Abstract
Autoimmunity results from a breakdown in tolerance mechanisms that regulate autoreactive lymphocytes. We recently showed that during innate immune responses, secretion of IL-6 by dendritic cells (DCs) maintained autoreactive B cells in an unresponsive state. In this study, we describe that TLR4-activated DCs from lupus-prone mice are defective in repressing autoantibody secretion, coincident with diminished IL-6 secretion. Reduced secretion of IL-6 by MRL/lpr DCs reflected diminished synthesis and failure to sustain IL-6 mRNA production. This occurred coincident with lack of NF-κB and AP-1 DNA binding and failure to sustain IκBα phosphorylation. Analysis of individual mice showed that some animals partially repressed Ig secretion despite reduced levels of IL-6. This suggests that in addition to IL-6, DCs secrete other soluble factor(s) that regulate autoreactive B cells. Collectively, the data show that MRL/lpr mice are defective in DC/IL-6-mediated tolerance, but that some individuals maintain the ability to repress autoantibody secretion by an alternative mechanism. The Journal of Immunology, 2007, 178: 4803–4810.
Systemic lupus erythematosus (SLE)4 is a multiorgan autoimmune disease characterized by the production of autoantibodies to nuclear components. Alternating periods of flares and remissions are associated with an increased burden of apoptotic cells, the formation of immune complexes, and inflammation (1). The etiology of SLE remains unknown; however, multiple immunoregulatory defects have been identified in lupus-prone mice (2–13), including complement deficiencies, TCR signal transduction anomalies, and dysfunctional cytokine secretion by macrophages (Mφs). These defects contribute to the onset and/or pathogenesis of SLE, while a breakdown in tolerance leads to the formation of autoantibodies and immune complexes that may play a role in vasculitis, glomerulonephritis, and cerebritis (14).
Studies in Ig transgenic (Tg) mouse models have defined anergy as a state of unresponsiveness that regulates autoreactive B cells in the periphery (15–19). Anergic B cells fail to secrete Ab in response to LPS or Ag immunization due to receptor unresponsiveness (17, 18, 20). Some anergic B cells exhibit reduced surface IgM levels (21, 22), decreased lifespan (20, 23), and exclusion from the lymphoid follicle (23, 24). In the case of B cells specific for the lupus-associated Ag, Smith (Sm), a partially anergic phenotype is evident. Sm-specific B cells from 2-12H/Vκ8 Ig Tg mice are unable to secrete Ig in response to LPS, yet maintain surface IgM levels, exhibit a normal lifespan, and remain competent to enter the B cell follicle (18). Recently, we described that Sm-specific B cells purified from myeloid dendritic cells (myDCs) and Mφs regain the ability to secrete Ig in response to LPS (25). The data show that secretion of IL-6 by DC/Mφs represses LPS-induced Ig secretion by autoreactive B cells without repressing acutely stimulated naive B cells. This mechanism of tolerance is not limited to Sm-specific B cells as chronically Ag-experienced HEL- and Ars/A1-specific B cells are similarly affected (25). These findings identify a unique mechanism of B cell tolerance wherein DCs and Mφs play a central role in regulating autoimmunity during innate immune responses.
myDCs and plasmacytoid DCs have been described as positive regulators of immunity promoting growth and differentiation of some B cells through the secretion of IL-12, IL-6, BLyS, and APRIL (26–28). Specifically, IL-6 was found to promote plasma cell survival (29, 30). Although this seems paradoxical, the data indicate that IL-6 differentially regulates naive and chronically Ag-experienced B cells (25). Studies identifying IL-6 as a positive regulator focused on B cells from non-Tg mice where the proportion of autoreactive cells is low. In contrast, the studies showing that IL-6 represses autoantibody production used self-reactive Ig Tg models where the B cells were constantly exposed to self-Ag (25). Thus, IL-6 acts as a positive or negative regulator of B cells depending on the history of BCR ligation. We propose that chronic BCR ligation by self-Ag reprograms IL-6R-mediated outcomes allowing naive B cells to produce Ig in response to polyclonal stimulation while simultaneously repressing autoreactive B cells from producing autoantibody. These findings identify a novel B cell tolerance mechanism, and suggest that overcoming tolerance in SLE might be associated with defects in the repression of autoreactive B cells by myDCs and/or Mφs.
In this report, we show that LPS-activated DCs from MRL/lpr mice inefficiently repress Sm-specific Ig secretion, coincident with diminished IL-6 secretion. Mechanistically, diminished secretion of IL-6 resulted from decreased synthesis of IL-6 mRNA coincident with decreased IκBα phosphorylation and reduced DNA binding by NF-κB and AP-1. These data identify signal transduction defects in DCs that occur coincident with diminished IL-6 secretion and failure to repress Ig secretion by autoreactive B cells. Further analysis of DC-mediated tolerance mechanisms revealed that DC conditioned medium (CM) from some MRL/lpr mice repressed Ig secretion despite low levels of IL-6. This suggested that additional soluble factors are involved in repressing autoantibody secretion. These findings implicate DC defects in the breakdown of tolerance in lupus-prone mice and suggest that defects in multiple factors may be required for the complete breakdown of tolerance associated with autoimmunity.
Materials and Methods
Mice
2-12H/Vκ8/Cκ−/− Ig transgenic mice were previously described (18, 25). MRL/MpJ-Faslpr/J (MRL/lpr) and C57BL/6J (B6) mice were purchased from The Jackson Laboratory, and NZM2410 mice from Taconic Farms. NZBxNZWF1 mice were obtained from Trine Jorgensen (University of Colorado), MRL/MpJ (MRL) and B6.Faslpr (B6.lpr) from Stephen Clarke (University of North Carolina). 2-12H/Vκ8/Cκ−/− mice were used at 9 to 17 wk of age. All other mice were used at 6- to 10 wk old. All studies were approved by the Institutional Animal Care and Use Committee.
Reagents and antibodies
7-Aminoactinomycin D (7-AAD), rIL-6, and Abs to CD11c, CD11b, B220, and IL-6 were purchased from BD Biosciences, GR1 and TLR4 from eBio-sciences, phospho-IκBα from Cell Signaling, IκBα and β-tubulin from Santa Cruz Biotechnology, and IgG HRP from Promega. Streptavidin-alkaline phosphatase (AP) was purchased from Southern Biotech, anti-actin, TEPC 183, and Escherichia coli 055:B5 LPS from Sigma-Aldrich, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) from Calbiochem, E. coli 0111:B4 LPS from List Biological Laboratories, mouse GM-CSF and IL-4 from PeproTech, poly(I:C) and R848 from InvivoGen, and CpG oligodeoxynucleotides (ODN) and non-CpG ODN from Coley Pharmaceutical Group. JA12.5, 54.1, 187.1, HB100, and CRL 1969 were purified from hybridoma culture supernatant.
