Abstract
Background
Inferior lead early repolarization pattern (ERP) has recently been associated with sudden cardiac death. Although ERP is common among athletes, prevalence, ECG lead distribution, clinical characteristics, and effects of physical training remain uncertain. We sought to examine the non-anterior early repolarization pattern (ERP) in competitive athletes.
Methods and Results
ERP was assessed in a cross-sectional cohort of collegiate athletes (n=879). The relationship between ERP and cardiac structure were then examined in a longitudinal subgroup (n=146) before and after a 90-day period of exercise training. ERP was defined as J-point elevation ≥ 0.1 mV in at least two leads within a non-anterior territory (inferior [II, III, aVF] or lateral [I, aVL, V4-V6]). Non-anterior ERP was present in 25.1% (221/879) of athletes including the inferior subtype in 3.8% (33/879). Exercise training led to significant increases in the prevalence of ERP and the inferior subtype but there were no associations between ERP and echocardiographic measures of left ventricular remodeling. In a multivariable model, ERP was associated with black race (OR 5.84, CI 3.54-9.61, p<0.001), increased QRS voltage (OR 2.08, CI 1.71-2.52, p<0.001), and slower HR (OR 1.54, CI 1.26-1.87, p<0.001).
Conclusions
Non-anterior ERP including the inferior subtype are common and have strong clinical associations among competitive athletes. The finding of increased ERP prevalence following intense physical training establishes a strong association between exercise and the ERP.
Keywords: exercise, electrocardiography, electrophysiology athlete’s heart, early repolarization
Introduction
Since its initial description nearly seventy-five years ago,1 the early repolarization pattern (ERP) has been considered a normal variant.2-4 However, emerging evidence from case-control5, 6 and prospective cohort7 studies suggest that ERP in the inferior leads is associated with an increased risk of sudden cardiac death (SCD).
The prevalence, clinical associations, and underlying mechanisms of this potentially malignant ERP subtype remains largely unstudied in young competitive athletes. Although SCD in athletes is relatively rare, it is an important clinical problem with devastating impact on families and communities. Valuable athlete SCD registry data have shown that the majority of athlete SCD is attributable to cardiac causes but that an identifiable cardiac disorder is absent roughly one third of the time.8 Mechanisms and markers of electrical instability in the absence of identifiable heart disease are lacking in this population.
It is well established that ERP is more common among young athletes (prevalence estimates of 20 to 90%) than in the general population.6,9 However, the recently identified and potentially malignant inferior ERP pattern has not been thoroughly examined in this population. Further, there are no available longitudinal data documenting the impact of exercise training on ERP. Accordingly, we conducted the current study to examine the prevalence, ECG lead distribution, clinical characteristics, effect of physical training, and structural LV parameters associated with ERP and the inferior subtype in young competitive athletes.
Methods
Study design
We utilized cross-sectional and prospective longitudinal study designs to examine specific aspects of ERP in competitive athletes. A large cross-sectional cohort comprised of competitive collegiate athletes was recruited to examine the demographic and clinical features associated with the presence of ERP. A sub-group of this cohort was studied in a prospective and longitudinal fashion to examine the effects of exercise training and exercise-induced cardiac remodeling on ERP.
Study subjects
Athletes enrolled in the Harvard Athletics Initiative, an on-going effort designed to address numerous aspects of student-athlete cardiovascular health, were studied. For the present study, male and female student athletes ≥ 18 years old who were official participants in competitive inter-collegiate athletics were enrolled between 2006 and 2010. Written informed consent was obtained from all participants before involvement. The Harvard University Institutional Review Board and the Partner’s Healthcare Human Research Committee approved the protocol before study initiation.
Study protocol
The cross-sectional cohort consisted of newly matriculated student athletes who were recruited for participation in varsity-level (intercollegiate) competitive athletics. Participants were enrolled and studied during pre-participation screening sessions as previously reported.10 This study excluded individuals with any cardiovascular disease that was detected during screening or previously known. Height, weight, resting vital signs, medication use, and personal/family medical history were obtained. Prior exercise training history was elicited by questionnaires and was confirmed by training logs. Exercise training history spanned the eight weeks prior to enrollment and was quantified as hours per week engaged in either endurance or strength training as previously detailed.11,12 A resting 12-lead electrocardiogram (ECG) was obtained in each participant.
A sub-group of participants were studied longitudinally to examine the impact of exercise training on ERP prevalence and to assess the relationship between ERP and exercise-induced cardiac remodeling. This subgroup included male rowers and football players. These 2 athlete groups were chosen based on previously published data which documented sport-specific cardiac remodeling among these forms of endurance- (rowers) and strength- (football players) trained athletes using a similar study design.12 Each athlete was studied with 12-lead electrocardiography and echocardiography before and after a 90-day session of team-based training and competition. Detailed records of exercise training during this 90-day study period were recorded.
Clinical Follow-up
All athletes in both the cross-sectional and longitudinal cohorts have been followed prospectively from the time of enrollment for the occurrence of adverse cardiac events. This follow-up period captured each individual’s participation in organized intercollegiate athletics, the critical time period during which sport-related adverse outcomes would be most likely to occur. Because enrollment occurred over 4 consecutive years, follow-up duration was variable. No participants were lost to clinical follow-up.
Twelve-Lead ECG adjudication
Electrocardiography was performed using standard 12-lead placement and equipment (MAC 5500, GE Healthcare, Milwaukee, Wisconsin). Individuals were studied in the supine position after >5 minutes of quiet rest. ECGs were adjudicated by two independent reviewers who were blinded to all subject characteristics. In ambiguous cases, final adjudication was achieved by consensus with a third reviewer. Early repolarization within a single lead was defined as elevation of the J-point (offset of QRS complex) of at least 0.1 mV. The amplitude of J-point elevation was semi-quantitatively coded as 0.1mV-<0.2mV or ≥0.2mV. Reviewers categorized the presence of ERP in each of the twelve leads separately and then by territory (anterior [V1-V3], inferior [II, III, aVF], lateral [V4-V6,I, aVL]). In order to be consistent with prior studies,5-7 anterior lead (V1-V3) early repolarization was recorded, but was not included in the definition of an ECG that was positive for ERP to avoid confusion with the Brugada syndrome. The ECG was considered to exhibit the ERP if early repolarization was present in at least two leads within a non-anterior territory (inferior [II, III, aVF] or lateral [V4-V6,I, aVL]).
