Abstract
Objective
Subantimicrobial dose doxycycline (SDD) treatment has been reported to reduce the severity of chronic inflammation and to increase serum HDL cholesterol. We determined in a double-blind, placebo-controlled clinical trial, whether SDD affects the ability of serum to facilitate cholesterol removal from macrophages.
Methods
Forty-five postmenopausal osteopenic women with periodontitis were randomly assigned to take placebo (n=26) or doxycycline hyclate (20 mg, n=19) tablets twice daily for 2 years. Serum samples were collected at baseline, 1-year, and 2-year appointments. The cholesterol efflux capacity of serum from cultured human macrophages (THP-1) was measured.
Results
SDD subjects demonstrated a significant increase in serum-mediated cholesterol efflux from macrophages at both time points, compared to baseline (p < 0.04 for each). Mean cholesterol efflux levels over the first year of follow-up were 3.0 percentage points (unit change) higher among SDD subjects compared to placebo subjects (p = 0.010), while there was no significant difference in 2-year changes. There were no significant differences in the changes of apolipoprotein A-I, apolipoprotein A-II, or serum amyloid A levels between the groups.
Conclusions
Our results suggest that SDD treatment may reduce the risk of cardiovascular disease in this patient group by increasing the cholesterol efflux capacity of serum.
Keywords: Atherosclerosis, Clinical trials, Inflammation, Lipids, Macrophages
Introduction
Periodontitis is a bacterial infection of the tooth-supporting tissues leading to chronic inflammation. It is one of the most common chronic inflammatory diseases in the adult population; a recent study from the United States revealed that 47% of adults have periodontitis, and of adults aged 65 years and older, 64% suffer from moderate or severe periodontitis [1]. According to present knowledge, chronic periodontitis is associated with an increased risk for atherosclerotic coronary artery disease (CAD), although a causal relationship has not yet been proven [2].
As a systemic response to periodontitis, patients suffer from low-grade chronic inflammation and display elevated serum levels of inflammatory biomarkers, such as C-reactive protein (CRP), interleukin-6 (IL-6) [3,4], and matrix metalloproteinases [5]. These inflammatory mediators are also regarded as biomarkers of CAD risk [6].
Decreased serum high density lipoprotein (HDL) cholesterol concentration is a significant independent risk factor for CAD. Low levels of HDL cholesterol and apolipoprotein A-I (apoA-I), the major protein component of HDL particles, have been associated with CAD incidence in numerous studies [7]. Among the several anti-atherogenic properties of HDL, the major one is considered to be its ability to facilitate the efflux of cholesterol from macrophage foam cells in the arterial walls. Cholesterol efflux from cell membranes is the first and rate-limiting step of reverse cholesterol transport, the process in which excess cholesterol is transported from peripheral tissues back to the liver for excretion. According to in vitro studies, pro-inflammatory factors such as bacterial endotoxins, tumor necrosis factor-alpha (TNF-α), and IL-1β, may impair cholesterol efflux from macrophages and reverse cholesterol transport [8]. Inflammation, including periodontitis, is associated with low serum HDL cholesterol concentration and an altered HDL composition [9]. The changes in HDL composition, especially replacement of apoA-I with serum amyloid A (SAA), induced by inflammation, may affect the cholesterol efflux capacity of HDL particles. The exact mechanisms, however, that generate the low HDL cholesterol profile during inflammation remain unclear.
Tetracyclines, in addition to their antimicrobial properties, also have immunomodulatory and anti-proteolytic effects [10] at both regular and low (sub-antimicrobial) concentrations [11]. Subantimicrobial dose doxycycline (SDD) treatment has been shown to reduce the severity of chronic inflammatory disorders such as periodontitis [12] and rheumatoid arthritis [13]. A meta-analysis of seven human studies indicated that, in addition to conventional periodontal therapy, the use of SDD demonstrated a significant positive effect in the treatment of chronic periodontitis [14]. SDD reduced high sensitivity (hs) -CRP and IL-6 levels in plasma as well as MMP-9 activity in patients with acute coronary syndrome [15]. SDD increased serum HDL cholesterol and apoA-I levels in a six-week trial of thirty-six patients [16]. SDD also significantly decreased MMP-9 and hs-CRP levels in post-menopausal osteopenic women with periodontitis, and increased HDL cholesterol among the subgroup of women more than 5 years postmenopausal [17].
With this background, our aim was to examine the effect of SDD therapy on the cholesterol efflux capacity of serum samples derived from a group of postmenopausal, osteopenic women with chronic periodontitis. The patients were at risk of developing CAD, but they had no history of myocardial infarction, angina or stroke. The data from this study represent secondary outcomes from a subgroup of patients who participated in a placebo-controlled, double-blind randomized clinical trial.
Methods
Study subjects
The details of the clinical trial and sample size estimation have been described earlier [18]. Briefly, the subjects were post-menopausal osteopenic females 45 to 70 years of age, and not receiving hormone replacement therapy. They had a history of moderate to advanced chronic periodontitis, and were undergoing periodontal maintenance therapy. Subjects had no history of myocardial infarction, angina or stroke. The study was a placebo-controlled, double-blind, two center (University of Nebraska Medical Center College of Dentistry and Stony Brook University School of Dental Medicine) randomized clinical trial. Only data from Stony Brook subjects were included in this paper as insufficient amount of serum remained from Nebraska subjects after completion of the previous biomarker analyses. Fifty-three subjects were randomized at Stony Brook; 46 Stony Brook subjects completed the trial and signed an addendum consent form to conduct additional serum analyses. Serum was not collected for one subject at the one year visit; thus, 45 subjects who had been randomly assigned to take placebo (n=26) or doxycycline hyclate (20 mg, n=19) tablets twice daily for 2 years were included in the analyses of cholesterol efflux and serum concentrations of apoA-I, apoA-II, and SAA. The study protocol was reviewed and approved by the Stony Brook Institutional Review Board.