Cell purification
B cells were purified from 2-12H/Vκ8/Cκ−/− spleens by negative selection (StemCell Technologies) (25). Biotinylated CD3 Ab was added to the Ab mixture to increase the efficiency of T cell depletion. B cells were 86–93% pure with <3% T cells and <7% DCs/Mφs. Splenic CD11c+ cells (~70% pure) were purified by positive selection (Miltenyi Biotech) and found to contain 20% lymphocytes and 10% Mφs.
Bone marrow-derived DC cultures
Bone marrow-derived DCs (BMDC) were generated as previously described (25). BMDCs were >95% CD11c+ (CRL 1969 hybridoma). CM was made from 1 × 104 BMDCs (0.2 ml) cultured for an additional 4 days with or without Sigma LPS (30 μg/ml). 5 × 105 BMDCs (0.2 ml) were cultured for an additional 4 days with or without poly(I:C) (50 μg/ml), R848 (10 μg/ml), CpG ODN (1 μg/ml), or non-CpG ODN (1 μg/ml). In experiments where RNA was isolated or nuclear extracts were prepared, BMDCs were stimulated with E. coli 0111:B4 LPS (List Biological Laboratories) that was re-purified (31) and confirmed to be unable to induce IL-6 secretion by TLR4−/− DCs.
B cell cultures
Splenocytes containing 1 × 105 B cells, or the equivalent number of purified B cells, were cultured with Sigma LPS (30 μg/ml) for 4 days. In the mixed B cell experiments, purified B6 (5 × 104; IgMb) and 2-12H/Vκ8 (5 × 104; IgMa) B cells were cocultured with LPS for 4 days as above. BMDCs, CD11c+ splenocytes, or BMDC CM (25% of final volume) were added to B cell cultures on day 0. The IL-6 in DC CM was neutralized with either anti-IL-6 Ab or a control rat IgG1 Ab (54.1).
ELISA
IgMa/κ (encoded by 2-12H/Vκ8/Cκ−/−) was captured with anti-κ (187.1), detected with biotinylated anti-IgMa (HB100) and streptavidin-AP as previously described (18). Purified mouse IgMa/κ (TEPC 183) served as the standard control. IgMa/κ levels were plotted as “percentage of control” defined by the level of Ig secretion in LPS-stimulated cultures of purified 2-12H/Vκ8/Cκ−/− B cells (100%). IL-6 was quantitated by capturing with anti-IL-6 (clone MP5-20F3) and detecting with biotinylated anti-IL-6 (clone MP5-32C11) and streptavidin-AP. rIL-6 served as the standard control.
Real-time RT-PCR
RNA was prepared from BMDCs treated with re-purified LPS (15 μg/ml) by solubilization in Trizol (Invitrogen Life Technologies) and treatment with Turbo DNase (Ambion). Reverse transcription with oligo(dT) primers was performed with Superscript II (Invitrogen Life Technologies). The amount of IL-6 message was determined using the TaqMan Assay-On-Demand primer-probe sets (Applied Biosystems) and the ABI 7000 sequence detection system. IL-6 mRNA transcript levels were normalized to the amount of 18S ribosomal RNA transcription according to the following equation: %18S = 2^ [-(IL-6 – 18S units)]. To measure IL-6 mRNA stability, BMDCs were stimulated with re-purified LPS (15 μg/ml) for 6 h and then treated with 50 μM DRB for 15, 30, and 60 min to block transcription. mRNA was quantitated by RT-PCR as described above.
EMSA
BMDCs were stimulated with re-purified LPS (15 μg/ml) and gel shift assays were performed as previously described (32).
Statistical analysis
Exact Wilcoxon rank sum test was used for most unpaired two-sample comparisons. When total sample size was small (<8), t test was used instead. For test differences between paired observations, exact Wilcoxon signed rank test was used. Values of p < 0.05 were considered significant and denoted by an asterisk (*).
Results
The frequencies of splenic myDCs and Mφs are not diminished in MRL/lpr mice
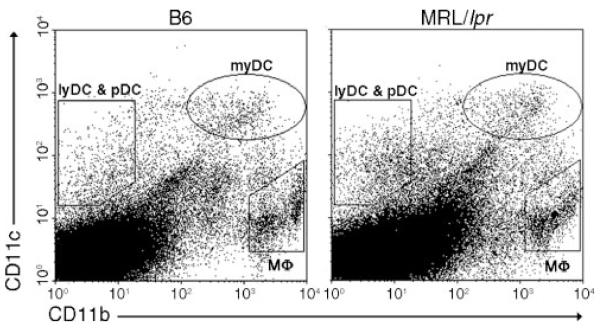
Maintaining B cell tolerance during activation of the innate immune system is crucial in preventing autoimmunity. We have previously shown that stimulation through TLR4 activates myDCs and Mφs to secrete soluble factors thereby repressing Ig secretion by chronically Ag-experienced (autoreactive) B cells (25). To determine whether the breakdown of tolerance in lupus-prone mice was associated with the lack of a repressive cell type, we compared the frequency of splenic Mφ and DC subsets in MRL/lpr and B6 mice. As shown in Fig. 1 and Table I, the frequencies of myDCs (CD11chigh/CD11bint/high) and plasmacytoid DCs (pDCs, CD11clow/CD11b−/B220+/GR1+) were not significantly different. The lymphoid DCs (lyDCs, CD11clow/CD11b−/B220−/GR1−) were significantly decreased in MRL/lpr; however, this population is not involved in DC/Mφ-mediated tolerance (25). The CD11c−/CD11bhigh and CD11c−/CD11blow populations were increased in MRL/lpr mice, raising the possibility that these populations might secrete an activator that enhances Ig secretion. However, when isolated by cell sorting, these populations did not augment LPS-induced Ig secretion or affect the ability of B6 DCs to regulate Ig secretion by Sm-specific B cells (data not shown), suggesting that neither population promotes the loss of B cell tolerance. Thus, neither diminished frequency of myDCs and Mφs nor secretion of an activator accounts for the loss of tolerance in MRL/lpr mice.
FIGURE 1.
The distribution of splenic myDCs and Mφs are comparable between B6 and MRL/lpr mice. DC and Mφ subsets were separated based on CD11c and CD11b expression. Dot plots are representative of nine mice each.
Table I.
