishing marker to identify DC progenitors in BM, as well as immature and mature DC in other peripheral locations [2, 18, 114]. CD11c, however, can also be moderately expressed on other immune cells, e.g., natural killer cells, B and activated T lymphocytes, monocytes, and microglia [70, 83, 131].
Results by Bulloch and colleagues have revealed a discrete network of putative EYFP-labeled DC within the steady-state brain of Cd11c/eyfp Tg (referred to as EYFP+ bDC) that co-localize with other immune markers commonly associated with both DC and microglia/macrophages (e.g. CD11b/Mac-1, Iba-1, CD45, and F4/80), but not with markers associated with neurons or glia (e.g. NeuN, DCX, NG2 proteoglycan and GFAP) [23]. EYFP+ bDC are evident in the CNS during embryonic day 6, postnatal day 2, and adult brains in regions of synaptic plasticity and neurogenesis. EYFP+ bDC are also noted in regions that lack the structural integrity of a BBB such as the circumventricular organs, olfactory and trigeminal-associated extracellular pathways, the meninges, choroid plexus, and in many fiber tracts that traverse the brain [100, 162]. More recently, Prodinger et al. [134] utilized an analogous Tg system in which the human diphtheria toxin receptor and enhanced green fluorescent protein (EGFP) are expressed under the CD11c promoter. In addition to the brain regions previously identified by Bulloch et al., EGFP-expressing cells were associated with juxtavascular parenchyma and were found integrated with the glia limitans. Interestingly, Prodinger et al. described an increase in EGFP+ expression in cultured brain slices highlighting a population of cells capable of up-regulating CD11c expression in response to trauma. This data corroborate studies carried out in vivo by Bulloch et al., Felger et al., and Gottfried-Blackmore et al., which identified an increase in Cd11c/eyfp expressing cells following brain trauma after seizures, stroke, and cytokine injections.
In addition to phenotypic evidence, recent work using both developmental and functional criteria identified DC that develop from pre-DC in the meninges and choroid plexus of mice [5]. As phenotypic markers (CD11c, CD11b, MHC II, DEC205) fail to distinguish DC adequately from pDC, monocytes, macrophages, and even activated microglia [51, 54], developmental criteria were examined. The development of meningeal/choroid DC (m/chDC), like cDC in the spleen, is fms-like tyrosine kinase 3 ligand (Flt3L) dependent. These cells repopulate from pre-DC with kinetics equal to spleen cDC. Other attributes they share with cDC include MHC II+ CD11c+ CD115/M-CSFRneg, and elevated expression of transcripts for CD103, CD24a, CD36a, and TLR 3. M/chDC also express several transcription factors common to DC lineages including ID2, RBP-J and BATF3. Functionally, m/chDC were similar to spleen CD8α+ DC based on antigen presentation of self-peptide complexes on the cell surface, and the ability to present CNS peptides and allo-antigens to T cells, unlike microglia which do not present antigens ex vivo [5]. Notably, these m/chDC are positioned along sites of activated T-cell entry into the brain during inflammation [138].
DC and neuroinflammation
The vascular endothelium and glia limitans change as a result of secreted cytokines and enhanced recruitment of leukocytes into perivascular spaces during neuroinflammation, leading to a compromised BBB [13]. Consequently, DC are prevalent within the brain parenchyma in response to auto-immunity, injury, aging, and infections. As of this review, most of what is known about DC within the CNS is limited to phenotypic evidence of cell populations that share cell surface attributes analogous with peripheral DC subsets, but in some cases, CNS DC have been functionally characterized as antigen presenters.
Experimental autoimmune encephalomyelitis
Multiple sclerosis (MS) is considered to be a chronic CNS inflammatory disease that is immune mediated [32]. Evidence for the immune origin of MS consists of: (1) the presence of activated myelin-specific lymphocytes, as well as other immune cells (e.g., activated microglia and macrophages) within MS lesions, and (2) genetic evidence of increased susceptibility correlating to mutations in MHC regions [137]. Despite its documented limitations, experimental autoimmune encephalomyelitis (EAE) is the most widely utilized animal model for MS, as each disease shares some clinical and pathological features [117, 118]. EAE is established by either immunizing animals with encephalitic peptides (most commonly myelin basic protein, myelin oligodendrocyte glycoprotein, and proteolipid protein) or through adoptive transfer of myelin-reactive T cells.
During EAE, DC isolated from the CNS at the onset of disease are found to prime inflammatory T cells, whereas DC isolated at the peak of disease seem to be poor APC and may support the development of regulatory T cells [8, 41, 115]. Other studies indicate that CNS DC can function as efficient APC and may serve to amplify intracerebral T-cell responses [46, 79]. In addition, strong evidence also supports peripheral (not resident) DC as being necessary and sufficient for the reactivation of encephalitogeninc T cells [58]. Although numerous studies emphasize the potential relevance of DC for CNS immune surveillance or autoimmune reactions, the contribution of DC in the initiation and perpetuation of neuroantigen-specific T-cell responses remains elusive [171]. Interestingly, while the trafficking of peripheral DC into the inflamed CNS is undisputed, their ability to exit the CNS and present CNS antigens to naive T cells in peripheral lymphoid organs is unresolved, and unlikely, given the lack of a typical lymphatic draining system [167].
Multiple mechanisms have been proposed to account for the accumulation of DC within the CNS during neuroinflammation caused by EAE, specifically the infiltration from peripheral bone-marrow-derived precursors, and/or the differentiation of resident microglia into DC. Granulocyte/macrophage colony-stimulating factor (GM-CSF), but not Flt3-ligand, is sufficient to recruit DC from the periphery via local expression of CC chemokine ligand 2 (CCL2). These recruited DC are phenotypically and functionally identical to DC found within the CNS during EAE [66]. GM-CSF, however, was not shown to induce significant brain resident DC [66], which is in contrast to in vitro studies showing up to 30 % differentiation of microglia into a DC phenotype [144]. Cultured microglia can be induced to become effective APC following stimulation with GM-CSF and CD40 ligation [47], suggesting that perhaps additional activation stimuli are required for microglia conversion. More recently, using a Cd11c/eyfp Tg mouse, Gottfried-Blackmore et al. [54] demonstrated that microglia can become EYFP+ MHC II+ DC in vivo following intracerebral injection of IFN-γ, and that these IFN-γ induced brain DC are potent activators of naïve T cells compared with their EYFPneg microglia counterparts. Interestingly, Flt-3 inhibition of microglia blocks IFN-γ induced expression of MHC II and CD86, suggesting that it is involved in regulating the capacity of microglia to become functional APC under inflammatory conditions [39]. Furthermore, although multiple models of brain injury/infection show that CD11c+ MHC II+ DC in the CNS arise from peripheral hematopoietic cells, the contribution of resident DC in these settings is likely to occur as well. In the spinal cord of EAE mice at the peak of disease, around 20 % of the DC present are CNS derived and show inhibition of T-cell proliferation [66].
