Abstract
Background
We have previously shown that the transcription factor AP-2α (Tcfap2a) is expressed in postmitotic developing amacrine cells in the mouse retina. Although retina-specific deletion of Tcfap2a did not affect retinogenesis, two other family members, AP-2β and AP-2γ, showed expression patterns similar to AP-2α.
Results
Here we show that, in addition to their highly overlapping expression patterns in amacrine cells, AP-2α and AP-2β are also co-expressed in developing horizontal cells. AP-2γ expression is restricted to amacrine cells, in a subset that is partially distinct from the AP-2α/β-immunopositive population. To address possible redundant roles for AP-2α and AP-2β during retinogenesis, Tcfap2a/b-deficient retinas were examined. These double mutants showed a striking loss of horizontal cells and an altered staining pattern in amacrine cells that were not detected upon deletion of either family member alone.
Conclusions
These studies have uncovered critical roles for AP-2 activity in retinogenesis, delineating the overlapping expression patterns of Tcfap2a, Tcfap2b, and Tcfap2c in the neural retina, and revealing a redundant requirement for Tcfap2a and Tcfap2b in horizontal and amacrine cell development.
Keywords: AP-2 transcription factors, retinal development, horizontal cell, amacrine cell, Tcfap2a/b double mutant, redundant roles
INTRODUCTION
The vertebrate neural retina (NR) is composed of six principal types of neurons and the Müller glia cells. During development, retinal cells are generated from multipotent progenitors that are instructed by both intrinsic and extrinsic factors to form the correct types and proportions of cells. Throughout neurogenesis, the differential expression of transcription factors is a defining feature of developing retinal cells as they proceed through cell fate determination and terminal differentiation (Livesey and Cepko, 2001). Amacrine and horizontal cells are retinal interneurons with tightly linked developmental pathways (Poche and Reese, 2009). Horizontal cell bodies reside in the outermost inner nuclear layer (INL) and make up only 3% of mouse INL cells (Jeon et al., 1998). Amacrine cells, a highly diverse class with approximately 30 morphologically distinct subtypes, are found in the inner portion of the INL as well as the ganglion cell layer (GCL), and in the mouse retina comprise approximately 40% of cells in both of these layers (Jeon et al., 1998; MacNeil et al., 1999). Nearly all amacrine cells contain either γ-aminobutyric acid (GABA) or glycine inhibitory neurotransmitters, and a range of additional neurotransmitters and neuropeptides have been localized to GABAergic amacrines (Vaney, 1990; Lam, 1997).
Gain- and loss-of-function studies in various animal models have identified key factors in the transcriptional hierarchy regulating amacrine and horizontal cell development. For example, Foxn4 is required for the generation of all horizontal and most amacrine cells and lies at the top of the hierarchy, activating expression of the downstream factors Math3 (Neurod4), NeuroD (Neurod1) and Ptf1a (Li et al., 2004; Fujitani et al., 2006). Math3/NeuroD double knockout (KO) mice exhibit a severe loss of amacrine cells, as do Ptf1a-null mice (Inoue et al., 2002; Fujitani et al., 2006; Nakhai et al., 2007). In the horizontal cell branch of the pathway, Ptf1a positively regulates the expression of Prox1, which is specifically required for horizontal cell genesis, and Lim1 (Lhx1), which is necessary for horizontal cell migration and differentiation (Dyer et al., 2003; Fujitani et al., 2006; Poche et al., 2007). Horizontal cell positioning and morphology are similarly affected by loss of the zinc finger transcription factor Sall3, which is required to maintain LIM1 expression in horizontal cells (de Melo et al., 2011). Downstream of early amacrine cell specification genes, additional transcription factors including Barhl2, Bhlhb5 (Bhlhe22), and Isl1 regulate the development of amacrine subpopulations (Feng et al., 2006; Elshatory et al., 2007b; Ding et al., 2009). Although much has been learned about the early regulators of retinal cell specification, retinogenesis is by no means complete upon generation of these retinal cell types and many of the regulators controlling the subsequent layering and mosaic spacing of retinal cell populations are not known.
The activating protein-2 (AP-2) transcription factors are a developmentally important family of genes that have been shown by our laboratory and others to play important roles in eye development (West-Mays et al., 1999; Dwivedi et al., 2005; Pontoriero et al., 2008; Bassett et al., 2010). The family currently includes five members, named Tcfap2a to Tcfap2e (encoding AP-2α, β, γ, δ, ε) in mice (Eckert et al., 2005). We have previously shown that AP-2α and AP-2β proteins are expressed in developing and mature mouse retinal amacrine cells of the INL and GCL, and we also detected transcripts of Tcfap2c (AP-2γ) and Tcfap2d (AP-2δ) in the embryonic and adult murine retina (Bassett et al., 2007). Tcfap2c appeared to be expressed in a pattern similar to Tcfap2a and Tcfap2b, whereas Tcfap2d, the most divergent AP-2 family member, was largely confined to the GCL (Bassett et al., 2007). Likewise, in the developing chick retina, AP-2δ is expressed in a subset of ganglion cells and shows no overlap with AP-2α and AP-2β expression (Bisgrove and Godbout, 1999; Li et al., 2008).
To examine a potential intrinsic role for AP-2α in retinogenesis, we previously generated a conditional KO of Tcfap2a in the developing retina (“Re-AP-2α” mice; Bassett et al., 2007). In Re-AP-2α mice, no apparent retinal defects were observed, suggesting that AP-2α alone is not intrinsically required for NR development. We therefore considered that the loss of AP-2α may have been compensated for by other AP-2 family member(s), the most likely candidates being AP-2β and/or AP-2γ. The purpose of this study was to investigate the potentially redundant roles of Tcfap2a, Tcfap2b, and Tcfap2c in the developing NR, through detailed expression analyses and generation of a double Tcfap2a/b mutant mouse model that was studied until its death at postnatal day 0 (P0). We show that, in addition to amacrine cells, AP-2α and AP-2β are also co-expressed in developing horizontal cells. AP-2γ expression is restricted to amacrine cells, in a subset that is partially distinct from the AP-2α/β-immunopositive population. Tcfap2a/b-deficient retinas show both amacrine and horizontal cell defects that are not detected upon deletion of either family member alone, uncovering critical roles for AP-2 activity in the development of these cell types.
RESULTS
AP-2α and AP-2β Are Expressed in Postmitotic Amacrine and Horizontal Cells
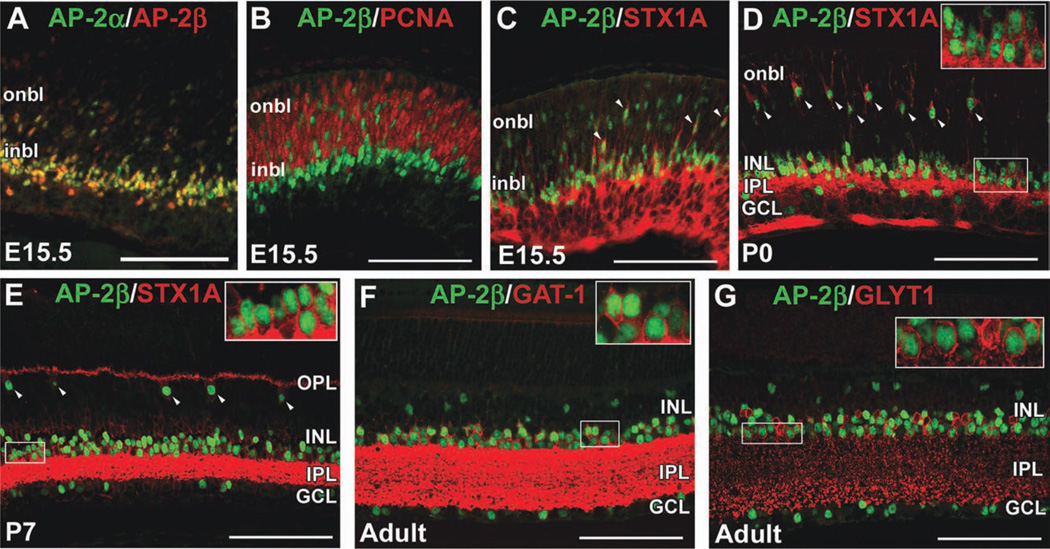
We previously showed that AP-2α is expressed in postmitotic developing amacrine cells, and that AP-2α and AP-2β are expressed in overlapping populations of cells in the neonatal and adult retina (Bassett et al., 2007). Here, we extend our previous work by examining earlier coexpression of AP-2α and AP-2β, where we detected extensive colocalization by embryonic day (E) 15.5 (Fig. 1A). We performed double immunostains for AP-2β and the proliferative markers phosphohistone H3 (PH3), which labels cells in late G2 and M phase (Hendzel et al., 1997), and proliferating cell nuclear antigen (PCNA), which labels cells in early G1 and S phase (Bravo et al., 1987). In E13.5, E15.5 and postnatal day (P) 0 retinas, AP-2β-positive cells did not colocalize with PCNA or PH3 (Fig. 1B and not shown). We also quantified the proportion of AP-2α-positive and AP-2β-positive cells colocalizing with Ki67, which is expressed by cells in all phases of the cell cycle (Scholzen and Gerdes, 2000; Supp. Fig. S1A, which is available online). The vast majority (>98%) of AP-2α or AP-2β-positive cells did not express Ki67, suggesting that both family members are a feature of postmitotic retinal cells. The synaptic vesicle docking protein syntaxin-1 (STX1A) is expressed in retinal progenitor cells (RPCs) that are heavily biased toward an amacrine or horizontal cell fate, in addition to postmitotic amacrine and horizontal cells in the developing and mature retina (Alexiades and Cepko, 1997). At E15.5, cells with AP-2β-positive nuclei also expressed STX1A (Fig. 1C). At birth, AP-2β and STX1A colocalized to amacrine cells in the INL and GCL, as well as a row of cells in the presumptive horizontal cell layer with the characteristic radial morphology of horizontal cells at this stage (Huckfeldt et al., 2009; Fig. 1D). By P7, AP-2β was clearly localized to STX1A-positive amacrine and horizontal cells (Fig. 1E). Amacrine cells can be broadly divided into two major subsets, glycinergic and GABAergic (Vaney, 1990), which can be detected by anti-GLYT1 (Slc6a9) and anti-GAT-1 (Slc6a1) antibodies, respectively. As is the case for AP-2α (Bassett et al., 2007), AP-2β expression was detected in both glycinergic and GABAergic amacrine cells in the adult retina (Fig. 1F,G).
Fig. 1. AP-2β is expressed in postmitotic amacrine and horizontal cells in the developing mouse retina.
A: Co-immunostain with anti-AP-2α (green) and anti-AP-2β (red) on horizontal section of E15.5 wild-type eye. B–G: Co-immunostain with anti-AP-2β (green) and either anti-PCNA (B), anti-STX1A (C–E), anti-GAT-1 (F), or anti-GLYT1 (G) antibodies (red) on horizontal sections of wild-type eyes, at the stages indicated. Arrowheads in C–E point to cells co-labeled by anti-AP-2β and anti-STX1A. Boxed areas in E–G are magnified in inset. i/onbl, inner/outer neuroblast layer; INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer; OPL, outer plexiform layer. Scale bars = 100 µm.