The frequencies of splenic myDCs and Mφs are not diminished in MRL/lpr micea
| B6 | MRL/lpr | p Value | |
|---|---|---|---|
| myDCs | 1.02 ± 0.12 | 0.86 ± 0.07 | 0.658 |
| lyDCs | 0.13 ± 0.01 | 0.25 ± 0.03 | 0.001b |
| pDCs | 0.03 ± 0.01 | 0.10 ± 0.02 | 0.168 |
| Mφs | 1.68 ± 0.25 | 4.38 ± 0.96 | 0.002b |
Splenic DC and Mφ subsets were analyzed based on CD11c, CD11b, B220, and GRI expression. The data depict the average percentage of total splenocytes ± SEM from nine mice. The average number of splenocytes from B6 was 1.4 × 108 ± 0.1 × 108 and from MRL/lpr was 1.3 × 108 ± 0.1 × 108.
Significantly different.
DCs from MRL/lpr mice fail to efficiently repress Sm-specific B cells
LPS-activated DCs from B6 mice regulate chronically Ag-experienced B cells (25). To assess whether DCs from MRL/lpr mice were capable of repressing Ig secretion, we cocultured Sm-specific B cells with BMDCs from B6 or MRL/lpr mice (Fig. 2A). Compared with B6 DCs, MRL/lpr DCs were less efficient at repressing Sm-specific B cells when cultured at B cell: DC ratios of 10:1, 20:1, and 100:1 (p = 0.016, 0.004, and 0.015, respectively). These differences were not due to contaminating cells, because BMDCs from B6 and MRL/lpr mice contained >95% myDCs, and sorted B cells compared with negatively selected B cells from 2-12H/Vκ8 mice cultured with DCs from MRL/lpr mice exhibited similar results (data not shown). To determine whether splenic DCs were also defective in repressing autoreactive B cells, splenic CD11c+ cells were isolated from B6 and MRL/lpr mice, and cocultured with B cells from 2-12H/Vκ8 mice (B cell: DC ratio 10:1). As shown in Fig. 2B, ex vivo B6 DCs repressed significantly better than DCs purified from MRL/lpr mice (p = 0.015), indicating that the defect was not specific to BMDCs. Collectively, the data indicate that myDCs from MRL/lpr mice are present at a normal frequency, but they are defective in repressing Ig secretion by autoreactive B cells.
FIGURE 2.
DCs from MRL/lpr mice fail to efficiently repress Sm-specific Ig secretion. LPS-stimulated (30 μg/ml) splenocytes (1 × 105 B cells) or purified B cells (1 × 105) were cocultured with the indicated ratios of BMDCs (A), or ex vivo splenic DCs (B). Secreted IgMa/κ levels were quantitated by ELISA from the day 4 culture supernatant. LPS-stimulated purified B cells (100%) secreted 1–10 μg/ml IgMa/κ. Data represent 14 (A) and 8 (B) MRL/lpr mice. (▲, Controls; ● B6, MRL/lpr).
DCs from MRL/lpr mice are defective in IL-6 secretion
We previously showed that IL-6 secreted by DCs repressed autoreactive B cells (25). To determine whether diminished IL-6 was associated with the inability of MRL/lpr DCs to repress Sm-specific Ig secretion, we measured IL-6 secretion. LPS-activated BMDCs (Fig. 3A) and splenic CD11c+ cells (Fig. 3B) from MRL/lpr mice secreted significantly less IL-6 compared with B6 controls (p < 0.001 and p = 0.003, respectively). To assess whether this defect was unique to MRL/lpr mice, we quantitated LPS-induced IL-6 secretion from BMDCs from several other lupus-prone models. As shown in Fig. 3A, BMDCs from MRL, NZM2410, and NZBxNZW F1 were defective in secreting IL-6 when compared with B6 (p < 0.0001, p < 0.0001, and p = 0.002, respectively). Interestingly, B6.lpr mice were not defective in secreting IL-6 (p = 0.932), suggesting that the inability to secrete IL-6 is associated with the MRL background. Defective IL-6 production was not secondary to IL-10 inhibiting TLR signaling, as MRL/lpr DCs secreted decreased levels of IL-10 and neutralizing IL-10 did not restore IL-6 levels (data not shown). To determine whether defective IL-6 secretion was limited to stimulation through TLR4, we measured IL-6 secretion from MRL/lpr-derived DCs in response to other TLR ligands. As shown in Fig. 3, C–E, IL-6 secretion was increased when MRL/lpr BMDCs were stimulated through TLR3 (poly(I:C), p = 0.006); however, secretion was defective when stimulated through TLR7 (R848, p = 0.028) and TLR9 (CpG ODN, p = 0.016). This indicates that not all TLRs are affected by this defect and that mutation within the IL-6 structural gene is unlikely to explain the reduced levels of IL-6. Collectively, the data indicate that DCs from multiple strains of autoimmune mice exhibit defects in cytokine secretion induced through some TLRs.
FIGURE 3.
DCs from MRL/lpr mice are defective in IL-6 secretion upon TLR4, 7, and 9 stimulation, but not upon TLR3 stimulation. 1 × 104 BMDCs (A) or 1 × 105 ex vivo splenic DCs (B) were stimulated with LPS (30 μg/ml). 5 × 105 BMDCs were stimulated with poly(I:C) (50 μg/ml) (C), R848 (10 μg/ml) (D), and non-CpG ODN (●/□) or CpG ODN (●/○) (1 μg/ml) (E). IL-6 was quantitated by ELISA from the day 4 culture supernatants. Data represent at least 5 mice per group. (●, B6; ○, MRL/lpr; ▲, MRL; △, B6.lpr; ◆, NZM2410; ◇, NZBxNZW F1).
Diminished IL-6 secretion is not due to decreased TLR4 expression or survival
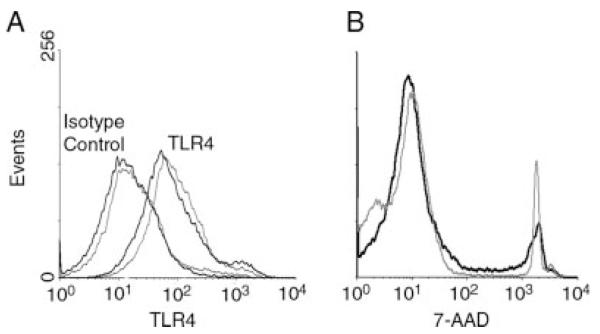
Expression of TLRs ensures that DCs are activated during innate immune responses. It was possible that the decreased secretion of IL-6 from MRL/lpr DCs reflected a reduced expression of surface TLR4. As shown in Fig. 4A, the expression of TLR4 on myDCs from B6 (MFI 58.9 ± 12.6) and MRL/lpr (MFI 68.1 ± 10.9) mice was not significantly different. Likewise, BMDCs from B6 and MRL/lpr mice did not differ in TLR4 expression (data not shown), nor did they differ in viability as determined by 7-AAD staining at day 4 (Fig. 4B). Thus, diminished surface expression of TLR4 or decreased survival do not account for the decreased IL-6 secretion by LPS-activated DCs from MRL/lpr mice.