CNS injury
Several traditional injury models have been employed to study DC responses to neuronal trauma from strokes or seizures. Neuroinflammation, resulting from ischemic injury by permanent middle cerebral artery occlusion in rats, has demonstrated a population of OX62+(an antibody that recognizes the rat ortholog of CD103) and MHC II+ DC around the periphery of ischemic lesions hours after the stroke and within the core of the lesion 6 days later [89]. The OX62+ DC were also associated with the production of pro-inflammatory cytokines within the lesion, suggesting a functional DC response to the injury. These findings were later expanded upon in a kainic acid excitotoxicity (seizure) model, where OX62+ MHC II+ DC near the lesion site were shown to arise from a BM-derived population [121]. In addition, kainic acid-induced seizures in the Cd11c/eyfp Tg mouse also revealed EYFP-expressing cells responding to damage of the hippocampus and, although less in number, their aggregation displayed morphologies similar to those described for seizure-activated microglia in the hippocampus of EGFP+ cfms (CSF-1R) mice [23].
Other work using photothrombosis-induced focal ischemia described a CD45hi CD11b+ CD11c+ brain DC population that could be isolated from infarcted tissue 6 days post-ischemia with a mature phenotype, i.e., CD8αneg DEC-205neg MHC II+ CD80+ CD86+ [139]. While in the Cd11c/eyfp transgenic mouse 24 h after stroke injury, 80 % of EYFP+ cells are resident derived, at 72 h there is a one-to-one distribution between peripheral and resident DC [45]. Further, the peripherally derived DC were concentrated in the necrotic infarct zone, while the resident DC were confined to the penumbral zone in association with infiltrating T cells, suggesting their possible immunomodulatory role [45].
Aging and CNS
The aging process has been linked to a progressive accumulation of cytokines, suggestive of a heightened pro-inflammatory state within the CNS that mostly has been attributed to an elevated level of microglia activation [30, 42]. A competing hypothesis revolves around the concept of age-related microglial senescence leading to impaired neuroprotection and increase neurodegeneration [30, 158]. Following the immunological link to age-related neurodegeneration, several studies have explored an additional correlation between aging and the accumulation of DC. Stichel and Luebbert [157] found CD11c+ cells with morphologies consistent with DC prevalent throughout the brains of mice that were 12 months or older, while similarly labeled cells were only evident within the meninges and choroid plexus of younger mice. These cells were also characterized by a CD11b+ DEC205+ phenotype, and were found to co-label with cathepsin S (a cysteine protease implicated in antigenic MHC II processing). These findings were further expanded upon by Kaunzner et al. [81], who showed an accumulation of both resident and peripherally derived EYFP+ cells in aged brains when compared to younger control animals. Quantification of these EYFP+ cells in the cortex, corpus callosum, and cerebellum of the aged brain revealed a two to fivefold increase in cell number. In contrast, either no change or a decrease in the number of EYFP+ cells was noted in regions of adult neurogenesis, e.g., the hippocampus. In parallel, there was a significant increase in the expression of DC maturation markers (e.g., MHC II and CD86) on these cells. Collectively, the numerical and phenotypic changes in EYFP+ cells indicate that these APC coincide with the alteration in CNS inflammatory tone associated with aging [81].
CNS infections
Pathogens capable of establishing CNS infections arise from fungal, bacterial, protozoal, viral, and prion origins. Such infections can give rise to inflammation of the brain (encephalitis), meninges (meningitis), and spinal cord (myelitis). Complications resulting from CNS infections range from headaches, nausea and vomiting, deficits in motor control, seizures, and behavioral abnormalities. Treatments usually follow a two-pronged approach involving the administration of biostatic (if available) and anti-inflammatory agents to avoid sequelae. Consequently, understanding the contributions DC may make to host defense against various CNS pathogens is very important for the design of more efficacious therapies for such diseases.
Protozoal infections
Toxoplasma gondii is a protozoan intracellular parasite that infects all animals and most birds [77], and is largely considered the most prevalent zoonotic disease in the world [161]. The greatest impact T. gondii has on human health is congenital transmission, which may lead to serious morbidity or mortality in utero or among newborns [113]. T. gondii is currently thought to gain access to the brain by crossing the BBB using migratory leukocytes (e.g. DC) as “Trojan horses” [31, 90]. Studies of T. gondii-induced encephalitis (TE) have revealed the presence of CD11c+ 33D1+ CD11b+ F4/80+ DC responding at the peak of acute infection [46], a phenotype similar to CD11b+ tissue DC [52]. DC found in the TE brain were mature, capable of presenting antigen to naïve T cells, major producers of IL-12, and directed a TH1 CD4+ T-cell bias [46, 47]. Interestingly, using the Cd11c/eyfp mouse, it has been reported that the majority (91 %) of the microglia (CD11b+ and CD45int) during TE are EYFP+ and efficient APCs; however, two-thirds of these cells are bone-marrow derived [76].
Bacterial infections
Relatively few bacteria are capable of causing disease within the CNS; however, bacterial meningitis can be more severe than other etiologies and can result in severe sequelae including neurologic disabilities, stroke, hydrocephalus, loss of limb, herniation, sepsis, and death [166]. The leading causes of bacterial meningitis are Streptococcus pneumonia and Neisseria meningitides, which are capable of infecting both healthy and immunologically compromised individuals [166]. Other bacteria capable of causing human disease include Group B Streptococci, Escherichia coli, Listeria monocytogenes, Haemophilus influenza, and Borrelia burgdorferi [72].
Early work by Pashenkov et al. [127] showed that CSF from patients suffering from bacterial meningitis caused by S. pneumonia and N. meningitides, as well as patients with Lyme meningoencephalitis from B. burgdorferi, possessed higher levels of both myeloid DC (CD11c+ HLA-DR+) and pDC (CD123hi HLA-DR+) than patients suffering from non-inflammatory neurological diseases. The CSF from these patients was further characterized by high levels of inflammatory chemokines, with stromal cell-derived factor-1α playing a prominent role. In a separate study, the CSF from B. burgdorferi infected patients resulted in the maturation of human monocyte-derived DC (as defined by higher levels of HLA-DR, CD80, and CD86) isolated from healthy donors, which led to better induction of TH1 responses and an increased ability to act as stimulators in mixed leukocyte reactions (MLR) [126]. This data suggest that DC utilize the ventricular system as a means of conveyance during inflammation.
In a rodent model of CNS listeriosis, low numbers of CD45hi CD11c+ DC were observed within the brain by 7 days post-infection (dpi) just before a CD8α+ T-cell dominated immune response led to bacterial clearance. This data suggest that DC were involved in the antigen uptake and processing responsible for T-cell activation [63]. Likewise, in another animal model of neonatal meningitis induced by intranasal infection with E. coli K1, a link between bacterial load/host survival and the expression of CD47 on DC was established. E. coli K1 lead to increased expression of CD47 [21], an integrin-associated protein that has been implicated in immune tolerance through its interactions with signal regulatory protein α [9, 104, 165], on the surface of monocyte-derived DC. CD47 upregulation coincided with both an immature DC status and refractory antigen presentation behavior during MLR, even in the presence of lipopolysaccharide. When the ability of E. coli K1 to upregulate CD47 on DC was countered with siRNA, DC matured and presented antigen in a MLR as expected. Furthermore, when monocyte-derived DC were pre-treated with CD47 siRNA and adoptively transferred into wild-type mice, an E. coli K1 CNS infection was cleared and no signs of meningitis were found when compared to mice adoptively transferred with control treated monocyte-derived DC. These data imply that the efficacious infiltration of DC into the CNS of E. coli K1 hinged upon the ability of the DC to circumvent this bacterial strain’s mechanism of host immune evasion.