Blood samples were drawn at baseline, 1-year, and 2-year appointments. Blood (15 ml) was drawn from the antecubital fossa using standard venipuncture technique. The blood was spun at 1000 g in a refrigerated centrifuge for 10 minutes after remaining at room temperature for 20 minutes. Serum samples were then aliquoted and frozen at -80°C until analyzed.
Isolation and radiolabeling of LDL
Low density lipoprotein (LDL) was isolated from human citrate plasma as a density cut-off 1.019 < d < 1.063 g/ml by sequential ultracentrifugation in a Beckman Ti 50.2 rotor at a speed of 193,000 g for 24 h using KBr to adjust the density [19]. Isolated LDL was acetylated in the presence of acetic anhydride [20] and sterile filtered. Thereafter, acetyl-LDL (acLDL) was labeled by incubating with [1α,2α(n)3H] cholesteryl oleate (PerkinElmer, Waltham, MA) dissolved in dimethyl sulfoxide. The specific activity of the [1α,2α(n)3H] cholesteryl oleate −acLDL was 18000 dpm/μg.
Cell Culture and Loading of the Cells with Cholesteryl Esters
Human THP-1 monocytes were purchased from American Type Culture Collection (ATCC). The monocytes were maintained in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS) (Lonza, Basel, Switzerland), 25 mM HEPES, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. The cells were used for the experiments between passages 7 and 12. Lipoprotein-deficient serum (LPDS; d > 1.25 g/ml) was prepared from FBS by ultracentrifugation in a TL-100.3 rotor at a speed of 541,000 g for 18 h at 10°C in an Optima TL-100 Tabletop Ultracentrifuge (Beckman) using solid KBr to adjust the density [21] and sterile filtered prior to use.
To differentiate the cells into macrophages, they were plated onto 24-well plates at a density of 500,000 cells/ml and treated with 100 nM phorbol 12-myristate 13-acetate (PMA) in the growth medium for 72 h. The macrophages were washed twice with PBS and loaded with [1α,2α(n)-3H] cholesteryl oleate −acLDL (10 μg of protein/ml) in RPMI 1640 medium containing 5% LPDS, 25 mM HEPES, 100 U/ml penicillin and 100 μg/ml streptomycin for 48 h. The cells were washed twice with PBS and the medium was changed to DMEM supplemented with 25 mM HEPES and the antibiotics.
Cholesterol Efflux Assay
The ability of the serum samples to remove cholesterol from acLDL-loaded THP-1 macrophages was measured as follows: serum (1% v/v) from placebo or SDD treated subjects was added to triplicate wells containing acLDL-loaded macrophages and the cells were incubated for 16 h. The control cells were incubated in the absence of serum to measure the spontaneous cholesterol diffusion. After incubation, the medium was collected and centrifuged to remove any detached cells. The cells were washed twice with PBS and lysed with 0.2 M NaOH. The radioactivity in the medium and cell lysates was analyzed by liquid scintillation counting (Wallac WinSpectral 1414, Wallac, Turku, Finland). Cholesterol efflux was calculated as the percentage of the medium [3H] activity of the total [3H] activity (medium + cells). Efflux values to incubation medium in the absence of serum were subtracted from those in the presence of serum. The experiments were performed in triplicate wells. The samples (baseline, 1-year, and 2-year) of each patient were measured on the same plate in adjacent wells. Each plate included samples from both placebo and SDD subjects.
Measurement of apoA-I, apoA-II and SAA
ApoA-I was quantified from serum samples by an ELISA-based assay [22]. Of other HDL-associated proteins, the concentrations of apoA-II and SAA were measured with commercial ELISA kits (apoA-II: Abnova, Taipei City, Taiwan; SAA: Invitrogen, Carlsbad, CA, USA). The concentrations of HDL cholesterol, total cholesterol, triglycerides, IL-6, MMP-8, MMP-9, TIMP-1, TNFα, and hsCRP were measured from the serum samples previously [17].
Statistical analyses
Analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA, version 9.1.3). These data represent secondary analyses from a randomized clinical trial. Demographic, clinical and medication use distributions were compared between SDD and placebo groups using a 2-sample t-test for continuous measures and a Chi-square test for categorical measures. Repeated measurements over time were analyzed using subject-level data. Linear regression models were fit, using generalized estimating equations methodology to account for the correlation among the repeated measurements for each subject. Changes in the follow-up outcome measures relative to baseline were modeled as a function of the study drug with adjustment for the baseline outcome measure, visit (one-year or two-year), and baseline smoking status (a randomization stratification factor) as independent variables [23]. A natural log transformation was applied to the outcome measure to satisfy linear model assumptions for models of triglyceride and MMP-8 levels. A visit-by-study-drug interaction was investigated. Given the limited power to detect differences in time trends between treatment groups, analyses were stratified by study visit. The distribution of one- and two-year changes in the outcome measures relative to baseline were compared to a null value of 0 using a one-sample t-test for within-group analyses. The estimated mean change in cholesterol efflux from baseline at a given visit is expressed in the original measurement units, percentage points, and not as a relative change. Similarly, model estimates of the effect sizes for other measures are expressed in the original measurement units. Linear mixed effects models, with random subject and tooth terms, were used to estimate the standard deviation of baseline site-level periodontal measures.
