Abstract
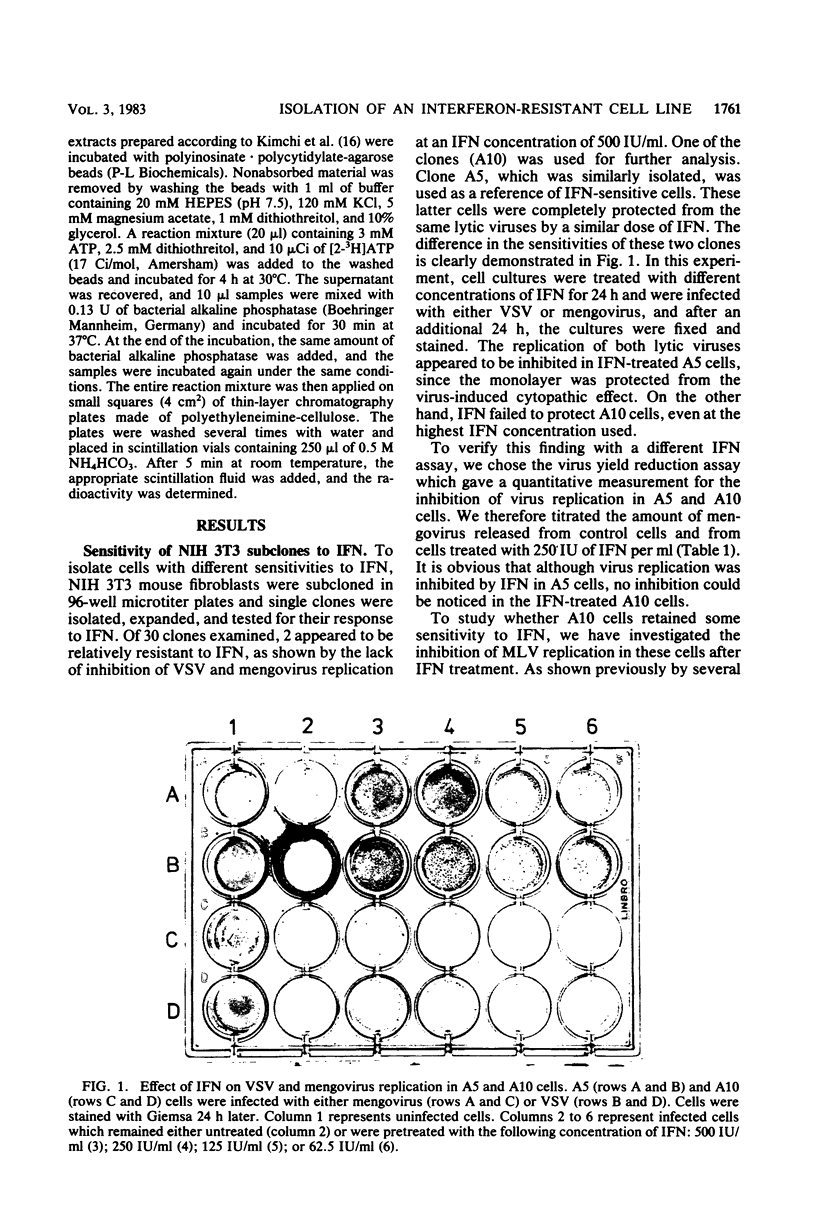
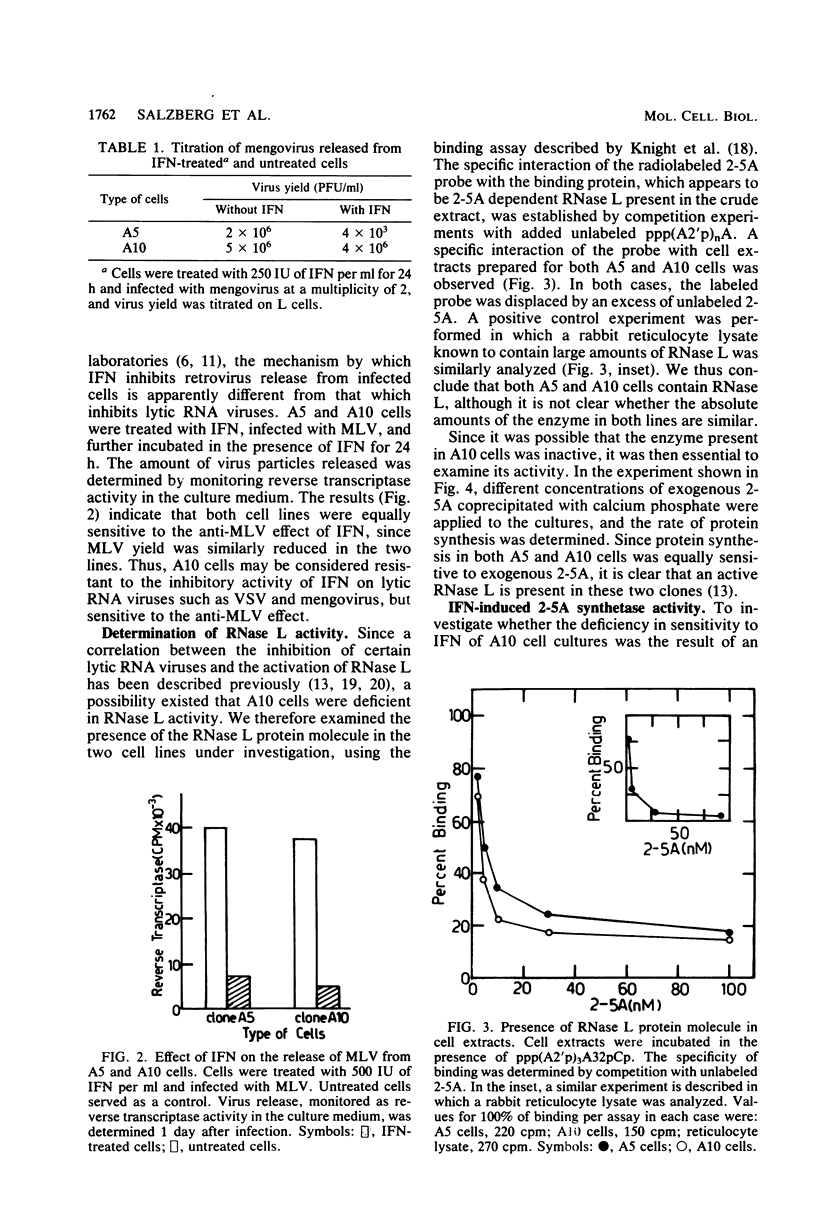
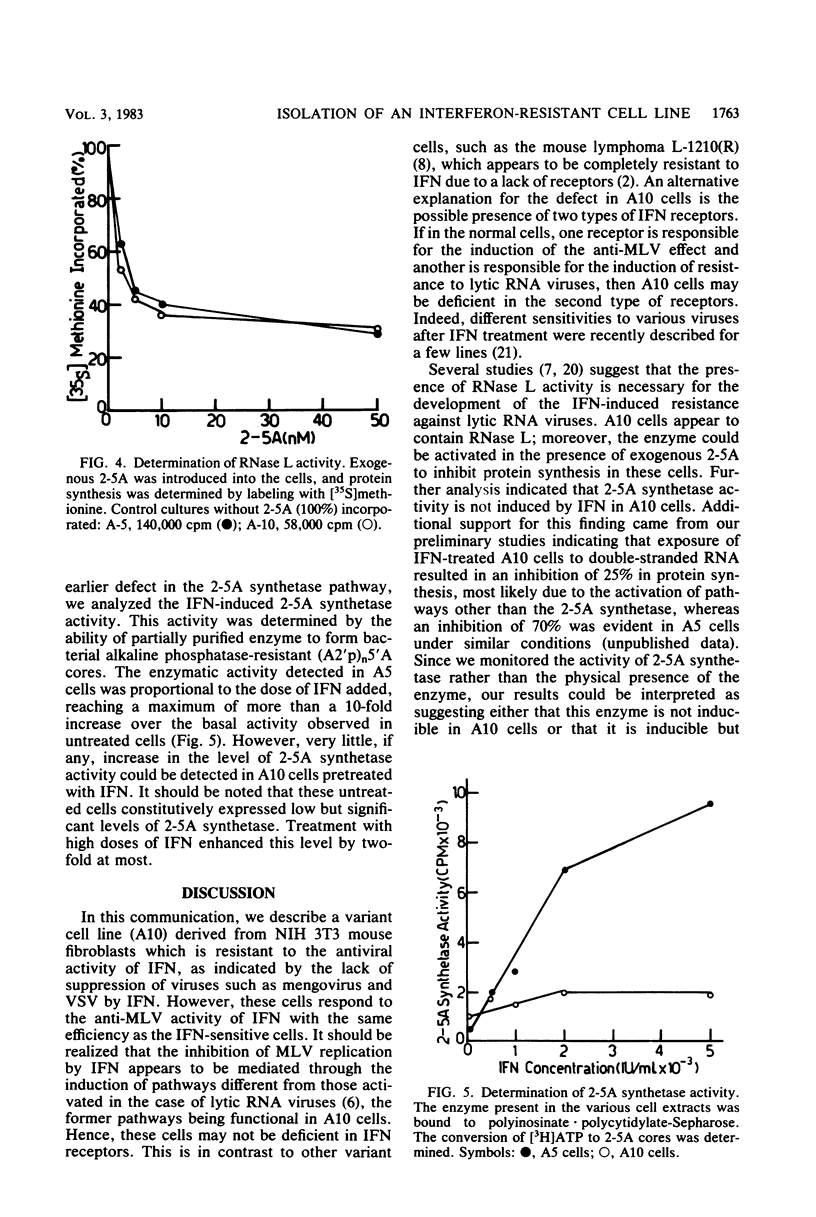
To screen for cells with different sensitivities to interferon (IFN), NIH 3T3 mouse fibroblasts were subcloned and examined for their response to IFN treatment. Of 30 clones tested, 2 appeared to be relatively resistant to IFN, since the replication of both vesicular stomatitis virus and mengovirus was not inhibited, even in the presence of 1,000 U of IFN per ml. One resistant (A10) and one sensitive (A5) clone were further analyzed. In both clones, murine leukemia virus replication was equally inhibited by IFN, indicating the presence of functional receptors for IFN in the resistant clone. Using the (2'-5')oligoadenylate (2-5A) radiobinding assay, we could demonstrate that both clones contained the RNase L protein. Furthermore, this enzyme appears to be active, since a similar reduction in the rate of protein synthesis was evident after the introduction of exogenous 2-5A to the cells. We also analyzed the activity of another enzyme in the 2-5A pathway, namely, 2-5A synthetase. In the sensitive cells (A5), the induction of enzyme activity was proportional to the IFN concentration used, reaching a maximum of more than a 10-fold increase over the background of untreated cells. However, little if any induction over the basal activity was observed in the resistant cells (A10) when similar doses of IFN were used. It is thus probable that the lack of induction of 2-5A synthetase activity by IFN in A10 cells is at least partly responsible for their relative resistance to IFN treatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Weiss O., Salzberg S. Rapid quantitation of interferon with chronically oncornavirus-producing cells. Infect Immun. 