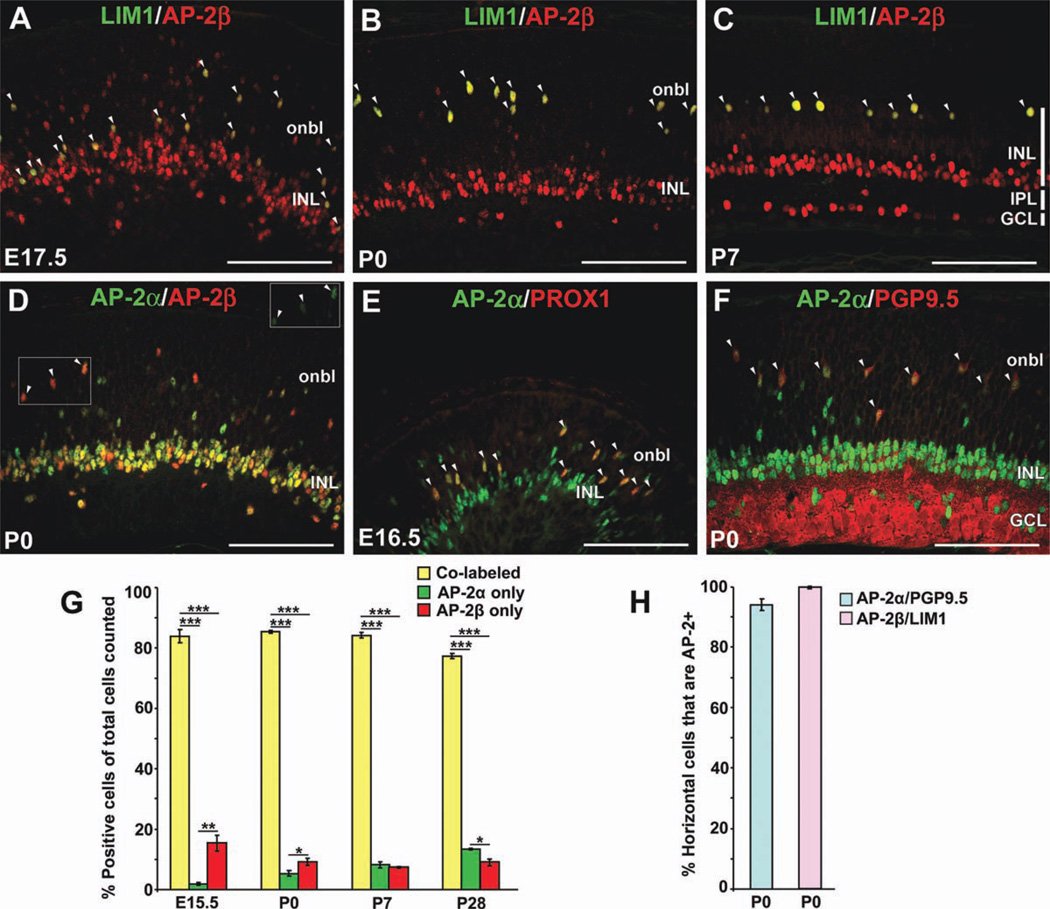
To further examine the expression of AP-2 proteins in horizontal cells, we used antibodies for known horizontal cell markers: homeobox transcription factors LIM1 (LHX1), expressed within the retina exclusively in horizontal cells (Liu et al., 2000; Poche et al., 2007) and PROX1, expressed in differentiating horizontal cells and populations of amacrine, bipolar and Müller glial cells (Dyer et al., 2003; Cid et al., 2010), as well as protein gene product 9.5 (PGP9.5; encoded by Uchl1), a deubiquitinating enzyme expressed in horizontal, amacrine and ganglion cells (Chen et al., 1994). At E17.5, a significant proportion of horizontal cells have yet to complete their migration to the outer retina and are intermingled among the developing amacrine cell population (Poche et al., 2007; Huckfeldt et al., 2009). At this stage, 100% of LIM1-positive cells in both the inner and outer retina were immunoreactive for AP-2β (Fig. 2A, arrowheads). At all subsequent stages examined (P0, P7, and adult), we detected AP-2β expression in 100% of LIM1-positive cells (Fig. 2B,C,H and not shown). Expression analyses have shown that LIM1 appears to be expressed in all mouse horizontal cells (Liu et al., 2000); therefore, the complete overlap of AP-2β and LIM1 in developing and mature horizontal cells suggests that AP-2β is also a pan-horizontal cell marker in the mouse retina.
Fig. 2. Both AP-2α and AP-2β are expressed in developing mouse horizontal cells, although AP-2α expression is transient.
A–F: Co-immunolabeling with anti-LIM1 (green) and anti-AP-2β (red; A–C); anti-AP-2α (green) and anti-AP-2β (red; D); anti-AP-2α (green) and anti-PROX1 (red; E); or anti-AP-2α (green) and anti-PGP9.5 (red; F) on horizontal sections of wild-type eyes, at the stages indicated. All LIM1-positive horizontal cells are AP-2β-positive (A–C, arrowheads). Arrowheads in D denote cells that are strongly AP-2β-immunoreactive and also more weakly AP-2α-immunoreactive (inset). Arrowheads in E and F point to PROX1-positive or PGP9.5-positive horizontal cells that co-express AP-2α. G: Quantification of the proportions of co-labeled versus singly labeled cells in the total AP-2 population examined (AP-2α and/or AP-2β-positive), at the stages indicated. Note that the relative proportions of co-labeled versus singly labeled cells were not significantly affected by the stage examined. H: Quantification of the proportion of PGP9.5-positive horizontal cells expressing AP-2α and proportion of LIM1-positive cells expressing AP-2β at P0. Bars represent the mean ±SEM of counts from 3 animals. onbl, outer neuroblast layer; INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer. ***P < 0.001; **P < 0.01; *P < 0.05. Scale bars = 100 µm.
AP-2α has been widely used as an amacrine cell marker; however studies in the chick retina have also revealed AP-2α expression in developing horizontal cells (Edqvist and Hallbook, 2004; Edqvist et al., 2008). To investigate the possibility of AP-2α expression in mouse horizontal cells, we first performed double immunostains for AP-2α and AP-2β at birth, when strongly immunoreactive AP-2β-positive cells are present in the future horizontal cell layer. In general, we detected more extensive overlap between AP-2α- and AP-2β-immunoreactive cells than previously appreciated in the neonatal retina (Fig. 2D), possibly due to the stage examined and the fact that we find our anti-AP-2 antibodies to work particularly well on P0 enucleated eyes. We then quantified the percent overlap of AP-2α-positive and AP-2β-positive populations in the developing and adult retina, which showed that over 80% of AP-2α and/or AP-2β-positive cells were co-labeled during development, while adult retinas exhibited 77 ± 1% overlap (Fig. 2G). At all stages examined, the proportion of co-labeled cells was significantly greater than the proportion of singly stained cells (Fig. 2G). In the P0 outer retina, putative horizontal cells exhibiting a strong signal for AP-2β were also more weakly immunoreactive to anti-AP-2α (Fig. 2D, arrowheads and inset), suggesting that AP-2α is co-expressed with AP-2β in developing horizontal cells. Given that the anti-AP-2α and anti-LIM1 antibodies are not compatible for double labeling, AP-2α expression in developing horizontal cells was verified by colocalization with PROX1 at E16.5 (Fig. 2E, arrowheads) and with PGP9.5 in the P0 presumptive horizontal cell layer (Fig. 2F, arrowheads), where 94 ± 1.9% of PGP9.5-positive cells expressed AP-2α (Fig. 2H). However, by P7 AP-2α protein was undetectable in horizontal cells (not shown), suggesting that it is only transiently expressed in this cell type during development.
AP-2γ Is Absent From Horizontal Cells and Expressed in Postmitotic Amacrine Cells
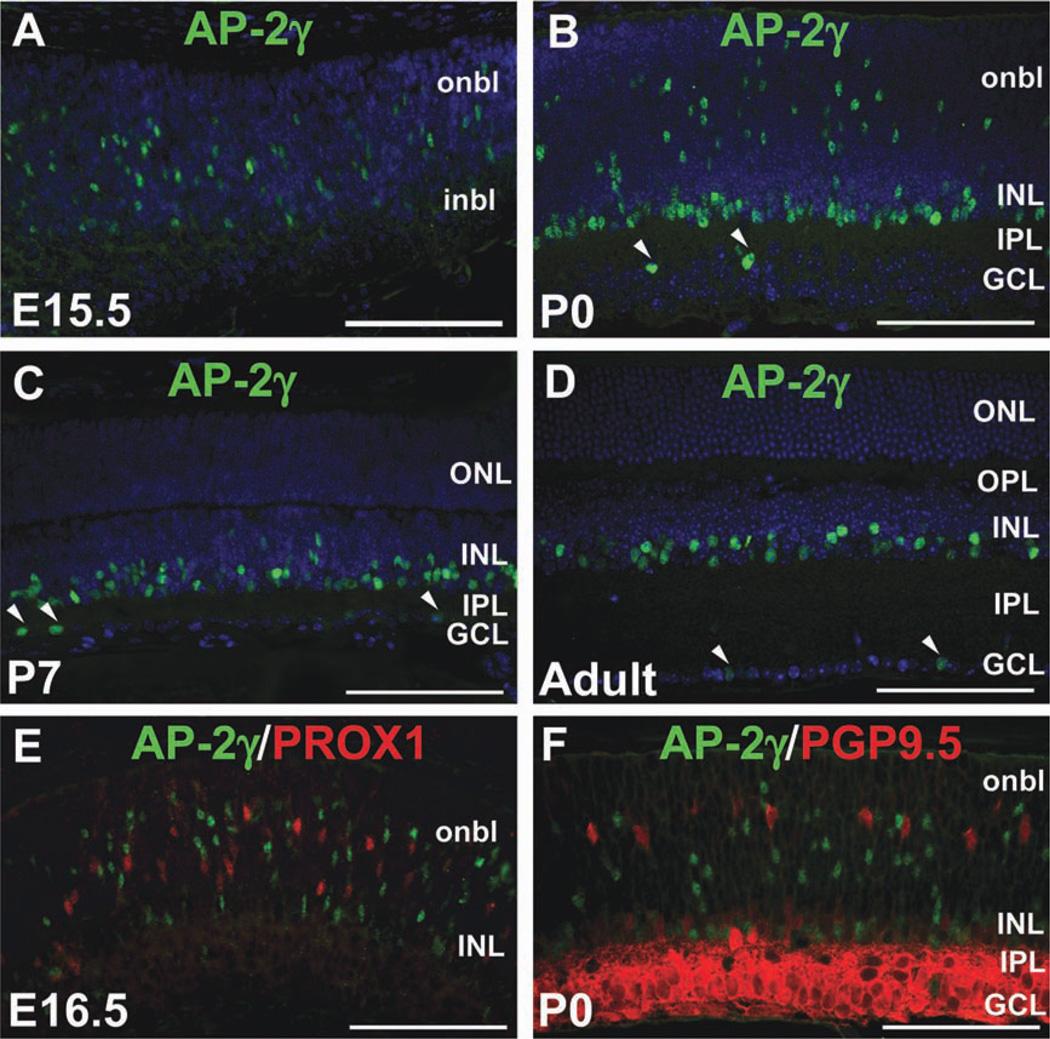
Given our previous data demonstrating similar retinal expression patterns of Tcfap2a, Tcfap2b and Tcfap2c transcripts (Bassett et al., 2007), we examined AP-2γ protein expression in the developing and mature mouse retina. We used a mouse monoclonal anti-AP-2γ antibody (6E4) previously shown to specifically detect human and mouse AP-2γ protein (Gee et al., 2009; Jager et al., 2010; Kuckenberg et al., 2010). In contrast to AP-2α and AP-2β, AP-2γ expression was not detected in the retina at E12.5 (not shown); however by E15.5, AP-2γ-immunoreactive cells were scattered in the inner and outer neuroblast layers (Fig. 3A). At P0, AP-2γ-positive cells were found in the neuroblast layer, a row of cells in the developing INL, and infrequently in the GCL (Fig. 3B). In the P7 and adult retina, AP-2γ was expressed in the inner INL and very few cells of the GCL (Fig. 3C,D). Although AP-2γ was clearly not expressed in mature horizontal cells, we determined whether it was transiently expressed in developing cells of this type, by co-immunolabeling with anti-PROX1 and anti-PGP9.5. We failed to detect co-expression of AP-2γ and PROX1 at E16.5 (Fig. 3E), and quantification of sections from five retinas co-labeled with AP-2γ and PGP9.5 at P0 showed that only 0.92 ± 0.2% of PGP9.5-positive cells expressed AP-2γ (Fig. 3F and not shown), suggesting that AP-2γ is virtually absent from horizontal cells. We also failed to detect AP-2γ expression in retinal ganglion cells labeled by anti-POU4F1/2/3 (BRN3A/B/C), indicating that the AP-2γ-positive cells in the GCL are displaced amacrine cells (not shown).
Fig. 3. AP-2γ is not a horizontal cell marker.
A–D: Immunofluorescence using anti-AP-2γ clone 6E4 (green) on horizontal sections of wild-type mouse heads or eyes counterstained with DAPI (4,6-diamino-2-phenylindole (DAPI; blue), at the stages indicated. Arrowheads in B–D indicate AP-2γ-immunoreactive cells in the GCL. E,F: Co-immunostain with anti-AP-2γ (green) and either anti-PROX1 (E) or anti-PGP9.5 (F) antibodies (red) on horizontal sections of wild-type eyes, at the stages indicated. i/onbl, inner/outer neuroblast layer; INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer; OPL, outer plexiform layer. Scale bars = 100 µm.