Viral infections
Neurotropic viruses represent another pathogenic class capable of causing CNS disease. Viruses enter the CNS either through infections that alter the vascular endothelium of the brain or by crossing the BBB via an infected leukocyte “Trojan horse” [107]. In addition, infection of the peripheral nervous system followed by hijacking of cellular retrograde transport systems can result in CNS viral infiltration [107]. Encephalitogenic viruses can be transmitted by several vectors, and arthropod-borne viruses (arboviruses) represent the most prominently contracted form [73]. Arboviruses are zoonotic and those with the highest degree of prevalence fall within the Flaviviridae, Togoviridae, and Bunyaviridae families of RNA viruses. Additional neurotropic viruses, not transmitted by biting insects, include both DNA- and RNA-based viruses, such as herpes simplex virus-1 (HSV-1), varicella zoster virus, rabies virus, human immunodeficiency virus, influenza virus, measles virus, lymphocytic choriomeningitis virus (LCMV), Epstein–Barr virus, cytomegalovirus, and enteroviruses [107].
Unfortunately, despite the existence of established animal models for many of the aforementioned neurotropic viruses, there is a paucity of in vivo experimental evidence describing the role of DC in neuroinflammation. Instead, most studies of DC function within the CNS are restricted to ex vivo examination of BM or spleen-derived DC in response to these pathogens [3, 25, 35, 36, 65, 94, 160]. Conversely, a vast amount of information exists pertaining to the role microglia and PVM play in response to viral infections of the CNS [82]. However, given the ongoing discovery of new DC subsets [64], the contribution of DC-mediated immune responses to neurotropic viruses is important to consider in the context of human disease.
Several in vivo studies have provided valuable insight into elucidating the role of DC in response to CNS viral infection. Work with intracranial murine hepatitis virus (MHV) infections has identified two CD11c+ DC subtypes within the brains of wild-type and CCL3−/− mice, which were differentiated by CD11bneg CD8α+ DEC205+ and CD11b+ CD8αneg DEC205neg phenotypes [164]. Of these two populations, the CD11b+ CD8 αneg DC subtype proved to be the most prominent and mature based on its expression of co-stimulatory molecules (CD40, CD80, and CD86). Interestingly, even though both the CD8α+ and CD8αneg DC populations accumulated to similar levels by 5 dpi, the arrival of the CD8αneg DC was delayed in the brain within the CCL3−/− mouse, suggesting these CD8αneg DC utilize either CC chemokine receptor 1 (CCR1) or CCR5 for tissue homing. In addition, in a West Nile virus infection model, the most prominent DC subset found within the brain of mice between 4 and 6 dpi were of a CD11c+ BST-2/PDCA-1+ B220+ CD45RA+ Ly-6C+ pDC phenotype, and their locations within the infected brain were paralleled by IFNα mRNA tissue distribution patterns [20]. Other chemokines/chemokine receptor pairings have also been implicated in the recruitment of pDC, as well as cDC, during HSV-1 infections of CXCL10−/− and CXCR3−/− deficient mice; in each case “knocking out” the respective chemokine receptors correlated with higher susceptibility to the virus [169]. Aside from chemokine gradients, leukocytes recognize inflammation-induced variations in cell-adhesion molecule expression during the tissue homing process. In this regard, VCAM-1 has been shown to be important for DC infiltration in the brains of LCMV-infected mice [123]. These examples illustrate how disparate the types of DC subsets can be in response to different viral pathogens, reacting perhaps to the various methods each virus employs to evade host immunity.
In addition to the identification of DC phenotypes within the virally infected CNS, several investigations have examined the functional properties of these cells under those conditions. For example, CD11c+ CD45hi F4/80neg DC, isolated from mouse brains and spinal cords after intracranial infection with Theiler’s murine encephalomyelitis virus, are able to present antigen to naïve proteolipid (PLP139–151) T-cell receptor-restricted CD4+ T cells leading to their proliferation and production of IL-2 [108], a property not shared by macrophages (CD45hi F4/80+) and microglia (CD45low F4/80+). Work employing immunocytotherapy as a means of treating chronic LCMV infection, further demonstrated that only the accumulation of CD45hi CD11c+ DC within the CNS (not CNS macrophages or microglia) was effective in stimulating the production of antiviral cytokines from LCMV-specific memory CD8+ T cells [92]. This result was punctuated by the inability of adoptively transferred LCMV-specific memory T cells to resolve the viral infection when these DC were depleted from Cd11c/dtr prior to immunocytotherapy.
Intranasal (i.n.) vesicular stomatitis virus (VSV) infections represent another well-established encephalitis model [28, 59, 74, 101, 140]. Using i.n. VSV infections, Steel et al. [153] have observed a small spike in cDC (CD45hi CD11c+ PDCA-1neg) infiltration within the brain between 6 and 10 dpi; experimental depletion of these DC correlated with an elevation of viral titers in the brain and increased mortality. In more recent work, D’Agostino and colleagues [34] employed i.n. VSV infection of the Cd11c/eyfp reporter mouse to focus on the early interactions of DC in the olfactory bulbs during the acute viral infection. In this work, it was demonstrated that EYFP+ cells at 4 dpi amassed around VSV-infected neurophil in the olfactory nerve and in glomerular layers of the olfactory bulb (OB). These EYFP+ cells displayed an activated morphology, and projected their processes into VSV-infected neuronal tissue. EYFP+ cells harvested from the OB were further characterized by flow cytometry into three CD45+ CD11b+ populations. One population, identified as CD45int CD11bhi, was shown to emanate from the brain and contained a CD11c+ CD103+ MHC IIneg phenotype, which was distinct from the major CD11clow/neg CD103neg MHC IIneg microglia. A second population, identified as CD45hi CD11bhi, was peripherally derived and phenotypically resembled a mucosal DC subset as it was CD11b+ CD103+ CD115+ Gr1+ Ly-6c+ [34]. The third population, identified as CD45hi CD11bint, had attributes most akin to monocyte-derived DC as it was CD11b+ CD103neg CD115neg Gr1neg Ly-6c+ [34]. These latter two DC populations found in the OB were phenotypically mature (MHC II+ CD80+ CD86+) and capable of functionally promoting ex vivo T lymphocyte proliferation and TH1 cytokine production, contrary to the CD45int CD11bhi microglial population. These data suggest that DC are both present early on, and participate in immune surveillance and activation within the parenchyma of the virally infected brain, before the infiltration of peripheral immune cells, e.g., T lymphocytes (Fig. 1).
Fig. 1.
