Abstract
The U.S. HIV epidemic has evolved over the past 30 years and is now concentrated in socially marginalized and disenfranchised communities. The health disparities in this epidemic are striking, with most HIV infections occurring in sexual minorities and communities of color. While widely recognized, the health disparities in HIV and AIDS are not often discussed. In this paper, we examine the factors underlying health disparities in the U.S. HIV epidemic. We first discuss the interlocking relationships between biological, social, and behavioral factors that drive HIV epidemics. Guided by a well-established conceptual model of health disparities, we then describe the social positions of those most affected by HIV and AIDS, particularly racial and gender groups. Structural and economic conditions including environmental resources, constraints, access to care, and psychosocial influences are examined in relation to HIV disease trajectories. Greater attention to contextual factors and co-morbidities is needed to reduce the health disparities in HIV infection.
Introduction
The 1988 November issue of the American Psychologist was dedicated to what was then the emerging HIV epidemic in the United States. In that issue, Stephen Morin of the University of California at San Francisco described three separate but linked epidemics (Morin, 1988). Morin distinguished the HIV (viral) epidemic from the subsequent AIDS (disease) epidemic, foreseeing the ultimate convergence of preventing the spread of the virus and managing the disease it causes. Morin also discussed a third epidemic, “the social, cultural, economic, and political reaction to the HIV and AIDS epidemics.” He went on to say that “this third epidemic of reaction, which is just beginning, is as much a part of the pathology of AIDS as the virus itself.” Twenty-five years later, Morin’s prescient analysis has been confirmed. The three epidemics he foresaw are no less relevant today and the impact of the social context on the course and distribution of HIV infection shines a glaring light on the inequalities of these epidemics.
The Centers for Disease Control and Prevention (CDC) estimates that in 2008 over one million Americans were living with HIV infection, and over one million Americans have now been diagnosed with AIDS (CDC, 2011a). In contrast to the generalized epidemics of sub-Saharan Africa, the U.S. HIV epidemic is concentrated in distinct geographical regions, with most affected Americans either living in urban centers of the east and west coasts or in major cities and small towns throughout the south. Within the cities hit hardest by HIV infection, impoverished neighborhoods are far more affected than are more affluent areas. Overall, the U.S. HIV epidemic is characterized by low HIV prevalence in the general population, with cases densely concentrated in local hotspots that primarily impact the most socially disenfranchised and marginalized populations (El-Sadr, Mayer, & Hodder, 2010).
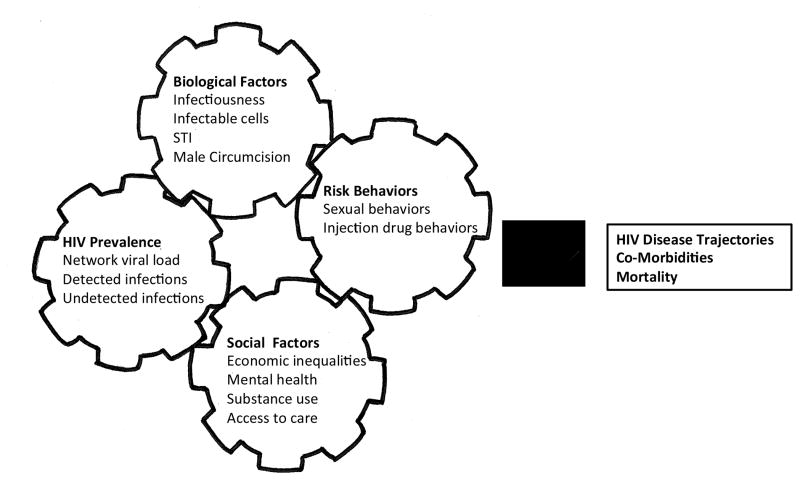
HIV transmission is a biological event that is entirely dependent on social context and behavioral practices. It has long been known that HIV transmission is a function of four concomitant interrelated factors: local HIV prevalence, individual behaviors, biological factors, and social conditions. Previous authors have conceptualized the complex factors that make up HIV epidemics as interlocking gears, where changes in one cog invariably shift the next (Strathdee et al., 2012). Figure 1 illustrates our adaptation of this concept for the purpose of this discussion.
Figure 1.
Interlocking factors that drive HIV epidemics. Adapted from Strathdee et al. (2012).
The spread of HIV requires a minimal prevalence in a population to maintain sufficient exposure for transmission. The most relevant portion of local HIV prevalence occurs at the social network level. For HIV transmission to occur the virus must have direct contact with cells that are susceptible to infection, namely those cells that carry the specific surface membrane molecules to which the virus attaches and thereby infects the host cell. The degree of risk for HIV transmission conferred by a given behavior is determined by the extent that both the virus and infectable cells are immediately present. Because HIV transmission is a biological event, multiple factors can facilitate or impede HIV transmission. HIV infected persons vary in their infectiousness depending on stage of disease and health status. Specifically, the amount of virus in blood plasma, or viral load, is highest in the first days of acute infection and again at the end stages of AIDS. In addition to stage of disease, concentrations of infectable cells present when exposure to the virus occurs also influences the likelihood of transmission.
Co-occurring sexually transmitted infections (STI), especially those that degrade mucous linings and cause genital ulcers, are also critical determinants of both HIV infectiousness and susceptibility. Studies show that the probability of HIV transmission during a single act of vaginal intercourse increases more than five times when there is a co-occurring genital ulcer disease (Boily et al., 2009). Conversely, male circumcision removes infectable cells in the penile foreskin and reduces HIV transmission by as much as half (Byakika-Tusiime, 2008).
Poverty, discrimination, inequality and other social conditions facilitate HIV transmission by influencing local HIV prevalence as well as an individual’s risk behaviors. For example, substance use can both reduce the likelihood that a person will take protective actions, such as using condoms, and substance use can stimulate HIV replication and therefore increases infectiousness (Kapadia, Vlahov, Donahoe, & Friedland, 2005). Relationship instability caused by economic stress, stigma, discrimination, domestic violence, migration, and incarceration also contribute to sexual partner mixing patterns that foster HIV transmission. Access to health care offers the potential to alleviate multiple sources of HIV transmission risk by reducing infectiousness through antiretroviral therapy and decreasing susceptibility through mental health, substance use, and STI treatment.
Each year, since the late 1990s, an estimated 56,000 Americans have become infected with HIV. The U.S. HIV epidemic disproportionately affects men who have sex with men (MSM) and ethnic and racial minorities. The group consistently at greatest risk for HIV infection represents the intersection of sexual orientation and racial disparities; MSM are by far the most HIV affected Americans and African American MSM are at six times the risk for HIV than white MSM (CDC, 2011d). AIDS is the third leading cause of death among Black men and women between ages 35 and 44, and the fourth leading cause of death among Latinos of the same age group. AIDS remains a mostly urban disease in the United States, with nearly half of all people living with AIDS residing in ten metropolitan areas. Furthermore, different ethnic groups account for the preponderance of AIDS cases in the ten metropolitan areas, e.g. Puerto Ricans in New York City, Haitians in Miami. There are HIV infection sub-epidemics also occurring throughout southern sub-urban and rural America.
Disparities are evident not only in the transmission of HIV, but in treatment and course of disease. While the successes of HIV treatments have significantly improved the health and life span of people living with HIV infection, these benefits are not equally shared across segments of the U.S. population (Losina et al., 2009; Chen et al., 2012). Death rates are declining in some places, remaining unchanged in others, and even increasing in others (Chiu, Hsu, Wang & Nkhoma, 2008). The health disparities in HIV and AIDS are quite striking and yet under-researched.
A Health Disparities Framework for HIV Infection
Health disparities in the U.S. are now widely recognized, with disease burden and mortality greatest among the poor and among racial and ethnic minorities. Within minority groups, diseases are concentrated along socioeconomic lines; those at the lowest rungs of the social ladder bear the greatest disease burden. Socio-economic status (SES) and chronic diseases rather consistently fall on a gradient, where those of relatively lower SES have poorer health and are more often afflicted by multiple diseases than those above them on the SES ladder (Adler & Ostrove, 1999; Adler & Stewart, 2010). For example, although osteoarthritis, cervical cancer cardiovascular disease and other chronic diseases affect individuals across all socio-economic levels, those at the lower end of SES have somewhat greater prevalence. HIV infection, however, does not show a graded association across the SES range; rather, HIV infections are concentrated among the poor with very few people in the middle and upper social strata contracting HIV.