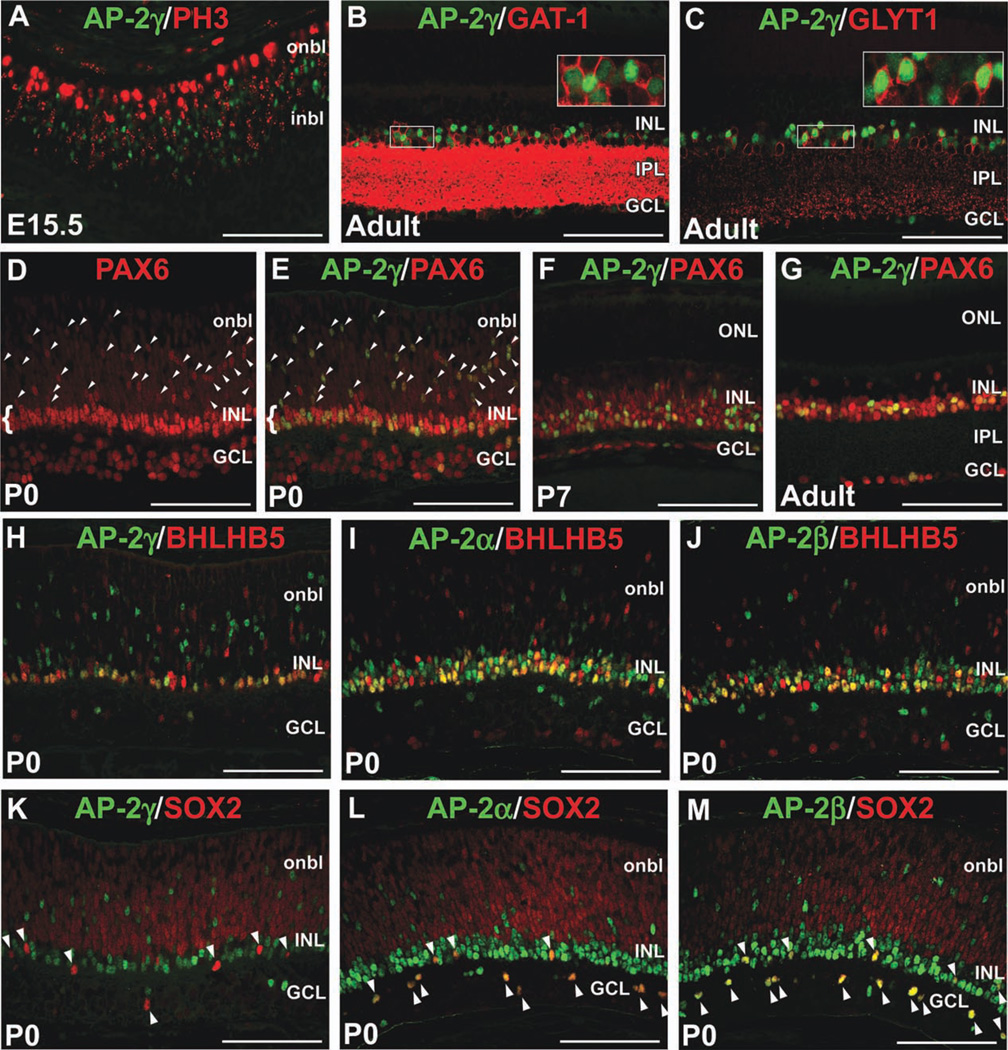
To assess whether AP-2γ is expressed in mitotic or postmitotic retinal cells, we co-immunolabeled retinas for AP-2γ and PH3 at E15.5, and did not observe double-labeled cells (Fig. 4A). Similarly, quantification of cell staining in retinal sections showed that only 1.51 ± 0.36% of AP-2γ-positive cells expressed Ki67 (Supp. Fig. S1A). Therefore, akin to AP-2α and AP-2β, AP-2γ expression is not a feature of mitotic RPCs. Also like AP-2α and AP-2β, AP-2γ expression was detected in both the GABAergic (GAT-1-positive) and glycinergic (GLYT1-positive) amacrine cell subsets in the mature mouse retina (Fig. 4B,C). Given the distribution of AP-2γ-immunoreactive cells in the neuroblast layer at birth (Fig. 3B,F), we considered the possibility that it is expressed in other developing INL or outer nuclear layer (ONL) cell types. However, in P0, P7 and P21 retinas, we did not detect co-expression of AP-2γ and markers of photoreceptor (OTX2, CRX), bipolar (VSX2) or Müller glial (SOX2) cells (not shown). Our immunofluorescent analyses therefore indicated that AP-2γ expression is restricted to amacrine cells. To confirm this, we double-immunolabeled retinas for AP-2γ and PAX6, which is initially expressed in all RPCs but persists only in differentiating and mature ganglion, amacrine, horizontal, and some Müller glial cells (de Melo et al., 2003; Roesch et al., 2008). At birth, strongly immunoreactive PAX6-positive cells in the neuroblast layer are presumptive amacrine or horizontal cells (Fig. 4D, arrowheads), while those in the INL are differentiating amacrine cells (Fig. 4D, bracket). As suspected, all AP-2γ-positive cells at this stage overlapped with brightly stained PAX6-positive cells, whether in the neuroblast layer (Fig. 4E, arrowheads) or INL (Fig. 4E, bracket). Quantification of P0 retinas revealed that 38 ± 0.9% of PAX6-positive cells expressed AP-2γ, while 81 ± 1.8% of these cells expressed AP-2α, confirming that the AP-2α/β-positive population is larger than the AP-2γ-positive one (Supp. Fig. S1B). Similarly, in P7 and adult retinas, AP-2γ continued to be confined to PAX6-positive cells and comprised a smaller proportion of the PAX6-positive population than AP-2α (Fig. 4F,G and Supp. Fig. S1B). We compared the expression patterns of AP-2α, AP-2β, and AP-2γ in amacrine cell subpopulations at birth. While all three family members were expressed in BHLHB5-positive GABAergic amacrines (Fig. 4H–J), only AP-2α and AP-2β were detected in SOX2-positive cholinergic amacrines (Fig. 4K–M, arrowheads).
Fig. 4. AP-2γ is expressed in postmitotic amacrine cells.
A–C: Co-immunostain with anti-AP-2γ (green) and either anti-PH3 (A), anti-GAT-1 (B), or anti-GLYT1 (C) antibodies (red) on horizontal sections of wild-type eyes, at the stages indicated. Boxed areas in B and C are magnified in inset. D–G: Co-immunostain with anti-AP-2γ (green) and anti-PAX6 (red) on horizontal sections of wild-type eyes at the stages indicated. D shows PAX6 alone while E shows double merge of the same image. Arrowheads in D and E denote strongly immunoreactive PAX6-positive cells in the neuroblast layer. Brackets in D and E indicate PAX6-positive amacrine cells in the INL. H–M: Co-immunostains of different AP-2 family members indicated (green) with BHLHB5 or SOX2 (red) at P0. Arrowheads in K–M point to SOX2-positive cholinergic amacrine cells, which co-express AP-2α and AP-2β (L,M) but not AP-2γ (K). i/onbl, inner/outer neuroblast layer; INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer; ONL, outer nuclear layer. Scale bars = 100 µm.
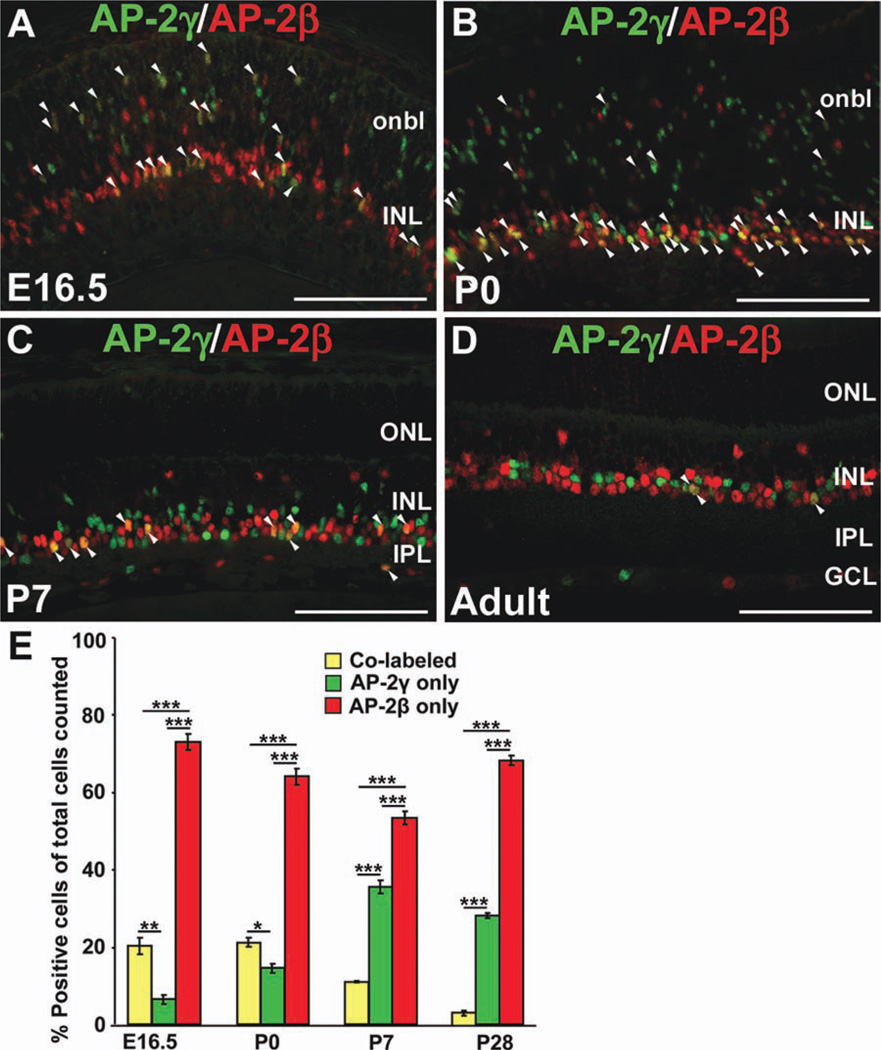
We also examined the extent to which the AP-2γ expression domain overlaps with that of AP-2α/β. Due to antibody limitations (the validated anti-AP-2α 3B5 and anti-AP-2γ 6E4 are both mouse monoclonal antibodies), we focused on co-immunolabeling with anti-AP-2γ and anti-AP-2β. Although we detected cells expressing both AP-2γ and AP-2β at E16.5, P0, P7, and adult stages (Fig. 5A–D, arrowheads), a significant proportion of immunopositive cells were singly labeled (Fig. 5A–E). At all stages, the AP-2γ-positive population was significantly smaller than the AP-2β-positive population (Fig. 5E). Postnatally, there was a significant progressive decrease in the percent of co-labeled cells. Approximately 20% of the AP-2γ and/or AP-2β-positive population was co-labeled at E16.5 and P0, while only 3.4 ± 0.7% was co-labeled in the mature retina (Fig. 5E). Taken together, these expression studies show that AP-2γ is expressed in postmitotic developing and mature amacrine cells, in a pattern that only partially overlaps with the extensively colocalized AP-2α/β-positive amacrine cell population.
Fig. 5. The AP-2γ-immunopositive amacrine cell population only partially overlaps with the AP-2β-immunopositive amacrine cell population.
A–D: Co-immunolabeling with anti-AP-2γ (green) and anti-AP-2β (red) on horizontal sections of wild-type eyes, at the stages indicated. Arrowheads denote double-labeled cells. E: Quantification of the proportions of co-labeled versus singly labeled cells in the total AP-2 population examined (AP-2γ and/or AP-2β-positive), at the stages indicated. Bars represent the mean ±SEM of counts from 3 animals. ***P < 0.001; **P < 0.01; *P < 0.05. onbl, outer neuroblast layer; INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer; ONL, outer nuclear layer. Scale bars = 100 µm.