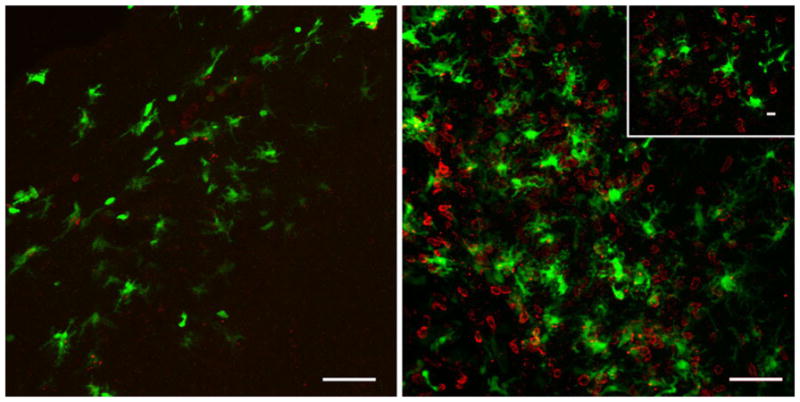
Putative DC within the brain can interact with CD4+ T lymphocytes following viral-induced neuroinflammation. Representative confocal Z-stack analysis of a VSV intranasally infected olfactory bulb at 4 (left) and 7 (right) days post-infection. CD11c/EYFP+ cells (green) are found throughout glomerular tissue richly populated with CD4+ T cells (red). Inset depicts a confocal section in which CD11c/EYFP+ cells are in physical contact with CD4+ T cells. Representative images from three experiments with an n =3; scale bar 50 μm (10 μm inset)
Prion infections
Transmissible spongiform encephalopathies (TSE) are subacute neurodegenerative diseases that affect animals and have been comprehensively reviewed by Mabbot and MacPherson [102]. The primary pathological features of TSE include a sponge-like tissue appearance, neuronal death, gliosis, and high levels of amyloidal protein aggregates. The causative agent of TSE found in the amyloidal aggregates is termed a prion, a non-nucleic acid-based infectious protein particle [135]. An overwhelming majority of the work involving prions and DC has either focused on the neuroinvasive transmission of the infectious agent into the CNS [55, 149], or used DC as vaccine vectors for the treatment/prophylaxis of TSE [7, 48, 119, 142]. However, one group has studied the potential recruitment of DC into the brain resulting from TSE. Using immunohistochemical (IHC) techniques, DEC205+ cells with DC morphology were found within spongiform-associated areas of the brain in mice infected with a prion, whereas these cells were prominently identified only within the meninges and choroid plexus of healthy controls [141]. These results again highlight that DC are associated with sites of neuroinflammation. It remains to be seen, however, if TSE-induced DEC205+ DC is in response to or contributes to the pathogenesis of this disease.
Future perspectives
Understanding the origins of DC found within the brain
Given that DC or “DC-like cells” have been identified in multiple models of neuroinflammation, it is now necessary to move beyond IHC/immunofluorescence phenotyping of these cells in order to assess their origins using the armamentarium of modern day immunologists. The more popular tools include gene deficiency models, both non-genetic and genetic depletion methods, cell type-specific gene expression (including reporter mice), and knockout models specifically designed for cells within the mononuclear phagocyte system. The utilities and caveats for each of the above methods have been extensively reviewed elsewhere [27]. In some instances, one or two of these methods have been applied to the study of DC [5, 23, 34, 45, 54, 81, 92, 153]. However, extending to these observations to include newer technologies will inevitably provide more in vivo developmental and functional insight to further define the role of DC during neuroinflammation. Understanding the ontogeny of DC involved in neuroinflammation could assist in identifying their functional role/s (which could be disease dependent), tissue reservoirs, and ports of entry/exit for access to the lymphatic system—each of which are active areas of CNS DC research.
Migration of DC between the CNS and peripheral compartments
How DC migrate from the brain to secondary lymphoid structures remains an intriguing question (see Weller et al. [167] for review). Most research has implicated the deep cervical lymph nodes as the primary drainage site from the brain, as demonstrated by intracranial injection of labeled antigen or preloaded DC [33, 80, 96]. However, given that CSF can drain through the cribriform plate into the lymphatic system, the critical role that the nasopharynx-associated lymphoid tissue (NALT; referred to as Waldyer’s ring in humans) may play in afferent immunity should also be explored [122]. Utilizing non-invasive imaging technologies like two-photon microscopy, positron emission tomography, and magnetic resonance imaging, methods of monitoring DC within the periphery have been developed and employed [11, 19]. The combination of these imaging methods and neuroinflammation models will undoubtedly provide new insight into the migration patterns used by DC to acquire, transport, and present CNS-derived antigens in lymphoid structures. Such a strategy was employed to study DC interactions within inguinal lymph nodes shortly after subcutaneous infection with vaccinia virus and VSV [69]. Analogous strategies have been implemented to follow DC migration in EAE and TE models of neuroinflammation [75, 76]. However, while these studies went a long way towards illuminating DC migration patterns during neuroinflammation, they either focused on phenomena within the site of inflammation or concentrated on the dynamics of DC movements in lymphoid organs.
Therapeutic employment of DC in the brain during CNS disease
Modulating the response of DC during neuroinflammation is currently being considered as a therapeutic approach toward ameliorating several CNS diseases. The most prominent area of research lies in the search for an efficacious immunotherapy for glioblastoma multiforme (GBM). GBM is an aggressive form of brain cancer with a dismal prognosis, even after initiating full treatment options with median survival ranging from 9 to 12 months after diagnosis [103]. At the time of writing this review, the use of DC-based vaccines represents roughly 20 % of active clinical trials for GBM-based immunotherapies [71]. DC-based vaccines for GBM commonly employ the loading (co-culturing) of autologous DC with either tumor lysates, apoptotic tumor cells or tumor-based cDNA [155]. Fusions between DC and tumor cells (to yield a multinucleated cell body) represent another method to generate such vaccines [88]. Recent studies have looked to enhance the efficacy of DC vaccines by implementing Flt3L gene therapy to increase the number of DC within tumors, or with thymidine kinase gene therapy coupled with ganciclovir to increase tumor cytotoxicity [4, 116]. In another approach, direct inoculation of the tumors with a DC vaccine, coupled with a subcutaneous injection regimen, led to significantly higher survival compared to controls [128].
Treatment modalities relating to other neuroinflammatory diseases are also being investigated. One example used the inhibition of DC functions with CEP-701 (a FLT3 inhibitor) as a treatment for EAE, which resulted in decreased numbers of brain DC infiltrates and a concomitant decrease in TH1 and TH17 responses [151]. In a spinal cord injury model, GM-CSF was used to increase the numbers of cells with DC morphology, along with neural stem/progenitor cells, which led to earlier recovery of locomotor function [62]. Lastly in an optic nerve injury model, immediate subcutaneous injection of glatiramer acetate loaded BM-derived DC, led to significant T-cell independent neuroprotection, believed to be associated with enhanced DC infiltration [97].
Conclusion
DC participation in brain immune responses is far from being completely understood, despite the progress that has been made since Medawar’s initial observations of an immunologically privilege status within the CNS [110–112]. We now know that CNS immune privilege mostly corresponds to a highly controlled condition of immunological surveillance, and that DC can be found in various tissue reservoirs within the steady-state CNS. The fact that some semblance of immunological surveillance (either directly by DC or indirectly mediated via microglia or PVM) occurs is unquestionable given the observations made with animal models of neuroinflammation and during human clinical studies. Figure 2 illustrates our current understanding of DC positioning in the steady-state and potential sites that DC are believed to migrate from during neuroinflammation. Despite this knowledge base, some questions that still remain unanswered include: (1) Can microglia differentiate into DC-like cells in a manner similar to inflammatory monocytes? (2) At what point does the tight control of immunological surveillance relax during the early stages of neuroinflammation? (3) How and where are effector T cells primed with CNS antigen? (4) What subsets of DC are the prominent players during CNS immune responses that allow T-cell entry into the CNS? Finally, (5) what are the dynamics of brain DC antigen presentation associated with secondary (and possibly tertiary) lymphoid structures? Attempting to answer these questions will undoubtedly improve our current understanding of CNS DC biology, as well as better our attempts to design treatment strategies for neuroinflammatory and immunologically linked neurodegenerative diseases.