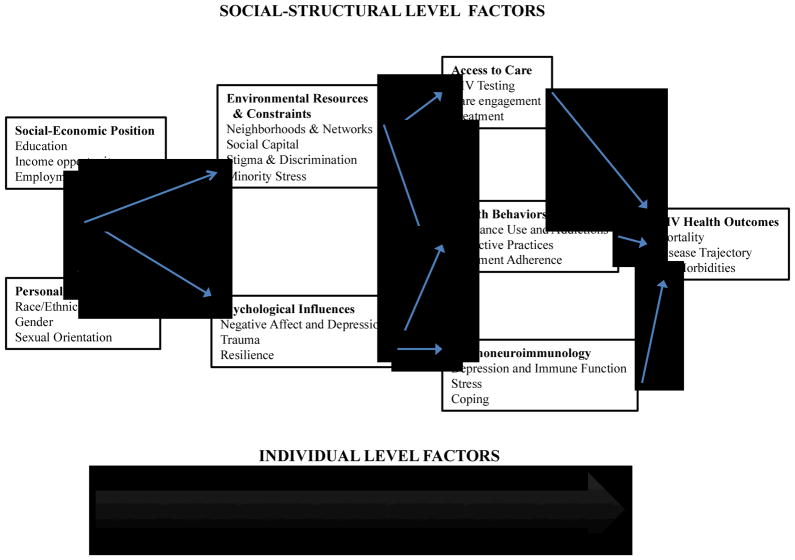
Adler and Stewart (2010) offered a framework to explain the major pathways by which SES can influence health. The model is developmental, illustrating individual, social, and structural influences on disease over the lifespan. Figure 2 shows their health disparities model, originally proposed to explain disparities in cancer, with only slight modifications to represent the U.S. HIV epidemic. In the current conceptual health framework, there are both individual level factors and social-structural level factors that influence HIV mortality. Starting at the left most side of the framework, social-economic position and personal characteristics influence each other as well as moderate the relationship between environmental resources and constraints, such as social capital, minority stress and stigma, and psychological influences including depression and trauma.
Figure 2.
A health disparities framework for HIV/AIDS. Adapted from Adler & Stewart (2010).
At the social structural level, environmental resources and psychological influences affect other social-structural and individual-level factors, namely access to care, health behaviors and psychoimmunology. We use this framework to guide our discussion of the individual, social and structural factors that influence HIV-related health disparities in the U.S. epidemic. At the individual level, the model highlights race, gender and sexual orientation as well as their intersections. In the words of Link and Phelan (1995), these constitute the “fundamental causes” of health inequality that apply just as well to HIV infection since they shape exposure to discrimination and limit economic opportunity (see also Phelan, Link & Tehranifar, 2010). Also at the individual level, these personal characteristics moderate the influence of psychological factors such as negative affect, trauma, and resilience. Psychological factors in turn influence health behaviors and physical processes that directly impact HIV infection trajectories and health outcomes.
Social-economic position, race, gender and sexual orientation
Unlike other chronic diseases that fall on the SES-health disparities gradient, HIV infection nearly exclusively impacts those who face economic adversity.b The CDC (2011b) reports that HIV prevalence is highest among people who are at or below the poverty level. In addition to the singular factor of income, education and employment also play important roles in the epidemic. HIV is concentrated among individuals who have less than a high school education and those who are unemployed (CDC, 2011b; Song et al., 2011). People with more advanced HIV infection and AIDS may qualify for disability benefits that cap their ability to earn additional income. Like so many other characteristics of people living with HIV, poverty is frequently a pre-morbid condition that is only exacerbated by an HIV diagnosis.
In 2009, African Americans accounted for 44% of all new HIV infections while making up only 14% of the U.S. population (CDC, 2011c). In one southern U.S. state, Georgia, 78% of HIV infections occur among African Americans, while African Americans comprise only 30% of the overall population. Additionally, a disproportionately greater number of Latinos are becoming HIV infected. Hall et al. (2008) found continued disparate incidence rates of HIV infection among blacks (83/100,000 population) as well as Latinos (29/100,000) as compared to whites (11/100,000). African Americans are also less likely to be aware of their HIV status and therefore less likely to be engaged in HIV-related care. In one of the largest studies to target HIV testing among MSM, 91% of African American MSM and 60% of White MSM who tested HIV-positive were unaware of their HIV infection (MacKellar et al., 2005).
The tangled implications of SES and ethnicity and race evident in the HIV epidemic mirror relationships found in other health conditions (Matthews & Gallo, 2011). The differential rates of infection based on race/ethnicity within SES groups signal that there is an underlying mechanism driving these relationships. Within the HIV epidemic the major underlying mechanism for this phenomenon occurs at the level of sexual networks. As stated before, HIV is transmitted through populations via sexual activity or injection drug use (Friedman & Aral, 2001). However, studies have shown that at least for African American MSM these sexual networks are mostly closed such that if HIV is introduced it is more likely to be transmitted to other African Americans than to outside groups (Friedman, Cooper & Osborne, 2009; Raymond & McFarland, 2009). HIV transmission within close knit sexual networks helps explain the differential rates of infection despite similar levels of socio-economic status.
In terms of gender, HIV in the U.S. has largely affected men in comparison to women; men comprise 78% of all new U.S. HIV infections. The greatest portion of HIV infections in men occurs among MSM, the most severely affected group in the U.S. HIV epidemic. Recent analyses show that HIV infection among MSM is now increasing at a rate faster than that which occurred in the late 1990s (Sullivan et al., 2009). In the state of Georgia, for example, MSM account for 48% of people living with HIV and 53% of incident HIV infections despite the fact that they comprise only 2% of the male population. As such, the rate of HIV diagnosis for MSM is 44 times that of other men (CDC, 2011d). Although women, make up only 23% of all new HIV infections, women of color are also disproportionately affected. Fifty-seven percent of new HIV infections among women occur in black women and sixteen percent among Hispanic women (CDC, 2011e).
Environmental resources and constraints
HIV in the U.S. is over-represented in places with wider income gaps and prevalence minority stress. In Washington DC, for example, the nations most wealthy and powerful live adjacent to neighborhoods with HIV infection rates that rival those seen only in southern Africa (El-Sadr et al., 2010). Similarly, the financial districts of New York City and San Francisco border neighborhoods ravaged by poverty and HIV. In a county level analysis, Gant et al. (2012) found a significant positive correlation between HIV infection rates and income inequality. Additionally, studies in urban health have shown that vacant buildings, broken windows, and high crime rates are all associated with the amplification of HIV infection (Fuller et al., 2005; Latkin, Williams, Wang, & Curry, 2005).
The poorest neighborhoods have the least social capital, another characteristic that predicts HIV infections. Social capital is generally defined as the value of a group’s social network, usually expressed in terms of trust, reciprocity and cooperation among network members who share common goals (Kreuter, Lezin, Young, & Koplan, 2001). However, there has been little research on social capital and HIV/AIDS. In one study, Holtgrave and Crosby (2003) conducted a state-level analysis of poverty, social capital and STI rates, including HIV infections. In this study, social capital was measured in an index of 14 variables that represented community organizational life, involvement in public affairs, volunteerism, informal sociability, and social trust. The results showed that low social capital was consistently associated with high rates HIV infection; an association that was significant over and above the relationship between poverty and disease (Holtgrave & Crosby, 2003). This analysis, however, does not account for the interrelatedness of income/poverty and race. It is possible that the association between social capital and rates HIV may be a by-product of segregation and race related social conditions, such as white privilege.
It is well known that minorities experience more discrimination and prejudice than do majority groups resulting in minority stress (Williams, 1999). These instances include exposure to structural barriers associated with residential social displacement and segregation, e.g. attending lower quality schools with less experienced teachers, exclusion from social networks that support career advancement (Fullilove & Wallace, 2011). Minorities are often concentrated in neighborhoods that have high crime rates and that lack stores for purchasing high-quality food or opportunities for physical activity and relaxation (Diez Roux & Mair, 2010; Massey & Denton, 1993). Many theorists have proposed that responses to race-based stressors range from sustained vigilance for threat to tremendous anxiety to internalizing negative stereotypes about race-based characteristics (Richman & Leary, 2009; Steele 1997), all of which have been posited to lead to poor mental and physical health (Hargrave, Fullilove, & Fullilove, 1991; Pascoe & Richman, 2009). Additionally, perceived racial privilege in favor of other races in the workplace has been associated with poorer health status among Blacks (Fujishiro, 2009). Although research is lacking demonstrating a direct relationship between minority stress alone to HIV contraction, there is some evidence for other “multiply stigmatized” groups to be at a higher risk for contracting HIV (Collins, von Unger & Armbrister, 2008).
For those already living with HIV infection, HIV-related stigma negatively impacts individuals along multiple dimensions. HIV stigma is associated with poorer mental health (Sayles et al., 2008), diminished social support (Kalichman et al., 2009), and poorer health outcomes (Visser, Kershaw, Makin, & Forsyth, 2008). People living with HIV who have internalized the degrading messages of HIV-related stigma experience increased depression (Berger, Ferrans, & Lashley, 2001), psychological distress (Mak et al., 2007), shame (Sayles et al., 2008), anxiety, and a loss of hope (Lee & Rotheram-Borus, 2001). In addition to impacting the health of people living with HIV, internalized stigma impacts an individual’s social spheres. For example, internalized stigma impedes social integration and increases relationship conflicts (Berger, 2001). Internalized stigma has the potential to undermine vital dimensions of the social support of people living with HIV, limiting resources that can often relieve the adverse effects of stress. Additionally, a large percent of African Americans have reported racial and SES based discrimination within health care settings (Bird & Bogart, 2001). Given the striking parallels between the processes underlying HIV stigma and race/ethnicity, it is likely that the stress exposures experienced by some minority groups may exacerbate the impact of HIV stigma.