Loss of Horizontal Cells in Tcfap2a/b-Deficient Retinas
As mentioned, we were unable to detect retinal defects in mice with retina-specific deletion of Tcfap2a, and hypothesized that another AP-2 family member may compensate for the loss of AP-2α (Bassett et al., 2007). Based on collective expression data showing that AP-2α and AP-2β clearly have the most similar retinal expression patterns among the AP-2 family members, we sought to determine the effects of combined Tcfap2a and Tcfap2b deletion in the developing retina. To generate these mutants using available lines of mice, retina-specific Tcfap2a conditional KO mice were crossed onto a Tcfap2b germ-line KO background to obtain Tcfap2aki7lacZ/lox/Tcfap2b−/−/α-Cre+/− mice, referred to herein as Tcfap2a/b mutants (Supp. Fig. S2). The α-Cre transgenic mice used to delete the floxed Tcfap2a allele express Cre recombinase in the peripheral retina while leaving a central and dorsal gap (Marquardt et al., 2001; Baumer et al., 2002). As such, peripheral retina regions from horizontal sections at or near the optic nerve head are always shown. Also, retinas were stained with anti-AP-2α and anti-AP-2β antibodies to confirm polymerase chain reaction (PCR) genotyping results (see example in Supp. Fig. S2).
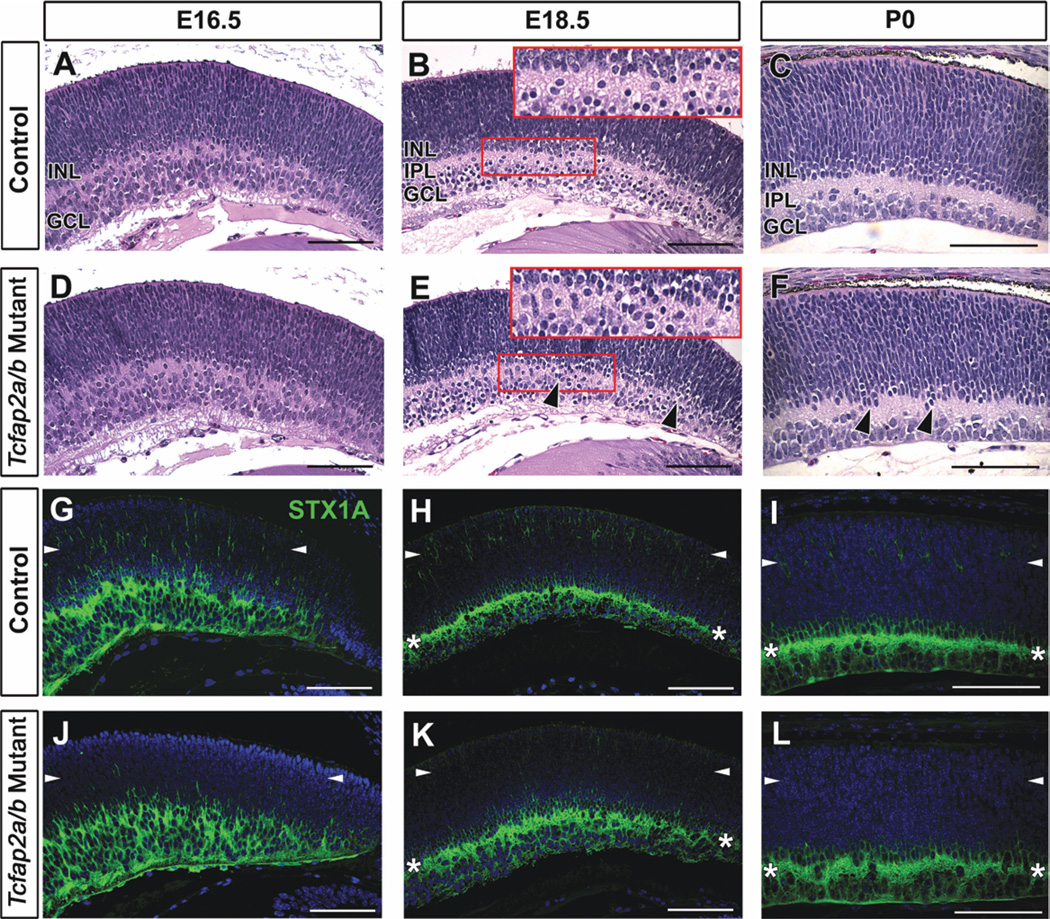
Histological inspection revealed subtle differences in Tcfap2a/b mutant retinal morphology that were apparent at E18.5 (Fig. 6). At E16.5, the emerging IPL was present, and no overt differences in the thickness or appearance of any retinal layers were noted (Fig. 6A,D). By E18.5 in control littermates, the GCL and INL were clearly separated by the inner plexiform layer (IPL), which at this stage contains amacrine and ganglion cell processes (Fig. 6B). In comparison, the inner retina of Tcfap2a/b mutants appeared slightly less organized, in that the general divisions between the INL, IPL and GCL were not as defined (Fig. 6E, arrowheads), a phenomenon that was also apparent at birth (Fig. 6, compare C with F, arrowheads). This disorganization was reflected in the staining pattern of STX1A. The band of strong STX1A expression representing amacrine cell neurites in the IPL was not as distinct in Tcfap2a/b mutants compared with controls (Fig. 6H,I,K,L, asterisks). In addition, the STX1A immunoreactivity in the outer retina was diminished in double mutants (Fig. 6G–L, arrowheads). By birth, the layer of STX1A-positive horizontal cells forming in the outer retina of control littermates was not detected in Tcfap2a/b mutants (Fig. 6I,L), suggesting that horizontal cell development might be severely affected. Because these mutants die at birth due to the Tcfap2b germ-line KO phenotype (Moser et al., 1997), in vivo analyses beyond embryonic and P0 stages could not be carried out.
Fig. 6.
Subtle disorganization and diminished syntaxin immunoreactivity in the retina in Tcfap2a/b mutants. A–F: Horizontal H&E-stained sections of control (A–C) and Tcfap2a/b mutant (D–F) peripheral retinas, at the stages indicated. Black arrowheads (E,F) show regions of mutant retinas that appear less organized than controls. Red boxes in B and E are magnified in insets. G–L: Horizontal sections of control (G–I) and Tcfap2a/b mutant (J–L) peripheral retinas immunolabeled with anti-STX1A (green). Asterisks (H,I,K,L) indicate the band of STX1A-labeled amacrine cell processes in the IPL. The STX1A immunoreactivity detected in the outer retina of control mice (G–I, arrowheads) was diminished in Tcfap2a/b mutants (J–L, arrowheads). INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer. Scale bars = 100 µm.
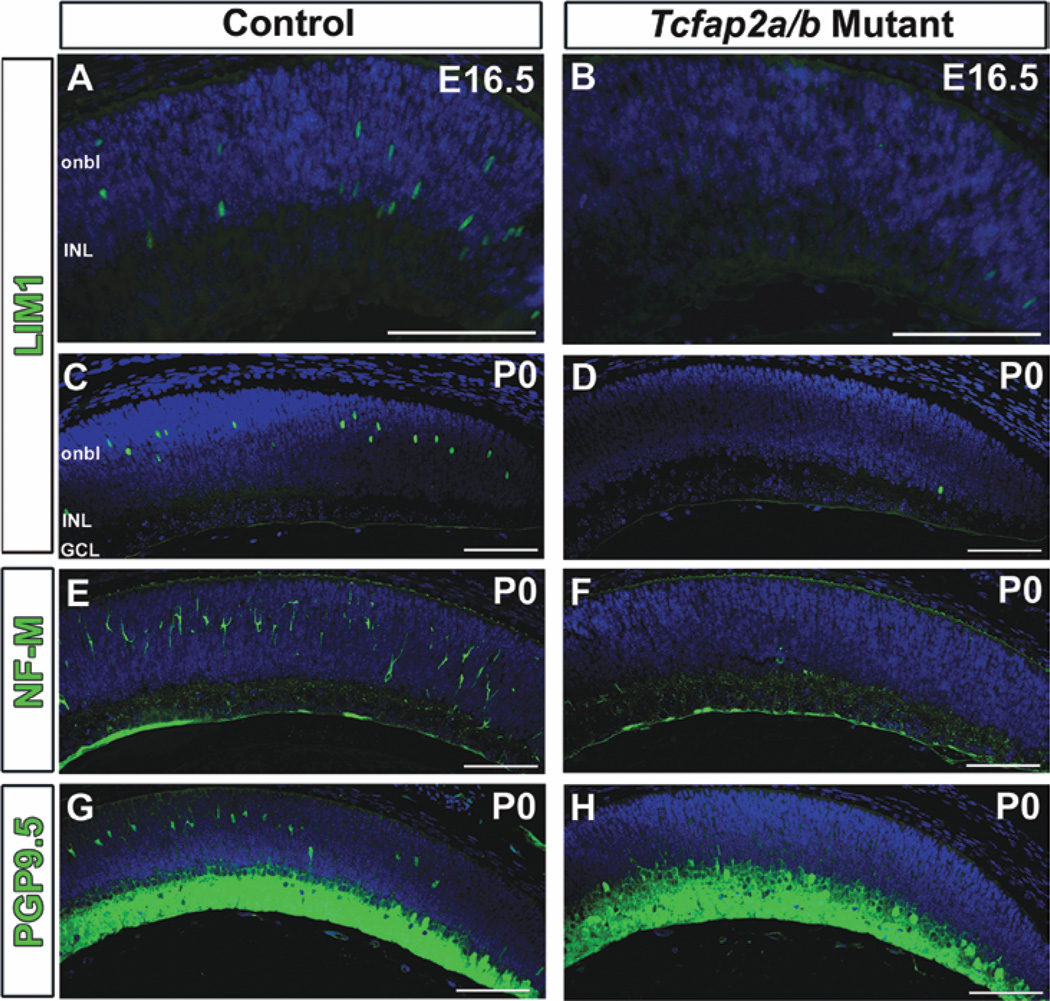
We used several retinal markers to further investigate horizontal cell development in Tcfap2a/b mutant retinas. At E16.5, when the first significant LIM1 expression occurs in postmitotic horizontal cells (Poche et al., 2007), the number of LIM1-positive cells in double mutant retinas was drastically reduced compared with controls (Fig. 7, compare A with B). At all subsequent stages examined (E17.5, 18.5, and P0), we rarely observed LIM1-expressing cells in Tcfap2a/b-deficient regions of the retina (Fig. 7C,D and not shown), suggesting a near loss of horizontal cells. Interestingly, we did not detect the same loss of LIM1-positive cells in single mutant retinas lacking only Tcfap2a or only Tcfap2b (not shown). The LIM1 results were corroborated by immunolabeling for PROX1, another early horizontal cell marker that was similarly absent from the double mutant outer retina at E18.5 and P0 (not shown). Developing and mature mouse horizontal cells exhibit strong neurofilament (NF) immunoreactivity (Peichl and Gonzalez-Soriano, 1993; Chien and Liem, 1995), which we readily detected in P0 control retinas using NF light chain (NF-L) and NF medium chain (NF-M) antibodies (Fig. 7E and not shown). In contrast, the strong NF-L and NF-M signals indicative of horizontal cells were not present in the Tcfap2a/b mutants (Fig. 7F and not shown). Similarly, PGP9.5-positive cells were missing from the presumptive horizontal cell layer in Tcfap2a/b mutants (Fig. 7, compare G with H). Notably, we observed LIM1- and PGP9.5-positive horizontal cells in the Tcfap2a/b mutant central retina, where the α-Cre activity (responsible for excising the Tcfap2alox allele) diminishes (Supp. Fig. S3A, and not shown). We also took advantage of the fact that Tcfap2a/b mutants carry one copy of the Tcfap2a floxed (Tcfap2alox), and one copy of the Tcfap2a IRES-lacZ knock-in null allele (Tcfap2aki7lacZ), which has been shown to faithfully express β-galactosidase in a pattern identical to that of endogenous AP-2α protein (Brewer et al., 2002). Given the extensive colocalization of AP-2α and AP-2β expression in the developing retina, a large proportion of the Tcfap2a-null cells expressing β-galactosidase would also normally co-express functional AP-2β protein and are, therefore, double “Tcfap2a/b null” cells. Marking these Tcfap2a/b null cells by X-gal staining demonstrated a lack of X-gal-positive cells in the outer retina of Tcfap2a/b mutants, showing that Tcfap2a/b null cells are missing from the presumptive horizontal cell layer, rather than present but failing to express horizontal cell markers (Supp. Fig. S3B). These results suggest that by birth, horizontal cells are virtually absent from retinas deficient in both Tcfap2a and Tcfap2b.