FIGURE 4.
myDCs from B6 and MRL/lpr mice have similar levels of TLR4 surface expression and no difference in survival. myDCs within the CD11c+ splenocyte population were gated as CD11chigh/CD11bint/high, and then analyzed for TLR4 expression (A). LPS-stimulated BMDCs were stained with 7-AAD on day 4 (B). The thick black line represents B6 mice. The thin gray line represents MRL/lpr mice. Histogram shows a representative plot from three experiments.
Defective IL-6 secretion is associated with failure to sustain IL-6 transcription
Transcriptional regulation of IL-6 depends on several signal transduction pathways that activate multiple transcriptional regulators including NF-κB and AP-1. To determine whether the diminished secretion of IL-6 by MRL/lpr DCs was due to defective transcriptional regulation, we LPS-stimulated BMDCs from B6 and MRL/lpr mice and quantitated IL-6 mRNA levels by real-time PCR. The basal level of IL-6 mRNA in the MRL/lpr mice was slightly lower than in B6 mice (Fig. 5A). Upon stimulation with LPS, IL-6 mRNA levels in B6 and MRL/lpr DCs were dramatically increased; however, the magnitude of the response by MRL/lpr BMDCs was 7-fold lower. Further, the sustained levels of IL-6 mRNA production were higher in B6 compared with MRL/lpr mice (24 h and 96 h time points). To determine whether decreased mRNA stability contributed to the decreased production of IL-6 message, BMDCs were LPS-stimulated for 6 h followed by pharmacological attenuation of transcription. The levels of IL-6 mRNA in BMDCs from B6 and MRL/lpr mice were quantitated by RT-PCR. As shown in Fig. 5B, the rates of mRNA degradation in the MRL/lpr DCs did not change over time; however, the IL-6 mRNA levels in B6 DCs were reduced by 3-fold within 15 min of attenuating new transcription. This indicates that IL-6 message is inherently unstable and that sustained production of IL-6 mRNA requires continual synthesis. Further, given that degradation was not observed in DCs from MRL/lpr mice, the data indicate that increased degradation does not contribute to the diminished IL-6 mRNA levels. This suggests that MRL/lpr DCs harbor a defect at or upstream of transcriptional initiation that reduces the level of IL-6 mRNA and protein.
FIGURE 5.
DCs from MRL/lpr mice show a decrease in synthesis and ability to sustain IL-6 mRNA levels. Real-time PCR was performed on RNA isolated from LPS-stimulated BMDCs untreated (A) or treated (B) with DRB at the indicated time points. The data from three individual B6 (●) and six MRL/lpr (○) mice are plotted as %18S.
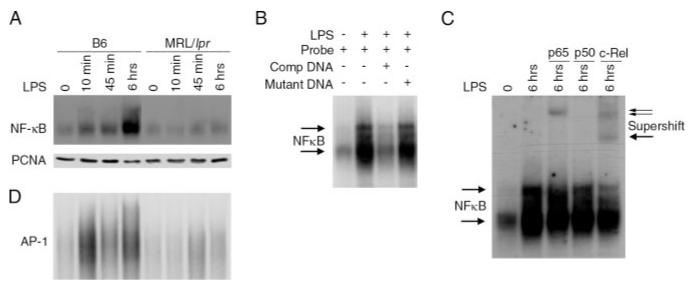
To assess whether decreased IL-6 mRNA levels were associated with defects in NF-κB or AP-1 activation, we compared the DNA binding activity in nuclear extracts prepared from B6 and MRL/lpr DCs. The DNA binding activity of NF-κB from LPS-stimulated B6 DCs occurred within 10 min, with robust binding at 6 h. In contrast, the DNA binding activity of NF-κB from MRL/lpr DCs was diminished at these same time points (Fig. 6A). This was not a reflection of unequal protein loading, as the levels of an unrelated nuclear protein (PCNA) were comparable. The specificity of NF-κB for the DNA probe was confirmed by diminished complex formation in the presence of unlabeled probe (competitor DNA), and failure of a mutant competitor DNA (mutant DNA) to reduce complex formation (Fig. 6B). To identify the NF-κB subunits involved in DNA binding, we supershifted the DNA/protein complex with subunit-specific Abs. As shown in Fig. 6C, p65 and c-Rel, but not p50, were identified as components of the NF-κB complex formed in B6 DCs following 6 h LPS stimulation. p65 and c-Rel anti-sera were specific for these components as preim-mune serum failed to supershift a protein/DNA complex (data not shown). Similar to NF-κB, DNA binding by AP-1 was also markedly diminished in DCs from MRL/lpr compared with B6 mice (Fig. 6D). Thus, LPS-stimulated MRL/lpr DCs fail to activate key transcriptional regulators required for IL-6 gene transcription.
FIGURE 6.
DCs from MRL/lpr mice fail to activate NF-κB and AP-1. BMDCs were stimulated with LPS (15 μg/ml) for the indicated times. Nuclear extracts were prepared, and NF-κB/DNA binding (A) or AP-1/DNA binding (D) was assessed by EMSA. Nuclear extracts prepared from unstimulated B6 BMDCs (lane 1) or from DCs stimulated 6 h with LPS (lanes 2–4) were incubated with radiolabeled DNA probe (lanes 1–4), unlabeled competitive DNA (lane 3), or mutant DNA (lane 4), and NF-κB DNA binding was assessed by EMSA (B). NF-κB/DNA complexes in the nuclear extracts from unstimulated B6 DCs (lane 1) or from DCs stimulated 6 h with LPS (lane 2–5) were supershifted using p65 (lane 3), p50 (lane 4), or c-Rel antiserum (lane 5) (C).
Nuclear translocation of NF-κB is dependent on phosphorylation and degradation of IκB (33). To assess whether the lack of NF-κB DNA binding was associated with defects in IκB phosphorylation/degradation, we immunoblotted whole cell lysates from LPS-stimulated B6 and MRL/lpr BMDCs. B6 DCs showed induced phosphorylation of IκBα at 5 min that was sustained through 6 h (Fig. 7A, left panel). In contrast, MRL/lpr DCs induced IκBα phosphorylation at 5 min with maximal phosphorylation at 15 min. Phosphorylation was not evident at 45 min or 6 h (Fig. 7A, right panel). Similarly, IκBα degradation was delayed following LPS stimulation of MRL/lpr DCs, indicating that defects in TLR4-induced signal transduction correlate with lack of IL-6 mRNA production and protein secretion. To assess whether other TLR pathways in MRL/lpr DCs were similarly affected, we assessed IκBα phosphorylation in response to TLR3 ligation. We showed in Fig. 3 that despite defects in TLR4-, TLR7-, and TLR9-induced IL-6 production, TLR3-induced IL-6 production was enhanced. This revealed that the defect in IL-6 production by MRL/lpr DCs did not affect all TLRs. To correlate TLR-induced protein secretion with TLR-mediated signal transduction, we assessed IκBα phosphorylation in response to poly(I:C). As shown in Fig. 7B, poly(I:C)-induced IκBα phosphorylation was comparable between DCs derived from B6 and MRL/lpr mice. Collectively, the data suggest failure to sustain IκBα phosphorylation reduces NF-κB activation, diminishes IL-6 transcription, and ultimately decreases IL-6 protein synthesis by MRL/lpr DCs. This supports the idea that continuous TLR4 signal transduction is required to maintain IL-6 secretion and suggests this is defective in DCs from lupus-prone mice (34).