Lee, Kochman, and Sikkema (2002) found that people recently diagnosed with HIV infection and characterized by having less supportive families, had not attended HIV support groups, knew fewer people living with HIV, and had a more negative image of themselves as persons living with HIV. In terms of health outcomes, less internalized stigma among people living with HIV is associated with engagement in health care and treatment (Roura et al., 2009). Socially enacted stigma is discrimination, which is also dependent on the social context (Earnshaw & Chaudoir, 2010). Additionally, stigma is a predictor of poor mental health, low self-esteem, and a personal sense of shame (Fife & Wright, 2000). Of particular importance is the association between stigma, discrimination, delays in seeking care when testing positive and poor HIV treatment adherence (Basta, Shacham, & Reece, 2009; Peretti-Watel, Spire, Pierret, Lert & Obadia, 2006).Living with HIV in an adverse social context therefore limits access to environmental resources and constrains opportunities for health improvement.
Psychological influences
Affective disorders are among the most common mental health problems facing people living with HIV. Estimates of depression in individuals living with HIV range from 5% to 20% depending on demographic characteristics, disease stage, and diagnostic methods (Cruess, et al., 2003). In a nationally representative sample of people receiving HIV treatment in the US, one in three had Major Depression and one in four had a less severe type of depression (Bing et al., 2001). Importantly, the chronic health problems seen in HIV infection do not account for higher depression in people living with HIV/AIDS. For example, a meta-analysis showed that depression in people living with HIV is not accounted for by their medical status (Ciesla & Roberts, 2001). Depression rates among people living with HIV are consistent with those seen in populations at risk for HIV infection. Because depression is a reliable predictor of HIV risk, it is expected that depression is also prevalent in people living with HIV. HIV-positive MSM show similar rates of depression as HIV-negative MSM, and both groups have higher rates of depression than observed in the general population (Perkins & Evans, 1991). Similar rates of depression have also been found among HIV-positive injection drug users and their uninfected counterparts (Lipsitz, et al., 1994). Additionally, although racial disparities are present in HIV treatment adherence, depression does not modify the relationship between suboptimal adherence and race (Kong, Nahata, Lacombe, Seiber & Balkrishnan, 2012). Indeed, depression has long been known to be a premorbid condition in a majority of depressed people living with HIV (Cruess et al., 2003).
Studies have also demonstrated that premorbid trauma experiences influence HIV disease progression. Most of the attention in trauma research among people living with HIV has focused on histories of child sexual abuse. In studies of HIV-positive individuals, rates of childhood sexual abuse range between 15% and 68% (Whetten, et al., 2006; Liebschutz, Feinman, Sullivan, Stein & Samet, 2000; O’Leary, Purcell, Remien, & Gomez, 2003). In a study of HIV-positive men, one-fifth reported pre-adolescence sexual abuse (Holmes, 1997). In terms of adolescent and adult abuse Kalichman, Sikkema, DiFonzo, Luke and Austin (2002) found that one in three men and two in three women with HIV reported sexual assault since age 15. These studies converge to show rates of childhood sexual abuse are much higher in people living with HIV than occurs in the general population (Felitti, et al., 1998). It should be noted that the majority of these studies were conducted with multiple races, however, research concerning racial/ethnic differences in the study of prior history of sexual abuse and/or assault for people living with HIV is lacking.
There are also long-term effects of childhood sexual abuse on later psychological functioning seen in people living with HIV, including depression, maladaptive coping, and re-victimization in adulthood (Johnsen & Harlow, 1996; Jumper, 1995). Early trauma experiences also predict health problems that overlap with the progression of HIV infection, including but not limited to illicit drug use, alcoholism and risks for co-occurring STI (Edwards, Iritani, & Hallfors, 2006). Sexual abuse at any point in life is related to subsequent sexual risk behaviors and substance use (Gore-Felton, Koopman, McGarvey, Hernandez & Canterbury, 2002; Thompson, Kao, & Thomas, 2005). Once again, because abuse experiences are reliable predictors of HIV infection, histories of abuse are prevalent in people living with HIV.
Research indicates that trauma severity also predicts mortality among patients with HIV disease. Leserman et al. (2007) found that lifetime trauma experiences were associated with AIDS-related mortality. Individuals who experience multiple lifetime traumas are nearly three times more likely to die of all-causes as compared to those who reported fewer stressors (Leserman et al., 2007). Evidence also suggests that trauma histories and posttraumatic stress symptoms are associated with poorer medication adherence and therefore disease progression (Boarts, Sledjeski, Bogart, & Delahanty, 2006). This evidence is important within our disparities framework because low SES has been associated with greater risk of exposure to traumatic acts of violence (Browne, Salomon & Bassuk, 1999). Untreated post-traumatic stress disorder (PTSD), a common outcome of violence, has been associated with lower levels of employment and annual income (Murdoch et al., 2011). Additionally, there are racial/ethnic differences in exposure to traumatic events and the development of PTSD. Exposure to household trauma such as child maltreatment and domestic violence has been shown to be highest among Blacks (Roberts, Gilman, Breslau, Breslau, & Koenen, 2011). However, the relationship between PTSD, trauma and HIV risk is complex within the context of racial disparities in HIV. Reisner, Falb and Mimiaga (2011) found racial/ethnic minority males who reported childhood violent events have substantially lower odds of becoming HIV infected compared to white males who also reported exposure to violent events in childhood.
Access to care
HIV testing is the essential first step to engaging infected persons in HIV-related health care. Testing later during the course of HIV infection translates to entering care later and therefore potential delays in treatment; delayed testing also has serious implications for HIV transmission risk. Examining low-income individuals in 16 US cities shows that African Americans and young adults are more likely to be tested later in HIV infection, putting these populations at a greater disadvantage for receiving necessary treatment (CDC, 2003). In 2008, one in three people diagnosed with HIV infection in the US were diagnosed with AIDS within 12-months. Indeed, an estimated 35% to 45% of people diagnosed with HIV infection are thought to have AIDS at the time they are tested (Gardner, McLees, Steiner, Del Rio, & Burman, 2011).
As many as half of people diagnosed with HIV infection fail to receive HIV care in any given year (Gardner et al., 2011). An estimated one in four of those who are in care are not being treated for their HIV infection although they meet criteria for recommended treatment. In addition, adherence to treatment is often poor, allowing HIV infection to progress. Although the overall literature seems to be inconclusive (Falagas, Zarkadoulia, Pliatsika, & Panos, 2008), several studies have shown that both lower SES and lower education have been associated with poorer medication adherence (Golin et al., 2002; Kleeberger et al., 2001). Additionally, racial differences in HIV care are mixed in the literature. A study done in the southeastern US showed that Latinos were more likely to enter into HIV care later in the course of infection (Dennis, Napravnik, Seña, & Eron, 2011). However, Sullivan et al.’s (2011) 10 U.S. city study found no relationship between delayed care and race/ethnicity.
Among individuals who test HIV positive, their access to quality health care is impacted by psychological, social, and economic factors. Just as one would expect, people with HIV who are more privileged and have health insurance as well as greater education also have better access to care (Shahani, Hartman, Troisi, Kapadia, & Giordano, 2012). Because they are often poor and unemployed, people living with HIV typically rely on public assistance for their health care. Although Medicare has been associated with more hospitalizations than private insurance, it does protect against suboptimal engagement in primary care. (Lafeuille et al., 2012) Shrinking budgets and health service cutbacks therefore have direct health implications for most people living with HIV. White privilege may also play a role, such that racial/ethnic minorities may be at an inherent disadvantage in obtaining quality care in a timely fashion simply because of their disadvantaged social status compared to Whites.
In addition to primary HIV health care, there is also a growing need for ongoing care for other chronic co-morbidities. There have been several estimations of psychiatric co-morbidities within the HIV positive population, frequently demonstrating to be significantly higher rates than observed in the general population (Kelly et al. 1998; Safren, Gershuny & Hendriksen, 2003, Evans et al., 2002). Specific to low income individuals, Soller et al. (2001) found that 56% had comorbid post-trauma symptoms and acute stress disorders. However, adherence to psychiatric care for low-income individuals living with HIV has been poor, with only about half of patients with psychiatric comorbidity following up with recommended psychiatric service referrals (Soller et al., 2001). Untreated psychiatric comorbidities can have serious impacts on regular care such as poor medication adherence. Not surprisingly, poor adherence to psychiatric medications is associated with non-adherence to antiretrovirals in people with co-morbid conditions such as depression (Bottonari et al., 2012; Cruess et al., 2012).