The morphology of the J-point was coded as notched (positive J deflection), slurred (terminal QRS slurring), discrete notched (notch is observed after signal return to baseline), or discrete (early repolarization after signal return to baseline). The ST segment was coded as ascending (after the J-point, signal ascends gradually until the peak of T-wave), descending (after the J-point, signal descends gradually until the T-wave), or horizontal (before T-wave, signal is horizontal). The predominant J-point and ST segment morphology were classified for each territory. Representative examples of these morphologies are shown in Figure 1.
Figure 1.
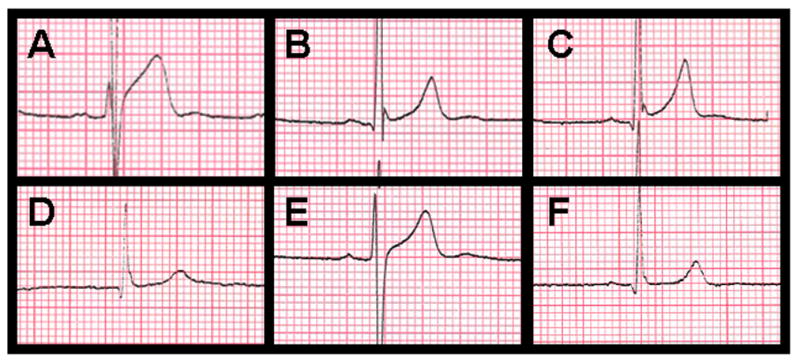
ERP morphologic classifications: (a) marked ERP (>0.2mV) with discrete J-point and ascending ST segment, (b) discrete notched J-point and ascending ST segment, (c) notched J-point and ascending ST segment, (d) slurred J-point and ascending ST segment, (e) discrete J-point and ascending ST segment (f) slurred J-point and horizontal ST segment.
Other ECG measurements included PR interval, QT interval, heart rate-corrected QT interval (Bazett’s correction), mean heart rate, and QRS duration. QRS amplitude was recorded as a continuous variable using the Sokolow-Lyon index (S in V1 + R in V5 or V6 [whichever is greater]).13 Left ventricular (LV) hypertrophy was recorded as a dichotomous variable as defined by a Sokolow-Lyon index ≥ 3.5mV. Presence of right bundle branch block (RBBB, defined as QRS complex ≥ 0.12 msec in duration with an rSR’ morphology in lead V1 and a qRS in lead V6), incomplete RBBB (IRBBB, defined as a RBBB morphology with a QRS duration of > 0.1 msec and < 0.12 msec), or left bundle branch block (LBBB, defined QRS complex ≥ 0.12 msec in duration with QS or rS complex in lead V1 and a monophasic R wave in leads I and V6) were noted.
Echocardiography
Echocardiography was performed using a commercially available system (Vivid-I, GE Healthcare, Milwaukee, WI) with a 1.9–3.8-mHz phased-array transducer. Participants were imaged at rest >12 h after the most recent training session. Imaging was performed to facilitate structural measurements and assessment for the presence of occult pathology of relevance to sport participation risk.10 Two-dimensional imaging was performed from standard parasternal and apical transducer positions with frame rates of 25–100 frames/s by trained sonographers. Each sonographer performed both baseline and post-study imaging on the same individuals. Cardiac structural measurements were made according to current guidelines.14 LV mass was estimated using the area-length formula and LV volumes were estimated using Simpson’s biplane technique. Relative wall thickness was calculated using the formula: [(septal thickness + posterior wall thickness) / left ventricular internal diameter at end diastole]. All data were stored digitally and post-hoc analyses were performed by cardiologists blinded to study time point (EchoPac, version 7.0, GE Healthcare).
Statistical analysis
In the cross-sectional cohort, the prevalence with 95% confidence intervals and clinical correlates of ERP and the inferior subtype were examined. A chi square test was performed to examine if the prevalence of ERP and the inferior subtype were distributed differently across sport types. Clinical characteristics were compared by ERP status (ERP positive vs. ERP negative, and inferior subtype ERP positive vs. ERP negative) using Fisher’s exact test for dichotomous covariates and T-test for independent samples for continuous variables. Univariable binary logistic regression models were used to test for association of clinical characteristics that differed based on ERP status (ERP positive vs. ERP negative, and inferior subtype ERP positive vs. ERP negative). A multivariable model was then built (forward stepwise selection) to determine the factors associated with ERP and specifically the inferior subtype using the significant covariates identified by univariable regression (threshold for entry and retention in the model p < 0.05).
In the longitudinal cohort, clinical and echocardiographic characteristics were compared pre- and post-season to characterize the nature of the training period using a paired-sample T-test. We then assessed changes in the prevalence of ERP and the inferior subtype after exercise training for rowers and football players pooled and by individual sport using McNemar’s test. Clinical and echocardiographic characteristics were compared pre- and post-season using a paired-sample T test. Echocardiographic characteristics were then compared (ERP positive vs. ERP negative, and inferior subtype positive ERP vs. ERP negative) in order to determine if ERP was associated with particular echocardiographic parameters using independent sample T test. Two-tailed p < 0.05 was defined as statistical significance throughout.
Results
Clinical characteristics of cross-sectional cohort
Characteristics of the athletes included in the cross-sectional cohort are shown in Table 1. This group was comprised of 879 athletes (64% male) and included representation from 20 sport types.
Table 1.