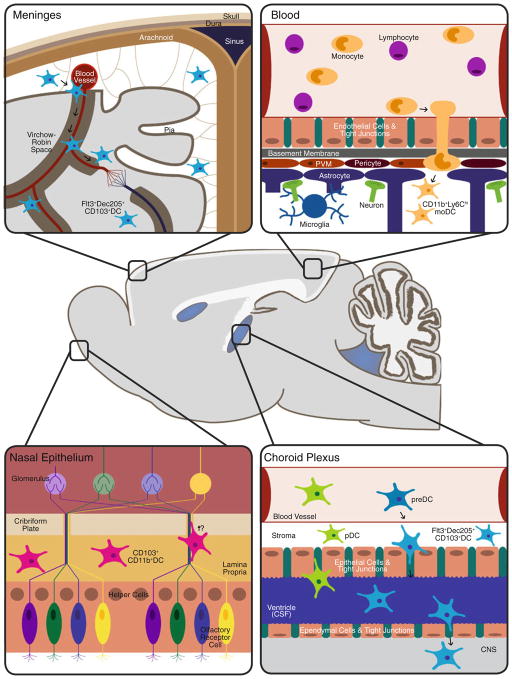
Fig. 2.
Schematic representation of tissue reservoirs believed to be the source of DC infiltration during neuroinflammation. In the steady-state brain Flt3+ DC are primarily found within the meninges and choroid plexus. Evidence for DC-like cells has also been found within circumventricular organs such as the area postrema, subfornical organ, median eminence, and pituitary (not depicted for simplicity). During neuroinflammation (e.g., of viral etiology) the DC found within these area are postulated to enter the brain parenchyma in response to cytokine/chemokine gradients. Other potential sources of infiltrating DC are the nasal mucosa, nasal-associated lymphoid tissue, and the blood, in the form of monocyte-derived DC (moDC). Therefore, in light of these various reservoirs, the methods of DC infiltration are believed to range from direct migration into brain tissue, diapedesis followed by crossing of the glia limitans from the Virchow–Robin spaces, and passage from CSF through the CVOs into the brain parenchyma; image not to scale
Acknowledgments
We would like to thank Dr. Judit Gal Toth and Haley A. Vecchiarelli for both contributing images used as figures in this review and for their comments and suggestions. Additionally, we are grateful to Drs. Bruce S. McEwen, Richard Hunter, and James M. Miller, as well as Changsoo Kwak and Zahrah Masheeb for their valuable recommendations. This work was supported by the Peter Deane Trust (K.B.).
Footnotes
Conflict of interest The authors have no conflicting financial interests.
Contributor Information
Paul M. D’Agostino, The Laboratories of Neuroendocrinology, The Rockefeller University, New York, NY 10065, USA
Andres Gottfried-Blackmore, The Laboratories of Neuroendocrinology, The Rockefeller University, New York, NY 10065, USA.
Niroshana Anandasabapathy, The Laboratories of Cellular Physiology and Immunology, The Rockefeller University, New York, NY 10065, USA.
Karen Bulloch, Email: bulloch@rockefeller.edu, The Laboratories of Neuroendocrinology, The Rockefeller University, New York, NY 10065, USA. The Laboratories of Cellular Physiology and Immunology, The Rockefeller University, New York, NY 10065, USA. The Laboratories of Molecular Immunology, The Rockefeller University, New York, NY 10065, USA. Neuroimmunology and Inflammation Program, The Rockefeller University, 1230 York Avenue, Box 165, New York, NY 10065, USA.
References
- 1.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 2.Agger R, Crowley MT, Witmer-Pack MD. The surface of dendritic cells in the mouse as studied with monoclonal antibodies. Int Rev Immunol. 1990;6(2–3):89–101. doi: 10.3109/08830189009056621. [DOI] [PubMed] [Google Scholar]
- 3.Aleyas AG, George JA, Han YW, Rahman MM, Kim SJ, Han SB, Kim BS, Kim K, Eo SK. Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. J Immunol. 2009;183(4):2462–2474. doi: 10.4049/jimmunol.0801952. [DOI] [PubMed] [Google Scholar]
- 4.Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, Liu C, Puntel M, Goverdhana S, Lowenstein PR, Castro MG. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10(6):1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208(8):1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axtell RC, Steinman L. Gaining entry to an uninflamed brain. Nat Immunol. 2009;10(5):453–455. doi: 10.1038/ni0509-453. [DOI] [PubMed] [Google Scholar]
- 7.Bachy V, Ballerini C, Gourdain P, Prignon A, Iken S, Antoine N, Rosset M, Carnaud C. Mouse vaccination with dendritic cells loaded with prion protein peptides overcomes tolerance and delays scrapie. J Gen Virol. 2010;91(Pt 3):809–820. doi: 10.1099/vir.0.013417-0. [DOI] [PubMed] [Google Scholar]
- 8.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8(2):172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 9.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6(6):457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 10.Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15(4):437–446. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- 11.Baumjohann D, Lutz MB. Non-invasive imaging of dendritic cell migration in vivo. Immunobiology. 2006;211(6–8):587–597. doi: 10.1016/j.imbio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Becher B, Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18(1):1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Bechmann I, Galea I, Perry VH. What is the blood–brain barrier (not)? Trends Immunol. 2007;28(1):5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168(2):242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- 15.Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14(10):1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 16.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12(2):101–113. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 17.Billingham RE, Boswell T. Studies on the problem of corneal homografts. Proc R Soc Lond B Biol Sci. 1953;141(904):392–406. doi: 10.1098/rspb.1953.0049. [DOI] [PubMed] [Google Scholar]
- 18.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T-cell tolerance. J Exp Med. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol. 2008;8(9):675–684. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- 20.Brehin AC, Mouries J, Frenkiel MP, Dadaglio G, Despres P, Lafon M, Couderc T. Dynamics of immune cell recruitment during West Nile encephalitis and identification of a new CD19+ B220-BST-2+ leukocyte population. J Immunol. 2008;180(10):6760–6767. doi: 10.4049/jimmunol.180.10.6760. [DOI] [PubMed] [Google Scholar]
- 21.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11(3):130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 22.Buller KM. Role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic-pituitary–adrenal axis. Clin Exp Pharmacol Physiol. 2001;28(7):581–589. doi: 10.1046/j.1440-1681.2001.03490.x. [DOI] [PubMed] [Google Scholar]
- 23.Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, Kaunzner UW, Liu K, Lindquist R, Nussenzweig MC, Steinman RM, McEwen BS. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508(5):687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 24.Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M. Glatiramer acetate fights against Alzheimer’s disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci USA. 2006;103(31):11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao S, Li Y, Ye J, Yang X, Chen L, Liu X, Chen H. Japanese encephalitis virus wild strain infection suppresses dendritic cells maturation and function, and causes the expansion of regulatory T cells. Virol J. 2011;8:39. doi: 10.1186/1743-422X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22(1):72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11(11):788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 28.Ciavarra RP, Stephens A, Nagy S, Sekellick M, Steel C. Evaluation of immunological paradigms in a virus model: are dendritic cells critical for antiviral immunity and viral clearance? J Immunol. 2006;177(1):492–500. doi: 10.4049/jimmunol.177.1.492. [DOI] [PubMed] [Google Scholar]
- 29.Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4(4):399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65(3):199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- 31.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107(1):309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coyle PK, Rizvi S. Clinical neuroimmunology: multiple sclerosis and related disorders. Current clinical neurology, Humana 2010 [Google Scholar]