1976 Jun;13(6):1626–1632. doi: 10.1128/iai.13.6.1626-1632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankel H., Krishnamurti C., Besançon F., Stefanos S., Falcoff E. Mouse fibroblast (type I) and immune (type II) interferons: pronounced differences in affinity for gangliosides and in antiviral and antigrowth effects on mouse leukemia L-1210R cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2528–2532. doi: 10.1073/pnas.77.5.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Bakhanashvili M., Wreschner D. H., Salzberg S. Specific antigrowth effect of interferon on mouse cells transformed by murine sarcoma virus. Cancer Res. 1983 Mar;43(3):1289–1294. [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Czarniecki C. W., Sreevalsan T., Friedman R. M., Panet A. Dissociation of interferon effects on murine leukemia virus and encephalomyocarditis virus replication in mouse cells. J Virol. 1981 Feb;37(2):827–831. doi: 10.1128/jvi.37.2.827-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein D. A., Czarniecki C. W., Jacobsen H., Friedman R. M., Panet A. A mouse cell line, which is unprotected by interferon against lytic virus infection, lacks ribonuclease F activity. Eur J Biochem. 1981 Aug;118(1):9–15. doi: 10.1111/j.1432-1033.1981.tb05479.x. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-Boyé D. Interferon and cell division. IX. Interferon-resistant L1210 cells: characteristics and origin. J Natl Cancer Inst. 1974 Feb;52(2):553–559. doi: 10.1093/jnci/52.2.553. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Holmes S. L., Mehra L. L. Interferon action against reovirus: activation of interferon-induced protein kinase in mouse L929 cells upon reovirus infection. Virology. 1982 Jul 30;120(2):495–499. doi: 10.1016/0042-6822(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Hicks N. J., Morris A. G., Burke D. C. Partial reversion of the transformed phenotype of murine sarcoma virus-transformed cells in the presence of interferon: a possible mechanism for the anti-tumour effect of interferon. J Cell Sci. 1981 Jun;49:225–236. doi: 10.1242/jcs.49.1.225. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Meurs E., Montagnier L. Lack of systematic correlation between the interferon mediated antiviral state and the levels of 2-5A synthetase and protein kinase in three different types of murine cells. J Interferon Res. 1981 Feb;1(2):179–190. doi: 10.1089/jir.1981.1.179. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Wood J. N. Anticellular and antiviral effects of pppA(2'p5'A)n. Virology. 1980 Feb;101(1):81–90. doi: 10.1016/0042-6822(80)90485-7. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Wood J., Meurs E., Montagnier L. Increased nuclease activity in cells treated with pppA2'p5'A2'p5' A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3261–3265. doi: 10.1073/pnas.76.7.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Lapidot Y., Rapoport S., Panet A., Revel M. Antimitogenic effects of interferon and (2'-5')-oligoadenylate in synchronized 3T3 fibroblasts. FEBS Lett. 1981 Nov 16;134(2):212–216. doi: 10.1016/0014-5793(81)80604-7. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Anti-mitogenic function of interferon-induced (2'-5')oligo(adenylate) and growth-related variations in enzymes that synthesize and degrade this oligonucleotide. Eur J Biochem. 1981;114(1):5–10. doi: 10.1111/j.1432-1033.1981.tb06163.x. [DOI] [PubMed] [Google Scholar]
- Knight M., Cayley P. J., Silverman R. H., Wreschner D. H., Gilbert C. S., Brown R. E., Kerr I. M. Radioimmune, radiobinding and HPLC analysis of 2-5A and related oligonucleotides from intact cells. Nature. 1980 Nov 13;288(5787):189–192. doi: 10.1038/288189a0. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Synthesis of (2'-5')oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J Virol. 