Characteristics of cross-sectional athlete cohort
|
|
|
|---|---|
| All sports pooled (n = 879)
|
|
| Demographics | |
| Age, years | 18.4 ± 0.8 |
| Sex, % male | 545/879 (62%) |
| Race | |
| Caucasian | 742 (84.3%) |
| Black | 92 (10.5%) |
| Asian | 36 (4.1%) |
| Latino | 6 (0.7%) |
| Anthropometrics | |
| Height, inches | 70.2 ± 3.0 |
| Weight, pounds | 167 ± 30 |
| BSA, m2 | 1.93 ± 0.21 |
| BMI, kg/m2 | 23.6 ± 2.7 |
| Clinical data | |
| HTN | 3 (0.3%) |
| Family history of HTN | 42 (7.4%) |
| Medication use | 149 (17%) |
| Resting HR | 60.3 ± 9.7 |
| Systolic BP, mmHg | 116.6 ± 8.6 |
| Diastolic BP, mmHg | 56.9 ± 11.5 |
| Training volume | |
| Total, hrs/w | 6.7 ± 3.8 |
| Strength, hrs/w | 2.9 ± 2.7 |
| Endurance, hrs/w | 3.9 ± 2.5 |
| Follow up | 18 ± 14 |
BSA: body surface area, BMI: body mass index, HTN: hypertension, HR: heart rate, BP: blood pressure, hrs/w: hours/week, continuous variables are shown as mean ± standard deviation.
Prevalence and characteristics associated with ERP
ERP was present in 221/879 [25.1 % (CI.95 = 21.4-28.9)] participants and ERP was significantly more common among male athletes (33%) than among female athletes (11%, p < 0.001). The inferior ERP subtype was observed in 33/879 [3.8% (CI.95 = 2.1-5.4)] which accounted for 14.9% of all ERP (Figure 2). The most common morphology of ERP in the lateral leads was a discrete J-point (101/199 [51%] discrete, 80/199 [40%] discrete notched, 14/199 [7%] notched, and 4/199 [2%] slurred) with an ascending ST segment (197/199 [99%] ascending and 2/199 [1%] horizontal). The most common morphology of ERP in the inferior leads was a notched J-point (19/33 [56%] notched, 10/33 [30%] slurred, 3/33 [9%] discrete notched, and 2/33 [6%] discrete) with an ascending ST segment (18/33 [55%] ascending and 15/33 [45%] horizontal). Marked J-point elevation (>0.2mV) was observed in two or more leads within an inferior or lateral territory in 18/879 (2%) of athletes. Isolated marked J-point elevation in a single lead, however, was more common. For example, isolated marked J-point elevation was observed in lead V4 in 199/879 (22.6%) of athletes.
Figure 2.
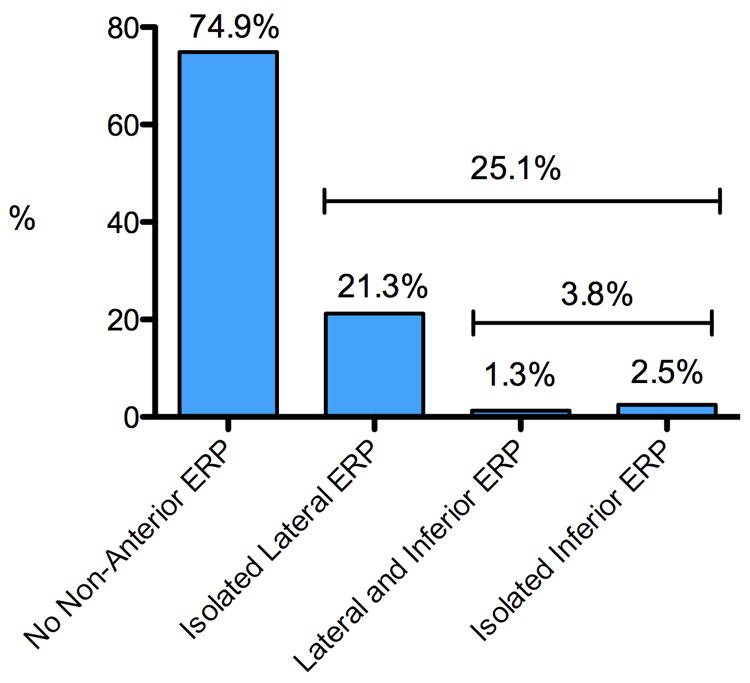
Prevalence and ECG lead distribution of non-anterior ERP among competitive intercollegiate athletes (n=879).
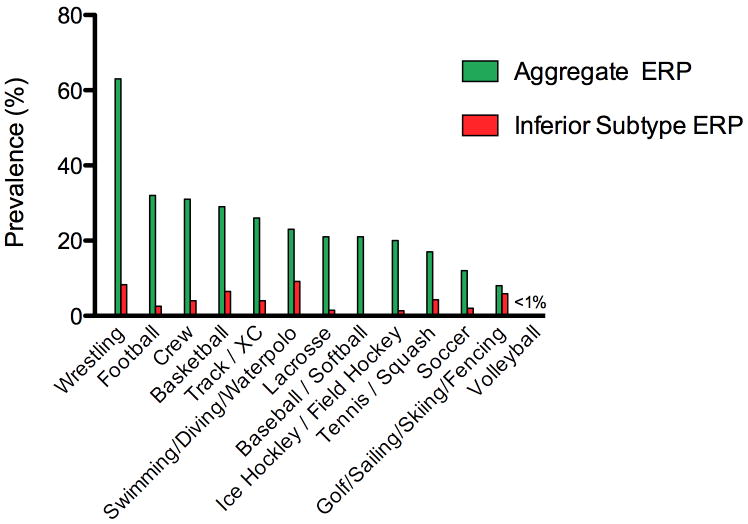
The prevalence of ERP varied by sport type ranging from 0% to 63% as shown in Figure 3. In chi-square analysis, the variability in ERP prevalence by sport type was driven by lateral ERP (p < 0.001), as the comparatively uncommon inferior ERP did not differ significantly by sport (p = 0.14).