FIGURE 7.
TLR4-stimulated DCs from MRL/lpr mice are unable to sustain IκBα phosphorylation. BMDCs (2 × 106) from B6 and MRL/lpr mice were stimulated with LPS (15 μg/ml) (A) or poly(I:C) (50 μg/ml) (B) for the indicated time points. Phospho-IκBα, IκBα, and β-tubulin (A) or β-actin (B) expression in whole cell lysates was determined by immunoblotting. Data represent seven (A) and three (B) experiments.
Autoantibody secretion is repressed by IL-6 and other soluble factors
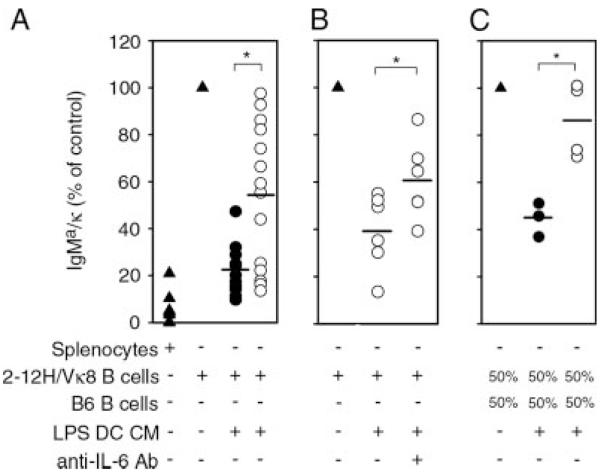
We have previously shown that IL-6 repressed 75% of Ig secretion by Sm-specific B cells (25). In this study, we show that DCs from lupus-prone MRL/lpr mice exhibit markedly decreased IL-6 levels coincident with their inability to regulate Ig secretion. To determine the importance of decreased IL-6 in the breakdown of tolerance, we assessed the ability of CM from B6 and MRL/lpr DCs to repress Ig secretion. CM allowed us to distinguish the effects of soluble mediators from the effects of cell contact. As shown in Fig. 8A, DC CM from most B6 mice repressed 70–90% of Ig secretion. In contrast, the ability of DC CM from individual MRL/lpr mice to repress Ig secretion was extremely variable (10–90% repression, p = 0.004). Given the central role for IL-6 in repressing autoantibody secretion (25), we reasoned that if IL-6 were the sole repressive factor, there would be a direct correlation between IL-6 in DC CM and Ig secretion. However, this broad range of repression only partially correlated with IL-6 levels (data not shown). Despite the fact that all MRL/lpr mice exhibited low levels of IL-6, four individuals still repressed 80–90% of Ig secretion (Fig. 8A). To assess whether the low levels of IL-6 secreted by MRL/lpr mice contributed to Ig repression, we neutralized any remaining IL-6 in the DC CM of mice retaining repressive function, then assessed the ability of the CM to regulate Ig secretion. As shown in Fig. 8B, neutralization partially restored Ig secretion (p = 0.031), confirming that the low levels of IL-6 regulated Ig secretion. Interestingly, secretion comparable to controls (100%) was never attained, suggesting that in addition to IL-6, other DC-derived soluble mediators regulate Ig secretion. It was possible that the variability in repression by MRL/lpr DC CM was due to the secretion of an activating factor by the MRL/lpr DCs. We addressed this in two ways. First, we added rIL-6 to the MRL/lpr DC CM, and then assessed Ig secretion by Sm-specific B cells. When added to the CM from three individual mice, rIL-6 repressed Ig secretion indicating that if activating factors were present, they did not override the repressive effect of IL-6 (data not shown). In a second experiment, we assessed whether MRL/lpr DCs secreted an activator by determining whether MRL/lpr DC CM activated naive B6 B cells. We previously showed that DC CM did not repress naive B cells (25); thus, the presence of an activator may be more evident when Ig secretion is not simultaneously being repressed by the low levels of IL-6 in the MRL/lpr DC CM. The data indicate that MRL/lpr DC CM did not increase Ig secretion of naive B6 B cells, indicating that the dysregulated production of an activator is unlikely (data not shown).
FIGURE 8.
In addition to IL-6, other soluble factors regulate autoantibody secretion. Purified B cells (1 × 105) from 2-12H/Vκ8 mice were stimulated with LPS (30 μg/ml) in the absence (▲) or presence of DC CM (25% of final volume) from B6 (●) or MRL/lpr (○) mice (A). DC CM from individual MRL/lpr mice (○) was untreated or neutralized with anti-IL-6 Ab (50 μg/ml) before coculture with B cells from 2-12H/Vκ8 mice (B). 5 × 104 purified B cells from 2-12H/Vκ8 and B6 mice were stimulated with LPS (30 μg/ml) in the absence (▲) or presence of DC CM from B6 (●) or MRL/lpr (○) mice (C). Secreted IgMa/κ levels were quantitated by ELISA from the day 4 culture supernatant. LPS-stimulated purified B cells (100%) secreted 1–10 μg/ml IgMa/κ. Data represent 15 (A), 5 (B), and 4 (C) MRL/lpr mice.
Collectively, the data indicate that during innate immune responses, IL-6 and another repressive factor(s) regulates B cells chronically exposed to Ag. Further, this mechanism appears defective in lupus-prone mice coincident with diminished secretion of IL-6. However, it remained unclear whether soluble factors secreted by LPS-activated DCs repressed autoreactive B cells when present in mixed populations with naive cells. To assess this, we cocultured naive (B6) and autoreactive (2-12H/Vκ8) B cells with DC CM prepared from B6 and MRL/lpr DCs. As shown in Fig. 8C, DC CM prepared from B6 cells, but not MRL/lpr cells, repressed Ig secretion in the mixed B cell cultures (p = 0.009) (Fig. 8C). The data suggest that DC-mediated repression regulates mixed populations of autoreactive and naive B cells.