The primary analysis was intent-to-treat, where data were analyzed according to randomized assignment (SDD=19; placebo=26). A secondary, per-protocol analysis included only measurements up to the time point at which lack of protocol adherence occurred (e.g., initiation of significant concomitant medications or pill count adherence rate below 80%) (SDD=11; placebo=14). Pre-specified subgroup analyses were performed based on time since menopause (within or beyond 5 years) and statin use, using tests of interactions in the regression models. No formal adjustment to the alpha level was made for multiple tests performed as the subgroup analyses are exploratory in nature. Linear regression models were created to describe the associations between serum cholesterol efflux capacity (outcome variable) and other serum measures, which included lipids (cholesterol, triglycerides), proteolytic enzymes (MMP-8, MMP-9, TIMP-1), inflammation markers (TNF-α, IL-6, hsCRP), and HDL-associated proteins (apoA-I, apoA-II, SAA, HDL total protein), at the same visit using a similar modeling approach as previously described to investigate modifying effects of SDD treatment and follow-up time.
Results
The baseline characteristics and medication use of the study subjects are presented in Table 1. The demographic characteristics, clinical characteristics and medication use did not differ between SDD and placebo groups.
Table 1.
Subject Demographics and Baseline Characteristicsa
| Characteristic | Placebo Group (n = 26) | SDD Group (n = 19) | p-value |
|---|---|---|---|
| Age (years) | 55.96 (5.44) | 58.07 (5.44) | 0.21 |
| Ethnicity | > 0.9 | ||
| Hispanic or Latino | 2 (8%) | 1 (5%) | |
| Not Hispanic or Latino | 24 (92%) | 18 (95%) | |
| Race | > 0.9 | ||
| Asian | 2 (8%) | 1 (5%) | |
| African American | 1 (4%) | ||
| White | 23 (88%) | 18 (95%) | |
| Years postmenopausal | 0.6 | ||
| 5 or fewer years | 13 (50%) | 8 (42%) | |
| More than 5 years | 13 (50%) | 11 (58%) | |
| Smoking status | 0.29 | ||
| Current smoker | 6 (23%) | 2 (11%) | |
| Former smoker | 7 (27%) | 9 (47%) | |
| Never smoker | 13 (50%) | 8 (42%) | |
| Dyslipidemiab | 23 (88%) | 17 (89%) | > 0.9 |
| Hypertensionc | 4 (15%) | 2 (11%) | > 0.9 |
| History of myocardial infarction, angina or stroke | 0 | 0 | |
| Diabetes | 0 | 0 | |
| Body Mass Index (kg/m2) | 27.41 (5.60) | 25.30 (3.39) | 0.12 |
| Number of teeth | 26.00 (2.37) | 25.63 (3.64) | 0.7 |
| Lumbar spine | |||
| Bone mineral density (g/cm2) | 0.88 (0.06) | 0.91 (0.06) | 0.15 |
| T-score | -1.52 (0.58) | -1.23 (0.53) | 0.1 |
| Femoral neck | |||
| Bone mineral density (g/cm2) | 0.73 (0.09) | 0.71 (0.07) | 0.54 |
| T-score | -1.16 (0.76) | -1.27 (0.67) | 0.62 |
| Alveolar bone height (mm) | 3.35 (1.43) | 3.59 (1.61) | 0.34 |
| Manual probing depth (mm) | 4.26 (1.39) | 4.18 (1.31) | 0.55 |
| Medication use | |||
| Aspirin (any dose ≤ 325 mg) | 1 (4%) | 1 (5%) | > 0.9 |
| Statins | 1 (4%) | 2 (11%) | 0.56 |
| Diuretics | 3 (13%) | 0 | 0.11 |
| Beta blockers | 2 (8%) | 1 (5%) | > 0.9 |
| Calcium channel blockers | 1 (4%) | 1 (5%) | > 0.9 |
| Angiotensin-converting enzyme inhibitors / angiotensin receptor blockers | 2 (8%) | 0 | 0.5 |
Data are expressed as count (%) for categorical variables and mean (standard deviation) for continuous measures. Standard deviation of alveolar bone height and probing depth was estimated using a linear mixed model.
Dyslipidemia is defined as total cholesterol > 5.17 mmol/l (200 mg/dl) or LDL cholesterol > 2.59 mmol/l (100 mg/dl) or HDL cholesterol < 1.29 mmol/l (50 mg/dl)
Hypertension was coded as present for any subject reporting use of a diuretic, calcium channel blocker, beta blocker, or angiotensin-converting enzyme inhibitor/angiotensin receptor blocker
The results of the cholesterol efflux experiments are illustrated in Figure 1. The mean cholesterol efflux % levels at baseline did not differ between the SDD and placebo groups (Table 2). In between-group comparisons, the data were analyzed as to whether the change from baseline differed between the SDD and the placebo groups. Mean cholesterol efflux % levels at the first year time point of follow-up were 3.0 percentage points (unit change) higher among SDD subjects compared to the placebo subjects (95% CI: 0.7-5.3 percentage points, p = 0.010) after adjustment for baseline cholesterol efflux levels and smoking status, while there was no significant difference based on 2-year changes (0.7 percentage point increase associated with SDD, 95% CI: 1.8 decrease to 3.1 increase, p=0.61). When the change in cholesterol efflux levels from baseline was analyzed for the SDD and the placebo groups separately, SDD subjects demonstrated a significant increase in cholesterol efflux at the 1-year and 2-year time points compared to baseline (mean unit change from baseline at 12-month time point: 2.83 percentage points; mean unit change from baseline at 24-month time point: 2.66 percentage points; p < 0.04 for each) (Table 2, Figure 1). There were no significant changes in cholesterol efflux in the placebo group during this time period.