Figure 3.
Prevalence of ERP and the inferior ERP subtype as function of sport type among competitive intercollegiate athletes (n=879).
Compared to ERP negative athletes, those with ERP were more likely to be male, black, taller, have slower heart rates and have performed more previous exercise training (Table 2). In addition, those with ERP had longer QRS-complex duration and higher QRS voltage. Athletes with the inferior ERP subtype were also more likely to be black, to have lower heart rates and to have increased QRS voltage compared with ERP negative athletes.
Table 2.
Means and SD of covariates by ERP status among pre-season athletes (cross-sectional cohort)
| All sports pooled, preseason (n=879)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| ERP neg (n = 658) | ERP positive (n = 221) | Inferior ERP pos (n = 33) | ||||||
| Mean or N | SD or % | Mean or N | SD or % | p* | Mean or N | SD or % | p* | |
|
|
|
|
||||||
| Demographics | ||||||||
| Age, years | 18.4 | 0.8 | 18.5 | 0.9 | 0.02 | 18.4 | 0.8 | 0.99 |
| Sex, % male | 358 | 54.4% | 187 | 84.2% | <0.001 | 10 | 30.3% | 0.23 |
| Black, % | 36 | 5.5% | 56 | 25.2% | <0.001 | 17 | 51.5% | <0.001 |
| Anthropometrics | ||||||||
| Height, inches | 70.0 | 2.9 | 71.0 | 3.0 | <0.001 | 71.0 | 2.3 | 0.05 |
| Weight, pounds | 165.3 | 30.6 | 171.2 | 29.9 | 0.12 | 166.9 | 23.9 | 0.75 |
| BMI, kg/m2 | 23.5 | 2.7 | 23.7 | 2.6 | 0.37 | 23.2 | 2.0 | 0.47 |
| Clinical data | ||||||||
| Diastolic BP, mmHg | 116.0 | 8.7 | 115.7 | 8.2 | 0.60 | 115.3 | 8.3 | 0.61 |
| Systolic BP, mmHg | 56.4 | 11.4 | 58.7 | 11.4 | 0.11 | 60.1 | 12.5 | 0.07 |
| ECG data (on post season ECG) | ||||||||
| HR bpm | 61.2 | 9.9 | 57.5 | 8.7 | <0.001 | 54.4 | 8.8 | <0.001 |
| PR interval, msec | 147.7 | 19.8 | 150.8 | 25.9 | 0.07 | 152.6 | 20.0 | 0.17 |
| QRS, msec | 92.4 | 11.5 | 95.1 | 9.1 | 0.001 | 93.6 | 8.5 | 0.54 |
| QTc, msec | 408.4 | 24.7 | 405.8 | 32.6 | 0.22 | 407.8 | 34.5 | 0.91 |
| Sokolow-Lyon index, mV | 2.81 | 0.95 | 3.65 | 0.98 | <0.001 | 3.51 | 1.18 | <0.001 |
| Sokolow-Lyon index, >3.5mV | 142 | 21.6% | 114 | 51.4% | <0.001 | 16 | 48.5% | <0.001 |
| LBBB | 0 | 0.0% | 0 | 0.0% | N/A | 0 | 0.0% | N/A |
| RBBB | 8 | 1.2% | 3 | 1.4% | 0.56 | 0 | 0.0% | 0.66 |
| IRBBB | 60 | 9.1% | 33 | 14.9% | 0.014 | 0 | 0.0% | 0.023 |
| Training characteristics | ||||||||
| Strength training, hrs | 2.8 | 2.7 | 3.1 | 2.8 | 0.082 | 2.9 | 2.7 | 0.67 |
| Endurance training, hrs | 3.8 | 2.3 | 4.2 | 3.0 | 0.033 | 3.8 | 2.4 | 0.99 |
| Total training, hrs | 6.5 | 3.8 | 7.3 | 4.0 | 0.009 | 6.3 | 2.8 | 0.75 |
| Medical history | ||||||||
| HTN | 3 | 0.5% | 0 | 0.0% | 0.42 | 0 | 0.0% | 0.89 |
| FH or HTN | 51 | 7.8% | 15 | 6.8% | 0.37 | 1 | 3.0% | 0.28 |
| Med use | 121 | 18.4% | 28 | 12.6% | 0.026 | 6 | 18.2% | 0.50 |
ERP includes both lateral and inferior subtypes. BMI: body mass index, BP: blood pressure, HTN: hypertension, FH: family history,
compared to ERP negative
These variables were each considered separately in univariable logistic regression models (Table 3A) and then collectively in a multivariable logistic regression model for association with ERP or the inferior subtype (Table 3B). Multivariable regression demonstrated that male sex, black race, slower heart rate and increased QRS voltage were significant associated with ERP while black race, slower heart rate and increased QRS voltage were significantly associated with the inferior ERP subtype. The covariate with the largest effect (highest odds ratio) on ERP prevalence was black race with odds ratios for ERP of 5.8 (3.54-9.61, p < 0.001) and for the inferior subtype of 21.1 (95% CI 9.1-49.1, p < 0.001, Table 3B).
Table 3.