Co-morbid medical conditions in people with HIV also stem from more generally problematic health behaviors, such as smoking and overeating. Behaviorally influenced diseases, such as diabetes and hypertension are represented on an SES gradient, with populations at the lowest level of SES being the most affected. Because people with HIV are concentrated in these lower SES groups, the prevalence of these co-morbid chronic conditions as well as the behaviors that influence them are high among people with HIV. Data from the U.S. Military HIV Natural History Study, for example, shows that 37% of people with HIV were overweight and 9% were obese. (Crum-Cianflone et al., 2010) Although this is not much higher than the U.S. population, obesity among people living with HIV can complicate care (Keithley, Duloy, Swanson, & Zeller, 2009; Shah et al., 2012). The majority of patients followed between 1985 and 2004 had gained weight during the course of their HIV infection and weight gain was not accounted for by medication side-effects (Crum-Cianflone et al., 2010). In addition, poorly controlled chronic co-morbidities are likely the result of poor adherence to multiple treatments. For example, suboptimal HIV suppression is associated with poorly controlled diabetes and hypertension (Monroe, Chander, & Moore, 2011) and poorly controlled diabetes has been associated with lower SES (Hassan, Loar, Anderson, & Heptulla, 2006).
Poor retention to care is especially problematic because of its direct link to adverse health outcomes. Studies have shown that missing three or more physician appointments is associated with a greater likelihood of not receiving antiretroviral medications. Furthermore, half of individuals who did not return to the clinic after their first visit ever received treatment (Giordano et al., 2003). Racial disparities also exist in terms of access to HIV-related health care and utilization. The National Health Disparities Report (2011) found that African Americans living with HIV are less likely to receive standard HIV care, including antiretroviral therapy, outpatient appointments and monitoring of immune functioning, as compared to White patients. Latinos also experience greater barriers to care. Turner (2000), for example, found that Latinos experience greater delays initiating care than African Americans and Whites. Although no studies have pinpointed the underlying cause for these discrepancies, discrimination, prejudice and privilege (or lack there of) are all possible explanations.
Gender differences also exist in access to quality HIV care. Studies show that women with HIV are more likely to attend outpatient appointments than are men (National Health Disparities Report, 2007). Conversely, women living with HIV were found to be less likely to receive antiretroviral therapy. This discrepancy may reflect men entering into care earlier in the HIV disease process and therefore being afforded more opportunities for early treatment. The observed gender differences also intersect with race and ethnicity. Women of color suffer disproportionately from poverty in America, with lower literacy and lack of economic opportunities manifesting in delayed entry to care and poorer adherence to health improving behaviors (Aziz & Smith, 2011).
HIV treatments have the potential to reduce HIV transmission risks and therefore offering public health benefits beyond those of improved personal health. Several antiretroviral medications penetrate the genital compartment of the immune system and reduce concentrations of HIV in semen and vaginal secretions, just as they do in peripheral blood (Kalichman, 2012; Vernazza et al., 2000). The potential preventive benefits of HIV treatment are therefore also threatened by lack of access to health care and health disparities (Gardner et al., 2011). Factors such as poor adherence and co-occurring STI are both influenced by access to health care and both significantly increase HIV infectiousness. A review of studies examining the concordance between HIV in blood plasma and HIV in semen found the strongest correlation of .67 (Gupta et al., 1997), with most studies reporting correlations closer to .40 (Eron et al., 2000), and some studies reporting virtually no association between the two compartments of the immune system (Kalichman, 2001; Medeiros, Munerato, & Diaz, 2004). Thus, at best only 45% of the variability in genital secretion viral load can be accounted for by blood plasma viral load (Kalichman, Di Berto, & Eaton, 2008). The public health, as well as personal health, benefits of HIV treatments are therefore unrealized for many Americans with HIV in poverty.
Health behaviors
Substance use and drug addiction are a driving force in the spread of HIV infection. However, not all drugs are equally represented in the HIV epidemic. Most obvious is the role that injection drug use has played in the U.S. HIV epidemic, where a substantial number of HIV infections have been directly linked to sharing contaminated injection drug equipment. Non-injection drug use also promotes the spread of HIV insofar as it influences sexual transmission. In addition, non-injection drug users’ sexual networks often overlap with the networks of injection drug users. A person who smokes crack cocaine, for example, does not risk HIV infection from the drug use itself, but will be at elevated risk if they trade sex for drugs or have sex partners who inject drugs.
One study found that HIV incidence rates were higher among recently infected individuals who reported amphetamine use within the past year (6.3% per year) as compared to non-users (2.1% per year; Buchacz, et al., 2005). Amphetamine and its derivative methamphetamine are, in particular, associated with high-risk sexual behaviors (Brewer, Golden & Handsfield, 2006). Of great concern is the association between methamphetamine use and a rapid turnover of multiple sexual partners (Halkitis, Shrem, & Martin, 2005). Research suggests that the probability of HIV infection increases as the number of sexual partners increases, particularly when sex partners are concurrent meaning overlapping in time. Having multiple sex partners increases risk of contracting a co-occurring STI as well as transmitting HIV (Mah & Halperin, 2010). Methamphetamine use has increased among gay and bisexual men, particularly in urban areas, and is prevalent in circuit parties and commercial sex environments (Halkitis, Green & Mourgues, 2005). Thus, methamphetamine is believed to fuel the spread of HIV in sex networks of MSM. It is also common for methamphetamine users to engage in other substance abuse, making the isolation of any one drug as an HIV risk factor exceedingly difficult.
There are other addictive behaviors that can have a significant impact on HIV risk and transmission. Research shows that lack of personal control over sexual impulses and sexual preoccupations are associated with sexual risks for HIV including multiple sex partners and infrequent condom use (Benotsch, Kalichman, & Kelly, 1999; Muench & Parsons, 2004; Semple, Strathdee, Zians, McQuaid, & Patterson, 2011). Another addictive behavior, alcoholism, increases HIV risks beyond those posed by specific sex acts by increasing the likelihood of intersecting sex networks with varied HIV prevalence.
In addition to facilitating high-risk sexual activity, some recreational drugs can also dangerously interact with antiretroviral medications. In particular, some antiretroviral medications, most notably drugs in the class of protease inhibitors, are metabolized through the same hepatic pathways as recreational drugs, increasing the potential for hazardous interactions. Regardless of the specific medication regimen, people who are co-infected with HIV and Hepatitis C virus, and take antiretrovirals, drinking alcohol is a significant risk for life threatening liver complications (Braithwaite et al., 2008; Braithwaite et al., 2007).
Another critical health behavior contributing to disparities in HIV infection progression is medication adherence. Close adherence to antiretroviral medications is necessary to achieve the life prolonging benefits of these drugs. Combination HIV treatments have had dramatic effects on reducing viral burden, improving health and quality of life of people living with HIV, and contributing directly to significant declines in HIV-related mortality (Greenberg et al., 1999;Gulick, 1998;Hammer,.Squires, & Hughes, 1997;Montainer et al., 1998). Unfortunately, not everyone who is prescribed antiretroviral therapies realizes the potential benefits of these medications. When efficacy of treatment is assessed in clinical samples, viral load remains detectable and therapeutic effects are suboptimal in 30% to 70% of HIV infected patients (Altice & Friedland, 1998;Eron, 2000). Although a number of factors contribute to HIV treatment failure, inconsistent adherence is one of the most critical factors implicated in suboptimal response to therapy (Bangsberg, 2006; Chesney,1999, 2003; Paterson et al., 2000). Adherence of at least 85% doses taken is often discussed as necessary for most treatment regimens to reach acceptable population based HIV suppression (Bangsberg, Moss & Deeks, 2004; Paterson et al., 2000). However, antiretroviral treatment adherence varies widely. Clinical studies report that 26% to 35% of HIV positive patients have difficulty maintaining even 80% adherence (Avants, Margolin, Warburton, Hawkins, & Shi, 2001).
HIV infections are concentrated in the most socially disadvantaged populations and are compounded by factors that impede adherence that are also prevalent among the least well resourced. For example, limited literacy skills are associated with misunderstanding HIV disease processes and HIV treatment non-adherence (Hicks, Barragan, Franco-Paredes, Williams, & del Rio, 2006; Wolf, Gazmararian, & Baker, 2005). The association between health literacy and antiretroviral adherence appears quite robust, with health literacy significantly predicting treatment adherence after controlling for social support, attitudes toward health care providers, HIV symptoms, income level, substance use, and even years of education. Studies find adverse health effects of poor treatment adherence in marginally literate people living with HIV, particularly with regard to viral burden, immune function, and HIV-related symptoms (Kalichman & Rompa, 2000).
Psychoneuroimmunology
Psychological, social, and physical stressors can alter immune functioning and undermine health (Kemeny, 2003; Vanable, Carey, Blair, & Littlewood, 2006). Of particular importance is depression, which has been linked to decrements in immune functioning that are relevant to persons with HIV. Most importantly, greater depressive symptoms have been associated with a greater decline in CD4 cells, the target of HIV (Burack et al., 1993; Leserman, 2000). These associations are also observed outside the US, with greater depressive symptoms associated with lower number of CD4 cells among HIV-positive individuals in rural Uganda (Kaharuza et al., 2006). Leserman et al. (2002) found that depressive symptoms associated with faster progression to the later stages of HIV infection. Individuals with a previous history of depression had a fivefold increased risk of developing a depressive episode during the study. Depressive symptoms predicted increased risk of developing AIDS-defining conditions, whereas the opposite was not observed (Leserman et al., 2002). Reducing depression has also been shown to enhance immunity in people with HIV. For example, a study of HIV-positive women found successfully treating depression and improved immune function, specifically natural killer cell activity (Cruess et al., 2005).