Fig. 1. The effect of SDD on the cholesterol efflux capacity of serum during the two-year clinical trial.
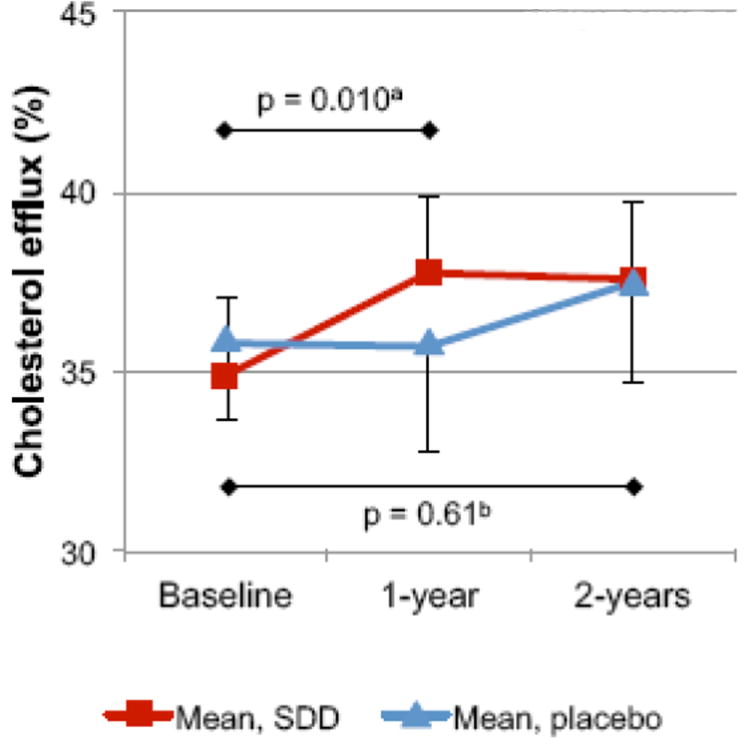
The efflux capacity of serum samples was measured by incubating acLDL-loaded macrophages in the presence of 1% serum. Cholesterol efflux was calculated as a percentage of [3H]cholesterol released into the medium of the total [3H]cholesterol in the medium and the cells. The efflux capacity of each serum sample was calculated as a mean of three incubations. The number of subjects was 26 in the placebo group and 19 in the SDD group
a p-value for 1-year change when comparing between SDD and placebo
b p-value for 2-year change when comparing between SDD and placebo
Table 2.
Serum cholesterol efflux capacity and the concentrations of apoA-I, apoA-II, SAA, HDL cholesterol, total cholesterol, and triglycerides in the study groups.
| Placebo group (n=26) | SDD group (n=19) | SDD versus placebo | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | p* | Mean | SD | p* | p ** | |
|
| |||||||
| Cholesterol efflux (%) | |||||||
| Baseline | 35.81 | 4.40 | 34.91 | 4.20 | |||
| One year | 35.67 | 5.74 | 37.75 | 4.33 | |||
| Two years | 37.45 | 5.56 | 37.58 | 4.23 | |||
| One-year Change from Baseline | -0.14 | 5.91 | 0.90 | 2.83 | 5.37 | 0.034 | 0.010 |
| Two-year Change from Baseline | 1.64 | 4.62 | 0.082 | 2.66 | 4.20 | 0.013 | 0.61 |
|
| |||||||
| ApoA-I (mg/ml) | |||||||
| Baseline | 1.59 | 0.46 | 1.56 | 0.54 | |||
| One year | 1.52 | 0.42 | 1.51 | 0.46 | |||
| Two years | 1.66 | 0.55 | 1.47 | 0.47 | |||
| One-year Change from Baseline | -0.07 | 0.30 | 0.23 | -0.05 | 0.44 | 0.63 | 0.89 |
| Two-year Change from Baseline | 0.06 | 0.40 | 0.43 | -0.09 | 0.38 | 0.32 | 0.11 |
|
| |||||||
| ApoA-II (mg/ml) | |||||||
| Baseline | 0.23 | 0.07 | 0.23 | 0.06 | |||
| One year | 0.25 | 0.09 | 0.22 | 0.06 | |||
| Two years | 0.24 | 0.08 | 0.25 | 0.10 | |||
| One-year Change from Baseline | 0.02 | 0.09 | 0.27 | -0.01 | 0.05 | 0.49 | 0.10 |
| Two-year Change from Baseline | 0.00 | 0.06 | 0.73 | 0.03 | 0.09 | 0.22 | 0.36 |
|
| |||||||
| SAA (μg/ml) | |||||||
| Baseline | 46.19 | 49.97 | 31.88 | 35.48 | |||
| One year | 35.69 | 26.98 | 31.05 | 27.90 | |||
| Two years | 41.06 | 33.18 | 34.12 | 27.23 | |||
| One-year Change from Baseline | -10.50 | 43.95 | 0.23 | -0.83 | 41.32 | 0.93 | 0.87 |
| Two-year Change from Baseline | -5.13 | 49.85 | 0.60 | 2.23 | 27.12 | 0.72 | 0.87 |
|
| |||||||
| HDL-C (mg/dl) | |||||||
| Baseline | 71.88 | 33.74 | 59.79 | 19.11 | |||
| One year | 73.23 | 28.25 | 62.26 | 18.67 | |||
| Two years | 70.69 | 25.29 | 64.32 | 16.29 | |||
| One-year Change from Baseline | 1.35 | 14.23 | 0.83 | 2.47 | 13.32 | 0.81 | 0.53 |
| Two-year Change from Baseline | -1.19 | 17.47 | 0.81 | 4.53 | 11.95 | 0.19 | 0.97 |
|
| |||||||
| Total Cholesterol (mg/dl) | |||||||
| Baseline | 235.12 | 57.7 | 210.26 | 42.73 | |||
| One year | 233.27 | 53.58 | 208.42 | 41.8 | |||
| Two years | 223.50 | 49.28 | 217.11 | 38.08 | |||
| One-year Change from Baseline | -1.85 | 42.74 | 0.58 | -1.84 | 39.85 | 0.59 | 0.41 |
| Two-year Change from Baseline | -11.62 | 61.21 | 0.63 | 6.84 | 33.65 | 0.48 | 0.79 |
|
| |||||||
| Triglycerides (mg/dl)*** | |||||||
| Baseline | 120.65 | 57.74 | 121.68 | 69.08 | |||
| One year | 125.85 | 70.37 | 123.32 | 71.28 | |||