Covariate relationships to ERP
| A. Univariate relationships between subject characteristics and ERP status | ||||
|---|---|---|---|---|
| All sports pooled (univariable model)
|
||||
| ERP | Inferior ERP subtype | |||
| OR | p* | OR | p* | |
|
|
|
|||
| Demographics | ||||
| Young age, per year increase | 0.80 (0.67-0.96) | 0.019 | --- | --- |
| Sex, male vs. female | 4.47 (3.03-6.61) | <0.001 | --- | --- |
| Black race, vs. non-black | 5.83 (3.72-9.15) | <0.001 | 18.32 (8.60-39.04) | <0.001 |
| Anthropometrics | ||||
| Height, per inches | 1.12 (1.06-1.18) | <0.001 | --- | --- |
| ECG data (on post season ECG) | ||||
| Heart rate, per 10bpm decrease | 1.52 (1.28-1.81) | <0.001 | 2.32 (1.51-3.56) | <0.001 |
| QRS, per 10 msec | 1.26 (1.10-1.44) | 0.001 | --- | --- |
| Sokolow-Lyon index, per mV | 2.36 (1.98-2.82) | <0.001 | 1.92 (1.38-2.67) | <0.001 |
| Sokolow-Lyon index, >3.5mV | 3.82 (2.77-5.26) | <0.001 | 3.45 (1.70-6.97) | 0.001 |
| Training characteristics | ||||
| Endurance training, per hour | 1.07 (1.00-1.13) | 0.034 | --- | --- |
| Total training, per hour | 1.05 (1.01-1.09) | 0.01 | --- | --- |
| Medical history | ||||
| Medication use | 0.64 (0.41-1.00) | 0.049 | --- | --- |
| B. Multivariable model describing associations with ERP status. | ||||
| All sports pooled (multi-variable model)
|
||||
| ERP | Inferior ERP subtype | |||
| OR | p* | OR | p* | |
|
|
|
|||
| Demographics | ||||
| Sex, male vs. female | 2.21 (1.40-3.47) | 0.001 | --- | --- |
| Black race, vs. non-black | 5.84 (3.54-9.61) | <0.001 | 21.09 (9.07-49.08) | <0.001 |
| ECG data (on post season ECG) | ||||
| Heart rate, per 10bpm decrease | 1.54 (1.26-1.87) | <0.001 | 2.41 (1.48-3.93) | <0.001 |
| Sokolow-Lyon index, per mV | 2.08 (1.71-2.52) | <0.001 | 2.44 (1.33-4.46) | 0.004 |
compared to ERP negative subjects
Characteristics of the longitudinal training cohort
The longitudinal training cohort was comprised of male football players (n = 78) and male rowers (n = 68) as described in table 4. Training during the 90-day study period (football = 19 ± 2 hours/week, crew = 21 ± 1 hours/week) was significantly higher than that experience by the athletes during the 8 weeks prior to enrollment. The football training regimen consisted predominantly of strength training (17 ± 1 hours/week) while the rowers engaged in mostly endurance training (19 ± 1 hours/week). At completion of the training period, football players demonstrated weight gain (219 ± 35 lbs to 223 ± 35 lbs, p < 0.001) and increased blood pressure (systolic from 116 ± 9 to 125 ± 13, p < 0.001; diastolic from 64 ± 11 to 67 ± 11, p = 0.008). In contrast, crew athletes demonstrated a drop in blood pressure (systolic from 114 ± 9 to 112 ± 8, p<0.001; diastolic from 61 ± 9 to 59 ± 8, p=0.006) and a drop in heart rate (62 ±6 to 56 ±4, p < 0.001, Table 4).
Table 4.
Clinical characteristics of the longitudinal training cohort
| Football (n = 78) | P* | Crew (n = 68) | p* | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pre-season | Post-season | Pre-season | Post-season | |||
| Age, years | 18.7 ± 0.6 | --- | 18.7 ± 0.6 | --- | ||
| Race | --- | --- | ||||
| Caucasian | 56 (71.8%) | --- | 60 (88.2%) | --- | ||
| Black | 19 (24.4%) | --- | 2 (2.9%) | --- | ||
| Asian | 0 | --- | 6 (8.8%) | --- | ||
| Latino | 3 (3.8%) | --- | 0 | --- | ||
| Height, inches | 73.6 ± 2.7 | --- | 73.6 ± 2.7 | --- | ||
| Weight, pounds | 219.1 ± 35.4 | 223.2 ± 35.2 | <0.001 | 182.7 ± 22.8 | 183.1 ± 22.7 | 0.31 |
| BMI | 28.3 ± 3.7 | 28.9 ± 3.7 | <0.001 | 23.6 ± 2.6 | 23.7 ± 2.5 | 0.35 |
| HTN | 0 | --- | 0 | --- | ||
| Family history of HTN | 21 (26.9%) | --- | 6 (8.8%) | --- | ||
| Medication use | 9 (11.5%) | --- | 9 (13.2%) | --- | ||
| Resting HR, bpm | 66.6 ± 6.6 | 66.5 ± 9.7 | 0.895 | 61.3 ± 6.3 | 55.7 ± 4.5 | <0.001 |
| Systolic BP, mmHg | 116.3 ± 9.0 | 125.3 ± 13.3 | <0.001 | 114.4 ± 8.7 | 111.5 ± 8.2 | 0.002 |
| Diastolic BP, mmHg | 64.7 ± 10.8 | 67.3 ± 10.8 | 0.008 | 60.8 ± 9.3 | 58.7 ± 7.8 | 0.006 |
| Prior training volume | ||||||
| Total, hrs/w | 9.8 ± 2.4 | --- | 10.9 ± 3.8 | |||
| Strength, hrs/w | 7.3 ± 2.6 | --- | 2.8 ± 3.0 | --- | ||
| Endurance, hrs/w | 2.6 ± 2.1 | --- | 7.8 ± 4.6 | --- | ||
BMI: body mass index, HTN: hypertension, FH: family history, HR: heart rate, BP: blood pressure, hrs/w: hours/week
compared to pre-season within a sport
Both football players and rowers experienced significant exercise-induced LV remodeling during the 90-day study period (Table 5). Football players experienced concentric hypertrophy as demonstrated by increases in LV mass, wall thickness, and relative wall thickness. LV mass increased by similar magnitude in rowers but was attributable to eccentric LV hypertrophy as characterized by LV chamber enlargement and unchanged relative wall thickness.
Table 5.