Stemming from the association between emotional distress and immune functioning, stress management interventions have been extensively tested in people living with HIV. A meta-analytic review integrated the results of 35 randomized controlled trials, which tested the effects of 46 separate stress-management interventions for HIV positive adults (Scott-Sheldon, Kalichman, Carey, & Fielder, 2008). The studies included over three thousand people living with HIV infection. Results showed that compared to control conditions, stress-management interventions reduce anxiety, depression, distress, and fatigue and improve quality of life. However, the interventions do not generally improve CD4 cell counts, viral load, or hormonal outcomes compared with various control groups. The degree to which stress management and other stress reducing interventions are available to people living with HIV is limited and is more likely to decline than improve with shrinking health care resources.
HIV-related health outcomes
Along with inequalities of health care coverage, disparities in health outcomes for people with HIV are apparent. In a recent analysis, unmet basic needs such as shelter and food were found to have the greatest impact on overall mental health and disease trajectories for HIV positive homeless and unstably housed women (Riley et al., 2011). In fact, unmet basic needs had a greater impact on overall health outcomes than medication non-adherence. With respect to food security, as many as half of all people with HIV in U.S. inner-cities lack sufficient food (Kalichman et al., 2010; Weiser et al., 2009a). Food insecurity in people with HIV is related to low medication adherence and poor health outcomes. In Vancouver, British Columbia, lacking adequate food and having a low body-mass index predicted mortality in people with HIV over and above medication adherence (Weiser et al., 2009b). As discussed earlier, many people living with HIV rely on public assistance for their health care. However, there is evidence that simply having Medicaid may not lead to better health outcomes. One study found that people living with HIV who received Medicaid reported similar levels of health status as those who did not have any insurance (Stoskopf, Richter, & Kim, 2001). Efforts to increase quality and access to health care through public programs are vulnerable to economic downturns and shifts in public policy.
Advances in HIV treatment have significantly improved and extended the lives of HIV positive persons. HIV infection is now recognized as a controllable chronic disease. A recent study, however, suggests that the introduction of antiretroviral therapy has also increased inequalities in AIDS-related mortality. Rubin (2009) found that the association between SES and AIDS-related mortality increased with the introduction of effective treatment, as persons at the higher end of SES have increasingly positive health outcomes. Although overall mortality was reduced dramatically for all individuals receiving antiretroviral medications, access to the latest treatments has not been equal. Advantages in accessing the best available treatment are likely due to resources such as knowledge, money, health insurance, power and social connections that often accompany increases in SES. Cunningham et al. (2005) found significantly greater risk of death for those with no accumulated financial assets and those with less than a high school education even when controlling for HIV treatment and clinical care. These disparate health outcomes will only be alleviated through changes in factors at the individual, social, and structural levels.
Conclusions
The greatest overall illness burden across all diseases occurs at the lowest levels of SES. These health disparities are exaggerated in people living with HIV who are primarily concentrated in the lowest SES strata among whom, racial and ethnic minorities are over-represented. Multiple co-morbidities are further amplified by high prevalence rates of smoking, obesity, empty calorie/low nutrient diets, substance abuse, and other poor health behaviors, which are also over-represented among the poor and disenfranchised. The factors that promote HIV infection and those that impede access to health care therefore parallel those identified for other chronic illnesses. Models of health disparities such as the one that we have highlighted in this article are useful when translated to the HIV/AIDS epidemic.
Addressing health disparities in HIV infection may, however, require different approaches than other chronic diseases. HIV infection is so closely enmeshed in conditions of poverty that it is indeed a pandemic of the poor. Changes in health behaviors alone will have significant impacts on HIV epidemics. Biological factors that promote and impede infectiousness and susceptibility require universal access to testing and treatment, a feat easier said than done. Substance abuse and mental health treatment should be considered direct strategies for both primary and secondary prevention in AIDS (Safren, Reisner, Harrick, Mimiaga & Stall, 2010; Sikkema et al., 2010). Providing adequate food and housing are health interventions as well as humanitarian imperatives. We must also acknowledge that expanding all kinds of access will mean nothing unless the barriers posed by racism, homophobia, stigmas and discrimination are lifted. Our best chance for reducing the health disparity that is HIV will be to address social and structural factors that underlie the HIV epidemic.
Contributor Information
Jennifer A. Pellowski, Department of Psychology, University of Connecticut
Seth C. Kalichman, Department of Psychology, University of Connecticut
Karen A. Matthews, Department of Psychiatry, University of Pittsburgh
Nancy Adler, Department of Psychiatry, University of California, San Francisco.
References
- Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Annals New York Academy of Sciences. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Annals New York Academy of Sciences. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- Agency for Healthcare Research and Quality. National Healthcare Disparities Report 2011. Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality; Mar, 2011. AHRQ Pub. No. 11-0005. [Google Scholar]
- Agency for Healthcare Research and Quality. National Healthcare Disparities Report 2007. Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality; Feb, 2008. AHRQ Pub. No. 08-0041. [Google Scholar]
- Avants SK, Margolin A, Warburton LA, Hawkins KA, Shi J. Predictors of nonadherence to HIV-related medication regimens during methadone stabilization. American Journal on Addictions. 2001;10(1):69–78. doi: 10.1080/105504901750160501. [DOI] [PubMed] [Google Scholar]
- Aziz M, Smith KY. Challenges and successes in linking HIV-infected women to care in the United States. Clin Infect Dis. 2011;52(Suppl 2):S231–S237. doi: 10.1093/cid/ciq047. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clinical Infectious Diseases. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. Journal of Antimicrobial Chemotherapy. 2004;53(5):696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- Basta TB, Shacham E, Reece M. Symptoms of psychological distress: a comparison of rural and urban individual enrolled in HIV-related mental health care. AIDS Patient Care STDS. 2009;23(12):1053–1057. doi: 10.1089/apc.2009.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benotsch EG, Kalichman SC, Kelly JA. Sexual compulsivity and substance use in HIV-seropositive men who have sex with men: prevalence and predictors of high-risk behaviors. Addictive Behaviors. 1999;24(6):857–868. doi: 10.1016/s0306-4603(99)00056-8. [DOI] [PubMed] [Google Scholar]
- Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Research in Nursing & Health. 2001;24(6):518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry. 2001;58(8):721–78. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Bird ST, Bogart LM. Perceived race-based and socioeconomic status (SES)-based discrimination in interactions with health care providers. Ethnicity & Disease. 2001;11(3):554–563. [PubMed] [Google Scholar]
- Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, Alary M. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. The Lancet of Infectious Diseses. 2009;9(2):118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarts JM, Sledjeski EM, Bogart LM, Delahanty DL. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS and Behavior. 2006;10(3):253–261. doi: 10.1007/s10461-006-9069-7. [DOI] [PubMed] [Google Scholar]
- Bottonari KA, Tripathi SP, Fortney JC, Curran G, Rimland D, Rodriguez-Barradas M, Pyne JM. Correlates of antiretroviral and antidepressant adherence among depressed HIV-infected patients. AIDS Patient Care and STDs. 2012;26(5):265–273. doi: 10.1089/apc.2011.0218. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, McGinnis KA, Maisto SA, Bryant K, Justice AC. Adjusting alcohol quantity for mean consumption and intox3ication threshold improves prediction of nonadherence in HIV patients and HIV-negative controls. Alcoholism: Clinical and Experimental Research. 2008;32(9):1645–1651. doi: 10.1111/j.1530-0277.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, Justice AC. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19(4):459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer DD, Goldern MR, Handsfield HH. Unsafe sexual behavior and correlates of risk in a probability sample of men who have sex with men in the era of highly active antiretroviral therapy. Sexually Transmitted Disease. 2006;33(4):250–255. doi: 10.1097/01.olq.0000194595.90487.ed. [DOI] [PubMed] [Google Scholar]
- Browne A, Salomon A, Bassuk SS. The impact of recent partner violence on poor women’s capacity to maintain work. Violence Against Women. 1999;5:393–426. doi: 10.1177/10778019922181284. [DOI] [Google Scholar]
- Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, Klausner JD. Amphetamine use us associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19(13):1423–1424. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. The Journal of the American Medical Association. 1993;270(21):2568–2573. doi: 10.1001/jama.270.21.2568. [DOI] [PubMed] [Google Scholar]
- Byakika-Tusiime J. Circumcision and HIV infection: assessment of causality. AIDS and Behavior. 2008;12(6):835–841. doi: 10.1007/s10461-008-9453-6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Late versus early testing of HIV -16 sites, United States, 2000–2003. Morbidity and Mortality Weekly Report. 2003;52:581–586. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV Surveillance- United States, 1981–2008. Morbidity and Mortality Weekly Report. 2011a;60(21):689–693. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Characteristics associated with HIV Infection among heterosexuals in urban areas with high AIDS prevalence –24 Cities, United States, 2006–2007. Morbidity and Mortality Weekly Report. 2011b;60 (31):1045–1049. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV Among African Americans. 2011c Retrieved from http://www.cdc.gov/hiv/topics/aa/PDF/aa.pdf.