1982 Jun;42(3):1039–1045. doi: 10.1128/jvi.42.3.1039-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Herz R. E. Differential antiviral effects of interferon in three murine cell lines. J Virol. 1983 Mar;45(3):1017–1027. doi: 10.1128/jvi.45.3.1017-1027.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R. H., Watling D., Balkwill F. R., Trowsdale J., Kerr I. M. The ppp(A2'p)nA and protein kinase systems in wild-type and interferon-resistant Daudi cells. Eur J Biochem. 1982 Aug;126(2):333–341. doi: 10.1111/j.1432-1033.1982.tb06783.x. [DOI] [PubMed] [Google Scholar]
- Vandenbussche P., Divizia M., Verhaegen-Lewalle M., Fuse A., Kuwata T., De Clercq E., Content J. Enzymatic activities induced by interferon in human fibroblast cell lines differing in their sensitivity to the anticellular activity of interferon. Virology. 1981 May;111(1):11–22. doi: 10.1016/0042-6822(81)90649-8. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Brown R. E., Gilbert C. S., Golgher R. R., Wreschner D. H., Roberts W. K., Silverman R. H., Kerr I. M. Assay of (2'-5')-oligo(A) synthesized in vitro and the analysis of naturally occurring (2'-5')-oligo(A) from intact cells. Methods Enzymol. 1981;79(Pt B):199–208. doi: 10.1016/s0076-6879(81)79030-x. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Kerr I. M., Gilbert C. S., White C. N., Ball L. A. Synthesis and breakdown of pppA2'p5'A2'p5'A and transient inhibiton of protein synthesis in extracts from interferon-treated and control cells. Eur J Biochem. 1978 Dec;92(2):455–462. doi: 10.1111/j.1432-1033.1978.tb12767.x. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., McCauley J. W., Skehel J. J., Kerr I. M. Interferon action--sequence specificity of the ppp(A2'p)nA-dependent ribonuclease. Nature. 1981 Jan 29;289(5796):414–417. doi: 10.1038/289414a0. [DOI] [PubMed] [Google Scholar]