Echocardiographic left ventricular measures before and after training
| Football (n = 78)
|
p* | Crew (n = 68)
|
p* | |||
|---|---|---|---|---|---|---|
| Pre-season | Post-season | Pre-season | Post-season | |||
| LV function | ||||||
| LVEF | 64.9% ± 6.7% | 65%± 6.6% | 0.757 | 60.2 5.5 | 59.8 5.4% | 0.42 |
| LV mass measurements | ||||||
| LV mass | 222.5 ± 28.6 | 248.1 ± 35.3 | <0.001 | 217.5 ± 33.1 | 242.2 ± 34.7 | <0.001 |
| LV mass index | 98.8 ± 13.6 | 109.0 ± 15.0 | <0.001 | 104.9 ± 12.4 | 116.9 ± 13.3 | <0.001 |
| IVS | 9.4 ± 0.8 | 10.7 ± 1.0 | <0.001 | 9.9 ± 1.1 | 10.6 ± 1.2 | <0.001 |
| IVS index | 4.2 ± 0.5 | 4.7 ± 0.6 | <0.001 | 4.8 ± 0.6 | 5.1 ± 0.7 | <0.001 |
| PWT | 9.9 ± 0.9 | 11.1 ± 1.0 | <0.001 | 10.1 ± 1.0 | 10.7 ± 1.1 | <0.001 |
| PWT index | 4.4 ± 0.6 | 4.9 ± 0.7 | <0.001 | 4.9 ± 0.6 | 5.2 ± 0.6 | <0.001 |
| LV size/volume measurements | ||||||
| LVIDd | 51.4 ± 3.7 | 51.9 ± 3.7 | 0.26 | 53.8 ± 4.5 | 55.7 ± 4.4 | <0.001 |
| LVIDd index | 22.9 ± 2.2 | 22.9 ± 2.3 | 0.954 | 26.1 ± 2.3 | 27.0 ± 2.2 | <0.001 |
| LVEDV | 147.3 ± 21.0 | 145.6 ± 24.2 | 0.277 | 166.8 ± 27.6 | 183.7 ± 26.5 | <0.001 |
| LVEDV index | 65.5 ± 10.2 | 64.0 ± 10.2 | 0.042 | 80.5 ± 11.3 | 88.7 ± 11.1 | <0.001 |
| Composite measurements | ||||||
| Relative wall thickness | 0.37 ± 0.042 | 0.42 ± 0.050 | <0.001 | 0.37 ± 0.039 | 0.38 ± 0.046 | 0.006 |
LV: left ventricle, IVS: interventricular septal thickness, PWT: posterior wall thickness, IDd: internal dimension in diastole, IDs: internal dimension in systole, EDV: end diastolic volume,
compared to preseason within a sport using a paired-sample T test.
ERP and exercise training
The prevalence of ERP and the inferior subtype before and after exercise training is shown in Table 6. At the conclusion of the training period, the prevalence of ERP increased from 37.2% (55/148) to 52.7% (78/148) (p = 0.003). This included a significant increase in the prevalence of the inferior ERP subtype from 4.1% (6/148) to 8.1% (12/148) (p = 0.031). A representative pre- and post- training ECG is shown in Figure 4. There were no significant associations between LV parameters (baseline, post-training, or change from baseline to post-training) and either ERP subtype.
Table 6.
Changes in ERP prevalence with training
| All sports (n = 148)
|
|||||
|---|---|---|---|---|---|
| Pre-season | Post-season | p | |||
| N | % | N | % | ||
| ERP | 55 | 37.2% | 78 | 52.7% | 0.003 |
| Inferior ERP subtype | 6 | 4.1% | 12 | 8.1% | 0.031 |
|
|
|||||
| Football (n = 78)
|
|||||
| Pre-season | Post-season | ||||
| N | % | N | % | ||
| ERP | 28 | 35.9% | 35 | 44.9% | 0.25 |
| Inferior ERP subtype | 3 | 3.8% | 6 | 7.7% | 0.25 |
|
|
|||||
| Crew (n = 68)
|
|||||
| Pre-season | Post-season | ||||
| N | % | N | % | ||
| ERP | 27 | 39.7% | 43 | 63.2% | 0.03 |
| Inferior ERP subtype | 3 | 4.4% | 6 | 8.8% | 0.21 |
p compares pre-season to post-season
Figure 4.
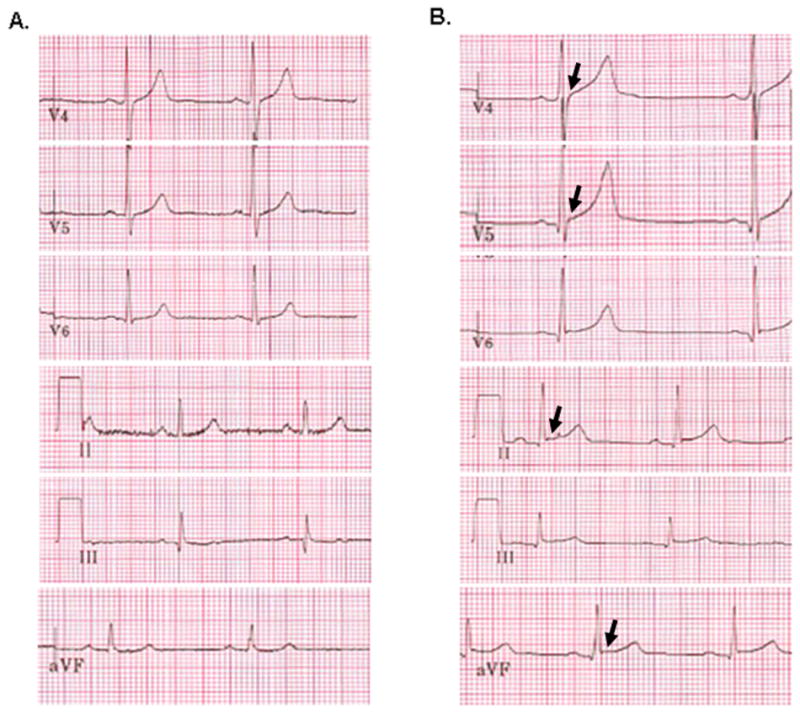
Lateral (V4-6) and inferior (II, III, aVF) 12-lead ECG leads in a collegiate rower before (A.) and after (B.) 90-days of team-based exercise training demonstrating the development of non-anterior early repolarization pattern (arrows).