Fig. 7.
Deletion of Tcfap2a and Tcfap2b leads to loss of retinal horizontal cells. A–H: Horizontal sections of control (A,C,E,G) and Tcfap2a/b mutant (B,D,F,H) peripheral retinas immunolabeled (green) with anti-LIM1 (A–D), anti-neurofilament medium chain (NF-M; E,F) or anti-PGP9.5 (G,H) antibodies and counterstained with DAPI (4,6-diamino-2-phenylindole; blue) at E16.5 (A,B) or P0 (C–H). onbl, outer neuroblast layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bars = 100 µm.
The apparent loss of an entire retinal cell type in Tcfap2a/b mutants prompted us to examine programmed cell death and proliferation during retinogenesis; however, from E16.5 to P0, we did not detect observable differences in the numbers of apoptotic (TUNEL [Terminal uridine deoxynucleotidyl transferase-mediated dUTP Nick End Labeling] -labeled) cells, or the staining patterns of the proliferative markers PH3, PCNA and Cyclin D1 (Supp. Fig. S4, and not shown). Taken together, our analyses demonstrate a redundant requirement for AP-2α and AP-2β activity for horizontal cell development.
Combined Deletion of Tcfap2a and Tcfap2b Causes Amacrine Cell Defects
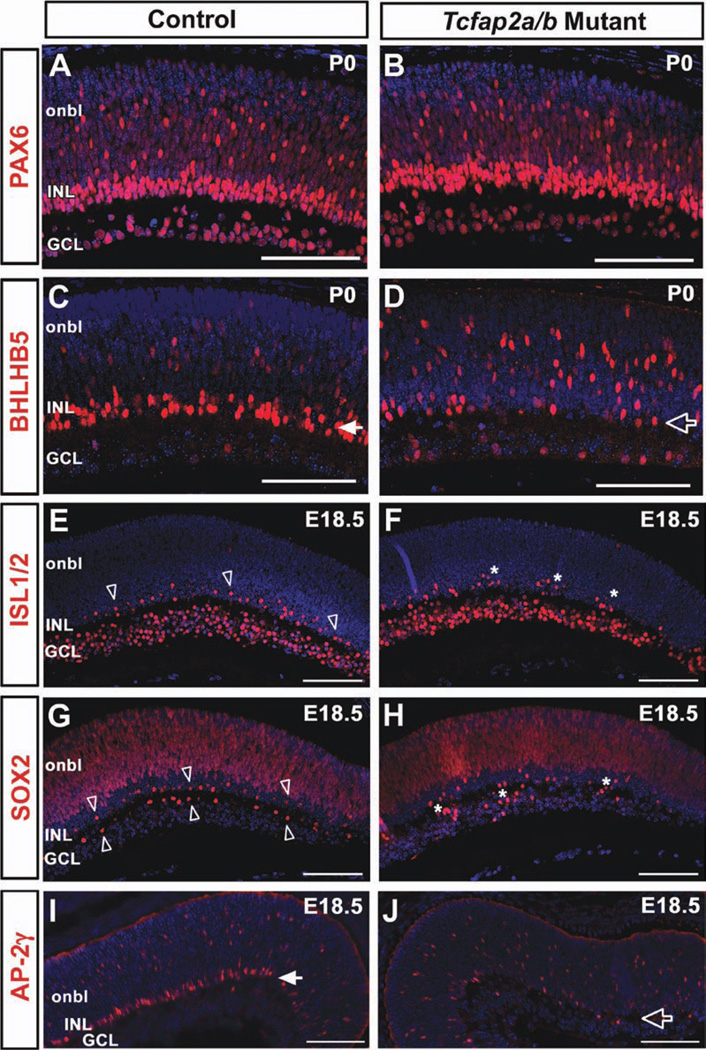
Given the overlapping expression of AP-2α and AP-2β in developing amacrine cells, we examined Tcfap2a/b mutant retinas for amacrine cell abnormalities. The pan-amacrine cell marker PAX6 showed comparable immunostaining patterns in Tcfap2a/b mutants and controls at E16.5, E18.5 and P0 (Fig. 8A,B and not shown). Although GABAergic amacrine cell birth peaks around mouse E14–E16, significant birth of glycinergic amacrines begins during late embryogenesis with a peak around P0, and is not detectable by glycinergic-specific marker expression until postnatal stages (Cherry et al., 2009; Voinescu et al., 2009). Given the death of Tcfap2a/b mutants at birth, our assessment of amacrine cell markers was limited to those expressed in the embryonic or neonatal retina, which restricted our subtype analysis to GABAergic populations. Transcription factor Bhlhb5 (Bhlhe22) is the earliest subtype-specific amacrine marker, expressed in postmitotic GABAergic amacrines starting at E11.5 (Feng et al., 2006). The BHLHB5 expression pattern was initially similar in Tcfap2a/b mutants and controls at E15.5 (not shown); however, while BHLHB5-positive cells in control retinas were clearly concentrated in the developing INL by birth, this distinct band of cells was not observed in double mutant littermates (Fig. 8, compare C with D). We did not observe this altered BHLHB5 staining pattern in retinas deficient in Tcfap2a alone or Tcfap2b alone (not shown).
Fig. 8.
Altered immunostaining patterns of amacrine cell markers in Tcfap2a/b mutant retinas. A–J: Horizontal sections of control (A,C,E,G,I) and Tcfap2a/b mutant (B,D,F,H,J) peripheral retinas immunolabeled (red) with anti-PAX6 (A,B), anti-BHLHB5 (C,D), anti-ISL1/2 (E,F), anti-SOX2 (G,H) or anti-AP-2γ (I,J) and counterstained with DAPI (4,6-diamino-2-phenylindole; blue), at the stages indicated. Closed arrows in C and I denote the band of BHLHB5-positive or AP-2γ-positive cells, respectively, clearly forming in the control INL, which was not observed in mutants (open arrows in D,J). Open arrowheads in E and G point to the developing cholinergic amacrine mosaic in control retinas, whereas asterisks in F and H indicate cholinergic cells that are failing to exhibit regular spacing in mutant retinas. onbl, outer neuroblast layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bars = 100 µm.
The cholinergic amacrine cells are one of the earliest born subsets of the GABAergic population, and form a nonrandom distribution in the late embryonic retina (Galli-Resta et al., 1997; Voinescu et al., 2009). Bhlhb5 is not expressed in cholinergic amacrines (Feng et al., 2006); therefore, this subpopulation was examined with other markers. We used an antibody that detects the ISL1 and ISL2 proteins (Tsuchida et al., 1994), and labels retinal ganglion cells (RGCs) and cholinergic amacrine cells at embryonic and neonatal stages (Elshatory et al., 2007a). By E18.5, the ISL1-positive cholinergic amacrine mosaic was evident in the developing INL of control retinas (Fig. 8E, open arrowheads). In contrast, although immunolabeled cells were present in the INL of Tcfap2a/b mutants, they did not exhibit the same regular arrangement (Fig. 8F, asterisks). The transcription factor SOX2 is expressed in retinal progenitors, but is also a marker for cholinergic amacrine cells and Müller glia (Taranova et al., 2006; Cherry et al., 2009; Lin et al., 2009). Its lack of expression in RGCs allowed us to visualize the presumptive cholinergic amacrine distributions in both the INL and GCL. Similar to the results obtained with anti-ISL1/2, SOX2-immunoreactive cells were present in both controls and double mutants at E18.5 (Fig. 8G,H). However, the orderly distribution of labeled cells in the INL and GCL of controls (Fig. 8G, open arrowheads) was not detected in Tcfap2a/b mutants (Fig. 8H, asterisks). We asked whether the expression pattern of AP-2γ was altered in Tcfap2a/b mutant retinas. Based on immunostaining, there was no compensatory expansion of the AP-2γ-immunopositive population in Tcfap2a/b mutants, but rather an expression pattern reflective of BHLHB5, in which the distinct INL band of AP-2γ-positive cells observed in controls (Fig. 8I, closed arrow) was lacking in double mutants (Fig. 8J, open arrow).
To investigate whether the loss of Tcfap2a and Tcfap2b impacted the development of cell types other than amacrine and horizontal cells, we analyzed the expression of additional retinal markers. We saw no differences between Tcfap2a/b mutant and control retinas immunolabeled at E17.5 and P0 for POU4F1/2/3 (BRN3A/B/C; ganglion cells), CRX and OTX2 (photoreceptors), and VSX2 (CHX10), which is expressed in progenitors and is maintained in the bipolar cell lineage (Supp. Fig. S5, and not shown). Thus, the current analyses show that, in addition to horizontal cells, AP-2α and AP-2β are redundantly required for amacrine cell development.
DISCUSSION
The AP-2 transcription factors have emerged as key regulators of eye morphogenesis in several species, including humans (Gestri et al., 2009; Dumitrescu et al., 2011; Milunsky et al., 2011). In this report, we have investigated the roles of multiple AP-2 family members in mouse retinal neurogenesis, and revealed their importance in the amacrine and horizontal cell lineages. We showed that AP-2α and AP-2β exhibit highly overlapping expression patterns in postmitotic amacrine and horizontal cells. AP-2γ expression was detected in developing and mature amacrine cells, in a population that only partially overlapped with AP-2α/β-expressing cells. Deletion of both Tcfap2a and Tcfap2b from the developing retina caused a striking loss of horizontal cells and defective amacrine cell development.
Expression Patterns of AP-2 Proteins in Amacrine and Horizontal Cells
Previous studies in mouse and chick have indicated that four of the five known AP-2 family members (AP-2α, AP-2β, AP-2γ, and AP-2δ) are expressed in the developing retina, and that three of these (AP-2α, AP-2β, and AP-2γ) are present in INL cell types while AP-2δ is confined to RGCs (Bisgrove and Godbout, 1999; Zhao et al., 2003; Bassett et al., 2007; Trimarchi et al., 2007; Li et al., 2008). Here, we clarified the expression patterns of AP-2α and AP-2β in horizontal cells of the mouse retina (Figs. 1, 2). While both family members were expressed in developing horizontal cells, AP-2α expression diminished shortly after birth and only AP-2β protein was retained in mature horizontal cells, a finding that concurs with data from the chick retina (Bisgrove and Godbout, 1999; Edqvist et al., 2008). We also showed that, in the developing and adult retina, a considerably higher proportion of cells are co-labeled for AP-2α and AP-2β compared with those singly labeled for AP-2α or AP-2β (Fig. 2), thereby demonstrating their extensive overlap and supporting compensation by one protein when the other is deficient in the retina. Recently, the expression patterns of TFAP2A and TFAP2B in the human fetal and mature retina have been examined, and closely resemble those in chick and mouse retinas (Li et al., 2010). Thus, AP-2α and AP-2β are key markers of amacrine and horizontal cells in the avian and mammalian retina, and, as the current study shows, they have overlapping roles in the development of both cell types.
Our spatiotemporal analysis of AP-2γ protein expression in the developing mouse retina showed that like AP-2α and AP-2β, this family member is expressed in postmitotic amacrine cells of the GABAergic and glycinergic classes (Figs. 3, 4). While the vast majority of cells expressing any of these three AP-2 family members were Ki67-negative (Supp. Fig. S1A), Ki67 immunoreactivity was found in a very small percentage of AP-2-positive cells. However, low levels of Ki67 protein have been reported in quiescent cells (Bullwinkel et al., 2006), and our data could reflect the presence of Ki67, perhaps transiently, in postmitotic AP-2-positive neurons. Unlike AP-2α and AP-2β, AP-2γ expression initiated later and was not a marker of developing horizontal cells (Fig. 3). We noted that cells expressing AP-2γ did not form an obvious band in the developing INL until late embryogenesis, and a significant proportion of AP-2γ-immunopositive amacrine precursors were scattered in the outer neuroblast layer at early postnatal stages. This expression pattern, combined with the later onset of AP-2γ expression, suggests that it may be expressed in later-born amacrine cells compared with AP-2α and AP-2β. This idea is supported by recent evidence showing that final amacrine cell soma position correlates with birthdate, in that the earliest born amacrine cells (such as the cholinergic subtype) reside in the GCL or inner INL, while later-born amacrine cells are found more toward the outer retina (Voinescu et al., 2009). In agreement with this finding, AP-2γ was not detected in the early-born cholinergic amacrines, whereas AP-2α and AP-2β were expressed in this subtype (Fig. 4). Double immunolabeling with anti-AP-2γ and anti-AP-2β (Fig. 5) also emphasized the fact that the AP-2γ-positive amacrine cell population is partially distinct from the extensively colocalized AP-2α/β-positive population, particularly in the adult retina where less than 4% of the AP-2γ and/or AP-2β-positive population overlaps (Fig. 5). This result is supported by a study in which gene expression profiles were obtained from individual developing retinal cells. Of the six amacrine cells profiled, all expressed Tcfap2b, five expressed Tcfap2a, and only one expressed Tcfap2c (Trimarchi et al., 2007).