- Centers for Disease Control and Prevention. HIV Among Gay, Bisexual and Other Men Who Have Sex with Men (MSM) 2011d Retrieved from http://www.cdc.gov/hiv/topics/msm/index.htm.
- Centers for Disease Control and Prevention. HIV Among Women. 2011e Retrieved from http://www.cdc.gov/hiv/topics/women/index.htm.
- Chesney M. The challenge of adherence. BETA. 1999;12(1):10–13. [PubMed] [Google Scholar]
- Chesney M. Adherence to HAART regimens. AIDS Patient Care and STDS. 2003;17(4):169–177. doi: 10.1089/108729103321619773. [DOI] [PubMed] [Google Scholar]
- Chen M, Rhodes PH, Hall IH, Kilmarx PH, Branson BM, Valleroy LA Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. Prevalence of undiagnosed HIV infection among persons aged ≥13 years- National HIV Surveillance System, United States, 2005–2008. Morbidity and Mortality Weekly Report Surveillance Summary. 2012;61(2):57–64. [PubMed] [Google Scholar]
- Chiu YW, Hsu CE, Wang MQ, Nkhoma ET. Examining geographic and temporal variations of AIDS mortality: evidence of racial disparities. Journal of the National Medical Association. 2008;100(7):788–796. doi: 10.1016/s0027-9684(15)31372-9. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Collins PY, von Unger H, Armbrister A. Church ladies, good girls, and locas: stigma and the intersection of gender, ethnicity, mental illness, and sexuality in relation to HIV risk. Social Science & Medicine. 2008;67(3):389–397. doi: 10.1016/j.socscimed.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruess DG, Douglas SD, Petitto JM, Have TT, Gettes D, Dube B, Evans DL. Association of resolution of major depression with increased natural killer cell activity among HIV-seropositive women. American Journal of Psychiatry. 2005;162(11):2125–2130. doi: 10.1176/appi.ajp.162.11.2125. [DOI] [PubMed] [Google Scholar]
- Cruess DG, Evans DL, Repetto MJ, Gettes D, Douglas SD, Petitto JM. Prevalence, diagnosis, and pharmacological treatment of mood disorders in HIV disease. Biological Psychiatry. 2003;54:307–316. doi: 10.1016/S0006-3223(03)00318-4. [DOI] [PubMed] [Google Scholar]
- Cruess DG, Kalichman SC, Amaral C, Swetzes C, Cherry C, Kalichman MO. Benefits of adherence to psychotopic medications on depressive symptoms and antiretroviral medication adherence among men and women living with HIV/AIDS. Annals of Behavioral Medicine. 2012;43(2):189–197. doi: 10.1007/s12160-011-9322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone NF, Roediger M, Eberly LE, Vyas K, Landrum ML, Ganesan A Infectious Disease Clinical Research Program HIV Working Group. Obesity among HIV-infected persons: impact of weight on CD4 cell count. AIDS. 2010;24(7):1069–1072. doi: 10.1097/QAD.0b013e328337fe01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WE, Hays RD, Duan N, Andersen R, Nakazono TT, Bozzette SA, Shapiro MF. The effect of socioeconomic status on the survival of people receiving care for HIV infection in the United States. J Health Care Poor Underserved. 2005;16 (4):655–676. doi: 10.1353/hpu.2005.0093. [DOI] [PubMed] [Google Scholar]
- Dennis AM, Napravnik S, Seña AC, Eron JJ. Late entry to HIV care among Latinos compared with non-Latinos in a southeastern US cohort. Clinical Infectious Diseases. 2011;53(5):480–487. doi: 10.1093/cid/cir434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Chapter in Biology of Disadvantage. In: Adler NE, Stewart J, editors. Annals of the New York Academy of Sciences. 2010. pp. 125–145. [DOI] [PubMed] [Google Scholar]
- Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS and Behavior. 2009;13(6):1160–1177. doi: 10.1007/s10461-009-9593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JM, Iritani BJ, Hallfors DD. Prevalence and correlates of exchanging sex for drugs or money among adolescents in the United States. Sexually Transmitted Infections. 2006;82(5):354–358. doi: 10.1136/sti.2006.020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America--forgotten but not gone. New England Journal of Medicine. 2010;362(11):967–970. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron JJ, Smeaton LM, Fiscus SA, Gulick RM, Currier JS, Lennox JL, Murphy RL. The effects of protease inhibitor therapy on human immunodeficiency virus type 1 levels in semen (AIDS clinical trials group protocol 850) Journal of Infectious Diseases. 2000;181(5):16222–1628. doi: 10.1086/315447. [DOI] [PubMed] [Google Scholar]
- Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, Petitto JM. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. 2002;159 (10):1752–1759. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Zarkadoulia EA, Pliatsika PA, Panos G. Socioeconomic status (SES) as a determinant of adherence to treatment in HIV infected patients: a systematic review of the literature. Retrovirology. 2008;5:13. doi: 10.1186/1742-4690-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventative Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fife BL, Wright ER. The dimensionality of stigma: a comparison of its impact on the self of persons with HIV/AIDS and cancer. Journal of Health and Social Behavior. 2000;41(1):50–67. doi: 10.2307/2676360. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Aral S. Social networks, risk-potential networks, health, and disease. Journal of Urban Health. 2001;78(3):411–418. doi: 10.1093/jurban/78.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Cooper HL, Osborne AH. Structural and social contexts of HIV risk among African Americans. American Journal of Public Health. 2009;99(6):1002–1008. doi: 10.2105/AJPH.2008.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro K. Is perceived racial privilege associated with health? Findings from the Behavioral Risk Factor Surveillance System. Social Science & Medicine. 2009;68(5):840–844. doi: 10.1016/j.socscimed.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Fuller CM, Borrell LN, Latkin CA, Galea S, Ompad DC, Strathdee SA, Vlahov D. Effects of race, neighborhood, and social network on age at initiation of injection drug use. American Journal of Public Health. 2005;95(4):689–695. doi: 10.2105/AJPH.2003.02178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullilove MT, Wallace R. Serial forced displacement in American cities, 1916–2012. Journal of Urban Health. 2011;88(3):381, 389. doi: 10.1007/s11524-011-9585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant Z, Lomotey M, Hall HI, Guo X, Song R. A county-level examination of the relationship between HIV and social determinants of health: 40 states, 2006–2008. The Open AIDS Journal J. 2012;6:1–7. doi: 10.2174/1874613601206010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infectious Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, White AC, Jr, Sajja P, Graviss EA, Arduino RC, Adu-Oppong A, Visnegarwala F. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32 (4):399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- Golin EC, Liu H, Hays RD, Miller LD, Beck CK, Ickovics J, Wenger NS. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of General Internal Medicine. 2002;16:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore-Felton C, Koopman C, McGarvey E, Hernandez N, Canterbury RJ., Jr Relationships of sexual, physical, and emotional abuse to emotional and behavioral problems among incarcerated adolescents. Journal of Child Sexual Abuse. 2001;10(1):73–88. doi: 10.1300/J070v10n01_04. [DOI] [PubMed] [Google Scholar]
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. Journal of the American Medical Association. 1998;280(1):35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- Gupta P, Mellors J, Kingsley L, Riddler S, Singh MK, Schreiber S, Rinaldo CR. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. Journal of Virology. 1997;71(8):6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis PN, Green KA, Mourgues P. Longitudinal investigation of methamphetamine use among gay and bisexual men in New York City: findings from Project BUMPS. Journal of Urban Health. 2005;82 (Suppl 1):18–25. doi: 10.1093/jurban/jti020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis PN, Shrem MT, Martin FW. Sexual behavior patterns of methamphetamine-using gay and bisexual men. Substance Use & Misuse. 2005;40(5):703–719. doi: 10.1081/JA-200055393. [DOI] [PubMed] [Google Scholar]
- Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, Janssen RS. Estimation of HIV Incidence in the United States. JAMA. 2008;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Fischi MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. New England Journal of Medicine. 1997;337(11):725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- Hargrave R, Rullilove M, Fullilove RE. Defining mental health needs for black patients with AIDS in Alameda County. Journal of the National Medical Association. 1991;83(9):801–804. [PMC free article] [PubMed] [Google Scholar]
- Hassan K, Loar R, Anderson BJ, Heptulla RA. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. The Journal of Pediatrics. 2006;149(4):526–531. doi: 10.1016/j.jpeds.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Hicks G, Barragan M, Franco-Paredes C, Williams MV, del Rio C. Health literacy is a predictor of HIV/AIDS knowledge. Family Medicine Journal. 2006;38(10):717–723. [PubMed] [Google Scholar]