Discussion
The defects leading to the breakdown in B cell tolerance remain a central focus in understanding SLE. Previous studies showed that during innate immunity Sm-specific B cells were regulated by myDCs and Mφs through the secretion of soluble mediators (25). We propose a model where polyclonal activators stimulate myDCs and Mφs to secrete IL-6, which selectively represses autoreactive B cells, while naive B cells mount a polyclonal Ab response to bac-terial and viral Ags. In this report, we show that DCs from lupus-prone mice are less efficient at repressing autoreactive B cells coincident with a defect in secreting IL-6. This DC defect was not due to decreased survival or TLR4 expression, lack of a regulatory DC subpopulation, or the secretion of factors that enhance Ig secretion. Instead, the reduced IL-6 secretion resulted from the inability of MRL/lpr DCs to induce or maintain IL-6 transcription in response to LPS. Analysis of upstream signaling effectors showed that, although LPS induced IκBα phosphorylation, it was not sustained. Further, DNA binding by NF-κB and AP-1 were markedly decreased. These findings indicate that MRL/lpr DCs exhibit a TLR4 signal transduction defect at, or upstream of, IκB kinase (IKK)/IκB/NF-κB activation that results in diminished IL-6 mRNA production and protein secretion.
Previous data showed that rIL-6 effectively regulated chronically Ag-experienced B cells (25). At several B cell: DC ratios, MRL/lpr DCs were less efficient at repressing Ig secretion compared with B6 DCs. However, despite significant defects in IL-6 secretion, they still repressed 53% of anti-Sm secretion at a ratio of 100:1 (Fig. 2A). Further, DC CM was less efficient at repressing Ig secretion compared with intact DCs indicating that a contact-dependent mechanism might partially regulate Ig secretion. In support of this, we have observed that DCs deficient in TLR4 partially repressed LPS-induced Ig secretion; however, repression was lost when the cells were separated in a Transwell apparatus (M. A. Kilmon and B. J. Vilen, unpublished observations).
The finding that repression of Ig secretion by DCs is multifaceted fits well with the heterogeneity of human disease. We propose that defects in any regulatory component may predispose to autoimmunity, but complete loss of tolerance requires multiple defects. Our data show that the repressive ability of LPS-activated MRL/lpr DCs was variable. Some DCs efficiently repressed Ig secretion, despite diminished IL-6 production (Figs. 2, 3, A and B, and 8A), while others failed to repress secretion coincident with reduced IL-6 levels. Compared with the contact-dependent mechanism described above, this repressive activity was apparent in the CM from some MRL/lpr mice, indicating that DCs secrete additional repressive factors that contribute to the regulation of Ig secretion. Thus, despite markedly decreased IL-6 secretion by DCs from all mice analyzed, some likely harbor defects in another repressive factor(s) making them more susceptible to autoimmunity during innate stimulation. Although a direct correlation between Ig secretion and IL-6 levels in MRL/lpr mice was not evident, we favor the interpretation that IL-6 and another repressive factor regulates Ig secretion because IL-6-deficient DCs repress LPS-induced Ig secretion (unpublished observations) and neutralizing IL-6 only partially restored Ig secretion (Fig. 8B). This indicates that the low levels of IL-6 secreted by MRL/lpr DCs partially represses Ig secretion, but that IL-6 is not the sole means of regulating autoimmunity during innate immune responses.
The inability of LPS-stimulated MRL/lpr DCs to produce IL-6 and efficiently repress Ig secretion suggests that defects in innate immune responses contribute to autoimmunity. Our data show that DCs derived from MRL/lpr mice are unable to sustain IκB phosphorylation, thereby reducing NF-κB DNA binding and IL-6 mRNA synthesis. This suggests an intrinsic defect where lack of sustained TLR-mediated signal transduction leads to decreased IL-6 protein secretion. This could reflect a defect in the TLR signaling pathway or possibly the selective formation of NF-κB complexes that are less transcriptionally active. Aberrant cytokine production and abnormal NF-κB activity in T cells and Mφs from lupus-prone mice and lupus patients have been associated with decreased p65, increased p50 homodimers which are more inhibtory to gene transcription, reduced binding of p50/c-Rel and p65 NF-κB complexes, and increased activity of histone deacetylases (35, 36). Unfortunately we could not identify the NF-κB subunits formed by MRL/lpr DCs because DNA binding was not observed at levels sufficient for supershifting.
MyD88-dependent, TLR-induced activation of NF-κB and AP-1 is mediated through TRAF6 (37). Thus, the findings that both NF-κB and AP-1 DNA binding activity are reduced (Fig. 6), and that IL-6 secretion and IκBα phosphorylation are defective only upon stimulation through MyD88-dependent TLRs (TLR4, 7, and 9, but not TLR3), suggest a defect in the MyD88-dependent signaling pathway possibly at or upstream of TRAF6. Alternatively, a defect at the level of the TLR4 receptor may occur. Yang et al. (34) showed that persistent TLR4 signals are required for normal DC secretion of IL-6. In the case of dysfunctional MRL/lpr DCs, the TLR4 receptor may become desensitized to LPS following an initial stimulus, mimicking LPS removal and causing the decreased phospho-IκBα and IL-6 mRNA levels seen at later time points (Figs. 5 and 7). In addition, exposure to apoptotic cells may affect the TLR4 response. Apoptotic cells fail to induce inflammatory responses, in part by repressing DC activation (38). Thus, the increased burden of apoptotic cells associated with SLE may dysregulate some of the TLRs, rendering them incapable of secreting cytokines that are needed to repress autoantibody secretion. In support of this, others have shown that apoptotic cells cause defective IL-6 secretion by macrophages (10), and mice functionally deficient in the phagocytosis of apoptotic cells get a lupus-like disease (11).
Increased production of proinflammatory cytokines such as IL-6, contribute to the inflammatory response and pathogenesis of lupus nephritis (39, 40). SLE patients (41–44) and diseased lupus-prone mice (45–47) exhibit elevated serum IL-6 levels (2–19 pg/ml) but fail to repress Ig secretion. Although elevated, this level of systemic IL-6 is insufficient to repress autoreactive B cells in vitro (25). Therefore, we propose that colocalization of DCs and B cells is necessary to provide sufficient IL-6 to repress Ig secretion. Our findings showed that DCs derived from MRL/lpr mice secrete reduced levels of IL-6, coincident with lack of Ig repression. We propose that once tolerance is overcome, autoantibody secretion and immune complex formation induce systemic production of proinflammatory mediators, promoting inflammation and pathogenesis. Consistent with this model, CpG-stimulated DCs from SLE patients produced lower levels of IL-6 (48), whereas endothelial cells (49–51), mesangial cells in the kidney (52, 53), and infiltrating monocytes/macrophages (54) secrete elevated levels of IL-6. This suggests that IL-6 plays a beneficial role when released in a local microenvironment between myDCs and autoreactive B cells, but when elevated systemically, it induces inflammation, tissue destruction, and spontaneous Ig production by activated B cells (41, 55–57). Therapies aimed at neutralizing the inflammatory effects of IL-6 may have short-term benefits in treating lupus nephritis; however, they are likely to promote loss of tolerance in newly emerging B cells during innate immune activation.