Requirement for Tcfap2a/b in Horizontal Cell Development
We showed here that like AP-2α, AP-2β is expressed in postmitotic transition cells during retinogenesis (Fig. 1). The temporal and spatial expression patterns of AP-2α and AP-2β are well correlated with amacrine and horizontal cell differentiation (Young, 1985). In contrast to amacrine cells, which are clearly present in Tcfap2a/b mutant retinas, horizontal cells are lost (Fig. 7). We failed to detect the expression of several horizontal cell markers, including the early markers LIM1 and PROX1. We suspect that the rare occurrence of a LIM1-positive horizontal cell in the peripheral retina of Tcfap2a/b mutants may be a result of incomplete Cre-mediated excision of Tcfap2a, which is supported by the fact that we detected horizontal cells in the Tcfap2a/b mutant central retina, where the α-Cre activity diminishes (Supp. Fig. S3). We also detected LIM1-positive cells in the retinas of embryos with no intact Tcfap2b alleles and only one intact Tcfap2a allele, or no intact Tcfap2a alleles and only one intact Tcfap2b allele (not shown). Therefore a single functional copy of Tcfap2a or Tcfap2b appears to rescue horizontal cells to some extent. Taken together, these results suggest that Tcfap2a and Tcfap2b are redundantly required for the determination or maintenance of the entire horizontal cell population. The underlying cause for the absence of horizontal cells in Tcfap2a/b mutant retinas is not known. Considering that horizontal cells comprise only approximately 0.2% of all mouse retinal cells (Ajioka et al., 2007), it is difficult to determine whether these cells have adopted an alternative fate or were initially generated but quickly died by apoptosis. Given their expression in postmitotic cells, we favor the idea that AP-2 proteins are not involved in retinal cell fate determination, but rather in aspects of differentiation or survival. The specific roles for AP-2α/β in horizontal cell development can be further clarified by experiments in which the Tcfap2a/b-null cells are traced and co-labeled with different retinal markers, as well as by gain-of-function studies.
Impaired Amacrine Cell Development in Tcfap2a/b-Deficient Retinas
Tcfap2a and Tcfap2b are expressed in glycinergic and GABAergic amacrine cells and are also co-expressed with markers of many other smaller amacrine subpopulations including cholinergic (ISL1-positive, SOX2-positive), NR4A2-positive, CALB2-positive, and AII (DAB1-positive) amacrines (Bassett et al., 2007; Trimarchi et al., 2007; Cherry et al., 2009). Rather than being concentrated in the ventricular half of the embryonic retina like early amacrine determination factors such as FOXN4 or PTF1A (Li et al., 2004; Fujitani et al., 2006), AP-2α and AP-2β are expressed in newly generated amacrine transition cells as they approach the presumptive INL, and both proteins are maintained in amacrine cells throughout postnatal development and into adulthood. Before their death at P0, our assessment of different amacrine cell populations in Tcfap2a/b mutants was confined to the earlier born GABAergic types. We noted altered staining patterns of the early GABAergic marker BHLHB5, cholinergic (ISL1, SOX2) markers, and AP-2γ (Fig. 8). These altered staining patterns were indicative of abnormal amacrine cell positioning, suggesting that migration and consequent mosaic formation could be affected by loss of AP-2. The Tcfap2a/b mutants die around P0, and at this early time point we were unable to determine if BHLHB5-positive and AP-2γ-positive amacrine cell numbers were also significantly altered in the mutant compared with the control (data not shown). Ultimately, generation of a retinal-specific conditional Tcfap2a/b-deficient mouse model that survives postnatally will be required to investigate the final outcome of Tcfap2a/b deletion on the differentiation and numbers of amacrine cells, and to determine whether the loss of Tcfap2a/b permanently affects the morphology and/or positioning of amacrine cells, or possibly delays differentiation.
AP-2 and Neural Development
Although all five AP-2 family members are expressed in specific regions of the embryonic CNS, their intrinsic roles in the development of neuroectoderm-derived tissues are not well studied. In vitro studies have shown that transfection of neuroectodermal cells with Tcfap2a, or neural crest progenitors with Tcfap2b, induces neuronal differentiation (Paggi et al., 2001; Hong et al., 2008). Tcfap2a has been shown to regulate the migration of gonadotropin-releasing hormone neurons (Orso et al., 2009), which undergo extensive movement from the nasal placode to the hypothalamus during development (Cariboni et al., 2007). The work presented here adds to a limited number of in vivo studies that have revealed cell autonomous roles for AP-2 in neurogenesis within the mammalian CNS (Hong et al., 2008; Feng et al., 2009; Pinto et al., 2009; Hesse et al., 2011; Schmidt et al., 2011). In neural tissues where the expression and function of AP-2 proteins have been examined in detail, they are not broadly distributed but rather exhibit cell type-specific expression in restricted subsets of neural progenitors or postmitotic developing neurons. These include AP-2α, AP-2β, and AP-2γ expression in the retinal amacrine/horizontal lineage, AP-2γ expression in apical (radial glia) progenitors of the cerebral cortex (Pinto et al., 2009), and AP-2ε expression in the output neurons (mitral and tufted cells) of the olfactory bulb (Feng et al., 2009). In regions of the brain and olfactory bulb, AP-2 proteins are required to drive the formation or differentiation of particular neuronal types, thereby conferring neuronal identity or downstream characteristics such as correct morphology or positioning. These include a requirement for AP-2γ in the specification of basal progenitors from apical progenitors (and consequent production of layer II/III pyramidal neurons) in the visual cortex (Pinto et al., 2009), and a role for AP-2ε in the arrangement and dendrite outgrowth of olfactory bulb output neurons (Feng et al., 2009). Complementary to these studies, AP-2α and AP-2β have also been implicated in the development of noradrenergic neurons within the mouse and zebrafish hindbrain (Holzschuh et al., 2003; Hong et al., 2008), and AP-2δ is required for cell survival in the posterior midbrain (Hesse et al., 2011). Furthermore, functional overlap of AP-2 family members was recently reported in the developing peripheral nervous system, where AP-2α and AP-2β have partially redundant roles in the survival of neural crest-derived sympathetic neurons (Schmidt et al., 2011). Given the early lethality associated with loss of the three AP-2 genes studied in this report (Schorle et al., 1996; Zhang et al., 1996; Moser et al., 1997; Auman et al., 2002; Werling and Schorle, 2002), tissue-specific KO mouse models will provide a means to examine the developmental roles of Tcfap2 family members in particular regions of the nervous system in vivo, and it will be of interest to compare their functions in the retina with those in other neural tissues.
This study has provided insight into the roles of Tcfap2a, Tcfap2b, and Tcfap2c in retinogenesis, delineating their overlapping and unique expression patterns in developing horizontal and amacrine cell populations, and revealing a redundant requirement for Tcfap2a and Tcfap2b in horizontal and amacrine cell development. In future studies, it will be important to examine the outcome of Tcfap2 gain-of-function in the retina, as well as Tcfap2c deletion from the retina, either alone or in combination with other AP-2 family member(s). In addition, once suitable mouse genetic resources become available, it will be important to extend the studies on AP-2α and AP-2β redundancy into the postnatal period to analyze the morphology, positioning, and population sizes of a spectrum of amacrine cell subtypes, and also to assess visual function. Nevertheless, our current results demonstrate that the AP-2 transcription factors are required for specific aspects of retinal neurogenesis, broadening their roles as regulatory molecules with respect to both eye and neural development.
EXPERIMENTAL PROCEDURES
Generation of Mouse Lines
All animal procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were maintained on mixed C57B/l6 and FVB/N backgrounds. To monitor genotypes by PCR, DNA was extracted from embryonic/neonatal tail samples, or ear clips of adult breeders using the DNeasy tissue kit (Qiagen).
Tcfap2a/b mutants
The series of crosses used to generate “Tcfap2a/b mutants” are diagrammed in Supp. Fig. S2A. Two different Tcfap2 alleles were used: A Tcfap2aki7lacZ null allele [due to a germ-line IRES-lacZ knock-in insertion disrupting exon 7 (Brewer et al., 2002); MGI: 2183192/Tcfap2atm1Hsv], and a Tcfap2b− null allele [due to a germ-line insertion of a PGK-neo cassette disrupting exon 4 (Moser et al., 1997); MGI: 2180473/Tcfap2btm1Rbu]. Tcfap2aki7lacZ/+ mice were crossed with α-Cre+/− transgenic mice (Marquardt et al., 2001) expressing Cre recombinase under control of the retina-specific “Pax6 α enhancer,” from the murine Pax6 gene [MGI: 3052661; Tg(Pax6-cre,GFP)2Pgr]. Tcfap2aki7lacZ/+/α-Cre+/− mice were crossed with Tcfap2b+/− mice to generate mice that retained the α-Cre transgene, and had only one functional copy of the Tcfap2a and Tcfap2b genes (Tcfap2aki7lacZ/+/Tcfap2b+/−/ α-Cre+/−). This cross is expected (based on Mendelian genetics) to result in 12.5% of the offspring being Tcfap2aki7lacZ/+/Tcfap2b+/−/α-Cre+/−; however, unpublished observations in the West-Mays and Williams laboratories have shown that the low frequency of neural tube closure defects seen in Tcfap2a heterozygotes (Kohlbecker et al., 2002) is exacerbated in Tcfap2a/Tcfap2b double heterozygotes. Thus, we generated fewer Tcfap2aki7lacZ/+/Tcfap2b+/−/α-Cre+/− mice for the final cross (see below) than expected.
In a separate cross, Tcfap2b+/− mice were bred with mice homozygous for the Tcfap2alox allele (Brewer et al., 2004), in which exons 5 and 6 are flanked by single loxP sites (MGI: 3038304/Tcfap2atm2Will), to obtain Tcfap2b+/− mice that were also heterozygous for the Tcfap2alox allele. These Tcfap2b+/−/Tcfap2a+/lox mice were back-crossed to Tcfap2alox/lox mice to regain homozygosity of the Tcfap2alox allele, and the resulting desired progeny (Tcfap2b+/−/Tcfap2alox/lox) were continually crossed with Tcfap2alox/lox mice to maintain this line. In the final cross, mice from the Tcfap2b+/−/Tcfap2alox/lox line were bred with the Tcfap2aki7lacZ/+/Tcfap2b+/−/α-Cre+/− mice, resulting in double α/β mutants that are heterozygous for the Tcfap2aki7lacZ null allele in all tissues, have the second copy of Tcfap2a conditionally deleted only from the developing retina, and lack both functional copies of Tcfap2b in all tissues (Tcfap2aki7lacZ/lox/Tcfap2b−/−/α-Cre+/−, referred to for simplicity as Tcfap2a/b mutants). This final cross in the breeding scheme is expected (based on Mendelian genetics) to result in 6.25% (1/16th) of the offspring being double α/β mutants, therefore numerous breedings were required. At birth, however, we found that double α/β mutants were often stillborn or died immediately, making the P0 stage particularly difficult to obtain. Littermates used as controls either contained two functional copies of Tcfap2a and two functional copies of Tcfap2b in the retina, or were missing one functional copy of either Tcfap2a or Tcfap2b if the former was not available (Table 1).
TABLE 1.