- 33.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2(4):269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 34.D’Agostino PM, Kwak C, Vecchiarelli HA, Toth JG, Miller JM, Masheeb Z, McEwen BS, Bulloch K. Viral-induced encephalitis initiates distinct and functional CD103+ CD11b+ brain dendritic cell populations within the olfactory bulb. Proc Natl Acad Sci USA. 2012;109(16):6175–6180. doi: 10.1073/pnas.1203941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82(21):10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daffis S, Suthar MS, Szretter KJ, Gale M, Jr, Diamond MS. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog. 2009;5(10):e1000607. doi: 10.1371/journal.ppat.1000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danielyan L, Schafer R, von Ameln-Mayerhofer A, Bernhard F, Verleysdonk S, Buadze M, Lourhmati A, Klopfer T, Schaumann F, Schmid B, Koehle C, Proksch B, Weissert R, Reichardt HM, van den Brandt J, Buniatian GH, Schwab M, Gleiter CH, Frey WH., 2nd Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14(1):3–16. doi: 10.1089/rej.2010.1130. [DOI] [PubMed] [Google Scholar]
- 38.Danielyan L, Schafer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, Burkhardt U, Proksch B, Verleysdonk S, Ayturan M, Buniatian GH, Gleiter CH, Frey WH., II Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88(6):315–324. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 39.DeBoy CA, Rus H, Tegla C, Cudrici C, Jones MV, Pardo CA, Small D, Whartenby KA, Calabresi PA. FLT-3 expression and function on microglia in multiple sclerosis. Exp Mol Pathol. 2010;89(2):109–116. doi: 10.1016/j.yexmp.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dellmann HD. Structure of the subfornical organ: a review. Microsc Res Tech. 1998;41(2):85–97. doi: 10.1002/(SICI)1097-0029(19980415)41:2<85::AID-JEMT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 41.Deshpande P, King IL, Segal BM. Cutting edge: CNS CD11c+ cells from mice with encephalomyelitis polarize Th17 cells and support CD25+ CD4+ T cell-mediated immunosuppression, suggesting dual roles in the disease process. J Immunol. 2007;178(11):6695–6699. doi: 10.4049/jimmunol.178.11.6695. [DOI] [PubMed] [Google Scholar]
- 42.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84(4):932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D, Saper CB. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381(2):119–129. doi: 10.1002/(sici)1096-9861(19970505)381:2<119::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Engelhardt B, Wolburg-Buchholz K, Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech. 2001;52(1):112–129. doi: 10.1002/1097-0029(20010101)52:1<112::AID-JEMT13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 45.Felger JC, Abe T, Kaunzner UW, Gottfried-Blackmore A, Gal-Toth J, McEwen BS, Iadecola C, Bulloch K. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun. 2010;24(5):724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer HG, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164(9):4826–4834. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 47.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166(4):2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 48.Flores-Langarica A, Sebti Y, Mitchell DA, Sim RB, MacPherson GG. Scrapie pathogenesis: the role of complement C1q in scrapie agent uptake by conventional dendritic cells. J Immunol. 2009;182(3):1305–1313. doi: 10.4049/jimmunol.182.3.1305. [DOI] [PubMed] [Google Scholar]
- 49.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28(1):12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20(3):269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 51.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10(6):453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206(13):3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80(4):797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 54.Gottfried-Blackmore A, Kaunzner UW, Idoyaga J, Felger JC, McEwen BS, Bulloch K. Acute in vivo exposure to interferon-gamma enables resident brain dendritic cells to become effective antigen presenting cells. Proc Natl Acad Sci USA. 2009;106(49):20918–20923. doi: 10.1073/pnas.0911509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin JC, Mannel D, Zurzolo C. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11(3):328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 56.Graeber MB, Streit WJ, Buringer D, Sparks DL, Kreutzberg GW. Ultrastructural location of major histocompatibility complex (MHC) class II positive perivascular cells in histologically normal human brain. J Neuropathol Exp Neurol. 1992;51(3):303–311. doi: 10.1097/00005072-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Gregerson DS, Sam TN, McPherson SW. The antigen-presenting activity of fresh, adult parenchymal microglia and perivascular cells from retina. J Immunol. 2004;172(11):6587–6597. doi: 10.4049/jimmunol.172.11.6587. [DOI] [PubMed] [Google Scholar]
- 58.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11(3):328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 59.Grodums EI. Ultrastructure of mouse periventricular and choroid plexus tissues in experimental vesicular stomatitis virus infection. Arch Virol. 1976;51(1–2):75–85. doi: 10.1007/BF01317836. [DOI] [PubMed] [Google Scholar]
- 60.Hanly A, Petito CK. HLA-DR-positive dendritic cells of the normal human choroid plexus: a potential reservoir of HIV in the central nervous system. Hum Pathol. 1998;29(1):88–93. doi: 10.1016/s0046-8177(98)90395-1. [DOI] [PubMed] [Google Scholar]
- 61.Hart DN, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981;154(2):347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayashi K, Ohta S, Kawakami Y, Toda M. Activation of dendritic-like cells and neural stem/progenitor cells in injured spinal cord by GM-CSF. Neurosci Res. 2009;64(1):96–103. doi: 10.1016/j.neures.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 63.Hayashi T, Nagai S, Fujii H, Baba Y, Ikeda E, Kawase T, Koyasu S. Critical roles of NK and CD8+ T cells in central nervous system listeriosis. J Immunol. 2009;182(10):6360–6368. doi: 10.4049/jimmunol.0803798. [DOI] [PubMed] [Google Scholar]
- 64.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234(1):55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 65.Her Z, Malleret B, Chan M, Ong EK, Wong SC, Kwek DJ, Tolou H, Lin RT, Tambyah PA, Renia L, Ng LF. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J Immunol. 2010;184(10):5903–5913. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- 66.Hesske L, Vincenzetti C, Heikenwalder M, Prinz M, Reith W, Fontana A, Suter T. Induction of inhibitory central nervous system-derived and stimulatory blood-derived dendritic cells suggests a dual role for granulocyte-macrophage colony-stimulating factor in central nervous system inflammation. Brain. 2010;133(Pt 6):1637–1654. doi: 10.1093/brain/awq081. [DOI] [PubMed] [Google Scholar]
- 67.Hickey WF. Migration of hematogenous cells through the blood–brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1(2):97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 68.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 69.Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9(2):155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- 70.Hogg N, Takacs L, Palmer DG, Selvendran Y, Allen C. The p150,95 molecule is a marker of human mononuclear phagocytes: comparison with expression of class II molecules. Eur J Immunol. 1986;16(3):240–248. doi: 10.1002/eji.1830160306. [DOI] [PubMed] [Google Scholar]
- 71. [Accessed 4 Apr 2012]; http://clinicaltrials.gov/ct2/results?term=glioblastoma+multiforme+immunotherapy.
- 72. [Accessed 23 Mar 2012]; http://www.cdc.gov/meningitis/bacterial.html.
- 73. [Accessed 29 Feb 2012]; http://www.cdc.gov/ncidod/dvbid/arbor/arbdet.htm.