Ig secretion by B cells is induced by ligation of the TLR and/or BCR. BCR-induced Ig secretion is regulated by lack of T cell help and sustained BCR-induced calcium signaling and prolonged Erk activation (20, 58–60). In contrast, TLR-induced Ig secretion is regulated by soluble factors secreted from DCs and Mφs (25). Although the mechanisms regulating the BCR and TLR are unique, signals derived from chronic BCR stimulation impact TLR-induced activation. For example, the chronic Erk activation associated with continuous exposure to self-Ags represses TLR9-induced Ig secretion, whereas, acute Erk activation following BCR stimulation of naive B cells promotes TLR9-induced Ig secretion (61, 62). Similarly, chronic BCR exposure to self-Ag reprograms IL-6R signal transduction to repress Ig secretion (25). However, B cells that have been acutely stimulated and exposed to IFN-αβ induce Ig secretion in response to IL-6 (27). Our data expand our understanding of IL-6 to include a role in repressing Ig secretion by autoreactive B cells. During autoimmunity, the tolerance mechanisms that regulate autoreactive B cells become dysregulated. For many B cells with autoreactive specificities, it remains unclear whether BCR and/or TLR responses facilitate autoantibody production. Our studies of TLR-mediated responses in Sm-, HEL-, and Ars/A1-specific autoreactive B cells identify DCs and Mφs as key regulatory cells during innate immune responses, and show that DC-mediated tolerance is defective in lupus-prone MRL/lpr mice. These findings implicate dysregulated innate immune responses in the autoantibody production associated with SLE.
Acknowledgments
We thank Dirk Dittmer for help with the analysis of the real-time data, and Jeff Frelinger and Stephen Clarke for critically reviewing this manuscript.
Footnotes
M.G. was supported by a minority supplement to R01 AI53266 and F31 AI71292. This study was supported by the Lupus Research Institute and by U.S. Public Health Service Grant AI53266.
Abbreviations used in this paper: SLE, systemic lupus erythematosus; Mφ, macrophage; Tg, transgenic; Sm, Smith; 7-AAD, 7-aminoactinomycin D; AP, alkaline phosphatase; DRB, 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole; ODN, oligodeoxynucleotides; DC, dendritic cell; BMDC, bone marrow-derived DC; CM, conditioned medium; lyDC, lymphoid DC; myDC, myeloid DC; pDC, plasmacytoid DC.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J. Clin. Pathol. 2003;56:481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vratsanos GS, Jung S, Park YM, Craft J. CD4+ T cells from lupus-prone mice are hyperresponsive to T cell receptor engagement with low and high affinity peptide antigens: a model to explain spontaneous T cell activation in lupus. J. Exp. Med. 2001;193:329–337. doi: 10.1084/jem.193.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monk CR, Spachidou M, Rovis F, Leung E, Botto M, Lechler RI, Garden OA. MRL/Mp CD4+,CD25− T cells show reduced sensitivity to suppression by CD4+,CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1180–1184. doi: 10.1002/art.20976. [DOI] [PubMed] [Google Scholar]
- 4.Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J. Clin. Invest. 1998;101:1448–1457. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brundula V, Rivas LJ, Blasini AM, Paris M, Salazar S, Stekman IL, Rodriguez MA. Diminished levels of T cell receptor ζ chains in peripheral blood T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42:1908–1916. doi: 10.1002/1529-0131(199909)42:9<1908::AID-ANR17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Nambiar MP, Enyedy EJ, Warke VG, Krishnan S, Dennis G, Wong HK, Kammer GM, Tsokos GC. T cell signaling abnormalities in systemic lupus erythematosus are associated with increased mutations/polymorphisms and splice variants of T cell receptor ζ chain messenger RNA. Arthritis Rheum. 2001;44:1336–1350. doi: 10.1002/1529-0131(200106)44:6<1336::AID-ART226>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Bouzahzah F, Jung S, Craft J. CD4+ T cells from lupus-prone mice avoid antigen-specific tolerance induction in vivo. J. Immunol. 2003;170:741–748. doi: 10.4049/jimmunol.170.2.741. [DOI] [PubMed] [Google Scholar]
- 8.Yi Y, McNerney M, Datta SK. Regulatory defects in Cbl and mitogen-activated protein kinase (extracellular signal-related kinase) pathways cause persistent hyperexpression of CD40 ligand in human lupus T cells. J. Immunol. 2000;165:6627–6634. doi: 10.4049/jimmunol.165.11.6627. [DOI] [PubMed] [Google Scholar]
- 9.Alleva DG, Kaser SB, Beller DI. Aberrant cytokine expression and autocrine regulation characterize macrophages from young MRL+/+ and NZB/W F1 lupus-prone mice. J. Immunol. 1997;159:5610–5619. [PubMed] [Google Scholar]
- 10.Koh JS, Wang Z, Levine JS. Cytokine dysregulation induced by apoptotic cells is a shared characteristic of murine lupus. J. Immunol. 2000;165:4190–4201. doi: 10.4049/jimmunol.165.8.4190. [DOI] [PubMed] [Google Scholar]
- 11.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 12.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 13.Boackle SA, Holers VM, Chen X, Szakonyi G, Karp DR, Wakeland EK, Morel L. Cr2, a candidate gene in the murine Sle1c lupus susceptibility locus, encodes a dysfunctional protein. Immunity. 2001;15:775–785. doi: 10.1016/s1074-7613(01)00228-x. [DOI] [PubMed] [Google Scholar]
- 14.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat. Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 15.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in nonautoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 16.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 17.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 18.Borrero M, Clarke SH. Low-affinity anti-Smith antigen B cells are regulated by anergy as opposed to developmental arrest or differentiation to B-1. J. Immunol. 2002;168:13–21. doi: 10.4049/jimmunol.168.1.13. [DOI] [PubMed] [Google Scholar]
- 19.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J. Immunol. 2001;167:5535–5538. doi: 10.4049/jimmunol.167.10.5535. [DOI] [PubMed] [Google Scholar]
- 20.Noorchashm H, Bui A, Li HL, Eaton A, Mandik-Nayak L, Sokol C, Potts KM, Pure E, Erikson J. Characterization of anergic anti-DNA B cells: B cell anergy is a T cell-independent and potentially reversible process. Int. Immunol. 1999;11:765–776. doi: 10.1093/intimm/11.5.765. [DOI] [PubMed] [Google Scholar]
- 21.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 22.Heltemes-Harris L, Liu X, Manser T. Progressive surface B cell antigen receptor down-regulation accompanies efficient development of antinuclear antigen B cells to mature, follicular phenotype. J. Immunol. 2004;172:823–833. doi: 10.4049/jimmunol.172.2.823. [DOI] [PubMed] [Google Scholar]