| Two years | 108.73 | 44.43 | 106.58 | 49.36 | |||
| One-year Change from Baseline | 5.19 | 62.69 | 0.87 | 1.63 | 43.06 | 0.93 | 0.81 |
| Two-year Change from Baseline | -11.92 | 47.89 | 0.33 | -15.11 | 51.07 | 0.37 | 0.74 |
|
| |||||||
| MMP-8 (ng/ml) | |||||||
| Baseline | 41.67 | 28.82 | 37.42 | 31.71 | |||
| One year | 32.37 | 19.11 | 24.8 | 15.14 | |||
| Two years | 46.15 | 42.93 | 32.26 | 21.83 | |||
| One-year Change from Baseline | -9.31 | 24.48 | 0.21 | -12.62 | 31.62 | 0.20 | 0.16 |
| Two-year Change from Baseline | 4.47 | 31.53 | 0.93 | -4.61 | 35.09 | 0.73 | 0.86 |
p-value comparing mean change to 0
p-value comparing mean change between SDD and placebo after adjustment for baseline measures and smoking status
Hypothesis testing based on natural log transformed values
In addition to cholesterol efflux, we analyzed the concentrations of certain HDL associated proteins. No significant differences in the changes of apoA-I, apoA-II, or SAA levels over the follow-up time between the SDD and the placebo groups were observed (Table 2). Furthermore, no significant differences were found in HDL cholesterol, total cholesterol, triglyceride, or MMP-8 concentrations between the groups (Table 2).
In the subgroup of women more than five years post-menopausal, the 1-year changes in cholesterol efflux % levels were 3.96 percentage points higher in the SDD group (n = 11) compared to the placebo group (n = 13) (p = 0.012), although statistically, this effect was not different from that seen among women within 5 years of menopause (p=0.17)(Table 3).
Table 3.
Cholesterol efflux capacities of serum and the effect of SDD treatment in subgroups of subjects defined by time since menopause
| Cholesterol efflux (%) | Placebo group | SDD group | Estimated difference in mean change from baseline between groups* | 95% CI | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | Mean | SD | n | Mean | SD | ||||
|
| |||||||||
| Subjects within 5 years of menopause | |||||||||
| Baseline | 13 | 36.03 | 2.97 | 8 | 35.17 | 5.38 | |||
| One year change from baseline | 13 | 0.28 | 7.73 | 8 | 1.51 | 4.67 | 1.24 | -1.88 to 4.35 | 0.44 |
| Two year change from baseline | 13 | 1.71 | 4.89 | 8 | 1.5 | 3.62 | -0.63 | -3.87 to 2.61 | 0.70 |
|
| |||||||||
| Subjects more than 5 years postmenopausal | |||||||||
| Baseline | 13 | 35.59 | 5.61 | 11 | 34.73 | 3.38 | |||
| One year change from baseline | 13 | -0.57 | 3.56 | 11 | 3.79 | 5.86 | 3.96 | 0.87 to 7.05 | 0.012 |
| Two year change from baseline | 13 | 1.57 | 4.52 | 11 | 3.51 | 4.55 | 1.75 | -1.98 to 5.47 | 0.36 |
Difference in mean change from baseline calculated as (SDD minus Placebo) and estimated based on a linear regression model with adjustment for baseline cholesterol efflux and smoking status.
The association between cholesterol efflux and certain lipids, inflammation markers, and proteolytic enzymes was studied. The cholesterol efflux capacities of serum were significantly associated with total cholesterol (positive association, at the 2-year visit only) and the proinflammatory cytokine IL-6 (inverse association, among placebo subjects only) (Table 4). There was no association between cholesterol efflux capacity and serum HDL cholesterol, LDL cholesterol, triglyceride, MMP-8, MMP-9, TIMP-1, TNFα, or hsCRP concentrations.
Table 4.