Clinical follow-up
All participants were followed for significant cardiac events for 21 ± 13 months (range 7 to 50 months) during organized intercollegiate sport participation. During this follow-up period, there were no cases of SCD, unexplained syncope, or hospitalization with a cardiovascular diagnosis.
Discussion
Recent studies suggest that ERP, specifically the inferior lead pattern, is associated with an increased risk of cardiac death.7 This observation is seemingly at odds with the fact that that ERP is particularly common in young, competitive athletes with prevalence estimates ranging from 20-90%.15-20 To our knowledge, ERP lead distribution in athletes has been addressed in only two prior studies. Specifically, Rosso et al utilized a cross-sectional, case-control approach to show that ERP (both inferior and lateral) was more common in young athletes (n = 132) than among sedentary controls but less common than among idiopathic ventricular fibrillation victims.6 Most recently, Junttila et al presented data documenting a non-anterior ERP prevalence of 30% in a sample of 503 collegiate athletes and demonstrated associations between ERP and both male gender and ECG correlates of LV mass.21
The present study provides additive information about ERP in competitive athletes. Our data are in accord with the recent report by Junttila et al and confirm that a significant percentage of young competitive athletes (~25% in the present study) show ERP in either the inferior or lateral ECG leads. While our data demonstrate that the inferior ERP subtype is comparatively uncommon, we found that it is associated with the same athlete characteristics (ethnicity, heart rate, etc.) as the more common lateral subtype. Importantly, the current study is the first to demonstrate that exercise training is causal in the development of ERP. Specifically, both ERP and the inferior subtype increased in prevalence after intense physical training. Interestingly and somewhat surprisingly, neither ERP nor the inferior subtype was associated with the structural left ventricular measures, including chamber volume or wall thickness, that are well established components of the “athlete’s heart.” These data suggest that exercise-related ERP may be an isolated electrical phenomenon that develops in parallel but not as a result of structural myocardial remodeling. In aggregate, our data suggest that ERP (including the inferior subtype) is a common finding in young healthy competitive athletes that is a direct result of exercise training.
Mechanistic explanations for the ERP are an area of active investigation but at present remain incompletely understood. In general, ERP is visible on the surface electrocardiogram when adjacent areas of myocardium repolarize at slightly different rates causing intramyocardial current flow and upward displacement of the ST segment.22 ERP can be thought of as an electrocardiographic signature of repolarization dispersion and can arise in numerous conditions including myocardial injury,23 extremes of temperature such as hypothermia, local differences in Ito current density,24 and primary heritable disorders (e.g. mutations in KCNJ8,25 CACNA1C, CACNB2B, and CACNA2D1 26). In athletes, it has been proposed that parasympathetic modulation increases regional electrophysiological differences and repolarization dispersion resulting in ST elevation, J-waves, and prominent T-waves.27 Although this study was not intended to examine mechanisms of ERP, several issues deserve mention. First, the finding that ERP (and the inferior subtype) were more common in bradycardic athletes supports a causal link between an alteration in the autonomic balance favoring parasympathetic dominance and these ERP subtypes. It should be noted, however, that other investigators have found that ST segment elevation is not attenuated by concomitant atropine and propranolol administration.28 Second, ERP shared no significant associations with any aspect of structural LV remodeling. As such, it appears that while ERP may develop in parallel with structural features of the athlete’s heart, it is not clearly caused by exercise-induced LV hypertrophy or dilation.
Findings from the current study have important implications with respect to the care of the athletic patient. Our data demonstrate that non-anterior ERP, including the inferior subtype, is a common characteristic of the healthy athlete ECG and does not indicate an underlying structural abnormality. Thus, when found on an ECG that is obtained during pre-participation screening, preoperative clearance, or routine health maintenance physical examinations in an asymptomatic athlete, ERP should not necessarily mandate further costly diagnostic testing. In addition, practitioners should recognize that ERP is a dynamic process that is more likely to be observed in healthy athletes at times of peak fitness.
Several limitations of this study are noteworthy. First, this study was not adequately powered to assess the relationship between ERP and sudden cardiac death in athletes and thus we cannot make definitive conclusions about this important topic. However, it is noteworthy that no athlete with ERP, including those with the inferior subtype, experienced an adverse cardiovascular event during an average follow-up period of roughly 2 years. This follow-up period is short but included the critical period of safety concern during which athletes were participating in intercollegiate athletics. Second, although we detected no association between ERP and underlying cardiac structure, we acknowledge that the echocardiographic assessment was limited to basic LV parameters. It is possible that ERP may be a reflection of structural features not included in this protocol such as those that define the right ventricle. Finally, we chose to study collegiate athletes, a fairly homogeneous population with respect to age, heart rate, blood pressure, and co-morbid medical conditions thereby limiting power to detect associations between clinical variables and ERP. However, we chose this group as it represents a large percentage of the real world competitive athletic population.
In conclusion, non-anterior ERP including the inferior subtype is common among young competitive athletes. Importantly, the inferior and more common lateral ERP subtypes are associated with similar athlete characteristics and both increase in prevalence with intense exercise training. These data establish a causal link between exercise training and ERP. Further investigation is warranted to fully characterize the prognostic implications of ERP in competitive athletes.
Acknowledgments
Funding Sources: This work was supported by the Max Schaldach Fellowship in Cardiac Pacing and Electrophysiology (P.A.N.), the NIH/NHLBI (HL080025,C.N.-C.), the Doris Duke Charitable Foundation (C.N.-C.), the Burroughs Wellcome Fund (C.N.-C.)., and the American Heart Association (09FTF2220328, A.L.B.)