Genotyping
| Alleles | Primers | Conditions | Products |
|---|---|---|---|
| Tcfap2aki7lacZ versus | Alpha 6/7 5′-GAA AGG TGT AGG CAG | 45 s at 95°C, 1 min at 67°C, | Tcfap2aki7lacZ 300 bp |
| Tcfap2a+ | AAG TTT GTC AGG GC -3′ | 1 min 10 sec at 72°C | |
| Alpha 3′KO 5′-CGT GTG GCT GTT | for 33 cycles | ||
| GGG GTT GTT GCT GAG GTA C -3′ | |||
| IRESUP 5′-GCT AGA CTA GTC TAG | Tcfap2a+ 500 bp | ||
| CTA GAG CGG CCC GGG -3′ | |||
| Tcfap2b− versus | 4 Exon DW 5′-CCT CCC AAA TCT | 45 s at 95°C, 45 s at 58°C, | Tcfap2b−380 bp |
| Tcfap2b+ | GTG ACT TCT-3′ | 1 min at 72°C for 37 cycles | |
| PGK-PolyA DW 5′-CTG CTC TTT ACT | |||
| GAA GGC TCT TT-3’ | |||
| 4 Exon Rev 5′-TTC TGA GGA CGC | Tcfap2b+ 221 bp | ||
| CGC CCA GG-3′ | |||
| α-Cre transgene | Cre1 5′-GCT GGT TAG CAC CGC | 45 s at 95°C, 1 min at 67°C, | Presence of α-Cre |
| AGG TGT AGA G-3′ | 1 min 10 sec at 72°C | transgene 420 bp | |
| Cre3 5′-CGC CAT CTT CCA GCA | for 33 cycles | ||
| GGC GCA CC-3′ | |||
| Tcfap2alox versus | Alflox4 5′-CCC AAA GTG CCT | 45 s at 95°C, 45 s at 65°C, | Tcfap2alox 560 bp |
| Tcfap2a+ | GGG CTG AAT TGA C-3′ | 1 min at 72°C for 39 cycl | |
| Alfscsq 5′-GAA TCT AGC TTG | Tcfap2a+490 bp | ||
| GAG GCT TAT GTC-3′ |
Histology
Dissected embryo heads or eyes were either fixed in 10% neutral buffered formalin (Sigma-Aldrich, Oakville, ON) overnight at room temperature, processed and embedded in paraffin (Paraplast tissue embedding media, Fisher Scientific, Waltham, MA), or fixed in 4% PFA overnight at 4°C, cryoprotected overnight in 30% sucrose/PBS, and embedded in Tissue-Tek OCT. Horizontal sections were cut 4 µm in thickness (paraffin) or 8 µm in thickness (frozen) and used for hematoxylin and eosin (H&E) staining, immunofluorescent analysis, or the Terminal uridine deoxynucleotidyl transferase-mediated dUTP Nick End Labeling (TUNEL) assay. We did not obtain large sample sizes of Tcfap2a/b mutants at each stage examined, due to the breeding scheme and viability issues caused by reduced Tcfap2a/b dosage (described above). The numbers of Tcfap2a/b mutant eyes examined at each stage were as follows: Two at E15.5; four at E16.5; two at E17.5; five at E18.5; two at P0. Results were consistent between the limited samples.
Immunofluorescence, TUNEL Assay, and Quantification of Immunolabeled Cells
Indirect immunofluorescence was performed using the primary antibodies listed below. To detect AP-2β, we primarily used a rabbit polyclonal anti-AP-2β at 1:80 (Cell Signaling Technology, Inc., Danvers, MA); however if co-staining with a primary antibody made in rabbit, we used a mouse polyclonal anti-AP-2β at 1:400 (Abnova Corporation, Taipei City, Taiwan). Note that these two AP-2β antibodies gave identical staining patterns. Additional antibodies were as follows: Mouse monoclonal anti-AP-2α (clone 3B5, supernatant) used undiluted (developed by Dr. Trevor Williams, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); mouse monoclonal anti-AP-2γ (clone 6E4/4) at 1:800 (Sigma-Aldrich, Oakville, ON); goat polyclonal anti-Bhlhb5 (E-17) at 1:400 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); goat polyclonal anti-Brn3 (C-13) at 1:200 (Santa Cruz Biotechnology); rabbit polyclonal anti-Crx (H-120) at 1:100 (Santa Cruz Biotechnology); mouse monoclonal anti-CyclinD1 at 1:100 (Santa Cruz Biotechnology); goat polyclonal anti-glycine transporter 1 (GlyT1) at 1:5,000 (Millipore-Chemicon, Billerica, MA); rabbit polyclonal anti-γ-aminobutyric acid (GABA) transporter 1 (GAT-1) at 1:250 (Abcam, Cambridge, MA); rabbit polyclonal anti-Islet1/2 (K5) at 1:500 (Dr. Thomas Jessell lab, Columbia University, New York, NY); mouse monoclonal anti-Ki67 (clone B56) at 1:100 (BD Biosciences, San Jose, CA); rabbit polyclonal anti-Ki67 at 1:500 (Novocastra, Newcastle, UK); mouse monoclonal anti-Lim1/2 (clone 4F2) at 1:50 (developed by Dr. Thomas Jessell and Susan Brenner-Morton, Developmental Studies Hybridoma Bank, University of Iowa); mouse monoclonal anti-Neurofilament 160 (clone NN18) at 1:300 (Sigma-Aldrich); mouse monoclonal anti-Neurofilament 68 (clone NR4) at 1:300 (Sigma-Aldrich); rabbit polyclonal anti-Otx2 at 1:100 (Abcam); rabbit polyclonal anti-Pax6 at 1:200 (Covance, Princeton, NJ); mouse monoclonal anti-PCNA (clone PC10) at 1:800 (Dako Canada, Mississauga, ON); rabbit polyclonal antiphospho-histone H3 (PH3) at 1:100 (Millipore-Upstate); rabbit polyclonal anti-PGP9.5 at 1:500 (Ultraclone Ltd., Isle of Wight, UK); rabbit polyclonal anti-Prox1 at 1:500 (AngioBio, Del Mar, CA); rabbit polyclonal anti-Sox2 at 1:1,000 (Millipore-Chemicon); mouse monoclonal anti-syntaxin-1 (clone HPC-1) at 1:2,000 (Sigma-Aldrich); sheep polyclonal anti-Vsx2/Chx10 at 1:100 (Exalpha Biologicals, Inc., Watertown, MA). Fluorescent secondary antibodies were Alexa Flour 488 or 568 (Invitrogen – Molecular Probes, Burlington, ON) used 1:200 for 1 hr at room temperature. For all primary antibodies, paraffin-embedded sections were deparaffinized in xylene, hydrated (through 100%, 95% and 70% ethanol, followed by water), treated for antigen retrieval with 10 mM sodium citrate buffer pH 6.0, boiling for 20 min, blocked with 5% normal serum from the host of the secondary antibody and incubated with primary antibodies overnight at 4°C. Frozen sections were rinsed in PBS, treated for antigen retrieval and blocked as above, and incubated with primary antibodies overnight at 4°C. For all co-immunostains, both primaries and both secondaries were mixed and incubated simultaneously. The TUNEL assay was performed using the ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (Millipore-Chemicon), according to the manufacturer’s instructions for fluorescent staining of paraffin-embedded tissue or cryosections. Following immunofluorescence or the TUNEL assay, stains were mounted with Vectashield mounting medium containing 4,6-diamino-2-phenylindole (DAPI; Vector Laboratories, Burlington, ON) or Pro-Long Gold antifade reagent with DAPI (Invitrogen – Molecular Probes). All stains were visualized with a microscope (Leica, Deerfield, IL) equipped with a fluorescence attachment, and images were captured with a high-resolution camera and associated software (Open-Lab; Improvision, Lexington, MA). Images were reproduced for publication with image-management software (Photoshop 7.0; Adobe Systems Inc., Mountain View, CA). To quantify immunolabeled cells, a 320 µm length of retinal section equidistant from the optic nerve head was scored on a minimum of 3 different sections from 3 animals at each stage counted, and expressed as the mean ±SEM. Data were tested for significance by two-way ANOVA or Student’s t-test where appropriate (SPSS 19.0 Software). Least significant difference post hoc comparisons were made when statistical significance (P < 0.05) was found between observations.
X-gal Staining
Dissected E18.5 control and Tcfap2a/b mutant heads were fixed in 4% PFA for 1 hr at 4°C, rinsed several times in PBS, cryoprotected overnight in 30% sucrose/PBS, and embedded in Tissue-Tek OCT. Horizontal sections were cut 14µm in thickness. X-Gal dilution buffer (5 mM potassium ferricyanide crystalline, 5 mM potassium ferricyanide trihydrate and 2 mM magnesium chloride in 1× PBS) was warmed to 37°C, and used to dilute (1:40) the X-Gal stock solution (40 mg/ml X-Gal in N,N-dimethylformamide) to create a 1 mg/ml X-Gal working solution. After rinsing cryosections in PBS to remove OCT, the warmed X-gal working solution was applied directly to the slides, which were incubated overnight at 37°C. Slides were rinsed in PBS and dehydrated in an ethanol series (70%, 95%, 100%), then placed in xylene before mounting with Permount (Fisher Scientific, Pittsburgh, PA).
Supplementary Material
Key findings.
Three AP-2 (Tcfap2) transcription factor family members, AP-2α, AP-2β and AP-2γ, are expressed in the amacrine/horizontal cell lineages in the developing mouse retina.
AP-2α and AP-2β are co-expressed in postmitotic horizontal and amacrine cells, while AP-2γ expression is restricted to a subset of postmitotic amacrine cells that is partially distinct from the AP-2α/β-immunopositive population.
Tcfap2a/b-deficient retinas exhibit horizontal and amacrine cell defects that are not detected upon deletion of either family member alone, revealing critical redundant roles for AP-2α and AP-2β in horizontal and amacrine cell development.