- 23.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 24.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J. Exp. Med. 1997;186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilmon MA, Rutan JA, Clarke SH, Vilen BJ. Cutting edge: low-affinity, smith antigen-specific B cells are tolerized by dendritic cells and macrophages. J. Immunol. 2005;175:37–41. doi: 10.4049/jimmunol.175.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 27.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 28.MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 29.Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J. Exp. Med. 1988;167:332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J. Immunol. 2002;169:4213–4221. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 31.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 32.Haskill S, Beg AA, Tompkins SM, Morris JS, Yurochko AD, Sampson-Johannes A, Mondal K, Ralph P, Baldwin AS., Jr. Characterization of an immediate-early gene induced in adherent monocytes that encodes I κB-like activity. Cell. 1991;65:1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- 33.Karin M. How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat. Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 35.Wong HK, Kammer GM, Dennis G, Tsokos GC. Abnormal NF-κB activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. J. Immunol. 1999;163:1682–1689. [PubMed] [Google Scholar]
- 36.Liu J, Beller D. Aberrant production of IL-12 by macrophages from several autoimmune-prone mouse strains is characterized by intrinsic and unique patterns of NF-κB expression and binding to the IL-12 p40 promoter. J. Immunol. 2002;169:581–586. doi: 10.4049/jimmunol.169.1.581. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect. 2004;6:1333–1338. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 39.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J. Exp. Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus: a putative role in pathogenesis. J. Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 42.Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2000;18:565–570. [PubMed] [Google Scholar]
- 43.Peterson E, Robertson AD, Emlen W. Serum and urinary interleukin-6 in systemic lupus erythematosus. Lupus. 1996;5:571–575. doi: 10.1177/096120339600500603. [DOI] [PubMed] [Google Scholar]
- 44.Davas EM, Tsirogianni A, Kappou I, Karamitsos D, Economidou I, Dantis PC. Serum IL-6, TNFα, p55 srTNFα, p75srTNFα, srIL-2α levels and disease activity in systemic lupus erythematosus. Clin. Rheumatol. 1999;18:17–22. doi: 10.1007/s100670050045. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi I, Matsuda T, Saito T, Yasukawa K, Kikutani H, Hirano T, Taga T, Kishimoto T. Abnormal distribution of IL-6 receptor in aged MRL/lpr mice: elevated expression on B cells and absence on CD4+ cells. Int. Immunol. 1992;4:1407–1412. doi: 10.1093/intimm/4.12.1407. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H, Yasukawa K, Saito T, Narazaki M, Hasegawa A, Taga T, Kishimoto T. Serum soluble interleukin-6 receptor in MRL/lpr mice is elevated with age and mediates the interleukin-6 signal. Eur. J. Immunol. 1993;23:1078–1082. doi: 10.1002/eji.1830230515. [DOI] [PubMed] [Google Scholar]
- 47.Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, Kishimoto T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int. Immunol. 1991;3:273–278. doi: 10.1093/intimm/3.3.273. [DOI] [PubMed] [Google Scholar]
- 48.Zeuner RA, Klinman DM, Illei G, Yarboro C, Ishii KJ, Gursel M, Verthelyi D. Response of peripheral blood mononuclear cells from lupus patients to stimulation by CpG oligodeoxynucleotides. Rheumatology (Oxford) 2003;42:563–569. doi: 10.1093/rheumatology/keg191. [DOI] [PubMed] [Google Scholar]
- 49.Lai KN, Leung JC, Lai KB, Wong KC, Lai CK. Upregulation of adhesion molecule expression on endothelial cells by anti-DNA autoantibodies in systemic lupus erythematosus. Clin. Immunol. Immunopathol. 1996;81:229–238. doi: 10.1006/clin.1996.0183. [DOI] [PubMed] [Google Scholar]
- 50.Lai KN, Leung JC, Lai KB, Lai FM, Wong KC. Increased release of von Willebrand factor antigen from endothelial cells by anti-DNA autoantibodies. Ann. Rheum Dis. 1996;55:57–62. doi: 10.1136/ard.55.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neng Lai K, Leung JC, Bik Lai K, Li PK, Lai CK. Anti-DNA autoantibodies stimulate the release of interleukin-1 and interleukin-6 from endothelial cells. J. Pathol. 1996;178:451–457. [PubMed] [Google Scholar]
- 52.Kuroiwa T, Lee EG, Danning CL, Illei GG, McInnes IB, Boumpas DT. CD40 ligand-activated human monocytes amplify glomerular inflammatory responses through soluble and cell-to-cell contact-dependent mechanisms. J. Immunol. 1999;163:2168–2175. [PubMed] [Google Scholar]
- 53.Yu CL, Sun KH, Tsai CY, Hsieh SC, Yu HS. Anti-dsDNA antibody up-regulates interleukin 6, but not cyclo-oxygenase, gene expression in glomerular mesangial cells: a marker of immune-mediated renal damage? Inflamm. Res. 2001;50:12–18. doi: 10.1007/s000110050718. [DOI] [PubMed] [Google Scholar]
- 54.Takemura T, Yoshioka K, Murakami K, Akano N, Okada M, Aya N, Maki S. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994;424:459–464. doi: 10.1007/BF00191429. [DOI] [PubMed] [Google Scholar]
- 55.Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J. Clin. Invest. 1994;94:585–591. doi: 10.1172/JCI117373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryffel B, Car BD, Gunn H, Roman D, Hiestand P, Mihatsch MJ. Interleukin-6 exacerbates glomerulonephritis in (NZB × NZW)F1 mice. Am. J. Pathol. 1994;144:927–937. [PMC free article] [PubMed] [Google Scholar]
- 57.Tomita M, Holman BJ, Williams LS, Pang KC, Santoro TJ. Cerebellar dysfunction is associated with overexpression of proinflammatory cytokine genes in lupus. J. Neurosci. Res. 2001;64:26–33. doi: 10.1002/jnr.1050. [DOI] [PubMed] [Google Scholar]
- 58.Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkelman FD, Linsley PS, Howard M, Goodnow CC. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J. Exp. Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 60.Vilen BJ, Nakamura T, Cambier JC. Antigen-stimulated dissociation of BCR mIg from Ig-α/Ig-β: implications for receptor desensitization. Immunity. 1999;10:239–248. doi: 10.1016/s1074-7613(00)80024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 62.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat. Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]