- Holmes WC. Association between a history of childhood sexual abuse and subsequent, adolescent psychoactive substance use disorder in a sample of HIV seropositive men. Journal of Adolescent Health. 1997;20(6):414–419. doi: 10.1016/S1054-139X(96)00278-9. [DOI] [PubMed] [Google Scholar]
- Holtgrave DR, Crosby RA. Social capital, poverty, and income inequality as predictors of gonorrhoea, syphilis, chlamydia and AIDS case rates in the United States. Sexually Transmitted Infections. 2003;79(1):62–64. doi: 10.1136/sti.79.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen LW, Harlow LL. Childhood sexual abuse linked with adult substance use, victimization, and AIDS-risk. AIDS Education and Prevention. 1996;8(1):44–57. [PubMed] [Google Scholar]
- Jumper SA. A meta-analysis of the relationship of child sexual abuse to adult psychological adjustment. Child Abuse and Neglect. 1995;19:715–728. doi: 10.1016/0145-2134(95)00029-8. [DOI] [PubMed] [Google Scholar]
- Kaharuza FM, Bunnell R, Moss S, Purcell DW, Bikaako-Kajura W, Wamai N, Mermin J. Depression and CD4 cell count among persons with HIV infection in Uganda. AIDS and Behavior. 2006;10(4 Suppl):S105–111. doi: 10.1007/s10461-006-9142-2. [DOI] [PubMed] [Google Scholar]
- Kalichman SC. HIV Treatment as Prevention (TasP): Primer for Behavior-Based Implementation. New York: Springer; 2012. [Google Scholar]
- Kalichman SC, Cage M, Barnett T, Tharnish P, Rompa D, Austin J, Schinazi RF. Human immunodeficiency virus in semen and plasma: investigation of sexual transmission risk behavioral correlates. AIDS Research and Human Retroviruses. 2001;17(18):1695–1703. doi: 10.1089/08892220152741397. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Cherry C, Amaral C, White D, Kalichman MO, Pope H, Macy R. Health and treatment implications of food insufficiency among people living with HIV/AIDS, Atlanta, Georgia. Journal of Urban Health. 2010;87(4):631–641. doi: 10.1007/s11524-010-9446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sexually Transmitted Diseases. 2008;35(1):55–60. doi: 10.1097/OLQ.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Rompa D. Functional health literacy is associated with health status and health-related knowledge in people living with HIV-AIDS. Journal of Acquired Immune Deficiency Syndromes. 2000;25(4):337–344. doi: 10.1097/00042560-200012010-00007. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Sikkema KJ, DiFonzo K, Luke W, Austin J. Emotional adjustment in survivors of sexual assult living with HIV-AIDS. Journal of Traumatic Stress. 2002;15(4):289–296. doi: 10.1023/A:1016247727498. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009;21(1):87–93. doi: 10.1080/09540120802032627. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clinical Infectious Diseases. 2005;41(7):1027–1034. doi: 10.1086/433175. [DOI] [PubMed] [Google Scholar]
- Keithley JK, Duloy AMS, Swanson B, Zeller JM. HIV infection and obesity: a review of the evidence. Journal of the Association of Nurses in AIDS Care. 2009;20(4):260–274. doi: 10.1016/j.jana.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Kelly B, Raphael B, Judd F, Perdices M, Kernutt G, Burnett P, Burrows G. Posttraumatic stress disorder in response to HIV infection. Gen Hosp Psychiatry. 1998;20 (6):345–352. doi: 10.1016/s0163-8343(98)00042-5. [DOI] [PubMed] [Google Scholar]
- Kemeny ME. An interdisciplinary research model to investigate psychosocial cofactors in disease: Application to HIV-1 pathogenesis. Brain, Behavioe and Immunity. 2003;17(Suppl 1):S62–72. doi: 10.1016/S0889-1591(02)00069-7. [DOI] [PubMed] [Google Scholar]
- Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. Journal of Acquired Immune Deficiency Syndromes. 2001;26:82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- Kong MC, Nahata MC, Lacombe VA, Seiber EE, Balkrishnan R. Association between race, depression, and antiretroviral therapy adherence in a low-income population with HIV infection. Journal of General Internal Medicine. 2012;27(9):1159–1164. doi: 10.1007/s11606-012-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter MW, Lezin NA, Young L, Koplan AN. Social capital: evaluation implications for community health promotion. WHO Regional Publications European Series. 2001;92:439–462. [PubMed] [Google Scholar]
- Lafeuille MH, Vekeman F, Wang ST, Kerrigan M, Menditto L, Duh MS. Lifetime costs to Medicare of providing care to patients with chronic lymphocytic leukemia. Leukemia & Lymphoma. 2012 doi: 10.3109/10428194.2011.643405. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Williams CT, Wang J, Curry AD. Neighborhood social disorder as a determinant of drug injection behaviors: a structural equation modeling approach. Health Psychology. 2005;24(1):96–100. doi: 10.1037/0278-6133.24.1.96. [DOI] [PubMed] [Google Scholar]
- Lee M, Rotheram-Borus MJ. Challenges associated with increased survival among parents living with HIV. American Journal of Public Health. 2001;91(8):1303–9. doi: 10.2105/AJPH.91.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Kochman A, Sikkema KJ. Internalized stigma among people living with HIV-AIDS. AIDS and Behavior. 2002;6(4):309–319. doi: 10.2105/AJPH.91.8.1303. [DOI] [Google Scholar]
- Leserman J. The effects of depression, stressful life events, social support, and coping on the progression of HIV infection. Current Psychiatry Reports. 2000;2(6):495–502. doi: 10.1007/s11920-000-0008-4. [DOI] [PubMed] [Google Scholar]
- Leserman J, Pence BW, Whetten K, Mugavero MJ, Theilman NM, Swartz MS, Stangi D. Relation of lifetime trauma and depressive symptoms to mortality in HIV. American Journal of Psychiatry. 2007;164(11):1707–1713. doi: 10.1176/appi.ajp.2007.06111775. [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, Evans DL. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychological Medicine. 2002;32(6):1059–1073. doi: 10.1017/S0033291702005949. [DOI] [PubMed] [Google Scholar]
- Liebschutz JM, Feinman G, Sullivan L, Stein M, Samet J. Physical and sexual abuse in women infected with the human immunodeficiency virus: increased illness and health care utilization. Archives of Internal Medicine. 2000;160(11):1659–64. doi: 10.1001/archinte.160.11.1659. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan JC. Social conditions as fundamental cause of disease. Journal of Health and Social Behavior. 1995;35:80–94. doi: 10.2307/2626958. [DOI] [PubMed] [Google Scholar]
- Lipsitz JD, Williams JB, Rabkin JG, Remien RH, Bradbury M, el Sadr W, Gorman JM. Psychopathology in male and female intravenous drug users with and without HIV infection. American Journal of Psychiatry. 1994;151(11):1662–1668. doi: 10.1176/ajp.151.11.1662. [DOI] [PubMed] [Google Scholar]
- Logie CH, James L, Tharao W, Loutfy MR. HIV, gender, race, sexual orientation, and sex work: a qualitative study of intersectional stigma experienced by HIV-positive women in Ontaio, Canada. PLoS Medicine. 2011;8(11):e1001124. doi: 10.1371/journal.pmed.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, Freedberg KA. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clinical Infectious Diseases. 2009;49(10):1570–8. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKellar DA, Valleroy LA, Secura GM, Behel S, Bingham T, Celentano DD Young Men’s Survey Study Group. Unrecognized HIV infection, risk behaviors, and perceptions of risk among young men who have sex with men: opportunities for advancing HIV prevention in the third decade of HIV/AIDS. Journal of Acquired Immune Deficiency Syndromes. 2005;38(5):603–614. doi: 10.1097/01.qai.0000141481.48348.7e. [DOI] [PubMed] [Google Scholar]
- Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS and Behavior. 2010;14(1):11–16. doi: 10.1007/s10461-008-9433-x. [DOI] [PubMed] [Google Scholar]
- Mak WW, Cheung RY, Law RW, Woo J, Li PC, Chung RW. Examining attribution model of self-stigma on social support and psychological well-being among people with HIV+/AIDS. Social Sciences & Medicine. 2007;64(8):1549–1559. doi: 10.1016/j.socscimed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Massey DS, Denton NA. American apartheid: Segregation and the making of the underclass. Harvard University Press; Cambridge: 1993. [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros RP, Munerato P, Diaz RS. HIV-1 viral load in blood and semen plasma of Brazilian patients under antiretroviral therapy. Journal of Clinical Virology. 2004;30(4):346–347. doi: 10.1016/j.jcv.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Monroe AK, Chander G, Moore RD. Control of medical comorbidities in individuals with HIV. Journal of Acquired Immune Deficiency Syndromes. 2011;58(5):458–462. doi: 10.1097/QAI.0b013e31823801c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner JS, Montessori V, Harrigan R, O’Shaughnessy M, Hogg R. Antiretroviral therapy: ‘the state of the art’. Biomedicine & Pharmacotherapy. 1999;53(2):63–72. doi: 10.1016/S0753-3322(99)80062-6. [DOI] [PubMed] [Google Scholar]