Coefficients from the regression models describing the associations between serum cholesterol efflux capacity (outcome variable) and other serum measures.
| Analysis Seta | Regression coefficient | p-value | |
|---|---|---|---|
| Total cholesterol | All subjects, Baseline visit | 0.012 | 0.38 |
| All subjects, 1-year visit | -0.026 | 0.15 | |
| All subjects, 2-year visit | 0.034 | 0.024 | |
|
| |||
| HDL Cholesterol | All subjects, All visits | -0.023 | 0.19 |
|
| |||
| LDL Cholesterol | All subjects, All visits | 0.015 | 0.13 |
|
| |||
| Triglycerides | All subjects, All visits | 0.0022 | 0.70 |
|
| |||
| MMP-8 | All subjects, All visits | -0.007 | 0.70 |
|
| |||
| MMP-9 | All subjects, All visits | -0.012 | 0.15 |
|
| |||
| TIMP-1 | All subjects, All visits | 0.22 | 0.11 |
|
| |||
| TNF-α | All subjects, All visits | 0.10 | 0.91 |
|
| |||
| IL-6 | Placebo, All visits | -3.03 | 0.0004 |
| SDD, All visits | 1.19 | 0.30 | |
|
| |||
| hsCRP | All subjects, All visits | 0.11 | 0.70 |
|
| |||
| ApoA-I | All subjects, All visits | 3.54 | 0.0006 |
|
| |||
| ApoA-II | All subjects, All visits | -1.79 | 0.69 |
|
| |||
| SAA | All subjects, All visits | 0.023 | 0.15 |
|
| |||
| Total HDL protein b | All subjects, All visits | 3.49 | 0.0005 |
Analyses are stratified based on significant subgroup effects as indicated by tests of interactions (p=0.025 total cholesterol, p=0.0093 IL-6).
Sum of apoA-I, apoA-II and SAA.
However, a significant positive association between apoA-I levels and serum cholesterol efflux capacity was demonstrated (regression coefficient 3.54, p = 0.0006) (Table 4). There was no association between apoA-II or SAA levels and cholesterol efflux. Finally, total HDL protein (the sum of apoA-I, apoA-II and SAA) was positively associated with cholesterol efflux when summarizing across all study visits (regression coefficient 3.49, p = 0.0005) (Table 4).
Discussion
In this study, mean cholesterol efflux capacity of serum samples over the first year of the clinical trial was significantly higher among SDD-treated subjects compared to placebo subjects after adjustment for baseline cholesterol efflux levels and smoking status. However, the difference between SDD patients and placebo patients was not maintained through the second year of the study. The cholesterol efflux capacity of serum was directly associated with the concentration of apoA-I and the sum of apoA-I, apoA-II and SAA proteins and, in the placebo group only, inversely associated with serum IL-6.
Previous studies have shown that, in addition to its antimicrobial activity, doxycycline also exhibits anti-inflammatory and anticollagenase properties [10,11]. SDD therapy for 6 months lowered the plasma levels of CRP and IL-6, and MMP-9 activity [15]. SDD treatment for 2 years decreased serum hsCRP and MMP-9 levels in the overall population of postmenopausal osteopenic women and increased serum HDL cholesterol levels in a subgroup of women more than five years postmenopausal [17]. According to our results, SDD also affects the capacity of serum to remove excess cholesterol from macrophages. Interestingly, SDD treatment especially affected the serum efflux capacity of women more than 5 years postmenopausal.
Based on between-treatment group comparisons, mean cholesterol efflux levels increased significantly over the first year of follow-up for SDD patients relative to placebo patients, while there was no significant difference between treatment groups based on 2-year changes from baseline. When considering within-group comparisons relative to baseline, the increase in cholesterol efflux observed among SDD patients at the one-year time point was sustained through the two-year study period. Cholesterol efflux levels were significantly increased at the 1-year and 2-year time points, relative to baseline, for SDD subjects. However, there was an increase in mean cholesterol efflux levels, although not statistically significant, in the placebo group at the 2-year time point relative to baseline, rendering the between-treatment group comparison of 2-year changes non-significant. This increase observed among placebo patients may be due to lipid-lowering medication used. Post-baseline initiation of or an increase in the dose of a statin was slightly more common among the placebo than the SDD patients during the course of the 2-year study: 3 of the placebo patients (12 %) either started a statin or increased the dose after the 6-month visit compared to 0% of the SDD patients, which may have influenced the results in this relatively small sample.
Since the best known promoters of cholesterol efflux are HDL and lipid-poor apoA-I, most efflux studies are carried out using isolated HDL as cholesterol acceptor. However, the serum environment containing the HDL with all its subpopulations in their native proportions and compositions as cholesterol acceptors is physiologically the most relevant one. Naturally, serum ability to remove cholesterol from macrophage-foam cells depends on multiple factors. In addition to lipoprotein subclass distribution and composition, the activities of cholesteryl ester transfer protein (CETP), lecithin-cholesterol acyltransferase (LCAT), and phospholipid transfer protein (PLTP) affect the macrophage cholesterol efflux [24]. We demonstrated that cholesterol efflux was correlated with the concentration of apoA-I and total HDL protein (calculated as a sum of apoA-I, apoA-II, and SAA). We did not observe a correlation between serum HDL cholesterol levels and cholesterol efflux. Importantly, however, there was a negative correlation between serum IL-6 and cholesterol efflux among placebo subjects, which is in agreement with our earlier study where cholesterol efflux had a negative association with CRP levels in periodontitis patients [9]. The present correlation with IL-6 was not observed among subjects treated with SDD, which is logical since this treatment has an effect on systemic inflammation [17].