- 74.Huneycutt BS, Plakhov IV, Shusterman Z, Bartido SM, Huang A, Reiss CS, Aoki C. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis. Brain Res. 1994;635(1–2):81–95. doi: 10.1016/0006-8993(94)91426-5. [DOI] [PubMed] [Google Scholar]
- 75.Jain P, Coisne C, Enzmann G, Rottapel R, Engelhardt B. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood–brain barrier during experimental autoimmune encephalomyelitis. J Immunol. 2010;184(12):7196–7206. doi: 10.4049/jimmunol.0901404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.John B, Ricart B, Tait Wojno ED, Harris TH, Randall LM, Christian DA, Gregg B, De Almeida DM, Weninger W, Hammer DA, Hunter CA. Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis. PLoS Pathog. 2011;7(9):e1002246. doi: 10.1371/journal.ppat.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joiner KA, Dubremetz JF. Toxoplasma gondii: a protozoan for the nineties. Infect Immun. 1993;61(4):1169–1172. doi: 10.1128/iai.61.4.1169-1172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaminski M, Bechmann I, Pohland M, Kiwit J, Nitsch R, Glumm J. Migration of monocytes after intracerebral injection at entorhinal cortex lesion site. J Leukoc Biol. 2012;92(1):31–39. doi: 10.1189/jlb.0511241. [DOI] [PubMed] [Google Scholar]
- 79.Karman J, Chu HH, Co DO, Seroogy CM, Sandor M, Fabry Z. Dendritic cells amplify T cell-mediated immune responses in the central nervous system. J Immunol. 2006;177(11):7750–7760. doi: 10.4049/jimmunol.177.11.7750. [DOI] [PubMed] [Google Scholar]
- 80.Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173(4):2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 81.Kaunzner UW, Miller MM, Gottfried-Blackmore A, Gal-Toth J, Felger JC, McEwen BS, Bulloch K. Accumulation of resident and peripheral dendritic cells in the aging CNS. Neurobiol Aging. 2012;33(4):681–693. e681. doi: 10.1016/j.neurobiolaging.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Kaushik DK, Gupta M, Basu A. Microglial response to viral challenges: every silver lining comes with a cloud. Front Biosci. 2012;17:2187–2205. doi: 10.2741/3847. [DOI] [PubMed] [Google Scholar]
- 83.Keizer GD, Borst J, Visser W, Schwarting R, de Vries JE, Figdor CG. Membrane glycoprotein p150,95 of human cytotoxic T cell clone is involved in conjugate formation with target cells. J Immunol. 1987;138(10):3130–3136. [PubMed] [Google Scholar]
- 84.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 85.Kida S, Steart PV, Zhang ET, Weller RO. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 1993;85(6):646–652. doi: 10.1007/BF00334675. [DOI] [PubMed] [Google Scholar]
- 86.Kivisakk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100(14):8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6. doi: 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koido S, Ohana M, Liu C, Nikrui N, Durfee J, Lerner A, Gong J. Dendritic cells fused with human cancer cells: morphology, antigen expression, and T cell stimulation. Clin Immunol. 2004;113(3):261–269. doi: 10.1016/j.clim.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke. 2002;33(4):1129–1134. doi: 10.1161/hs0402.105379. [DOI] [PubMed] [Google Scholar]
- 90.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8(10):1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 91.Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7(1):19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- 92.Lauterbach H, Zuniga EI, Truong P, Oldstone MB, McGavern DB. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J Exp Med. 2006;203(8):1963–1975. doi: 10.1084/jem.20060039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Xu M, Chen L, Zhu J, Ye J, Liu X, Sun Y, Chen H, Cao S. Evaluation of murine bone marrow-derived dendritic cells loaded with inactivated virus as a vaccine against Japanese encephalitis virus. Vaccine. 2009;27(43):6004–6010. doi: 10.1016/j.vaccine.2009.07.078. [DOI] [PubMed] [Google Scholar]
- 95.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5(12):1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 96.Ling C, Sandor M, Fabry Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol. 2003;141(1–2):90–98. doi: 10.1016/s0165-5728(03)00249-2. [DOI] [PubMed] [Google Scholar]
- 97.Liu J, Johnson TV, Lin J, Ramirez SH, Bronich TK, Caplan S, Persidsky Y, Gendelman HE, Kipnis J. T cell independent mechanism for copolymer-1-induced neuroprotection. Eur J Immunol. 2007;37(11):3143–3154. doi: 10.1002/eji.200737398. [DOI] [PubMed] [Google Scholar]
- 98.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234(1):45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 99.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324(5925):392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH., 2nd Intranasal administration of insulin-like growth factor-I bypasses the blood–brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187(1–2):91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 101.Lundh B, Kristensson K, Norrby E. Selective infections of olfactory and respiratory epithelium by vesicular stomatitis and Sendai viruses. Neuropathol Appl Neurobiol. 1987;13(2):111–122. doi: 10.1111/j.1365-2990.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 102.Mabbott NA, MacPherson GG. Prions and their lethal journey to the brain. Nat Rev Microbiol. 2006;4(3):201–211. doi: 10.1038/nrmicro1346. [DOI] [PubMed] [Google Scholar]
- 103.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 104.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19(2):72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 105.Matyszak MK, Perry VH. A comparison of leucocyte responses to heat-killed bacillus Calmette-Guerin in different CNS compartments. Neuropathol Appl Neurobiol. 1996;22(1):44–53. [PubMed] [Google Scholar]
- 106.Matyszak MK, Perry VH. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience. 1996;74(2):599–608. doi: 10.1016/0306-4522(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 107.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11(5):318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11(3):335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 109.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405(4):553–562. [PubMed] [Google Scholar]
- 110.Medawar PB. The behaviour and fate of skin autografts and skin homografts in rabbits: a report to the War Wounds Committee of the Medical Research Council. J Anat. 1944;78(Pt 5):176–199. [PMC free article] [PubMed] [Google Scholar]
- 111.Medawar PB. A second study of the behaviour and fate of skin homografts in rabbits: a report to the War Wounds Committee of the Medical Research Council. J Anat. 1945;79(Pt 4):157–176. [PubMed] [Google Scholar]
- 112.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 113.Meerburg BG, Kijlstra A. Changing climate-changing pathogens: Toxoplasma gondii in North-Western Europe. Parasitol Res. 2009;105(1):17–24. doi: 10.1007/s00436-009-1447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171(5):1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller SD, McMahon EJ, Schreiner B, Bailey SL. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 2007;1103:179–191. doi: 10.1196/annals.1394.023. [DOI] [PubMed] [Google Scholar]
- 116.Mineharu Y, King GD, Muhammad AK, Bannykh S, Kroeger KM, Liu C, Lowenstein PR, Castro MG. Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: implications for clinical trial design. Clin Cancer Res. 2011;17(14):4705–4718. doi: 10.1158/1078-0432.CCR-11-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mix E, Meyer-Rienecker H, Hartung HP, Zettl UK. Animal models of multiple sclerosis—potentials and limitations. Prog Neurobiol. 2010;92(3):386–404. doi: 10.1016/j.pneurobio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mix E, Meyer-Rienecker H, Zettl UK. Animal models of multiple sclerosis for the development and validation of novel therapies—potential and limitations. J Neurol. 2008;255(Suppl 6):7–14. doi: 10.1007/s00415-008-6003-0. [DOI] [PubMed] [Google Scholar]
- 119.Mohan J, Hopkins J, Mabbott NA. Skin-derived dendritic cells acquire and degrade the scrapie agent following in vitro exposure. Immunology. 2005;116(1):122–133. doi: 10.1111/j.1365-2567.2005.02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nagra G, Koh L, Zakharov A, Armstrong D, Johnston M. Quantification of cerebrospinal fluid transport across the cribriform plate into lymphatics in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1383–1389. doi: 10.1152/ajpregu.00235.2006. [DOI] [PubMed] [Google Scholar]