- Morin SF. AIDS: the challenge to psychology. American Psychologist. 1988;43(11):838–842. doi: 10.1037//0003-066X.43.11.838. [DOI] [PubMed] [Google Scholar]
- Muench F, Parsons JT. Sexual compulsivity and HIV: identification and treatment. Focus. 2004;19(6):1–5. [PubMed] [Google Scholar]
- Murdoch M, Sayer NA, Spoont MR, Rosenheck R, Noorbaloochi S, Griffin JM, Hagel EM. Long-term outcomes of disability benefits in US veterans with posttraumatic stress disorder. Archives of General Psychiatry. 2011;68(10):1072–1080. doi: 10.1001/archgenpsychiatry.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary A, Purcell D, Remien RH, Gomez C. Childhood sexual abuse and sexual transmission risk behaviour among HIV-positive men who have sex with men. AIDS Care. 2003;15(1):17–26. doi: 10.1080/0954012021000039725. [DOI] [PubMed] [Google Scholar]
- Pascoe EA, Richman LA. Perceived discrimination and health: A meta-analytic reviews. Psychological Bulletin. 2009;135:531–544. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Peretti-Watel P, Spire B, Pierret J, Lert F, Obadia Y. Management of HIV-related stigma and adherence to HAART: evidence from a large representative sample of outpatients attending French hospitals (ANRS-EN12-VESPA 2003) AIDS Care. 2006;18(3):254–261. doi: 10.1080/09540120500456193. [DOI] [PubMed] [Google Scholar]
- Perkins D, Evans DL. HIV-related major depression: response to zidovudine treatment. Psychosomatics. 1991;32(4):451–4. doi: 10.1016/S0033-3182(91)72051-3. [DOI] [PubMed] [Google Scholar]
- Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence and policy implications. Journal of Health and Social Behavior. 2010;51:S28–S40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- Raymond HF, McFarland W. Racial mixing and HIV risk among men who have sex with men. AIDS and Behavior. 2009;13(4):630–637. doi: 10.1007/s10461-009-9574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner SL, Falb KL, Mimiaga MJ. Early life traumatic stressors and the mediating role of PTSD in incident HIV infection among US men, comparisons by sexual orientation and race/ethnicity: results from the NESARC, 2004–2005. Journal of Acquired Immune Deficency Syndromes. 2011;57(4):340–50. doi: 10.1097/QAI.0b013e31821d36b4. [DOI] [PubMed] [Google Scholar]
- Riley ED, Moore K, Sorensen JL, Tulsky JP, Bangsberg DR, Neilands TB. Basic subsistence needs and overall health among human immunodeficiency virus-infected homeless and unstably housed women. American Journal of Epidemiology. 2011;174(5):515–522. doi: 10.1093/aje/kwr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC. Race/ethnic difference in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychological Medicine. 2011;41:71–83. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura M, Urassa M, Busza J, Mbata D, Wringe A, Zaba B. Scaling up stigma? The effects of antiretroviral roll-out on stigma and HIV testing. Early evidence from rural Tanzania. Sexually Transmitted Infections. 2009;85(4):308–312. doi: 10.1136/sti.2008.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MS, Colen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. American Journal of Public Health. 2009;100 (6):1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Gershuny BS, Hendriksen E. Symptoms of posttraumatic stress and death anxiety in person with HIV and medication adherence difficulties. AIDS Patient Care STDS. 2003;17 (12):657–664. doi: 10.1089/108729103771928717. [DOI] [PubMed] [Google Scholar]
- Safren SA, Reisner SL, Harrick A, Mimiaga MJ, Stall RD. Mental health and HIV risk in men who have sex with men. Journal of Acquired Immune Deficiency Syndromes. 55(Suppl 2):S74–S77. doi: 10.1097/QAI.0b013e3181fbc939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayles JN, Hays RD, Sarkisian CA, Mahajan AP, Spritzer KL, Cunningham WE. Development and psychometric assessment of a multidimensional measure of internalized HIV stigma in a sample of HIV-positive adults. AIDS and Behavior. 2008;12(5):748–758. doi: 10.1007/s10461-008-9375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LA, Kalichman SC, Carey MP, Fielder RL. Stress management interventions for HIV+ adults: a meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychology. 2008;27(2):129–139. doi: 10.1037/0278-6133.27.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Strathdee SA, Zians J, McQuaid J, Patterson TL. Correlates of obsessive-compulsive disorder in a sample of HIV-positive, methamphetamine-using men who have sex with men. AIDS and Behavior. 2011;15(6):1153–1160. doi: 10.1007/s10461-010-9719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. Journal of the American Geriatric Society. 2012;60(3):545–549. doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahani L, Hartman C, Troisi C, Kapadia A, Giordano TP. Causes of hospitalization and perceived access to care among persons newly diagnosed with HIV infection: implications for HIV testing programs. AIDS Patient Care and STDs. 2012;26(2):81–86. doi: 10.1089/apc.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema KJ, Watt MH, Drabkin AS, Meade CS, Hansen NB, Pence BW. Mental health treatment to reduce HIV transmission risk behavior: a positive prevention model. AIDS and Behavior. 2010;14(2):252–262. doi: 10.1007/s10461-009-9650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller M, Kharrazi N, Prentiss D, Cummings S, Balmas G, Koopman C, Israelski D. Utilization of psychiatric services among low-income HIV infected patients and psychiatric comorbidity. AIDS Care. 23(11):1351–1359. doi: 10.1080/09540121.2011.565024. [DOI] [PubMed] [Google Scholar]
- Song R, Hall HA, Harrison KM, Sharpe TT, Lin LS, Dean HD. Identifying the impact of social determinants of health on disease rates using correlation analysis of area-based summary information. Public Health Reports. 2011;126(Suppl 3):70–80. doi: 10.1177/00333549111260S312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoskopf CH, Richter DL, Kim YK. Factors affecting health status in African Americans living with HIV/AIDS. AIDS Patient Care and STDs. 2001;15(6):331–338. doi: 10.1089/108729101750279704. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Shoptaw S, Dyer TP, Quan VM, Aramrattana A for the Substance Use Scientific Committee of the HIV Prevention Trials Network. Towards combination HIV prevention for injection drug users: addressing addictophobia, apathy and inattention. Current Opinions in HIV and AIDS. 2012;7(4):320–325. doi: 10.1097/COH.0b013e32835369ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Hamouda O, Delpech V, Geduld JE, Prejean J, Semaille C, Annecy MSM Epidemiology Study Group. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Annals of Epidemiology. 2009;19(6):423–431. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Sullivan PS, Juhasz M, McNaghten AD, Frankel M, Bozzette S, Shapiro M. Time to first annual HIV care visit and associated factors for patients in care for HIV infection in 10 US cities. AIDS Care. 2011;23(10):1314–1320. doi: 10.1080/09540121.2011.555746. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Kao TC, Thomas RJ. The relationship between alcohol use and risk-taking sexual behaviors in a large behavioral study. Preventative Medicine. 2005;41(1):247–252. doi: 10.1016/j.ypmed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Cunningham WE, Duan N, Andersen RM, Shapiro MF, Bozzette SA, Zierler S. Delayed medical care after diagnosis in a U.S. national probability sample of persons infected with human immunodeficiency virus. Arch Intern Med. 2000;160(17):2614–2622. doi: 10.1001/archinte.160.17.2614. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS and Behavior. 2006;10(5):473–482. doi: 10.1007/s10461-006-9099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, Eron JJ. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS. 2000;14(2):117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- Visser MJ, Kershaw T, Makin JD, Forsyth BW. Development of parallel scales to measure HIV-related stigma. AIDS and Behavior. 2008;12(5):759–771. doi: 10.1007/s10461-008-9363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Bangsberg DR, Kegeles S, Ragland K, Kushel MB, Fongillo EA. Food insecurity among homeless and maginally housed individuals living with HIV/AIDS in San Francisco. AIDS and Behavior. 2009a;13(5):841–848. doi: 10.1007/s10461-009-9597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Fernandes KA, Brandson EK, Lima VD, Anema A, Bangsberg DR, Hogg RS. The association between food insecurity and mortality among HIV-infected individuals on HAART. Journal of Acquired Immune Deficiency Syndromes. 2009b;52(3):342–349. doi: 10.1097/QAI.0b013e3181b627c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten K, Leserman J, Lowe K, Stangi D, Thielman N, Swartz M, Van Scoyoc L. Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. American Journal of Public Health. 2006;96(6):1028–1030. doi: 10.2105/AJPH.2005.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MS, Gazmararian JA, Baker DW. Health literacy and functional health status among older adults. Archives of Internal Medicine. 2005;165(17):1946–1952. doi: 10.1001/archinte.165.17.1946. [DOI] [PubMed] [Google Scholar]