HDL is a heterogeneous population of particles that differ in particle size, charge, and lipid/protein composition. The functional capacity of HDL may vary significantly among these subpopulations [25]. In this context, our study was limited since the composition or functional capacity of HDL or its subclasses was not determined, and neither were the activities of major HDL-modifying factors, such as CETP, LCAT, or PLTP. Even though there was an association between cholesterol efflux capacity and apoA-I concentration, the modifying effect of SDD on cholesterol efflux of serum could not be explained by the changes in apoA-I levels. Thus, the detailed mechanisms responsible for the increase of cholesterol efflux during SDD therapy require further investigation.
Other limitations of our study include the small sample size and the study population being comprised of women only. The potential effect of SDD on men should be studied separately.
It has been suggested [26,27] that plasma is better than serum in analyzing circulating MMPs and TIMPs, as blood cells, platelets, and the coagulation process contribute to the concentrations of MMPs and TIMPs during serum preparation. The MMP-8, MMP-9, and TIMP-1 concentrations that were analyzed in this study were measured from serum samples [17]. MMP-8 concentrations between these two sample materials have a strong correlation with each other [28] and particularly serum MMP-8 levels associate with future CVD events [29,30].
Patients with chronic periodontitis suffer from low-grade systemic inflammation, as reflected by elevated levels of serum inflammation markers [31]. The levels of HDL cholesterol and apoA-I are decreased during both acute and chronic inflammations [32,33], such as periodontitis. Periodontitis causes changes in HDL metabolism and decreases its antiatherogenic potency and cholesterol efflux capacity [9]. Keeping in mind that five out of ten adults display signs of chronic periodontitis, their “dysfunctional” HDL may be associated with a considerably increased long-term atherosclerosis risk. In the SDD treatment group, the cholesterol efflux increased 2.83 percentage units relative to baseline over the first year, representing an 8.1% relative increment, which may have substantial long-term antiatherogenic effects. SDD therapy increased the cholesterol efflux capacity of serum to an even greater extent among women who were more than five years postmenopausal. In previous reports, it was found that SDD also decreased biomarkers of systemic inflammation (hsCRP [15,17] and IL-6 [15]) and mediators of atheromatous plaque destabilization (MMP-9 [15,17]), and increased cardio-protective HDL cholesterol in a subgroup of women more than five years postmenopausal [17]. The results from this and the previous studies suggest that SDD therapy may reduce the risk of cardiovascular disease in this vulnerable group of postmenopausal women with significantly reduced protective endogenous estrogen levels.
Acknowledgments
The project was supported by Grant Numbers R01DE012872 (Dr. Jeffrey Payne, PI and Dr. Lorne Golub, Co-PI) and R03DE019805 (Dr. Julie Stoner PI) from the National Institute of Dental and Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health.
The study was supported by the Finnish Dental Society Apollonia, the Sigrid Juselius Foundation, and the Paulo Foundation (grants to Pirkko Pussinen), and the Academy of Finland, and Helsinki University Central Hospital Research Foundation (grants to Timo Sorsa).
Footnotes
Author disclosures
Dr. Golub is listed as an inventor on patents on the test medication in this trial and those have been fully assigned to his institution, Stony Brook University. He is also a consultant to Galderma Research and Development (Lausanne, Switzerland) which has licensed a series of tetracycline patents from the State University of New York. Drs. Payne and Golub have a patent pending for treatment of postmenopausal women with non-antibacterial tetracyclines.
References
- 1.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, et al. Periodontal Disease and Atherosclerotic Vascular Disease: Does the Evidence Support an Independent Association?: A Scientific Statement From the American Heart Association. Circulation. 2012;125:2520–44. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 3.Loos BG, Craandijk J, Hoek FJ, Werteim-van Dillen PM, van der Velden U. Elevation of Systemic Markers Related to Cardiovascular Diseases in the Peripheral Blood of Periodontitis Patients. J Periodontol. 2000;71:1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 4.Joshipura KJ, Wand HC, Merchant AT, Rimm EB. Periodontal Disease and Biomarkers Related to Cardiovascular Disease. J Dent Res. 2004;83:151–5. doi: 10.1177/154405910408300213. [DOI] [PubMed] [Google Scholar]