ACKNOWLEDGMENTS
We thank Dr. Rod Bremner and Dr. Marek Pacal for helpful discussions. This work was supported by the National Eye Institute/National Institutes of Health (EY11910 to J.W.-M.); National Institute of Dental and Craniofacial Research/National Institutes of Health (DE12728 to T.W.); The Foundation Fighting Blindness to J.W.-M.; and a Natural Sciences and Engineering Research Council of Canada – Postgraduate Scholarship to E.A.B. The monoclonal antibodies 3B5 (developed by Dr. Trevor Williams) and 4F2 (developed by Dr. Thomas M. Jessell and Susan Brenner-Morton) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Ajioka I, Martins RA, Bayazitov IT, Donovan S, Johnson DA, Frase S, Cicero SA, Boyd K, Zakharenko SS, Dyer MA. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. doi: 10.1242/dev.124.6.1119. [DOI] [PubMed] [Google Scholar]
- Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- Bassett EA, Pontoriero GF, Feng W, Marquardt T, Fini ME, Williams T, West-Mays JA. Conditional deletion of activating protein 2alpha (AP-2alpha) in the developing retina demonstrates non-cell-autonomous roles for AP-2alpha in optic cup development. Mol Cell Biol. 2007;27:7497–7510. doi: 10.1128/MCB.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett EA, Williams T, Zacharias AL, Gage PJ, Fuhrmann S, West-Mays JA. AP-2alpha knockout mice exhibit optic cup patterning defects and failure of optic stalk morphogenesis. Hum Mol Genet. 2010;19:1791–1804. doi: 10.1093/hmg/ddq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer N, Marquardt T, Stoykova A, Ashery-Padan R, Chowdhury K, Gruss P. Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development. 2002;129:4535–4545. doi: 10.1242/dev.129.19.4535. [DOI] [PubMed] [Google Scholar]
- Bisgrove DA, Godbout R. Differential expression of AP-2alpha and AP-2beta in the developing chick retina: repression of R-FABP promoter activity by AP-2. Dev Dyn. 1999;214:195–206. doi: 10.1002/(SICI)1097-0177(199903)214:3<195::AID-AJA3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev Biol. 2004;267:135–152. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Brewer S, Jiang X, Donaldson S, Williams T, Sucov HM. Requirement for AP-2alpha in cardiac outflow tract morphogenesis. Mech Dev. 2002;110:139–149. doi: 10.1016/s0925-4773(01)00579-2. [DOI] [PubMed] [Google Scholar]
- Bullwinkel J, Baron-Luhr B, Ludemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206:624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- Cariboni A, Maggi R, Parnavelas JG. From nose to fertility: the long migratory journey of gonadotropin-releasing hormone neurons. Trends Neurosci. 2007;30:638–644. doi: 10.1016/j.tins.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Chen ST, von Bussmann KA, Garey LJ, Jen LS. Protein gene product 9.5-immunoreactive retinal neurons in normal developing rats and rats with optic nerve or tract lesion. Brain Res Dev Brain Res. 1994;78:265–272. doi: 10.1016/0165-3806(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Cherry TJ, Trimarchi JM, Stadler MB, Cepko CL. Development and diversification of retinal amacrine inter-neurons at single cell resolution. Proc Natl Acad Sci U S A. 2009;106:9495–9500. doi: 10.1073/pnas.0903264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CL, Liem RK. The neuronal intermediate filament, alpha-internexin is transiently expressed in amacrine cells in the developing mouse retina. Exp Eye Res. 1995;61:749–756. doi: 10.1016/s0014-4835(05)80026-0. [DOI] [PubMed] [Google Scholar]
- Cid E, Santos-Ledo A, Parrilla-Monge M, Lillo C, Arevalo R, Lara JM, Aijon J, Velasco A. Prox1 expression in rod precursors and Muller cells. Exp Eye Res. 2010;90:267–276. doi: 10.1016/j.exer.2009.10.015. [DOI] [PubMed] [Google Scholar]
- de Melo J, Qiu X, Du G, Cristante L, Eisenstat DD. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J Comp Neurol. 2003;461:187–204. doi: 10.1002/cne.10674. [DOI] [PubMed] [Google Scholar]
- de Melo J, Peng GH, Chen S, Blackshaw S. The Spalt family transcription factor Sall3 regulates the development of cone photoreceptors and retinal horizontal interneurons. Development. 2011;138:2325–2336. doi: 10.1242/dev.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Chen H, Xie X, Libby RT, Tian N, Gan L. BARHL2 differentially regulates the development of retinal amacrine and ganglion neurons. J Neurosci. 2009;29:3992–4003. doi: 10.1523/JNEUROSCI.5237-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu AV, Milunsky JM, Longmuir SQ, Drack AV. A family with branchio-oculo-facial syndrome with primarily ocular involvement associated with mutation of the TFAP2A gene. Ophthalmic Genet. 2011 doi: 10.3109/13816810.2011.634878. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Dwivedi DJ, Pontoriero GF, Ashery-Padan R, Sullivan S, Williams T, West-Mays JA. Targeted deletion of AP-2alpha leads to disruption in corneal epithelial cell integrity and defects in the corneal stroma. Invest Ophthalmol Vis Sci. 2005;46:3623–3630. doi: 10.1167/iovs.05-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- Eckert D, Buhl S, Weber S, Jager R, Schorle H. The AP-2 family of transcription factors. Genome Biol. 2005;6:246. doi: 10.1186/gb-2005-6-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edqvist PH, Hallbook F. Newborn horizontal cells migrate bi-directionally across the neuroepithelium during retinal development. Development. 2004;131:1343–1351. doi: 10.1242/dev.01018. [DOI] [PubMed] [Google Scholar]
- Edqvist PH, Lek M, Boije H, Lindback SM, Hallbook F. Axon-bearing and axon-less horizontal cell subtypes are generated consecutively during chick retinal development from progenitors that are sensitive to follistatin. BMC Dev Biol. 2008;8:46. doi: 10.1186/1471-213X-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory Y, Deng M, Xie X, Gan L. Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J Comp Neurol. 2007a;503:182–197. doi: 10.1002/cne.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci. 2007b;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Simoes-de-Souza F, Finger TE, Restrepo D, Williams T. Disorganized olfactory bulb lamination in mice deficient for transcription factor AP-2epsilon. Mol Cell Neurosci. 2009;42:161–171. doi: 10.1016/j.mcn.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, Long Q, Kawaguchi Y, Edlund H, MacDonald RJ, Furukawa T, Fujikado T, Magnuson MA, Xiang M, Wright CV. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development. 2006;133:4439–4450. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J Neurosci. 1997;17:7831–7838. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Eloranta JJ, Ibbitt JC, Robertson JF, Ellis IO, Williams T, Nicholson RI, Hurst HC. Overexpression of TFAP2C in invasive breast cancer correlates with a poorer response to anti-hormone therapy and reduced patient survival. J Pathol. 2009;217:32–41. doi: 10.1002/path.2430. [DOI] [PubMed] [Google Scholar]
- Gestri G, Osborne RJ, Wyatt AW, Gerrelli D, Gribble S, Stewart H, Fryer A, Bunyan DJ, Prescott K, Collin JR, Fitzgerald T, Robinson D, Carter NP, Wilson SW, Ragge NK. Reduced TFAP2A function causes variable optic fissure closure and retinal defects and sensitizes eye development to mutations in other morphogenetic regulators. Hum Genet. 2009;126:719–803. doi: 10.1007/s00439-009-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hesse K, Vaupel K, Kurt S, Buettner R, Kirfel J, Moser M. AP-2delta is a crucial transcriptional regulator of the posterior midbrain. PLoS One. 2011;6:e23483. doi: 10.1371/journal.pone.0023483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschuh J, Barrallo-Gimeno A, Ettl AK, Durr K, Knapik EW, Driever W. Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development. 2003;130:5741–5754. doi: 10.1242/dev.00816. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Lardaro T, Oh MS, Huh Y, Ding Y, Kang UJ, Kirfel J, Buettner R, Kim KS. Regulation of the noradrenaline neurotransmitter phenotype by the transcription factor AP-2beta. J Biol Chem. 2008;283:16860–16867. doi: 10.1074/jbc.M709106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckfeldt RM, Schubert T, Morgan JL, Godinho L, Di Cristo G, Huang ZJ, Wong RO. Transient neurites of retinal horizontal cells exhibit columnar tiling via homotypic interactions. Nat Neurosci. 2009;12:35–43. doi: 10.1038/nn.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Jager R, Schafer S, Hau-Liersch M, Schorle H. Loss of transcription factor AP-2gamma/TFAP2C impairs branching morphogenesis of the murine mammary gland. Dev Dyn. 2010;239:1027–1033. doi: 10.1002/dvdy.22239. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlbecker A, Lee AE, Schorle H. Exencephaly in a subset of animals heterozygous for AP-2alpha mutation. Teratology. 2002;65:213–218. doi: 10.1002/tera.10037. [DOI] [PubMed] [Google Scholar]
- Kuckenberg P, Buhl S, Woynecki T, van Furden B, Tolkunova E, Seiffe F, Moser M, Tomilin A, Winterhager E, Schorle H. The transcription factor TCFAP2C/AP-2gamma cooperates with CDX2 to maintain trophectoderm formation. Mol Cell Biol. 2010;30:3310–3320. doi: 10.1128/MCB.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DM. Neurotransmitters in the vertebrate retina. Invest Ophthalmol Vis Sci. 1997;38:553–556. [PubMed] [Google Scholar]
- Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807. doi: 10.1016/j.neuron.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Li X, Glubrecht DD, Mita R, Godbout R. Expression of AP-2delta in the developing chick retina. Dev Dyn. 2008;237:3210–3221. doi: 10.1002/dvdy.21744. [DOI] [PubMed] [Google Scholar]
- Li X, Glubrecht DD, Godbout R. AP2 transcription factor induces apoptosis in retinoblastoma cells. Genes Chromosomes Cancer. 2010;49:819–830. doi: 10.1002/gcc.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Ouchi Y, Satoh S, Watanabe S. Sox2 plays a role in the induction of amacrine and Muller glial cells in mouse retinal progenitor cells. Invest Ophthalmol Vis Sci. 2009;50:68–74. doi: 10.1167/iovs.07-1619. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang JH, Xiang M. Specific expression of the LIM/homeodomain protein Lim-1 in horizontal cells during retinogenesis. Dev Dyn. 2000;217:320–325. doi: 10.1002/(SICI)1097-0177(200003)217:3<320::AID-DVDY10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgistained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Milunsky JM, Maher TM, Zhao G, Wang Z, Mulliken JB, Chitayat D, Clemens M, Stalker HJ, Bauer M, Burch M, Chenier S, Cunningham ML, Drack AV, Janssens S, Karlea A, Klatt R, Kini U, Klein O, Lachmeijer AM, Megarbane A, Mendelsohn NJ, Meschino WS, Mortier GR, Parkash S, Ray CR, Roberts A, Reardon W, Schnur RE, Smith R, Splitt M, Tezcan K, Whiteford ML, Wong DA, Zori R, Lin AE. Genotype-phenotype analysis of the branchio-oculo-facial syndrome. Am J Med Genet A. 2011;155A:22–32. doi: 10.1002/ajmg.a.33783. [DOI] [PubMed] [Google Scholar]
- Moser M, Pscherer A, Roth C, Becker J, Mucher G, Zerres K, Dixkens C, Weis J, Guay-Woodford L, Buettner R, Fassler R. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev. 1997;11:1938–1948. doi: 10.1101/gad.11.15.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhai H, Sel S, Favor J, Mendoza-Torres L, Paulsen F, Duncker GI, Schmid RM. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 2007;134:1151–1160. doi: 10.1242/dev.02781. [DOI] [PubMed] [Google Scholar]
- Orso F, Jager R, Calogero RA, Schorle H, Sismondi P, De Bortoli M, Taverna D. AP-2alpha regulates migration of GN-11 neurons via a specific genetic programme involving the Axl receptor tyrosine kinase. BMC Biol. 2009;7:25. doi: 10.1186/1741-7007-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paggi MG, Bonetto F, Severino A, Baldi A, Battista T, Bucci F, Felsani A, Lombardi D, Giordano A. The retinoblastoma-related Rb2/p130 gene is an effector downstream of AP-2 during neural differentiation. Oncogene. 2001;20:2570–2578. doi: 10.1038/sj.onc.1204356. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Unexpected presence of neurofilaments in axon-bearing horizontal cells of the mammalian retina. J Neurosci. 1993;13:4091–4100. doi: 10.1523/JNEUROSCI.13-09-04091.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Drechsel D, Schmid MT, Ninkovic J, Irmler M, Brill MS, Restani L, Gianfranceschi L, Cerri C, Weber SN, Tarabykin V, Baer K, Guillemot F, Beckers J, Zecevic N, Dehay C, Caleo M, Schorle H, Gotz M. AP2gamma regulates basal progenitor fate in a region- and layer-specific manner in the developing cortex. Nat Neurosci. 2009;12:1229–1237. doi: 10.1038/nn.2399. [DOI] [PubMed] [Google Scholar]
- Poche RA, Reese BE. Retinal horizontal cells: challenging paradigms of neural development and cancer biology. Development. 2009;136:2141–2151. doi: 10.1242/dev.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poche RA, Kwan KM, Raven MA, Furuta Y, Reese BE, Behringer RR. Lim1 is essential for the correct laminar positioning of retinal horizontal cells. J Neurosci. 2007;27:14099–14107. doi: 10.1523/JNEUROSCI.4046-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoriero GF, Deschamps P, Ashery-Padan R, Wong R, Yang Y, Zavadil J, Cvekl A, Sullivan S, Williams T, West-Mays JA. Cell autonomous roles for AP-2alpha in lens vesicle separation and maintenance of the lens epithelial cell phenotype. Dev Dyn. 2008;237:602–617. doi: 10.1002/dvdy.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Huber L, Majdazari A, Schutz G, Williams T, Rohrer H. The transcription factors AP-2beta and AP-2alpha are required for survival of sympathetic progenitors and differentiated sympathetic neurons. Dev Biol. 2011;355:89–100. doi: 10.1016/j.ydbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–238. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Roska B, Billings N, Sun B, Bartch B, Cepko CL. Molecular heterogeneity of developing retinal ganglion and amacrine cells revealed through single cell gene expression profiling. J Comp Neurol. 2007;502:1047–1065. doi: 10.1002/cne.21368. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Retin eye Res. 1990;9:49–100. [Google Scholar]
- Voinescu PE, Kay JN, Sanes JR. Birthdays of retinal amacrine cell subtypes are systematically related to their molecular identity and soma position. J Comp Neurol. 2009;517:737–750. doi: 10.1002/cne.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling U, Schorle H. Transcription factor gene AP-2 gamma essential for early murine development. Mol Cell Biol. 2002;22:3149–3156. doi: 10.1128/MCB.22.9.3149-3156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Zhang J, Nottoli T, Hagopian-Donaldson S, Libby D, Strissel KJ, Williams T. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206:46–62. doi: 10.1006/dbio.1998.9132. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–241. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- Zhao F, Lufkin T, Gelb BD. Expression of Tfap2d, the gene encoding the transcription factor Ap-2 delta, during mouse embryogenesis. Gene Expr Patterns. 2003;3:213–217. doi: 10.1016/s1567-133x(02)00067-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.