- 121.Newman TA, Galea I, van Rooijen N, Perry VH. Blood-derived dendritic cells in an acute brain injury. J Neuroimmunol. 2005;166(1–2):167–172. doi: 10.1016/j.jneuroim.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 122.Okada K, Yamasoba T, Kiyono H. Craniofacial mucosal immune system: importance of its unique organogenesis and function in the development of a mucosal vaccine, vol 72. Recent advances in tonsils and mucosal barriers of the upper airways. Proceedings of the 7th International Symposium on Tonsils and Mucosal Barriers of the Upper Airways; 2010; Asahikawa, Japan. Basel, New York: Karger; 2011. [DOI] [PubMed] [Google Scholar]
- 123.Ou R, Zhang M, Huang L, Flavell RA, Koni PA, Moskophidis D. Regulation of immune response and inflammatory reactions against viral infection by VCAM-1. J Virol. 2008;82(6):2952–2965. doi: 10.1128/JVI.02191-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pachter JS, de Vries HE, Fabry Z. The blood–brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62(6):593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- 125.Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124(Pt 3):480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- 126.Pashenkov M, Soderstrom M, Huang YM, Link H. Cerebrospinal fluid affects phenotype and functions of myeloid dendritic cells. Clin Exp Immunol. 2002;128(2):379–387. doi: 10.1046/j.1365-2249.2002.01850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pashenkov M, Teleshova N, Kouwenhoven M, Smirnova T, Jin YP, Kostulas V, Huang YM, Pinegin B, Boiko A, Link H. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J Neuroimmunol. 2002;122(1–2):106–116. doi: 10.1016/s0165-5728(01)00451-9. [DOI] [PubMed] [Google Scholar]
- 128.Pellegatta S, Poliani PL, Stucchi E, Corno D, Colombo CA, Orzan F, Ravanini M, Finocchiaro G. Intra-tumoral dendritic cells increase efficacy of peripheral vaccination by modulation of glioma microenvironment. Neuro Oncol. 2010;12(4):377–388. doi: 10.1093/neuonc/nop024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peters A, Palay SL, Webster Hd. The fine structure of the nervous system: the neurons and supporting cells. Saunders; Philadelphia: 1976. [Google Scholar]
- 130.Platten M, Steinman L. Multiple sclerosis: trapped in deadly glue. Nat Med. 2005;11(3):252–253. doi: 10.1038/nm0305-252. [DOI] [PubMed] [Google Scholar]
- 131.Postigo AA, Corbi AL, Sanchez-Madrid F, de Landazuri MO. Regulated expression and function of CD11c/CD18 integrin on human B lymphocytes. Relation between attachment to fibrinogen and triggering of proliferation through CD11c/CD18. J Exp Med. 1991;174(6):1313–1322. doi: 10.1084/jem.174.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Press R, Nennesmo I, Kouwenhoven M, Huang YM, Link H, Pashenkov M. Dendritic cells in the cerebrospinal fluid and peripheral nerves in Guillain–Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neuroimmunol. 2005;159(1–2):165–176. doi: 10.1016/j.jneuroim.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 133.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14(10):1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 134.Prodinger C, Bunse J, Kruger M, Schiefenhovel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121(4):445–458. doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 135.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 136.Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999;30(1):77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 137.Ramagopalan SV, Knight JC, Ebers GC. Multiple sclerosis and the major histocompatibility complex. Curr Opin Neurol. 2009;22(3):219–225. doi: 10.1097/WCO.0b013e32832b5417. [DOI] [PubMed] [Google Scholar]
- 138.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C–C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 139.Reichmann G, Schroeter M, Jander S, Fischer HG. Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. J Neuroimmunol. 2002;129(1–2):125–132. doi: 10.1016/s0165-5728(02)00184-4. [DOI] [PubMed] [Google Scholar]
- 140.Rodriguez Boulan E, Sabatini DD. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci USA. 1978;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rosicarelli B, Serafini B, Sbriccoli M, Lu M, Cardone F, Pocchiari M, Aloisi F. Migration of dendritic cells into the brain in a mouse model of prion disease. J Neuroimmunol. 2005;165(1–2):114–120. doi: 10.1016/j.jneuroim.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 142.Rosset MB, Sacquin A, Lecollinet S, Chaigneau T, Adam M, Crespeau F, Eloit M. Dendritic cell-mediated-immunization with xenogenic PrP and adenoviral vectors breaks tolerance and prolongs mice survival against experimental scrapie. PLoS One. 2009;4(3):e4917. doi: 10.1371/journal.pone.0004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 144.Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA. 2001;98(11):6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22(13):5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schulz M, Engelhardt B. The circumventricular organs participate in the immunopathogenesis of experimental auto-immune encephalomyelitis. Cerebrospinal Fluid Res. 2005;2:8. doi: 10.1186/1743-8454-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Serot JM, Foliguet B, Bene MC, Faure GC. Ultrastructural and immunohistological evidence for dendritic-like cells within human choroid plexus epithelium. NeuroReport. 1997;8(8):1995–1998. doi: 10.1097/00001756-199705260-00039. [DOI] [PubMed] [Google Scholar]
- 148.Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65(1):94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sethi S, Kerksiek KM, Brocker T, Kretzschmar H. Role of the CD8+ dendritic cell subset in transmission of prions. J Virol. 2007;81(9):4877–4880. doi: 10.1128/JVI.02345-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 151.Skarica M, Wang T, McCadden E, Kardian D, Calabresi PA, Small D, Whartenby KA. Signal transduction inhibition of APCs diminishes Th17 and Th1 responses in experimental autoimmune encephalomyelitis. J Immunol. 2009;182(7):4192–4199. doi: 10.4049/jimmunol.0803631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Steel CD, Hahto SM, Ciavarra RP. Peripheral dendritic cells are essential for both the innate and adaptive antiviral immune responses in the central nervous system. Virology. 2009;387(1):117–126. doi: 10.1016/j.virol.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13(10):1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 155.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94(4):459–473. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 156.Stevenson PG, Hawke S, Sloan DJ, Bangham CR. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol. 1997;71(1):145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28(10):1507–1521. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 158.Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29(9):506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 159.Streit WJ, Graeber MB. Heterogeneity of microglial and perivascular cell populations: insights gained from the facial nucleus paradigm. Glia. 1993;7(1):68–74. doi: 10.1002/glia.440070112. [DOI] [PubMed] [Google Scholar]
- 160.Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Daffis S, Rudensky AY, Bevan MJ, Clark EA, Kaja MK, Diamond MS, Gale M., Jr IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog. 2010;6(2):e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12–13):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 163.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31(45):16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trifilo MJ, Lane TE. The CC chemokine ligand 3 regulates CD11c+ CD11b+ CD8alpha− dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 2004;327(1):8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175(12):7781–7787. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 166.van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354(1):44–53. doi: 10.1056/NEJMra052116. [DOI] [PubMed] [Google Scholar]
- 167.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117(1):1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 168.Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36(2):156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- 169.Wuest TR, Carr DJ. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J Immunol. 2008;181(11):7985–7993. doi: 10.4049/jimmunol.181.11.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17(1):6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zozulya AL, Ortler S, Lee J, Weidenfeller C, Sandor M, Wiendl H, Fabry Z. Intracerebral dendritic cells critically modulate encephalitogenic versus regulatory immune responses in the CNS. J Neurosci. 2009;29(1):140–152. doi: 10.1523/JNEUROSCI.2199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]