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Shipley R, Hallaran W. The four lead electrocardiogram in 200 normal men and women. Am Heart J. 1936;11:325–345. [Google Scholar]
- 2.Myers GB, Klein HA, Stofer BE, Hiratzka T. Normal variations in multiple precordial leads. Am Heart J. 1947;34:785–808. doi: 10.1016/0002-8703(47)90144-0. [DOI] [PubMed] [Google Scholar]
- 3.Goldman MJ. RS-T segment elevation in mid- and left precordial leads as a normal variant. Am Heart J. 1953;46:817–820. doi: 10.1016/0002-8703(53)90080-5. [DOI] [PubMed] [Google Scholar]
- 4.Wasserburger RH, Alt WJ. The normal RS-T segment elevation variant. Am J Cardiol. 1961;8:184–192. doi: 10.1016/0002-9149(61)90204-1. [DOI] [PubMed] [Google Scholar]
- 5.Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquie JL, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O’Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clementy J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 6.Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M, Katz A, Viskin S. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 9.Crouse SF, Meade T, Hansen BE, Green JS, Martin SE. Electrocardiograms of collegiate football athletes. Clin Cardiol. 2009;32:37–42. doi: 10.1002/clc.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggish AL, Hutter AM, Jr, Wang F, Yared K, Weiner RB, Kupperman E, Picard MH, Wood MJ. Cardiovascular screening in college athletes with and without electrocardiography: A cross-sectional study. Ann Intern Med. 152:269–275. doi: 10.7326/0003-4819-152-5-201003020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Weiner RB, Hutter AM, Jr, Wang F, Kim J, Weyman AE, Wood MJ, Picard MH, Baggish AL. The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging. 3:1001–1009. doi: 10.1016/j.jcmg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM, Jr, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol. 2008;104:1121–1128. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- 13.Sokolow M, Lyon TP. Criteria for the diagnosis of right ventricular hypertrophy using unipolar limb and precordial leads. Am J Med. 1947;3:125. doi: 10.1016/0002-9343(47)90136-8. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Bianco M, Zeppilli P. Early repolarization in the athlete. J Am Coll Cardiol. 2009;53:2199–2200. doi: 10.1016/j.jacc.2009.01.070. [DOI] [PubMed] [Google Scholar]
- 16.Boraita Perez A, Serratosa Fernandez L. “The athlete’s heart”: most common electrocardiographic findings. Rev Esp Cardiol. 1998;51:356–368. doi: 10.1016/s0300-8932(98)74759-1. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP. Normal resting electrocardiographic variants in young athletes. Phys Sportsmed. 2008;36:69–75. doi: 10.3810/psm.2008.12.14. [DOI] [PubMed] [Google Scholar]
- 18.Pelliccia A, Culasso F, Di Paolo FM, Accettura D, Cantore R, Castagna W, Ciacciarelli A, Costini G, Cuffari B, Drago E, Federici V, Gribaudo CG, Iacovelli G, Landolfi L, Menichetti G, Atzeni UO, Parisi A, Pizzi AR, Rosa M, Santelli F, Santilio F, Vagnini A, Casasco M, Di Luigi L. Prevalence of abnormal electrocardiograms in a large, unselected population undergoing pre-participation cardiovascular screening. Eur Heart J. 2007;28:2006–2010. doi: 10.1093/eurheartj/ehm219. [DOI] [PubMed] [Google Scholar]
- 19.Serra-Grima R, Estorch M, Carrio I, Subirana M, Berna L, Prat T. Marked ventricular repolarization abnormalities in highly trained athletes’ electrocardiograms: clinical and prognostic implications. J Am Coll Cardiol. 2000;36:1310–1316. doi: 10.1016/s0735-1097(00)00853-6. [DOI] [PubMed] [Google Scholar]
- 20.Swiatowiec A, Krol W, Kuch M, Braksator W, Krysztofiak H, Dluzniewski M, Mamcarz A. Analysis of 12-lead electrocardiogram in top competitive professional athletes in the light of recent guidelines. Kardiol Pol. 2009;67:1095–1102. [PubMed] [Google Scholar]
- 21.Junttila MJ, Sager SJ, Freiser M, McGonagle S, Castellanos A, Myerburg RJ. Inferolateral early repolarization in athletes. J Interv Card Electrophysiol. doi: 10.1007/s10840-010-9528-y. [DOI] [PubMed] [Google Scholar]
- 22.Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol. 56:1177–1186. doi: 10.1016/j.jacc.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 23.Shinde R, Shinde S, Makhale C, Grant P, Sathe S, Durairaj M, Lokhandwala Y, Di Diego J, Antzelevitch C. Occurrence of “J waves” in 12-lead ECG as a marker of acute ischemia and their cellular basis. Pacing Clin Electrophysiol. 2007;30:817–819. doi: 10.1111/j.1540-8159.2007.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 25.Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kaab S, Koster J, Rudy Y, Le Marec H, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 26.Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpon E, Hu D, Desai M, Borggrefe M, Haissaguerre M, Kanter R, Pollevick GD, Guerchicoff A, Laino R, Marieb M, Nademanee K, Nam GB, Robles R, Schimpf R, Stapleton DH, Viskin S, Winters S, Wolpert C, Zimmern S, Veltmann C, Antzelevitch C. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbosa EC, Bomfim Ade S, Benchimol-Barbosa PR, Ginefra P. Ionic mechanisms and vectorial model of early repolarization pattern in the surface electrocardiogram of the athlete. Ann Noninvasive Electrocardiol. 2008;13:301–307. doi: 10.1111/j.1542-474X.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endres S, Mayuga KA, de Cristofaro A, Taneja T, Goldberger JJ, Kadish AH. Age and gender difference in ST height at rest and after double autonomic blockade in normal adults. Ann Noninvasive Electrocardiol. 2006;11:253–258. doi: 10.1111/j.1542-474X.2006.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]