- 5.Marcaccini AM, Novaes AB, Jr, Meschiari CA, Souza SL, Palioto DB, Sorgi CA, Faccioli LH, Tanus-Santos JE, Gerlach RF. Circulating matrix metalloproteinase-8 (MMP-8) and MMP-9 are increased in chronic periodontal disease and decrease after non-surgical periodontal therapy. Clin Chim Acta. 2009;409:117–22. doi: 10.1016/j.cca.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb Haemost. 2006;95:511–8. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- 7.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J Lipid Res. 2003;44:1728–36. doi: 10.1194/jlr.M300100-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Pussinen PJ, Jauhiainen M, Vilkuna-Rautiainen T, Sundvall J, Vesanen M, Mattila K, Palosuo T, Alfthan G, Asikainen S. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J Lipid Res. 2004;45:139–147. doi: 10.1194/jlr.M300250-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Golub LM, Lee H-, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines Inhibit Connective Tissue Breakdown by Multiple Non-Antimicrobial Mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 11.Golub LM, Sorsa T, Lee HM, Ciancio S, Sorbi D, Ramamurthy NS, Gruber B, Salo T, Konttinen YT. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Periodontol. 1995;22:100–9. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 12.Golub LM, Lee HM, Stoner JA, Sorsa T, Reinhardt RA, Wolff MS, Ryan ME, Nummikoski PV, Payne JB. Subantimicrobial-Dose Doxycycline Modulates Gingival Crevicular Fluid Biomarkers of Periodontitis in Postmenopausal Osteopenic Women. J Periodontol. 2008;79:1409–18. doi: 10.1902/jop.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Dell JR, Elliott JR, Mallek JA, Mikuls TR, Weaver CA, Glickstein S, Blakely KM, Hausch R, Leff RD. Treatment of early seropositive rheumatoid arthritis: Doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum. 2006;54:621–627. doi: 10.1002/art.21620. [DOI] [PubMed] [Google Scholar]
- 14.Reddy MS, Geurs NC, Cunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- 15.Brown DL, Desai KK, Vakili BA, Nouneh C, Lee H, Golub LM. Clinical and Biochemical Results of the Metalloproteinase Inhibition with Subantimicrobial Doses of Doxycycline to Prevent Acute Coronary Syndromes (MIDAS) Pilot Trial. Arterioscler Thromb Vasc Biol. 2004;24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 16.Tüter G, Kurtiş B, Serdar M, Aykan T, Okyay K, Yücel A, Toyman U, Cemri M, Cengel A, Walker SG, Golub LM. Effects of scaling and root planing and sub-antimicrobial dose doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. J Clin Periodontol. 2007;34:673–681. doi: 10.1111/j.1600-051X.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 17.Payne JB, Golub LM, Stoner JA, Lee HM, Reinhardt RA, Sorsa T, Slepian MJ. The effect of subantimicrobial-dose doxycycline periodontal therapy on serum biomarkers of systemic inflammation: a randomized, double-masked, placebo-controlled clinical trial. Journal Am Dent Assoc. 2011;142:262–273. doi: 10.14219/jada.archive.2011.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne JB, Stoner JA, Nummikoski PV, Reinhardt RA, Goren AD, Wolff MS, Lee HM, Lynch JC, Valente R, Golub LM. Subantimicrobial dose doxycycline effects on alveolar bone loss in post-menopausal women. J Clin Periodontol. 2007;34:776–787. doi: 10.1111/j.1600-051X.2007.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pussinen P, Jauhiainen M, Metso J, Tyynelä J, Ehnholm C. Pig plasma phospholipid transfer protein facilitates HDL interconversion. J Lipid Res. 1995;36:975–85. [PubMed] [Google Scholar]
- 22.Siggins S, Jauhiainen M, Olkkonen VM, Tenhunen J, Ehnholm C. PLTP secreted by HepG2 cells resembles the high-activity PLTP form in human plasma. J Lipid Res. 2003;44:1698–1704. doi: 10.1194/jlr.M300059-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 24.Ohashi R, Mu H, Wang X, Yao Q, Chen C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM. 2005;98:845–56. doi: 10.1093/qjmed/hci136. [DOI] [PubMed] [Google Scholar]
- 25.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung K, Lein M. By mistakes we learn: determination of matrix metalloproteinase-8 and tissue inhibitor of matrix metalloproteinase-1 in serum yields doubtful results. J Clin Periodontol. 2009;36:34–35. doi: 10.1111/j.1600-051X.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuvaja P, Talvensaari-Mattila A, Turpeenniemi-Hujanen T. The Sample Type used Affects the Levels of Gelatinases (MMP-2 and -9) and their Inhibitors (TIMP-1 and -2) in Circulating Blood of Healthy Controls and Breast Cancer Patients. Biomarker Insights. 2007:117–127. [PMC free article] [PubMed] [Google Scholar]
- 28.Tuomainen AM, Nyyssönen K, Tervahartiala T, Sorsa T, Pussinen PJ. Letters to the Editor, Response: Matrix Metalloproteinase-8 and Tissue Inhibitor of Metalloproteinase-1 in Serum Do Not Reflect the Analytes Circulating in Blood. Arterioscler Thromb Vasc Biol. 2008;28:e17. doi: 10.1161/ATVBAHA.107.159277. [DOI] [PubMed] [Google Scholar]
- 29.Tuomainen AM, Nyyssönen K, Laukkanen JA, Tervahartiala T, Tuomainen TP, Salonen JT, Sorsa T, Pussinen PJ. Serum matrix metalloproteinase-8 concentrations are associated with cardiovascular outcome in men. Arterioscler Thromb Vasc Biol. 2007;27:2722–8. doi: 10.1161/ATVBAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 30.Tuomainen AM, Kormi I, Havulinna AS, Tervahartiala T, Salomaa V, Sorsa T, Pussinen PJ. Serum tissue-degrading proteinases and incident cardiovascular disease events. Eur J Prev Cardiol. 2012 Oct 18; doi: 10.1177/2047487312465524. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251–64. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- 32.Sammalkorpi K, Valtonen V, Kerttula Y, Nikkilä E, Taskinen MR. Changes in serum lipoprotein pattern induced by acute infections. Metabolism. 1988;37:859–65. doi: 10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 33.Laurila A, Bloigu A, Näyhä S, Hassi J, Leinonen M, Saikku P. Chronic Chlamydia pneumoniae infection is associated with a serum lipid profile known to be a risk factor for atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2910–3. doi: 10.1161/01.ATV.17.11.2910. [DOI] [PubMed] [Google Scholar]


